Medicinally Significant Enantiopure Compounds from Garcinia Acid Isolated from Garcinia gummi-gutta
Abstract
1. Introduction
2. Traditional Uses of Garcinia gummi-gutta Extracts
3. Plant Constituents of Garcinia gummi-gutta
4. Medicinal Properties
5. Toxicity Studies
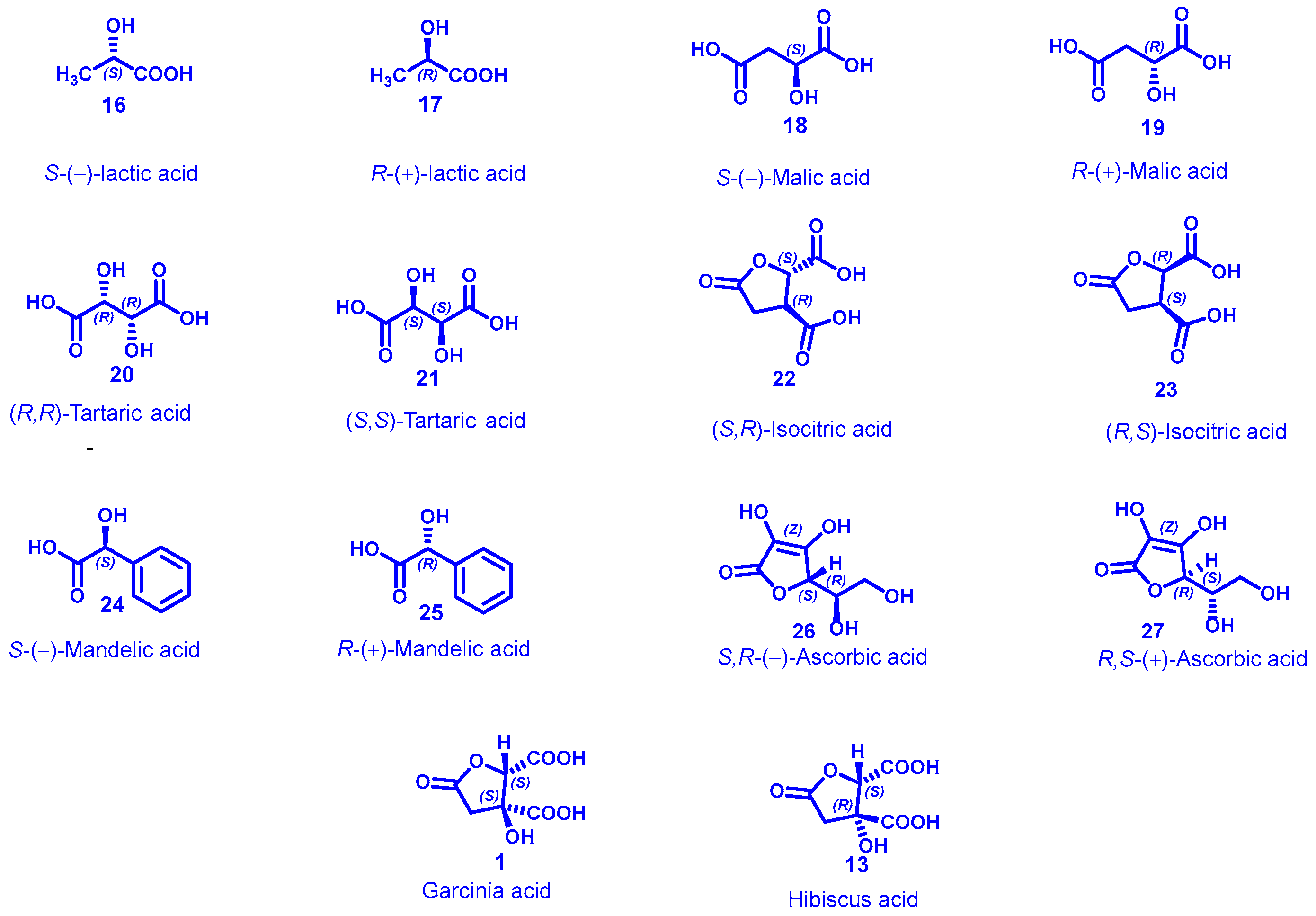
6. Hydroxycitric Acids
7. Garcinia Acid as Chiral Building Block: A Value Addition to India’s Natural Resources
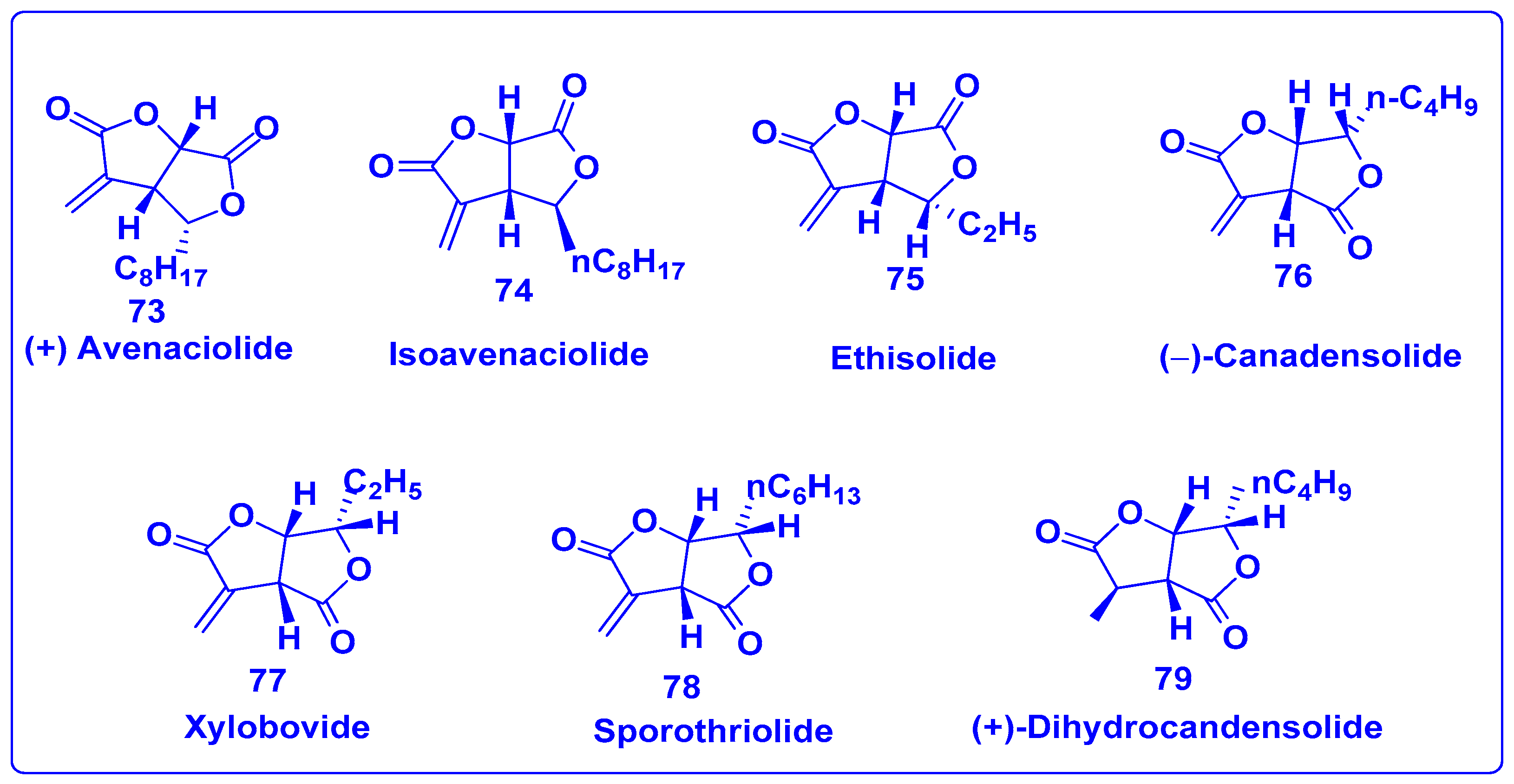
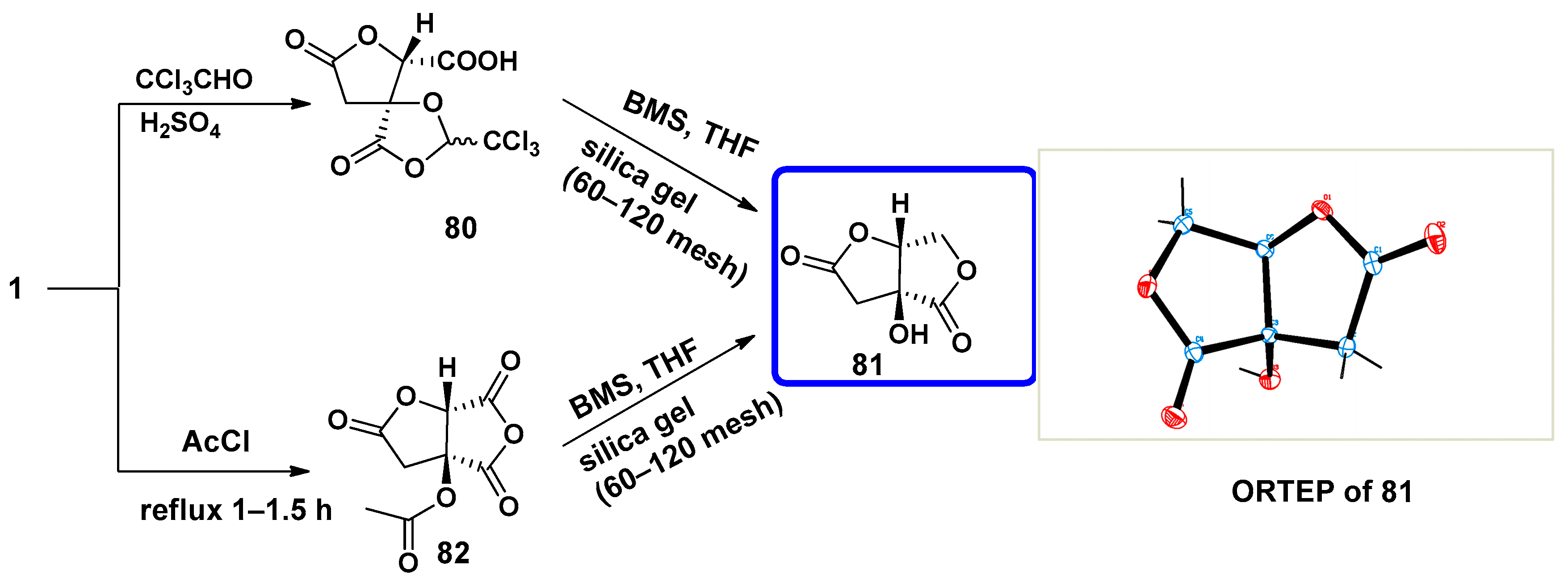
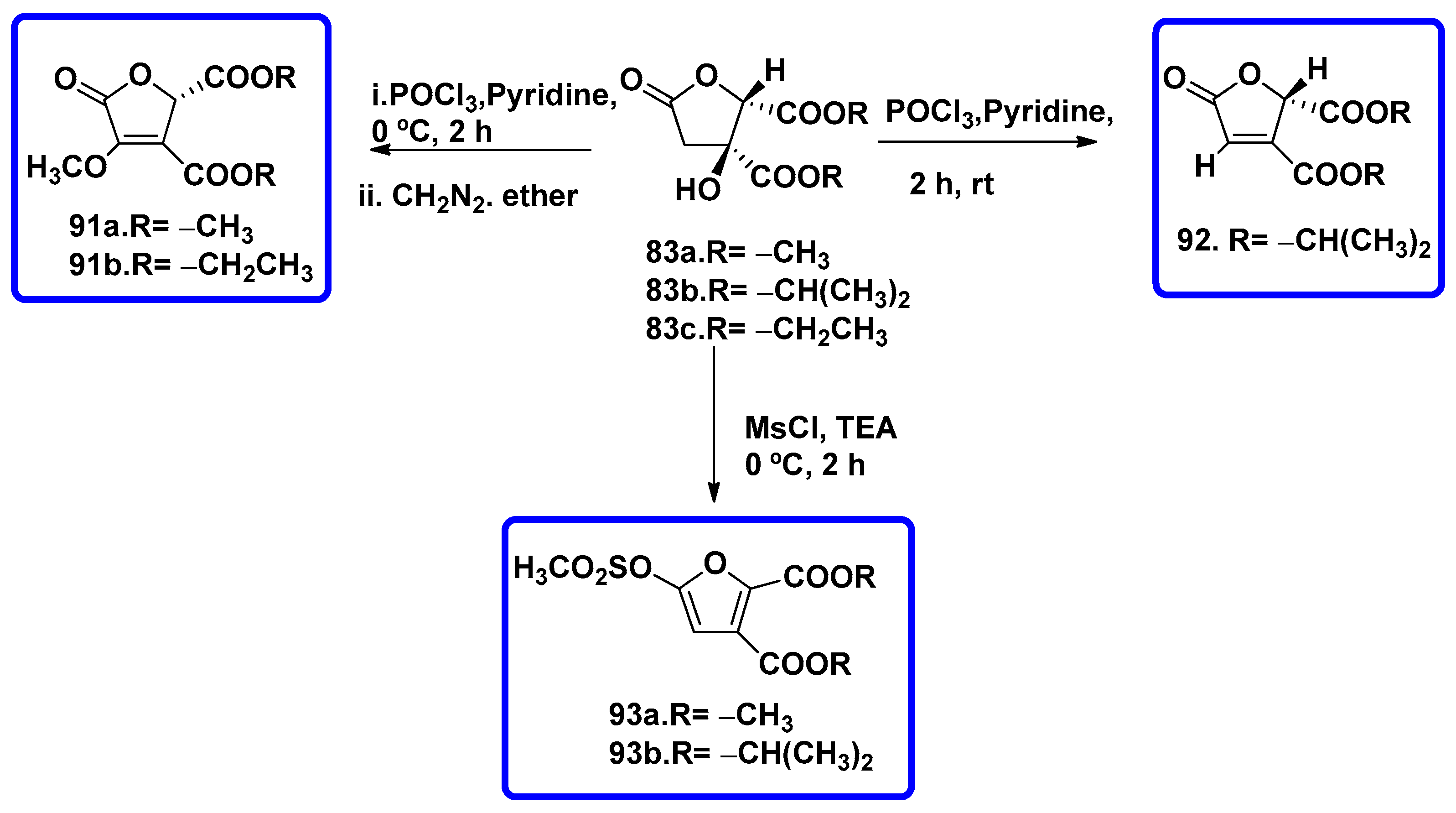
7.1. Synthesis of Bislactones
7.2. Synthesis of Analogues of the Quararibea Metabolite Chiral Enolic-γ-Lactones
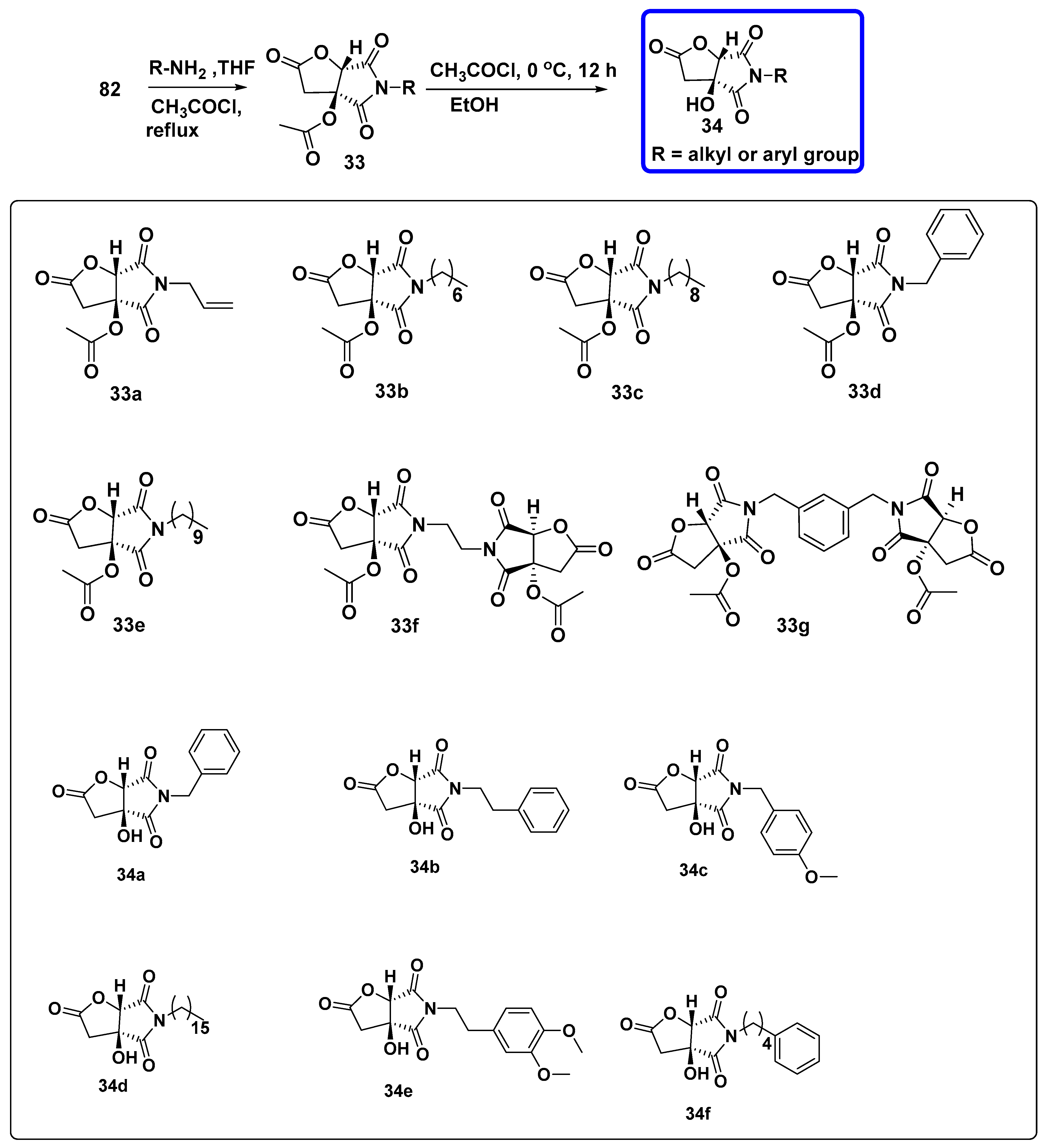
7.3. Synthesis of 3-Substituted and 3,4-Disubstituted Pyrrolidine-2-5-Diones
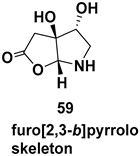
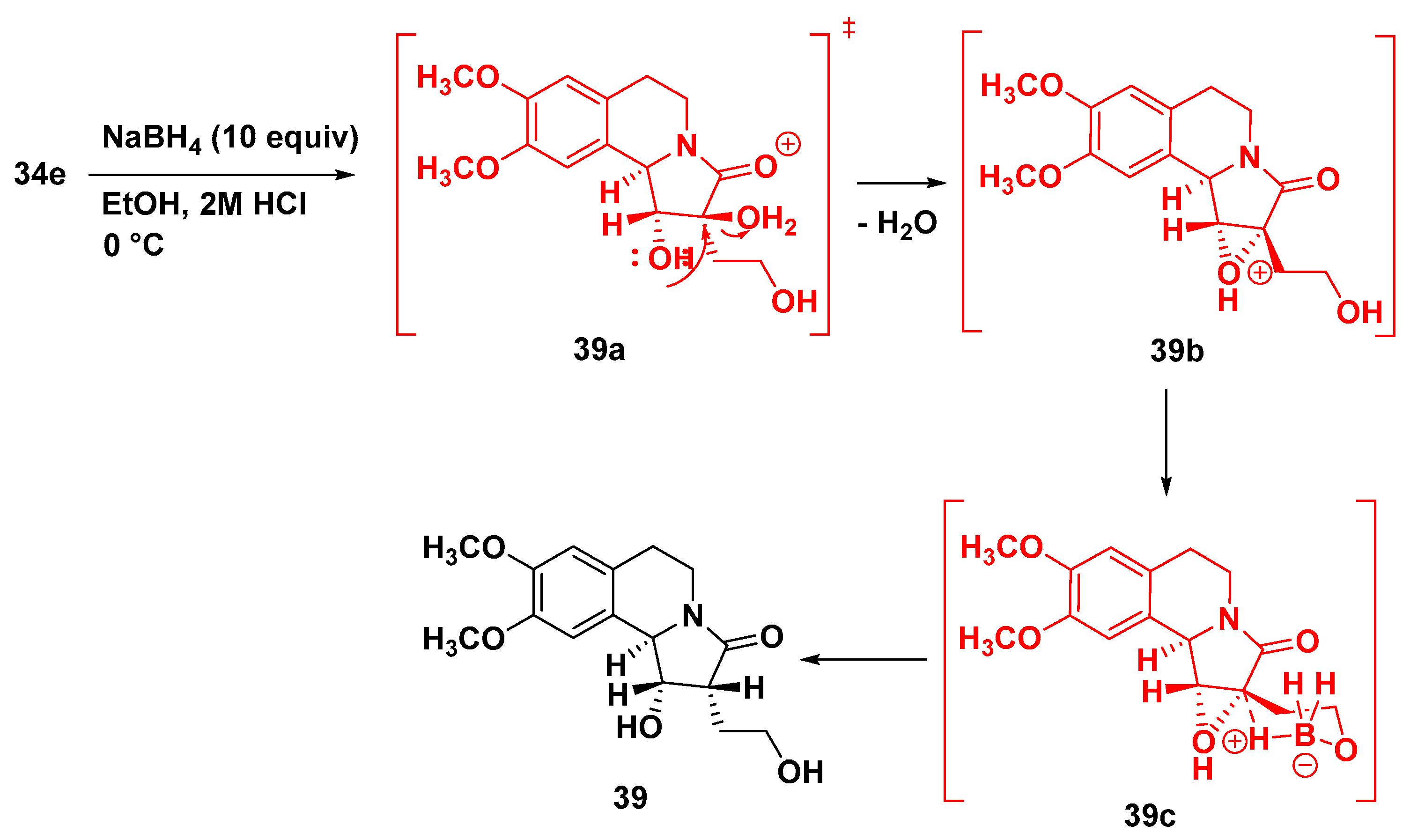
7.4. Synthesis of Tetrahydropyrrolo[2,1-a] Isoquinolinone, Hexahydroindolizino[8,7-b] Indolones and Furo[2,3-b]pyrroles
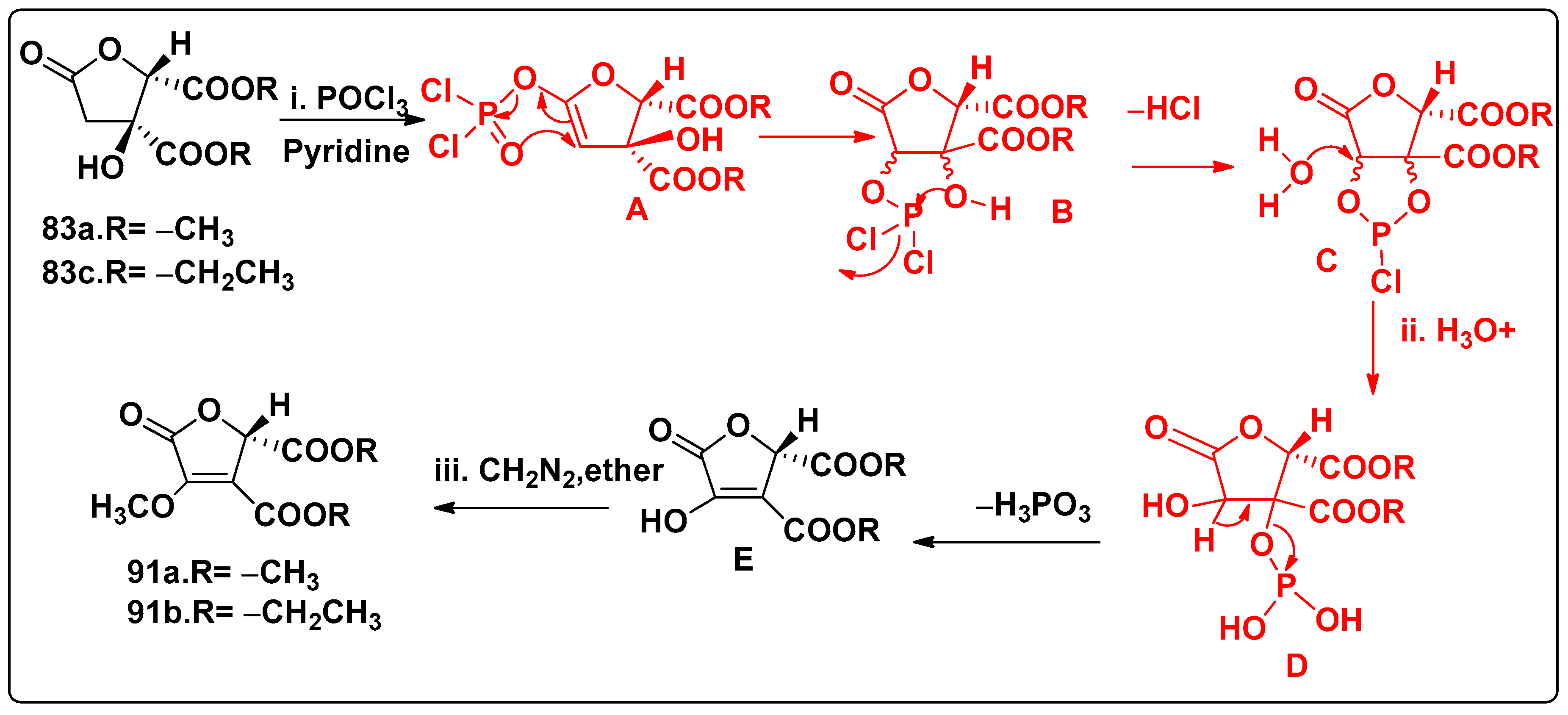
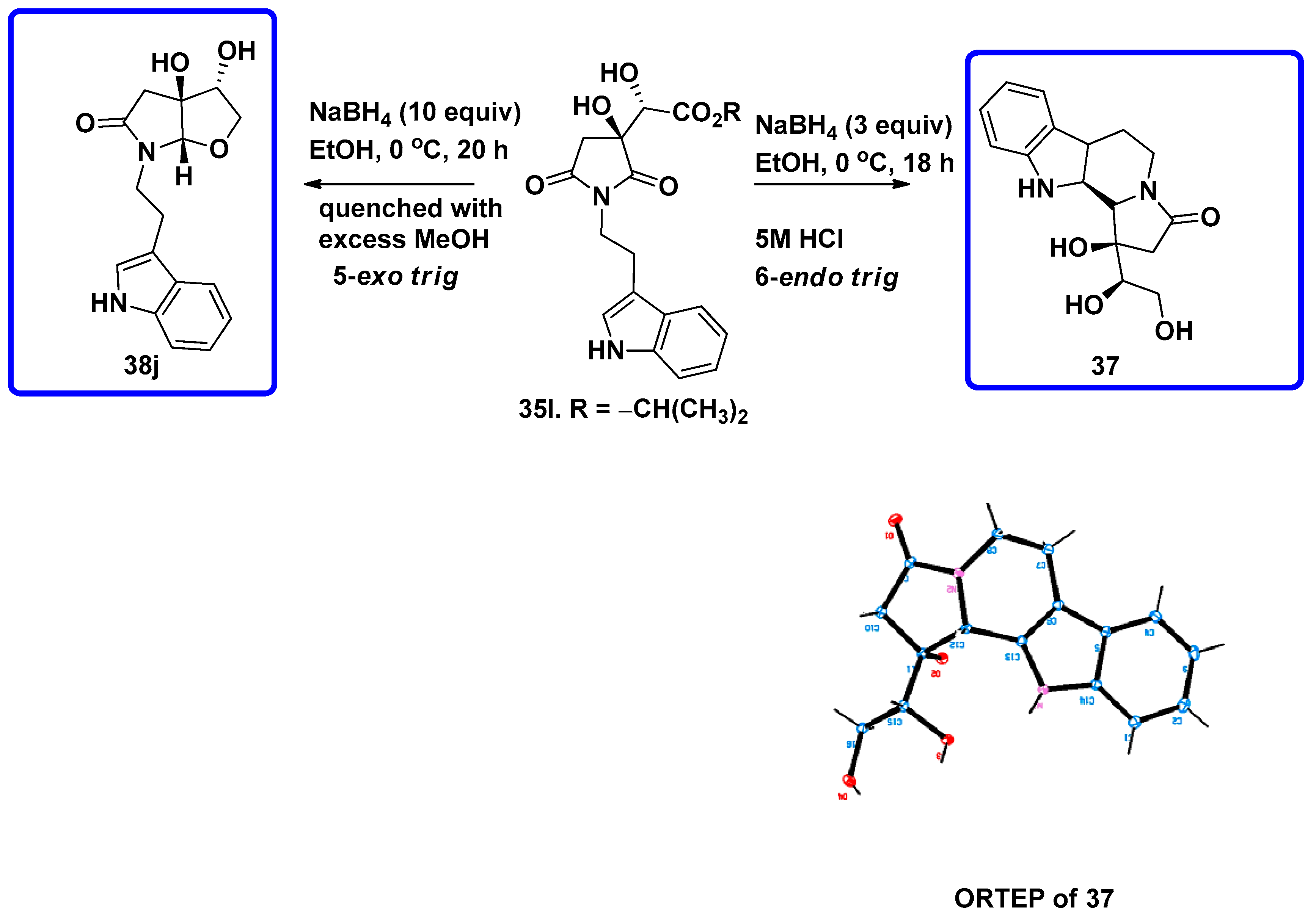
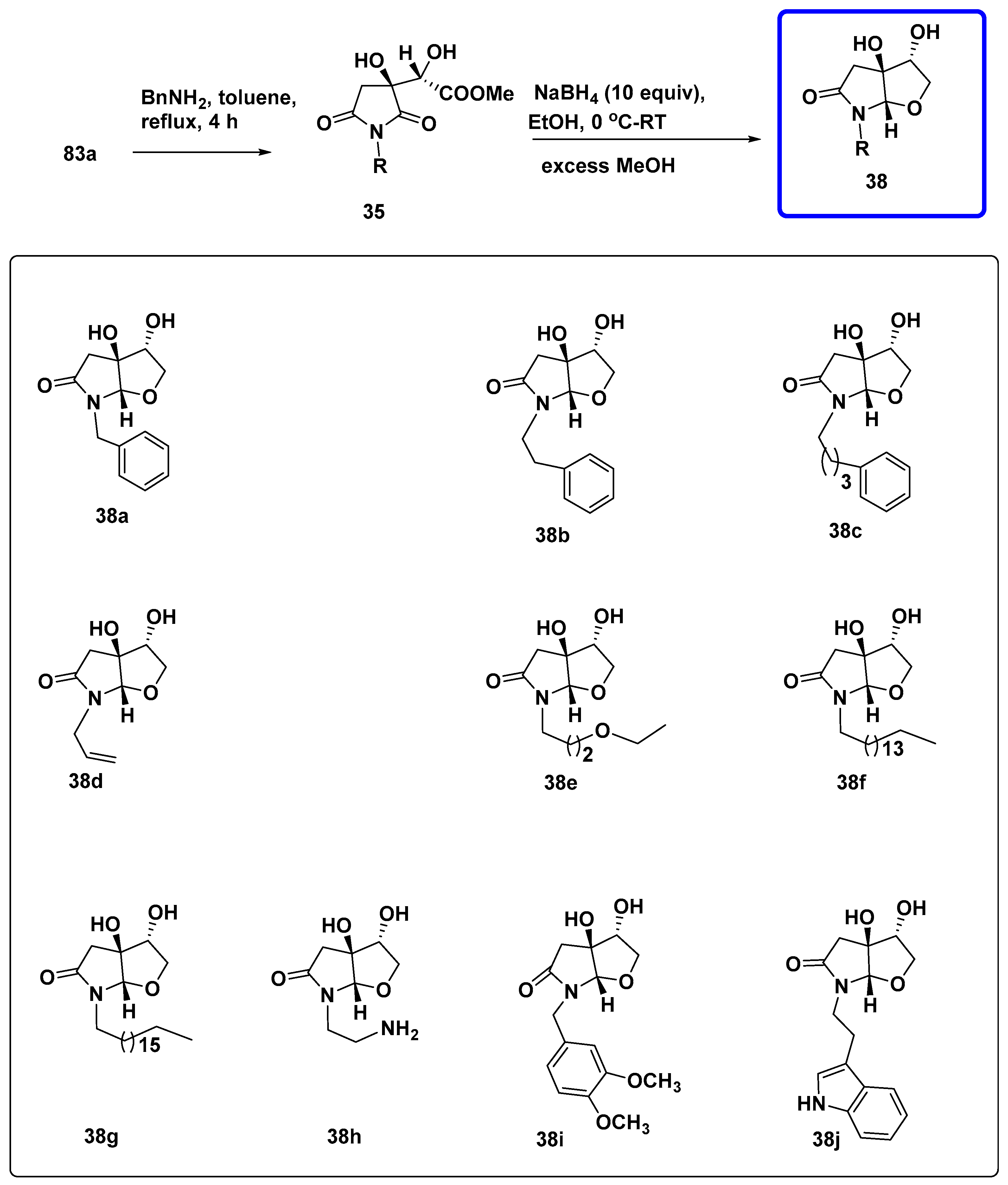
7.4.1. Synthesis of Furopyrroles
7.4.2. Synthesis of Pyrroloisoquinolinone from Bicyclic Anhydride
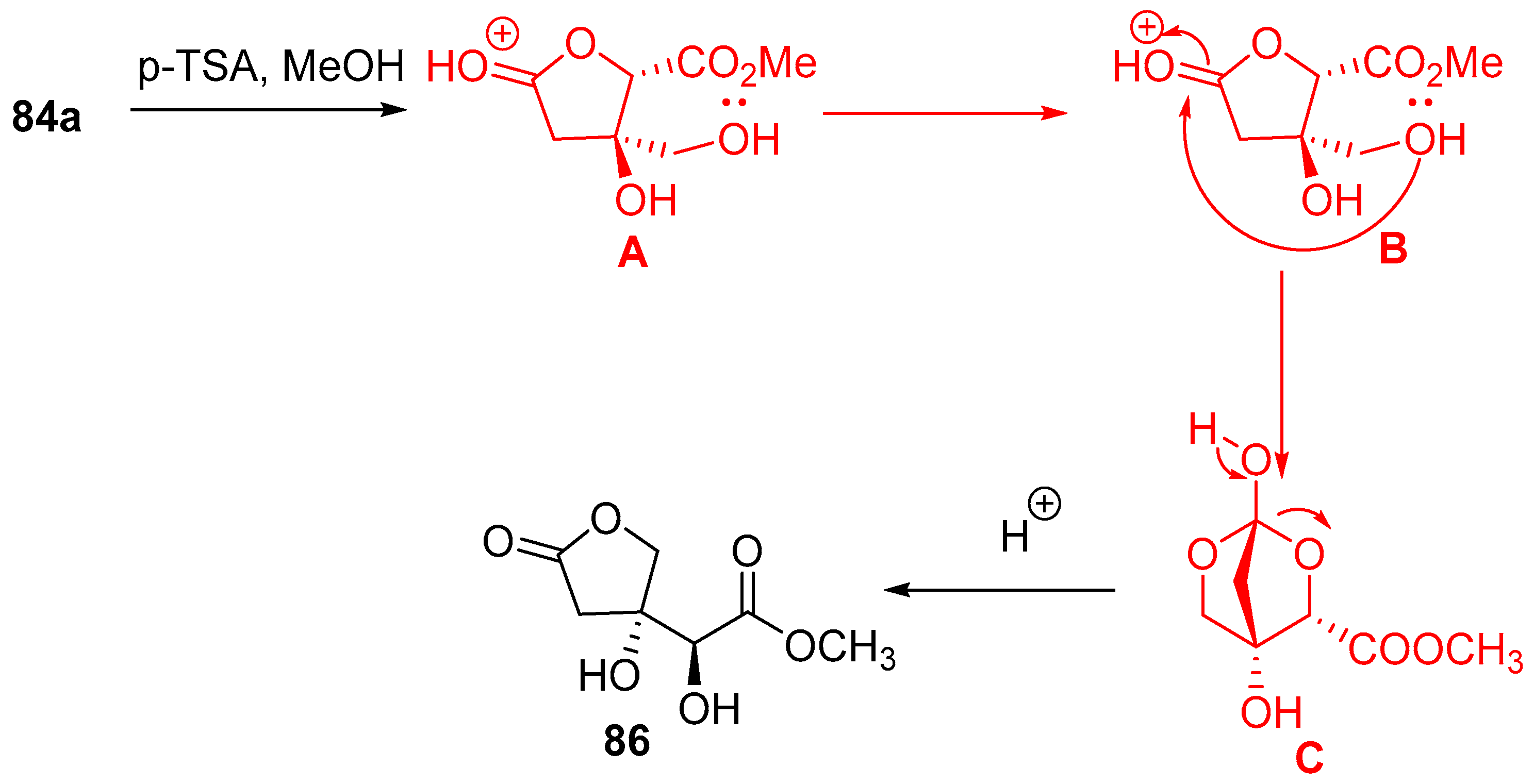
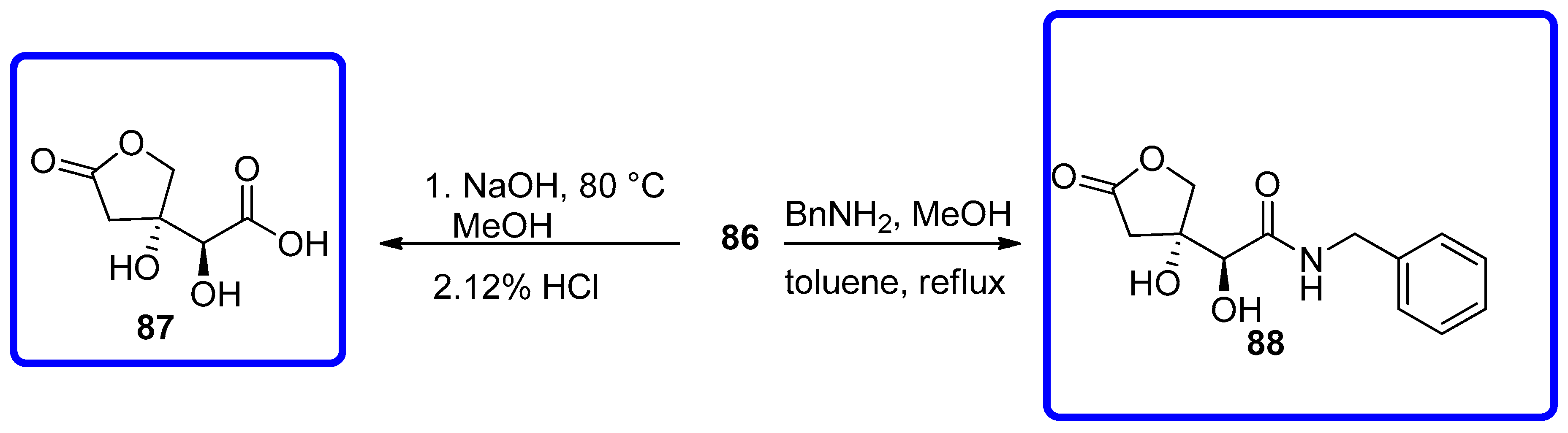
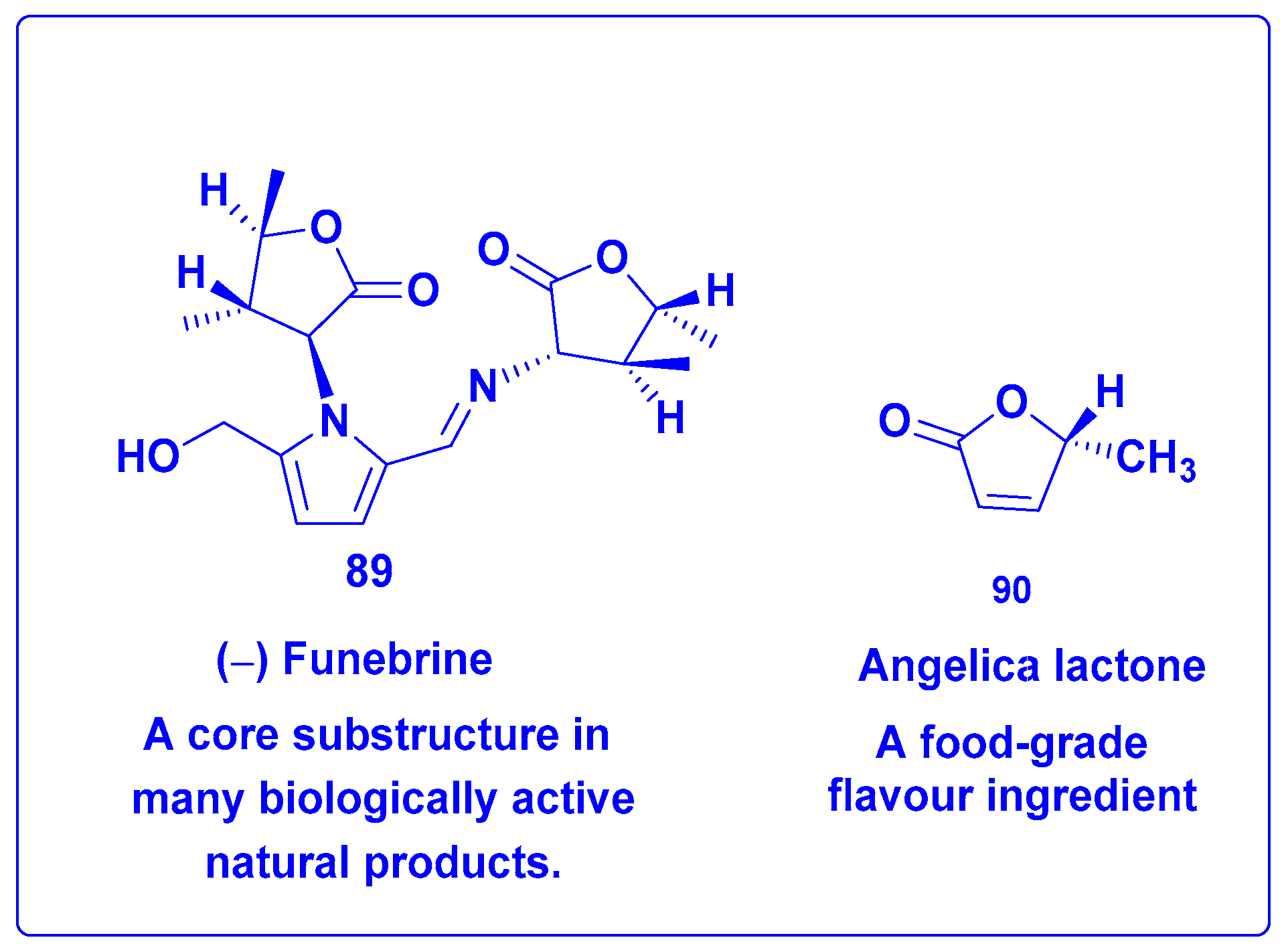
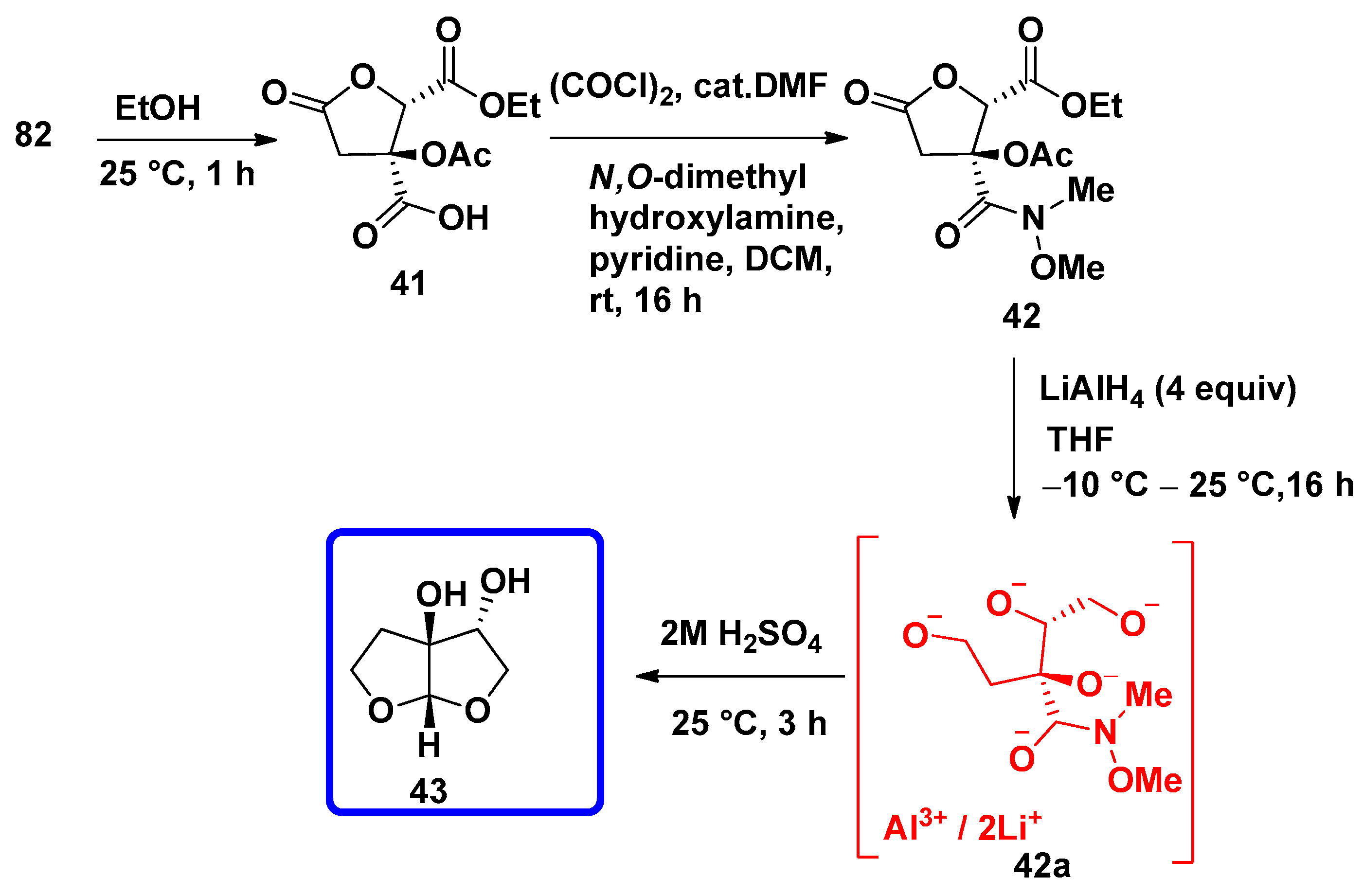
7.4.3. Synthesis of Furo[2,3-b]furanol Skeletons
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acetyl-CoA | Acetyl coenzyme A |
| BnNH2 | Benzyl amine |
| bis-THF | bis-tetrahydrofuran |
| BMS | Borane dimethyl sulphide |
| CH3COCl | Acetyl chloride |
| CH2N2 | Diazomethane |
| (COCl)2 | Oxalyl chloride |
| DCM | Dichloromethane |
| DMF | N,N-Dimethylformamide |
| EtOH | Ethanol |
| HCA | Hydroxycitric acid |
| HCl | Hydrochloric acid |
| HIV | Human immunodeficiency virus |
| HPLC | High-performance liquid chromatography |
| H3PO3 | Orthophosphorous acid |
| H2SO4 | Sulphuric acid |
| LiAlH4 | Lithium aluminum hydride |
| MeOH | Methanol |
| MsCl | Methane sulfonyl chloride |
| NaBH4 | Sodium borohydride |
| NaOH | Sodium hydroxide |
| ORTEP | Oak Ridge Thermal-Ellipsoid Plot Program |
| POCl3 | Phosphorus oxychloride |
| THF | Tetrahydrofuran |
| TEA | Triethyl amine |
| p-TSA | p-Toluene sulfonic acid |
References
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric Natural Products: Occurrence and Biogenesis. Angew. Chem. Int. Ed. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural Products from Marine Organisms and Their Associated Microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Yu, Z.-P.; Capon, R.J.; Zhang, H. Natural Enantiomers: Occurrence, Biogenesis and Biological Properties. Molecules 2022, 27, 1279. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Koca, C.; Dey, S.; Gehlot, P.; Yodkeeree, S.; Danda, D.; Sung, B.; Aggarwal, B.B. Traditional Uses of Spices: An Overview. In Molecular Targets and Therapeutic Uses of Spices; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2009; pp. 1–24. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.-E.; Leaman, D.J.; et al. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef]
- H. Baky, M.; Fahmy, H.; Farag, M.A. Recent Advances in Garcinia cambogia Nutraceuticals in Relation to Its Hydroxy Citric Acid Level. A Comprehensive Review of Its Bioactive Production, Formulation, and Analysis with Future Perspectives. ACS Omega 2022, 7, 25948–25957. [Google Scholar] [CrossRef]
