Ocean-Floor Sediments as a Resource of Rare Earth Elements: An Overview of Recently Studied Sites
Abstract
:1. Introduction
2. The Rare Earth Elements (REE)
2.1. The Chemistry of REE
2.2. The Abundance and Applications of REE
2.3. Economic Value of REE
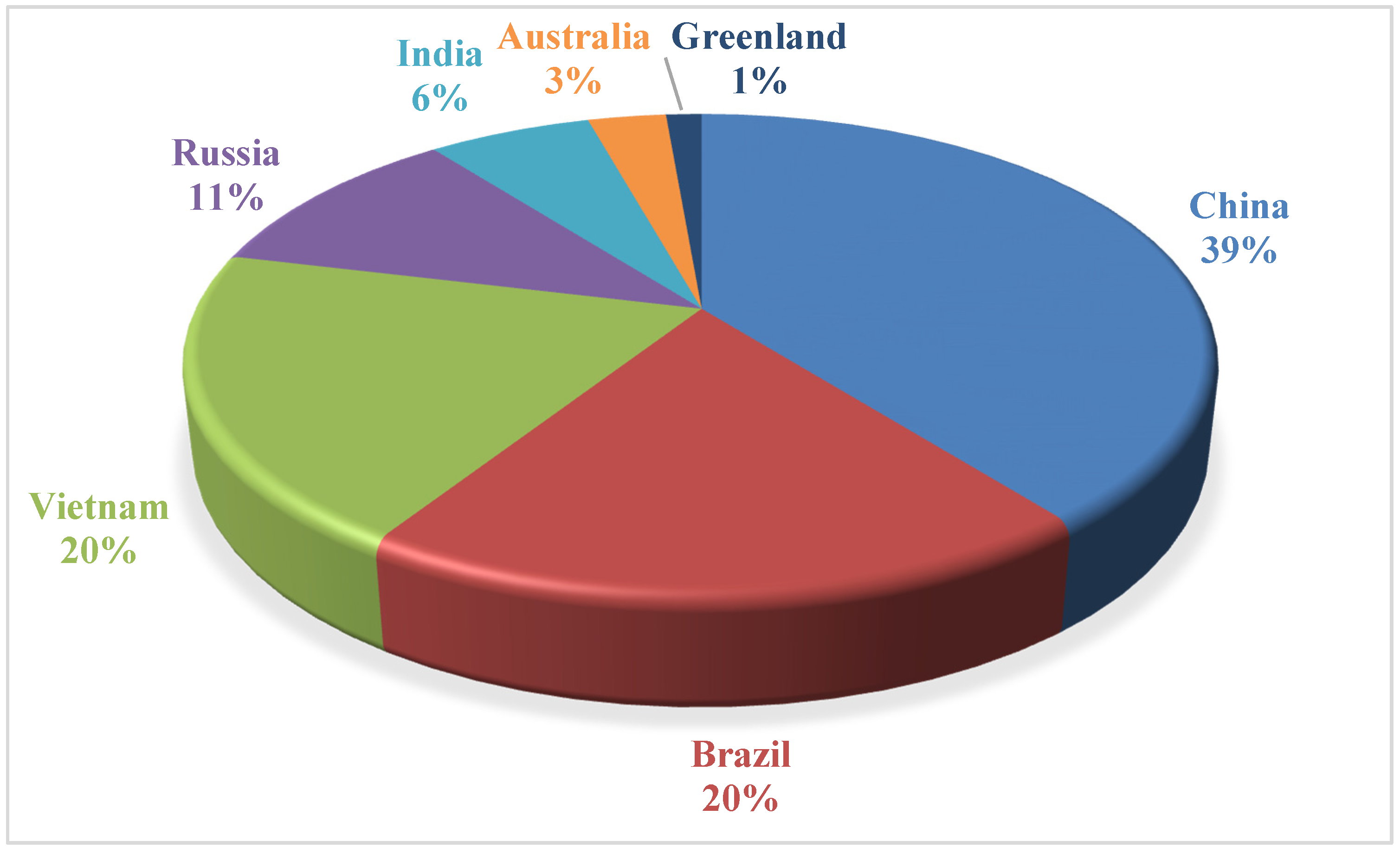
3. Ocean-Floor Sediments as a Resource of REE
3.1. Onshore Methods vs. Offshore Exploration of REE in Ocean-Floor Sediments
3.2. The Concentration of REE in Ocean-Floor Sediments
3.3. Economic Cost and Environmental Implications of REE Exploitation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hurst, C. China’s Rare Earth Elements Industry: What Can the West Learn? Institute for the Analysis of Global Security (IAGS): Washington, DC, USA, 2010; pp. 1–42. [Google Scholar]
- Balaram, V.; Banakar, V.K.; Subramanyam, K.S.V.; Roy, P.; Satyanarayanan, M.; Mohan, M.R.; Sawant, S.S. Yttrium and rare earth element contents in seamount cobalt crusts in the Indian Ocean. Curr. Sci. 2012, 103, 1334–1338. [Google Scholar]
- Folkersen, M.V.; Fleming, C.M.; Hasan, S. The economic value of the deep sea: A systematic review and meta-analysis. Mar. Policy 2018, 94, 71–80. [Google Scholar] [CrossRef]
- Mez, L. Rare earth strategies of Japan and EU/Germany. In The Ecological Modernization Capacity of Japan and Germany. Comparing Nuclear Energy, Renewables, Automobility and Rare Earth Policy. Energy Policy and Climate Protection; Mez, L., Okamura, L., Weidner, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 171–184. [Google Scholar]
- Preinfalk, C.; Morteani, G. The Industrial Applications of Rare Earth Elements. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; (Special Publication No. 7 of the Society for Geology Applied to Mineral Deposits); Springer: Berlin/Heidelberg, Germany, 1989; pp. 359–370. [Google Scholar]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green. Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- European Commission (EC), COM(2017) 490. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, on the 2017 List of Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2017; pp. 1–8. [Google Scholar]
- Van Gosen, B.S.; Verplanck, P.L.; Emsbo, P. Rare Earth Element Mineral Deposits in the United States; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2019; pp. 1–14.
