Dispersibility of Kaolinite-Rich Coal Gangue in Rubber Matrix and the Mechanical Properties and Thermal Stability of the Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Characterization of Starting Samples and Fillers
3.1.1. Composition and Structure of Starting Samples
3.1.2. Particles Size of Starting Samples before and after Grinding
3.1.3. Surface Properties of Fillers
3.2. Characterization of Coal Gangue/SBR Composites
3.2.1. Optimum Curing Time of Coal Gangue/SBR Composites
3.2.2. Dispersion of Coal Gangue in the Rubber Matrix
3.2.3. Mechanical Properties of Coal Gangue/SBR Composites
3.2.4. Thermal Stability of Coal Gangue/SBR Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Dong, X.; Zhu, Z.; Dong, Y. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite. J. Hazard. Mater. 2014, 273, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, D.; Xu, F.; Lai, J.; Shao, L. Literature overview of Chinese research in the field of better coal utilization. J. Clean. Prod. 2018, 185, 959–980. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Jablonska, B.; Kityk, A.V.; Busch, M.; Huber, P. The structural and surface properties of natural and modified coal gangue. J. Environ. Manag. 2017, 190, 80–90. [Google Scholar] [CrossRef]
- Gong, G.; Xie, B.; Yang, M.; Yang, W.; Zhang, W.; Zhao, M. Mechanical properties and fracture behavior of injection and compression molded polypropylene/coal gangue powder composites with and without a polymeric coupling agent. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1683–1693. [Google Scholar] [CrossRef]
- Zhao, M.; Xiang, Y. Natural rubber vulcanizate reinforced by modified coal-shale-based fillers. J. Appl. Polym. Sci. 2004, 93, 1397–1400. [Google Scholar] [CrossRef]
