Novel “Anti-Zeolite” Ba3Sr3B4O12: Eu3+ Phosphors: Crystal Structure, Optical Properties, and Photoluminescence
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
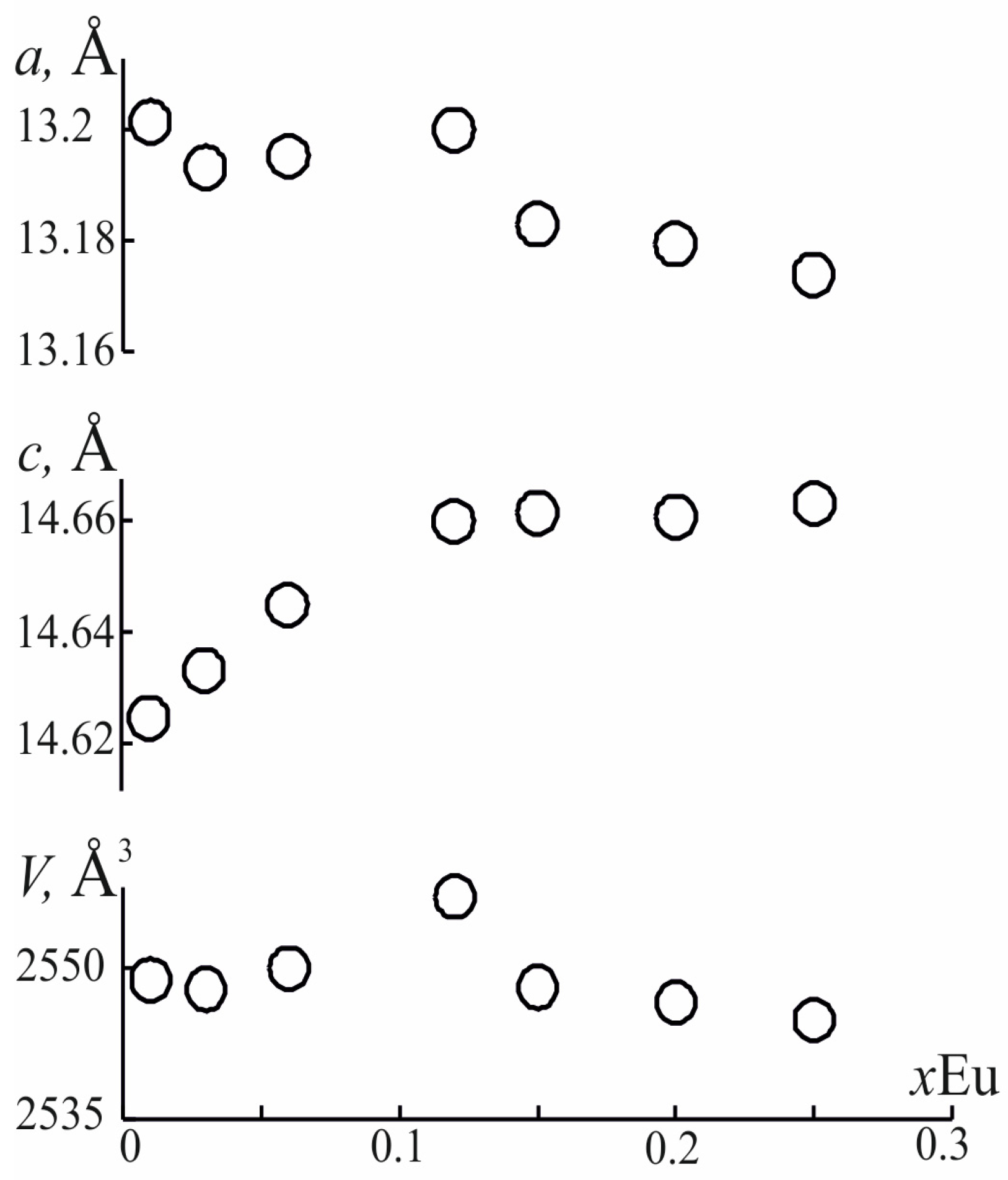
3.1. Powder X-ray Diffraction of the Ba3(Sr3−1.5xEux)B4O12 (x = 0.01, 0.03, 0.06, 0.12, 0.15, 0.20, 0.25) Phosphors
3.2. Thermal Analysis
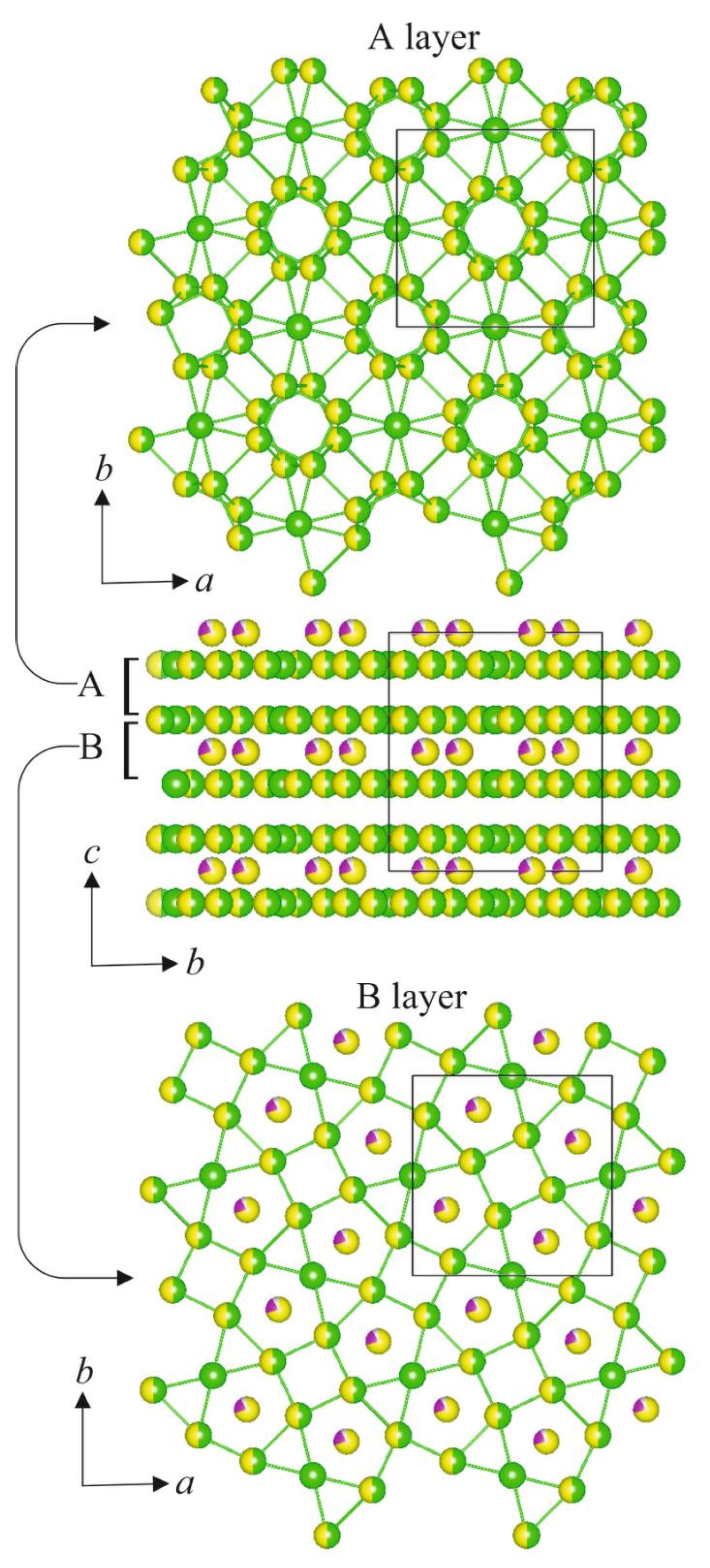
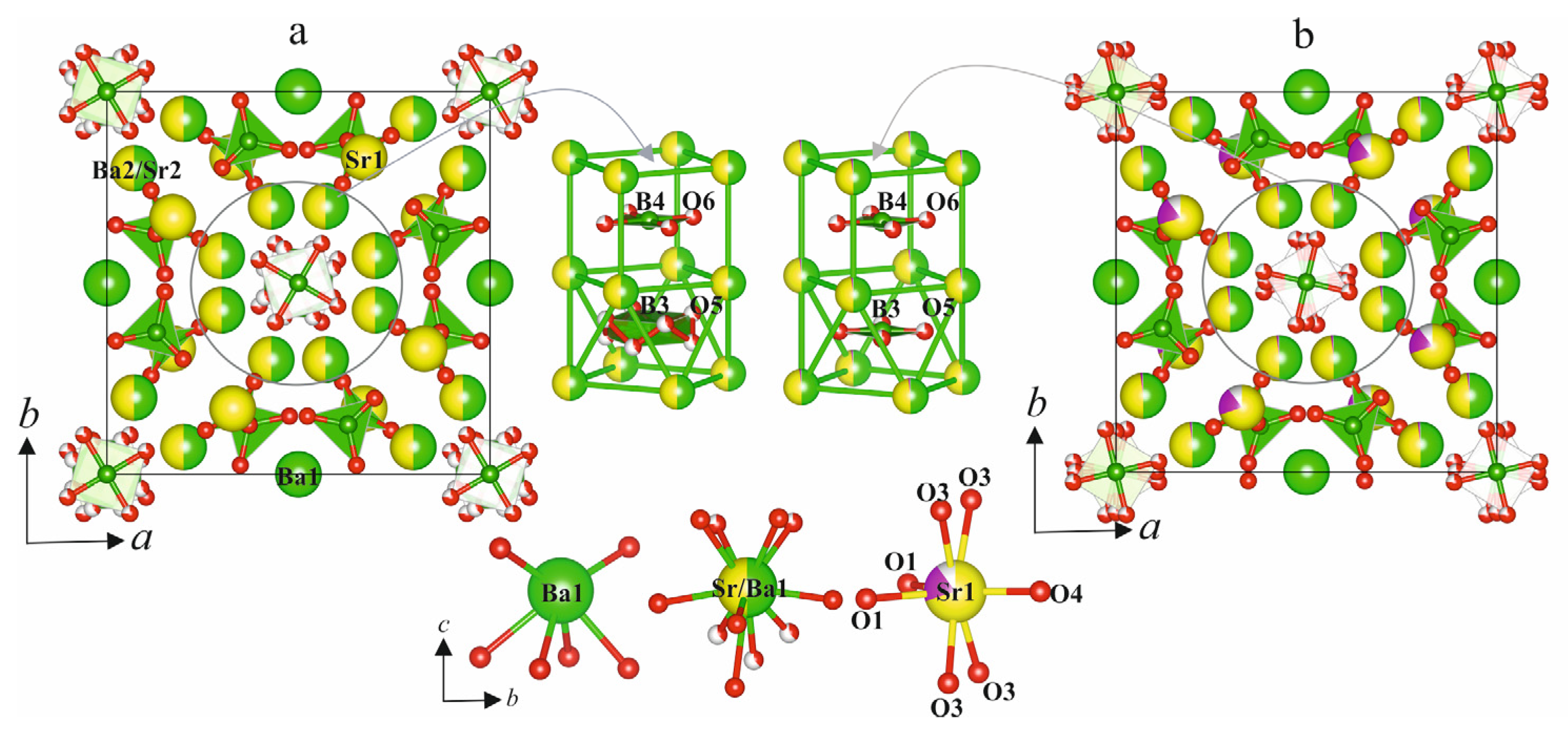
3.3. Crystal Structure Description
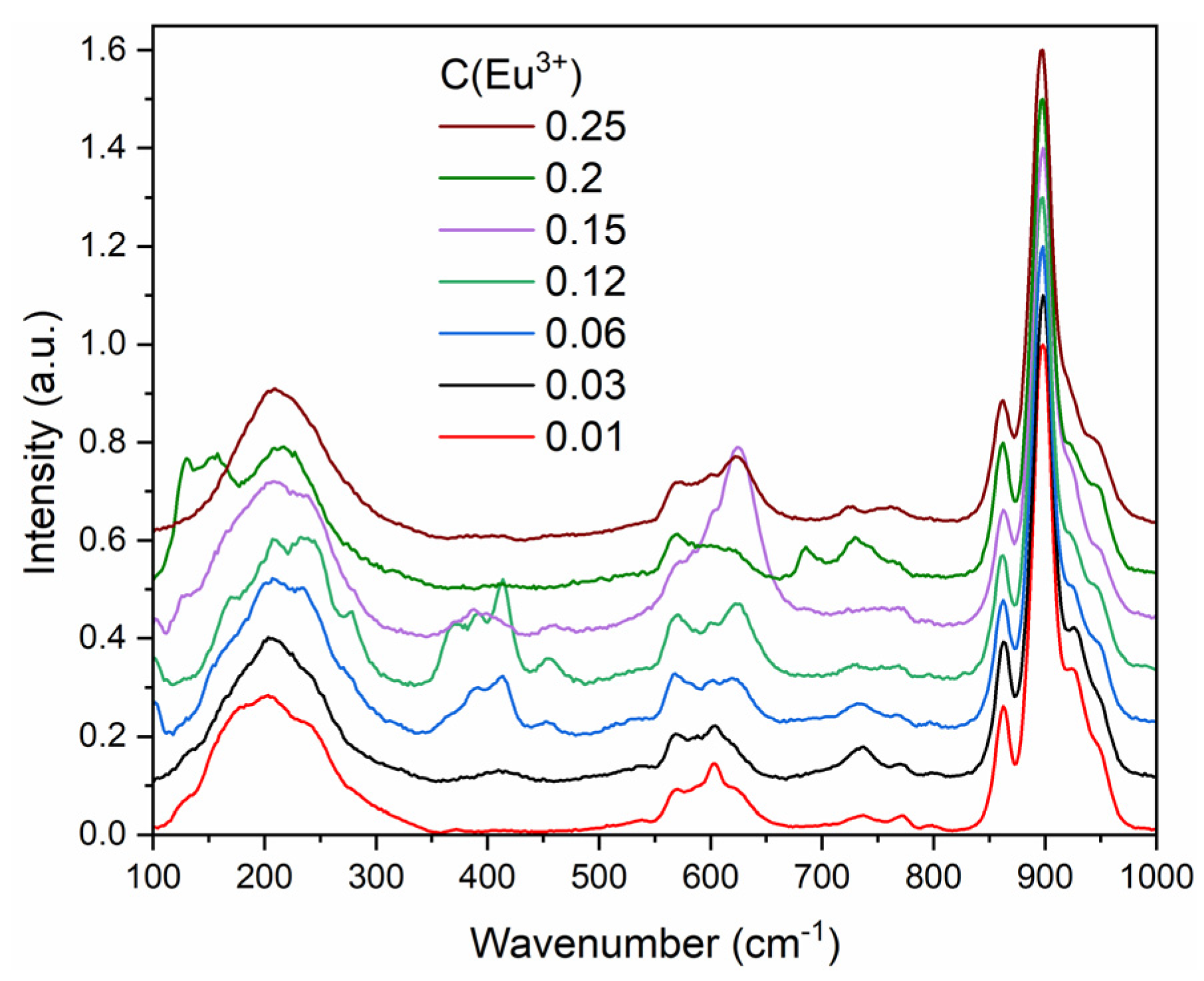
3.4. Raman Spectroscopy
3.5. Absorption Spectroscopy
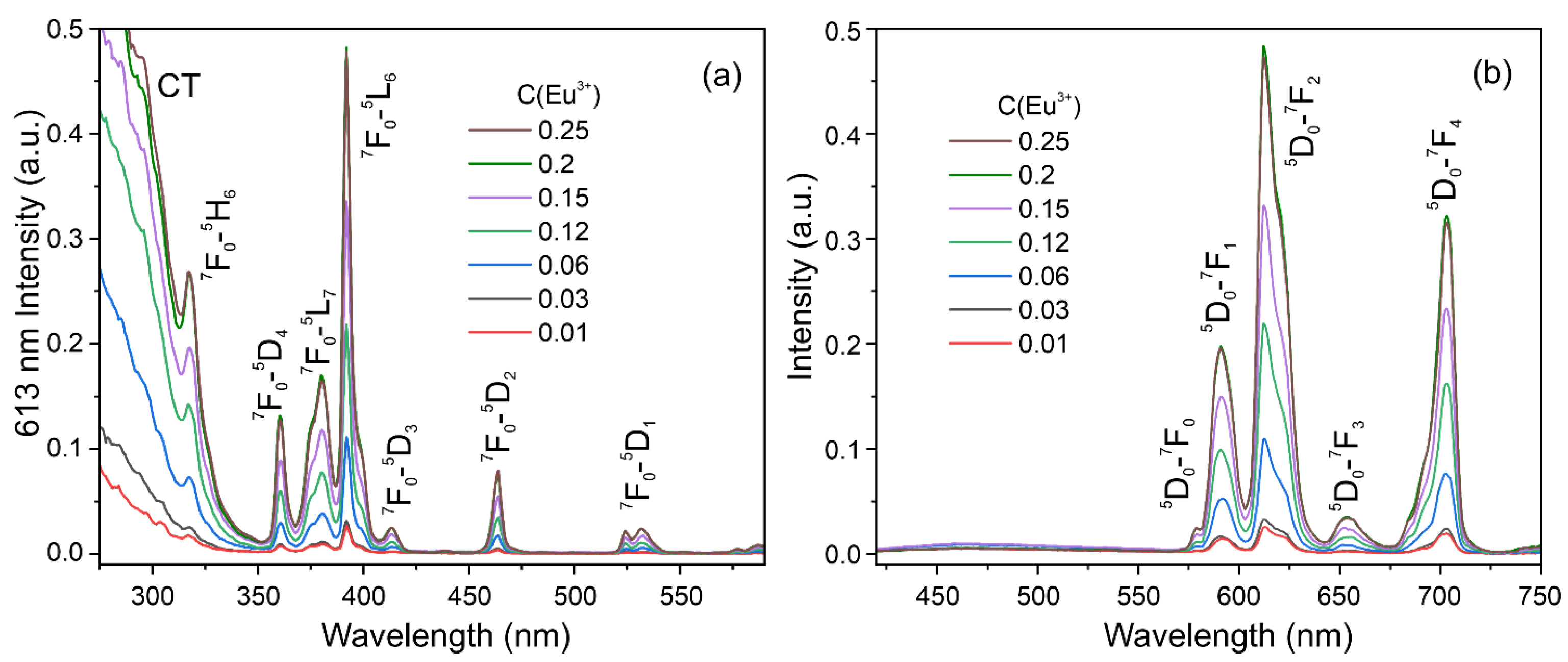
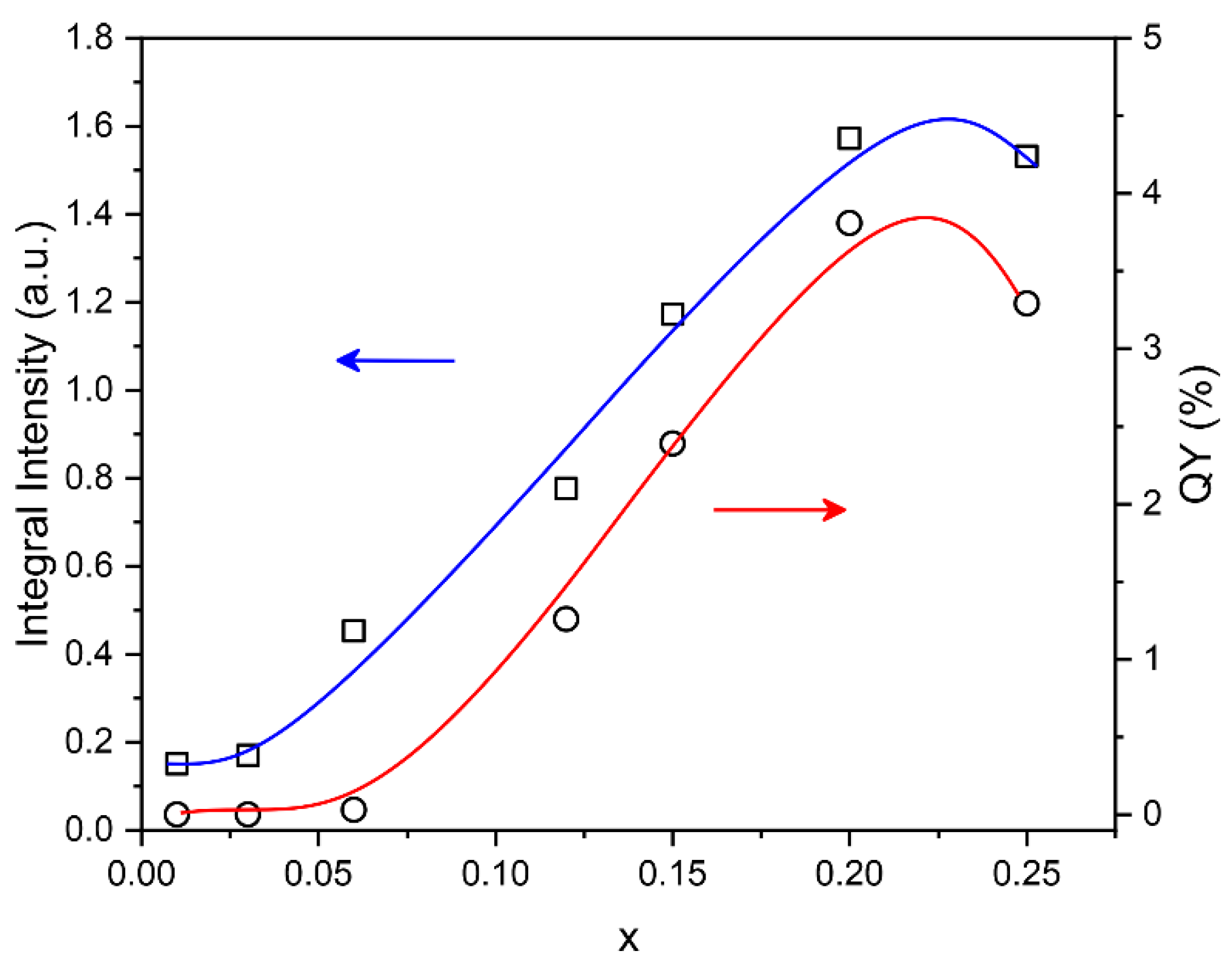
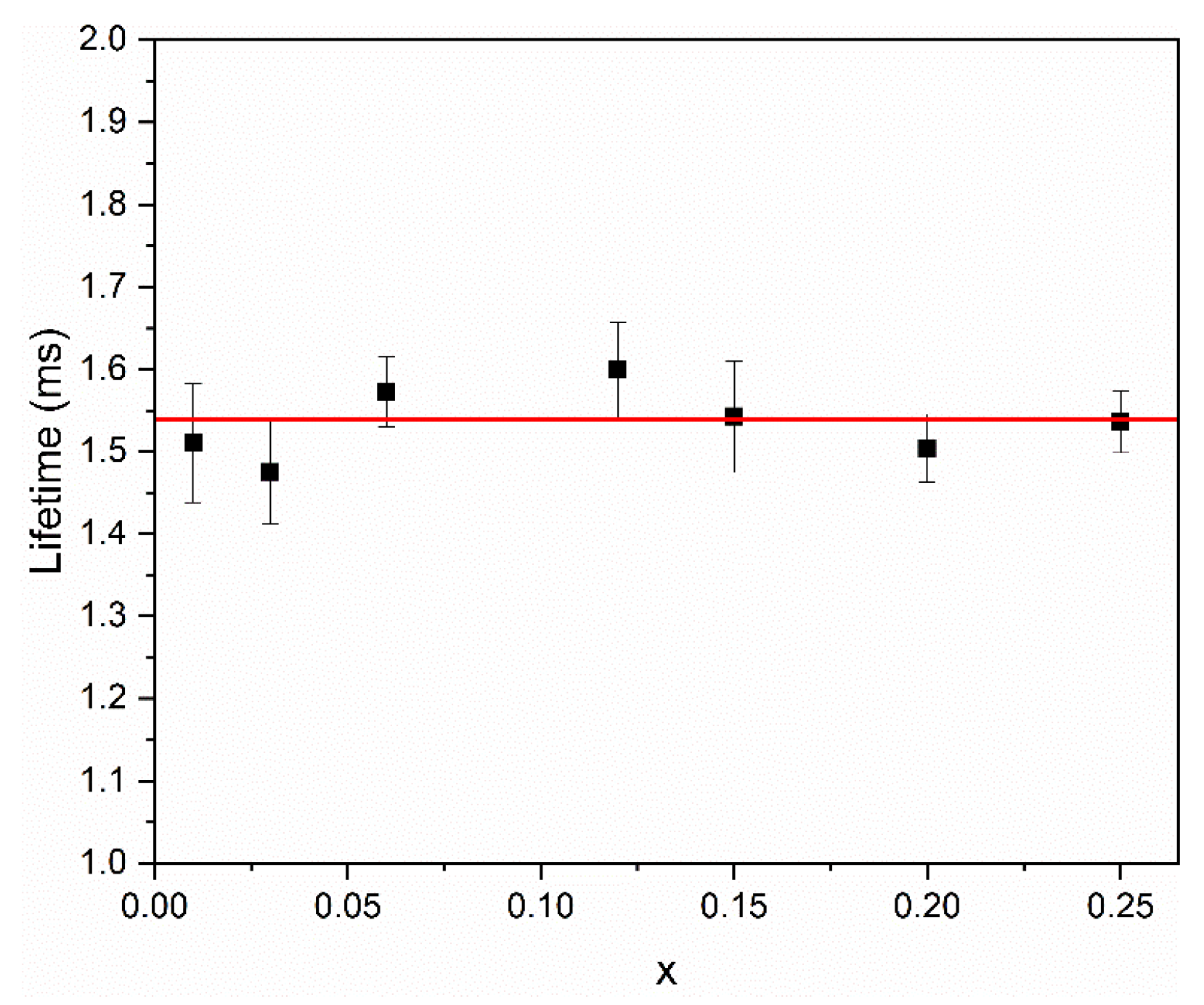
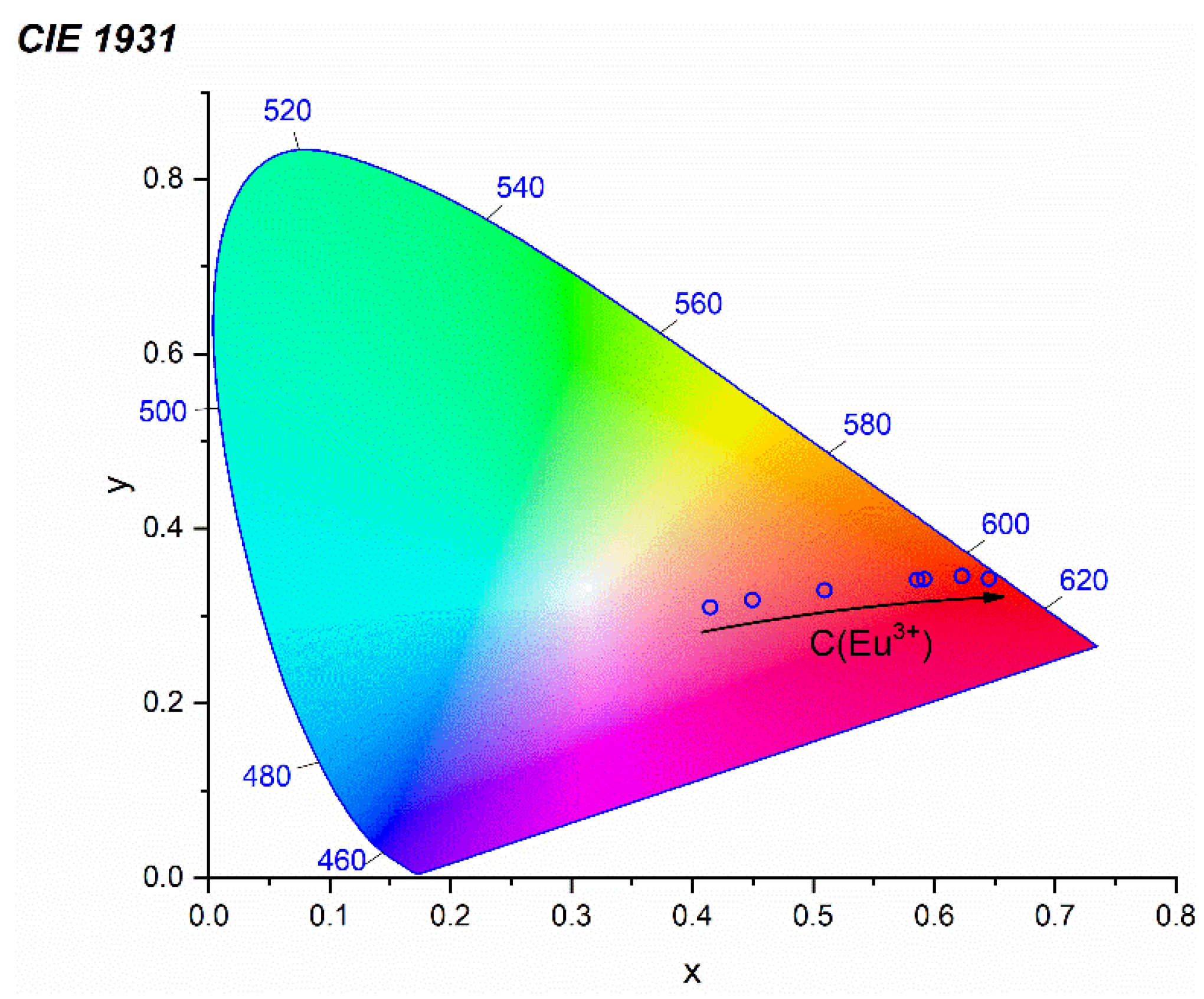
3.6. Photoluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawthorne, F.C.; Burns, P.C.; Grice, J.D. The crystal chemistry of boron. Rev. Miner. 1996, 33, 41–116. [Google Scholar]
- Touboul, M.; Penin, N.; Nowogrocki, G. Borates: A survey of main trends concerning crystal-chemistry, polymorphism and dehydration process of alkaline and pseudo-alkaline borates. Solid State Sci. 2003, 5, 1327–1342. [Google Scholar] [CrossRef]
- Yu, D.; Xue, D. Bond analyses of borates from the Inorganic Crystal Structure Database. Acta Crystallogr. 2006, B62, 702–709. [Google Scholar] [CrossRef]
- Yuan, G.; Xue, D. Crystal chemistry of borates: The classification and algebraic description by topological type of fundamental building blocks. Acta Crystallogr. 2007, B63, 353–362. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Filatov, S.K. High-temperature borate crystal chemistry. Z. Kristallogr. 2013, 228, 395–428. [Google Scholar] [CrossRef]