- Kynicky, J.; Smith, M.P.; Xu, C. Diversity of rare earth deposits: The key example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- Simandl, G.J. Geology and market-dependent significance of rare-earth element resources. Miner. Depos. 2014, 49, 889–904. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Ge, J.; Wu, S. Production forecast of China’s rare earths based on the Generalized Weng model and policy recommendations. Resour. Policy 2015, 43, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.; Krätschell, A.; Augustin, N.; Jamieson, J.; Hein, J.R.; Hannington, M.D. News from the seabed—Geological characteristics and resource potential of deep-sea mineral resources. Mar. Policy 2016, 70, 175–187. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries. Rare Earths. 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-rare-earths.pdf (accessed on 13 January 2021).
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.A.; Staudigel, H. Seamount mineral deposits, as a source of rare metals for high-technology industries. Oceanography 2010, 23, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Bern, C.R.; Shah, A.K.; Benzel, W.M.; Lowers, H.A. The distribution and composition of REE-bearing minerals in placers of the Atlantic and Gulf coastal plains, USA. J. Geochem. Explor. 2016, 162, 50–61. [Google Scholar] [CrossRef]
- Kato, Y.; Fujinaga, K.; Nakamura, K.; Takaya, Y.; Kitamura, K.; Ohta, J.; Toda, R.; Nakashima, T.; Iwamori, H. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat. Geosci. 2011, 4, 534–539. [Google Scholar] [CrossRef]
- Zawadzki, D.; Maciag, L.; Abramowski, T.; McCartney, K. Fractionation trends and variability of rare earth elements and selected critical metals in pelagic sediments from Abyssal Basin of NE Pacific (Clarion-Clipperton Fracture Zone). Minerals 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Pak, S.J.; Seo, I.; Lee, K.Y.; Hyeong, K. Rare earth elements and other critical metals in deep seabed mineral deposits: Composition and implications for resource potential. Minerals 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Takaya, Y.; Yasukawa, K.; Kawasaki, T.; Fujinaga, K.; Ohta, J.; Usui, Y.; Nakamura, K.; Kimura, J.I.; Chang, Q.; Hamada, G.; et al. The tremendous potential of deep-sea mud as a source of rare-earth elements. Sci. Rep. 2018, 8, 5763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasukawa, K.; Miyazaki, T.; Vaglarov, B.S.; Chang, Q.; Ueki, K.; Toyama, C.; Kimura, J.I.; Tanaka, E.; Nakamura, K.; Fujinaga, K.; et al. Statistic and isotopic characterization of deep-sea sediments in the Western North Pacific Ocean: Implications for genesis of the sediment extremely enriched in rare-earth elements. Geochem. Geophys. Geosyst. 2019, 20, 3402–3430. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Sun, X.; Wu, Z.; Sa, R.; Guan, Y.; Lu, Y.; Li, D.; Liu, Y.; Deng, Y.; Pan, Y. Fe-Mn (oxyhydr)oxides as an indicator of REY enrichment in the deep-sea sediments from the central North Pacific. Ore Geol. Rev. 2019, 112, 103044. [Google Scholar] [CrossRef]