- Li, B.; Gong, G.; Xie, B.; Yang, W.; Yang, M.; Lai, S. Fracture behaviour of polypropylene sheets filled with epoxidized natural rubber (ENR)-treated coal gangue powder. J. Mater. Sci. 2007, 42, 3856–3864. [Google Scholar] [CrossRef]
- Ye, T.; Min, X.; Li, X.; Zhang, S.; Gao, Y. Improved holding and releasing capacities of coal gangue toward phosphate through alkali-activation. Chemosphere 2021, 287, 132382. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Zhang, J.; Zhang, C.; Chen, J.; Liu, C. Effect of particle size and thermal activation on the coal gangue based geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.; Sun, H.; Li, L. Investigation on the activation of coal gangue by a new compound method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitious material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Algeo, T.J.; Zhang, K.; Hong, H.; Zhang, S.; Liu, Q. Control of coal-bearing claystone composition by sea level and redox conditions: An example from the Upper Paleozoic of the Datong Basin, North China. Appl. Clay Sci. 2021, 211, 106204. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Ma, S.; Frost, R.L. The thermal behavior of kaolinite intercalation complexes—A review. Thermochim. Acta 2012, 545, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhou, Y.; Liu, Q. Kaolinite Nanomaterials: Preparation, Properties and Functional Applications. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; p. 285. [Google Scholar]
- Sánchez-Soto, P.J.; Jiménez de Haro, M.C.; Pérez-Maqueda, L.; Varona, I.; Pérez-Rodríguez, J.L. Effects of Dry Grinding on the Structural Changes of Kaolinite Powder. J. Am. Ceram. Soc. 2000, 83, 1649–1657. [Google Scholar] [CrossRef]
