Synthesis, Crystal Structure, Local Structure, and Magnetic Properties of Polycrystalline and Single-Crystalline Ce2Pt6Al15
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
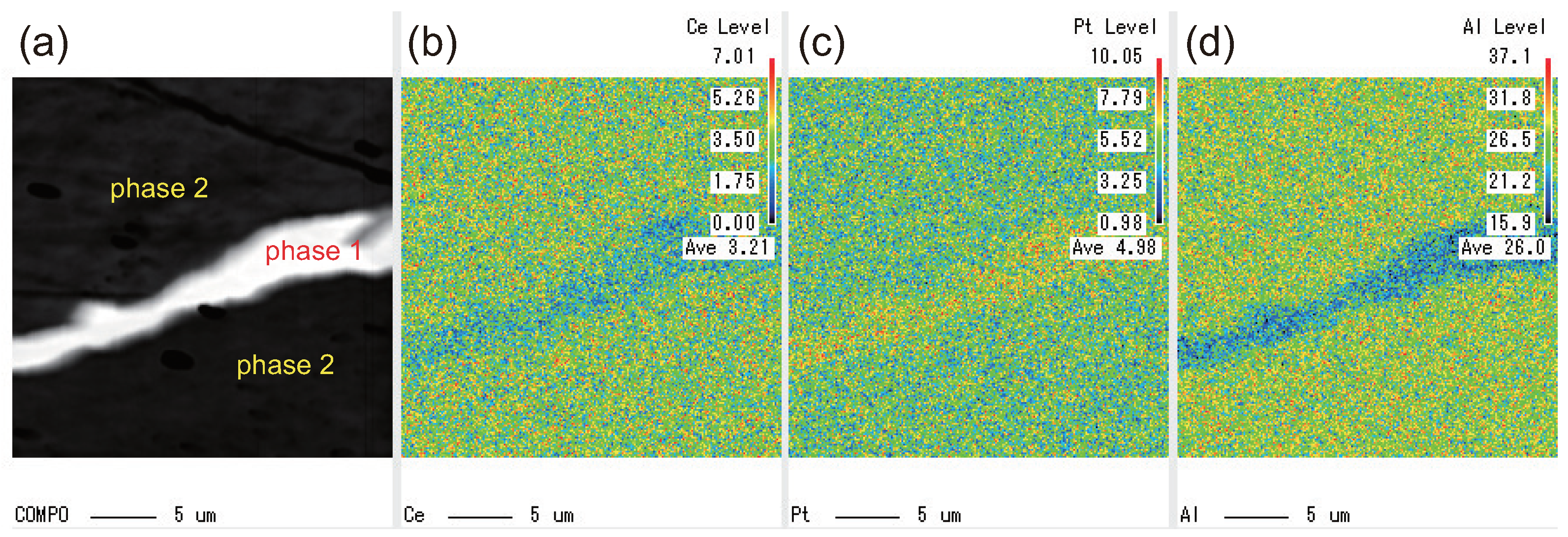
3.1. EPMA
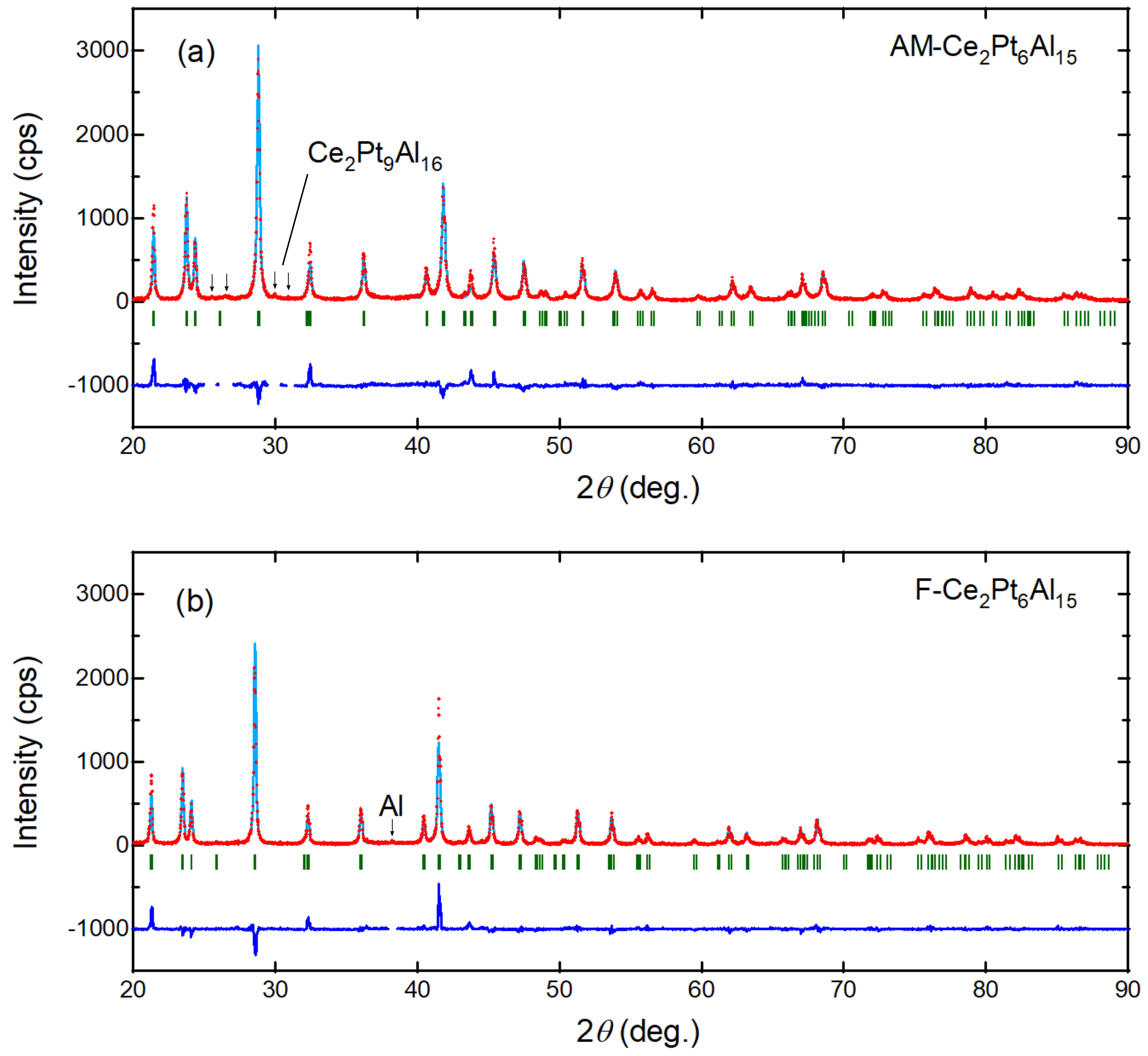
3.2. Powder X-ray Diffraction
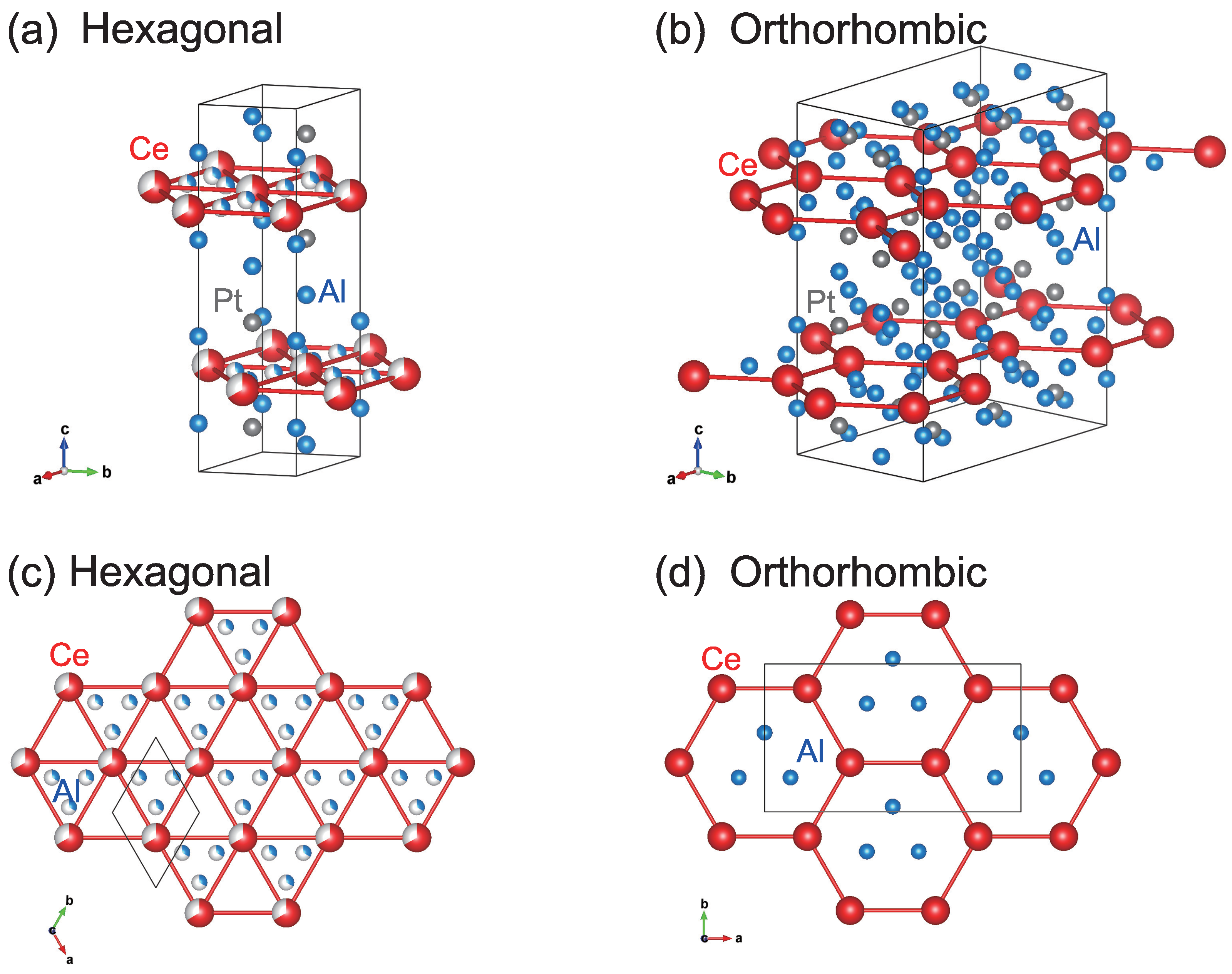
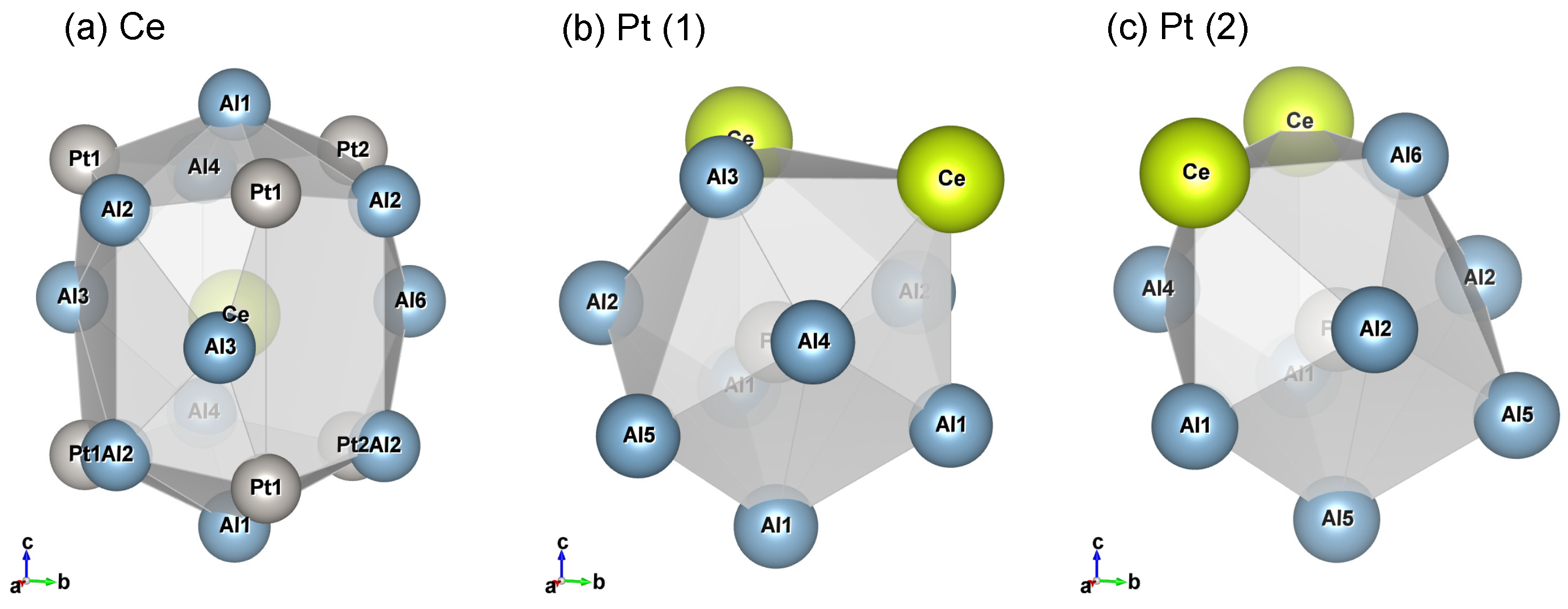
3.3. Single Crystal X-ray Diffraction
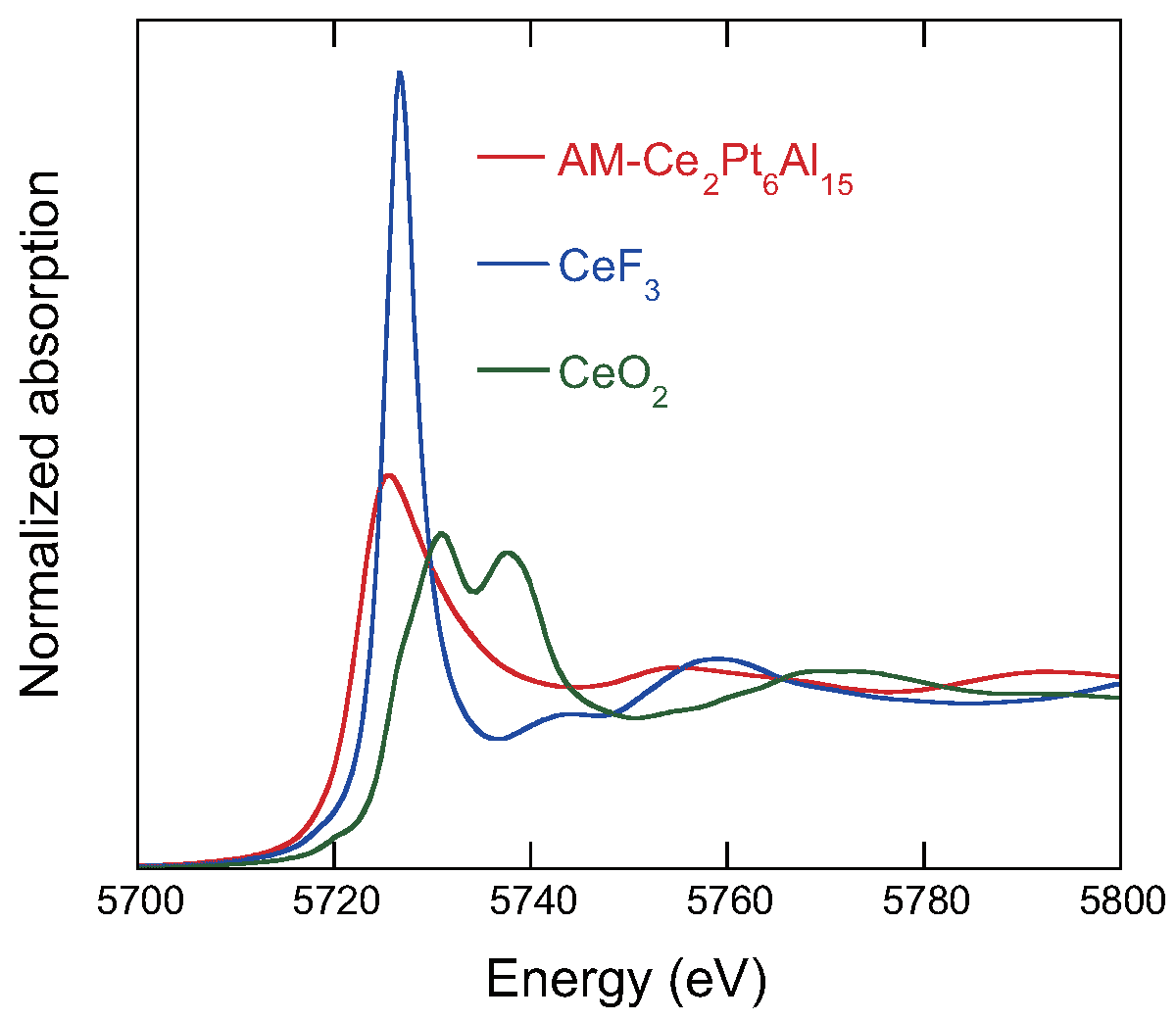
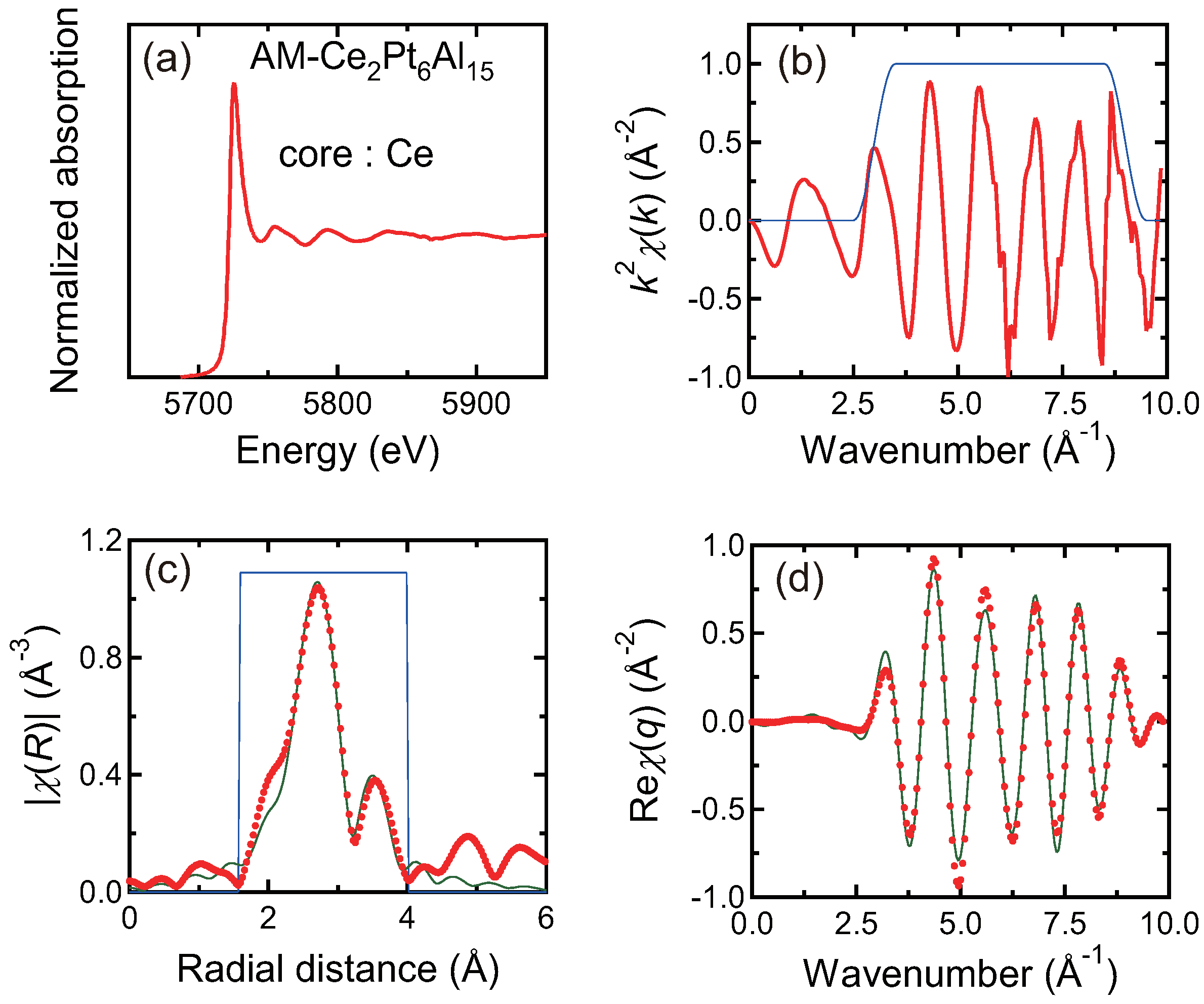
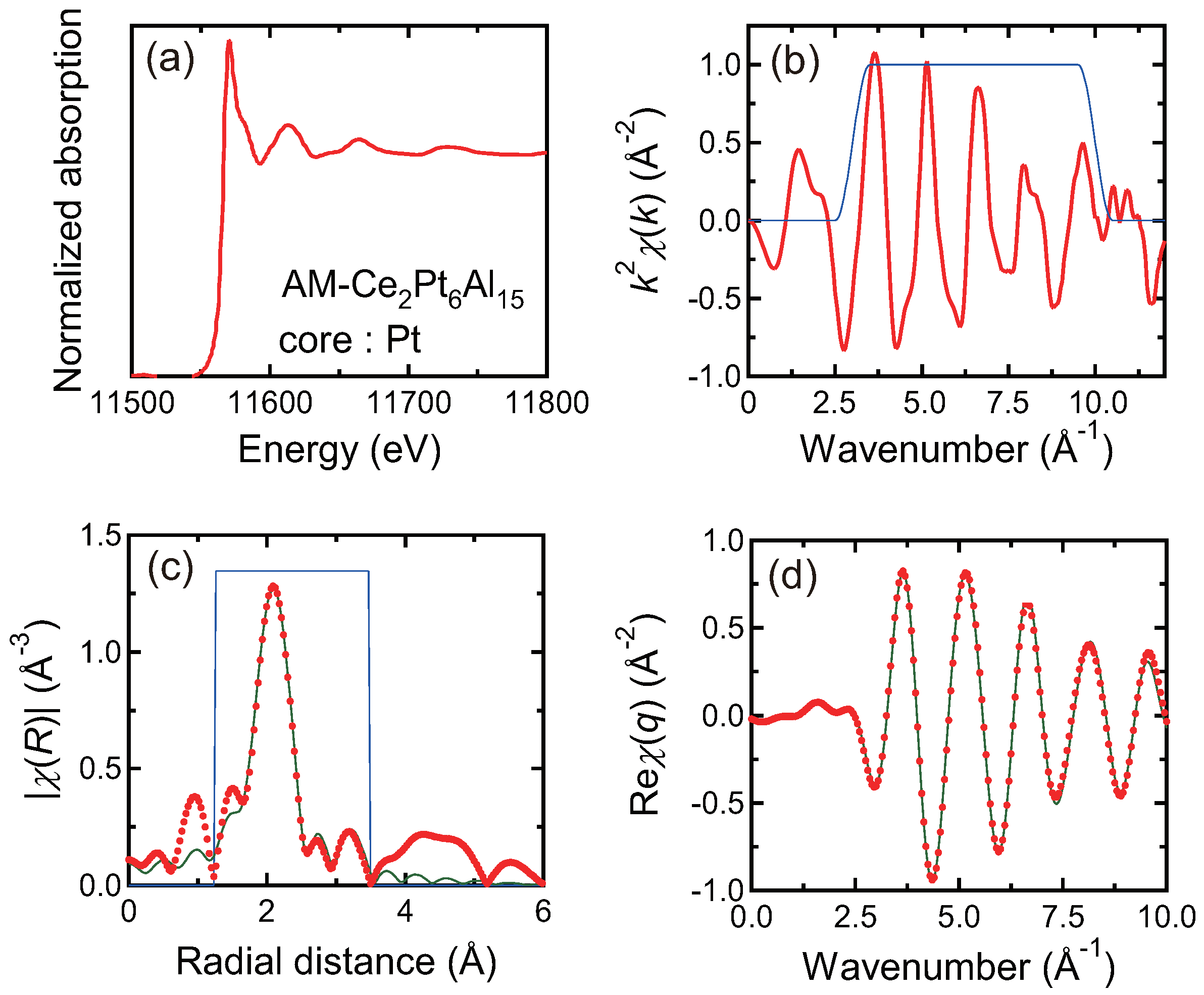
3.4. X-ray Absorption Spectroscopy
3.5. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPMA | electron micro-probe analysis |
| XAS | X-ray absorption spectroscopy |
| XAFS | X-ray absorption fine structure |
| EXAFS | extended X-ray absorption fine structure |
| NFL | non Fermi liquid |
| BSE | backscattered electron |
| FT | Fourier transform |
| AM-CePtAl | CePtAl prepared by arc melting method |
| F-CePtAl | CePtAl prepared by Al-flux method |
References
- Bud’ko, S.L.; Morosan, E.; Canfield, P.C. Magnetic field induced non-Fermi-liquid behavior in YbAgGe single crystals. Phys. Rev. B 2004, 69, 014415. [Google Scholar] [CrossRef] [Green Version]
- Gegenwart, P.; Custers, J.; Geibel, C.; Neumaier, K.; Tayama, T.; Tenya, K.; Trovarelli, O.; Steglich, F. Magnetic-Field Induced Quantum Critical Point in YbRh2Si2. Phys. Rev. Lett. 2002, 89, 056402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Custers, J.; Gegenwart, P.; Wilhelm, H.; Neumaier, K.; Tokiwa, Y.; Trovarelli, O.; Geibel, C.; Steglich, F.; Pépin, C.; Coleman, P. The break-up of heavy electrons at a quantum