Synthesis, X-ray Diffraction and Computational Druglikeness Evaluation of New Pyrrolo[1,2-a][1,10]Phenanthrolines Bearing a 9-Cyano Group
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentations
2.2. Synthesis and Characterization of Compounds 5a–c
2.3. X-ray Structural Analysis
2.4. Computational Details
3. Results
3.1. Synthesis
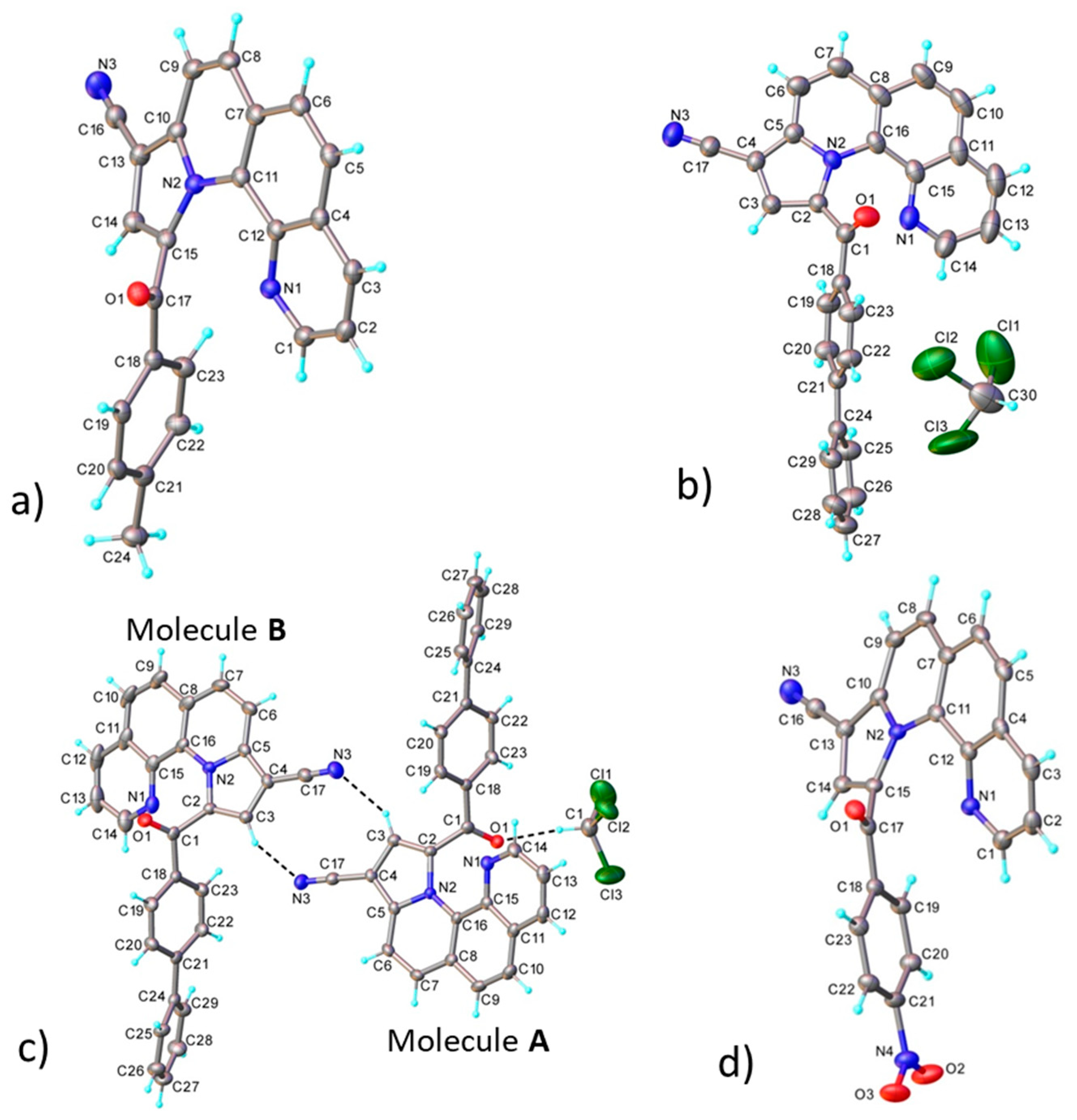
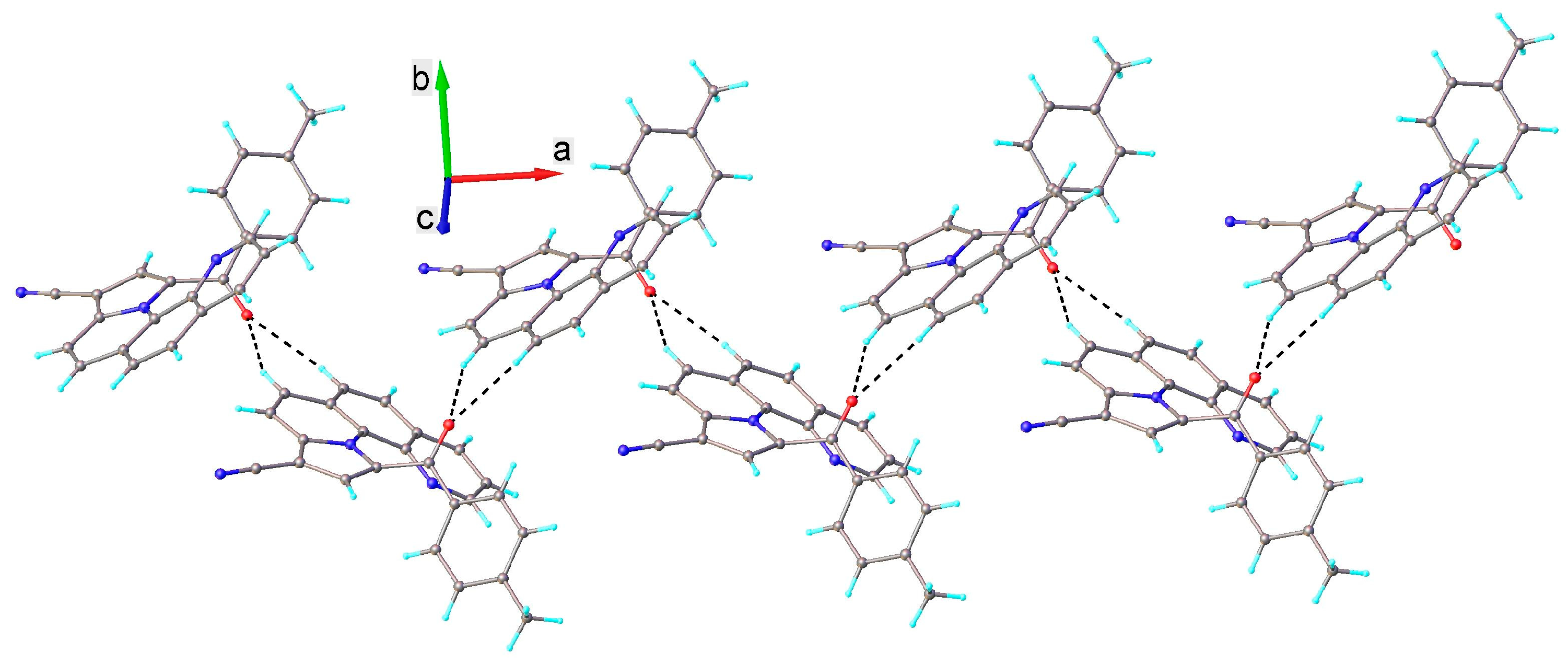
3.2. X-ray Crystallography
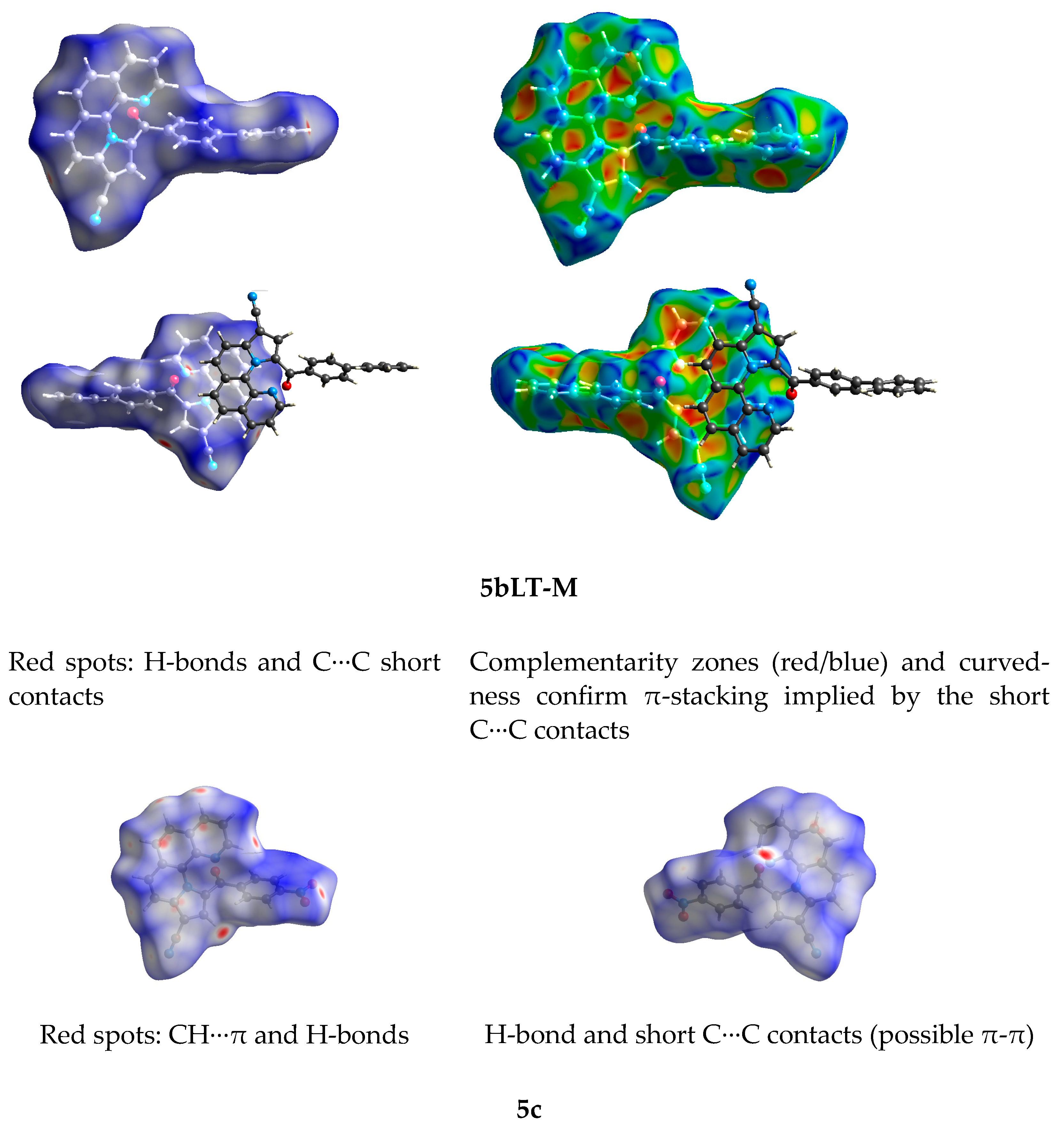
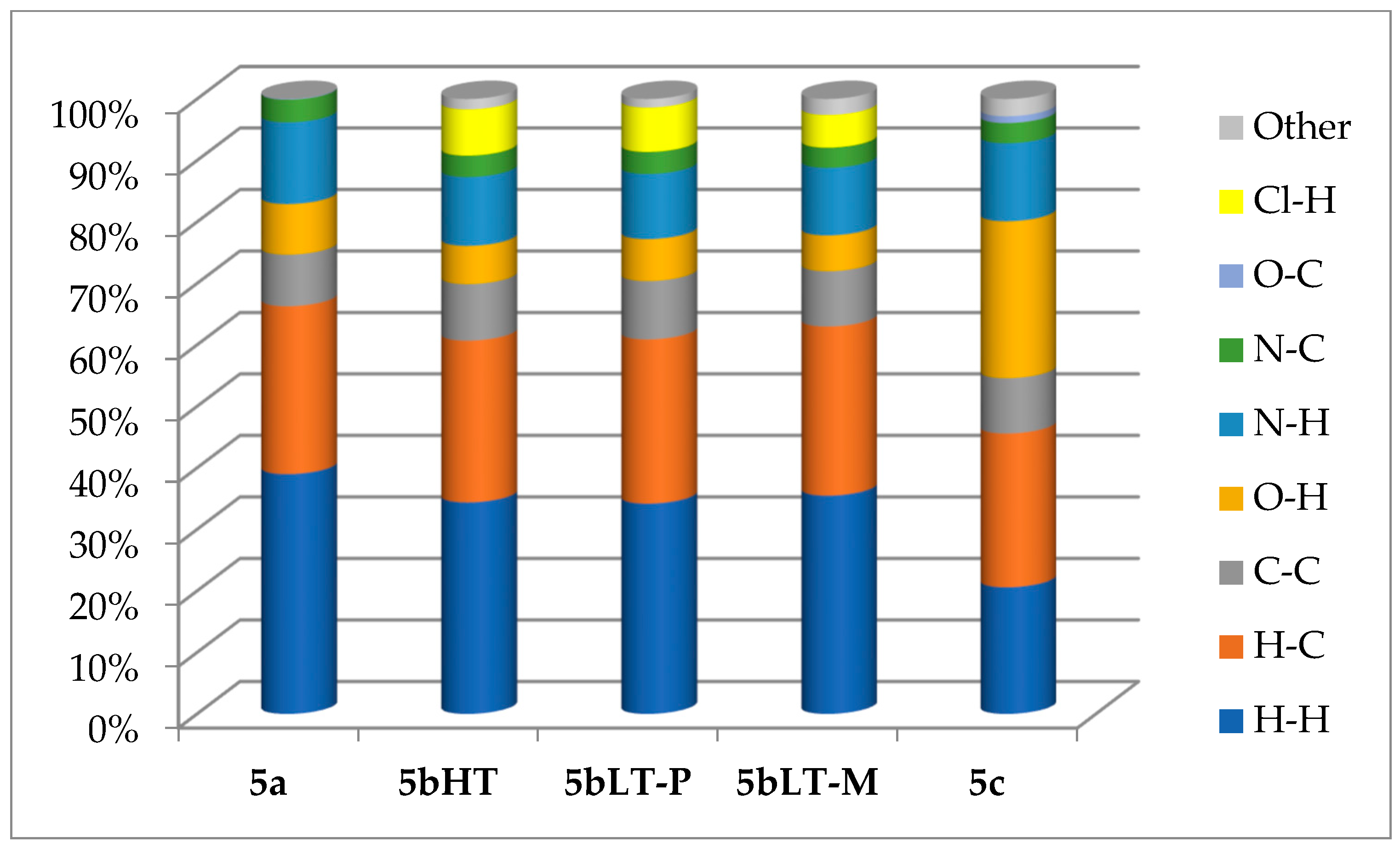
3.3. Hirshfeld Surface Analysis
3.4. Druglikeness Assessment
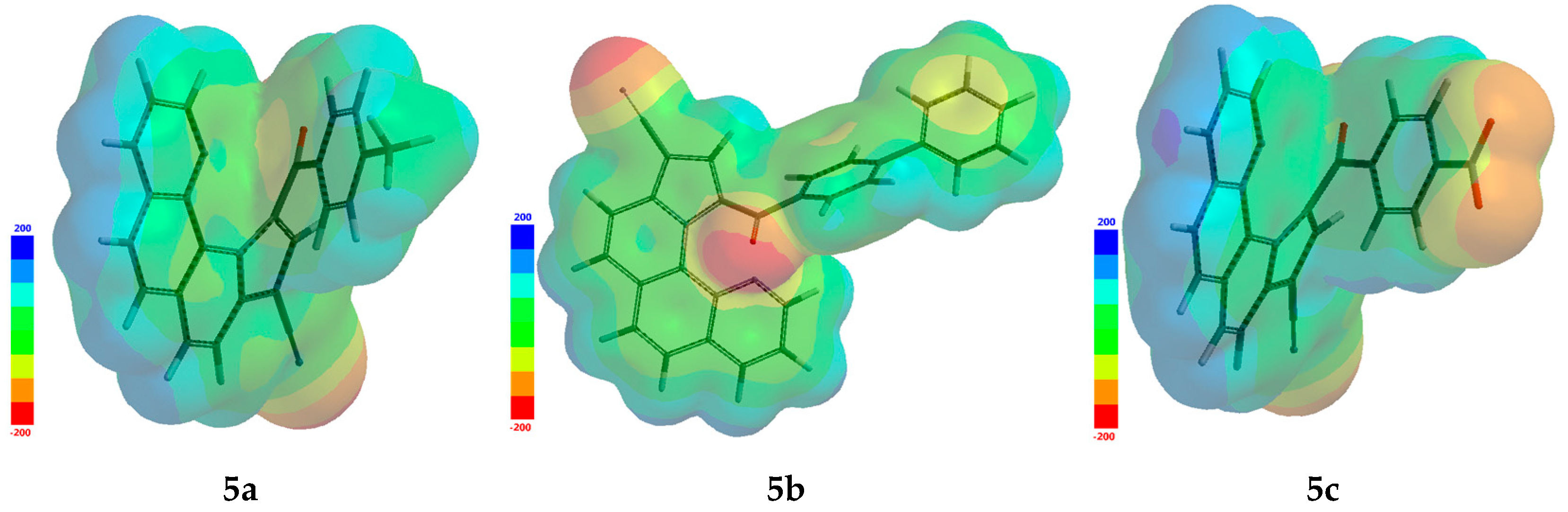
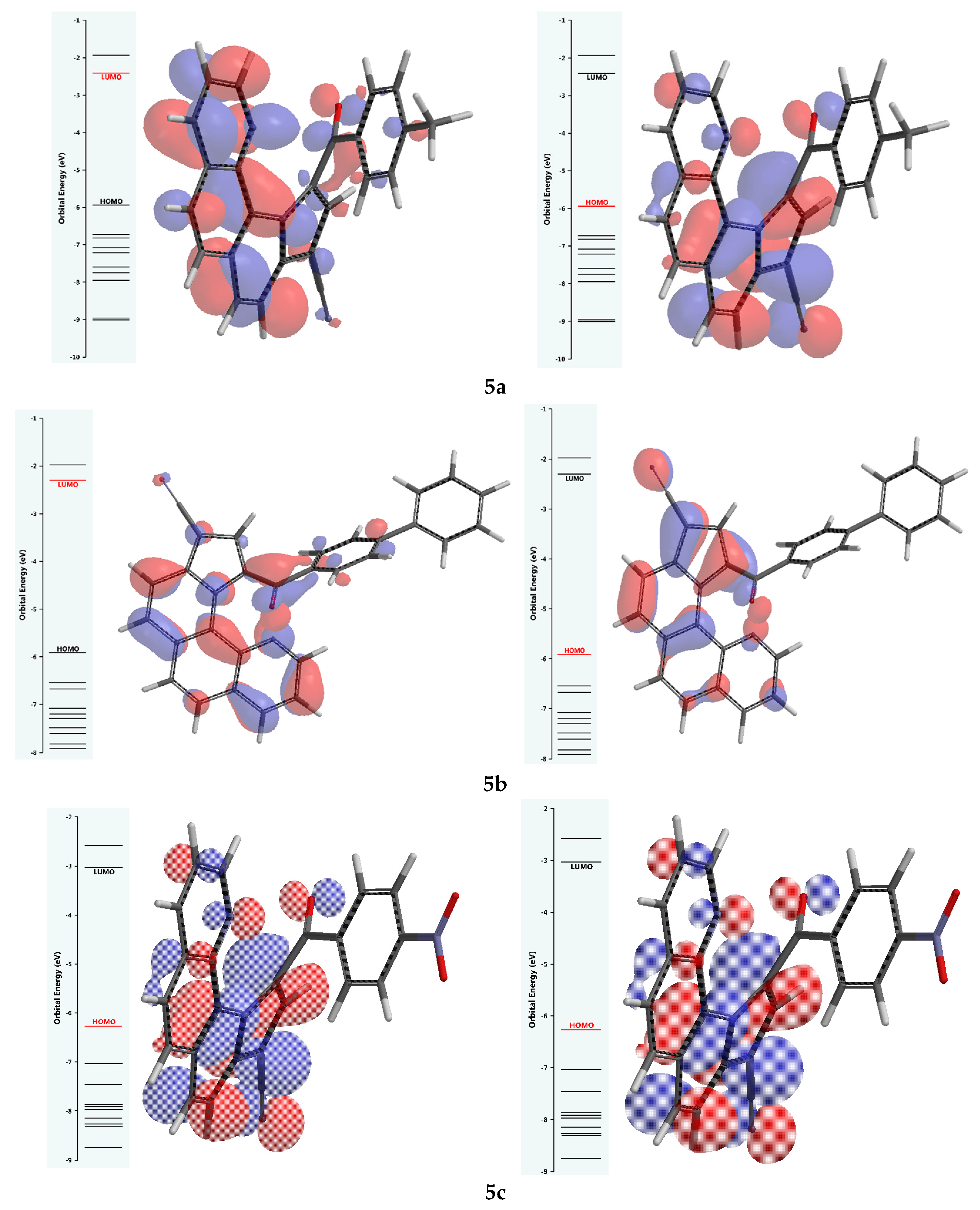
3.5. Quantum Reactivity Parameters Calculated from FMO Predicted Energy Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Dhinamkaran, I.; Padmini, V.; Ganesan, K.; Selvarasu, K. A-One Pot Four Component and Microwave-Assisted Synthesis of Pyrrolo[1,10]phenanthrolines. ChemistrySelect 2017, 2, 6154–6158. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Vysotsky, M.O. Phenanthroline ligands with divergent pyridine units. J. Chem. Res. 2009, 2009, 133–136. [Google Scholar] [CrossRef]
- Liu, T.-F.; Lin, H.-K.; Chen, L.-X.; Zhu, S.-R.; Wang, Z.-M.; Su, X.-C.; He, X.-W. Syntheses of the novel thiamacrocycles containing the 1,10-phenanthroline unit. J. Chem. Res. 2004, 2004, 374–375. [Google Scholar] [CrossRef]
- Montalban, A.G.; Herrera, A.J.; Johannsen, J. Phenanthroline dipyrromethene conjugates: Potential building blocks for the construction of novel supramolecular architectures. Tetrahedron Lett. 2010, 51, 2917–2919. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; Habibi-Khorassani, S.M.; Hazeri, N.; Heydari, R.; Marandi, R.G.; Nassiri, M. The new γ-spiroiminolactone synthesis by reaction between alkyl or aryl isocyanides and 1,10-phenanthroline-5,6-dione in the presence of acetylenic esters. J. Chem. Res. 2006, 2006, 220–222. [Google Scholar] [CrossRef]
- Danac, R.; Al Matarneh, C.M.; Shova, S.; Daniloaia, T.; Balan, M.; Mangalagiu, I.I. New indolizines with phenanthroline skeleton: Synthesis, structure, antimycobacterial and anticancer evaluation. Bioorg. Med. Chem. 2015, 23, 2318–2327. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Shova, S.; Mangalagiu, I.I.; Danac, R. Synthesis, structure, antimycobacterial and anticancer evaluation of new pyrrolo-phenanthroline derivatives. J. Enz. Inhib. Med. Chem. 2016, 31, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, F.; Caira, M.R.; Draghici, C.; Caproiu, M.T.; Barbu, L.; Miu, B. New 1,10-phenanthroline derivatives with potential antitumoral activity. Rev. Roum. Chim. 2008, 53, 183–187. [Google Scholar]
