Effect of Commercial Amendments on Immobilization of Arsenic, Copper, and Zinc in Contaminated Soil: Comprehensive Assessing to Plant Uptake Combined with a Microbial Community Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Amendments Preparation
2.2. Greenhouse Experiment
2.3. Samples Analysis
2.4. Microbial Metabolic Activity
2.5. Statistical Analysis
3. Results and Discussions
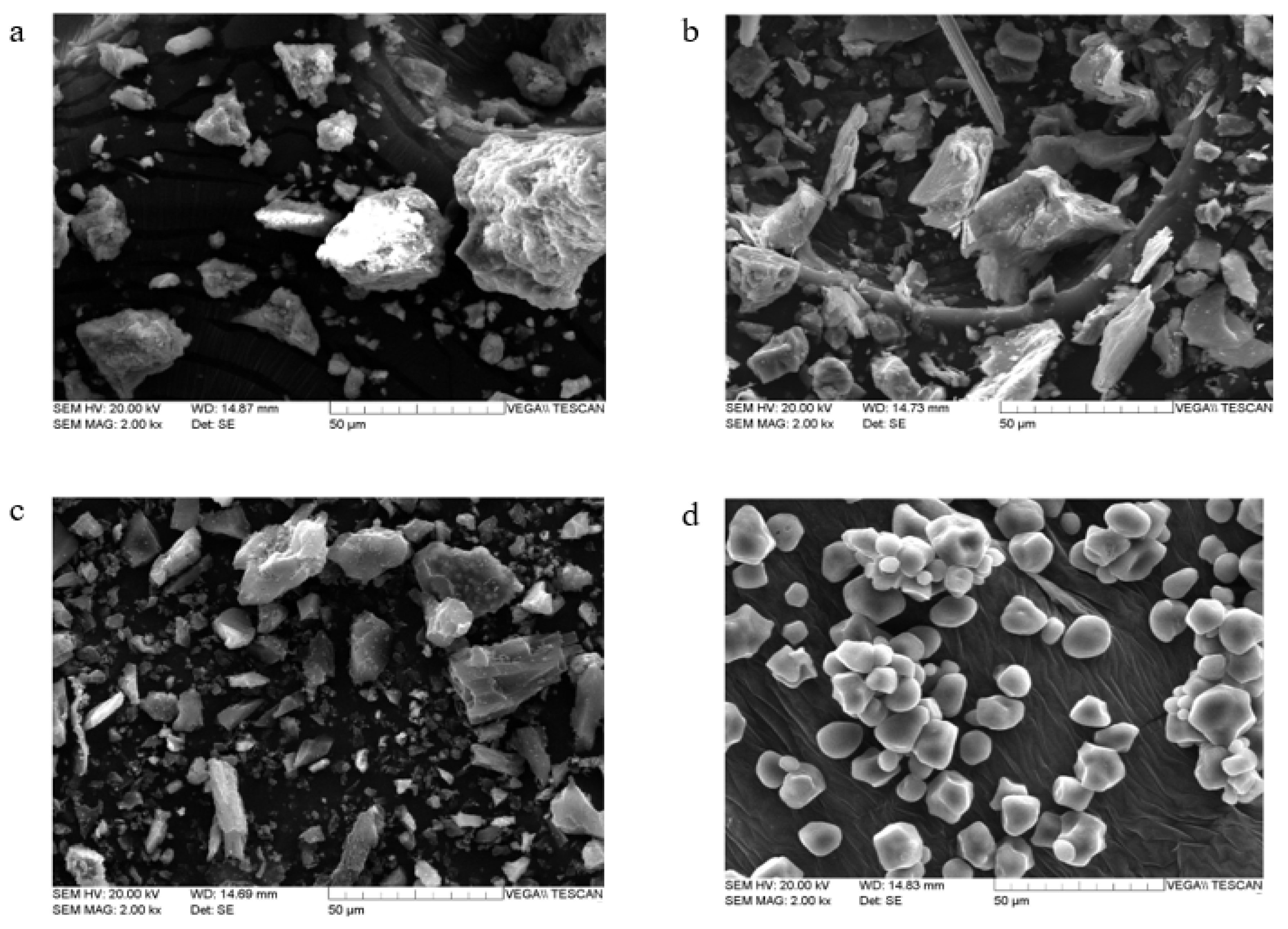
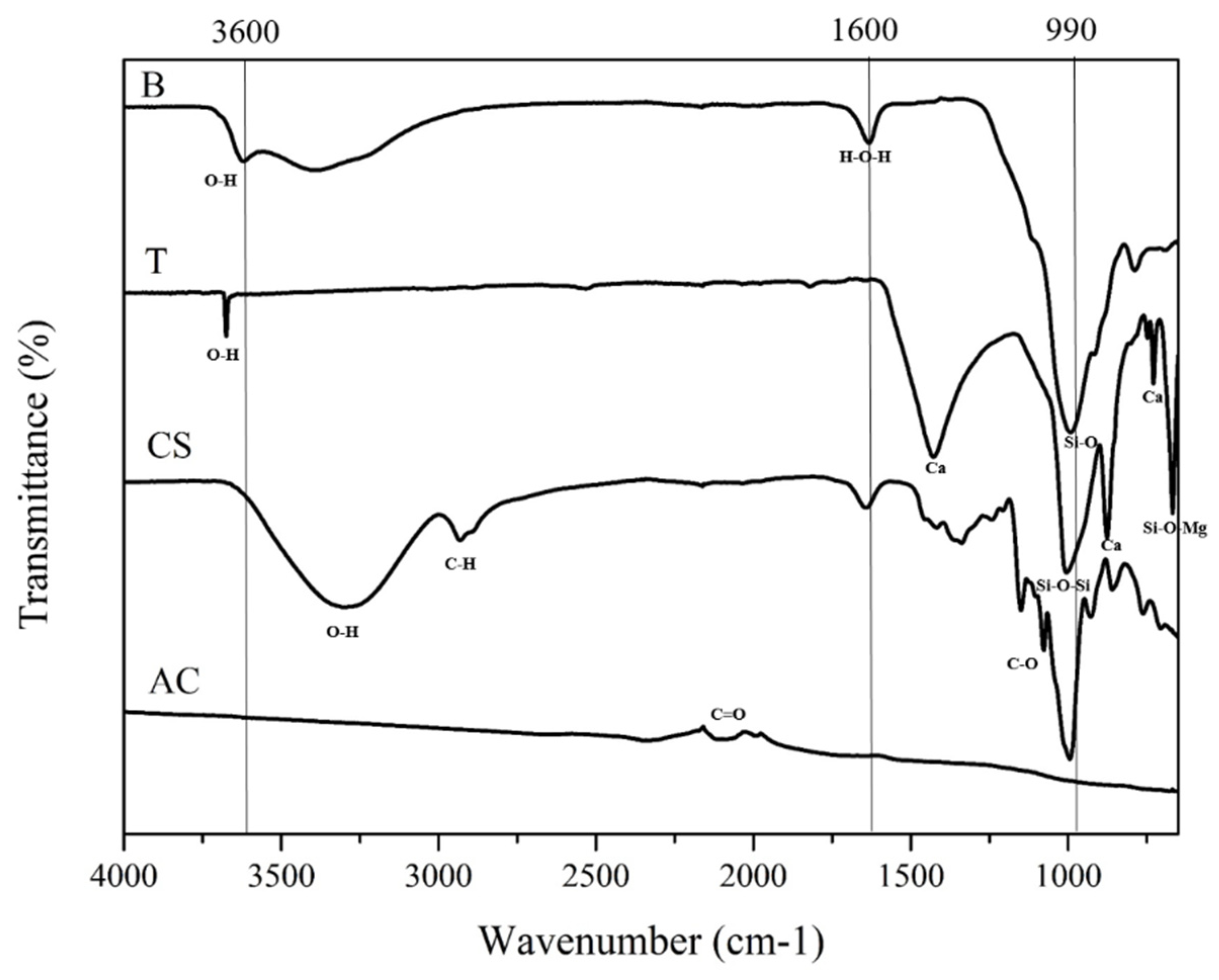
3.1. Physicochemical Properties of Initial Soil and Amendments