- Stepkowska, E.T.; Perez-rodriguze, J.L.; de Haro, M.J.; Sánchez–Soto, P.J.; Maqueda, C. Effect of grinding and water vapour on the particle size of kaolinite and pyrophyllite. Clay Min. 2001, 36, 105–114. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Han, Y.; Gao, P. Effects of carbonaceous matter additives on kinetics, phase and structure evolution of coal-series kaolin during calcination. Appl. Clay Sci. 2018, 165, 124–134. [Google Scholar] [CrossRef]
- Maia, A.Á.B.; Angélica, R.S.; de Freitas Neves, R.; Pöllmann, H.; Straub, C.; Saalwächter, K. Use of 29Si and 27Al MAS NMR to study thermal activation of kaolinites from Brazilian Amazon kaolin wastes. Appl. Clay Sci. 2014, 87, 189–196. [Google Scholar] [CrossRef]
- He, Y.; Liu, L.; He, L.; Cui, X. Characterization of chemosynthetic H3PO4–Al2O3–2SiO2 geopolymers. Ceram. Int. 2016, 42, 10908–10912. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Xu, H. Properties of vulcanized rubber nanocomposites filled with nanokaolin and precipitated silica. Appl. Clay Sci. 2008, 42, 232–237. [Google Scholar] [CrossRef]
- Ptáček, P.; Frajkorová, F.; Šoukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Chen, J.; Xu, K.; Rongkai, P.; Peng, G.; Ma, L.; Zheng, L. Effect of Activated and Modified Conditions on Properties of Coal Gangue Powder Reinforced Natural Rubber. Polym. Mater. Sci. Eng. 2013, 29, 59–62. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-GFZC201308014.htm (accessed on 5 December 2021). [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Zhang, Y.; Liang, P. Characterization of kaolinite/styrene butadiene rubber composite: Mechanical properties and thermal stability. Appl. Clay Sci. 2016, 124–125, 167–174. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zhang, K.; Liu, L.; Ji, L.; Liu, Q. Vulcanization, interfacial interaction, and dynamic mechanical properties of in-situ organic amino modified kaolinite/SBR nanocomposites based on latex compounding method. Appl. Clay Sci. 2020, 185, 105366. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Cheng, H. Gas barrier properties and mechanism of kaolin/styrene–butadiene rubber nanocomposites. Appl. Clay Sci. 2015, 111, 37–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, Q.; Lu, Y. Gas barrier properties of natural rubber/kaolin composites prepared by melt blending. Appl. Clay Sci. 2010, 50, 255–259. [Google Scholar] [CrossRef]
- Yahaya, L.E.; Adebowale, K.O.; Menon, A.R.R. Mechanical properties of organomodified kaolin/natural rubber vulcanizates. Appl. Clay Sci. 2009, 46, 283–288. [Google Scholar] [CrossRef]
- Sukumar, R.; Menon, A.R.R. Organomodified kaolin as a reinforcing filler for natural rubber. J. Appl. Polym. Sci. 2008, 107, 3476–3483. [Google Scholar] [CrossRef]
- Wu, W.; Tian, L. Formulation and morphology of kaolin-filled rubber composites. Appl. Clay Sci. 2013, 80–81, 93–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Xiang, J.; Frost, R.L. Thermal stability and decomposition kinetics of styrene-butadiene rubber nanocomposites filled with different particle sized kaolinites. Appl. Clay Sci. 2014, 95, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Zhang, Y.; Zhang, Y.; Gong, Y. Efficient preparation of coal-series kaolinite intercalation compounds via a catalytic method and their reinforcement for styrene butadiene rubber composite. Appl. Clay Sci. 2021, 213, 106237. [Google Scholar] [CrossRef]
