Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Multiple Fly Ash Products
3.2. Individual Runs
3.3. Analysis of Variance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connelly, N.G.; Hartshorn, R.M.; Damhus, T.; Hutton, A.T. Nomenclature of Inorganic Chemistry: IUPAC Recommendations 2005; Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Greene, J. Digging for rare earths: The mine where iPhones are born. 2012. Available online: http://www.cnet.com/news/digging-for-rare-earths-the-mines-where-iphones-are-born/ (accessed on 25 January 2020).
- Hatch, G.P. Dynamics in the Global Market for Rare Earths. Elements 2012, 8, 341–346. [Google Scholar] [CrossRef]
- Dobransky, S. Rare Earth Elements and U.S. Foreign Policy: The Critical Ascension of REEs in Global Politics and U.S. National Security. American Diplomacy. 2013. Available online: http://www.unc.edu/depts/diplomat/item/2013/0912/ca/dobransky_rareearth.html (accessed on 25 January 2020).
- Swift, T.K.; Moore, M.G.; Rose-Glowacki, H.R.; Sanchez, E. The Economic Benefits of the North American Rare Earths Industry. American Chemistry Council: Washington, WA, USA, 2014. Available online: http://www.rareearthtechalliance.com/ (accessed on 25 January 2020).
- U.S. Geological Survey. The Rare-Earth Elements—Vital to Modern Technologies and Lifestyles. U.S. Geological Survey Fact Sheet 2014-3078. 2014. Available online: https://pubsusgsgov/fs/2014/3078/ (accessed on 25 January 2020).
- Watson, B. Rare earth hunting in US coal country. 18 October 2018. Available online: https://www.stitcher.com/podcast/defense-one-radio/e/56804885?refid=stpr&autoplay=true (starts at about 28:50) (accessed on 25 January 2020).
- U.S. Department of Energy, National Energy Technology Laboratory. Rare Earth Elements from Coal and Coal Byproducts. 2018. Available online: https://www.netl.doe.gov/research/coal/rare-earth-elements (accessed on 25 January 2020).
- Clarke, L.B.; Sloss, L.L. Trace elements—Emissions from Coal Combustion and Gasification; IEACR/49; IEA Coal Research: London, UK, 1992; 111p. [Google Scholar]
- Ratafia-Brown, J.A. Overview of trace element partitioning in flames and furnaces of utility coal-fired boilers. Fuel Process. Technol. 1994, 39, 139–157. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Rare elements in coal ashes. Ind. Eng. Chem. 1935, 27, 1100–1102. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as a potential alternative source for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Seredin, V.V. Rare earth element-bearing coals from the Russian Far East deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Balashov, Y.A. Geochemistry of Rare Earth elements; Nauka: Moscow, Russia, 1976; 267p. (In Russian) [Google Scholar]
- Seredin, V.V. A new method for primary evaluation of the outlook for rare earth element ores. Geol. Ore Depos. 2010, 52, 428–433. [Google Scholar] [CrossRef]
- Ward, C.R.; French, D. Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffractometry. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Hower, J.C. Petrographic examination of coal-combustion fly ash. Int. J. Coal Geol. 2012, 92, 90–97. [Google Scholar] [CrossRef]
- Kolker, A. Topics in coal geochemistry- Short course U.S. Geological Survey Open File Report 2018-1145. 2018. Available online: https://pubs.er.usgs.gov/publication/ofr20181145 (accessed on 25 January 2020).