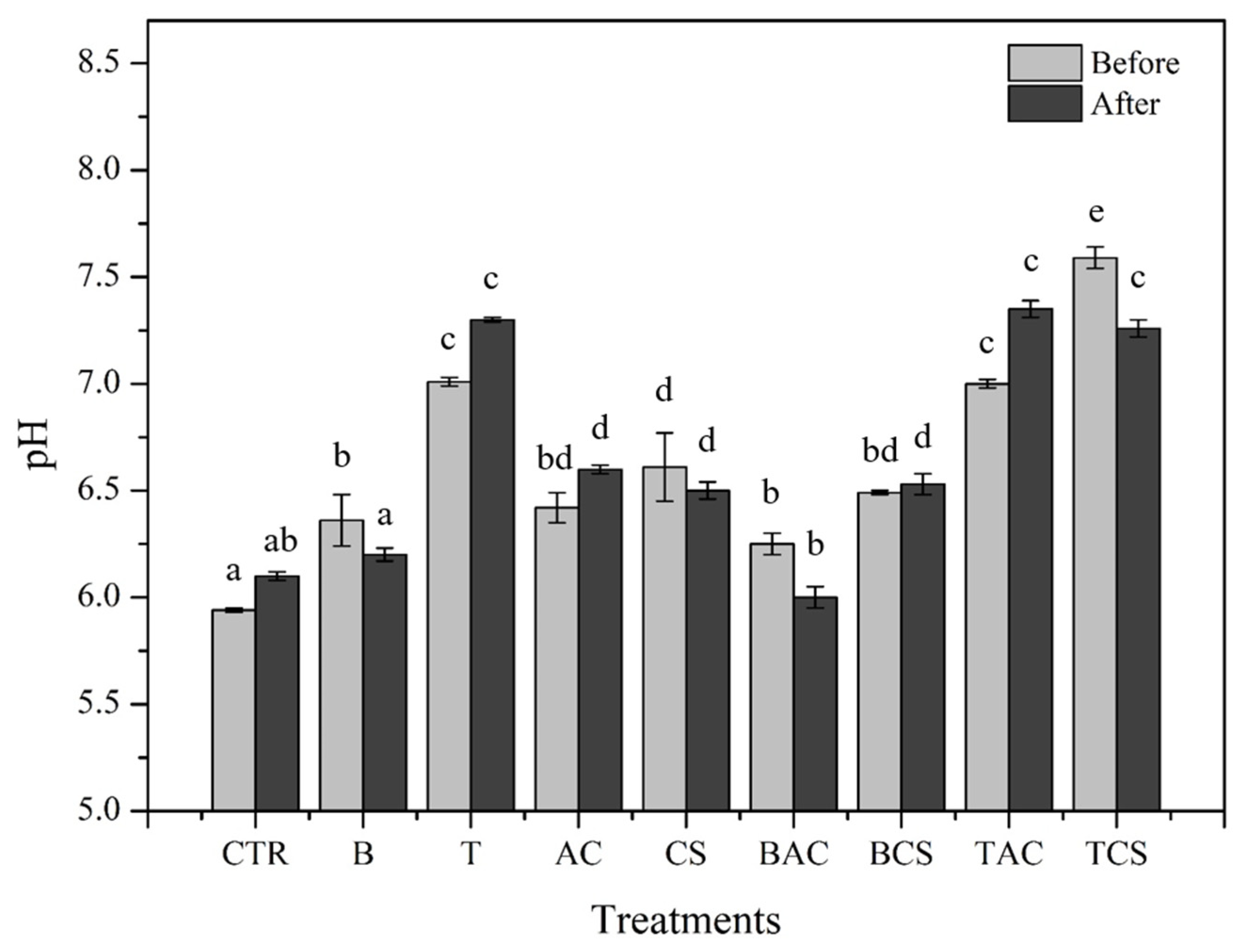
3.2. Effects of Amendments on Soil Properties
3.3. Effects of Amendments on As, Cu, and Zn Fractional Distribution in Soil
3.4. Effects of Amendments on Plant Growth Index and Phytotoxicity
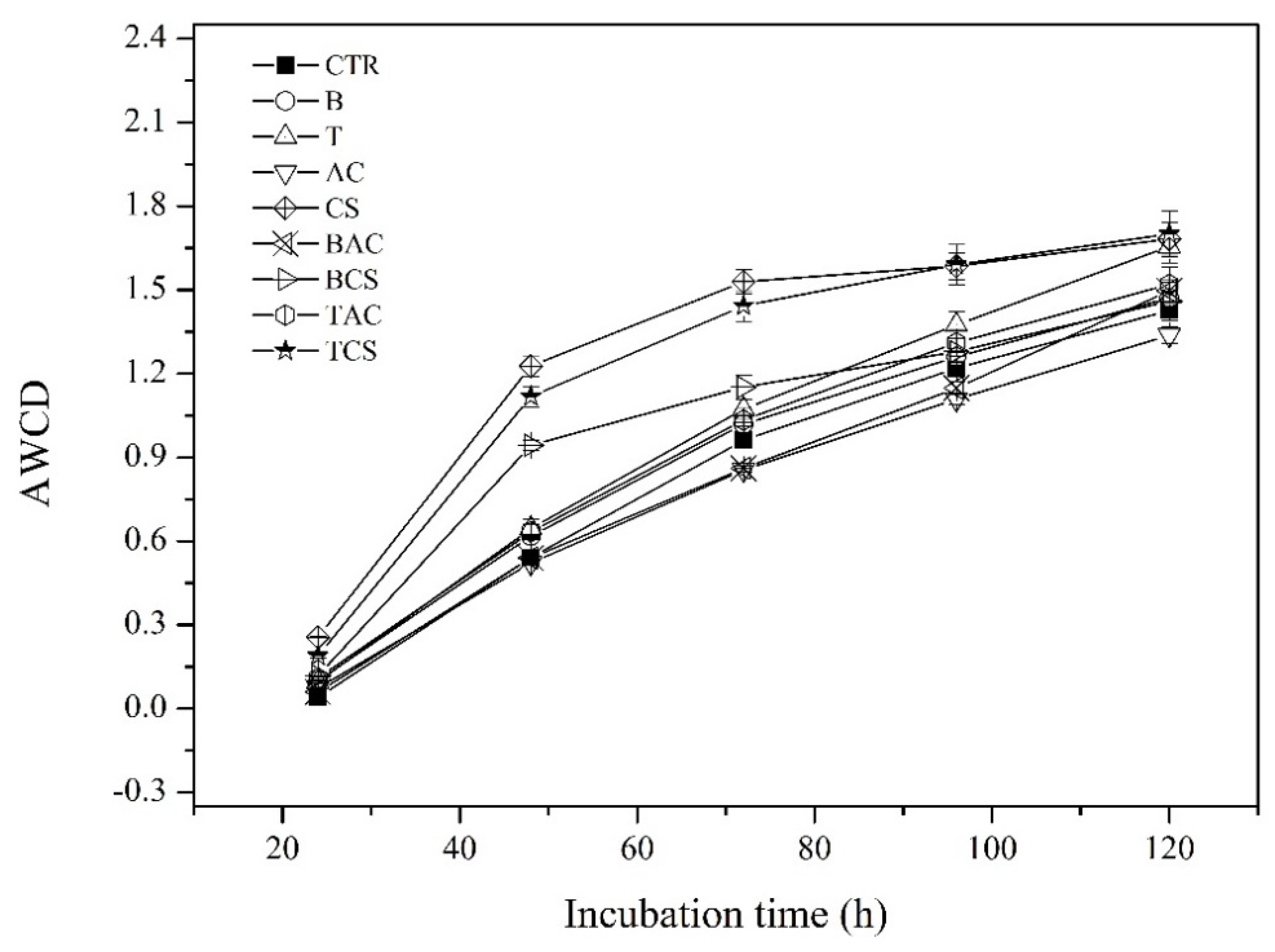
