Determination of Na+ Cation Locations in Nanozeolite ECR-1 Using a 3D ED Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 15 December 2023).
- Frising, T.; Leflaive, P. Extraframework cation distributions in X and Y faujasite zeolites: A review. Microporous Mesoporous Mater. 2008, 114, 27–63. [Google Scholar] [CrossRef]
- Chao, C.C. Process for Separating Nitrogen from Mixtures Thereof with Less Polar Substances. U.S. Patent 4,859,217, 22 August 1989. [Google Scholar]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.W.; Strohmaier, K.G. Crystalline Zeolite (ECR-1) and Process for Preparing It. U.S. Patent 4,657,748, 14 April 1987. [Google Scholar]
- Database of Zeolite Structures—Building Scheme for EON. Available online: http://www.iza-structure.org/databases/ModelBuilding/EON.pdf (accessed on 15 December 2023).
- Hsia Chen, C.S.; Schlenker, J.L.; Wentzek, S.E. Synthesis and characterization of synthetic zeolite ECR-1. Zeolites 1996, 17, 393–400. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Ferrari, S.; Galli, E.; Di Renzo, F.; van Beek, W. Rietveld structure refinement of zeolite ECR-1. Chem. Mater. 2006, 18, 76–84. [Google Scholar] [CrossRef]
- Song, J.; Dai, L.; Ji, Y.; Xiao, F.-S. Organic template free synthesis of aluminosilicate zeolite ECR-1. Chem. Mater. 2006, 18, 2775–2777. [Google Scholar] [CrossRef]
- Lu, T.; Wang, Z.; Zhang, H.; Qin, J.; Yang, Y.; Cheng, P.; Zhao, Z. Radicalized seeds-assist route for the rapid synthesis of zeolite ECR-1 in the absence of organic templates. Microporous Mesoporous Mater. 2022, 341, 112071. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, H.; He, P.Y.; Juan Li, C. Developing silica fume-based self-supported ECR-1 zeolite membrane for seawater desalination. Mater. Lett. 2019, 236, 538–541. [Google Scholar] [CrossRef]
- Leonowicz, M.E.; Vaughan, D.E.W. Proposed synthetic zeolite ECR-1 structure gives a new zeolite framework topology. Nature 1987, 329, 819–821. [Google Scholar] [CrossRef]
- Chatelard, C.; Dodin, M.; Martinez-Franco, R.; Tuel, A. Di- and trioxacyclohexane as structure directing molecules in the synthesis of zeolites omega and ECR-1. Microporous Mesoporous Mater. 2021, 318, 111015. [Google Scholar] [CrossRef]
- Weirich, T.E.; Lábár, J.L.; Zou, X. Electron Crystallography: Novel Approaches for Structure Determination of Nanosized Materials; Springer: Dordrecht, The Netherlands, 2006; p. 536. [Google Scholar]
- Vincent, R.; Midgley, P.A. Double conical beam-rocking system for measurement of integrated electron diffraction intensities. Ultramicroscopy 1994, 53, 271–282. [Google Scholar] [CrossRef]
- Midgley, P.A.; Eggeman, A.S. Precession electron diffraction—A topical review. IUCrJ 2015, 2, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mugnaioli, E.; Gorelik, T.; Kolb, U. “Ab initio” structure solution from electron diffraction data obtained by a combination of automated diffraction tomography and precession technique. Ultramicroscopy 2009, 109, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Sun, J.; Su, J.; Hovmoller, S.; Zou, X. Three-dimensional rotation electron diffraction: Software RED for automated data collection and data processing. J. Appl. Cryst. 2013, 46, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zou, X.; Smeets, S. Automated serial rotation electron diffraction combined with cluster analysis: An efficient multi-crystal workflow for structure determination. IUCrJ 2019, 6, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Petříček, V.; Corrêa, C.A. Structure refinement using precession electron diffraction tomography and dynamical diffraction: Theory and implementation. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 235–244. [Google Scholar] [CrossRef]
