Characterization of Fibrous Wollastonite NYAD G in View of Its Use as Negative Standard for In Vitro Toxicity Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of the Sample and Geological Overview
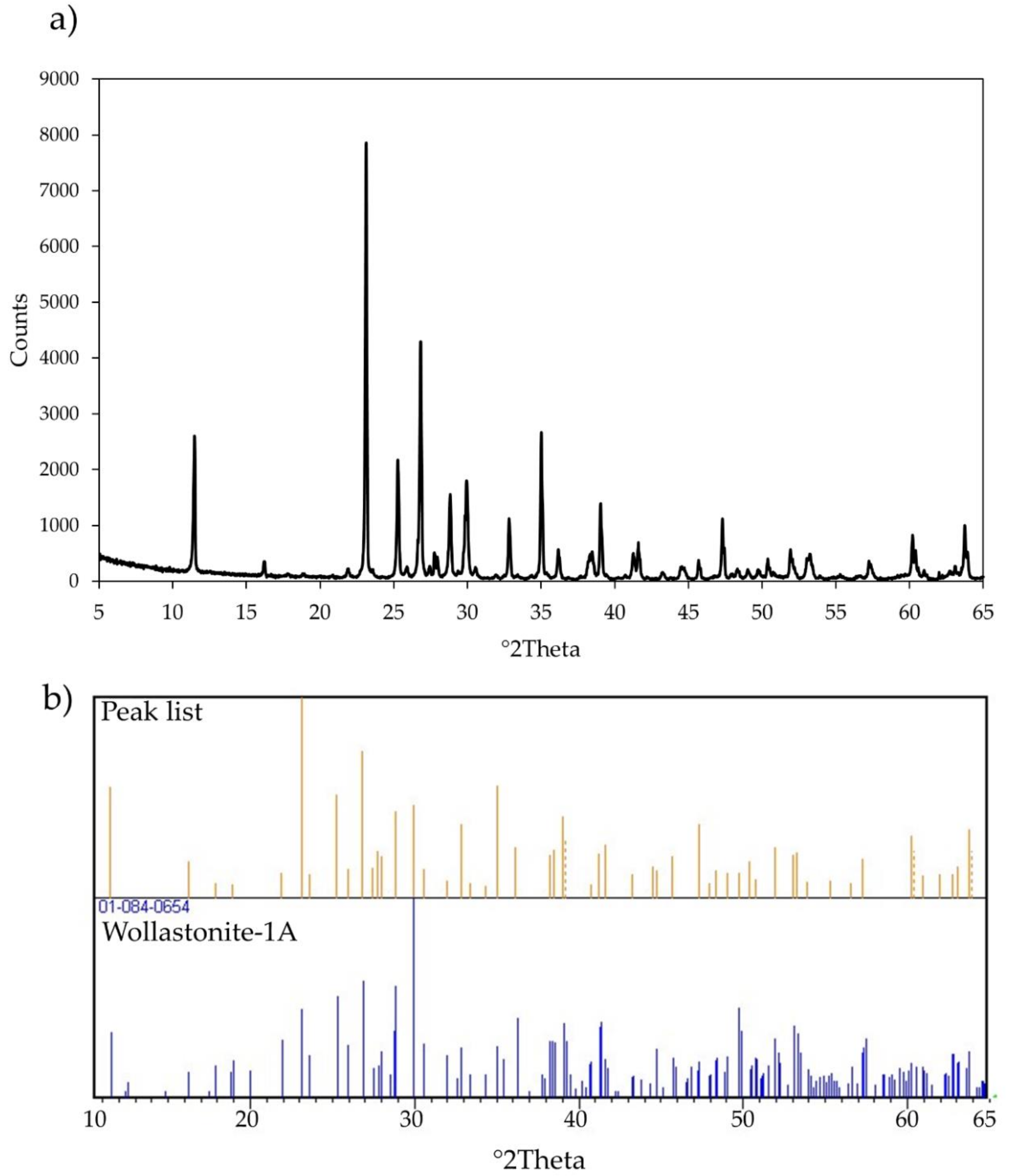
2.2. X-ray Powder Diffraction (XRPD)
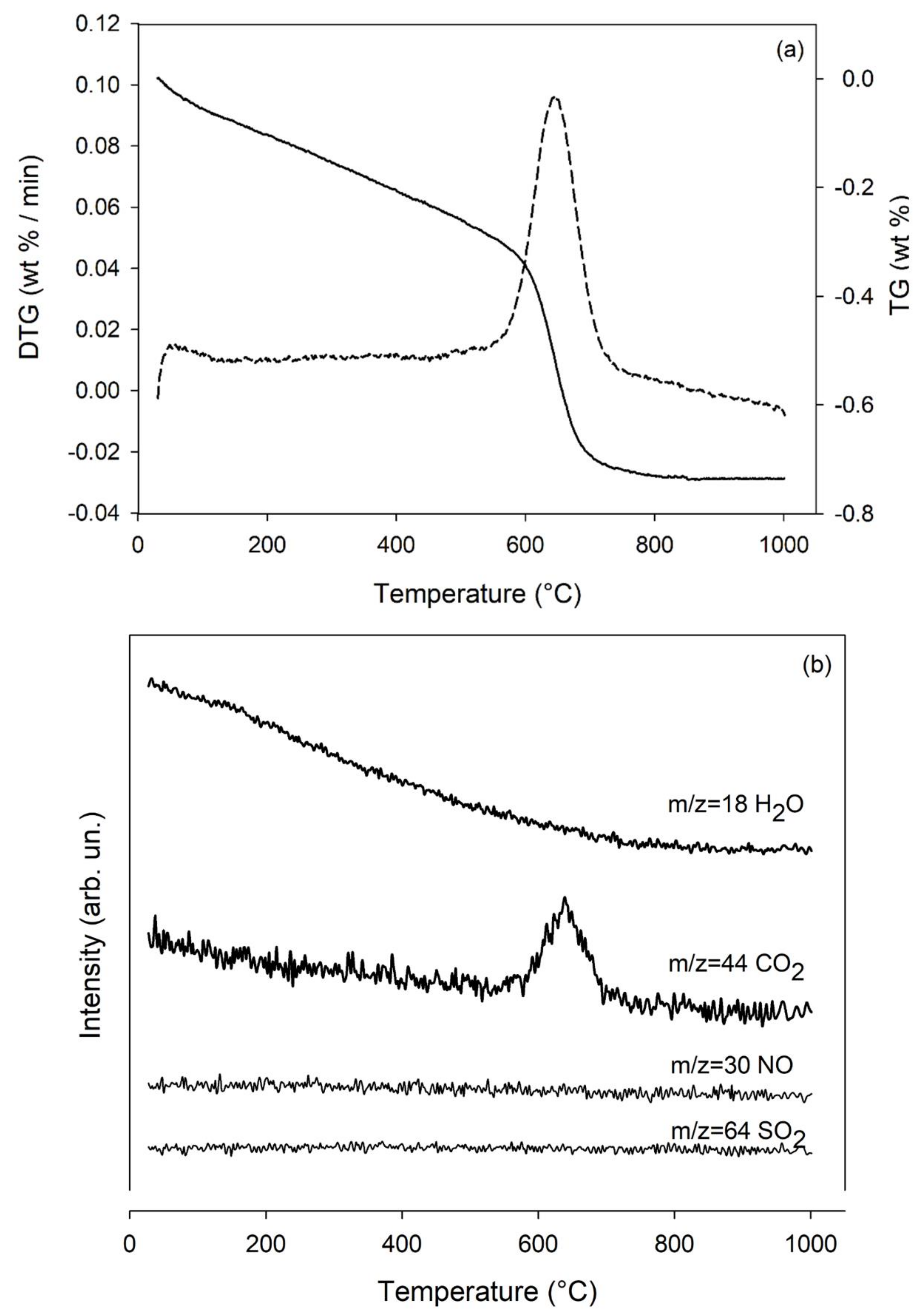
2.3. Thermogravimetric Measurements and Analysis of the Evolved Gases with Mass Spectrometry
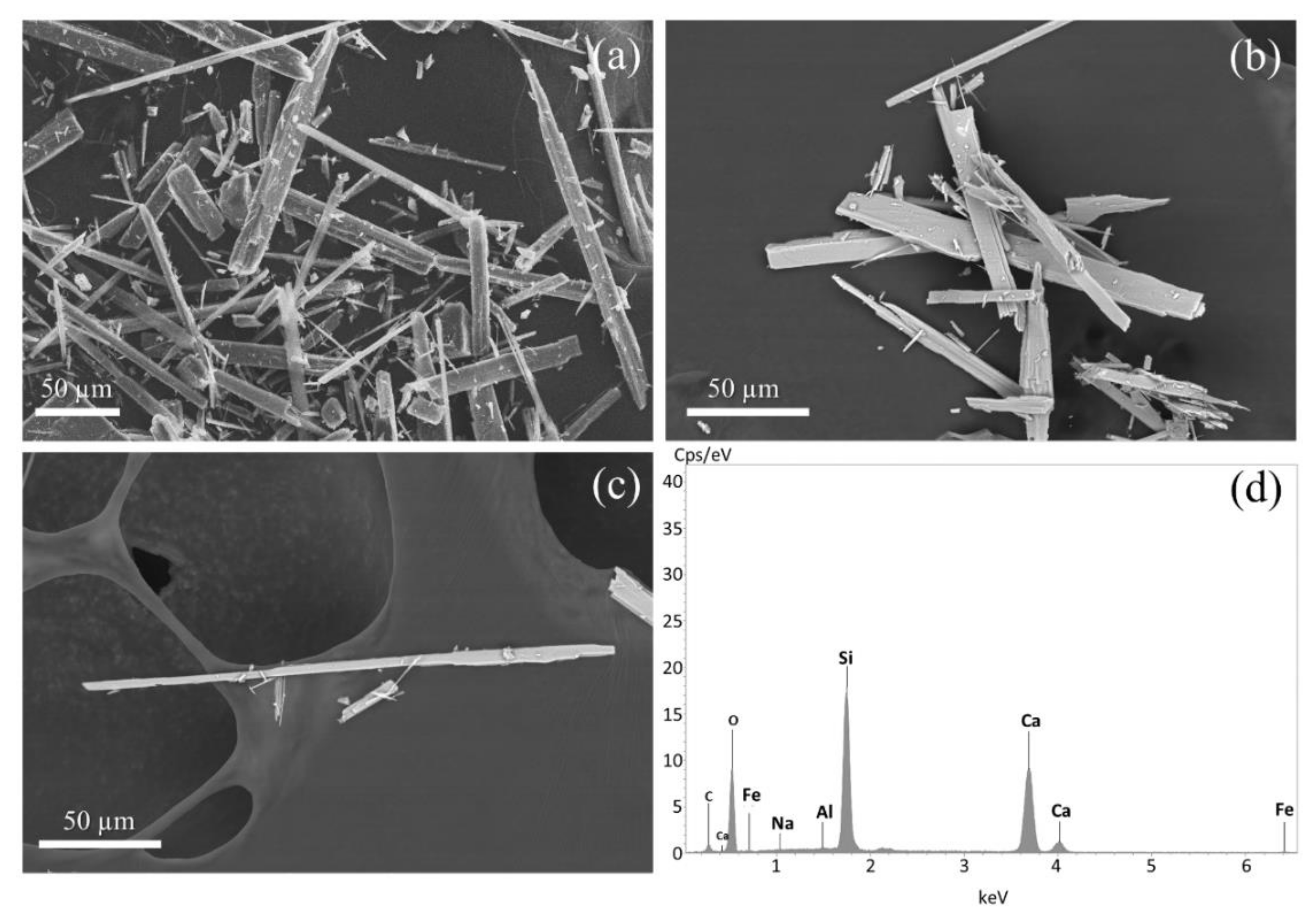
2.4. Scanning Electron Microscopy
2.5. Determination of the Specific Surface Area
2.6. Determination of the Density
2.7. Determination of the Zeta Potential
2.8. Electron Probe Micro Analysis (EPMA)
2.9. Mössbauer Spectroscopy
2.10. X-ray Photoelectron Spectroscopy (XPS)
2.11. Ultraviolet-Visible (UV-Vis) Spectroscopy
2.12. Biodurability
2.13. Determination of the Fibre Potential Toxicity Index (FPTI) of Wollastonite
2.14. In Vitro DNA Damage Quantification
3. Results
3.1. Characteristics of Wollastonite NYAD G
| Wt% | EMPA | TDS NYCO Minerals |
|---|---|---|
| SiO2 | 50.76 (0.25) | 51.60 |
| FeO | 0.33 (0.09) | - |
| Fe2O3 | 0.17 (0.09) | 0.77 |
| MnO | 0.15 (0.04) | 0.15 |
| MgO | 0.03 (0.02) | 0.15 |
| CaO | 48.27 (0.24) | 46.36 |
| Na2O | * bdl | - |
| Al2O3 | bdl | 0.40 |
| K2O | bdl | 0.02 |
| TiO2 | bdl | 0.05 |
| L.o.I. | ** 0.30 | 0.50 |
| Tot | 100.0 | 100.0 |
| Doublet | δ (mm/s) | Δ (mm/s) | Г+ (mm/s) | A (%) | Attributions |
|---|---|---|---|---|---|
| Db1 | 0.41 ± 0.01 | 0.56 ± 0.03 | 0.12 ± 0.01 | 31 ± 2 | Fe3+ octahedral (M1,M2,M3) |
| Db2 | 1.09 ± 0.04 | 2.26 ± 0.09 | 0.31 ± 0.08 | 46 ± 2 | Fe2+ octahedral (M3) |
| Db3 | 1.26 ± 0.01 | 2.76 ± 0.04 | 0.11 ± 0.02 | 23 ± 2 | Fe2+ octahedral (M1) |
3.2. Toxicity/Pathogenicity Potential of Wollastonite NYAD G
3.3. DNA Damage Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baumann, F.; Ambrosi, J.P.; Carbone, M. Asbestos is not just asbestos: An unrecognised health hazard. Lancet Oncol. 2013, 14, 576–578. [Google Scholar] [CrossRef]
- Gunter, M.E. Elongate mineral particles in the natural environment. Toxicol. Appl. Pharmacol. 2018, 361, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Harper, M.; Bailey, M.; Erskine, B.; Della Ventura, G.; Ardit, M.; Pasquali, L.; Tomaino, G.; Ray, R.; Mason, H.; et al. Characterization and assessment of the potential toxicity/pathogenicity of fibrous glaucophane. Environ. Res. 2019, 178, 108723. [Google Scholar] [CrossRef]
- Mattioli, M.; Giordani, M.; Dogan, M.; Cangiotti, M.; Avella, G.; Giorgi, R.; Dogan, A.U.; Ottaviani, M.F. Morpho-chemical characterization and surface properties of carcinogenic zeolite fibers. J. Hazard. Mater. 2016, 306, 140–148. [Google Scholar] [CrossRef]