- Choppa, T.; Selvaraj, C.I.; Zachariah, A. Evaluation and Characterization of Malabar Tamarind [Garcinia cambogia (Gaertn.) Desr.] Seed Oil. J. Food Sci. Technol. 2015, 52, 5906–5913. [Google Scholar] [CrossRef]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Indian Traditional Ayurvedic System of Medicine and Nutritional Supplementation. Evid.-Based Complement. Altern. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef]
- Espirito Santo, B.L.S.d.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.d.O.; Bogo, D.; Freitas, K.d.C.; Guimarães, R.d.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.d.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Sakariah, K.K. Organic Acids from Leaves, Fruits, and Rinds of Garcinia cowa. J. Agric. Food Chem. 2002, 50, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Masullo, M.; Bassarello, C.; Bifulco, G.; Piacente, S. Polyisoprenylated benzophenone derivatives from the fruits of Garcinia cambogia and their absolute configuration by quantum chemical circular dichroism calculations. Tetrahedron 2010, 66, 139–145. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Lewis, Y.S.; Ravindranath, B. On the structures of garcinol, isogarcinol and camboginol. Tetrahedron Lett. 1981, 22, 793–796. [Google Scholar] [CrossRef]
- Sullivan, C.; Triscari, J. Metabolic regulation as a control for lipid disorders. I. Influence of (—)-hydroxycitrate on experimentally induced obesity in the rodent. Am. J. Clin. Nutr. 1977, 30, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.A.; Fang, M.; Lowenstein, J.M. Tricarballylate and hydroxycitrate: Substrate and inhibitor of ATP: Citrate oxaloacetate lyase. Arch. Biochem. Biophys. 1969, 135, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.O.; Ho, W.Y.; Beh, B.K.; Yeap, S.K. Updates on Antiobesity Effect of Garcinia Origin (-)-HCA. Evid.-Based Complement. Altern. Med. eCam 2013, 2013, 751658. [Google Scholar] [CrossRef]
- Hayamizu, K.; Tomi, H.; Kaneko, I.; Shen, M.; Soni, M.G.; Yoshino, G. Effects of Garcinia cambogia extract on serum sex hormones in overweight subjects. Fitoterapia 2008, 79, 255–261. [Google Scholar] [CrossRef]
- Iinuma, M.; Ito, T.; Miyake, R.; Tosa, H.; Tanaka, T.; Chelladurai, V. A xanthone from Garcinia cambogia. Phytochemistry 1998, 47, 1169–1170. [Google Scholar] [CrossRef]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Adverse events of herbal food supplements for body weight reduction: Systematic review. Obes. Rev. 2005, 6, 93–111. [Google Scholar] [CrossRef]
- Saito, M.; Ueno, M.; Ogino, S.; Kubo, K.; Nagata, J.; Takeuchi, M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food Chem. Toxicol. 2005, 43, 411–419. [Google Scholar] [CrossRef]
- Ranjith, D.; Prakash, S.S.; Karunakara, A.C.; Diwakar, L.; Reddy, G.C. Issue of testicular toxicity of hydroxycitric acid lactone. Curr. Sci. 2011, 100, 24–27. [Google Scholar]
- Chuah, L.O.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Alitheen, N.B. In vitro and in vivo toxicity of garcinia or hydroxycitric Acid: A review. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 197920. [Google Scholar] [CrossRef] [PubMed]
- von Lippmann, E.O. Uber eine neue, im Ruben-saft vorkommende Saure. Ber. Dtsch Chem. Ges. 1883, 16, 1078–1081. [Google Scholar] [CrossRef]
- Martius, C.; Maué, R. Darstellung, physiologisches Verhalten und Bedeutung der (+)-Oxycitronensäure und ihrer Isomeren. Biol. Chem. 1941, 269, 33–40. [Google Scholar] [CrossRef]
- Lewis, Y.S.; Neelakantan, S. (−)-Hydroxycitric acid—The principal acid in the fruits of Garcinia cambogia desr. Phytochemistry 1965, 4, 619–625. [Google Scholar] [CrossRef]