- Roy, S.; Hagan, K.D.; Maheswari, P.U.; Lutz, M.; Spek, A.L.; Reedijk, J.; van Wezel, G.P. Phenanthroline derivatives with improved selectivity as DNA-targeting anticancer or antimicrobial drugs. ChemMedChem 2008, 3, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Sall, C.; Yapi, A.-D.; Desbois, N.; Chevalley, S.; Chezal, J.-M.; Tan, K.; Teulade, J.-C.; Valentin, A.; Blache, Y. Design, synthesis, and biological activities of conformationally restricted analogs of primaquine with a 1,10-phenanthroline framework. Bioorg. Med. Chem. Lett. 2008, 18, 4666–4669. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Larsen, A.F.; Abdikadir, F.H.; Ulven, T. Phenanthroline- 2,9-bistriazoles as selective G-quadruplex ligands. Eur. J. Med. Chem. 2014, 72, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wesselinova, D.; Neykov, M.; Kaloyanov, N.; Toshkova, R.; Dimitrov, G. Antitumour activity of novel 1,10-phenanthroline and 5-amino-1,10-phenanthroline derivatives. Eur. J. Med. Chem. 2009, 44, 2720–2723. [Google Scholar] [CrossRef] [PubMed]
- Leontie, L.; Druta, I.; Danac, R.; Rusu, G.I. On the electronic transport properties of pyrrolo[1,2-a][1,10]phenanthroline derivatives in thin films. Synth. Met. 2005, 155, 138–145. [Google Scholar] [CrossRef]
- Leontie, L.; Druta, I.; Danac, R.; Prelipceanu, M.; Rusu, G.I. Electrical properties of some new high resistivity organic semiconductors in thin films. Prog. Org. Coat. 2005, 54, 175–181. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Danac, R.; Leontie, L.; Tudorache, F.; Petrila, I.; Iacomi, F.; Carlescu, A.; Nedelcu, G.; Mangalagiu, I. Synthesis and electron transport properties of some new 4,7-phenanthroline derivatives in thin films. Environ. Eng. Manag. J. 2015, 14, 421–431. [Google Scholar]
- Prelipceanu, M.; Prelipceanu, O.S.; Leontie, L.; Danac, R. Photoelectron spectroscopy investigations of pyrrolo[1,2-a][1,10]phenanthroline derivatives. Phys. Lett. A 2007, 368, 331–335. [Google Scholar] [CrossRef]