- Pacella, A.; Ballirano, P. Chemical and structural characterization of fibrous richterite with high environmental and health relevance from Libby, Montana (USA). Period. Mineral. 2016, 85, 169–177. [Google Scholar]
- Cametti, G.; Pacella, A.; Mura, F.; Rossi, M.; Ballirano, P. New morphological, chemical, and structural data of woolly erionite-Na from Durkee, Oregon, USA. Am. Mineral. 2013, 98, 2155–2163. [Google Scholar] [CrossRef]
- Petriglieri, J.R.; Laporte-Magoni, C.; Salvioli-Mariani, E.; Ferrando, S.; Tomatis, M.; Fubini, B.; Turci, F. Morphological and chemical properties of fibrous antigorite from lateritic deposit of New Caledonia in view of hazard assessment. Sci. Total Environ. 2021, 777, 146185. [Google Scholar] [CrossRef]
- Comba, P.; Gianfagna, A.; Paoletti, L. Pleural mesothelioma cases in Biancavilla are related to a new fluoro-edenite fibrous amphibole. Arch. Environ. Health 2003, 58, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.C.; Antao, V.C.; Bove, F.J. Vermiculite worker mortality: Estimated effects of occupational exposure to Libby amphibole. J. Occup. Environ. Med. 2010, 52, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y. Carcinogenic and fibrogenic effects of zeolites: Preliminary observations. Environ. Res. 1982, 27, 433–445. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 2012; Volume 100, pp. 219–309. [Google Scholar]
- International Agency for Research on Cancer. Erionite. In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 2012; Volume 100, pp. 311–315. [Google Scholar]
- International Agency for Research on Cancer. Fluoro-edenite. In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 2017; Volume 111, pp. 215–242. [Google Scholar]
- Carbone, M.; Emri, S.; Dogan, A.U.; Steele, I.; Tuncer, M.; Pass, H.I.; Baris, Y.I. A mesothelioma epidemic in Cappadocia: Scientific developments and unexpected social outcomes. Nat. Rev. Cancer 2007, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Sepiolite. In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 1997; Volume 68, pp. 267–282. [Google Scholar]
- International Agency for Research on Cancer. Wollastonite. In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 1997; Volume 68, pp. 283–306. [Google Scholar]
- International Agency for Research on Cancer. Zeolite other than erionite. In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 1997; Volume 68, pp. 307–336. [Google Scholar]
- International Agency for Research on Cancer. Palygorskite (Attapu1gite). In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 1997; Volume 68, pp. 245–266. [Google Scholar]