- Huang, C.; Mutailipu, M.; Zhang, F.; Griffith, K.J.; Hu, C.; Yang, Z.; Griffin, J.M.; Poeppelmeier, K.R.; Pan, S. Expanding the chemistry of borates with functional [BO2]− anions. Nat. Commun. 2021, 12, 2597. [Google Scholar] [CrossRef] [PubMed]
- Becker, P. Borate materials in nonlinear optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Mutailipu, M.; Poeppelmeier, K.R.; Pan, S.L. Borates: A rich source for optical materials. Chem. Rev. 2021, 121, 1130–1202. [Google Scholar] [CrossRef]
- Guo, J.R.; Huang, X.; Chen, Q.; Luo, L.; Luo, Y.; Xiong, Y.; Zhang, S. Pechini sol-gel synthesis of La2CaB8O16: Eu3+ red phosphor and its photoluminescence spectral properties. J. Lumin. 2019, 206, 15–20. [Google Scholar] [CrossRef]
- Hermus, M.; Phan, P.C.; Duke, A.C.; Brgoch, J. Tunable optical properties and increased thermal quenching in the blue-emitting phosphor series: Ba2(Y1–x Lux)5B5O17: Ce3+ (x = 0–1). Chem. Mater. 2017, 29, 5267–5275. [Google Scholar] [CrossRef]
- Yang, L.; Wan, Y.; Huang, Y.; Chen, C.; Seo, H.J. Development of YK3B6O12: RE (RE = Eu3+, Tb3+, Ce3+) tricolor phosphors under near-UV light excitation. J. Alloy. Comp. 2016, 684, 40–46. [Google Scholar] [CrossRef]
- Cao, M.; Tian, J.H.; Zhuang, W.D.; Liu, R.H.; Liu, Y.H.; Chen, G.T.; Zhou, G.G.; Wang, L.M.; Wang, J.W. Multisite Cation Regulation of Broadband Cyan-Emitting (Ba1–xSrx)9Lu2Si6O24:Eu2+ Phosphors for Full-Spectrum wLEDs. Inorg. Chem. 2022, 61, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, Z.Y.; Yun, X.H.; Yang, H.; Liu, Y.X.; Li, G.G. Abnormal Bi3+-activated NIR emission in highly symmetric XAl12O19 (X = Ba, Sr, Ca) by selective sites occupation. Chem. Mater. 2020, 32, 8747–8753. [Google Scholar] [CrossRef]