- Gemmi, M.; Mugnaioli, E.; Gorelik, T.E.; Kolb, U.; Palatinus, L.; Boullay, P.; Hovmöller, S.; Abrahams, J.P. 3D electron diffraction: The nanocrystallography revolution. ACS Cent. Sci. 2019, 5, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Gruene, T.; Mugnaioli, E. 3D electron diffraction for chemical analysis: Instrumentation developments and innovative applications. Chem. Rev. 2021, 121, 11823–11834. [Google Scholar] [CrossRef]
- Samperisi, L.; Zou, X.; Huang, Z. Three-dimensional electron diffraction: A powerful structural characterization technique for crystal engineering. CrystEngComm 2022, 24, 2719–2728. [Google Scholar] [CrossRef]
- Klar, P.B.; Krysiak, Y.; Xu, H.; Steciuk, G.; Cho, J.; Zou, X.; Palatinus, L. Accurate structure models and absolute configuration determination using dynamical effects in continuous-rotation 3D electron diffraction data. Nat. Chem. 2023, 15, 848–855. [Google Scholar] [CrossRef]
- Cho, J.; Willhammar, T.; Zou, X. The synergistic development of electron crystallography and zeolite discovery. Microporous Mesoporous Mater. 2023, 358, 112400. [Google Scholar] [CrossRef]
- Lorgouilloux, Y.; Dodin, M.; Mugnaioli, E.; Marichal, C.; Bats, N.; Caullet, P.; Kolb, U.; Paillaud, J.-L. IM-17: A new zeolitic material, synthesis and structure elucidation from electron diffraction ADT data and Rietveld analysis. RSC Adv. 2014, 4, 19440–19449. [Google Scholar] [CrossRef]
- Cichocka, M.O.; Lorgouilloux, Y.; Smeets, S.; Su, J.; Wan, W.; Caullet, P.; Bats, N.; McCusker, L.B.; Paillaud, J.-L.; Zou, X. Multidimensional disorder in zeolite IM-18 revealed by combining transmission electron microscopy and X-ray powder diffraction analyses. Cryst. Growth Des. 2018, 18, 2441–2451. [Google Scholar] [CrossRef]
- Steciuk, G.; Schäf, O.; Tortet, L.; Pizzala, H.; Hornfeck, W.; Palatinus, L.; Paillaud, J.-L. A new lithium-rich zeolitic 10-MR zincolithosilicate MZS-1 hydrothermally synthesized under high pressure and characterized by 3D electron diffraction. Eur. J. Inorg. Chem. 2021, 2021, 628–638. [Google Scholar] [CrossRef]
- Krysiak, Y.; Barton, B.; Marler, B.; Neder, R.B.; Kolb, U. Ab initio structure determination and quantitative disorder analysis on nanoparticles by electron diffraction tomography. Acta Crystallogr. Sect. A Found. Adv. 2018, 74, 93–101. [Google Scholar] [CrossRef]
- Krysiak, Y.; Marler, B.; Barton, B.; Plana-Ruiz, S.; Gies, H.; Neder, R.B.; Kolb, U. New zeolite-like RUB-5 and its related hydrous layer silicate RUB-6 structurally characterized by electron microscopy. IUCrJ 2020, 7, 522–534. [Google Scholar] [CrossRef]
- Fitch, A.; Dejoie, C.; Covacci, E.; Confalonieri, G.; Grendal, O.; Claustre, L.; Guillou, P.; Kieffer, J.; de Nolf, W.; Petitdemange, S.; et al. ID22—The high-resolution powder-diffraction beamline at ESRF. J. Synchrotron Radiat. 2023, 30, 1003–1012. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Palatinus, L.; Brázda, P.; Jelínek, M.; Hrdá, J.; Steciuk, G.; Klementová, M. Specifics of the data processing of precession electron diffraction tomography data and their implementation in the program PETS2.0. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Palatinus, L.; Plášil, J.; Dušek, M. Jana2020—A new version of the crystallographic computing system Jana. Z. Kristallogr. Cryst. Mater. 2023, 238, 271–282. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond—Crystal and Molecular Structure Visualization; Version 3.2k4; Crystal Impact: Bonn, Germany, 2018; Available online: https://www.crystalimpact.de/diamond (accessed on 3 December 2023).