- International Agency for Research on Cancer. Preamble. In IARC Monographs; International Agency for Research on Cancer: Lyon Cedex, France, 2012; Volume 100, pp. 11–34. [Google Scholar]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, M.; Yang, H. Biological activities of asbestos and other mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 435–445. [Google Scholar]
- Jablonski, R.P.; Kim, S.J.; Cheresh, P.; Kamp, D.W. Insights into mineral fibre-induced lung epithelial cell toxicity and pulmonary fibrosis. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 447–500. [Google Scholar]
- Xue, J.; Patergnani, S.; Giorgi, C.; Suarez, J.; Goto, K.; Bononi, A.; Tanji, M.; Novelli, F.; Pastorino, S.; Xu, R.; et al. Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc. Natl. Acad. Sci. USA 2020, 117, 25543–25552. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Towards a quantitative model to predict the toxicity/pathogenicity potential of mineral fibers. Toxicol. Appl. Pharmacol. 2018, 361, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. Natl. Cancer Inst. 1981, 67, 965–975. [Google Scholar]
- Krombach, F.; Münzing, S.; Allmeling, A.M.; Gerlach, J.T.; Behr, J.; Dörger, M. Cell size of alveolar macrophages: An interspecies comparison. Environ. Health Persp. 1997, 105, 1261–1263. [Google Scholar]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part. Fibre Tox. 2010, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Bio. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Lusvardi, G.; Zoboli, A.; Di Giuseppe, D.; Lassinantti Gualtieri, M. Biodurability and release of metals during the dissolution of chrysotile, crocidolite and fibrous erionite. Environ. Res. 2019, 171, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Turci, F.; Tomatis, M.; Lesci, I.S.; Roveri, N.; Bice, F. The iron-related molecular toxicity mechanism of synthetic asbestos nanofibres: A model study for high-aspect-ratio nanoparticles. Chem. Eur. J. 2011, 17, 250–358. [Google Scholar] [CrossRef]
- Mossman, B.T.; Pugnaloni, A. In vitro biological activity and mechanisms of lung and pleural cancers induced by mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 261–306. [Google Scholar]
- Capella, S.; Belluso, E.; Bursi Gandolfi, N.; Tibaldi, E.; Mandrioli, D.; Belpoggi, F. In vivo biological activity of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 307–346. [Google Scholar]