- Du, F.; Zhuang, W.D.; Liu, R.H.; Zhong, J.Y.; Liu, Y.H.; Hu, Y.S.; Gao, W.; Zhang, X.; Chen, L.; Lin, K. Site occupancy and photoluminescence tuning of La3Si6-xAlxN11-x/3: Ce3+ phosphors for high power white light-emitting diodes. CrystEngComm 2017, 19, 2836–2843. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Povolotskiy, A.V.; Biryukov, Y.P.; Kolesnikov, I.E.; Volkov, S.N.; Filatov, S.K. Cation sites occupation and luminescence of novel red-emitting phosphors Ba6(Lu1–xEux)5B9O27 (x = 0.02–0.2). Ceram. Int. 2022, 48, 15966–15974. [Google Scholar] [CrossRef]
- Tian, J.; Xie, J.; Zhuang, W. Recent Advances in Multi-Site Luminescent Materials: Design, Identification and Regulation. Materials 2023, 16, 2179. [Google Scholar] [CrossRef]
- Shablinskii, A.P.; Kolesnikov, I.E.; Bubnova, R.S.; Povolotskiy, A.V.; Lähderantac, E.; Filatov, S.K. A novel thermally stable Ba3Bi2(BO3)4:Eu3+ red phosphor for solid state lighting application. J. Lumin. 2019, 216, 116714. [Google Scholar] [CrossRef]
- Shablinskii, A.P.; Povolotskiy, A.V.; Kolesnikov, I.E.; Biryukov, Y.P.; Bubnova, R.S.; Avdontceva, M.S.; Demina, S.V.; Filatov, S.K. Novel red-emitting color-tunable phosphors BaBi2−xEuxB2O7 (x = 0–0.40): Study of the crystal structure and luminescence. J. Solid State Chem. 2022, 307, 122837. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Bubnova, R.S.; Povolotskiy, A.V.; Biryukov, Y.P.; Povolotckaia, A.V.; Shorets, O.Y.; Filatov, S.K. Europium-activated phosphor Ba3Lu2B6O15: Influence of isomorphic substitution on photoluminescence properties. Ceram. Int. 2021, 47, 8030–8034. [Google Scholar] [CrossRef]
- Wang, G.F.; Huang, Q.Z.; Liang, J.K. Studies on phase equilibrium relation in binary section BaB2O4-SrB2O4 and BaB2O4-SrO. Acta Chim. Sin. 1984, 42, 503–508. [Google Scholar]
- Huang, Q.; Huang, L.; Dai, G.; Liang, J. Structure of SrxBa3–x(B3O6)2 in a Solid Solution. Acta Crystallogr. 1992, C48, 539–541. [Google Scholar] [CrossRef]