- Zawadzki, D.; Maciag, L.; Kotlinski, R.A.; Borówka, R.K. Rare earth elements and selected major elements distribution in the siliceous-clayey silts from the NE Pacific abyssal Basin (Clarion-Clipperton Fracture Zone). Harvesting seabed minerals resources in harmony with nature UMI 2014. In Proceedings of the 43rd Underwater Mining Institute Conference, Lisbon, Portugal, 21–28 September 2014. [Google Scholar]
- Maciag, L.; Zawadzki, D. Spatial variability and resources estimation of selected critical metals and rare earth elements in surface sediments from the Clarion-Clipperton Fracture Zone, equatorial Pacific Ocean, Interoceanmetal claim area. In Proceedings of the 20th Annual Conference of the International Association for Mathematical Geosciences, IAMG, State College, PA, USA, 10–16 August 2019. [Google Scholar]
- Iijima, K.; Yasukawa, K.; Fujinaga, K.; Nakamura, K.; Machida, S.; Takaya, Y.; Ohta, J.; Haraguchi, S.; Nishio, T.; Usui, Y.; et al. Discovery of extremely REY_rich mud in the western North Pacific Ocean. Geochem. J. 2016, 50, 557–573. [Google Scholar] [CrossRef] [Green Version]
- Barriga, F.J.A.S. Can recycling and the circular economy render seafloor mining unnecessary? In Economical, Technological and Environmental Aspects: Cooperative Solutions for Future Deep-Sea Mining; UMC, Federation of German Industries (BDI): Berlin, Germany, 2017. [Google Scholar]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical characterization of mine waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Petersen, S. RV METEOR Fahrtbericht/Cruise Report M127 Metal fluxes and Resource Potential at the Slow-spreading TAG Mid-Ocean Ridge Segment (26° N, MAR)—Blue Mining@Sea, Bridgetown (Barbados)—Ponta Delgada (Portugal), 25.05.–28.06. 2016 (extended version). GEOMAR Helmholtz-Zent. Ozeanforschung Kiel 2016. [Google Scholar] [CrossRef]
- Milinovic, J.; Dias, Á.A.; Janeiro, A.I.; Pereria, M.F.C.; Martins, S.; Petersen, S.; Barriga, F.J.A.S. XRD identification of ore minerals during cruises: Refinement of extraction procedure with sodium acetate buffer. Minerals 2020, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, A.V. Geochemistry of rare earth elements in the ocean. Lithol. Miner. Resour. 2004, 39, 289–307. [Google Scholar] [CrossRef]
- Yasukawa, K.; Liu, H.; Fujinaga, K.; Machida, S.; Haraguchi, S.; Ishii, T.; Nakamura, K.; Kato, Y. Geochemistry and mineralogy of REY-rich mud in the eastern Indian Ocean. J. Asian Earth Sci. 2014, 93, 25–36. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry (IUPAC). Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005; Connelly, N.G., Damhus, T., Hartshorn, R.M., Hutton, A.T., Eds.; IUPAC: Cambridge, UK, 2005; p. 51. [Google Scholar]
