Abstract
The spin transition of iron can greatly affect the stability and various physical properties of iron-bearing carbonates at high pressure. Here, we reported laser Raman measurements on iron-bearing dolomite and siderite at high pressure and room temperature. Raman modes of siderite FeCO3 were investigated up to 75 GPa in the helium (He) pressure medium and up to 82 GPa in the NaCl pressure medium, respectively. We found that the electronic spin-paring transition of iron in siderite occurred sharply at 42–44 GPa, consistent with that in the neon (Ne) pressure medium in our previous study. This indicated that the improved hydrostaticity from Ne to He had minimal effects on the spin transition pressure. Remarkably, the spin crossover of siderite was broadened to 38–48 GPa in the NaCl pressure medium, due to the large deviatoric stress in the sample chamber. In addition, Raman modes of iron-bearing dolomite Ca1.02Mg0.76Fe0.20Mn0.02(CO3)2 were explored up to 58 GPa by using argon as a pressure medium. The sample underwent phase transitions from dolomite-Ⅰ to -Ⅰb phase at ~8 GPa, and then to -Ⅱ at ~15 and -Ⅲb phase at 36 GPa, while no spin transition was observed in iron-bearing dolomite up to 58 GPa. The incorporation of FeCO3 by 20 mol% appeared to marginally decrease the onset pressures of the three phase transitions aforementioned for pure dolomite. At 55–58 GPa, the ν1 mode shifted to a lower frequency at ~1186 cm−1, which was likely associated with the 3 + 1 coordination in dolomite-Ⅲb. These results shed new insights into the nature of iron-bearing carbonates at high pressure.
1. Introduction
Carbon mainly exists as accessory minerals (e.g., carbonates, diamond, graphite, and carbides) in the deep mantle due to its relatively low solubility in silicates [1]. Carbonates are considered to be one of the most important carbon carriers from the crust to deep mantle [2,3,4]. Given carbonate inclusions in ultra-deep diamonds originating from the deep mantle, carbonates can descend to the Earth’s deep interior [5,6]. The presence of carbonates may dramatically affect the physical and chemical properties (e.g., melting, viscosity, electrical conductivity, thermal conductivity, and elasticity) of the deep mantle [7,8,9]. More importantly, knowledge of the stability of carbonates is indispensable to interpret the deep carbon cycle.
Iron plays a fundamental role in the behavior of carbonates at extreme conditions, relevant to the Earth’s lower mantle [7,10]. In particular, iron substitution can greatly change the thermodynamic stability of MgCO3 and other mantle phases at high pressure and high temperature [11,12,13,14]. Siderite is considered to be more stable than magnesite due to Fe2+ in the low-spin (LS) state with a radius smaller than Mg2+. It could be preserved in relatively cold subducting slabs down to the lower mantle [15,16,17,18]. Most of the previous studies about iron-bearing carbonates have concentrated on the spin transition of iron in FeCO3 [10,17,18,19,20,21,22,23]. The onset pressure of spin transition, as well as the width of spin crossover, appear to be greatly affected by hydrostatic conditions of the sample chamber. Intriguingly, it is still unclear how the helium or NaCl pressure medium influences the behavior of siderite at high pressure.
Iron-bearing dolomite has also been suggested to enter the Earth’s interior through subduction slabs. It adopts a rhombohedral structure (space group R) with alternating layers of CaO6 and MgO6 octahedra stacked along the c-axis at ambient conditions [24]. The stability of dolomite has been investigated at lower mantle conditions and most of the previous studies have concentrated on its phase stability and vibrational properties by using a battery of experimental methods (e.g., X-ray diffraction, Raman, and infrared) [24,25,26,27,28,29,30]. Further investigation is needed to determine how iron substitution affects the nature of dolomite at a high pressure.
In the present work, we collected the high-pressure Raman spectra of siderite FeCO3 and iron-bearing dolomite Ca1.02Mg0.76Fe0.20Mn0.02(CO3)2 natural samples at high pressure in diamond anvil cells (DACs). The siderite sample was compressed in the He pressure medium to 75 GPa and in the NaCl pressure medium to 82 GPa, respectively. The use of He and NaCl allowed us to better understand how hydrostatic and non-hydrostatic conditions affected the stability and spin transition of FeCO3 at high pressures. As compared with our previous results, there was a negligible impact on the spin transition in FeCO3 between Ne and He pressure-transmitting media. We also carried out laser Raman measurements on iron-bearing dolomite at high pressure up to 58 GPa at room temperature by using argon as a pressure-transmitting medium. In this study, we found that there was no spin transition that occurred in the iron-bearing dolomite sample. These results improved the knowledge about phase stability and vibrational properties of iron-bearing carbonates at high pressure.
2. Experimental Methods
2.1. Sample Characterization
The starting materials were natural siderite Fe0.998Mn0.002CO3 single-crystal samples obtained from the mineralogical collection of the Department of Mineral Sciences, Smithsonian Institution (collection no. NMNH R11313). The chemical composition of the siderite sample contained less than 0.2 mol% of MnCO3, which was determined using electron microprobe analyses (JEOL JXA-8200, The University of Texas at Austin, USA). For simplicity, we neglected the minor impurity, and thus referred to the composition of the sample as FeCO3 thereinafter. FeCO3 has the crystal structure of calcite (CaCO3). Single-crystal X-ray diffraction (XRD) patterns confirmed the Rc structure with lattice parameters a = 4.6909(5) Å and c = 15.3687(49) Å for FeCO3 under ambient conditions, in good agreement with previous studies [31]. For the dolomite sample, based on electron microprobe analyses (JEOL JXA-8230, Northwest University, China), the chemical composition was Ca1.02Mg0.76Fe0.20Mn0.02(CO3)2.