- Ammouli, T.; Paillaud, J.-L.; Nouali, H.; Stephan, R.; Hanf, M.-C.; Sonnet, P.; Deroche, I. Insights into water adsorption in potassium-exchanged X-type faujasite zeolite: Molecular simulation and experiment. J. Phys. Chem. C 2021, 125, 19405–19416. [Google Scholar] [CrossRef]
- Martin, M.G. MCCCS Towhee: A tool for Monte Carlo molecular simulation. Mol. Simul. 2013, 39, 1212–1222. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. Available online: https://pubs.geoscienceworld.org/msa/ammin/article-abstract/39/1-2/92/539331 (accessed on 21 October 2023).
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Mahoney, M.W.; Jorgensen, W.L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 2000, 112, 8910–8922. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Newsam, J.M.; Deem, M.W.; Treacy, M.M.J.; Newsam, J.M.; Deem, M.W. A General recursion method for calculating diffracted intensities from crystals containing planar faults. Proc. Math. Phys. Sci. 1991, 433, 499–520. [Google Scholar] [CrossRef]
- Kolb, U.; Gorelik, T.E.; Mugnaioli, E.; Stewart, A. Structural characterization of organics using manual and automated electron diffraction. Polym. Rev. 2010, 50, 385–409. [Google Scholar] [CrossRef]