- Boll, P.M.S.; SØrensen, E.; Balieu, E.; Theander, O.; Jansen, G.; Lamm, B.; Samuelsson, B. Naturally Occurring Lactones and Lactames. III. The Absolute Configuration of the Hydroxycitric Acid Lactones: Hibiscus Acid and Garcinia Acid. Acta Chem. Scand. 1969, 23, 286–293. [Google Scholar] [CrossRef][Green Version]
- Glusker, J.P.; Minkin, J.A.; Casciato, C.A. The structure and absolute configuration of the calcium salt of garcinia acid, the lactone of (-)-hydroxycitric acid. Acta Cryst. 1971, 27, 1284–1293. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Sakariah, K.K. Determination of organic acids in Garcinia cambogia (Desr.) by high-performance liquid chromatography. J. Chromatogr. A 1998, 806, 337–339. [Google Scholar] [CrossRef]
- Abhijith, B.L.; Mohan, M.; Joseph, D.; Haleema, S.; Aboul-Enein, H.Y.; Ibnusaud, I. Capillary zone electrophorsis for the analysis of naturally occurring 2-hydroxycitric acids and their lactones. J. Sep. Sci. 2017, 40, 3351–3357. [Google Scholar] [CrossRef]
- Juaristi, E.; Soloshonok, V.A. (Eds.) Enantioselective Synthesis of Beta-Amino Acids, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 2005; p. 656. [Google Scholar]
- Gary, M. Coppola., H.F.S. α-Hydroxy Acids in Enantioselective Syntheses. In α-Hydroxy Acids in Enantioselective Syntheses, 1st ed.; Wiley-VCH: Weinheim, Germany, 1997; Volume 524. [Google Scholar]
- Ibnusaud, I.; Thomas, P.T.; Rani, R.N.; Sasi, P.V.; Beena, T.; Hisham, A. Chiral γ-butyrolactones related to optically active 2-hydroxycitric acids. Tetrahedron 2002, 58, 4887–4892. [Google Scholar] [CrossRef]
- Ibnusaud, I.; Thomas, G. Biologically interesting chiral 3,4-disubstituted pyrrolidines from optically active hydroxycitric acid lactones. Tetrahedron Lett. 2003, 44, 1247–1249. [Google Scholar] [CrossRef]
- Varugese, S.; Thomas, S.; Haleema, S.; Puthiaparambil, T.; Ibnusaud, I. Synthesis of enantiopure concave (+)-avenaciolide and (−)-canadensolide skeletons. Tetrahedron Lett. 2007, 48, 8209–8212. [Google Scholar] [CrossRef]
- Gopinath, C.; Thomas, S.; Nair, M.S.; Ibnusaud, I. Analogues of the Quararibea metabolite chiral enolic-γ-lactone from (2S,3S)- and (2S,3R)-tetrahydro-3-hydroxy-5-oxo-2,3-furandicarboxylic acids. Tetrahedron Lett. 2006, 47, 7957–7960. [Google Scholar] [CrossRef]
- CP, P.; Joseph, E.; DS, N.; Ibnusaud, I.; Raskatov, J.; Singaram, B. Stabilization of NaBH4 in Methanol Using a Catalytic Amount of NaOMe. Reduction of Esters and Lactones at Room Temperature without Solvent-Induced Loss of Hydride. J. Org. Chem. 2018, 83, 1431–1440. [Google Scholar] [CrossRef]
- Polavarapu, P.L.; Donahue, E.A.; Shanmugam, G.; Scalmani, G.; Hawkins, E.K.; Rizzo, C.; Ibnusaud, I.; Thomas, G.; Habel, D.; Sebastian, D. A Single Chiroptical Spectroscopic Method May Not Be Able To Establish the Absolute Configurations of Diastereomers: Dimethylesters of Hibiscus and Garcinia Acids. J. Phys. Chem. A 2011, 115, 5665–5673. [Google Scholar] [CrossRef]
- Haleema, S.; Sasi, P.V.; Ibnusaud, I.; Polavarapu, P.L.; Kagan, H.B. Enantiomerically pure compounds related to chiral hydroxy acids derived from renewable resources. RSC Adv. 2012, 2, 9257–9285. [Google Scholar] [CrossRef]
- Skarżewski, J.; Gupta, A. Synthesis of C2 symmetric primary vicinal diamines. Double stereospecific mitsunobu reaction on the heterocyclic diols derived from tartaric acid. Tetrahedron Asymmetry 1997, 8, 1861–1867. [Google Scholar] [CrossRef]
- Jenkinson, S.F.; Best, D.; Saville, A.W.; Mui, J.; Martínez, R.F.; Nakagawa, S.; Kunimatsu, T.; Alonzi, D.S.; Butters, T.D.; Norez, C.; et al. C-Branched Iminosugars: α-Glucosidase Inhibition by Enantiomers of isoDMDP, isoDGDP, and isoDAB–l-isoDMDP Compared to Miglitol and Miglustat. J. Org. Chem. 2013, 78, 7380–7397. [Google Scholar] [CrossRef]