2.2. High-Pressure Raman Spectroscopy
High-pressure Raman spectra of FeCO3 were collected by using a Renishaw Raman spectroscopy (RM1000, Center for High Pressure Science and Technology Advanced Research, China) excited by a 532 nm wavelength of an Ar+ laser. The spectral resolution was about 2 cm−1 with the holographic diffraction grating of 1800 lines/mm. High pressures were produced by a symmetric diamond anvil cell (DAC) mounted with a pair of 200 μm diamond anvils. A ~30 μm thickness of pre-indented tungsten gasket with a 120 μm hole was used as a sample chamber. Together with two ruby spheres, a platelet of single-crystal FeCO3 was loaded into the sample chamber using He as a pressure medium. The use of He can maintain the hydrostatic conditions at 50 GPa [32], and thus avoid the influence of deviatoric stress. For more detailed experimental information, one can refer to our previous study [33]. Additionally, the Raman spectra of iron-bearing dolomite were collected by using an eXcelon digital CCD spectroscopy system (PIXIS 400, Princeton Instruments co., USA) coupled with an 1800 G/mm ruled grating with 532 nm blaze wavelength. It was equipped with a Coherent Verdi V2 laser with a wavelength of 532 nm. Pressure was determined by using multiple measurements of the ruby fluorescence before and after each experimental run in the He [34], NaCl, or argon pressure-transmitting medium [35]. Raman spectra fitting was carried out using the software PeakFit v4.12 with the Voigt area method.
3. Results and Discussion
Raman spectra of siderite FeCO3 and iron-bearing dolomite Ca1.02Mg0.76Fe0.20Mn0.02(CO3)2 were collected in varying pressure-transmitting media at high pressure and room temperature. Rhombohedral carbonates (e.g., siderite, calcite, magnesite, and dolomite) with the space group Rc or R have two lattice modes (T and L modes) and four internal modes (in-plane bend internal (ν4), symmetric stretch internal (ν1), anti-symmetric stretch (ν3), and out-of-plane bend (2ν2) modes) [36]. At ambient conditions, the four Raman modes of T, L, ν4, and ν1 were collected in FeCO3 and Ca1.02Mg0.76Fe0.20Mn0.02(CO3)2 from 100 to 1300 cm−1. These Raman mode values are consistent with literature values [31,36,37].
3.1. Spin Transition of FeCO3
Representative Raman spectra of siderite FeCO3 at high pressure up to 75 GPa were observed, as shown in Figure 1, by using He as a pressure-transmitting medium. The inset in Figure 1 illustrates optical images of the single-crystal siderite in the DAC. The color of siderite platelet is colorless and transparent in the high-spin (HS) state. At 42 GPa, part of the crystallite changes from transparent to green and the spectral features of siderite changes significantly due to the spin transition of Fe2+ [18,38]. The emergence of L′, ν4′, and ν1′ modes was observed in nearly pure FeCO3 at 42 GPa, suggesting coexistence of the two species with different unit-cell volumes that correspond to the HS and LS domains, respectively [20,39]. The ν1′ mode that occurred at the left of the original ν1 mode provided strong evidence of the spin transition for iron-bearing carbonates [19,20,22,40]). The L and ν4 modes of FeCO3 in the LS state jump to higher wavenumbers because of the reduced distance between the CO32− groups and the cations, and the shortening of O-O distances, respectively [17,40]). Meanwhile, the ν1 mode shifts to lower wavenumbers from the HS to LS states, due to an increase in the C-O bond lengths across the spin transition [19,20,39,40]. The lengthening of the C-O bond and the contraction of the O-O distances were reported in the single-crystal XRD study across the spin transition by Lavina et al. (2010) [17].
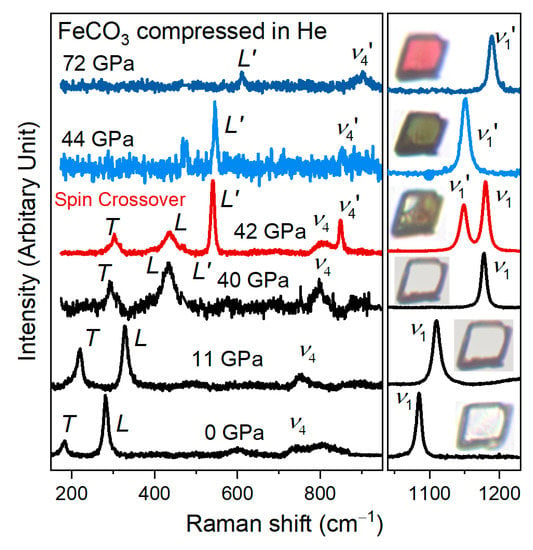
Figure 1.
Representative Raman spectra of siderite FeCO3 at high pressures. The Raman modes are labeled as T, L, ν4, and ν1 based on Rividi et al. (2010) [36]. The T, L, and ν4 modes were simultaneously enlarged to illustrate changes in Raman spectra of siderite. At 42 GPa, the splitting of L, ν4, and ν1 modes into L′, ν4′, and ν1′ indicates the occurrence of spin transition of Fe2+ in siderite. Insets, the color evolution of siderite sample captured through optical microscope images.