- Hood, M.M.; Taggart, R.K.; Smith, R.C.; Hsu-Kim, H.; Henke, K.R.; Graham, U.M.; Groppo, J.G.; Unrine, J.M.; Hower, J.C. Rare earth element distribution in fly ash derived from the Fire Clay coal, Kentucky. Coal Combust. Gasif. Prod. 2017, 9, 22–33. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Henke, K.R.; Graham, U.M.; Hood, M.M.; Joshi, P.; Preda, D.V. Ponded and landfilled fly ash as a source of rare earth elements from a Kentucky power plant. Coal Combust. Gasif. Prod. B 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Hower, J.C.; Qian, D.; Briot, N.J.; Santillan-Jimenez, E.; Hood, M.M.; Taggart, R.K.; Hsu-Kim, H. Nano-scale rare earth distribution in fly ash derived from combustion of the Fire Clay coal, Kentucky. Minerals 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. A. 2017, 179, pp. 11–27. [CrossRef]
- Hower, J.C.; Robertson, J.D.; Thomas, G.A.; Wong, A.S.; Schram, W.H.; Graham, U.M.; Rathbone, R.F.; Robl, T.L. Characterization of fly ash from Kentucky power plants. Fuel 1996, 75, 403–411. [Google Scholar] [CrossRef]
- Hower, J.C.; Robl, T.L.; Thomas, G.A. Changes in the Quality of Coal Combustion By-products Produced by Kentucky Power Plants, 1978 to 1997: Consequences of Clean Air Act Directives. Fuel 1999, 78, 701–712. [Google Scholar] [CrossRef]
- Ardini, F.; Soggia, F.; Rugi, F.; Udisti, R.; Grotti, M. Comparison of inductively coupled plasma spectrometry techniques for the direct determination of rare earth elements in digests from geological samples. Anal. Chim. Acta 2010, 678, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, N.S.; Shaverina, A.V.; Tsygankova, A.R.; Saprykin, A.I. Comparison of analytical performances of inductively coupled plasma mass spectrometry and inductively coupled plasma atomic emission spectrometry for trace analysis of bismuth and bismuth oxide. Spectrochim. Acta Part B At. Spectrosc. 2018, 142, 23–28. [Google Scholar] [CrossRef]
- McLennan, S.M.; Taylor, S.R. Sedimentary rocks and crustal evolution: Tectonic setting and secular trends. J. Geol. 1991, 99, 1–21. [Google Scholar] [CrossRef]




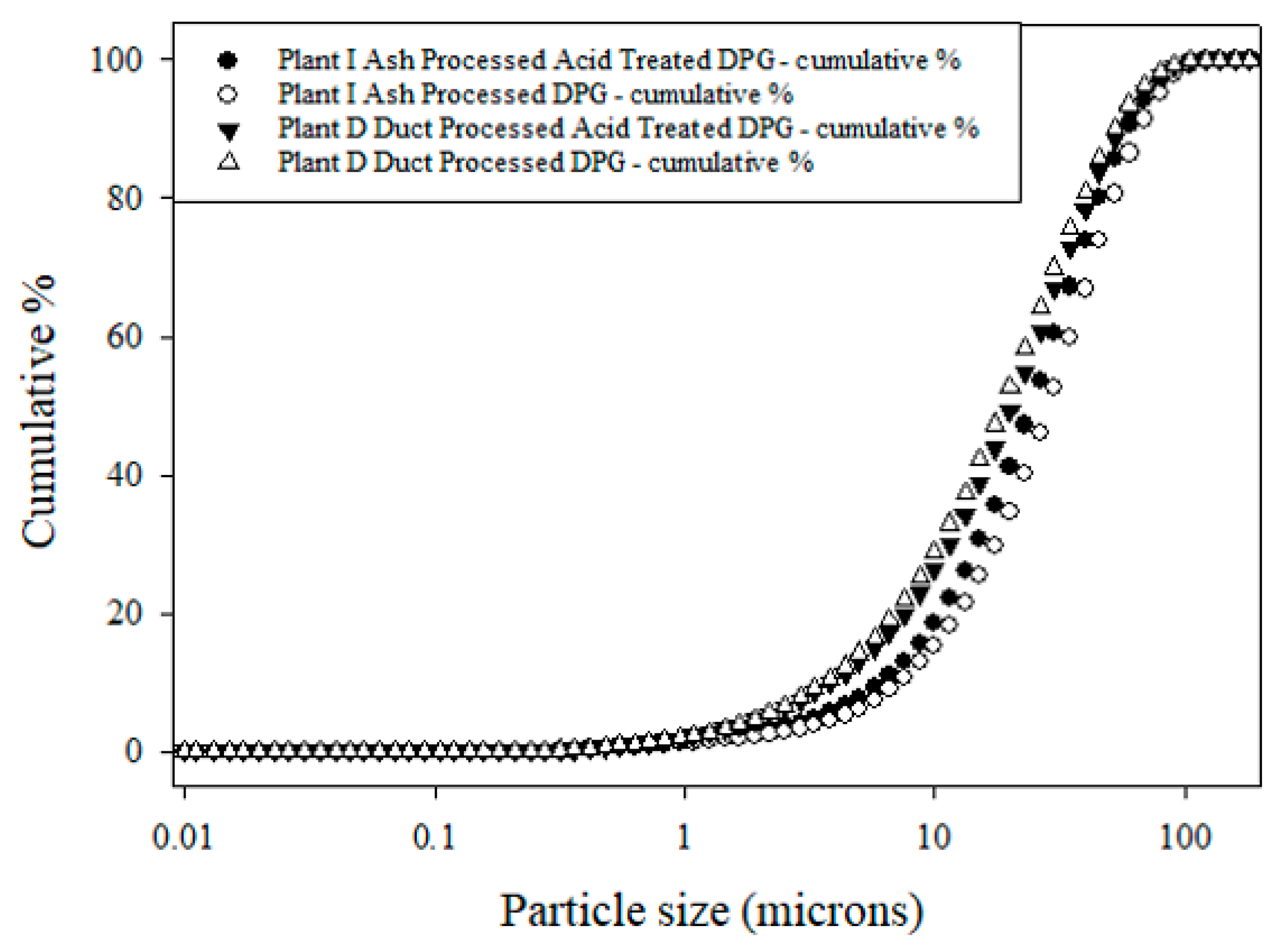




| Sample Type | Plant D Run 11 | Plant I Run 42 | ||||||