- Palatinus, L.; Jacob, D.; Cuvillier, P.; Klementova, M.; Sinkler, W.; Marks, L.D. Structure refinement from precession electron diffraction data. Acta Crystallogr. Sect. A Found. Cryst. 2013, 69, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Wang, Y.; Carraro, F.; Liang, W.; Roostaeinia, M.; Siahrostami, S.; Proserpio, D.M.; Doonan, C.; Falcaro, P.; Zheng, H.; et al. High-throughput electron diffraction reveals a hidden novel metal–organic framework for electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 11391–11397. [Google Scholar] [CrossRef] [PubMed]
- Girelli Consolaro, V.; Rouchon, V.; Ersen, O. Electron beam damages in zeolites: A review. Microporous Mesoporous Mater. 2024, 364, 112835. [Google Scholar] [CrossRef]
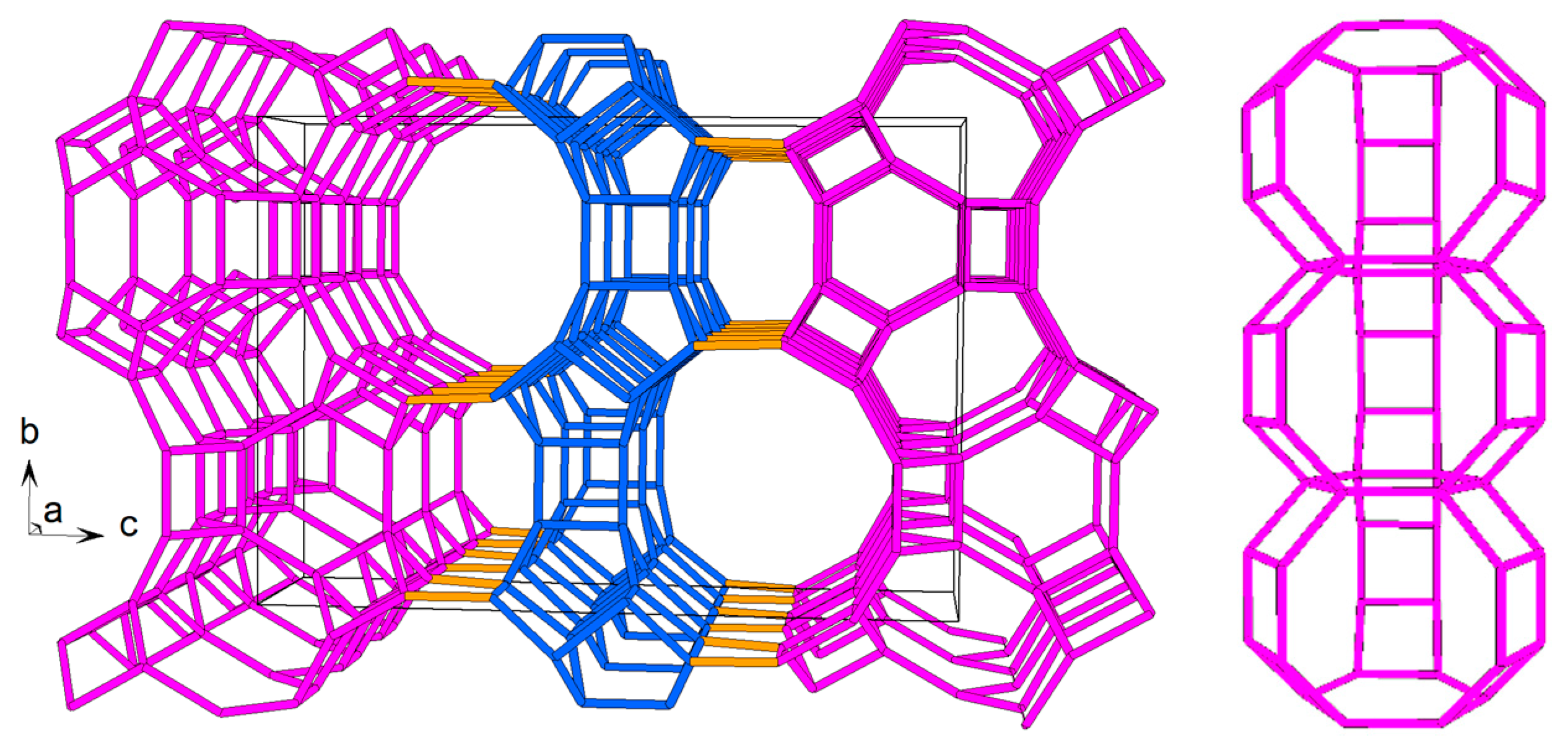

 zeolites. The Miller indices are indicated alongside the FWHM values in parentheses for representative reflections.
zeolites. The Miller indices are indicated alongside the FWHM values in parentheses for representative reflections.
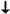 zeolites. The Miller indices are indicated alongside the FWHM values in parentheses for representative reflections.
zeolites. The Miller indices are indicated alongside the FWHM values in parentheses for representative reflections.
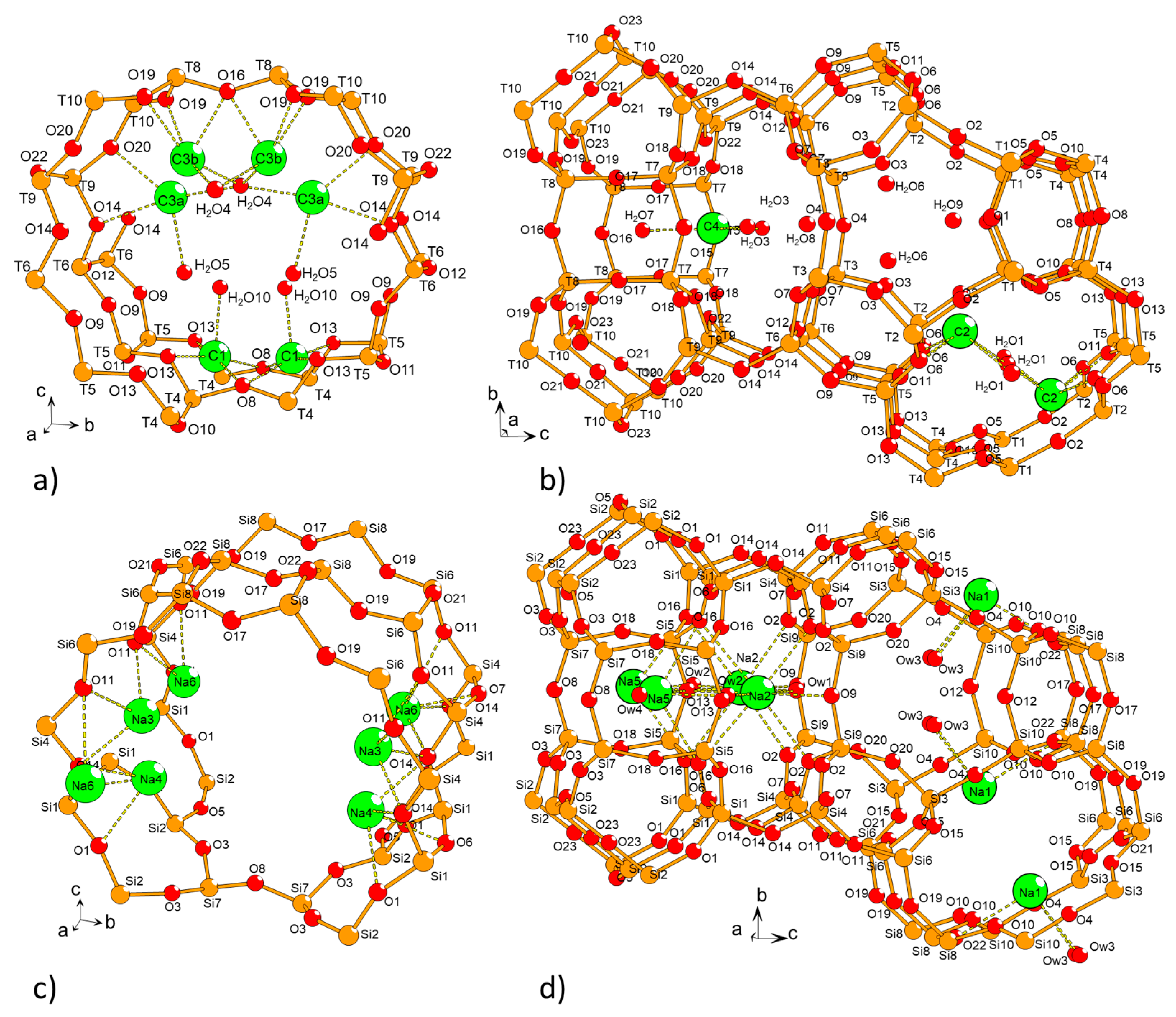
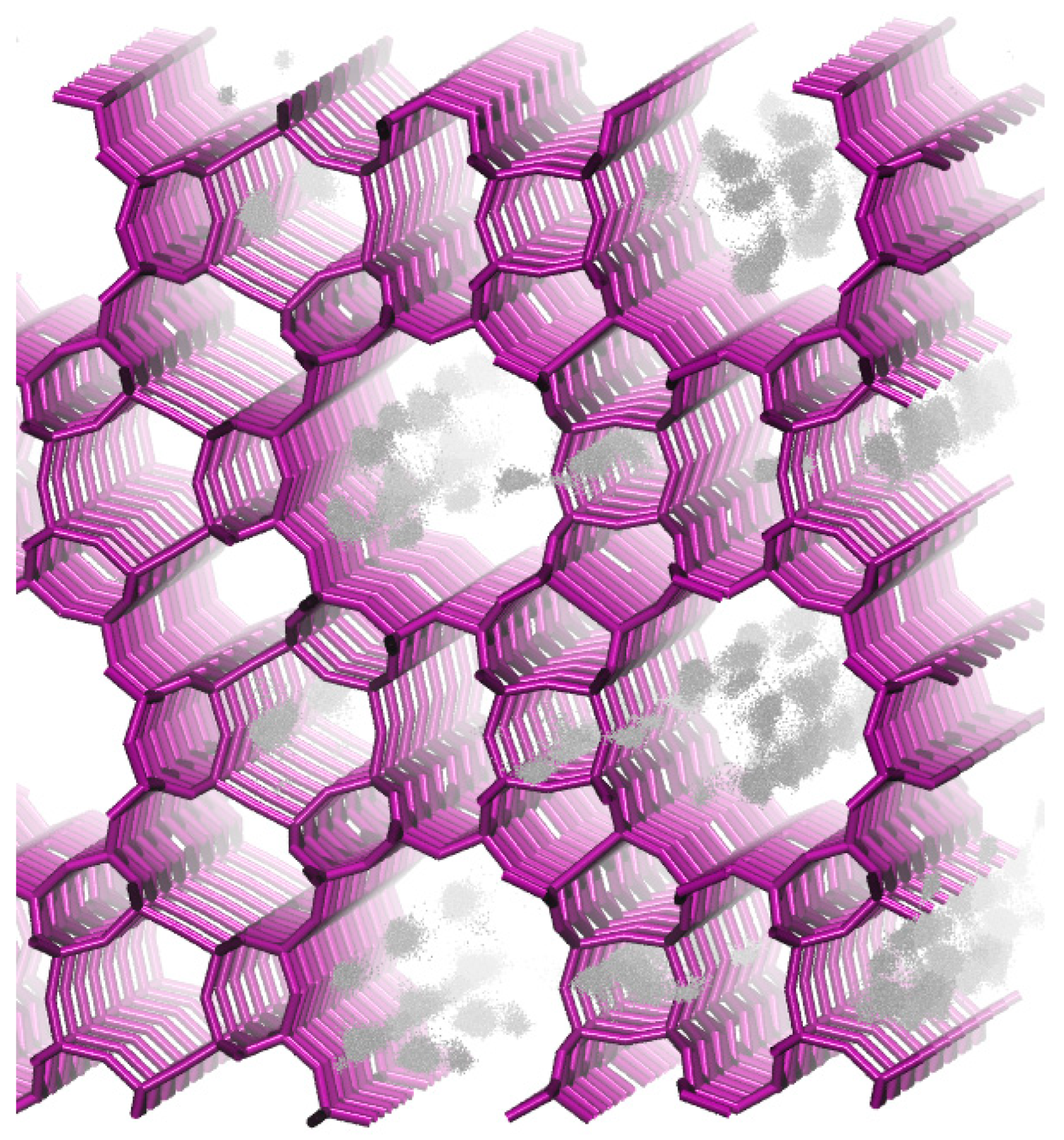
| Parameters for the Non-Bonded Interactions | |||
|---|---|---|---|
| Atom | ε/kB (K) | σ (Å) | q (e) |
| H(H2O) | 0 | 0 | 0.241 |
| O(H2O) | 80.52 | 3.12 | 0 |
| L(H2O) (lone pair interaction site) | 0 | 0 | −0.241 |
| O (zeolite) | 78.20 | 3.17 | −1.094 |