- Xing, W.; Tang, M.; Wu, J.; Huang, G.; Li, H.; Lei, Z.; Fu, X.; Li, H. Multifunctional properties of graphene/rubber nanocomposites fabricated by a modified latex compounding method. Compos. Sci. Technol. 2014, 99, 67–74. [Google Scholar] [CrossRef]
- Tang, K.; Wang, J. Chlorinated butyl rubber/two-step modified montmorillonite nanocomposites: Mechanical and damping properties. Chin. J. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- He, S.; He, T.; Wang, J.; Wu, X.; Xue, Y.; Zhang, L.; Lin, J. A novel method to prepare acrylonitrile-butadiene rubber/clay nanocomposites by compounding with clay gel. Compos. B Eng. 2019, 167, 356–361. [Google Scholar] [CrossRef]
- Sarkarat, M.; Lanagan, M.; Ghosh, D.; Lottes, A.; Budd, K.; Rajagopalan, R. High field dielectric properties of clay filled silicone rubber composites. Mater. Today Commun. 2020, 23, 100947. [Google Scholar] [CrossRef]
- Dai, J.C.; Huang, J.T. Surface modification of clays and clay–rubber. Appl. Clay Sci. 1999, 15, 51–65. [Google Scholar] [CrossRef]
- Derouiche, R.; Baklouti, S. Phosphoric acid based geopolymerization: Effect of the mechanochemical and the thermal activation of the kaolin. Ceram. Int. 2021, 47, 13446–13456. [Google Scholar] [CrossRef]
- Zribi, M.; Samet, B.; Baklouti, S. Mechanical, microstructural and structural investigation of phosphate-based geopolymers with respect to P/Al molar ratio. J. Solid State Chem. 2020, 281, 121025. [Google Scholar] [CrossRef]