- Sun, T.Q.; Pan, F.; Wang, X.Q.; Shen, G.Q.; Shen, D.Z. Growth, structure and properties of barium strontium borate (Ba0.87Sr3.13B14O25) crystal. Rengong Jingti Xuebao 2004, 33, 935–939. [Google Scholar]
- Volkov, S.N.; Bubnova, R.S.; Povolotskiy, A.V.; Ugolkov, V.L.; Arsent’ev, M.Y. Two novel centrosymmetric barium strontium borates with a deep-UV cut-off edge: Ba2Sr3B4O11 and Ba3Sr3B4O12. J. Solid State Chem. 2020, 281, 121023. [Google Scholar] [CrossRef]
- Furmanova, N.G.; Maksimov, B.A.; Molchanov, V.N.; Kokh, A.E.; Kononova, N.G.; Fedorov, P.P. Crystal structure of the novel barium borate Ba5(BO3)2(B2O5). Crystallogr. Rep. 2006, 51, 219–224. [Google Scholar] [CrossRef]
- Bekker, T.B.; Rashchenko, S.V.; Seryotkin, Y.V.; Kokh, A.E.; Davydov, A.V.; Fedorov, P.P. BaO-B2O3 system and its mysterious member Ba3B2O6. J. Am. Ceram. 2018, 101, 450–457. [Google Scholar] [CrossRef]
- Rashchenko, S.V.; Bekker, T.B.; Bakakin, V.V.; Seryotkin, Y.V.; Simonova, E.A.; Goryainov, S.V. New fluoride borate with ‘anti-zeolite’ structure: A possible link to Ba3(BO3)2. J. Alloys Compd. 2017, 694, 1196–1200. [Google Scholar] [CrossRef]
- Solntsev, V.P.; Bekker, T.B.; Davydov, A.V.; Yelisseyev, A.P.; Rashchenko, S.V.; Kokh, A.E.; Grigorieva, V.D.; Park, S.-H. Optical and Magnetic Properties of Cu-Containing Borates with “Antizeolite” Structure. J. Phys. Chem. C 2019, 123, 4469–4474. [Google Scholar] [CrossRef]
- Bekker, T.B.; Rashchenko, S.V.; Solntsev, V.P.; Yelisseyev, A.P.; Kragzhda, A.A.; Bakakin, V.V.; Seryotkin, Y.V.; Kokh, A.E.; Kokh, K.A.; Kuznetsov, A.B. Growth and Optical Properties of LixNa1–xBa12(BO3)7F4 Fluoride Borates with “Antizeolite” Structure. Inorg. Chem. 2017, 56, 5411–5419. [Google Scholar] [CrossRef]