- Cristea, M.; Răducă, M.; Shova, S.; Drăghici, C.; Neacşu, V.A.; Maganu, M.; Loredana Albotă (Barbu), L.; Dumitrescu, D.; Dumitrascu, F. Synthesis, Crystal Structure and Photoluminescent Properties of Novel 9-Cyano-Pyrrolo[1,2-a][1,10]Phenanthrolines. Crystals 2024, 14, 67. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Mitan, C.I.; Draghici, C.; Caproiu, M.T.; Raileanu, D. Primary cycloadducts of 1,10-phenanthrolinium and phthalazinium phenacylides with DMAD. Tetrahedron Lett. 2001, 42, 8379–8382. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Caira, M.R.; Draghici, C.; Caproiu, M.T.; Barbu, L.; Dumitrescu, D.G. Enhancing the helical distortion in pyrrolo[1,2-a][1,10]phenanthrolines. Arkivoc 2010, 9, 97–107. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Dumitrescu, D.G.; Aron, I. Azahelicenes and other similar tri and tetracyclic helical molecules. Arkivoc 2010, 1, 1–32. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; Wiley: New York, NY, USA, 1994; pp. 1163–1166. [Google Scholar]
- Dumitrascu, F.; Mitan, C.I.; Drăghici, C.; Căproiu, M.T. New pyrrolo[1,2-a][1,10]phenanthrolines with helical chirality. Rev. Roum. Chim. 2002, 47, 881–884. [Google Scholar]
- Dumitrascu, F.; Mitan, C.I.; Drăghici, C.; Căproiu, M.T.; Răileanu, D. Helical chirality of new pyrrolo [1,2-a]([1,10]) phenanthrolines. Rev. Chim. 2002, 53, 787. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Caira, M.R.; Draghici, C.; Caproiu, M.T.; Badoiu, A. 1,3-Dipolar Cycloaddition Reactions of 1-(4-Phenylphenacyl)-1,10-phenanthrolinium N-Ylide with Activated Alkynes and Alkenes. Molecules 2005, 10, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, F.; Draghici, C.; Caira, M.R.; Badoiu, A.; Barbu, L.; Cristea, M. 1,3-Dipolar cycloaddition reactions of 1-(3-nitrophenacyl)-1,10-phenanthrolinium N-ylide with activated alkynes. Arkivoc 2005, 10, 165–173. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Caira, M.R.; Drăghici, C.; Căproiu, M.T.; Barbu, L.; Bădoiu, A. Helical chirality of pyrrolo[1,2-a][1,10]phenanthroline derivatives. J. Chem. Crystallogr. 2005, 35, 361–365. [Google Scholar] [CrossRef]
- Dumitrascu, F.; Caira, M.R.; Draghici, C.; Caproiu, M.T. Crystal Structure of a New Pyrrolo[1,2-a][1,10]phenanthroline Derivative. Anal. Sci. X-ray Struct. Anal. Online 2007, 23, X13–X14. [Google Scholar] [CrossRef][Green Version]