| Si (zeolite) | 0.93 × 10−3 | 3.30 | 2.10 |
| Al (zeolite) | 0.93 × 10−3 | 3.30 | 1.58 |
| Na+ | 65.47 | 2.35 | 1.00 |
| Kinematical Refinement | Dynamical Refinement | |
|---|---|---|
| Refined empirical formula | Na8.84Si60O120 | Na8.16Si60O124.52 |
| Formula weight | 3802.0 | 3869.5 |
| Temperature/K | 103.0 | |
| Crystal system | orthorhombic | |
| Space group | Pmmn (#59) | |
| a/Å | 7.487 (3) | |
| b/Å | 17.846 (12) | |
| c/Å | 25.655 (8) | |
| α = β = γ/° | 90 | |
| V/Å3 | 3427.60 (15) | |
| Z | 1 | |
| ρcalc g/cm3 | 1.842 | 1.875 |
| F(000) | 629.896 | 635.553 |
| Crystal size/nm | ~400 × ~200 | |
| Data collection | TEM Philips CM200, | |
| Collection mode | Precession-assisted 3D ED | |
| Source (wavelength) | electrons (λ = 0.02508 Å) | |
| 2θ range for data collection/° | 0.05 to 1.01 | |
| Index ranges | −8 ≤ h ≤ 9, −23 ≤ k ≤ 23, −31 ≤ l ≤ 26 | |
| Reflections collected | 4367 | 17,481 |
| Independent reflections | 438 (Rint = 0.1837) | 1040 (no averaging done) |
| Data coverage for sinθ/λ = 0.6 Å−1 | 92.4% | 93% |
| Data/restraints/constraints/parameters | 4367/0/137 | 17481/17/32/275 |
| Avg./Min/Max crystal thickness | - | 526/25/2386 Å |
| gmax, RSg(max) | - | 1.3, 0.5 |
| Goodness-of-fit on F2 | obs: 2.34, all: 1.281 | obs: 2.62, all: 0.7976 |
| Final R values [I ≥ 3σ(I)] | Robs = 0.169, wR2obs = 0.358 | Robs = 0.097, wR2obs = 0.1798 |