|---|---|---|---|---|---|---|---|---|
| Yield | LOI | REE | REE Recovery | Yield | LOI | REE | REE Recovery | |
| % | % | ppm w.b. | % | % | % | ppm w.b. | % | |
| Feed | 100 | 3.6 | 490 | 100 | 100 | 8.2 | 453 | 100 |
| Magnetics | 1.7 | 0.7 | 335 | 1.2 | 8.1 | 1 | 339 | 6.1 |
| Carbon Froth | - | - | - | - | 7.5 | 29 | 441 | 7.3 |
| >100 mesh | 1.3 | 11 | 365 | 1 | 1.6 | 12 | 360 | 1.2 |
| <100 x >200 mesh | 9.4 | 6.1 | 355 | 6.8 | 12.2 | 17 | 352 | 9.9 |
| <200-mesh product | 87.7 | 2.4 | 509 | 91.1 | 70.6 | 2.8 | 485 | 75.6 |
| Sample | Plant | Type | Ash | Mois | C | H | N | S | O | |||||
| 94093 | I | feed | 92.23 | 0.29 | 6.45 | 0.17 | <0.01 | <0.01 | 1.15 | |||||
| 94094 | I | C conc. | 80.86 | 0.41 | 17.50 | 0.04 | 0.06 | 0.01 | 1.53 | |||||
| 94095 | I | magnetics | 98.77 | 0.08 | 1.51 | 0.14 | <0.01 | <0.01 | <0.01 | |||||
| 94096 | I | >200 mesh | 81.04 | 0.62 | 14.80 | <0.01 | <0.01 | 0.02 | 4.14 | |||||
| 94097 | I | <200 mesh | 94.44 | 0.21 | 4.41 | <0.01 | <0.01 | <0.01 | 1.15 | |||||
| Sample | Plant | Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | P2O5 | TiO2 | SO3 | ||
| 94093 | I | feed | 51.65 | 29.21 | 11.60 | 1.06 | 0.98 | 0.24 | 2.80 | 0.33 | 1.43 | 0.13 | ||
| 94094 | I | C conc. | 49.39 | 29.63 | 12.50 | 1.42 | 1.03 | 0.27 | 2.81 | 0.50 | 1.52 | 0.26 | ||
| 94095 | I | magnetics | 22.32 | 13.66 | 60.18 | 0.78 | 0.54 | 0.09 | 0.98 | 0.31 | 0.58 | 0.14 | ||
| 94096 | I | >200 mesh | 56.74 | 29.24 | 7.03 | 0.79 | 0.89 | 0.21 | 2.78 | 0.30 | 1.28 | 0.20 | ||
| 94097 | I | <200 mesh | 54.78 | 30.81 | 6.86 | 0.99 | 1.01 | 0.26 | 3.02 | 0.33 | 1.38 | 0.08 | ||
| Sample | Plant | Type | Ash | V | Cr | Mn | Co | Ni | Cu | Zn | As | Sr | Ba | Pb |
| 94093 | I | feed | 92.23 | 287 | 152 | 168 | 76 | 133 | 178 | 144 | 123 | 816 | 1190 | 70 |
| 94094 | I | C conc. | 80.86 | 400 | 203 | 207 | 94 | 186 | 250 | 232 | 341 | 917 | 1424 | 106 |
| 94095 | I | magnetics | 98.77 | 260 | 165 | 401 | <37 | 169 | 189 | 156 | 83 | 425 | 632 | 45 |
| 94096 | I | >200 mesh | 81.04 | 240 | 138 | 123 | 47 | 97 | 117 | 85 | 131 | 785 | 1324 | 41 |
| 94097 | I | <200 mesh | 94.44 | 286 | 148 | 148 | 66 | 127 | 169 | 144 | 111 | 855 | 1243 | 74 |