- Bekker, T.B.; Solntsev, V.P.; Rashchenko, S.V.; Yelisseyev, A.P.; Davydov, A.V.; Kragzhda, A.A.; Kokh, A.E.; Kuznetsov, A.B.; Park, S. Nature of the Color of Borates with “Anti-Zeolite” Structure. Inorg. Chem. 2018, 57, 2744–2751. [Google Scholar] [CrossRef]
- Simonova, E.A.; Kuznetsov, A.B.; Svetlichnyi, V.A.; Kononova, N.G.; Shevchenko, V.S.; Nigmatulina, E.N.; Kolesnichenko, M.V.; Kokh, K.A.; Rashchenko, S.V.; Kokh, A.E. Nd3+ and Pr3+ doped anti-zeolite matrix-LiBa12(BO3)7F4: Crystal structures, luminescence properties. Mater. Chem. Phys. 2020, 247, 122612. [Google Scholar] [CrossRef]
- Petříček, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Bakakin, V.V. On a dual function of anions in crystallogenesis of compounds: Structure-directing and stabilizing. J. Struct. Chem. 2017, 58, 947–952. [Google Scholar] [CrossRef]
- Rashchenko, S.V.; Bekker, T.B. Crystal chemistry of novel “antizeolite” structures. J. Struct. Chem. 2021, 62, 1935–1945. [Google Scholar] [CrossRef]
- Ilyukhin, A.B.; Dzhurinskii, B.F. Crystal structures of Ln14(GeO4)2(BO3)6O8 (Ln = Nd, Sm) and Tb(3+)54Tb(4+)(GeO4)12O59. Zhurnal Neorganicheskoi Khimii 1994, 39, 556–563. (In Russian) [Google Scholar]
- Ma, R.R.; Yang, Y.; Hu, C.; Yang, Z.H.; Pan, S.L. The First Examples of Lithium Containing Mixed-Alkali Strontium Borates with Different Dimensional Anionic Architectures and Short Cutoff Edges. Chem. Eur. J. 2018, 24, 15355. [Google Scholar] [CrossRef] [PubMed]
- Filatov, S.K.; Biryukov, Y.P.; Bubnova, R.S.; Shablinskii, A.P. The novel borate Lu5Ba6B9O27 with a new structure type: Synthesis, disordered crystal structure and negative linear thermal expansion. Acta Cryst. 2019, B75, 697–703. [Google Scholar]