- Danac, R.; Rotaru, A.; Drochioiu, G.; Druta, I. Synthesis of Novel Phenanthroline Derivatives by 3+2 Dipolar Cycloadition Reaction. J. Heterocycl. Chem. 2003, 40, 283–287. [Google Scholar] [CrossRef]
- Danac, R.; Constantinescu, M.; Rotaru, A.; Vlahovici, A.; Cretescu, I.; Druta, I. Study of Dipolar 3+2 Cycloaddition Reaction of 1,10-Phenanthrolinium Ylides to Activated Alkenes. Rev. Chim. 2005, 56, 85–88. [Google Scholar]
- Dumitrascu, F.; Caira, M.R.; Draghici, C.; Caproiu, M.T.; Barbu, L. Isolation and X-Ray Structure of an Intermediate in 1,3-Dipolar Cycloaddition of 1,10-Phenanthrolinium N-Ylides with Alkynes: 1,2-Dihydropyrrolo-[1,2-a][1,10]phenanthroline. Rev. Chim. 2009, 60, 851–854. [Google Scholar]
- Dürüst, Y.; Sağırlı, A.; Fronczek, F.R. Regioselective 1,3-dipolar cycloaddition of phenanthrolinium N-ylides to substituted arylidene oxazolones. Mol. Divers. 2011, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Fang, J.; Yan, C.G. Diastereoselective Synthesis of 1,10-Dihydropyrrolo[1,2-a][1,10]phenanthroline Derivatives via 1,3-Dipolar Cycloaddition Reaction. Chem. Res. Chin. Univ. 2013, 29, 1089–1093. [Google Scholar] [CrossRef]
- Paira, R.; Anwar, T.; Banerjee, M.; Bharitkar, Y.P.; Mondal, S.; Kundu, S.; Hazra, A.; Maulik, P.R.; Nirup, B.; Mondal, N.B. Copper–phenanthroline catalysts for regioselective synthesis of pyrrolo[3′,4′:3,4]pyrrolo[1,2-a]furo-quinolines/phenanthrolines and of pyrrolo[1,2-a]phenanthrolines under mild conditions. Beilstein J. Org. Chem. 2014, 10, 692–700. [Google Scholar]
- Al Matarneh, C.M.; Ciobanu, C.I.; Apostu, M.O.; Mangalagiu, I.I.; Danac, R. Cycloaddition versus amidation in reactions of 2-amino-2-oxoethyl-phenanthrolinium ylides to activated alkynes and alkenes. C. R. Chim. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Al-Matarneh, C.M.; Rosca, I.; Shova, S.; Danac, R. Synthesis and properties of new fused pyrrolo-1,10-phenanthroline type derivatives. J. Serb. Chem. Soc. 2021, 86, 901–915. [Google Scholar] [CrossRef]
- Marandi, G.; Hazeri, N.; Maghsoodlou, M.T.; Habibi-Khorassani, S.M.; Torbati, N.A.; Rostami-Charati, F.; Skelton, B.W.; Makhad, M. Synthesis of Cyano-pyrrolo[1,2-a][1,10]phenanthroline Derivatives Using a Multicomponent Condensation. J. Heterocycl. Chem. 2013, 50, 568–572. [Google Scholar] [CrossRef]