- Cardinali, G.; Kovacs, D.; Maresca, V.; Flori, E.; Dell’Anna, M.L.; Campopiano, A.; Casciardi, S.; Spagnoli, G.; Torrisi, M.L.; Picardo, M. Differential in vitro cellular response induced by exposure to synthetic vitreous fibers (SVFs) and asbestos crocidolite fibers. Exp. Mol. Pathol. 2006, 81, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Zoboli, A.; Filaferro, M.; Benassi, M.; Scarfì, S.; Mirata, S.; Avallone, R.; Vitale, G.; Bailey, M.; Harper, M.; et al. In vitro toxicity of fibrous glaucophane. Toxicology 2021, 454, 152743. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Renis, M.; Scifo, C.; Lombardo, L.; Gulino, R.; Mancari, B.; Panico, A. Behaviour of the new asbestos amphibole fluoro-edenite in different lung cell systems. Int. J. Biochem. Cell Biol. 2004, 36, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.M.; Pavlisko, E.N. Differential pathological response and pleural transport of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 2417–2434. [Google Scholar]
- Bernstein, D.M.; Toth, B.; Rogers, R.A.; Kling, D.E.; Kunzendorf, P.; Phillips, J.I.; Ernst, H. Evaluation of the exposure, dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to TiO2, chrysotile, crocidolite or amosite asbestos in a 90-day quantitative inhalation toxicology study—Interim results Part 1: Experimental design, aerosol exposure, lung burdens and BAL. Toxicol. Appl. Pharmacol. 2020, 387, 11487. [Google Scholar]
- Gavett, S.H.; Parkinson, C.U.; Willson, G.A.; Wood, C.E.; Jarabek, A.M.; Roberts, K.C.; Kodavani, U.P.; Dodd, D.E. Persistent effects of Libby amphibole and amosite asbestos following subchronic inhalation in rats. Part. Fibre Toxicol. 2015, 13, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.K.; MacPherson, M.B.; Beuschel, S.L.; Shukla, A. Asbestos-induced mesothelial to fibroblastic transition is modulated by the inflammasome. Am. J. Pathol. 2017, 187, 665–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, A.; Pellegrini, L.; Dey, A.; Larson, D.; Tanji, M.; Flores, E.G.; Kendrick, B.; Lapid, D.; Powers, A.; Kanodia, S.; et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016, 35, 1996–2002. [Google Scholar] [CrossRef]
- Eskes, C.; Boström, A.C.; Bowe, G.; Coecke, S.; Hartung, T.; Hendriks, G.; Pamies, D.; Piton, A.; Rovida, C. Good cell culture practices & in vitro toxicology. Toxicol. Vitr. 2017, 45, 272–277. [Google Scholar]
- Lipsitch, M.; Tchetgen, E.T.; Cohen, T. Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 2010, 21, 383–388. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 3rd ed.; Mineralogical Society of Great Britain and Ireland: London, UK, 2013; pp. 132–135. [Google Scholar]
- Angel, R.J. Structural variation in wollastonite and bustamite. Min. Mag. 1985, 49, 37–48. [Google Scholar] [CrossRef]