- Song, J.L.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. A facile synthetic route to a new SHG material with two types of parallel π-conjugated planar triangular units. Angew. Chem. Int. Ed. 2015, 54, 3679. [Google Scholar] [CrossRef]
- Niu, X.; Wang, L. Synthesis, characterization and crystal structure of a new mixed alkali and alkaline-earth metal borate Rb9Ba24(BO3)19. New J. Chem. 2020, 44, 3882–3887. [Google Scholar] [CrossRef]
- Biryukov, Y.P.; Bubnova, R.S.; Krzhizhanovskaya, M.G.; Filatov, S.K.; Povolotskiy, A.V.; Ugolkov, V.L. Thermal behavior of polymorphic modifications of LuBO3. Solid State Sci. 2020, 99, 106061. [Google Scholar] [CrossRef]



| Eux | 0.03 | 0.06 | 0.15 | 0.20 | 0.25 |
|---|---|---|---|---|---|
| Mr | 910.7 | 911.3 | 913.2 | 914.2 | 915.4 |
| Crystal system, space group | Tetragonal, I4/mcm | ||||
| Temperature (°C) | 25 | ||||
| a, Å | 13.163 (3) | 13.154 (3) | 13.167 (3) | 13.151 (3) | 13.132 (3) |
| c, Å | 14.608 (4) | 14.612 (4) | 14.634 (4) | 14.653 (4) | 14.633 (4) |
| V, Å3 | 2531.1 (11) | 2528.3 (11) | 2537.0 (11) | 2534.5 (11) | 2523.5 (11) |
| Z | 8 | ||||
| Radiation type | Mo Kα | ||||
| µ (mm−1) | 21.76 | 21.74 | 21.54 | 21.49 | 21.52 |
| Crystal size (mm) | 0.08 × 0.13 × 0.15 | 0.07 × 0.12 × 0.13 | 0.10 × 0.12 × 0.14 | 0.10 × 0.09 × 0.15 | 0.11 × 0.13 × 0.17 |
| Diffractometer | Rigaku XtaLab Synergy-S | ||||
| No. of measured, independent, and observed [I > 3σ(I)] reflections | 24,289, 786, 533 | 26,613, 807, 512 | 27,486, 808, 615 | 24,411, 633, 451 | 9670, 776, 270 |
| Rint | 0.145 | 0.116 | 0.112 | 0.174 | 0.148 |
| (sin θ/λ)max (Å−1) | 0.781 | 0.777 | 0.776 | 0.777 | 0.777 |
| R (obs), wR(obs), S | 0.070, 0.070, 2.20 | 0.051, 0.059, 1.83 | 0.051, 0.069, 2.89 | 0.058, 0.069, 2.42 | 0.068, 0.093, 1.02 |
| No. of reflections | 787 | 807 | 808 | 633 | 776 |
| No. of parameters | 64 | 65 | 64 | 64 | 56 |