- Medri, V.; Fabbri, S.; Dedecek, J.; Sobalik, Z.; Tvaruzkova, Z.; Vaccari, A. Role of the morphology and the dehydroxylation of metakaolins on geopolymerization. Appl. Clay Sci. 2010, 50, 538–545. [Google Scholar] [CrossRef]
- Gharzouni, A.; Joussein, E.; Samet, B.; Baklouti, S.; Rossignol, S. Effect of the reactivity of alkaline solution and metakaolin on geopolymer formation. J. Non-Cryst Solids 2015, 410, 127–134. [Google Scholar] [CrossRef]
- Chakraborty, A.K. Phase Transformation of Kaolinite Clay; Springer India: New Delhi, India, 2013; p. 87. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Frost, R.L. Thermogravimetric analysis of selected coal-bearing strata kaolinite. Thermochim. Acta 2010, 507–508, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Havlica, J.; Brandštetr, J. Crystallization of spinel phase from metakaoline: The nonisothermal thermodilatometric CRH study. Powder Technol. 2013, 243, 40–45. [Google Scholar] [CrossRef]
- Ptáček, P.; Kubátová, D.; Havlica, J.; Brandštetr, J.; Šoukal, F.; Opravil, T. Isothermal kinetic analysis of the thermal decomposition of kaolinite: The thermogravimetric study. Thermochim. Acta 2010, 501, 24–29. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Wasserbauer, J.; Másilko, J.; Baráček, J. The influence of structure order on the kinetics of dehydroxylation of kaolinite. J. Eur. Ceram. Soc. 2013, 33, 2793–2799. [Google Scholar] [CrossRef]
- Yan, K.; Guo, Y.; Fang, L.; Cui, L.; Cheng, F.; Li, T. Decomposition and phase transformation mechanism of kaolinite calcined with sodium carbonate. Appl. Clay Sci. 2017, 147, 90–96. [Google Scholar] [CrossRef]