- Mazzucato, E.; Gualtieri, A.F. Wollastonite polytypes in the CaO-SiO2 system. Phys. Chem. Miner. 2000, 27, 565–574. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; Utell, M.J.; McConnell, E.E.; LaRosa, S.; Segrave, A.M. Wollastonite toxicity: An update. Inhal. Toxicol. 2014, 26, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Somtürk, S.M.; Emek, I.Y.; Senler, S.; Eren, M.; Kurt, S.Z.; Orbay, M. Effect of wollastonite extender on the properties of exterior acrylic paints. Prog. Org. Coat. 2016, 93, 34–40. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. In vivo evaluation of plasma-sprayed wollastonite coating. Biomaterials 2005, 26, 3455–3460. [Google Scholar] [CrossRef]
- Miu, D.M.; Jinga, S.I.; Voicu, G.; Iordache, F. Characteristics of wollastonite ceramic coatings obtained by pulsed laser deposition. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1601–1607. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Kalantari, A.; Ershad-Langroudi, A. Effects of wollastonite nanofibers on biological resistance of historical paper against Aspergillus niger. Lignocellulose 2014, 3, 111–118. [Google Scholar]
- Taghiyari, H.R.; Majidi, R.; Esmailpour, A.; Samadi, Y.S.; Jahangiri, A.; Papadopoulos, A.N. Engineering composites made from wood and chicken feather bonded with UF resin fortified with wollastonite: A novel approach. Polymers 2020, 12, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, A.; Misra, D.K.; Rohrmann, J. Scratch deformation characteristics of micrometric wollastonite-reinforced ethylene-propylene copolymer compositess. Polym. Eng. Sci. 2004, 9, 1738–1748. [Google Scholar] [CrossRef]
- Maxim, L.D.; McConnell, E.E. A review of the toxicology and epidemiology of wollastonite. Inhal. Toxicol. 2005, 17, 451–466. [Google Scholar] [CrossRef]
- Amin, A.M.; El-Amir, A.A.; Karunakaran, G.; Kuznetsov, D.; Ewais, E.M. In-vitro evaluation of wollastonite nanopowder produced by a facile process using cheap precursors for biomedical applications. Ceram. Int. 2021, 47, 18684–18692. [Google Scholar] [CrossRef]
- Nikhil, P.S.; Ravichandran, P.T.; Krishnan, K.D. Stabilisation and characterisation of soil using wollastonite powder. Mater. Today Proc. 2021, 40, S161–S166. [Google Scholar] [CrossRef]
- Hayder, A.; Vanderburgt, S.; Santos, R.M.; Chiang, Y.W. Phosphorous runoff risk assessment and its potential management using wollastonite according to geochemical modeling. Open Agric. 2019, 4, 787–794. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils—An Ontario field study. Int. J. Greenh. Gas Control 2020, 97, 103017. [Google Scholar] [CrossRef]
- USGS National Minerals Information Center. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-wollastonite.pdf (accessed on 26 July 2021).
- Vitra, B.R.; Wollastonite. U.S. Geological Survey Minerals Yearbook. 2000, pp. 84.1–84.2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/wollastonite/860400.pdf (accessed on 26 July 2021).