| No. of restraints | 3 | 2 | 2 | 2 | 4 |
| x | 0.03 | 0.06 | 0.15 | 0.2 | 0.25 |
|---|---|---|---|---|---|
| Ba(1)–O(2) × 2 | 2.640 (12) | 2.616 (9) | 2.645 (9) | 2.671 (13) | 2.665 (15) |
| Ba(1)–O(1) × 4 | 2.791 (10) | 2.814 (7) | 2.814 (7) | 2.816 (10) | 2.822 (15) |
| <Ba(1)–O>6 | 2.74 | 2.75 | 2.76 | 2.77 | 2.77 |
| Ba(1)–O(2) | 3.271 (14) × 2 | 3.266 (11) × 2 | 3.295 (10) × 2 | 3.270 (15) × 2 | 3.277 (18) × 2 |
| <Ba(1)–O>8 | 2.87 | 2.88 | 2.89 | 2.89 | 2.90 |
| Sr(1)–O(4) | 2.435 (10) | 2.428 (10) | 2.460 (11) | 2.484 (16) | 2.511 (18) |
| Sr(1)–O(1) × 2 | 2.595 (15) | 2.586 (10) | 2.588 (11) | 2.586 (15) | 2.541 (16) |
| Sr(1)–O(3) × 4 | 2.686 (14) | 2.655 (10) | 2.641 (10) | 2.660 (15) | 2.669 (16) |
| <Sr(1)–O>7 | 2.62 | 2.60 | 2.60 | 2.61 | 2.61 |
| Sr/Ba(1)–O(5) | 2.42 (5) | 2.33 (4) | 2.45 (3) | 2.42 (5) | 2.297 (11) |
| Sr/Ba(1)–O(5) | 2.49 (4) | 2.56 (4) | 2.51 (4) | 2.60 (6) | 3.167 (4) |
| Sr/Ba(1)–O(5) | 2.57 (5) | 2.57 (3) | 2.50 (4) | 2.48 (6) | - |
| Sr/Ba(1)–O(3) | 2.616 (13) | 2.604 (9) | 2.608 (10) | 2.593 (14) | 2.627 (16) |
| Sr/Ba(1)–O(2) | 2.641 (12) | 2.641 (9) | 2.628 (9) | 2.635 (13) | 2.631 (15) |
| Sr/Ba(1)–O(3) | 2.710 (15) | 2.740 (11) | 2.713 (12) | 2.738 (16) | 2.690 (16) |
| Sr/Ba(1)–O(6) | 2.84 (2) | 2.908 (18) | 2.88 (2) | 2.88 (3) | 2.62 (4) |
| Sr/Ba(1)–O(1) | 2.854 (10) | 2.828 (7) | 2.829 (7) | 2.826 (10) | 2.821 (15) |
| Sr/Ba(1)–O(6) | 2.86 (3) | 2.771 (17) | 2.81 (2) | 2.83 (3) | 2.62 (4) |
| Sr/Ba(1)–O(4) | 2.901 (10) | 2.897 (8) | 2.897 (8) | 2.889 (11) | 2.892 (14) |
| Sr/Ba(1)–O(3) | 3.083 (14) | 3.117 (11) | 3.142 (11) | 3.133 (15) | 3.115 (17) |
| <Sr/Ba(1)–O>11 | 2.73 | 2.72 | 2.72 | 2.73 | 2.75 * |
| B(1)–O(2) | 1.36 (3) | 1.37 (2) | 1.363 (18) | 1.30 (4) | 1.37 (4) |