- Heretsch, P.; Thomas, F.; Aurich, A.; Krautscheid, H.; Sicker, D.; Giannis, A. Syntheses with a Chiral Building Block from the Citric Acid Cycle: (2R,3S)-Isocitric Acid by Fermentation of Sunflower Oil. Angew. Chem. Int. Ed. 2008, 47, 1958–1960. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, P.-Q.; Ruan, Y.-P.; Lee, A.; Chan, W.H. A New Approach for Asymmetric Synthesis of (R)-3-Methylpyrrolidine Alkaloids from (S)-Malic Acid. Nat. Prod. Lett. 2002, 16, 53–56. [Google Scholar] [CrossRef]
- Shibuguchi, T.; Fukuta, Y.; Akachi, Y.; Sekine, A.; Ohshima, T.; Shibasaki, M. Development of new asymmetric two-center catalysts in phase-transfer reactions. Tetrahedron Lett. 2002, 43, 9539–9543. [Google Scholar] [CrossRef]
- Dang, T.P.; Kagan, H.B. The asymmetric synthesis of hydratropic acid and amino-acids by homogeneous catalytic hydrogenation. J. Chem. Soc. D Chem. Commun. 1971, 10, 481. [Google Scholar] [CrossRef]
- Kagan, H.B.; Dang, T.-P. Asymmetric catalytic reduction with transition metal complexes. I. Catalytic system of rhodium(I) with (-)-2,3-0-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane, a new chiral diphosphine. J. Am. Chem. Soc. 1972, 94, 6429–6433. [Google Scholar] [CrossRef]
- Sinou, D.; Kagan, H.B. Catalyse asymetrique par le complexe cationique [Rh(COD)(+)Diop]+ClO4−. J. Organomet. Chem. 1976, 114, 325–337. [Google Scholar] [CrossRef]
- Galeazzi, R.; Martelli, G.; Orena, M.; Rinaldi, S.; Sabatino, P. Conformationally restricted analogues of both (S)-β-homoserine and (S)-aspartic acid from chiral 3-acylamino pyrrolidin-2-ones. Tetrahedron 2005, 61, 5465–5473. [Google Scholar] [CrossRef]
- Brandi, A.; Cardona, F.; Cicchi, S.; Cordero, F.M.; Goti, A. Stereocontrolled Cyclic Nitrone Cycloaddition Strategy for the Synthesis of Pyrrolizidine and Indolizidine Alkaloids. Chem.—A Eur. J. 2009, 15, 7808–7821. [Google Scholar] [CrossRef]
- Asano, N.; Nishida, M.; Miyauchi, M.; Ikeda, K.; Yamamoto, M.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Fleet, G.W.J. Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulaceae). Phytochemistry 2000, 53, 379–382. [Google Scholar] [CrossRef]
- Mao, B.; Fañanás-Mastral, M.; Feringa, B.L. Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev. 2017, 117, 10502–10566. [Google Scholar] [CrossRef]
- Nair, D.S.; Ibnusaud, I. Synthesis of enantiopure furo[2,3-b]pyrroles. Tetrahedron Lett. 2014, 55, 5822–5824. [Google Scholar] [CrossRef]
- Habel, D.; Nair, D.S.; Kallingathodi, Z.; Mohan, C.; Pillai, S.M.; Nair, R.R.; Thomas, G.; Haleema, S.; Gopinath, C.; Abdul, R.V.; et al. Natural Product-Derived Chiral Pyrrolidine-2,5-diones, Their Molecular Structures and Conversion to Pharmacologically Important Skeletons. J. Nat. Prod. 2020, 83, 2178–2190. [Google Scholar] [CrossRef]
- Pässler, U.; Knölker, H.-J. Chapter 2—The Pyrrolo[2,1-a]isoquinoline Alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 70, pp. 79–151. [Google Scholar]
- Black, D.M.; Davis, R.; Doan, B.D.; Lovelace, T.C.; Millar, A.; Toczko, J.F.; Xie, S. Highly diastereo- and enantioselective catalytic synthesis of the bis-tetrahydrofuran alcohol of Brecanavir and Darunavir. Tetrahedron Asymmetry 2008, 19, 2015–2019. [Google Scholar] [CrossRef]
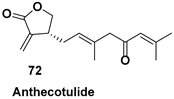