- Whitney, P.R.; Olmsted, J.F. Wollastonite Deposits of the Northeastern Adirondacks. In Field Trip Guidebook for 67th Annual Meeting of the New York State Geological Association; Union College: Schenectady, NY, USA, 1995; pp. 25–38. Available online: https://ottohmuller.com/nysga2ge/Files/1995/NYSGA%201995%20A2%20-%20Wollastonite%20Deposits%20of%20the%20Northeastern%20Adirondacks.pdf (accessed on 26 July 2021).
- Peck, W.H.; Bailey, E. Origin of the Lewis wollastonite deposit. In Field Trip Guidebook for the 80th Annual Meeting of the New York State Geological Association: September 26–28, 2008; New York State Geological Association: New York, NY, USA, 2008; pp. 130–135. Available online: https://ottohmuller.com/nysga2ge/Files/2008/NYSGA%202008%2011.%20%20Origin%20of%20the%20Lewis%20Wollastonite%20Deposit.pdf (accessed on 26 July 2021).
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore Suite. Powder Diffr. 2014, 29, 13–18. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Mental Health. ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 14 July 2021).
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Zoboli, A.; Nodari, L.; Pasquali, L.; Sala, O.; Ballirano, P.; Malferrari, D.; Raneri, S.; Hanuskova, M.; Gualtieri, A.F. Characterization and assessment of the potential toxicity/pathogenicity of Russian commercial chrysotile. Am. Min. 2021, 106, 1606–1621. [Google Scholar] [CrossRef]
- Marques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Rancourt, D.; McDonald, A.M.; Lalonde, A.E.; Ping, J.Y. Mössbauer absorber thicknesses for accurate site populations in Fe-bearing minerals. Am. Min. 1993, 78, 1–7. [Google Scholar]
- Lagarec, K.; Rancourt, D.G. Recoil—Mössbauer Spectral Analysis Software for Windows; Department of Physics, University of Ottawa: Ottawa, ON, USA, 1998. [Google Scholar]
- Pirngruber, G.D.; Roy, P.K.; Prins, R. On determining the nuclearity of iron sites in Fe-ZSM-5—A critical evaluation. Phys. Chem. Chem. Phys. 2006, 8, 3939–3950. [Google Scholar] [CrossRef]
- Borghi, E.; Occhiuzzi, M.; Foresti, E.; Lesci, I.G.; Roveri, N. Spectroscopic characterization of Fe-doped synthetic chrysotile by EPR, DRS and magnetic susceptibility measurements. Phys. Chem. Chem. Phys. 2010, 12, 227–238. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Pollastri, S.; Bursi Gandolfi, N.; Lassinantti Gualtieri, M. In vitro acellular dissolution of mineral fibres: A comparative study. Sci. Rep. 2018, 8, 7071. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Mossman, B.T.; Roggli, V.L. Towards a general model for predicting the toxicity and pathogenicity of minerals fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 501–526. [Google Scholar]
- Garcia-Canton, C.; Anadón, A.; Meredith, C. γH2AX as a novel endpoint to detect DNA damage: Applications for the assessment of the in vitro genotoxicity of cigarette smoke. Toxicol. Vitr. 2012, 26, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary—Kiadja a Magyar Állami Földtani Intézet: Budapest, Hungary, 2011; p. 180. [Google Scholar]
- World Health Organization. Determination of Airborne Fibre Number Concentrations; A Recommended Method, by Phase Contrast Optical Microscopy (Membrane Filter Method); World Health Organization: Geneva, Switzerland, 1997; pp. 1–53. [Google Scholar]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy. Available online: http://www.handbookofmineralogy.org (accessed on 26 August 2021).
- Buthelezi, N. An Investigation into the Flotation Response of Wollastonite and Silicate Gangue Minerals. Master’s Thesis, McGill University, Montreal, QC, Canada, January 2020. [Google Scholar]
- Van Oss, C.J.; Giese, R.F. The hydrophilicity and hydrophobicity of clay minerals. Clays Clay Miner. 1995, 43, 474–477. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Hassan, A.; Mohamad, Z.; Othman, N. Mechanical properties of wollastonite reinforced thermoplastic composites: A review. Polym. Compos. 2020, 41, 395–429. [Google Scholar] [CrossRef]
- Yuhaida, I.; Salmah, H.; Hanafi, I.; Firuz, Z. The effect of acrylic acid on tensile and morphology properties of wollastonite filled high density polyethylene/natural rubber composites. Procedia Chem. 2016, 19, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, D.C.; Hawthorne, F.C. A structure hierarchy for silicate minerals: Chain, ribbon, and tube silicates. Min. Mag. 2020, 84, 165–244. [Google Scholar]
- Ohashi, Y.; Finger, L.W. The role of octahedral cations in pyroxenoid crystal chemistry; I, Bustamite, wollastonite, and the pectolite-schizolite-serandite series. Am. Min. 1978, 63, 274–288. [Google Scholar]
- Matsueda, H. Iron-wollastonite from the Sampo mine showing properties distinct from those of wollastonite. Min. J. 1973, 7, 180–201. [Google Scholar] [CrossRef] [Green Version]
- Nechita, M.T.; Berlier, G.; Ricchiardi, G.; Bordiga, S.; Zecchina, A. New precursor for the post-synthesis preparation of Fe-ZSM-5 zeolites with low iron content. Catal. Lett. 2005, 103, 33–41. [Google Scholar] [CrossRef]
- Ohashi, T. Polysynthetically-twinned structures of enstatite and wollastonite. Phys. Chem. Miner. 1984, 10, 217–229. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Dove, P.M. Mineral/solution reaction rates in a mixed flow reactor: Wollastonite hydrolysis. Geochim. Cosmochim. Acta 1986, 50, 2509–2516. [Google Scholar] [CrossRef]
- Wollastonite NYAD G Technical Data Sheet. Available online: https://www.chem-on.com.sg/image/catalog/product_catalog/TDS%20-%20WOLLASTONITE%20NYAD%20G.pdf (accessed on 29 July 2021).