critical point. Nature 2003, 424, 524–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, Q.; Rabello, S.; Ingersent, K.; Smith, J.L. Locally critical quantum phase transitions in strongly correlated metals. Nature 2001, 413, 804–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dönni, A.; Ehlers, G.; Maletta, H.; Fischer, P.; Kitazawa, H.; Zolliker, M. Geometrically frustrated magnetic structures of the heavy-fermion compound CePdAl studied by powder neutron diffraction. J. Phys. Condens. Matter 1996, 8, 11213–11230. [Google Scholar] [CrossRef]
- Kimura, N.; Ito, K.; Saitoh, K.; Umeda, Y.; Aoki, H.; Terashima, T. Pressure-induced superconductivity in noncentrosymmetric heavy-fermion CeRhSi3. Phys. Rev. Lett. 2005, 95, 247004. [Google Scholar] [CrossRef]
- Kasahara, Y.; Sugii, K.; Ohnishi, T.; Shimozawa, M.; Yamashita, M.; Kurita, N.; Tanaka, H.; Nasu, J.; Motome, Y.; Shibaushi, T.; et al. Unusual thermal Hall effect in a Kitaev spin liquid candidate α-RuCl3. Phys. Rev. Lett. 2018, 120, 217205. [Google Scholar] [CrossRef] [Green Version]
- Saito, H.; Uenishi, K.; Miura, N.; Tabata, C.; Hidaka, H.; Yanagisawa, T.; Amitsuka, H. Evidence of a new current-induced magnetoelectric effect in a toroidal magnetic ordered state of Uni4B. J. Phys. Soc. Jpn. 2018, 87, 033702. [Google Scholar] [CrossRef]
- Lutsyshyn, Y.; Tokaychuk, Y.; Gladyshevskii, R. Ternary compounds with Sc0.6Fe2Si4.9-type structure in the systems Sm–{Pd, Pt}–Al. Chem. Met. Alloys 2012, 5, 98–102. [Google Scholar] [CrossRef]
- Lutsyshyn, Y.; Tokaychuk, Y.; Gladyshevskii, R. Crystal structure of the compound Tb0.67Pt2Al5. Chem. Met. Alloys 2009, 2, 75–82. [Google Scholar] [CrossRef]