- Hodgson, D.M.; Talbot, E.P.A.; Clark, B.P. Catalytic Asymmetric Synthesis of (+)-Anthecotulide Using Enyne and Meyer–Schuster Rearrangements. Org. Lett. 2011, 13, 5751–5753. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.M.; Talbot, E.P.A.; Clark, B.P. Stereoselective Synthesis of β-(Hydroxymethylaryl/alkyl)-α-methylene-γ-butyrolactones. Org. Lett. 2011, 13, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Brookes, D.; Tidd, B.K.; Turner, W.B. 1028. Avenaciolide, an antifungal lactone from Aspergillus avenaceus. J. Chem. Soc. (Resumed) 1963, 5385–5391. [Google Scholar] [CrossRef]
- Nubbemeyer, U. Diastereoselective Zwitterionic Aza-Claisen Rearrangement: The Synthesis of Bicyclic Tetrahydrofurans and a Total Synthesis of (+)-Dihydrocanadensolide. J. Org. Chem. 1996, 61, 3677–3686. [Google Scholar] [CrossRef]
- Lertvorachon, J.; Thebtaranonth, Y.; Thongpanchang, T.; Thongyoo, P. Stereoselective Synthesis of Naturally Occurring α-Methylenebis-γ-butyrolactones: An Application of Novel Oxiranyl “Remote” Anions. J. Org. Chem. 2001, 66, 4692–4694. [Google Scholar] [CrossRef]
- Rodríguez, C.M.; Martín, T.; Martín, V.S. A New Stereoselective Synthesis of (−)-Isoavenaciolide and (−)-Avenaciolide. J. Org. Chem. 1996, 61, 8448–8452. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Davies, P.W.; Schmidt, A.T. Asymmetric synthesis of avenaciolide via cascade palladium catalysed cyclisation–carbonylation of bromodienes. Chem. Commun. 2004, 10, 1232–1233. [Google Scholar] [CrossRef]
- Labeeuw, O.; Blanc, D.; Phansavath, P.; Ratovelomanana-Vidal, V.; Genêt, J.-P. An Efficient Ruthenium-Catalyzed Formal Synthesis of (−)-Isoavenaciolide. European J. Org. Chem. 2004, 2004, 2352–2358. [Google Scholar] [CrossRef]
- Alcázar, E.; Kassou, M.; Matheu, I.; Castillón, S. The Enantioselective Formal Synthesis of (+)-Avenaciolide and (+)-Isoavenaciolide from Tri-O-acetyl-D-glucal Using a Ring Contraction Reaction as the Key Step. Eur. J. Org. Chem. 2000, 2000, 2285–2289. [Google Scholar] [CrossRef]
- Yu, M.; Lynch, V.; Pagenkopf, B.L. Intramolecular Cyclopropanation of Glycals: Studies toward the Synthesis of Canadensolide, Sporothriolide, and Xylobovide. Org. Lett. 2001, 3, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.L.; Berger, M.H.; Schlessinger, R.H. A total synthesis of racemic avenaciolide. J. Am. Chem. Soc. 1979, 101, 1544–1549. [Google Scholar] [CrossRef]
- Kallmerten, J.; Gould, T.J. A short, stereocontrolled synthesis of avenaciolide. J. Org. Chem. 1985, 50, 1128–1131. [Google Scholar] [CrossRef]
- Burke, S.D.; Pacofsky, G.J.; Piscopio, A.D. Synthesis of ethisolide, isoavenaciolide, and avenaciolide. J. Org. Chem. 1992, 57, 2228–2235. [Google Scholar] [CrossRef]
- Buser, H.P.; Pugin, B.; Spindler, F.; Sutter, M. Two enantioselective syntheses of a precursor of the biologically most active isomer of CGA 80000 (clozylacon). Tetrahedron 1991, 47, 5709–5716. [Google Scholar] [CrossRef]
- Saito, S.; Ishikawa, T.; Kuroda, A.; Koga, K.; Moriwake, T. A revised mechanism for chemoselective reduction of esters with borane-dimethyl sulfide complex and catalytic sodium tetrahydroborate directed by adjacent hydroxyl group. Tetrahedron 1992, 48, 4067–4086. [Google Scholar] [CrossRef]
- K. Banerjee, A.; Laya Mimo, M.S.; Vera Vegas, W.J. Silica gel in organic synthesis. Russ. Chem. Rev. 2001, 70, 971–990. [Google Scholar] [CrossRef]
- Simimole, H. Chiral Pool Approach Towards the Synthesis of a Few Optically Active Γ-Butyrolactone Based Natural Products. Ph.D. Thesis, Mahatma Gandhi University, Kottayam, India, 2015. [Google Scholar]
- Cren, S.; Gurcha, S.S.; Blake, A.J.; Besra, G.S.; Thomas, N.R. Synthesis and biological evaluation of new inhibitors of UDP-Galf transferase—A key enzyme in M. tuberculosis cell wall biosynthesis. Org. Biomol. Chem. 2004, 2, 2418–2420. [Google Scholar] [CrossRef]