- Droop, G.T.R. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Gonda, I.; Abd El Khalik, A.F. On the calculation of aerodynamic diameters of fibers. Aerosol. Sci. Tech. 1985, 4, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Pollastri, S.; D’Acapito, F.; Trapananti, A.; Colantoni, I.; Andreozzi, G.B.; Gualtieri, A.F. The chemical environment of iron in mineral fibres. A combined X-ray absorption and Mössbauer spectroscopic study. J. Hazard. Mater. 2015, 298, 282–293. [Google Scholar] [CrossRef]
- Pollastri, S.; Gualtieri, A.F.; Lassinantti Gualtieri, M.; Hanuskova, M.; Cavallo, A.; Gaudino, G. The zeta potential of mineral fibres. J. Hazard. Mater. 2014, 276, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.; Pokrovsky, O.S.; Spalla, O.; Devreux, F.; Gloter, A.; Mielczarski, J.A. Formation, growth and transformation of leached layers during silicate minerals dissolution: The example of wollastonite. Geochim. Cosmochim. Acta 2012, 98, 259–281. [Google Scholar] [CrossRef]
- Ptáček, P.; Nosková, M.; Brandštetr, J.; Šoukal, F.; Opravil, T. Mechanism and kinetics of wollastonite fibre dissolution in the aqueous solution of acetic acid. Powder Technol. 2011, 206, 338–344. [Google Scholar] [CrossRef]
- Weissbart, E.J.; Rimstidt, J.D. Wollastonite: Incongruent dissolution and leached layer formation. Geochim. Cosmochim. Acta 2000, 64, 4007–4016. [Google Scholar] [CrossRef]
- Xue, H.; Wang, G.; Hu, M.; Chen, B. Modification of wollastonite by acid treatment and alkali-induced redeposition for use as papermaking filler. Powder Technol. 2015, 276, 193–199. [Google Scholar] [CrossRef]
- Peters, S.C.; Blum, J.D.; Driscoll, C.T.; Likens, G.E. Dissolution of wollastonite during the experimental manipulation of Hubbard Brook Watershed 1. Biogeochemistry 2004, 67, 309–329. [Google Scholar] [CrossRef] [Green Version]
- Bellmann, B.; Muhle, H. Investigation of the biodurability of wollastonite and xonotlite. Environ. Health Perspect. 1994, 102, 191–195. [Google Scholar] [PubMed] [Green Version]
- Macdonald, J.L.; Kane, A.B. Mesothelial cell proliferation and biopersistence of wollastonite and crocidolite asbestos fibers. Fund. Appl. Toxicol. 1997, 38, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F. Bridging the gap between toxicity and carcinogenicity of mineral fibres by connecting the fibre crystal-chemical and physical parameters to the key characteristics of cancer. Cur. Res. Toxicol. 2021, 2, 42–52. [Google Scholar] [CrossRef]


| Parameters | Classes | Normalized Score FPTIi | Chrysotile, Balangero [65] | Asbestos Tremolite, Val D’Ala [24] | Wollastonite NYAD G [24] | Wollastonite NYAD G [This Study] |
|---|---|---|---|---|---|---|
| Length | >5 μm and <10 μm >10 μm and <20 μm >20 μm | 0.10 0.20 0.40 | 0.40 | 0.40 | 0.20 | 0.40 |
| Diameter | >1 μm and <3 μm >0.25 μm and <1μm <0.25 μm | 0.10 0.20 0.40 | 0.10 | 0.10 | 0.10 | 0.00 |
| Crystal curvature | Flat surface Altered surface Cylindrical surface | 0.05 0.10 0.20 | 0.20 | 0.05 | 0.05 | 0.05 |
| Crystal habit | Curled Mixed curled/acicular Acicular | 0.10 0.20 0.40 | 0.10 | 0.40 | 0.40 | 0.40 |
| Fibre density | <2.75 g/cm3 >2.75 and <3.5 g/cm3 >3.5 g/cm3 | 0.05 0.10 0.20 | 0.05 | 0.10 | 0.10 | 0.10 |
| Hydrophobic character of the surface | Hydrophobic Amphiphilic Hydrophilic | 0.05 0.10 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Surface area | >25 m2/g <25 and >5 m2/g <5 m2/g | 0.05 0.10 0.20 | 0.05 | 0.20 | 0.20 | 0.20 |
| Total iron content | Fe2O3 + FeO wt% < 1 1 < Fe2O3+FeO wt% < 10 Fe2O3 + FeO wt% > 10 | 0.05 0.10 0.20 | 0.10 | 0.10 | 0.05 | 0.05 |