| B(1)–O(3) × 2 | 1.37 (3) | 1.37 (2) | 1.394 (17) | 1.39 (3) | 1.35 (4) |
| <B(1)–O>3 | 1.36 | 1.37 | 1.383 | 1.36 | 1.36 |
| B(2)–O(1) × 2 | 1.39 (2) | 1.36 (4) | 1.36 (2) | 1.33 (3) | 1.36 (3) |
| B(2)–O(4) | 1.30 (2) | 1.38 (5) | 1.35 (2) | 1.38 (3) | 1.36 (3) |
| <B(2)–O>3 | 1.36 | 1.37 | 1.36 | 1.35 | 1.36 |
| B(4)–O(6) × 4 | 1.40 (3) | 1.45 (2) | 1.42 (2) | 1.41 (3) | 1.51 (5) |
| <B(4)–O>4 | 1.40 | 1.45 | 1.42 | 1.41 | 1.51 |
| B(3)–O(5) | 1.37 (3) × 8 | 1.41 (3) × 8 | 1.41 (3) × 8 | 1.39 (4) × 8 | 1.40 (2) × 4 |
| <B(3)–O>8 | 1.37 | 1.41 | 1.41 | 1.39 | 1.40 ** |
| C (Eu3+) | x | y |
|---|---|---|
| 0.01 | 0.449 | 0.319 |
| 0.03 | 0.415 | 0.310 |
| 0.06 | 0.509 | 0.330 |
| 0.12 | 0.591 | 0.343 |
| 0.15 | 0.586 | 0.342 |
| 0.2 | 0.623 | 0.346 |
| 0.25 | 0.623 | 0.346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubnova, R.S.; Shablinskii, A.P.; Povolotskiy, A.V.; Shorets, O.Y.; Ugolkov, V.L.; Volkov, S.N.; Yukhno, V.A.; Filatov, S.K. Novel “Anti-Zeolite” Ba3Sr3B4O12: Eu3+ Phosphors: Crystal Structure, Optical Properties, and Photoluminescence. Symmetry 2023, 15, 1399. https://doi.org/10.3390/sym15071399
Bubnova RS, Shablinskii AP, Povolotskiy AV, Shorets OY, Ugolkov VL, Volkov SN, Yukhno VA, Filatov SK. Novel “Anti-Zeolite” Ba3Sr3B4O12: Eu3+ Phosphors: Crystal Structure, Optical Properties, and Photoluminescence. Symmetry. 2023; 15(7):1399. https://doi.org/10.3390/sym15071399
Chicago/Turabian StyleBubnova, Rimma S., Andrey P. Shablinskii, Alexey V. Povolotskiy, Olga Yu. Shorets, Valery L. Ugolkov, Sergey N. Volkov, Valentina A. Yukhno, and Stanislav K. Filatov. 2023. "Novel “Anti-Zeolite” Ba3Sr3B4O12: Eu3+ Phosphors: Crystal Structure, Optical Properties, and Photoluminescence" Symmetry 15, no. 7: 1399. https://doi.org/10.3390/sym15071399