- Ilić, B.; Radonjanin, V.; Malešev, M.; Zdujić, M.; Mitrović, A. Effects of mechanical and thermal activation on pozzolanic activity of kaolin containing mica. Appl. Clay Sci. 2016, 123, 173–181. [Google Scholar] [CrossRef]
- Xing, H.; Liu, H.; Zhang, X.; Deng, H.; Hu, H.; Yao, H. Enhanced sodium adsorption capacity of kaolinite using a combined method of thermal pre-activation and intercalation-exfoliation: Alleviating the problems of slagging and fouling during the combustion of Zhundong coal. Fuel 2019, 239, 312–319. [Google Scholar] [CrossRef]
- Liu, S.; Xiaowei, Z.; Zhou, H. Surface modification and application of kaolin filler. Conserv. Util. Min. Resour. 2010, 1, 24–29. [Google Scholar]
- Zhang, L. Rubber Nanocomposites: Fundamentals and Apllications, 1st ed.; Chemical Industry Press: Beijing, China, 2018; p. 92. [Google Scholar]
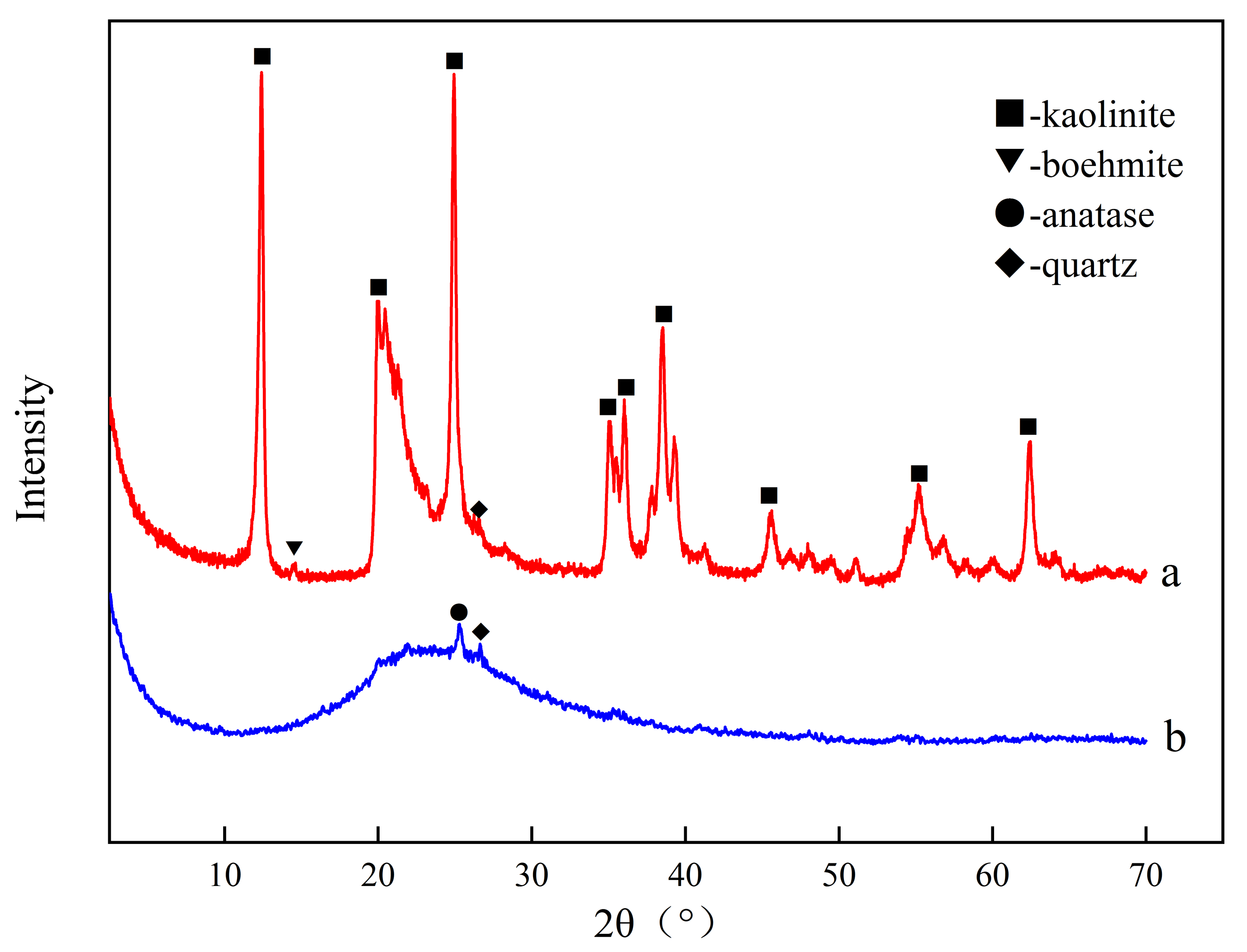
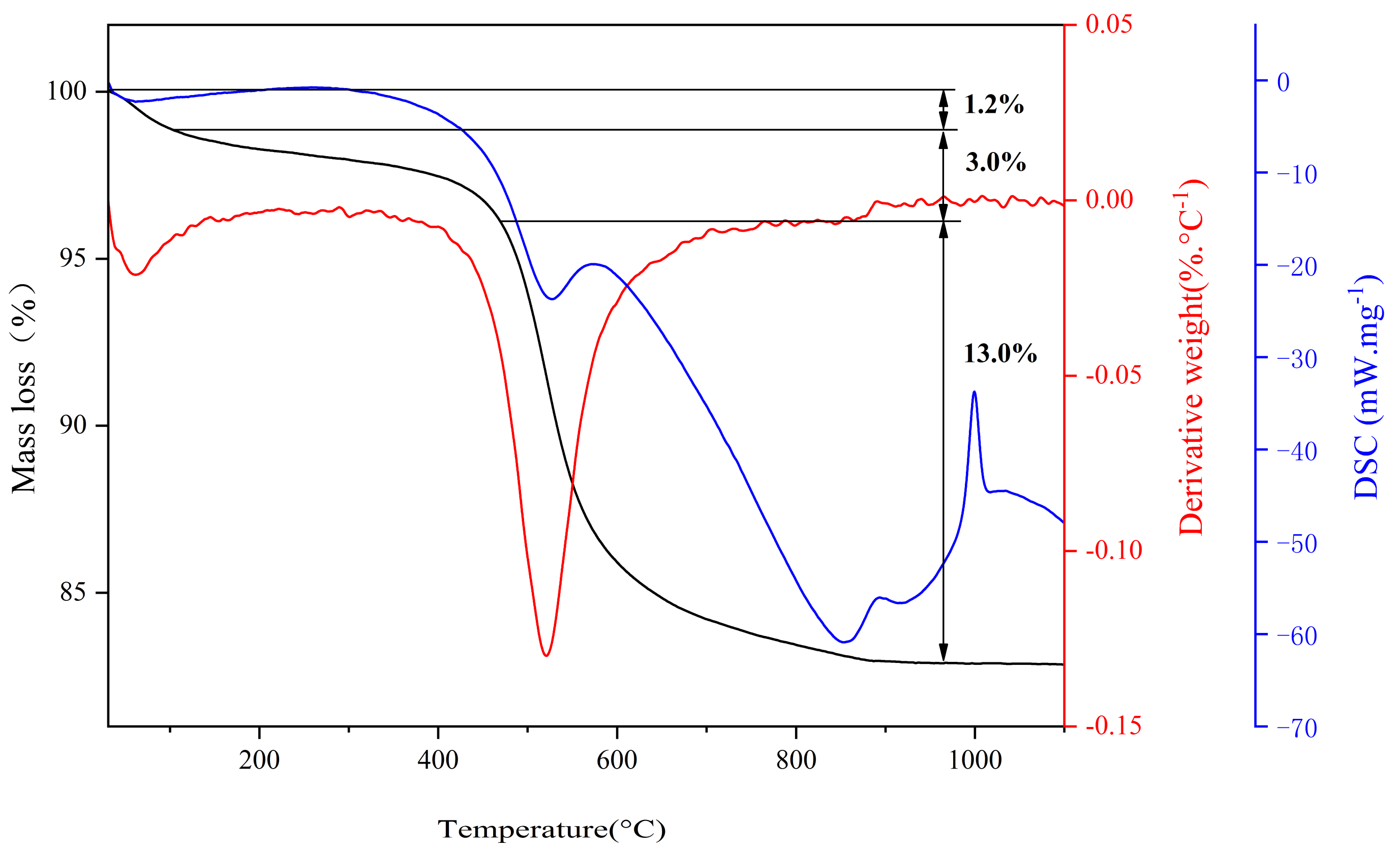
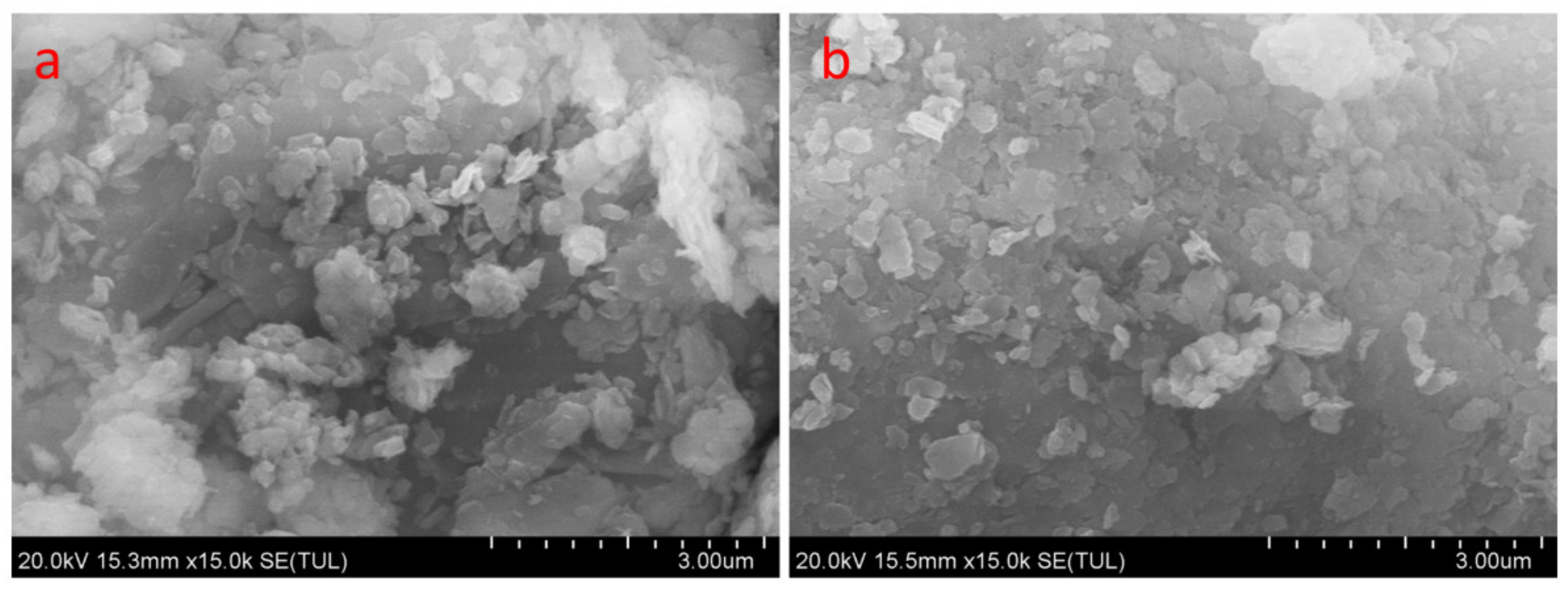
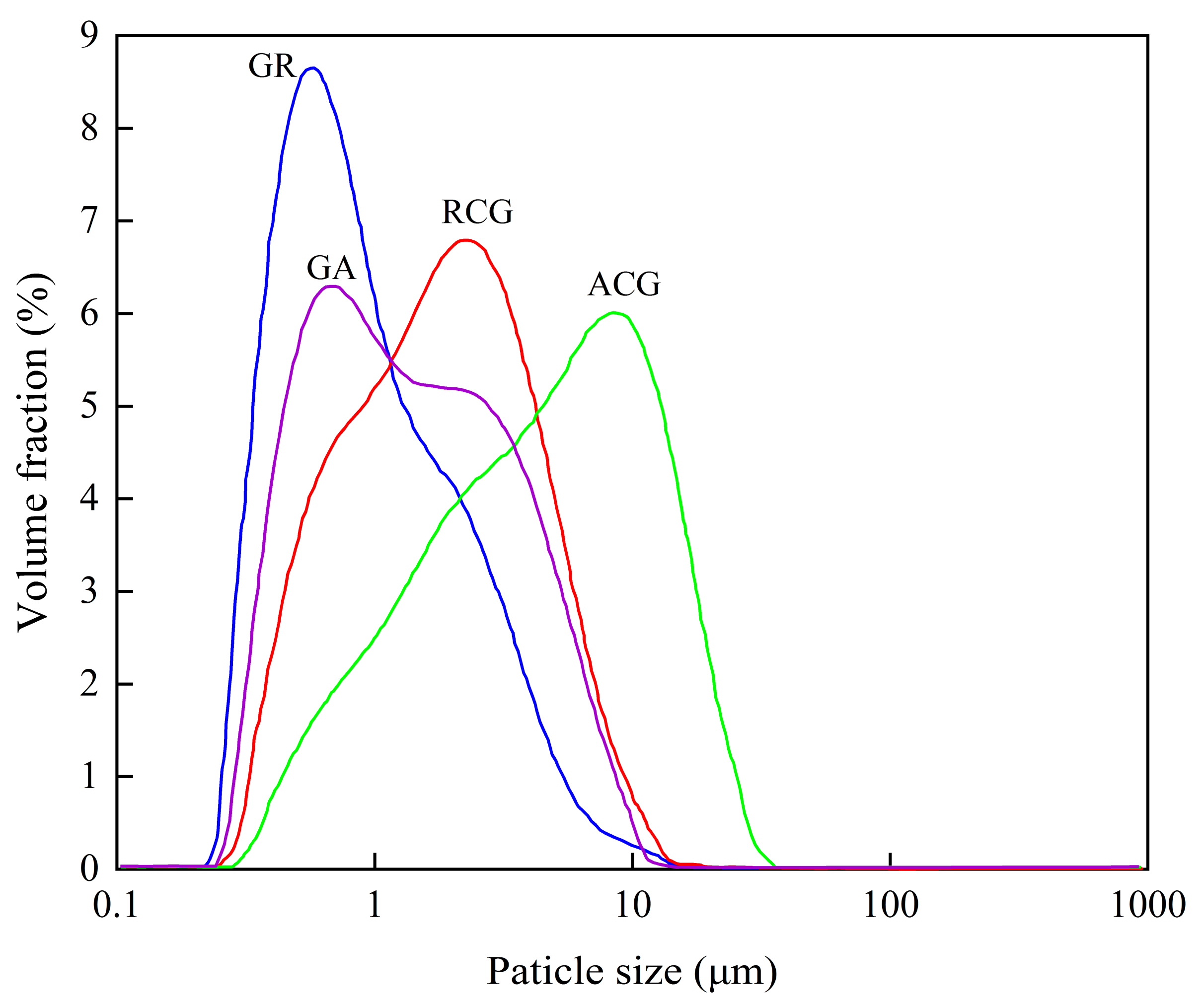

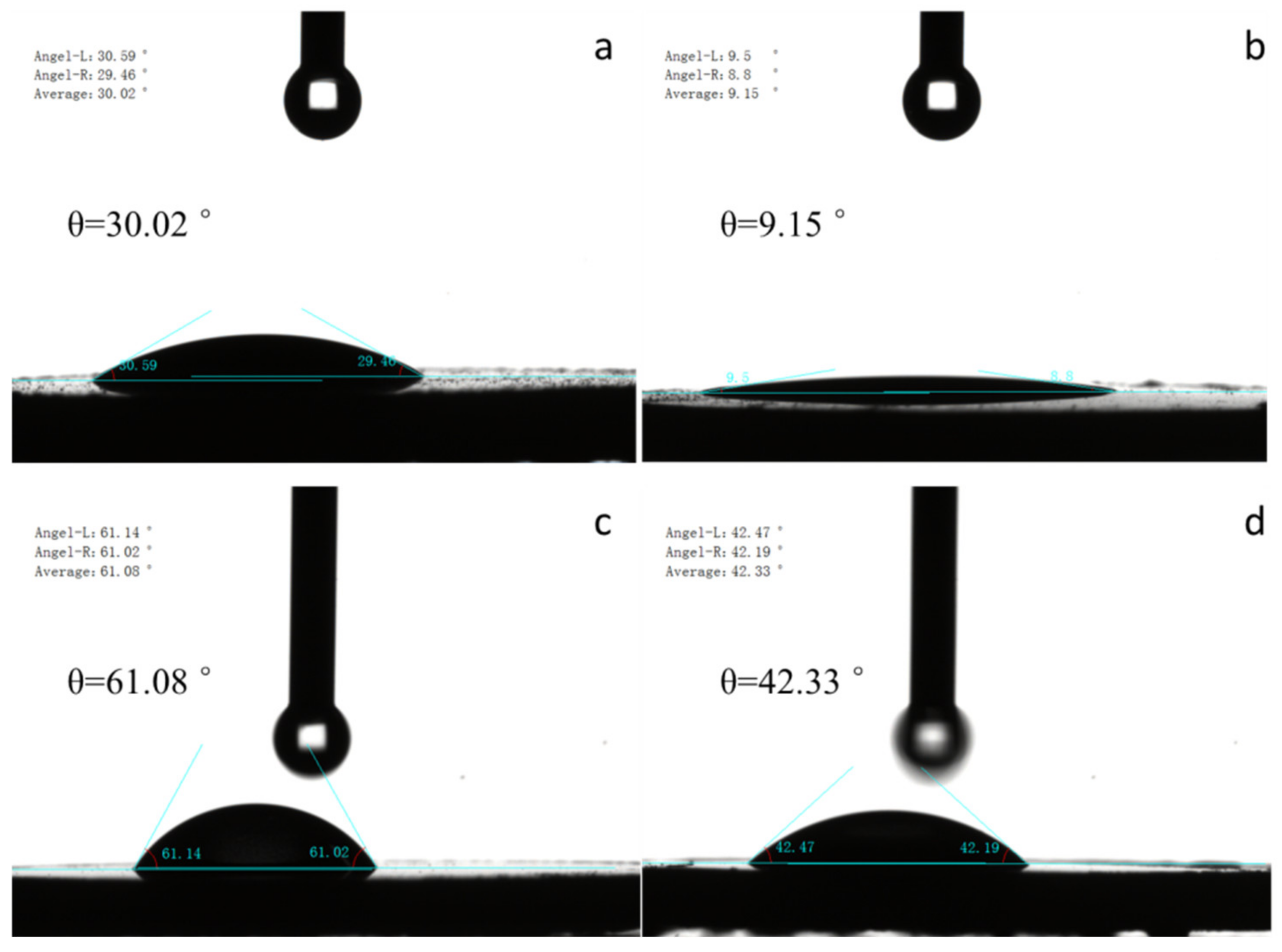

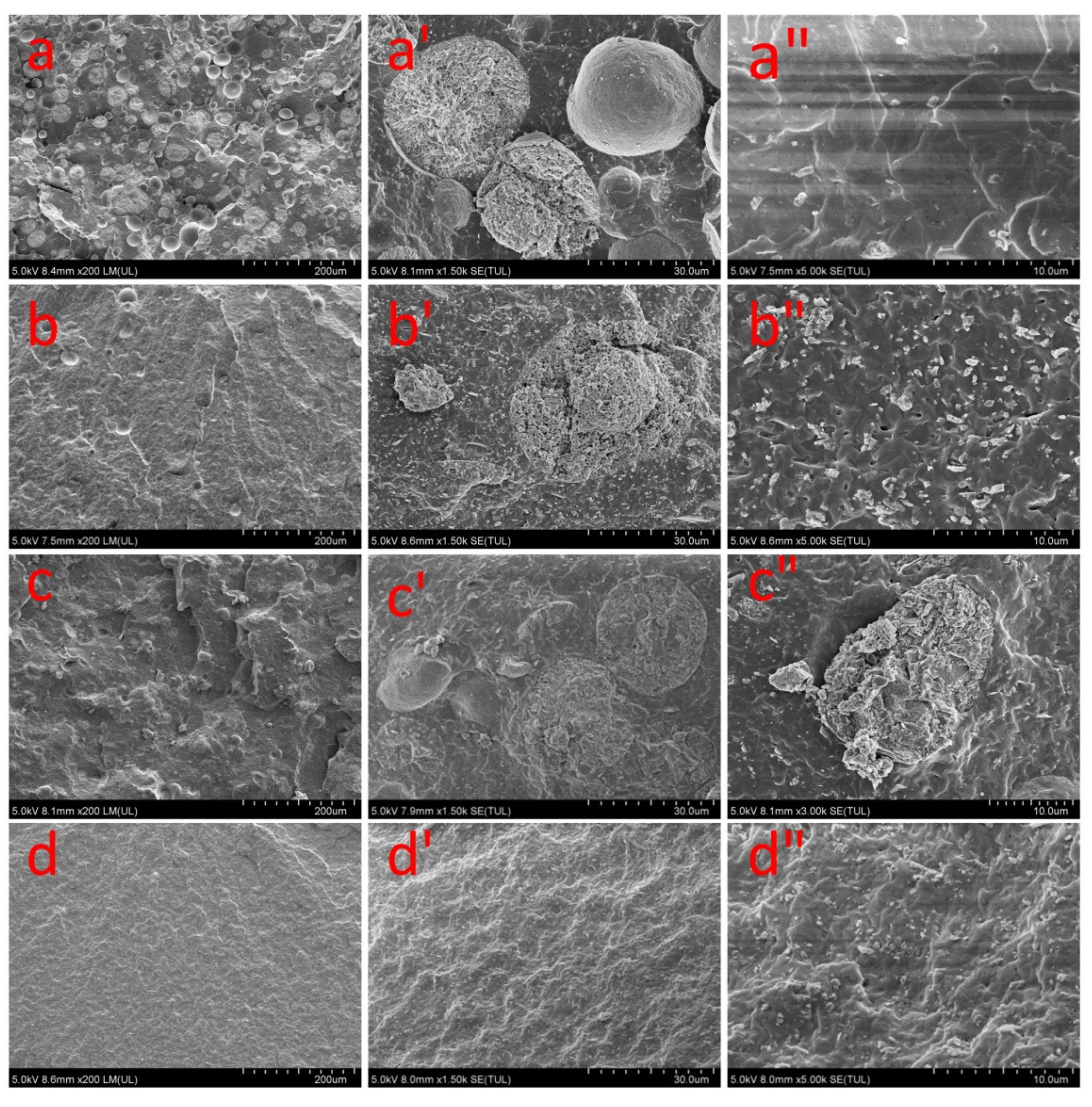
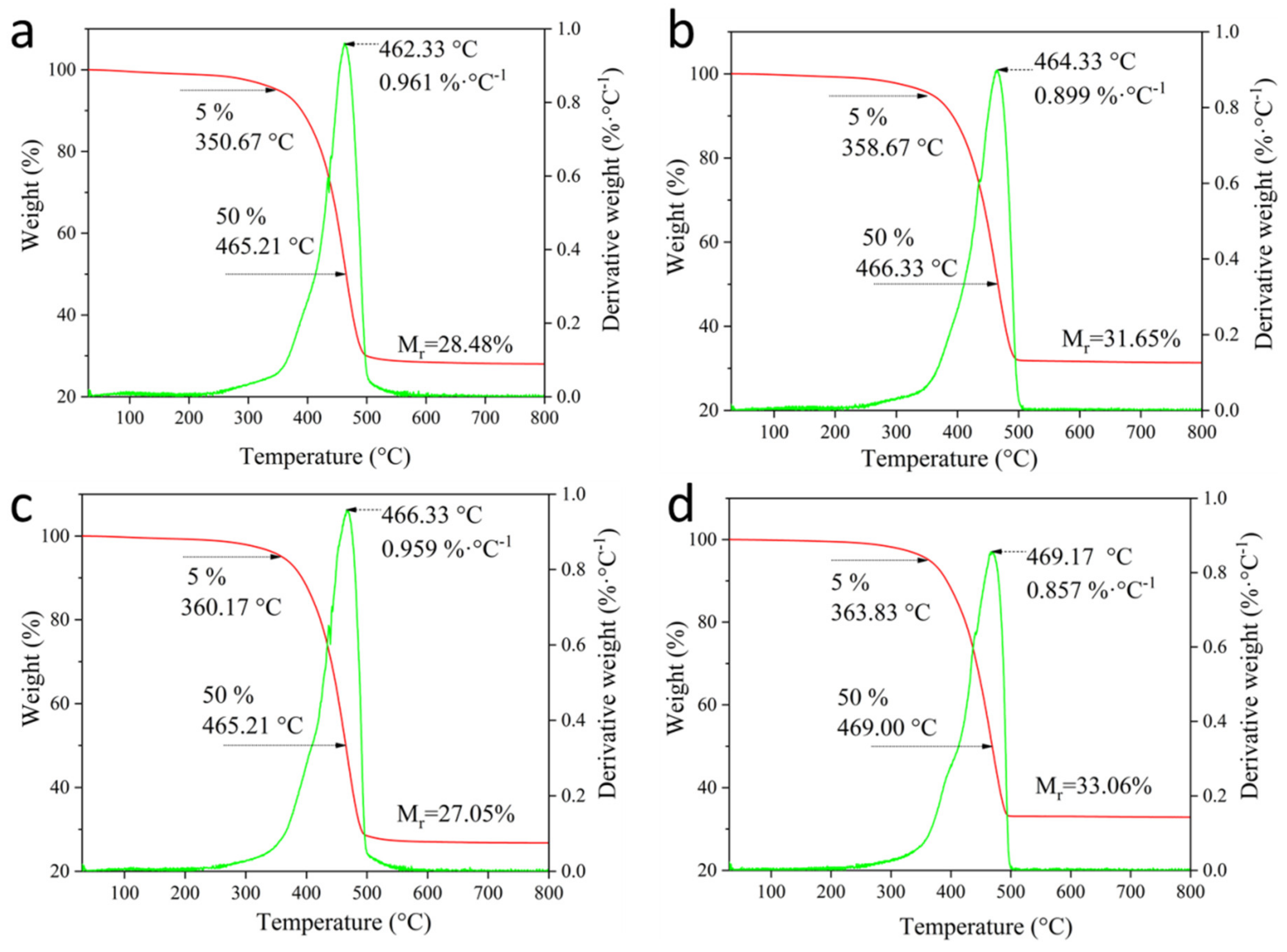
| Oxide (wt. %) | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | Na2O | K2O | MnO | MgO | P2O5 | Ignition Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coal gangue | 43.16 | 37.63 | 0.429 | 1.39 | 0.230 | 0.072 | 0.129 | 0.005 | 0.174 | 0.074 | 16.62 1 |
| Ingredient | SBR | Zinc Oxide | Stearic Acid | Accelerant (NS) | Sulphur | Fillers |
|---|---|---|---|---|---|---|
| Content/phr 1 | 100.00 | 3.00 | 1.00 | 1.00 | 1.75 | 50.00 |
| Samples | D10 (μm) | D50 (μm) | D90 (μm) | Specific Surface Area 1 (m2 · g−1) |
|---|---|---|---|---|
| RCG | 0.572 | 1.832 | 5.135 | 4.67 |