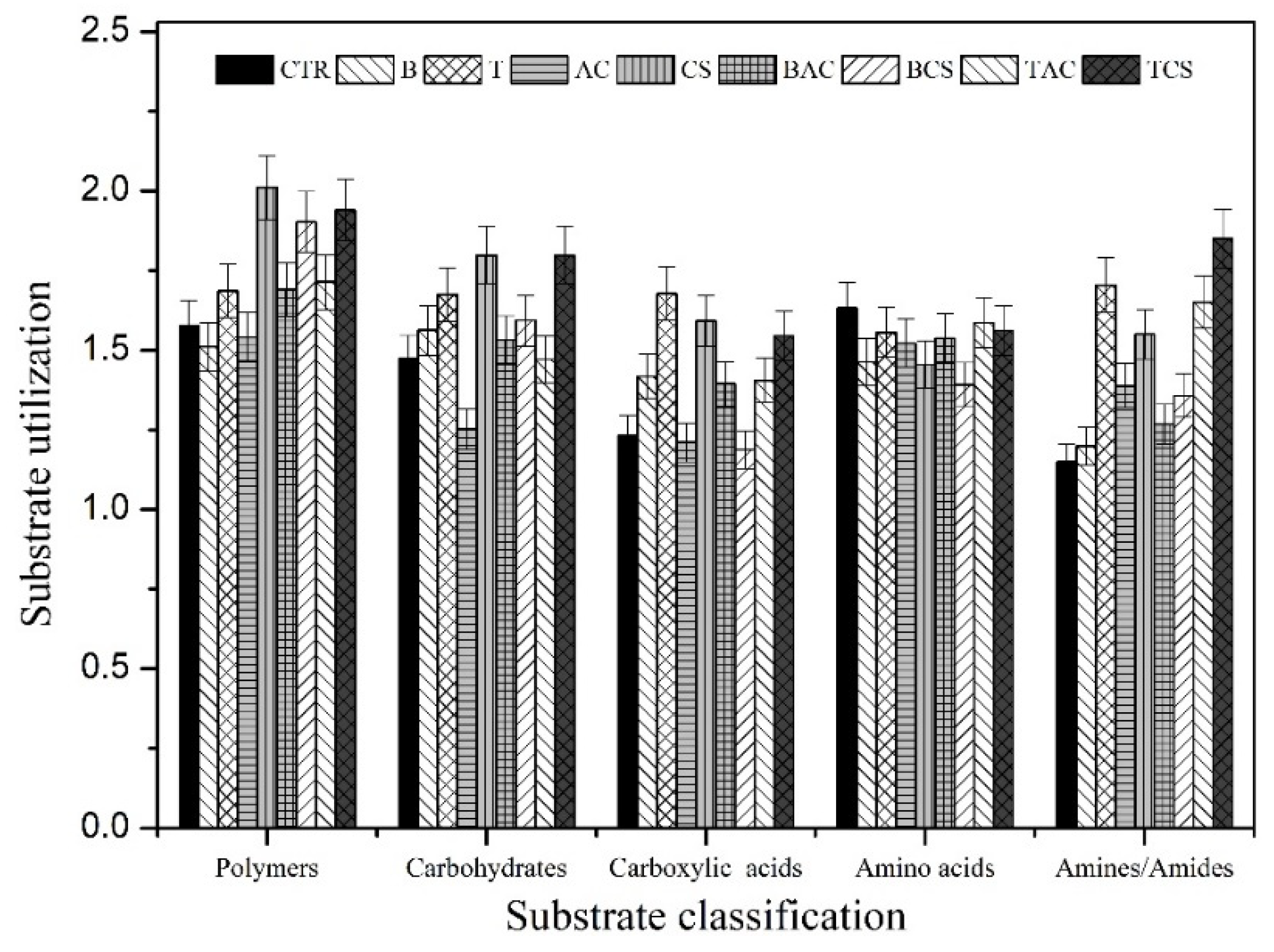
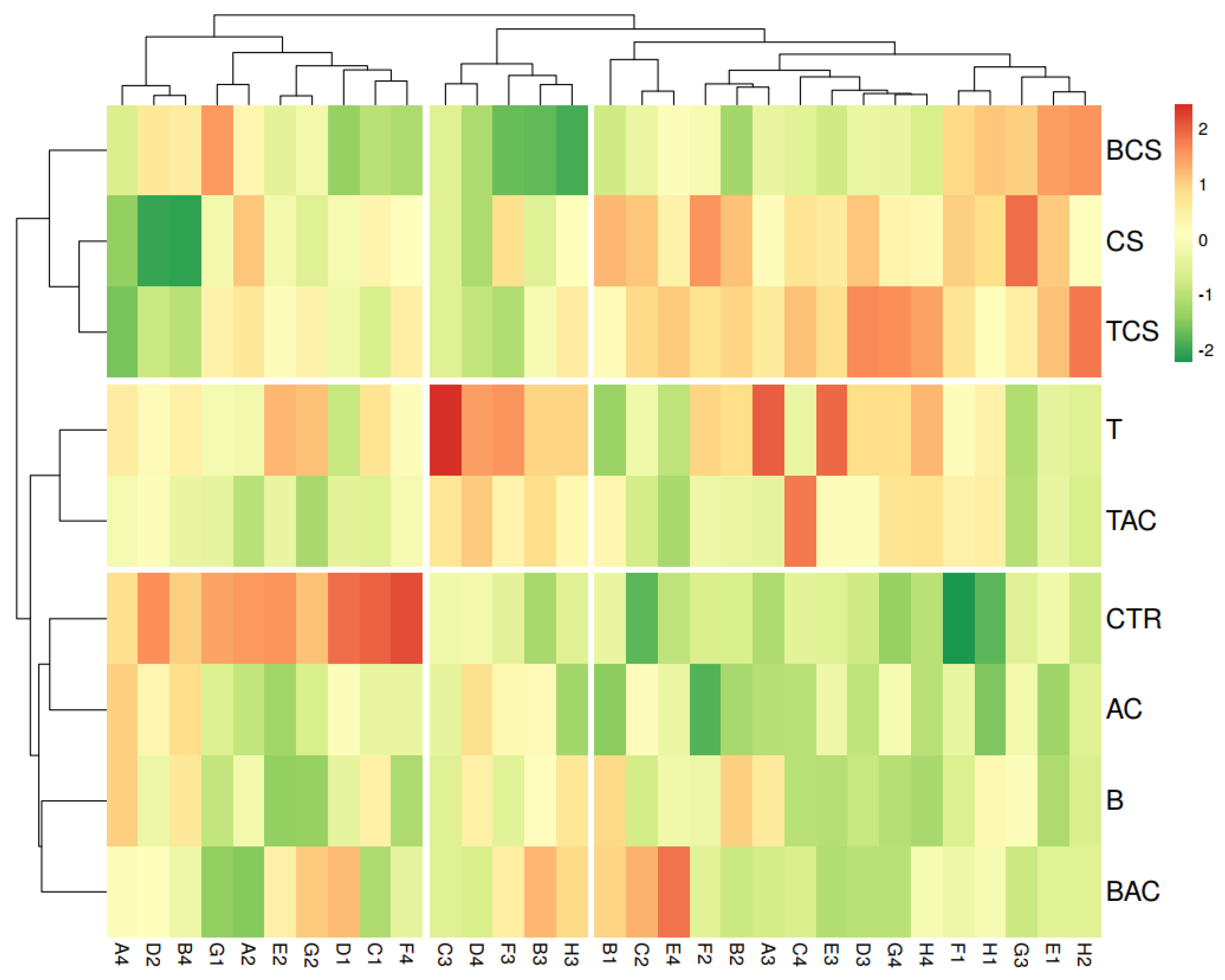
3.5. Effect of Amendments on Metabolic Function of Soil Microbial Communities
4. Conclusions
- (1)
- Amending soil-induced pH caused a heavy metal(loid)s immobilization.