- Kitahara, Y.; Mizuno, T.; Kubo, A. Synthetic studies of benzo[b]pyrrolo[4,3,2-de][1,10]phenanthroline. Tetrahedron 2004, 60, 4283–4288. [Google Scholar] [CrossRef]
- Li, M.; Lv, X.-L.; Wen, L.-R.; Hu, Z.-Q. Direct solvent-free regioselective construction of pyrrolo[1,2-a][1,10]phenanthrolines based on isocyanide-based multicomponent reactions. Org. Lett. 2013, 15, 1262–1265. [Google Scholar] [CrossRef]
- Heydari, R.; Tahamipour, B. Highly regioselective synthesis of dicyano-8a,10,11-tetrahydropyrrolo [1,2-a][1,10]phenanthrolines via a domino-Knoevenagel-cyclization. Chin. Chem. Lett. 2011, 22, 1281–1284. [Google Scholar] [CrossRef]
- Tahamipour, B.; Heydari, R.; Torbati, N.A.; Ziyaadini, M.; Graif, C. Diastereoselective synthesis and X-ray structure of new stable dicyano(8aRS,10SR,11SR)-9,9-dicyano-10-aryl-11-benzoyl-8a,9,10,11-tetrahydropyrrolo[1,2-a][1,10]phenanthrolines. J. Chem. Res. 2011, 35, 329–332. [Google Scholar] [CrossRef]
- Heydari, R.; Tahamipour, B.; Torbati, N.A.; Graiff, C.; Ziyaadini, M. One-pot synthesis and X-ray structure of new, stable tetrahydropyrrolo [1,2-a][1,10]phenanthrolines with four diastereoisomeric centers. Synth. Commun. 2013, 43, 2031–2041. [Google Scholar] [CrossRef]
- Al-Matarneh, C.M.; Ciobanu, C.-I.; Mangalagiu, V.; Zbancioc, G.; Danac, R. Microwave assisted synthesis of six-member ring azaheterocycles and their antimycobacterial and anticancer evaluation. Rev. Chim. 2020, 71, 287–293. [Google Scholar] [CrossRef]
- Al Matarneh, C.M.; Amarandi, R.M.; Craciun, A.M.; Mangalagiu, I.I.; Zbancioc, G.; Danac, R. Design, Synthesis, Molecular Modelling and Anticancer Activities of New Fused Phenanthrolines. Molecules 2020, 25, 527. [Google Scholar] [CrossRef]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-containing pharmaceuticals: Efficacious roles of the nitrile pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Li, X.; Yu, Z.; Song, C.; Du, Y. Nitrile-containing pharmaceuticals: Target, mechanism of action, and their SAR studies. RSC Med. Chem. 2021, 12, 1650–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hong, L. Application of nitrile in drug design. Chin. J. Org. Chem. 2012, 32, 1643–1652. [Google Scholar] [CrossRef]
- Vasile (Corbei), A.-A.; Ungureanu, E.-M.; Stanciu, G.; Cristea, M.; Stefaniu, A. Evaluation of (Z)-5-(Azulen-1-ylmethylene)-2-thioxothiazolidin-4-ones Properties Using Quantum Mechanical Calculations. Symmetry 2021, 13, 1462. [Google Scholar] [CrossRef]