| Sample | Plant | Type | Ash | Sc | Y | La | Ce | Pr | Nd | Sm | ||||
| 94093 | I | feed | 92.23 | 26 | 49 | 67 | 187 | 19 | 83 | 12 | ||||
| 94094 | I | C conc. | 80.86 | 34 | 63 | 72 | 195 | 21 | 91 | 14 | ||||
| 94095 | I | magnetics | 98.77 | 16 | 30 | 31 | 102 | 35 | 45 | 11 | ||||
| 94096 | I | >200 mesh | 81.04 | 22 | 42 | 65 | 178 | 16 | 78 | 11 | ||||
| 94097 | I | <200 mesh | 94.44 | 29 | 54 | 71 | 195 | 19 | 88 | 12 | ||||
| Sample | Plant | Type | Ash | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||
| 94093 | I | feed | 92.23 | 1 | 22 | 2 | 9 | <0.1 | 8 | <0.1 | 5 | <0.1 | ||
| 94094 | I | C conc. | 80.86 | 1 | 24 | 2 | 12 | <0.1 | 10 | <0.1 | 7 | <0.1 | ||
| 94095 | I | magnetics | 98.77 | <0.1 | 55 | 5 | 4 | <0.1 | 3 | <0.1 | 6 | <0.1 | ||
| 94096 | I | >200 mesh | 81.04 | 1 | 16 | 2 | 8 | <0.1 | 7 | <0.1 | 4 | <0.1 | ||
| 94097 | I | <200 mesh | 94.44 | 1 | 18 | 2 | 11 | <0.1 | 9 | <0.1 | 5 | <0.1 | ||
| Sample | Plant | Type | REE | REY | REYSc | REYSc | ||||||||
| ash | whole | |||||||||||||
| 94093 | I | feed | 415 | 464 | 490 | 452 | ||||||||
| 94094 | I | C conc. | 449 | 512 | 546 | 441 | ||||||||
| 94095 | I | magnetics | 297 | 327 | 343 | 339 | ||||||||
| 94096 | I | >200 mesh | 386 | 428 | 450 | 364 | ||||||||
| 94097 | I | <200 mesh | 431 | 485 | 514 | 485 |
| Sample # | Plant | Run | Ash | C | Fe2O3 | CaO | SO3 | REYSc |
|---|---|---|---|---|---|---|---|---|
| ash | ||||||||
| 94101 | D | 1 | 94.51 | 1.72 | 6.96 | 6.85 | 4.57 | 464 |
| 94102 | D | 2 | 96.46 | 1.19 | 6.04 | 4.57 | 2.92 | 480 |
| 94103 | D | 3 | 95.29 | 1.41 | 6.59 | 5.78 | 3.64 | 497 |
| 94104 | D | 4 | 94.13 | 1.55 | 7.20 | 6.56 | 4.35 | 465 |
| 94105 | D | 5 | 97.57 | 0.77 | 5.43 | 3.14 | 2.00 | 494 |
| group avg. | 95.59 | 1.33 | 6.44 | 5.38 | 3.50 | 480 | ||
| group st. dev | 1.42 | 0.37 | 0.72 | 1.53 | 1.06 | 16 | ||
| 94132 | D | 11 | 97.17 | 0.96 | 6.08 | 3.14 | 2.29 | 554 |
| 94133 | D | 12 | 96.78 | 1.11 | 6.39 | 3.86 | 2.81 | 546 |
| 94134 | D | 13 | 97.48 | 0.84 | 6.05 | 2.67 | 1.99 | 558 |
| 94135 | D | 14 | 97.63 | 0.85 | 5.84 | 2.34 | 1.62 | 556 |
| 94136 | D | 15 | 97.69 | 0.71 | 6.00 | 2.30 | 1.94 | 548 |