| Ferrous iron | 0 < FeO wt% < 0.25 0.25 < FeO wt% < 1 FeO wt% > 1 | 0.05 0.10 0.20 | 0.20 | 0.20 | 0.05 | 0.10 |
| Surface ferrous iron/iron nuclearity | Fe2+ nuclearity > 2 Fe2+ nuclearity = 2 Fe2+ nuclearity = 1 | 0.02 0.033 0.067 | 0.033 | 0.033 | 0.02 | 0.067 |
| Content of metals other than iron * | < 1 < 5 > 5 | 0.10 0.20 0.40 | 0.40 | 0.40 | 0.10 | 0.10 |
| Dissolution rate log(R) ** | <1 y >1 and <40 y >40 y | 0.05 0.10 0.20 | 0.05 | 0.20 | 0.05 | 0.05 |
| Velocity of iron release *** | <0.1 >0.1 and <1 >1 | 0.033 0.067 0.133 | 0.133 | 0.067 | 0.033 | 0.067 |
| Velocity of silica dissolution **** | <0.5 >0.5 and <1 >1 | 0.02 0.033 0.067 | 0.067 | 0.033 | 0.067 | 0.067 |
| Velocity of release of metals ***** | <1 >1 and <10 >10 | 0.033 0.067 0.133 | 0.133 | 0.133 | 0.067 | 0.033 |
| Zeta potential | Negative at pH = 4.5 Negative at both pH = 4.5 and 7 | 0.10 0.20 | 0.10 | 0.20 | 0.20 | 0.20 |
| Fibres’ aggregation | Zeta potential > |20| |10| < Zeta potential < |20||0| < Zeta potential < |10| | 0.033 0.067 0.133 | 0.033 | 0.067 | 0.033 | 0.033 |
| Cation exchange (in zeolites) | Cation Exchange No cation exchange | 0.067 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| FPTI (error) | 2.35(0.22) | 2.88(0.43) | 1.92(0.30) | 2.12(0.18) |
| Percentiles * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | 5th | 25th | 50th | 75th | 95th | Max | Average | σ | |
| L (µm) | 8.17 | 13.6 | 21.5 | 32.7 | 57.4 | 132 | 228 | 46.6 | 38.1 |
| W (µm) | 1.09 | 1.40 | 2.42 | 3.17 | 4.46 | 7.20 | 18.1 | 3.74 | 2.29 |
| SSA (m2g−1) | k (s−1) | R (mol·m−2s−1) | t(d) | t (y) | |
|---|---|---|---|---|---|
| Wollastonite NYAD G | 0.50(0.01) | 1.98(3) × 10−6 | 1.59(3) × 10−4 | 30 (15) | 0.08 (4) |
| Balangero chrysotile | 42.0(1) | 1.8(6) × 10−10 | 1.7(6) × 10−10 | 124 (41) | 0.3 (1) |
| UICC amosite | 9.5(3) | 6.1(6) × 10−14 | 2.7(3) × 10−13 | 27,010 (2647) | 74 (7) |
| UICC anthophyllite asbestos | 4.4(2) | 1.2(3) × 10−13 | 1.0(3) × 10−13 | 83,950 (20,990) | 245 (64) |
| Val d’Ala tremolite asbestos | 9.2(3) | 5.4(9) × 10−14 | 4.5(7) × 10−13 | 17,885 (2981) | 49.0 (8) |
| UICC crocidolite | 16.1(6) | 1.3(3) × 10−13 | 3.2(7) × 10−13 | 24,090 (5840) | 66.0 (16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giuseppe, D.; Scognamiglio, V.; Malferrari, D.; Nodari, L.; Pasquali, L.; Lassinantti Gualtieri, M.; Scarfì, S.; Mirata, S.; Tessari, U.; Hanuskova, M.; et al. Characterization of Fibrous Wollastonite NYAD G in View of Its Use as Negative Standard for In Vitro Toxicity Tests. Minerals 2021, 11, 1378. https://doi.org/10.3390/min11121378
Di Giuseppe D, Scognamiglio V, Malferrari D, Nodari L, Pasquali L, Lassinantti Gualtieri M, Scarfì S, Mirata S, Tessari U, Hanuskova M, et al. Characterization of Fibrous Wollastonite NYAD G in View of Its Use as Negative Standard for In Vitro Toxicity Tests. Minerals. 2021; 11(12):1378. https://doi.org/10.3390/min11121378
Chicago/Turabian StyleDi Giuseppe, Dario, Valentina Scognamiglio, Daniele Malferrari, Luca Nodari, Luca Pasquali, Magdalena Lassinantti Gualtieri, Sonia Scarfì, Serena Mirata, Umberto Tessari, Miriam Hanuskova, and et al. 2021. "Characterization of Fibrous Wollastonite NYAD G in View of Its Use as Negative Standard for In Vitro Toxicity Tests" Minerals 11, no. 12: 1378. https://doi.org/10.3390/min11121378
APA StyleDi Giuseppe, D., Scognamiglio, V., Malferrari, D., Nodari, L., Pasquali, L., Lassinantti Gualtieri, M., Scarfì, S., Mirata, S., Tessari, U., Hanuskova, M., & Gualtieri, A. F. (2021). Characterization of Fibrous Wollastonite NYAD G in View of Its Use as Negative Standard for In Vitro Toxicity Tests. Minerals, 11(12), 1378. https://doi.org/10.3390/min11121378