- Flourat, A.L.; Haudrechy, A.; Allais, F.; Renault, J.-H. (S)-γ-Hydroxymethyl-α,β-butenolide, a Valuable Chiral Synthon: Syntheses, Reactivity, and Applications. Org. Process Res. Dev. 2020, 24, 615–636. [Google Scholar] [CrossRef]
- Tan, Z.; Yihuo, A.; Wu, Z.; Wang, F.; Dong, S.; Feng, X. Concise synthesis of chiral γ-butenolides via an allylation/lactonization cascade reaction. Chem. Commun. 2024, 60, 7926–7929. [Google Scholar] [CrossRef]
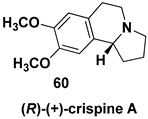
- Johnson, J.L.; Nair, D.S.; Pillai, S.M.; Johnson, D.; Kallingathodi, Z.; Ibnusaud, I.; Polavarapu, P.L. Dissymmetry Factor Spectral Analysis Can Provide Useful Diastereomer Discrimination: Chiral Molecular Structure of an Analogue of (−)-Crispine A. ACS Omega 2019, 4, 6154–6164. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Párraga, J.; Galán, A.; Cabedo, N.; Primo, J.; Cortes, D. Synthesis of new antimicrobial pyrrolo[2,1-a]isoquinolin-3-ones. Bioorganic Med. Chem. 2012, 20, 6589–6597. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, C.; Hanumanthappa, S.K.T.; Nagendrappa, G.; Ganapathy, P.S.S.; Shruthi, S.D.; More, S.S.; Jose, G.; Sowmya, H.B.V.; Kulkarni, R.S. Synthesis, ABTS-Radical Scavenging Activity, and Antiproliferative and Molecular Docking Studies of Novel Pyrrolo[1,2-a]quinoline Derivatives. Synth. Commun. 2015, 45, 2529–2545. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, D.W.; Lee, S.J.; Park, H. Asymmetric Synthesis of Both Enantiomers of Pyrrolidinoisoquinoline Derivatives from L-Malic Acid and L-Tartaric Acid. J. Org. Chem. 1995, 60, 7149–7152. [Google Scholar] [CrossRef]
- Allin, S.M.; Northfield, C.J.; Page, M.I.; Slawin, A.M.Z. A facile and highly stereoselective approach to a polycyclic isoindolinone ring system via an N-acyliminium ion cyclization reaction. Tetrahedron Lett. 1998, 39, 4905–4908. [Google Scholar] [CrossRef]
- Molina, A.; Pascual-Escudero, A.; Adrio, J.; Carretero, J.C. Catalytic Asymmetric 1,3-Dipolar Cycloaddition/Hydroamination Sequence: Expeditious Access to Enantioenriched Pyrroloisoquinoline Derivatives. J. Org. Chem. 2017, 82, 11238–11246. [Google Scholar] [CrossRef]
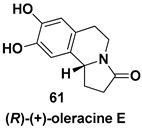
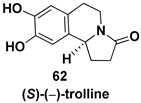
- Kawai, N.; Matsuda, M.; Uenishi, J.I. Stereoselective synthesis of tetrahydroisoquinoline alkaloids: (−)-trolline, (+)-crispin A, (+)-oleracein E. Tetrahedron 2011, 67, 8648–8653. [Google Scholar] [CrossRef]
- Allin, S.M.; Gaskell, S.N.; Towler, J.M.R.; Page, P.C.B.; Saha, B.; McKenzie, M.J.; Martin, W.P. A New Asymmetric Synthesis of the Anti-Tumor Alkaloid (R)-(+)-Crispine A. J. Org. Chem. 2007, 72, 8972–8975. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; Zhang, H.-C.; Cohen, J.H.; Turchi, I.J.; Maryanoff, C.A. Cyclizations of N-Acyliminium Ions. Chem. Rev. 2004, 104, 1431–1628. [Google Scholar] [CrossRef]
- Wijnberg, J.B.P.A.; Schoemaker, H.E.; Speckamp, W.N. A regioselective reduction of gem-disubstituted succinimides. Tetrahedron 1978, 34, 179–187. [Google Scholar] [CrossRef]
- Sunazuka, T.; Hirose, T.; Shirahata, T.; Harigaya, Y.; Hayashi, M.; Komiyama, K.; Ōmura, S.; Smith, A.B. Total Synthesis of (+)-Madindoline A and (−)-Madindoline B, Potent, Selective Inhibitors of Interleukin 6. Determination of the Relative and Absolute Configurations. J. Am. Chem. Soc. 2000, 122, 2122–2123. [Google Scholar] [CrossRef]