The optical image of siderite completely changes to green at 44 GPa when the original L, ν4, and ν1 modes disappear. It turns into red above 50–55 GPa. The change of crystal color can be assigned to a significant increase in the overall optical absorption of siderite in the LS state. The green color of siderite comes from the absorption minima of the 1Ag to 1T1g band, while the red color is due to the overlap of the crystal field band with the absorption edge [18]. The Raman spectra and optical images demonstrate that the electronic spin-paring of iron in FeCO3 occurs sharply at 42–44 GPa by using the He pressure medium, comparable to using the Ne pressure medium [16,18,40], that is, the enhanced hydrostaticity from Ne to He has a neglected effect on the spin transition pressure. In contrast, we observed a broadened spin crossover with the same siderite sample using NaCl as a pressure medium (Figure 2). Between 38 and 48 GPa, a weak shoulder is assigned as the ν1′ mode next to the initial ν1 mode. The obvious splitting of the ν1 mode at 41.5 and 44.8 GPa represents the mixed spin state of siderite. With further compression, the intensity of the two ν1 modes exchanges. The ν1 mode completely disappears at 48.4 GPa, indicating the loss of the HS state. Figure 3 shows the low-spin fraction of siderite as a function of pressure with the use of NaCl as a pressure-transmitting medium. The HS-LS fraction was determined on the basis of the ratio of the Raman peak areas between the ν1 and ν1′ modes. The low-spin fraction changes dramatically from 38 to 48 GPa corresponding to the spin crossover of siderite. Moreover, compared to the use of He as a pressure medium, the onset pressure of spin transition is lowered by ~4 GPa using NaCl as a pressure medium. The same effect of non-hydrostatic stress has also been observed for high-pressure phase transitions of other minerals, e.g., barite BaSO4 and rhodochrosite MnCO3 [41,42].
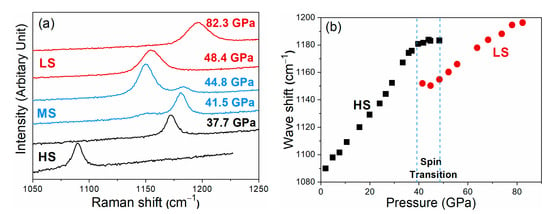
Figure 2.
Representative Raman spectra (a) and Raman shifts (b) of ν1 mode of siderite using NaCl as a pressure-transmitting medium. A weak shoulder assigned as the ν1′ mode near the initial ν1 mode was observed between 38 and 48 GPa. The ν1 and ν1′ modes correspond to the high- and low-spin (HS and LS) states, respectively. The obvious split of ν1 mode at 41.5 and 44.8 GPa represents the mixture spin state of siderite. With the further increase of pressure, the intensity of two ν1 modes is exchanged. The ν1 mode completely disappears at 48.4 GPa, indicating the completion of spin transition. The two blue dashed lines represent the spin crossover of siderite.
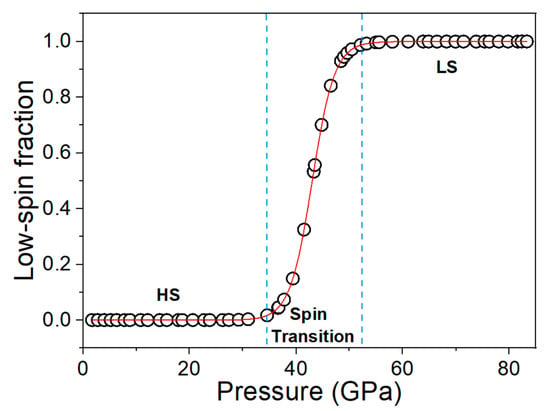
Figure 3.
The low-spin fraction of siderite as a function of pressure with the use of NaCl as a pressure-transmitting medium. The low-spin fraction changes dramatically at around 38 to 48 GPa, corresponding to the spin crossover of siderite.
The literature data on the spin crossover of (Mg,Fe)CO3 are summarized in Figure 4 and Table 1. In order to accurately estimate the effects of FeCO3 content on the spin crossover of (Mg,Fe)CO3, the datasets are grouped in the two categories, as shown in Figure 4, i.e., hydrostatic/quasi-hydrostatic (Ne or Ne as a pressure medium) and non-hydrostatic conditions (silicone oil, argon, NaCl, or no pressure medium). By considering the uncertainty induced by varying methods (e.g., Raman, XRD), the spin transition pressure of (Mg,Fe)CO3 is not sensitive to FeCO3 content under hydrostatic/quasi-hydrostatic conditions [10]. It can be explained by the relatively large Fe2+ to Fe2+ distance separated by (CO3)2− units in (Mg,Fe)CO3 [20].
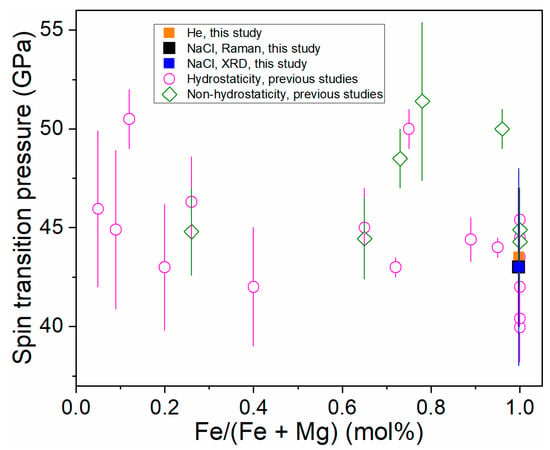
Figure 4.
The spin crossover of magnesite-siderite solid solutions with increasing iron content under hydrostatic and non-hydrostatic pressure conditions. The composition of (Mg,Fe)CO3 is the same as in Table 1. The black square is enlarged to differ from the overlapped blue square.

Table 1.
Spin transition of (Mg,Fe)CO3.