- Stefaniu, A.; Pirvu, L.C. In Silico Study Approach on a Series of 50 Polyphenolic Compounds in Plants; A Comparison on the Bioavailability and Bioactivity Data. Molecules 2022, 27, 1413. [Google Scholar] [CrossRef]
- Pirvu, L.C.; Neagu, G.; Albulescu, A.; Stefaniu, A.; Pintilie, L. Potential Benefits of Dietary Plant Compounds on Normal and Tumor Brain Cells in Humans: In Silico and In Vitro Approaches. Int. J. Mol. Sci. 2023, 24, 7404. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis Pro Software System; Rigaku Corporation: Oxford, UK, 2015; CrysAlis Pro v. 1.171.38.46. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.J. A Guide to Molecular Mechanics and Quantum Chemical Calculations; Wavefunction, Inc.: Irvine, CA, USA, 2003. [Google Scholar]
- Hu, Y.; Hu, H. A Versatile Oxidizing Agent: Tetrakis-pyridino-cobalt(II) Dichromate Py4Co(HCrO4)2 (TPCD). Oxidations of Alcohols, Halides and Amines to Their Corresponding Carbonyl Compounds. Synth. Commun. 1992, 22, 1491–1496. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.F. Helicenes: Synthesis and applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef] [PubMed]
- D’Arminio, N.; Ruggiero, V.; Pierri, G.; Marabotti, A.; Tedesco, C. Emerging role of carbonyl–carbonyl interactions in the classification of beta turns. Protein Sci. 2024, 33, e4868. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, J.L.; Thawani, A.R.; Fajardo-Ruiz, E.; Meiring, J.C.; Heise, C.; White, A.J.P.; Akhmanova, A.; Brandt, J.R.; Thorn-Seshold, O.; Fuchter, M.J. [5]-Helistatins: Tubulin-Binding Helicenes with Antimitotic Activity. JACS Au 2022, 2, 2561–2570. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Yankova, R.; Genieva, S.; Halachev, N.; Dimitrova, G. Molecular structure, vibrational spectra, MEP, HOMO-LUMO and NBO analysis of Hf(SeO3)(SeO4)(H2O)4. J. Mol. Struct. 2016, 1106, 82–88. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent Advances in the Concept of Hard and Soft Acids and Bases. J. Chem. Educ. 1987, 64, 561–567. [Google Scholar] [CrossRef]
- Pearson, R.G.; Songstad, J. Application of the Principle of Hard and Soft Acids and Bases to Organic Chemistry. J. Am. Chem. Soc. 1967, 89, 1827–1836. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]