- Rousuli, A.; Nakamura, S.; Sato, H.; Ueda, T.; Matsumoto, Y.; Ohara, S.; Schwier, E.F.; Nagasaki, T.; Mimura, K.; Anzai, H.; et al. Photoemission study of the electronic structure of the Kondo lattices Yb2Pt6X15 (X = Al, Ga). Phys. Rev. B 2017, 96, 045117. [Google Scholar] [CrossRef]
- Prots, Y.; Deppe, M.; Cardoso-Gil, R.; Cervellino, A.; Ormeci, A.; Geibel, C.; Grin, Y. Yb2Al15Pt6—The most ordered variety of the Sc1.2Fe4Si9.8 aristotype. Chem. Met. Alloys 2014, 7, 85–99. [Google Scholar] [CrossRef]
- Niermann, J.; Jeitschko, W. Ternary Aluminides with the Ideal Composition A2Pt6Al15(A = Y, Gd-Tm, Zr). Z. Anorg. Alleg. Chem. 2004, 630, 361–368. [Google Scholar] [CrossRef]
- Latturner, S.E.; Kanatzidis, M.G. Gd1.33Pt3(Al,Si)8 and Gd0.67Pt2(Al,Si)5: Two Structures Containing a Disordered Gd/Al Layer Grown in Liquid Aluminum. Inorg. Chem. 2002, 41, 5479–5486. [Google Scholar] [CrossRef]
- Murashova, E.V.; Tursina, A.I.; Bukhan’ko, N.G.; Gribanov, A.V.; Chernyshev, I.V.; Seropegin, Y.D. Ternary compounds Ce0.67Pt2Al5 and Ce1.33Pt3Al8. J. Alloys Compd. 2005, 398, 100–105. [Google Scholar] [CrossRef]
- Lacerda, A.; Canfield, P.C.; Beyermann, W.P.; Hundley, M.F.; Thompson, J.D.; Sparn, G.; Fisk, Z.; Burns, C.; Barnhart, D.; Lawson, A.C.; et al. Possible heavy-fermion behavior and field-induced transitions in new R-Pt-Ga compounds. J. Alloys Compd. 1992, 181, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Deppe, M.; Hartmann, S.; Macovei, M.E.; Oeschler, N.; Nicklas, M.; Geibel, C. Investigation of Yb2Pt6Al15 single crystals:heavy fermion system with a large local moment degeneracy. New J. Phys. 2008, 10, 093017. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Ueda, T.; Ohara, S. Single crystal growth and heat capacity measurements of triangular lattice R2Pt6Ga15 (R = rare earth). J. Phys. Conf. Ser. 2016, 683, 012035. [Google Scholar] [CrossRef]
- Kwei, G.H.; Lawson, A.C.; Larson, A.C.; Morosin, B.; Larson, E.M.; Canfield, P.C. Structure of Ce2Pt6Ga15: Interplanar Disorder from the Ce2Ga3 Layers. Acta Cryst. 1996, B52, 580–585. [Google Scholar] [CrossRef]
- Yamashita, T.; Ohara, S. Non-Fermi liquid behavior on heavy-fermion system Ce2Pt6Ga15. J. Phys. Conf. Ser. 2012, 400, 042074. [Google Scholar] [CrossRef] [Green Version]
- Radzieowski, M.; Stegemann, F.; Block, T.; Stahl, J.; Johrendt, D.; Janka, O. Abrupt Europium Valence Change in Eu2Pt6Al15 around 45 K. J. Am. Chem. Soc. 2018, 140, 8950–8957. [Google Scholar] [CrossRef]
- Radzieowski, M.; Stegemann, F.; Hoffmann, R.-D.; Janka, O. The monoclinic superstructure of the M2Pt6Al15 series (M = Ca, Sc, Y, La, Lu). Z. Kristallogr. 2017, 232, 2050. [Google Scholar]
- Macaluso, R.T.; Shatruk, M.; Chai, P.; Hong, H.; Wangeline, C.; Ryan, K.; Holton, P.; Allaz, J.; Morrison, G.; Fulfer, B.; et al. Synthesis, structure, and magnetic behavior of (LaxCe1−x)1.33Pt4Ga10 (0 ≤ x ≤ 1). J. Alloys Compd. 2014, 600, 193–198. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Ryan, K.; Chai, P.; Shatruk, M.; Xin, Y.; Chapman, K.W.; Chupas, P.J.; Fronczek, F.R.; Macaluso, R.T. Pr1.33Pt4Ga10: Superstructure and magnetism. J. Solid State Chem. 2014, 220, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Radzieowski, M.; Stegemann, F.; Janka, O. Magnetic Properties of the RE2Pt6X15 (RE = Y, La–Nd, Sm, Gd–Lu; X = Al, Ga) Series. Eur. J. Inorg. Chem. 2020, 2020, 1199–1210. [Google Scholar] [CrossRef]