- Weng, Z.H.; Jowitt, S.M.; Mudd, G.M.; Haque, N. Assessing rate earth element mineral deposit types and links to environmental impacts. Appl. Earth Sci. 2013, 122, 83–96. [Google Scholar] [CrossRef]
- Lide, D.R. Geophysics, Astronomy, and Acoustics; Abundance of elements in the Earth’s crust and in the sea. In CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Giese, E.C. Rare earth elements: Therapeutic and diagnostic applications in modern medicine. Clin. Med. Rep. 2018, 2, 1–2. [Google Scholar] [CrossRef]
- Barriga, F.J.A.S. Sustainable and responsible mining in the deep oceanic crust. In Colóquio Contaminação Ambiental; Neiva, A., Ed.; Academia das Ciências de Lisboa: Lisbon, Portugal, 2019; pp. 3–28. [Google Scholar]
- Dostal, J. Rare earth element deposits of alkaline igneous rocks. Resources 2017, 6, 34. [Google Scholar] [CrossRef]
- Jenness, J.E.; Ober, J.A.; Wilkins, A.M.; Gambogi, J. A world of minerals in your mobile device. US Geol. Surv. Gen. Inf. Prod. 2016, 167, 1–2. [Google Scholar]
- Statista. Global Smartphone Sales to End Users 2007–2021. Available online: https://www.statista.com/statistics/263437/global-smartphone-sales-to-end-users-since-2007/ (accessed on 28 December 2020).
- Rohrig, B. Smartphones: Smart Chemistry. Chem. Matters 2015, 10–12. [Google Scholar]
- Riba, J.R.; López-Torres, C.; Romeral, L.; Garcia, A. Rare-earth-free propulsion motors for electric vehicles: A technology review. Renew. Sustain. Energy Rev. 2016, 57, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Kramer, M.; Zhou, L.; Liu, F.; Gabay, A.; Hadjipanayis, G.; Balasubramanian, B.; Sellmyer, D. Current progress and future challenges in rare-earth-free permanent magnets. Acta Mater. 2018, 158, 118–137. [Google Scholar] [CrossRef]
- Kostova, I. Lanthanides as anticancer agents. Curr. Med. Chem. Anticancer Agents. 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, L.M.; Bhargava, P.; Essig, M.; Maki, J.H. Magnetic resonance imaging using gadolinium-based contrast agents. Top. Magn. Reson. Imaging 2014, 23, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Asian Metal. Rare Earths: Uses. Available online: http://metalpedia.asianmetal.com/metal/rare_earth/application.shtml (accessed on 24 April 2020).
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare earth elements: Industrial applications and economic dependency of Europe. ICOAE 2015, 24, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Mineral Prices. Rare Earth Metals. Available online: https://mineralprices.com/rare-earth-metals/ (accessed on 22 April 2020).