| group avg. | 97.35 | 0.89 | 6.07 | 2.86 | 2.13 | 552 | ||
| group st. dev | 0.38 | 0.15 | 0.20 | 0.65 | 0.45 | 5 | ||
| 94106 | D | 16 | 97.72 | 0.53 | 6.00 | 2.09 | 1.87 | 532 |
| 94107 | D | 17 | 97.79 | 0.55 | 5.82 | 1.95 | 1.69 | 546 |
| 94108 | D | 18 | 97.87 | 0.61 | 5.84 | 1.66 | 1.18 | 554 |
| 94109 | D | 19 | 97.87 | 0.63 | 6.42 | 1.59 | 1.13 | 585 |
| 94110 | D | 20 | 97.79 | 0.57 | 5.61 | 1.53 | 1.15 | 564 |
| group avg. | 97.81 | 0.58 | 5.94 | 1.76 | 1.40 | 556 | ||
| group st. dev | 0.06 | 0.04 | 0.30 | 0.24 | 0.35 | 20 | ||
| plant avg. | 96.92 | 0.93 | 6.15 | 3.34 | 2.34 | 530 | ||
| plant st.dev. | 1.26 | 0.38 | 0.48 | 1.81 | 1.10 | 39 | ||
| Sample # | Plant | run | Ash | C | Fe2O3 | CaO | SO3 | REYSc |
| ash | ||||||||
| 94111 | I | 36 | 97.13 | 2.70 | 6.34 | 0.98 | 0.08 | 508 |
| 94112 | I | 37 | 94.93 | 5.08 | 7.27 | 1.00 | 0.10 | 527 |
| 94113 | I | 38 | 94.98 | 4.88 | 6.71 | 1.02 | 0.13 | 530 |
| 94114 | I | 39 | 96.06 | 3.77 | 6.55 | 1.00 | 0.08 | 525 |
| 94115 | I | 40 | 95.98 | 3.88 | 6.47 | 0.98 | 0.08 | 533 |
| group avg. | 95.82 | 4.06 | 6.67 | 1.00 | 0.09 | 524 | ||
| group st. dev | 0.91 | 0.96 | 0.36 | 0.02 | 0.02 | 10 | ||
| 94141 | I | 46 | 97.62 | 3.66 | 6.45 | 0.94 | 0.05 | 525 |
| 94142 | I | 47 | 97.80 | 3.50 | 10.92 | 0.92 | 0.05 | 514 |
| 94143 | I | 48 | 98.72 | 1.65 | 6.13 | 0.91 | 0.03 | 522 |
| 94144 | I | 49 | 97.28 | 4.35 | 6.59 | 0.95 | 0.04 | 511 |
| 94145 | I | 50 | 96.44 | 5.57 | 6.58 | 0.98 | 0.07 | 544 |
| group avg. | 97.57 | 3.75 | 7.33 | 0.94 | 0.05 | 523 | ||
| group st. dev | 0.83 | 1.43 | 2.01 | 0.03 | 0.01 | 13 | ||
| 97171 | I | 51 | 95.93 | 4.30 | 6.27 | 0.95 | 0.04 | 493 |
| 97172 | I | 52 | 95.33 | 4.87 | 6.41 | 0.96 | 0.05 | 509 |
| 97173 | I | 53 | 96.69 | 3.62 | 6.26 | 0.92 | 0.04 | 496 |
| 97174 | I | 54 | 96.59 | 3.69 | 6.08 | 0.93 | 0.05 | 491 |
| 97175 | I | 55 | 97.53 | 2.92 | 6.10 | 0.95 | 0.03 | 492 |
| group avg. | 96.41 | 3.88 | 6.22 | 0.94 | 0.04 | 496 | ||
| group st. dev | 0.83 | 0.74 | 0.14 | 0.02 | 0.01 | 7 | ||
| plant avg. | 96.60 | 3.90 | 6.74 | 0.96 | 0.06 | 515 | ||
| plant st.dev. | 1.09 | 1.01 | 1.19 | 0.03 | 0.03 | 17 | ||