| GR | 0.384 | 0.822 | 2.938 | 8.12 |
| ACG | 0.970 | 4.814 | 14.569 | 2.42 |
| GA | 0.466 | 1.327 | 4.695 | 5.93 |
| Samples | Shore A Hardness (°) | Tensile Strength (MPa) | Elongation at Break (%) | Modulus (MPa) | Tear Resistance (kN/m) | |||
|---|---|---|---|---|---|---|---|---|
| 50% | 100% | 200% | 300% | |||||
| GR | 51 | 1.54 | 380.18 | 0.73 | 0.85 | 1.02 | 1.27 | 12.67 |
| GA | 54 | 4.10 | 447.24 | 1.15 | 1.50 | 1.98 | 2.53 | 15.48 |
| MGR | 49 | 4.55 | 633.28 | 0.92 | 1.18 | 1.57 | 1.90 | 20.34 |
| MGA | 51 | 10.24 | 509.36 | 1.07 | 1.55 | 3.00 | 4.92 | 22.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, H.; Liu, L.; Yang, Y.; Liu, L.; Liu, Q. Dispersibility of Kaolinite-Rich Coal Gangue in Rubber Matrix and the Mechanical Properties and Thermal Stability of the Composites. Minerals 2021, 11, 1388. https://doi.org/10.3390/min11121388
Zhang K, Zhang H, Liu L, Yang Y, Liu L, Liu Q. Dispersibility of Kaolinite-Rich Coal Gangue in Rubber Matrix and the Mechanical Properties and Thermal Stability of the Composites. Minerals. 2021; 11(12):1388. https://doi.org/10.3390/min11121388
Chicago/Turabian StyleZhang, Kenan, Hao Zhang, Linsong Liu, Yongjie Yang, Lihui Liu, and Qinfu Liu. 2021. "Dispersibility of Kaolinite-Rich Coal Gangue in Rubber Matrix and the Mechanical Properties and Thermal Stability of the Composites" Minerals 11, no. 12: 1388. https://doi.org/10.3390/min11121388
APA StyleZhang, K., Zhang, H., Liu, L., Yang, Y., Liu, L., & Liu, Q. (2021). Dispersibility of Kaolinite-Rich Coal Gangue in Rubber Matrix and the Mechanical Properties and Thermal Stability of the Composites. Minerals, 11(12), 1388. https://doi.org/10.3390/min11121388