- Balaram, V.; Rao, T.G. Rapid determination of REE and other trace elements in geological samples by microwave acid digestion and ICP-MS. Atom. Spectrosc. 2003, 24, 206–212. [Google Scholar]
- Amaral, C.D.B.; Machado, R.C.; Barros, J.A.V.A.; Virgilio, A.; Schiavo, D.; Nogueira, A.R.A.; Nobrega, J.A. Determination of rare earth elements in geological and agricultural samples by ICP-OES. Spectroscopy 2017, 32, 32–36. [Google Scholar]
- Bentlin, F.R.S.; Pozebon, D. Direct determination of lanthanides in environmental samples using ultrasonic nebulization and ICP OES. J. Braz. Chem. Soc. 2010, 21, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Nakamura, K.; Fujinaga, K.; Machida, S.; Ohta, J.; Takaya, Y.; Kato, Y. Rare-earth, major, and trace element geochemistry of deep-sea sediments in the Indian Ocean: Implications for the potential distribution of REY-rich mud in the Indian Ocean. Geochem. J. 2015, 49, 621–635. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakamura, T. X-ray fluorescence analysis of rare earth elements in rocks using low dilution glass beads. Anal. Sci. 2005, 21, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Milinovic, J.; Barriga, F.A.J.S.; Murton, B.J. Analysis of deep-ocean sediments from the TAG hydrothermal field (MAR, 26° N): Application of short-wave infrared reflectance (SWIR) spectra for offshore geochemical exploration. J. Soils Sediments 2020, 20, 3472–3486. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-ocean ferromanganese crusts and nodules. Treatise Geochem. 2014, 2, 273–291. [Google Scholar]
- Konstantinova, N.; Cherkashov, G.; Hein, J.R.; Mirão, J.; Dias, L.; Madureira, P.; Kuznetsov, V.; Maksimov, F. Composition and characteristics of the ferromanganese crusts from the western Arctic Ocean. Ore Geol. Rev. 2017, 87, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Marino, E.; González, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Strategic and rare elements in Cretaceous-Cenozoic cobalt rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (Northeastern tropical Atlantic. Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T. Mineralogy and chemistry of ferromanganese crusts from the Atlantic Ocean. Geochem. Int. 2011, 49, 578–593. [Google Scholar] [CrossRef]
- Zeng, Z.G.; Ma, Y.; Yin, X.B.; Selby, D.; Kong, F.C.; Chen, S. Factors affecting the rare earth element compositions in massive sulfides from deep-sea hydrothermal systems. Geochem. Geophy. Geosy. 2015, 16, 2679–2693. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Zhang, H.S.; Zhao, P.D. Rare earth element geochemistry of co-rich crust in the mid-Pacific and South China Sea. Mar. Geol. Quat. Geol. 2006, 26, 45–57. [Google Scholar]
- Jaireth, S.; Hoatson, D.M.; Miezitis, Y. Geological setting and resources of the major rare-earth-element deposits in Australia. Ore Geol. Rev. 2014, 62, 72–128. [Google Scholar] [CrossRef]
- Dekov, V.M.; Petersen, S.; Garbe-Schönberg, C.D.; Kamenov, G.D.; Perner, M.; Kuzmann, E.; Schmidt, M. Fe-Si-oxyhydroxide deposits at a slow-spreading centre with thickened oceanic crust: The Lilliput hydrothermal field (9°33′ S, Mid-Atlantic Ridge). Chem. Geol. 2010, 278, 186–200. [Google Scholar] [CrossRef]
- Dekov, V.; Boycheva, T.; Hålenius, U.; Petersen, S.; Billström, K.; Stummeyer, J.; Kamenov, G.; Shanks, W. Atacamite and paratacamite from the ultramafic-hosted Logatchev seafloor vent field (14°45′N, Mid-Atlantic Ridge). Chem. Geol. 2011, 86, 169–184. [Google Scholar] [CrossRef]