| Parameter | Source | DF | Sum sq. | Mean sq. | F Ratio | Prob>F |
|---|---|---|---|---|---|---|
| C | Plant | 1 | 65.83045 | 65.83045 | 102.6916 | <0.0001 |
| Composite of 5 runs | 2 | 1.22021 | 0.61010 | 0.9517 | 0.4014 | |
| Run | 4 | 0.99638 | 0.24910 | 0.3886 | 0.8145 | |
| Fe2O3 | Plant | 1 | 2.61665 | 2.61665 | 3.4072 | 0.0784 |
| Composite of 5 runs | 2 | 2.11373 | 1.05686 | 1.3762 | 0.2734 | |
| Run | 4 | 4.18923 | 1.04731 | 1.3637 | 0.2786 | |
| CaO | Plant | 1 | 42.34032 | 42.34032 | 36.2717 | <0.0001 |
| Composite of 5 runs | 2 | 17.74633 | 8.87316 | 7.6014 | 0.0031 | |
| Run | 4 | 2.27929 | 0.56982 | 0.4881 | 0.7443 | |
| SO3 | Plant | 1 | 39.05643 | 39.05643 | 86.0759 | <0.0001 |
| Composite of 5 runs | 2 | 5.93859 | 2.96929 | 6.5440 | 0.0059 | |
| Run | 4 | 1.13855 | 0.28464 | 0.6273 | 0.6481 | |
| REE | Plant | 1 | 1657.63330 | 1657.63330 | 2.0930 | 0.1621 |
| Composite of 5 runs | 2 | 6553.40000 | 3276.70000 | 4.1372 | 0.0298 | |
| Run | 4 | 951.53330 | 237.88333 | 0.3004 | 0.8745 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hower, J.C.; Groppo, J.G.; Joshi, P.; Preda, D.V.; Gamliel, D.P.; Mohler, D.T.; Wiseman, J.D.; Hopps, S.D.; Morgan, T.D.; Beers, T.; et al. Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals. Minerals 2020, 10, 105. https://doi.org/10.3390/min10020105
Hower JC, Groppo JG, Joshi P, Preda DV, Gamliel DP, Mohler DT, Wiseman JD, Hopps SD, Morgan TD, Beers T, et al. Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals. Minerals. 2020; 10(2):105. https://doi.org/10.3390/min10020105
Chicago/Turabian StyleHower, James C., John G. Groppo, Prakash Joshi, Dorin V. Preda, David P. Gamliel, Daniel T. Mohler, John D. Wiseman, Shelley D. Hopps, Tonya D. Morgan, Todd Beers, and et al. 2020. "Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals" Minerals 10, no. 2: 105. https://doi.org/10.3390/min10020105
APA StyleHower, J. C., Groppo, J. G., Joshi, P., Preda, D. V., Gamliel, D. P., Mohler, D. T., Wiseman, J. D., Hopps, S. D., Morgan, T. D., Beers, T., & Schrock, M. (2020). Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals. Minerals, 10(2), 105. https://doi.org/10.3390/min10020105