- Zhuravleva, M.A.; Chen, X.Z.; Wang, X.; Schultz, A.J.; Ireland, J.; Kannewurf, C.K.; Kanatzidis, M.G. X-ray and Neutron Structure Determination and Magnetic Properties of New Quaternary Phases RE0.67Ni2Ga5+n−xGex and RE0.67Ni2Ga5+n−xSix (n = 0, 1; RE = Y, Sm, Gd, Tb, Dy, Ho, Er, Tm) Synthesized in Liquid Ga. Chem. Mater. 2002, 14, 3066–3081. [Google Scholar] [CrossRef]
- Kotur, B.Y.; Bruvo, M. Crystal structure of the silicide Sc1.2Fe4Si9.8. Sov. Phys. Crystallogr. 1991, 36, 787–789. [Google Scholar]
- Noguchi, S.; Okuda, K.; Adachi, T.; Yoshida, T. Synthesis and characterization of new ternary silicide Er1.2Fe4Si9.8. Physica B 1997, 237, 612–615. [Google Scholar] [CrossRef]
- Han, M.K.; Wu, Y.Q.; Kramer, M.; Vatoves, B.; Grandjean, F.; Long, G.J.; Miller, G.J. Superstructure in RE2−xFe4Si14−y (RE = Y, Gd-Lu) Characterized by Diffraction, Electron Microscopy, and Mo1ssbauer Spectroscopy. Inorg. Chem. 2006, 45, 10503–10519. [Google Scholar] [CrossRef] [Green Version]
- Manni, S.; Lin, Q.; Bud’ko, S.L.; Canfield, P.C. Systematic investigation of structural, transport, magnetic and thermodynamic properties of hexagonal R2Pt6Al15 (R = Y, La-Nd, Sm-Lu) series. In Proceedings of the MAR16 Meeting of the American Physical Society, USA, 16 March 2015. [Google Scholar]
- Suzuki, H.; Yamamoto, R.; Onimaru, T.; Urushihara, D.; Nakamura, S.; Ohara, S. Single crystal growth and physical properties of heavy fermion Ce2P6Al15. In Proceedings of the the Autumn Meeting of the Japan Physical Society, Tokyo, Japan, 12 September 2022. 12pW541-10. [Google Scholar]