- Dias, Á.S.; Mills, R.A.; Costa, I.R.; Costa, R.; Taylor, R.N.; Cooper, M.J.; Barriga, F.J.A.S. Tracing fluid-rock reaction and hydrothermal circulation at the Saldanha hydrothermal fluid. Chem. Geol. 2010, 273, 168–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Y.; Gao, L.; Zhang, Y.; Shi, G.; Liu, C.; Zhang, P.; Duan, X. Enrichment of REEs in polymetallic nodules and crusts and its potential for exploitation. J. Rare Earths 2012, 30, 621–626. [Google Scholar] [CrossRef]
- Usui, A.; Bau, M.; Yamazaki, T. Manganese microchimneys buried in the Central Pacific pelagic sediments: Evidence of intraplate water circulation? Mar. Geol. 1997, 141, 269–285. [Google Scholar] [CrossRef]
- Haase, K.M.; Koschinsky, A.; Petersen, S.; Devey, C.W.; Geman, C.; Lackschewitz, K.S.; Melchert, B.; Seifert, R.; Borowski, C.; Giere, O.; et al. Diking, young volcanism and diffuse hydrothermal activity on the southern Mid-Atlantic Ridge: The Lilliput field at 9°33′ S. Mar. Geol. 2009, 266, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Barriga, F.J.A.S.; Fouquet, Y.; Almeida, A.; Biscoito, M.J.; Charlou, J.L.; Costa, R.L.P.; Dias, A.; Marques, A.M.S.F.; Miranda, J.M.A.; Olu, K.; et al. Discovery of the Saldanha hydrothermal field on the Famous Segment of the MAR (36°30′ N). Eos Trans. AGU 1998, 79, F67. [Google Scholar]
- Ardron, J.A.; Ruhl, H.A.; Jones, D.O.B. Incorporating transparency into governance of deep-seabed mining in the Area beyond national jurisdiction. Mar. Policy 2018, 89, 58–66. [Google Scholar] [CrossRef]
- Clark, M.R.; Durden, J.M.; Christiansen, S. Environmental impact assessments for deep-sea mining: Can we improve their future effectiveness? Mar. Policy 2020, 114. [Google Scholar] [CrossRef]
- Jones, D.O.B.; Durden, J.M.; Murphy, K.; Gjerde, K.M.; Gebicka, A.; Colaço, A.; Morato, T.; Cuvelier, D.; Billett, D.S.M. Existing environmental management approaches relevant to deep-sea mining. Mar. Policy 2019, 103, 172–181. [Google Scholar] [CrossRef]
- Tunnicliffe, V.; Metaxas, A.; Le, J.; Ramirez-Llodra, E.; Levin, L.A. Strategic environmental goals and objectives: Setting the basis for environmental regulation of deep seabed mining. Mar. Policy 2020, 114, 103347. [Google Scholar] [CrossRef]
- Durden, J.M.; Lallier, L.E.; Murphy, K.; Jaeckel, A.; Gjerde, K.; Jones, D.O.B. Environmental impact assessment process for deep-sea mining in ‘the Area’. Mar. Policy 2018, 87, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.; Ferreira, M.A.; Kenchington, E. Climate change is likely to severely limit the effectiveness of deep-sea ABMTs in the North Atlantic. Mar. Policy 2018, 87, 111–122. [Google Scholar] [CrossRef]

| REE | Atomic number (Z) | Oxidation State | Abundance * (ppm) | Applications ‡ |
|---|---|---|---|---|
| Lanthanum (La) | 57 | +3 | 39 | Camera lenses; catalysts for petroleum refining; MRI; new-generation electric vehicles; optical glass; rechargeable batteries |
| Cerium (Ce) | 58 | +3, +4 | 66.5 | Catalyst for oil refineries; color screen, LCD; metal alloys; PET; polishing powder¸ radiation shielding; UV filters in glass |
| Praseodymium (Pr) | 59 | +3 | 9.2 | Colorant in glasses; color screen LCD; cryogenic refrigerant; energy-efficient lighting; hard disk drives; lasers; magnets; pigments; portable electronics and small motors; radiotherapy techniques |
| Neodymium (Nd) | 60 | +3 | 41.5 | Hard disk drives; new-generation electric vehicles; permanent magnets; portable electronics and small motors; treatment of skin cancers, violet colors in glass and ceramics |
| Promethium (Pm) | 61 | +3 | trace † | Luminous paint; nuclear batteries |