| Compound | 5a | 5bHT | 5bLT | 5c |
|---|---|---|---|---|
| Emp. formula | C24H15N3O | C29H17N3O·0.5(CHCl3) | C29H17N3O·0.5(CHCl3) | C23H12N4O3 |
| Fw | 361.39 | 483.14 | 483.14 | 392.37 |
| T [K] | 100 | 293 | 160 | 100 |
| space group | Pbca | P-1 | P-1 | I2/a |
| a [Å] | 11.3181(2) | 8.0034(6) | 12.1617(5) | 24.8719(5) |
| b [Å] | 12.4550(2) | 12.3093(12) | 13.3458(5) | 5.80280(9) |
| c [Å] | 24.9296(4) | 12.4079(10) | 15.9128(7) | 27.2343(6) |
| α [°] | 90 | 77.473(8) | 114.311(4) | 90 |
| β [°] | 90 | 78.838(7) | 92.757(3) | 116.219(3) |
| γ [°] | 90 | 87.836(7) | 98.816(4) | 90 |
| V [Å3] | 3514.25(10) | 1170.70(18) | 2308.13(18) | 3526.21(13) |
| Z | 8 | 2 | 4 | 8 |
| ρcalcd [g cm−3] | 1.366 | 1.371 | 1.390 | 1.478 |
| μ [mm−1] | 0.681 | 0.249 | 0.253 | 0.835 |
| Crystal size [mm] | 0.23 × 0.12 × 0.03 | 0.35 × 0.30 × 0.30 | 0.25 × 0.20 × 0.15 | 0.35 × 0.03 × 0.02 |
| 2Θ range | 7.09 to 133.10 | 3.42 to 50.05 | 3.38 to 50.05 | 7.24 to 133.16 |
| Refls. collected | 13,627 | 10,142 | 21,753 | 11,594 |
| Indep. refls., Rint | 3096, 0.0281 | 4136, 0.0247 | 8145, 0.0373 | 3113, 0.0290 |
| Data/rests./params. | 3096/0/255 | 4136/0/335 | 8145/0/631 | 3113/0/272 |
| GOF | 1.048 | 1.032 | 1.026 | 1.065 |
| R1, wR2(all data) | 0.0350, 0.0967 | 0.0628, 0.1740 | 0.0631, 0.1629 | 0.040, 0.0956 |
| CCDC no. | 2352573 | 2354481 | 2354482 | 2352575 |
| Compound | Dihedral Angle ◦ | d (C, N), Å | % Sum of vdW Radii |
|---|---|---|---|
| 5a | 21.11 | 2.529 | 77.82 |
| 5bLT-A | 20.54 | 2.532 | 77.91 |
| 5bLT-B | 19.80 | 2.514 | 77.35 |
| 5c | 25.06 | 2.527 | 77.75 |
| 4-CNBenzoyl [19] | 21.74 | 2.532 | 77.91 |
| Benzoyl [19] | 20.53 | 2.553 | 78.55 |
| 4-MeOBenzoyl [19] | 20.82 | 2.551 | 78.49 |
| 4-HexylBenzoyl [19] | 26.62 | 2.585 | 79.54 |
| AQIKER [20b] | 24.16 | 2.436 | 74.95 |
| QAQCIV [21c] | 18.81 | 2.513 | 77.32 |
| ZIPQUM [24a] | 25.54 | 1.8513 | 67.32 |
| Property (Symbol), Units | 5a | 5b | 5c |
|---|---|---|---|
| Molecular weight (M) g·mol−1 | 361.404 | 423.475 | 392.374 |
| Area (A), Å2 | 366.81 | 431.64 | 371.88 |
| Volume (V), Å3 | 370.10 | 435.72 | 373.26 |
| Polar surface area (PSA), Å2 | 32.552 | 33.008 | 71.158 |
| Water-octanol partition coefficient (logP) | 5.53 | 6.72 | 3.33 |
| Ovality index (OI) | 1.47 | 1.55 | 1.48 |
| Polarizability (α), 10−30·m3 | 70.56 | 75.87 | 70.89 |