- Polavarapu, P.L.; Santoro, E.; Covington, C.L.; Johnson, J.L.; Puente, A.R.; Schley, N.D.; Kallingathodi, Z.; Prakasan, P.C.; Haleema, S.; Thomas, A.A.; et al. How important are the intermolecular hydrogen bonding interactions in methanol solvent for interpreting the chiroptical properties? Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119094. [Google Scholar] [CrossRef] [PubMed]
- Khmelnitsky, Y.L.; Michels, P.C.; Cotterill, I.C.; Eissenstat, M.; Sunku, V.; Veeramaneni, V.R.; Cittineni, H.; Kotha, G.R.; Talasani, S.R.; Ramanathan, K.K.; et al. Biocatalytic Resolution of Bis-tetrahydrofuran Alcohol. Org. Process Res. Dev. 2011, 15, 279–283. [Google Scholar] [CrossRef]
- Quaedflieg, P.J.; Kesteleyn, B.R.; Wigerinck, P.B.; Goyvaerts, N.M.; Vijn, R.J.; Liebregts, C.S.; Kooistra, J.H.; Cusan, C. Stereoselective and Efficient Synthesis of (3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-ol. Org. Lett. 2005, 7, 5917–5920. [Google Scholar] [CrossRef] [PubMed]
- Canoy, W.L.; Cooley, B.E.; Corona, J.A.; Lovelace, T.C.; Millar, A.; Weber, A.M.; Xie, S.; Zhang, Y. Efficient Synthesis of (3R,3aS,6aR)- Hexahydrofuro[2,3-b]furan-3-ol from Glycolaldehyde. Org. Lett. 2008, 10, 1103–1106. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Shin, D.; Swanson, L.; Krishnan, K.; Cho, H.; Hussain, K.A.; Walters, D.E.; Holland, L.; Buthod, J. Structure-based design of non-peptide HIV protease inhibitors. Il Farm. 2001, 56, 29–32. [Google Scholar] [CrossRef]

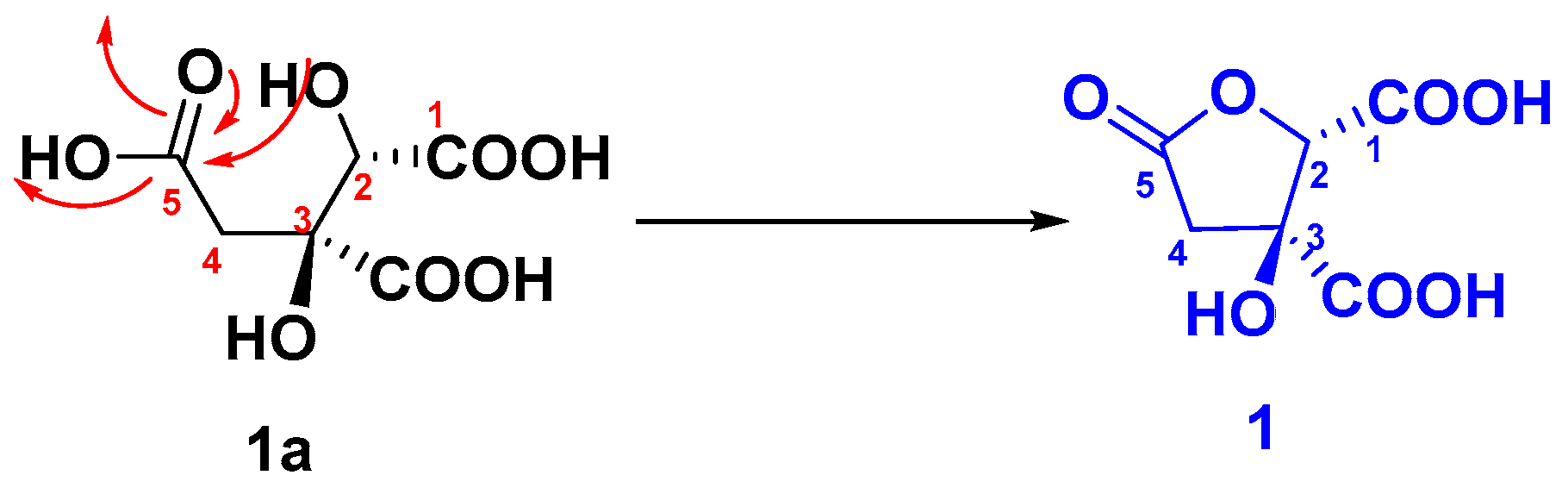







| Structure & Name | Plant Part | Biological Activity |
|---|---|---|
| Major organic acids | ||
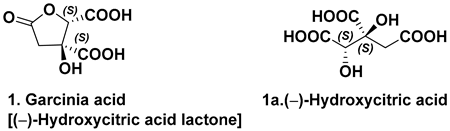 | Isolated from the fruit [7,11] | Inhibit the enzyme ATP citrate lyase |
| Polyisoprenylated Benzophenones | ||
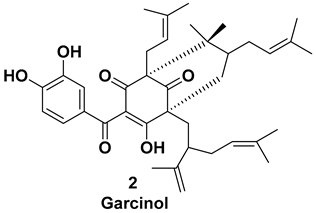 | Isolated from the peel [7,11] | Anti-inflammatory, antioxidant, anticancer, antiparasitic, action in nervous system |
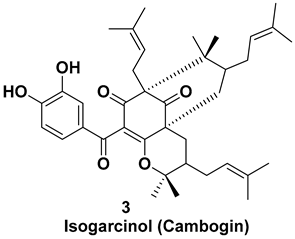 | Isolated from the peel [7,11] | Anti-inflammatory, antioxidant, anticancer, antiparasitic, action in nervous system |
 | Isolated from the fruit [7,11] | Unknown |
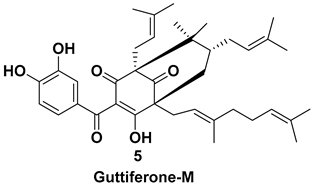 | Isolated from the fruit [7,11] | Inhibitor of topoisomerase II |
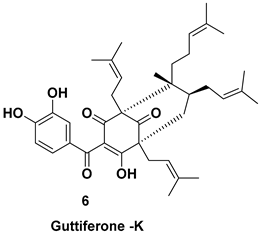 | Isolated from the fruit [7,11] | Inhibitor of topoisomerase II |
| Xanthones | ||
 | Isolated from the root [7,11] | Inhibition of α-glucosidase |
 | Isolated from the peel [7,11] | Unknown |
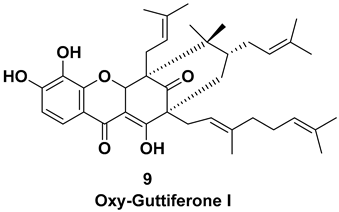 | Isolated from the fruit [7,11] | Unknown |
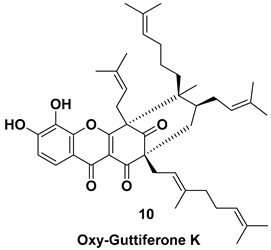 | Isolated from the fruit [7,11] | Unknown |
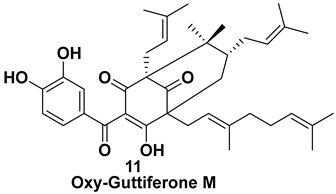 | Isolated from the fruit [7,11] | Unknown |
 | Isolated from the fruit [7,11] | Unknown |
| Structure of the Compound | Applications (Relevant Properties of the Derived Compounds) |
|---|---|
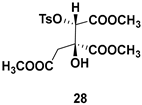 | Chiral synthon, building block used for the synthesis of pharmacologically important natural products like substituted indolizines and other heterocyclic scaffolds |
 | Chiral synthon, building block used for the synthesis of pharmacologically important natural products like substituted indolizines and other heterocyclic scaffolds |
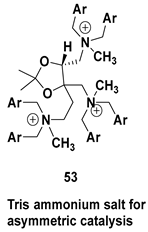
 | Chiral intermediate for the synthesis of trisammonium salt for asymmetric catalysis |
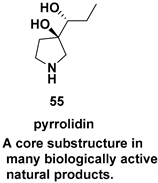
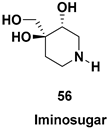
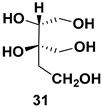 | Chiral intermediate for the synthesis of iminosugars |
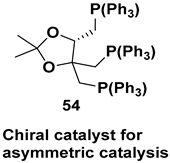
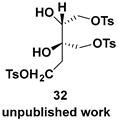 | Chiral intermediate for the synthesis of chiral catalysts |
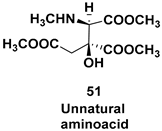