- (2)
- Among soil amendments, B and AC and their composites show a higher efficiency for As and Cu immobilization in soil with a great reduction in their easily bioavailable fractions.
- (3)
- T, CS, and their composites showed a great effect on Zn immobilization in soil.
- (4)
- The lettuce grew well in B, AC, and their composites amended soils with the minimum amount of As and Cu found in its tissue, while Zn concentration in plant tissue was higher.
- (5)
- No significant change in the microbial communities was found for the B, AC, and their composite amended soils, while T, CS, and their composites induced soil microbial diversity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derakhshan Nejad, Z.; Rezania, S.; Jung, M.C.; Al-Ghamdi, A.A.; Mustafa, A.E.-Z.M.A.; Elshikh, M.S. Effects of fine fractions of soil organic, semi-organic, and inorganic amendments on the mitigation of heavy metal(loid)s leaching and bioavailability in a post-mining area. Chemosphere 2021, 271, 129538. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Govarthanan, M.; Lee, G.-W.; Park, J.-H.; Kim, J.S.; Lim, S.-S.; Seo, S.-K.; Cho, M.; Myung, H.; Kamala-Kannan, S.; Oh, B.-T. Bioleaching characteristics, influencing factors of Cu solubilization and survival of Herbaspirillum sp. GW103 in Cu contaminated mine soil. Chemosphere 2014, 109, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, K.-W.; Lee, J.-U.; Lee, J.-S.; Cook, J. Assessment of As and Heavy Metal Contamination in the Vicinity of Duckum Au-Ag Mine, Korea. Environ. Geochem. Health 2002, 24, 213–225. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Derakhshan-Nejad, Z.; Jung, M.C. Remediation of multi-metal contaminated soil using biochars from rice husk and maple leaves. J. Mater. Cycles Waste Manag. 2019, 21, 457–468. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: Immobilization mechanism and long-term stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Govarthanan, M.; Park, S.-H.; Park, Y.-J.; Myung, H.; Krishnamurthy, R.R.; Lee, S.-H.; Lovanh, N.; Kamala-Kannan, S.; Oh, B.-T. Lead biotransformation potential of allochthonous Bacillus sp. SKK11 with sesame oil cake extract in mine soil. RSC Adv. 2015, 5, 54564–54570. [Google Scholar] [CrossRef]
- Govarthanan, M.; Selvankumar, T.; Mythili, R.; Srinivasan, P.; Ameen, F.; AlYahya, S.A.; Kamala-Kannan, S. Biogreen remediation of chromium-contaminated soil using Pseudomonas sp. (RPT) and neem (Azadirachta indica) oil cake. Int. J. Environ. Sci. Technol. 2019, 16, 4595–4600. [Google Scholar] [CrossRef]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T. A comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina. J. Hazard. Mater. 2008, 153, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Riedel, G.S.; Riedel, G.; Kwon, S.; Landis, R.; Brown, S.S.; Menzie, C.A.; Ghosh, U. Activated carbon mitigates mercury and methylmercury bioavailability in contaminated sediments. Environ. Sci. Technol. 2013, 47, 13001–13010. [Google Scholar] [CrossRef]
- Meynet, P.; Hale, S.E.; Davenport, R.J.; Cornelissen, G.; Breedveld, G.D.; Werner, D. Effect of activated carbon amendment on bacterial community structure and functions in a PAH impacted urban soil. Environ. Sci. Technol. 2012, 46, 5057–5066. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, K.; Sun, Y.; Gao, Y.; Yang, H.; Xu, H. Effects of amendments on heavy metal immobilization and uptake by Rhizoma chuanxiong on copper and cadmium contaminated soil. R. Soc. Open Sci. 2018, 5, 181138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Eyles, J.L.; Yupanqui, C.; Beckingham, B.; Riedel, G.; Gilmour, C.; Ghosh, U. Evaluation of biochars and activated carbons for in situ remediation of sediments impacted with organics, mercury, and methylmercury. Environ. Sci. Technol. 2013, 47, 13721–13729. [Google Scholar] [CrossRef]
- Wu, B.; Cheng, G.; Jiao, K.; Shi, W.; Wang, C.; Xu, H. Mycoextraction by Clitocybe maxima combined with metal immobilization by biochar and activated carbon in an aged soil. Sci. Total Environ. 2016, 562, 732–739. [Google Scholar] [CrossRef]
- Li, Y.; Liang, X.; Huang, Q.; Xu, Y.; Yang, F. Inhibition of Cd accumulation in grains of wheat and rice under rotation mode using composite silicate amendment. RSC Adv. 2019, 9, 35539–35548. [Google Scholar] [CrossRef] [Green Version]