- Izumi, F.; Momma, K. Three-Dimensional Visualization in Powder Diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tursina, A.I.; Murashova, E.V.; Noël, H.; Bukhan’ko, N.G.; Seropegin, Y.D. Crystal structure and magnetic properties of the new intermetallic Ce2Pt9Al16. Intermetallics 2009, 17, 780–783. [Google Scholar] [CrossRef]
- Strydom, A.M. The Cage Compound Ce2Pt9Al16: A Low-Temperature Magnetic Field Study. J. Low. Temp. Phys. 2013, 170, 334–339. [Google Scholar] [CrossRef]
- Haen, P.; Flouquet, J.; Lapierre, F.; Lejay, P.; Remenyi, G. Metamagnetic-Like Transition in CeRu2Si2? J. Low Temp. 1987, 67, 391–419. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Hanya, K.; Haga, Y.; Fisk, Z.; Ohara, S. Single crystal growth and physical properties of YbPd2Si2. J. Phys. Conf. Ser. 2015, 592, 012022. [Google Scholar] [CrossRef]
- Bonville, P.; Hammann, J.; Hodges, J.A.; Imbert, P.; Jéhanno, G.; Besnus, M.J.; Meyer, A. Hybridisation and crystal field in YbPd2Si2. Z. Phys. B 1991, 82, 267–274. [Google Scholar] [CrossRef]


| AM-CePtAl | F-CePtAl | |
|---|---|---|
| Space group | 194 | 194 |
| Lattice constants (Å) | a = 4.3127(7) | a = 4.3322(4) |
| c = 16.5156(13) | c = 16.4976(7) | |
| Formula units per cell, Z | 1 | 1 |
| Formula mass | 1855.50 | 1855.50 |
| Calculated density (g cm) | 11.581 | 11.490 |
| Absorption coefficient (mm) | 87.809 | 87.114 |
| Detector distance (mm) | 127.40 | 127.40 |
| range (deg.) | 2.46–34.88 | 2.47–34.89 |
| Range in | ||
| Total number of reflections | 3596 | 5905 |