 | Chiral pyrrolidine diones, a common structural subunit found in a variety of natural and unnatural bioactive compounds |
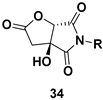 | Chiral pyrrolidine diones, a common structural subunit found in a variety of natural and unnatural bioactive compounds |
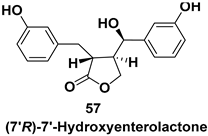
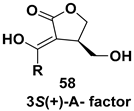
 | Chiral building blocks used for the syntheses of compounds having potent inhibitory activities against purine nucleoside phosphorylases, aldose reductase inhibitors, antibacterial activity etc. |
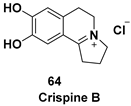
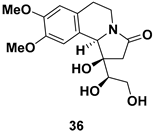 | Pyrrolo[2,1-a]isoquinoline alkaloid, an analogue of naturally occurring anti-tumor agent (−)-crispine A |
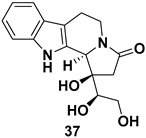 | Indolizino[8,7-b]indole alkaloid, an analogue of naturally occurring (−)-harmicine, known for its antileishmanial and antinociceptive activities |
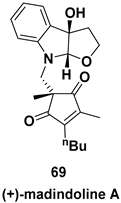
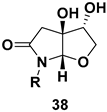 | Furo[2,3-b]pyrrolo skeleton, a rare class of concave cis-fused bicyclic nitrogen and oxygen heterocycles, subunit in complex natural products like millingtonine A, madindoline, as well as in several synthetic drugs |
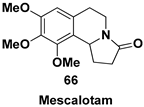
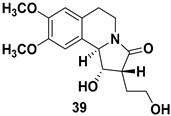 | Pyrrolo[2,1-a]isoquinoline alkaloid, fundamental structural component of many synthetic and biologically active compounds |
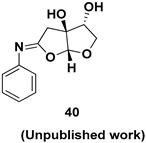 | Chiral intermediate |
 | Chiral intermediate for the synthesis of bis-tetrahydrofuran (bis-THF) alcohol moiety found in the structure of HIV protease inhibitors (PIs) like Darunavir, Brecanavir, GS-9005, and SPI-256 |
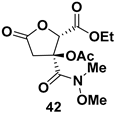 | Weinreb amide derivative, Chiral intermediate for the synthesis of bis-tetrahydrofuran (bis-THF) alcohol moiety found in the structure of HIV protease inhibitors (PIs) like Darunavir, Brecanavir, GS-9005, and SPI-256 |
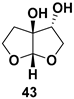 | Furo[2,3-b]furanol, part of anti-HIV drug Darunavir, Brecanavir, GS-9005, and SPI-256 |
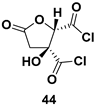 | Chiral synthon |
 | Weinreb amide derivative, chiral intermediate |
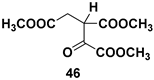 | Chiral intermediate |
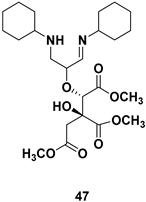 | Chiral intermediate |
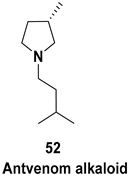
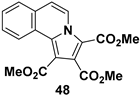 | Biologically and functionally important substituted indolizine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haleema, S.; Gopinath, C.; Kallingathodi, Z.; Thomas, G.; Polavarapu, P.L. Medicinally Significant Enantiopure Compounds from Garcinia Acid Isolated from Garcinia gummi-gutta. Symmetry 2024, 16, 1331. https://doi.org/10.3390/sym16101331
Haleema S, Gopinath C, Kallingathodi Z, Thomas G, Polavarapu PL. Medicinally Significant Enantiopure Compounds from Garcinia Acid Isolated from Garcinia gummi-gutta. Symmetry. 2024; 16(10):1331. https://doi.org/10.3390/sym16101331
Chicago/Turabian StyleHaleema, Simimole, Chithra Gopinath, Zabeera Kallingathodi, Grace Thomas, and Prasad L. Polavarapu. 2024. "Medicinally Significant Enantiopure Compounds from Garcinia Acid Isolated from Garcinia gummi-gutta" Symmetry 16, no. 10: 1331. https://doi.org/10.3390/sym16101331
APA StyleHaleema, S., Gopinath, C., Kallingathodi, Z., Thomas, G., & Polavarapu, P. L. (2024). Medicinally Significant Enantiopure Compounds from Garcinia Acid Isolated from Garcinia gummi-gutta. Symmetry, 16(10), 1331. https://doi.org/10.3390/sym16101331