Raman shifts of each mode in FeCO3 can be linearly fitted as a function of pressure before and after the spin transition, respectively, when He is used as a pressure-transmitting medium (Figure 5 and Table 2). We note that the T mode might be too weak to be observed in the LS state. The pressure dependences of L and ν1 modes are 3.65 and 2.28 cm−1/GPa, respectively, in the HS state. These modes in the LS state decrease by ~40% to 2.41 and 1.38 cm−1/GPa, respectively, indicating that siderite in the LS state is stiffer and less compressible than in the HS state. By comparison, the pressure dependences of Raman shifts (dν/dP) in FeCO3 (denoted as “sid100”) in this study are comparable to that in sid65 in the HS state [20]. However, the pressure dependence of Raman shifts of L′, ν4′, and ν1′ in sid100 are about 48%, 69%, and 47% greater than that in sid65 in the LS state. This is consistent with the observations that the bulk modulus of sid65 is higher than that of sid100 in the LS state at a given pressure [16,20]. We note that the discrepancy in the pressure dependence of Raman shifts between this study and Farsang et al. (2018) [31] may be related to the use of different pressure media and the pressure range of Raman spectroscopic measurements.
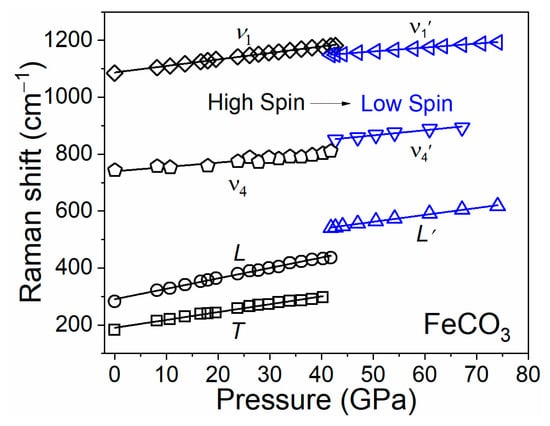
Figure 5.
Raman shifts of siderite at high pressures using He as a pressure-transmitting medium. Solid lines represent linear fits in the HS (black) and LS (blue) states, respectively. Among all four modes, the T mode was not observed in Raman spectra of siderite in the LS state. Error bars within symbols are not shown for clarity.

Table 2.
Vibrational parameters of siderite at high pressures.
Mode Grüneisen parameters provide important information about the relative contributions of each vibration to the thermochemical properties [50]. Combined with XRD and Raman results from previous work and this study, the mode Grüneisen parameters (γi) were derived according to the equation as follows:
where ν0, V, P, and KT are frequency at ambient conditions in the unit of cm−1, unit cell volume in the unit of Å3, pressure in the unit of GPa, and isothermal bulk model in the unit of GPa, respectively.
On the basis of the Raman shifts of each mode in this study and the equation of state reported by Liu et al. (2015) [16], the mode Grüneisen parameters (γi) of sid100 in the HS and LS states were derived (Table 2). The γi values for L, ν4, and ν1 modes are 1.50, 0.24, and 0.24, respectively, in the HS state, and change to 0.75, 0.36, and 0.20, respectively, in the LS state. The γi values of the L and ν1 modes decrease by approximate 50% and 17%, while that of the ν4 mode increases by 50% across the spin transition of iron in siderite, which should be attributed to the shrink of Fe-O octahedra [19,20]. By comparison, the mode Grüneisen parameters show a large difference among different studies, especially for T and L modes in the HS state, which are likely associated with pressure medium and compositional effects on the pressure dependence of Raman shifts. On the other hand, the γi values in the LS state are consistent with that in sid100 reported by Cerantola et al. (2015) [19], likely due to the similar chemical composition and compression environment.
3.2. Phase Transitions of Iron-Bearing Dolomite at High Pressure
Iron-bearing dolomite undergoes a series of phase transitions at the pressure range of this study (Figure 6 and Figure 7 and Table 3). The splitting of a low frequency mode around 201 cm−1 was observed at 7.8 GPa, which indicates the occurrence of dolomite-Ⅰb phase [25,26]. Moreover, the splitting of Raman mode at around 750 cm−1 and the several new Raman peaks observed at the low frequency of 200−600 cm−1 at 14.8 GPa are assigned as the onset of the dolomite-Ⅱ phase. These Raman modes further split at ~36.0 GPa, together with a weak shoulder in ν1 mode (shown as the black arrow in Figure 6), indicating the occurrence of dolomite-Ⅲ phase. On the basis of the previous XRD studies, the dolomite-Ⅲ is assigned as dolomite-Ⅲb for iron-rich dolomite, instead of dolomite-Ⅲc [29]. Given our work and previous studies on iron-bearing dolomite with varying iron contents at high pressure (Figure 7 and Table 3), we found that the onset pressure of phase transitions of dolomite-Ⅰ to -Ⅰb, -Ⅱ, and -Ⅲb phases were almost insensitive with the iron content at the expense of the tilting of the CO3 groups [24,25,26]. It should be mentioned that the phase transitions of iron-bearing dolomite are 2–4 GPa lower than that of iron-free dolomite CaMg(CO3)2 [25,28]. This means that the incorporation of iron (even though there is minimal iron) into pure dolomite endmember may decrease the onset phase transition of dolomite, likely due to the fact that iron substitution changes the ordered atom arrange of dolomite.