| Unique reflections | 266 | 267 |
| Reliability factor | 0.0258 | 0.0522 |
| Goodness-of-fit | 1.177 | 1.259 |
| Reflections with | 243 | 253 |
| Number of variables | 21 | 21 |
| 0.0109 | 0.0111 | |
| 0.0146 | 0.0208 | |
| Residual electron density ( e Å) | 0.73/−1.15 | 0.78/−0.94 |
| AM-CePtAl | |||||
|---|---|---|---|---|---|
| Atom | Occupancy | (Å) | |||
| Ce | 1/3 | 2/3 | 1/4 | 0.6715(17) | 0.00514(13) |
| Pt | 1/3 | 2/3 | 0.60755(2) | 1 | 0.00495(5) |
| Al(1) | 0.5354(4) | 0.0709(7) | 1/4 | 0.337(5) | 0.0064(8) |
| Al(2) | 0 | 0 | 0.13147(7) | 1.008(6) | 0.0078(4) |
| Al(3) | 1/3 | 2/3 | 0.04594(7) | 0.993(7) | 0.0061(4) |
| F-CePtAl | |||||
| Atom | Occupancy | (Å) | |||
| Ce | 1/3 | 2/3 | 1/4 | 0.6777(19) | 0.00603(14) |
| Pt | 1/3 | 2/3 | 0.60727(2) | 1 | 0.00598(6) |
| Al(1) | 0.5349(4) | 0.0699(8) | 1/4 | 0.343(5) | 0.0092(8) |
| Al(2) | 0 | 0 | 0.13227(7) | 1.065(6) | 0.0098(3) |
| Al(3) | 1/3 | 2/3 | 0.04672(7) | 1.074(7) | 0.0091(4) |
| Atom | x | y | z |
|---|---|---|---|
| Ce | 1/6 | 1/6 | 1/4 |
| Pt(1) | 1/3 | 1/3 | 0.1076 |
| Pt(2) | 0 | 1/3 | 0.1076 |
| Al(1) | 1/6 | 1/6 | 0.0459 |
| Al(2) | 1/3 | 0 | 0.1315 |
| Al(3) | 0.3990 | 0.2678 | 1/4 |
| Al(4) | 0 | 0 | 0.1315 |
| Al(5) | 0 | 1/3 | 0.5459 |
| Al(6) | 0 | 0.4646 | 1/4 |
| Ce | Pt(1) | Pt(2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Site | Distance (Å) | N | Site | Distance (Å) | N | Site | Distance (Å) | N |
| Al(3) | 3.098 | 1 | Al(2) | 2.521 | 2 | Al(4) | 2.521 | 1 |
| Al(6) | 3.099 | 1 | Al(4) | 2.521 | 1 | Al(2) | 2.521 | 2 |
| Al(3) | 3.099 | 1 | Al(1) | 2.535 | 1 | Al(5) | 2.535 | 1 |