- León, O.; Soto, D.; González, J.; Piña, C.; Muñoz-Bonilla, A.; Fernandez-García, M. Environmentally Friendly Fertilizers Based on Starch Superabsorbents. Materials 2019, 12, 3493. [Google Scholar] [CrossRef] [Green Version]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan Nejad, Z.; Jung, M.C. The effects of biochar and inorganic amendments on soil remediation in the presence of hyperaccumulator plant. Int. J. Energy Environ. Eng. 2017, 8, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Walter, W.; Kirchbaumer, N.; Prohaska, Y.; Stingeder, G.; Lombi, E.; Domy, A. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Chen, C.-X.; Huang, B.; Li, T.; Wu, G.-F. Preparation of phosphoric acid activated carbon from sugarcane bagasse by mechanochemical processing. BioResources 2012, 7, 5109–5116. [Google Scholar] [CrossRef] [Green Version]
- Gryta, A.; Frac, M.; Oszust, K. The application of the Biolog EcoPlate approach in ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Feigl, V.; Ujaczki, E.; Vaszita, E.; Molnar, M. Influence of red mud on soil microbial communities: Application and comprehensive evaluation of the Biolog EcoPlate approach as a tool in soil microbiological studies. Sci. Total Environ. 2017, 595, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Du, H.; Gao, Y.; Qiu, W. Analysis on Metabolic Functions of Stored Rice Microbial Communities by BIOLOG ECO Microplates. Front. Microbiol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenarova, A.; Radeva, G.; Traykov, I.; Boteva, S. Community level physiological profiles of bacterial communities inhabiting uranium mining impacted sites. Ecotoxicol. Environ. Saf. 2014, 100, 226–232. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: New York, NY, USA, 1979; p. 333. [Google Scholar]
- Jo, I.S.; Koh, M.H. Chemical changes in agricultural soils of Korea: Data review and suggested countermeasures. Environ. Geochem. Health 2004, 26, 105–117. [Google Scholar] [CrossRef]
- Que, W.; Zhou, Y.-H.; Liu, Y.-G.; Wen, J.; Tan, X.-F.; Liu, S.-J.; Jiang, L.-H. Appraising the effect of in-situ remediation of heavy metal contaminated sediment by biochar and activated carbon on Cu immobilization and microbial community. Ecol. Eng. 2019, 127, 519–526. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Suchismita, D.; Gress, J.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Arsenic uptake by lettuce from As-contaminated soil remediated with Pteris vittata and organic amendment. Chemosphere 2017, 176, 249–254. [Google Scholar] [CrossRef]
- Bejarano, A.; Sauer, U.; Mitter, B.; Preininger, C. Parameters influencing adsorption of Paraburkholderia phytofirmans PsJN onto bentonite, silica and talc for microbial inoculants. Appl. Clay Sci. 2017, 141, 138–145. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Characterization of char from biomass gasification and its similarities with activated carbon in adsorption applications. Appl. Energy 2018, 227, 92–99. [Google Scholar] [CrossRef]
- Ciesielskia, Y.; Lii, C.-y.; Yen, M.T.; Tomasikc, T. Interactions of starch with salts of metals from the transition groups. Carbohydr. Polym. 2003, 51, 47–56. [Google Scholar] [CrossRef]
- Zhirong, L.; Azhar Uddin, M.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Ossman, M.E.; Mansour, M.S.; Fattah, M.A.; Taha, N.; Kiros, Y. Peanut shells and talc powder for removal of hexavalent chromium from aqueous solutions. Bulg. Chem. Commun. 2014, 46, 629–639. [Google Scholar]
- Lagdic, L.; Mitchell, M.K.; Payne, B.D. Highly effective adsorption of heavy metal ions by a thiolfunctionalized magnesium phyllosilicate clay. Environ. Sci. Technol. 2001, 35, 84–90. [Google Scholar]
- Jovanovski, G.; Stefov, V.; Šoptrajanov, B.; Boev, B. Minerals from Macedonia. IV. Discrimination between some carbonate minerals by FTIR spectroscopy. Neues Jahrb. Mineral.-Abh. 2002, 177, 241–253. [Google Scholar] [CrossRef]
- Bayat, M.; Nasernejad, B.; Falamaki, C. Preparation and characterization of nano-galvanic bimetallic Fe/Sn nanoparticles deposited on talc and its enhanced performance in Cr(VI) removal. Sci. Rep. 2021, 11, 7715. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Guan, W.; Ji, F.; Song, Z.; Zhao, Y. Production of Biologically Activated Carbon from Orange Peel and Landfill Leachate Subsequent Treatment Technology. J. Chem. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Warton, B.; Matthiessen, J.N. The crucial role of calcium interacting with soil pH in enhanced biodegradation of metam-sodium. Pest Manag. Sci. 2005, 61, 856–862. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, W.; Ge, L.; Zhao, W.; Zhang, G.; Niu, Y. Immobilization properties and adsorption mechanism of nickel(II) in soil by biochar combined with humic acid-wood vinegar. Ecotoxicol. Environ. Saf. 2021, 215, 112159. [Google Scholar] [CrossRef] [PubMed]