| Final R values [all data] | Rall = 0.604, wR2all = 0.641 | Rall = 0.517, wR2all = 0.2245 |
| Gualtieri et al. [10] | This Work | ||||||
|---|---|---|---|---|---|---|---|
| C1 | O13 | 2× | 2.25(4) | Na1 | Ow3 | 2× | 2.48(8) |
| H2O10 | 1× | 2.36(9) | O22 | 1× | 2.72(4) | ||
| O8 | 2× | 2.36(5) | O10 | 2× | 2.98(2) | ||
| O5 | 2× | 2.95(7) | Na2 | Ow2 | 1× | 2.29(2) | |
| O11 | 1× | 3.03(7) | Ow1 | 1× | 2.32(3) | ||
| C2 | O11 | 1× | 2.28(4) | O2 | 2× | 2.64(1) | |
| H2O1 | 2× | 2.65(2) | O13 | 1× | 2.66(2) | ||
| O6 | 2× | 2.76(2) | O9 | 1× | 2.79(2) | ||
| H2O6 | 1× | 3.06(5) | O16 | 2× | 2.85(2) | ||
| C3 | H2O4 | 1× | 2.35(2) | Na3 | O14 | 2× | 2.41(6) |
| H2O5 | 1× | 2.49(4) | O11 | 2× | 2.59(5) | ||
| O20 | 1× | 2.58(3) | Na4 | O6 | 1× | 2.46(6) | |
| O14 | 1× | 2.62(3) | O14 | 2× | 2.52(5) | ||
| C3b | O16 | 1× | 2.40(4) | O1 | 2× | 2.57(5) | |
| H2O4 | 2× | 2.47(4) | Na5 | Ow2 | 1× | 2.44(1) | |
| O19 | 2× | 2.50(4) | O13 | 1× | 2.73(1) | ||
| C4 | H2O7 | 1× | 2.37(6) | O16 | 2× | 2.83(8) | |
| H2O3 | 2× | 2.51(4) | O18 | 2× | 3.08(1) | ||
| O22 | 2× | 2.954(4) | Na6 | O14 | 2× | 2.33(3) | |
| O7 | 1× | 2.41(6) | |||||
| O11 | 2× | 2.84(4) | |||||
| H2O/Unit Cell | Na1 | Na2 | Na3 | Na4 | Na5 | Na6 | Others | |
|---|---|---|---|---|---|---|---|---|
| 0 | Nb. of cations | 3.05 | 2.79 | 1.50 | 0.03 | 0.00 | 0.49 | 3.14 |
| s.o.f. (MC@103 K) a | 0.76 | 0.70 | 0.38 | 0.01 | 0.00 | 0.16 | ||
| 4 | Nb. of cations | 2.41 | 3.00 | 1.13 | 0.16 | 0.00 | 0.35 | 3.95 |