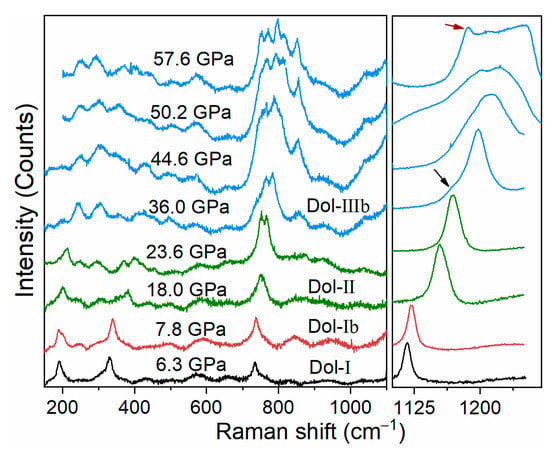
Figure 6.
Representative Raman spectra of iron-bearing dolomite with increasing pressure. The Dol-Ib, Dol-Ⅱ, and Dol-Ⅲb phases occur at around 7.8, 14.8 and 36.0 GPa, respectively. The new peak observed at 36.0 GPa (shown as the black arrow) represents the onset of Dol-Ⅲb phase. Meanwhile, one of the ν1 modes shifts to a lower frequency of ~1186 cm−1 at 57.6 GPa (shown as the red arrow), which may be related to the 3 + 1 coordination in Dol-Ⅲb [29].
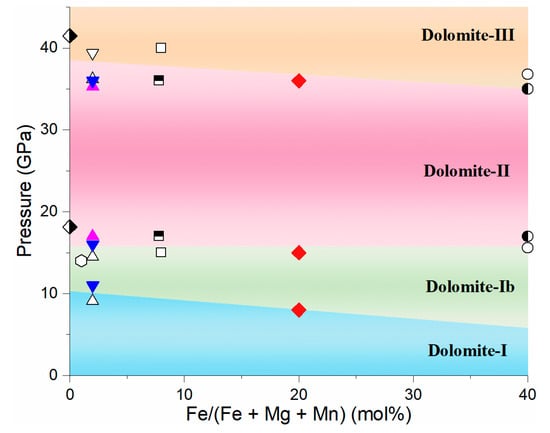
Figure 7.
Phase transitions of dolomite as a function of iron content. The cyan, green, magenta, and yellow areas represent the dolomite-Ib, -II, -III phases. Solid red diamonds, ◆: Ca1.02Mg0.76Fe0.20Mn0.02(CO3), this study; half open and half solid black diamonds, ⬗: CaMg(CO3)2 [28]; open black hexagon, ⬡: Ca1.001Mg0.987Fe0.01Mn0.002(CO3)2 [52]; open black up triangles, △: CaMg0.98Fe0.02(CO3)2 [25]; open black down triangle, ▽: CaMg0.98Fe0.02(CO3)2 [24]; solid magenta triangles, ⯅: CaMg0.98Fe0.02(CO3)2 [24]; half open and half solid black squares, ⬒: Ca0.988Mg0.918Fe0.078Mn0.016(CO3)2 [27]; open black squares, ☐: CaMg0.92Fe0.08(CO3)2 [29]; open black circles, ○: CaMg0.6Fe0.4(CO3)2; half open and half solid black circles, ◐: CaMg0.6Fe0.4(CO3)2 [28].

Table 3.
Overview of the current literature about dolomite and a comparison of the methods used, composition, and phase transition pressure.
Furthermore, one of the ν1 modes shifts to a lower frequency of ~1186 cm−1 at 57.6 GPa (shown as the red arrow in Figure 6). It may be related to the 3 + 1 coordination (three strong and one weaker C-O banding, more detailed information can refer to the reference therein) in dolomite-Ⅲb as illustrated by Raman spectroscopy on Ca1.00Mg0.92Fe0.08(CO3)2 [29] and XRD results on CaMg0.6Fe0.4(CO3)2 [30]. The onset phase transition pressure of the 3 + 1 coordination observed in this study is about 8 GPa lower than that in Ca1.00Mg0.92Fe0.08(CO3)2 [29], which may be attributed to either high iron content or the relatively large deviatoric stress induced by the argon pressure medium.
In this study, we observed the splitting and lower frequency of ν1 modes in the dolomite-Ⅲb phase, unlike the splitting of the ν1 modes into the two Raman peaks in siderite across the spin transition of iron (Figure 1 and Figure 2). It suggests that there is no spin transition of iron in Ca1.02Mg0.76Fe0.20Mn0.02(CO3)2 up to 58 GPa. Moreover, the pressure-volume profiles of iron-rich dolomite CaMg0.6Fe0.4(CO3)2 show that there is no volume collapse observed at the whole pressure range of dolomite-Ⅲb phase [28,30], suggesting that no spin transition occurs at 36−115 GPa. By contrast, Mao et al. (2011) [27] put forward that the spin transition of Ca0.988Mg0.918Fe0.078Mn0.016(CO3)2 was at ~47 GPa with a volume collapse of 2% based on the compression and decompression XRD data. In addition, theoretical calculations have predicted a higher spin transition pressure of 65−68 GPa for iron-rich dolomite [51]. To eliminate such discrepancy, further experiments are imperative with more sensitive probes including synchrotron Mössbauer spectroscopy and X-ray emission spectroscopy.
4. Conclusions
Two iron-bearing carbonates, i.e., siderite and iron-bearing dolomite, were investigated by Raman spectroscopy at high pressure and room temperature in DACs using varying pressure media. The electronic spin-paring transition of iron in siderite occurs sharply at 42–44 GPa using helium as the pressure medium, while it is ~38–48 GPa in the NaCl pressure medium. It suggests that the spin crossover of siderite is significantly influenced by large deviatoric stress. Considering the high temperature environment of the Earth’s interior, the stress field of the Earth’s interior should be close to a hydrostatic environment, which is considered to be a factor for modeling carbon subduction at deep mantle conditions [24].