- Brendova, K.; Zemanova, V.; Pavlikova, D.; Tlustos, P. Utilization of biochar and activated carbon to reduce Cd, Pb and Zn phytoavailability and phytotoxicity for plants. J. Environ. Manag. 2016, 181, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Wu, Y.; Cheng, T. Silicate Minerals as a Source of Arsenic Contamination in Groundwater. Water Air Soil Pollut. 2014, 225, 2201. [Google Scholar] [CrossRef]
- Huang, D.; Li, B.; Wu, M.; Kuga, S.; Huang, Y. Graphene Oxide-Based Fe-Mg (Hydr)oxide Nanocomposite as Heavy Metals Adsorbent. J. Chem. Eng. Data 2018, 63, 2097–2105. [Google Scholar] [CrossRef]
- Yi, X.; Sun, F.; Han, Z.; Han, F.; He, J.; Ou, M.; Gu, J.; Xu, X. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu(II) and U(VI) removal. Ecotoxicol. Environ. Saf. 2018, 158, 309–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, M.; Zhang, Z.; Marhaba, T.; Sun, C.; Zhang, W. In situ immobilization of heavy metals in contaminated sediments by composite additives of hydroxyapatite and oxides. Environ. Earth Sci. 2019, 78, 94. [Google Scholar] [CrossRef]
- Wu, L.; Forsling, W.; Schindler, P.W. Surface complexation of calcium minerals in aqueous solution: 1. Surface protonation at fluorapatite—water interfaces. J. Colloid Interface Sci. 1991, 147, 178–185. [Google Scholar] [CrossRef]
- Pourbeyram, S. Effective Removal of Heavy Metals from Aqueous Solutions by Graphene Oxide-Zirconium Phosphate (GO-Zr-P) Nanocomposite. Ind. Eng. Chem. Res. 2016, 55, 5608–5617. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Mizuta, K.; Taguchi, S.; Sato, S. Soil aggregate formation and stability induced by starch and cellulose. Soil Biol. Biochem. 2015, 87, 90–96. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Deng, L.; Zeng, G.; Fan, C.; Lu, L.; Chen, X.; Chen, M.; Wu, H.; He, X.; He, Y. Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Appl. Microbiol. Biotechnol. 2015, 99, 8259–8269. [Google Scholar] [CrossRef] [PubMed]
- Hmid, A.; Al Chami, Z.; Sillen, W.; De Vocht, A.; Vangronsveld, J. Olive mill waste biochar: A promising soil amendment for metal immobilization in contaminated soils. Environ. Sci. Pollut. Res. Int. 2015, 22, 1444–1456. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Han, Y.; Chen, L.; Wang, D. Responses of soil aggregates and bacterial communities to soil-Pb immobilization induced by biofertilizer. Chemosphere 2019, 220, 828–836. [Google Scholar] [CrossRef]
- Wu, W.; Wu, J.; Liu, X.; Chen, X.; Wu, Y.; Yu, S. Inorganic phosphorus fertilizer ameliorates maize growth by reducing metal uptake, improving soil enzyme activity and microbial community structure. Ecotoxicol. Environ. Saf. 2017, 143, 322–329. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Mellis, E.V.; Escalas, A.; Lemos, L.N.; Junior, J.L.; Quaggio, J.A.; Zhou, J.; Tsai, S.M. Zinc concentration affects the functional groups of microbial communities in sugarcane-cultivated soil. Agric. Ecosyst. Environ. 2017, 236, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Choubey, S.; Kulkarni, A.; Awale, K.; Godbole, S. Microbiological quality of different brands of talcum powder in India. World J. Pharm. Med. Res. 2017, 8, 206–211. [Google Scholar]
- Wang, X.; Conway, P.L.; Brown, I.L.; Evans, A.J. In vitro utilization of amylopectin and high-amylose maize (Amylomaize) starch granules by human colonic bacteria. Appl. Environ. Microbiol. 1999, 65, 4848–4854. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.P.; Legge, R.L. One-dimensional metric for tracking bacterial community divergence using sole carbon source utilization patterns. J. Microbiol. Methods 2009, 79, 55–61. [Google Scholar] [CrossRef] [PubMed]





| Methods | Target Phase | Reagents |
|---|---|---|
| Wenzel et al., 2001 for As fractionation | ||
| Fraction I. Exchangeable | Easily exchangeable | 0.05 M (NH4)2SO4; 4 h |
| Fraction II. Bound to carbonates | Phosphate competes with adsorbed As | 0.05 M (NH4)H2PO4; 16 h |
| Fraction III. Bound to Fe and Mn oxides | Oxalate competes with adsorbed As on amorphous Fe oxides | 0.2 M NH4-oxalate buffer, pH 3.25 in dark; 4 h |
| Fraction IV. Bound to organic matter | Associated with crystalline Fe oxides | 0.2 M NH4-oxalate buffer + 0.1 M ascorbic acid, pH 3.25; 30 min/96 °C |