| s.o.f. (MC@103 K) a | 0.60 | 0.75 | 0.28 | 0.04 | 0.00 | 0.09 | ||
| s.o.f. (3D ED) b | 0.507 | 0.789 | 0.212 | 0.209 | 0.082 | 0.240 |
| Ow1 | Ow2 | Ow3 | Ow4 | Na5 | Others | |
|---|---|---|---|---|---|---|
| Nb. of H2O molecules | 0.50 | 0.38 | 0.89 | 0.13 | 0.32 | 2.10 |
| s.o.f. (MC-103K) a | 0.25 | 0.19 | 0.11 | 0.06 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Örs, T.; Deroche, I.; Chatelard, C.; Dodin, M.; Martinez-Franco, R.; Tuel, A.; Paillaud, J.-L. Determination of Na+ Cation Locations in Nanozeolite ECR-1 Using a 3D ED Method. Symmetry 2024, 16, 477. https://doi.org/10.3390/sym16040477
Örs T, Deroche I, Chatelard C, Dodin M, Martinez-Franco R, Tuel A, Paillaud J-L. Determination of Na+ Cation Locations in Nanozeolite ECR-1 Using a 3D ED Method. Symmetry. 2024; 16(4):477. https://doi.org/10.3390/sym16040477
Chicago/Turabian StyleÖrs, Taylan, Irena Deroche, Corentin Chatelard, Mathias Dodin, Raquel Martinez-Franco, Alain Tuel, and Jean-Louis Paillaud. 2024. "Determination of Na+ Cation Locations in Nanozeolite ECR-1 Using a 3D ED Method" Symmetry 16, no. 4: 477. https://doi.org/10.3390/sym16040477
APA StyleÖrs, T., Deroche, I., Chatelard, C., Dodin, M., Martinez-Franco, R., Tuel, A., & Paillaud, J.-L. (2024). Determination of Na+ Cation Locations in Nanozeolite ECR-1 Using a 3D ED Method. Symmetry, 16(4), 477. https://doi.org/10.3390/sym16040477