For iron-bearing dolomite, the high-pressure phase (dolomite-Ⅲb) can be stable at least to 58 GPa at room temperature. It is a potential carbon carrier and could carry carbon down to the deep mantle via cold subduction slabs [27,28,30]. In addition, based on the vibrational properties of iron-bearing dolomite in this study and high-pressure XRD results from Merlini et al. (2017) [28], there is no spin transition of iron in dolomite with iron content up to 40 mol% at high pressure from 36 to 115 GPa. Further investigation is needed to clarify the spin transition of iron in dolomite by using more sensitive probes (e.g., X-ray emission spectroscopy).
Author Contributions
Data curation, J.L., C.Z.; Formal analysis, C.Z., L.X., and J.L.; Funding acquisition, J.L.; Investigation, C.Z.; Supervision, J.L.; Writing—original draft, C.Z.; Writing—review and editing, C.Z., L.X., W.G., and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The National Key Research and Development Program of China, 2019YFA0708502 and the National Natural Science Foundation of China, U1930401
Acknowledgments
We appreciate three anonymous reviewers for their constructive suggestions and comments, which helped improve the manuscript significantly. This study is funded by the National Key Research and Development Program of China (2019YFA0708502). J. Liu acknowledges support from the National Natural Science Foundation of China (grant no. U1930401).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shcheka, S.S.; Wiedenbeck, M.; Frost, D.J.; Keppler, H. Carbon solubility in mantle minerals. Earth Planet. Sci. Lett. 2006, 245, 730–742. [Google Scholar] [CrossRef]
- Boulard, E.; Guyot, F.; Fiquet, G. High-pressure transformations and stability of ferromagnesite in the Earth’s mantle. Carbon Earth’s Inter. 2020, 105–113. [Google Scholar] [CrossRef]
- Hazen, R.M.; Downs, R.T.; Jones, A.P.; Kah, L. Carbon mineralogy and crystal chemistry. Rev. Mineral. Geochem. 2013, 75, 7–46. [Google Scholar] [CrossRef]
- Hazen, R.M.; Schiffries, C.M. Why deep carbon? Rev. Mineral. Geochem. 2013, 75, 1–6. [Google Scholar] [CrossRef]
- Brenker, F.E.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; Dele-Duboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Fu, S.; Yang, J.; Lin, J.F. Abnormal elasticity of single-crystal magnesiosiderite across the spin transition in Earth’s lower mantle. Phys. Rev. Lett. 2017, 118, 036402. [Google Scholar] [CrossRef]
- Gaillard, F.; Malki, M.; Iacono-Marziano, G.; Pichavant, M.; Scaillet, B. Carbonatite melts and electrical conductivity in the asthenosphere. Science 2008, 322, 1363–1365. [Google Scholar] [CrossRef]
- Yao, C.; Wu, Z.; Zou, F.; Sun, W. Thermodynamic and elastic properties of magnesite at mantle conditions: First-principles calculations. Geochem. Geophys. Geosystems 2018, 19, 2719–2731. [Google Scholar] [CrossRef]
- Liu, J.; Lin, J.F.; Mao, Z.; Prakapenka, V.B. Thermal equation of state and spin transition of magnesiosiderite at high pressure and temperature. Am. Mineral. 2014, 99, 84–93. [Google Scholar] [CrossRef]
- Boulard, E.; Pan, D.; Galli, G.; Liu, Z.; Mao, W.L. Tetrahedrally coordinated carbonates in Earth’s lower mantle. Nat. Commun. 2015, 6, 6311. [Google Scholar] [CrossRef] [PubMed]
- Cerantola, V.; Wilke, M.; Kantor, I.; Ismailova, L.; Kupenko, I.; McCammon, C.; Pascarelli, S.; Dubrovinsky, L.S. Experimental investigation of FeCO3 (siderite) stability in Earth’s lower mantle using XANES spectroscopy. Am. Mineral. 2019, 104, 1083–1091. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Bi, W.; Yang, L.; Xiao, Y.; Chow, P.; Meng, Y.; Prakapenka, V.B.; Mao, H.K.; Mao, W.L. Altered chemistry of oxygen and iron under deep Earth conditions. Nat. Commun. 2019, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.; Lv, C.; Su, X.; Tang, R.; Chen, J.; Hu, Q.; Mao, H.-K.; Mao, W. Evidence for oxygenation of Fe-Mg oxides at mid-mantle conditions and the rise of deep oxygen. Natl. Sci. Rev. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Cerantola, V.; Bykova, E.; Kupenko, I.; Merlini, M.; Ismailova, L.; McCammon, C.; Bykov, M.; Chumakov, A.I.; Petitgirard, S.; Kantor, I.; et al. Stability of iron-bearing carbonates in the deep Earth’s interior. Nat. Commun. 2017, 8, 15960. [Google Scholar] [CrossRef]
- Liu, J.; Lin, J.F.; Prakapenka, V.B. High-pressure orthorhombic ferromagnesite as a potential deep-mantle carbon carrier. Sci. Rep. 2015, 5, 7640. [Google Scholar] [CrossRef]
- Lavina, B.; Dera, P.; Downs, R.T.; Yang, W.; Sinogeikin, S.; Meng, Y.; Shen, G.; Schiferl, D. Structure of siderite FeCO3 to 56 GPa and hysteresis of its spin-pairing transition. Phys. Rev. B 2010, 82, 064110. [Google Scholar] [CrossRef]