| Minimum electrostatic potential, kJ·mol−1 | −209.66 | −208.91 | −194.19 |
| Maximum electrostatic potential, kJ·mol−1 | 141.24 | 130.72 | 157.19 |
| Dipole moment (D), Debye | 9.79 | 7.01 | 7.92 |
| Number of hydrogen bond donors (HBD) | 0 | 0 | 0 |
| Number of hydrogen bond acceptors (HBA) | 4 | 4 | 7 |
| Parameter | Formula | 5a | 5b | 5c |
|---|---|---|---|---|
| Energy of the HOMO orbital (EHOMO), eV | −5.95 | −5.92 | −6.26 | |
| Energy of the LUMO orbital (ELUMO), eV | −2.40 | −2.30 | −3.03 | |
| Ionization potential (I), eV | I = −EHOMO | 5.95 | 5.92 | 6.26 |
| Electron affinity (A), eV | A = −ELUMO | 2.40 | 2.30 | 3.03 |
| FMO energy gap (ΔE), eV | ΔE = I − A | 3.55 | 3.62 | 3.23 |
| Electronegativity (χ), eV | χ = (I + A)/2 | 4.175 | 4.11 | 4.645 |
| Global hardness (η), eV | η = (I − A)/2 | 1.775 | 1.81 | 1.615 |
| Softness (σ), eV−1 | σ = 1/η | 0.563 | 0.553 | 0.619 |
| Global electrophilicity index, D·eV−1 | ω = μ2/2η | 26.998 | 13.575 | 19.420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristea, M.; Popa, M.M.; Shova, S.; Gdaniec, M.; Stefaniu, A.; Draghici, C.; Raduca, M.; Banu, N.D.; Dumitrascu, F. Synthesis, X-ray Diffraction and Computational Druglikeness Evaluation of New Pyrrolo[1,2-a][1,10]Phenanthrolines Bearing a 9-Cyano Group. Symmetry 2024, 16, 911. https://doi.org/10.3390/sym16070911
Cristea M, Popa MM, Shova S, Gdaniec M, Stefaniu A, Draghici C, Raduca M, Banu ND, Dumitrascu F. Synthesis, X-ray Diffraction and Computational Druglikeness Evaluation of New Pyrrolo[1,2-a][1,10]Phenanthrolines Bearing a 9-Cyano Group. Symmetry. 2024; 16(7):911. https://doi.org/10.3390/sym16070911
Chicago/Turabian StyleCristea, Mihaela, Marcel Mirel Popa, Sergiu Shova, Maria Gdaniec, Amalia Stefaniu, Constantin Draghici, Mihai Raduca, Nicoleta Doriana Banu, and Florea Dumitrascu. 2024. "Synthesis, X-ray Diffraction and Computational Druglikeness Evaluation of New Pyrrolo[1,2-a][1,10]Phenanthrolines Bearing a 9-Cyano Group" Symmetry 16, no. 7: 911. https://doi.org/10.3390/sym16070911
APA StyleCristea, M., Popa, M. M., Shova, S., Gdaniec, M., Stefaniu, A., Draghici, C., Raduca, M., Banu, N. D., & Dumitrascu, F. (2024). Synthesis, X-ray Diffraction and Computational Druglikeness Evaluation of New Pyrrolo[1,2-a][1,10]Phenanthrolines Bearing a 9-Cyano Group. Symmetry, 16(7), 911. https://doi.org/10.3390/sym16070911