| Al(2) | 3.167 | 4 | Al(3) | 2.549 | 1 | Al(6) | 2.549 | 1 |
| Al(4) | 3.167 | 2 | Al(1) | 2.690 | 2 | Al(1) | 2.690 | 2 |
| Al(1) | 3.370 | 2 | Al(5) | 2.690 | 1 | Al(5) | 2.690 | 1 |
| Pt(1) | 3.426 | 4 | Ce | 3.426 | 2 | Ce | 3.426 | 2 |
| Pt(2) | 3.426 | 2 |
| Neighbor | N | (Å) | R (Å) | |
|---|---|---|---|---|
| Ce L-edge | 1st: Al | 9 | 0.012 | 3.12 |
| 2nd: Pt | 6 | 0.003 | 3.41 | |
| Pt L-edge | 1st: Al | 5 | 0.005 | 2.54 |
| 2nd: Al | 3 | 0.013 | 2.71 | |
| 3rd: Ce | 2 | 0.011 | 3.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, K.; Watabe, Y.; Haga, Y.; Iesari, F.; Okajima, T.; Matsumoto, Y. Synthesis, Crystal Structure, Local Structure, and Magnetic Properties of Polycrystalline and Single-Crystalline Ce2Pt6Al15. Symmetry 2023, 15, 1488. https://doi.org/10.3390/sym15081488
Ota K, Watabe Y, Haga Y, Iesari F, Okajima T, Matsumoto Y. Synthesis, Crystal Structure, Local Structure, and Magnetic Properties of Polycrystalline and Single-Crystalline Ce2Pt6Al15. Symmetry. 2023; 15(8):1488. https://doi.org/10.3390/sym15081488
Chicago/Turabian StyleOta, Kyugo, Yuki Watabe, Yoshinori Haga, Fabio Iesari, Toshihiro Okajima, and Yuji Matsumoto. 2023. "Synthesis, Crystal Structure, Local Structure, and Magnetic Properties of Polycrystalline and Single-Crystalline Ce2Pt6Al15" Symmetry 15, no. 8: 1488. https://doi.org/10.3390/sym15081488
APA StyleOta, K., Watabe, Y., Haga, Y., Iesari, F., Okajima, T., & Matsumoto, Y. (2023). Synthesis, Crystal Structure, Local Structure, and Magnetic Properties of Polycrystalline and Single-Crystalline Ce2Pt6Al15. Symmetry, 15(8), 1488. https://doi.org/10.3390/sym15081488