- Lobanov, S.S.; Goncharov, A.F.; Litasov, K.D. Optical properties of siderite (FeCO3) across the spin transition: Crossover to iron-rich carbonates in the lower mantle. Am. Mineral. 2015, 100, 1059–1064. [Google Scholar] [CrossRef]
- Cerantola, V.; McCammon, C.; Kupenko, I.; Kantor, I.; Marini, C.; Wilke, M.; Ismailova, L.; Solopova, N.; Chumakov, A.; Pascarelli, S.; et al. High-pressure spectroscopic study of siderite (FeCO3) with a focus on spin crossover. Am. Mineral. 2015, 100, 2670–2681. [Google Scholar] [CrossRef]
- Lin, J.-F.; Liu, J.; Jacobs, C.; Prakapenka, V.B. Vibrational and elastic properties of ferromagnesite across the electronic spin-pairing transition of iron. Am. Mineral. 2012, 97, 583–591. [Google Scholar] [CrossRef]
- Nagai, T.; Ishido, T.; Seto, Y.; Nishio-Hamane, D.; Sata, N.; Fujino, K. Pressure-induced spin transition in FeCO3-siderite studied by X-ray diffraction measurements. J. Phys. Conf. Ser. 2010, 215, 012002. [Google Scholar] [CrossRef]
- Spivak, A.; Solopova, N.; Cerantola, V.; Bykova, E.; Zakharchenko, E.; Dubrovinsky, L.; Litvin, Y. Raman study of MgCO3–FeCO3 carbonate solid solution at high pressures up to 55 GPa. Phys. Chem. Miner. 2014, 41, 633–638. [Google Scholar] [CrossRef]
- Wei Chariton s, C.; Sternemann, C.; Cerantola, V.; Sahle, C.J.; Spiekermann, G.; Harder, M.; Forov, Y.; Kononov, A.; Sakrowski, R.; Yavas, H.; et al. Pressure driven spin transition in siderite and magnesiosiderite single crystals. Sci. Rep. 2017, 7, 16526. [Google Scholar]
- Efthimiopoulos, I.; Germer, M.; Jahn, S.; Harms, M.; Reichmann, H.J.; Speziale, S.; Schade, U.; Sieber, M.; Koch-Müller, M. Effects of hydrostaticity on the structural stability of carbonates at lower mantle pressures: The case study of dolomite. High Press. Res. 2018, 39, 1–14. [Google Scholar] [CrossRef]
- Binck, J.; Chariton, S.; Stekiel, M.; Bayarjargal, L.; Morgenroth, W.; Milman, V.; Dubrovinsky, L.; Winkler, B. High-pressure, high-temperature phase stability of iron-poor dolomite and the structures of dolomite-IIIc and dolomite-V. Phys. Earth Planet. Inter. 2020, 299, 106403. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Jahn, S.; Kuras, A.; Schade, U.; Koch-Müller, M. Combined high-pressure and high-temperature vibrational studies of dolomite: Phase diagram and evidence of a new distorted modification. Phys. Chem. Miner. 2017, 44, 465–476. [Google Scholar] [CrossRef]
- Mao, Z.; Armentrout, M.; Rainey, E.; Manning, C.E.; Dera, P.; Prakapenka, V.B.; Kavner, A. Dolomite III: A new candidate lower mantle carbonate. Geophys. Res. Lett. 2011, 38, L22303. [Google Scholar] [CrossRef]
- Merlini, M.; Cerantola, V.; Gatta, G.D.; Gemmi, M.; Hanfland, M.; Kupenko, I.; Lotti, P.; Müller, H.; Zhang, L. Dolomite-IV: Candidate structure for a carbonate in the Earth’s lower mantle. Am. Mineral. 2017, 102, 1763–1766. [Google Scholar] [CrossRef]
- Vennari, C.E.; Williams, Q. A novel carbon bonding environment in deep mantle high-pressure dolomite. Am. Mineral. 2018, 103, 171–174. [Google Scholar] [CrossRef]
- Merlini, M.; Crichton, W.A.; Hanfland, M.; Gemmi, M.; Muller, H.; Kupenko, I.; Dubrovinsky, L. Structures of dolomite at ultrahigh pressure and their influence on the deep carbon cycle. Proc. Natl. Acad. Sci. USA 2012, 109, 13509–13514. [Google Scholar] [CrossRef]
- Farsang, S.; Facq, S.; Redfern, S.A. Raman modes of carbonate minerals as pressure and temperature gauges up to 6 GPa and 500 °C. Am. Mineral. J. Earth Planet. Mater. 2018, 103, 1988–1998. [Google Scholar]
- Klotz, S.; Chervin, J.C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Zhao, C.S.; Lv, C.J.; Xu, L.X.; Liu, J. Raman signatures of the distortion and stability of MgCO3 to 75 GPa. Am. Mineral. 2021, 106. in press. [Google Scholar] [CrossRef]
- Shen, G.; Wang, Y.; Dewaele, A.; Wu, C.; Fratanduono, D.E.; Eggert, J.; Klotz, S.; Dziubek, K.F.; Loubeyre, P.; Fat’yanov, O.V.; et al. Toward an international practical pressure scale: A proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Press. Res. 2020, 40, 299–314. [Google Scholar] [CrossRef]
- Mao, H.; Xu, J.-A.; Bell, P. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Rividi, N.; van Zuilen, M.; Philippot, P.; Menez, B.; Godard, G.; Poidatz, E. Calibration of carbonate composition using micro-Raman analysis: Application to planetary surface exploration. Astrobiology 2010, 10, 293–309. [Google Scholar] [CrossRef]