| Fraction V. Residual | Bound to silicates | HCl/HNO3 3/1 v/v |
| Tessier et al., 1979 for Cu and Zn fractionation | ||
| Fraction I. Exchangeable | Adsorbed metals | 1 g sample, 0.5 M MgCl2; 20 min |
| Fraction II. Bound to carbonates | Specially adsorbed metals as well as carbonate-bound fractions | 1 M NaOAc, pH 5.0 with HOAc; 5 h |
| Fraction III. Bound to Fe and Mn oxides | Extraction of reducible Fe and Mn oxides | 0.04 M NH2∙OH∙HCl in 25% (v/v) HOAC; 6 h/96 °C |
| Fraction IV. Bound to organic matter | Bound to various forms of organic matter and sulfide minerals | 0.02 M HNO3 30% H2O2, pH 2.0 with HNO3, 3.2 M NH4OAc in 20% (v/v) HNO3; 3 h/85 °C |
| Fraction V. Residual | Detrital silicate minerals, resistant sulfides, and a small quantity of refractory organic material | HCl/HNO3 3/1 v/v |
| Sample | pH | As (mg/kg) | Cu (mg/kg) | Zn (mg/kg) | T-N (mg/kg) | T-P (mg/kg) | T-K (mg/kg) | OM (%) | Texture |
|---|---|---|---|---|---|---|---|---|---|
| Soil | 5.9 ± 0.01 | 39.45 ± 0.38 | 33.60 ± 0.52 | 108.5 ± 0.91 | 0.15 | 56.8 | 880.1 | 2.9 | Loamy sand |
| Sample | pH | As (mg/kg) | Cu (mg/kg) | Zn (mg/kg) | SSA(m2/g) |
|---|---|---|---|---|---|
| B | 7.8 ± 0.04 | 0.56 ± 0.01 | 33.20 ± 0.05 | 35.22 ± 0.04 | 60.56 |
| T | 8.8 ± 0.02 | 0.69 ± 0.02 | 1.40 ± 0.01 | 29.50 ± 0.06 | 1.80 |
| AC | 7.2 ± 0.04 | 9.06 ± 0.01 | 4.6 ± 0.03 | 1.12 ± 0.02 | 1081.9 |
| CS | 8.2 ± 0.01 | 0.44 ± 0.005 | 0.01 ± 0.002 | 0.58 ± 0.006 | 0.15 |
| Sample | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | CaO | TiO2 | MnO | SO3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| B | 67.8 | 15.8 | 6.94 | 2.60 | 2.13 | 2.12 | 0.66 | 0.25 | 0.1 | 0.07 |
| T | 35.3 | 0.65 | 1.59 | 0.03 | 34.9 | 26.9 | 0.1 | 0.06 | 0.02 | 0.05 |
| Well Number | Carbon Source | Category |
|---|---|---|
| B1 | Pyruvic acid and methyl ester | Carbohydrates |
| G1 | D-Cellobiose | Carbohydrates |
| H1 | α-D-Lactose | Carbohydrates |
| A2 | β-Methyl-D-glucoside | Carbohydrates |
| B2 | D-Xylose | Carbohydrates |
| C2 | i-Erythritol | Carbohydrates |
| D2 | D-Mannitol | Carbohydrates |
| E2 | N-Acetyl-D-glucosamine | Carbohydrates |
| G2 | Glucose-1-phosphate | Carbohydrates |
| H2 | D,L-α-Glycerol phosphate | Carbohydrates |
| C1 | Tween 40 | Polymers |
| D1 | Tween 80 | Polymers |
| E1 | α-Cyclodextrin | Polymers |
| F1 | Glycogen | Polymers |
| F2 | D-Glucosaminic acid | Carboxylic and ketonic acids |
| A3 | D-Galactonic acid-γ-lactone | Carboxylic and ketonic acids |
| B3 | D-Galacturonic acid | Carboxylic and ketonic acids |
| C3 | 2-Hydroxybenzoic acid | Carboxylic and ketonic acids |
| D3 | 4-Hydroxybenzoic acid | Carboxylic and ketonic acids |
| E3 | γ-Hydroxybutyric acid | Carboxylic and ketonic acids |
| F3 | Itaconic acid | Carboxylic and ketonic acids |
| G3 | α-Ketobutyric acid | Carboxylic and ketonic acids |
| H3 | D-Malic acid | Carboxylic and ketonic acids |
| A4 | L-Arginine | Amino acids |
| B4 | L-Asparagine | Amino acids |
| C4 | L-Phenylalanine | Amino acids |
| D4 | L-Serine | Amino acids |
| E4 | L-Threonine | Amino acids |
| F4 | Glycyl-L-glutamic acid | Amino acids |
| G4 | Phenylethylamine | Amines/amides |
| H4 | Putrescine | Amines/amides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen Quoc, T.; Nejad, Z.D.; Jung, M.C. Effect of Commercial Amendments on Immobilization of Arsenic, Copper, and Zinc in Contaminated Soil: Comprehensive Assessing to Plant Uptake Combined with a Microbial Community Approach. Minerals 2021, 11, 1143. https://doi.org/10.3390/min11101143
Nguyen Quoc T, Nejad ZD, Jung MC. Effect of Commercial Amendments on Immobilization of Arsenic, Copper, and Zinc in Contaminated Soil: Comprehensive Assessing to Plant Uptake Combined with a Microbial Community Approach. Minerals. 2021; 11(10):1143. https://doi.org/10.3390/min11101143
Chicago/Turabian StyleNguyen Quoc, Tuan, Zahra Derakhshan Nejad, and Myung Chae Jung. 2021. "Effect of Commercial Amendments on Immobilization of Arsenic, Copper, and Zinc in Contaminated Soil: Comprehensive Assessing to Plant Uptake Combined with a Microbial Community Approach" Minerals 11, no. 10: 1143. https://doi.org/10.3390/min11101143
APA StyleNguyen Quoc, T., Nejad, Z. D., & Jung, M. C. (2021). Effect of Commercial Amendments on Immobilization of Arsenic, Copper, and Zinc in Contaminated Soil: Comprehensive Assessing to Plant Uptake Combined with a Microbial Community Approach. Minerals, 11(10), 1143. https://doi.org/10.3390/min11101143