- Boulard, E.; Guyot, F.; Fiquet, G. The influence on Fe content on Raman spectra and unit cell parameters of magnesite–siderite solid solutions. Phys. Chem. Miner. 2012, 39, 239–246. [Google Scholar] [CrossRef]
- Lavina, B.; Dera, P.; Downs, R.T.; Prakapenka, V.; Rivers, M.; Sutton, S.; Nicol, M. Siderite at lower mantle conditions and the effects of the pressure-induced spin-pairing transition. Geophys. Res. Lett. 2009, 36, L23306. [Google Scholar] [CrossRef]
- Farfan, G.; Wang, S.; Ma, H.; Caracas, R.; Mao, W.L. Bonding and structural changes in siderite at high pressure. Am. Mineral. 2012, 97, 1421–1426. [Google Scholar] [CrossRef]
- Müller, J.; Speziale, S.; Efthimiopoulos, I.; Jahn, S.; Koch-Müller, M. Raman spectroscopy of siderite at high pressure: Evidence for a sharp spin transition. Am. Mineral. 2016, 101, 2638–2644. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; Gracia, L.; Garbarino, G.; Beltrán, A.; Chuliá-Jordán, R.; Gomis, O.; Errandonea, D.; Ferrer-Roca, C.; Martínez-García, D.; Segura, A. High-pressure study of the behavior of mineral barite by x-ray diffraction. Phys. Rev. B 2011, 84, 054102. [Google Scholar] [CrossRef]
- Zhao, C.S.; Li, H.P.; Jiang, J.J.; He, Y.; Liang, W. Phase transition and vibration properties of MnCO3 at high pressure and high-temperature by Raman spectroscopy. High Press. Res. 2018, 38, 212–223. [Google Scholar] [CrossRef]
- Chariton, S.; McCammon, C.; Vasiukov, D.M.; Stekiel, M.; Kantor, A.; Cerantola, V.; Kupenko, I.; Fedotenko, T.; Koemets, E.; Hanfland, M.; et al. Seismic detectability of carbonates in the deep Earth: A nuclear inelastic scattering study. Am. Mineral. 2020, 105, 325–332. [Google Scholar]
- Stekiel, M.; Nguyen-Thanh, T.; Chariton, S.; McCammon, C.; Bosak, A.; Morgenroth, W.; Milman, V.; Refson, K.; Winkler, B. High pressure elasticity of FeCO3-MgCO3 carbonates. Phys. Earth Planet. Inter. 2017, 271, 57–63. [Google Scholar] [CrossRef]
- Hsu, H.; Huang, S.-C. Spin crossover and hyperfine interactions of iron in (Mg,Fe)CO3 ferromagnesite. Phys. Rev. B 2016, 94, 060404. [Google Scholar] [CrossRef]
- Mattila, A.; Pylkkänen, T.; Rueff, J.P.; Huotari, S.; Vankó, G.; Hanfland, M.; Lehtinen, M.; Hämäläinen, K. Pressure induced magnetic transition in siderite FeCO3 studied by x-ray emission spectroscopy. J. Phys. Condens. Matter 2007, 19, 386206. [Google Scholar] [CrossRef]
- Chao, K.-H.; Hsieh, W.-P. Thermal conductivity anomaly in (Fe0.78Mg0.22)CO3 siderite across spin transition of iron. J. Geophys. Res. Solid Earth 2019, 124, 1388–1396. [Google Scholar] [CrossRef]
- Boulard, E.; Menguy, N.; Auzende, A.L.; Benzerara, K.; Bureau, H.; Antonangeli, D.; Corgne, A.; Morard, G.; Siebert, J.; Perrillat, J.P.; et al. Experimental investigation of the stability of Fe-rich carbonates in the lower mantle. J. Geophys. Res. Solid Earth 2012, 117, B02208. [Google Scholar] [CrossRef]
- Tsuchiya, J.; Nishida, R.; Tsuchiya, T. First Principles calculation of the stability of iron bearing carbonates at high pressure conditions. Minerals 2020, 10, 54. [Google Scholar] [CrossRef]
- Williams, Q.; Collerson, B.; Knittle, E. Vibrational spectra of magnesite (MgCO3) and calcite-III at high pressures. Am. Mineral. 1992, 77, 1158–1165. [Google Scholar]
- Solomatova, N.V.; Asimow, P.D. First-principles calculations of high-pressure iron-bearing monoclinic dolomite and single-cation carbonates with internally consistent Hubbard U. Phys. Chem. Miner. 2017, 45, 293–302. [Google Scholar] [CrossRef]
- Zucchini, A.; Prencipe, M.; Belmonte, D.; Paola, C. Ab initio study of the dolomite to dolomite-II high pressure phase transition. Eur. J. Mineral. 2017, 29, 227–238. [Google Scholar] [CrossRef]
- Santillán, J.; Williams, Q.; Knittle, E. Dolomite-II: A high-pressure polymorph of CaMg(CO3)2. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Fiquet, G.; Guyot, F.; Itie, J.-P. High-pressure X-ray diffraction study of carbonates MgCO3, CaMg(CO3)2, and CaCO3. Am. Mineral. 1994, 79, 15–23. [Google Scholar]
- Ross, N.L.; Reeder, R.J. High-pressure structural study of dolomite and ankerite. Am. Mineral. 1992, 77, 412–421. [Google Scholar]
- Gillet, P.; Biellmann, C.; Reynard, B.; McMillan, P. Raman spectroscopic studies of carbonates part I: High-pressure and high-temperature behaviour of calcite, magnesite, dolomite and aragonite. Phys. Chem. Miner. 1993, 20, 1–18. [Google Scholar] [CrossRef]
- Kraft, S.; Knittle, E.; Williams, Q. Carbonate stability in the Earth’s mantle: A vibrational spectroscopic study of aragonite and dolomite at high pressures and temperatures. J. Geophys. Res. 1991, 96, 17997. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).