| Samarium (Sm) | 62 | +3 | 7.05 | Lasers; magnets; new-generation electric vehicles; Sm-153 treatment of tumors |
| Europium (Eu) | 63 | +3, +2 | 2 | Bioimaging; color screen LCD; energy-efficient lighting; fluorescent lighting; lasers; portable electronics and small motors |
| Gadolinium (Gd) | 64 | +3 | 6.2 | Lasers; magnetostrictive alloys; memory chips; MRI; neutron capture; steel additive |
| Terbium (Tb) | 65 | +3 | 1.2 | Cancer therapies; color screen LCD; fuel cells; fluorescent lamps; high-powered electric motors; lasers; magnetostrictive alloys; optical computer memories |
| Dysprosium (Dy) | 66 | +3 | 5.2 | Dy-165 treatment of effusions; high-powered electric motors; lasers; lighter vehicles; magnetostrictive alloys |
| Holmium (Ho) | 67 | +3 | 1.3 | Cancer therapies; lasers; magnets; standards for optical spectrophotometers |
| Erbium (Er) | 68 | +3 | 3.5 | Fiber-optic technology; lasers; medical and dental practice; steel |
| Thulium (Tm) | 69 | +3 | 0.52 | Lasers; metal-halide lamps; Tm-167 in portable XRD-devices |
| Ytterbium (Yb) | 70 | +3 | 3.2 | Chemical reducing agent; infrared lasers; stainless steel; Yb-176 medical application |
| Lutetium (Lu) | 71 | +3 | 0.8 | Cancer therapies; catalyst; high-refractive-index glass; LED |
| Yttrium (Y) | 39 (66.5) ‖ | +3 | 33 | Cancer therapies; ceramics; color screen LCD; compact fluorescent lamps; high-temperature superconductors; laser; LED; microwave filters |
| Scandium (Sc) | 21 | +3 | 22 | Alloys for aerospace components; camera lighting; catalysts; color screen LCD; energy-efficient lighting; radioactive tracing agent in oil refineries; super alloys; X-ray tubes |
| Locality | Sediment Sample, Water Depth (m) | ΣREE, Min–Max (ppm) | Wet Digestion | Reference |
|---|---|---|---|---|
| Atlantic | ||||
| Main Lilliput, Roman City (9°32′–9°33′ S, 13°12′ W) | 1486–1495 | 14.4–16.6 | HF-HCl-HNO3 aqua regia | [63] |
| Logatchev (14°45′ N, 44°58′ W) | 2923 | 13.6–130 | HF-HCl-HNO3 aqua regia | [64] |
| Saldanha (36°34′ N, MAR) | 2125–2300 | 6.3–40.6 | HCl-aqua regia-HF-HBr | [65] |
| TAG (26° N, MAR) | 3407–3654 | 2.8–113.9 * | Na2O2-HNO3 | [55] |
| Pacific | ||||
| South East (5°–20° S, 90°–150° W) Central North (3°–20° N, 130° W–170° E) | 4000–5000 | 1000–2230 * 400–1000 * | HNO3-HF-HClO4 aqua regia | [19] |
| North East (5°–20° N, 119°–160° W) | 4313–4529 | 200–577 | HNO3-HF-HClO4 aqua regia | [20] |
| North East(9°30′–10°30′ N, 131°–133° W) | 4000–6000 | 150–1150 † | NP ‡ | [21] |
| North West (21°–27° N, 150°–158° E) | 5720–5735 | 299–6800 * | HNO3-HF-HClO4 aqua regiaHNO3-HCl-HF | [23,27] |
| North (21°48′–22°15′ N, 153°30′–154°07′ E) | 4700–6100 | 5000–22,000 * | HClO4-HF-HNO3 aqua regia | [22] |
| Indian | ||||
| ODP sites (5° N, 90° E) | 2924 | 0.5–1113 * | HNO3-HF-HClO4 aqua regia | [33] |
| DSDP and ODP sites (1°–30° S, 57°–112° E) | 2832–5609 | 0.5–920 * | HNO3-HF-HClO4 aqua regia | [53] |
| Southern | ||||
| DSDP site (59° S, 104° E) | 4522 | 72–114 * | HNO3-HF-HClO4 aqua regia | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milinovic, J.; Rodrigues, F.J.L.; Barriga, F.J.A.S.; Murton, B.J. Ocean-Floor Sediments as a Resource of Rare Earth Elements: An Overview of Recently Studied Sites. Minerals 2021, 11, 142. https://doi.org/10.3390/min11020142
Milinovic J, Rodrigues FJL, Barriga FJAS, Murton BJ. Ocean-Floor Sediments as a Resource of Rare Earth Elements: An Overview of Recently Studied Sites. Minerals. 2021; 11(2):142. https://doi.org/10.3390/min11020142
Chicago/Turabian StyleMilinovic, Jelena, Francisco J. L. Rodrigues, Fernando J. A. S. Barriga, and Bramley J. Murton. 2021. "Ocean-Floor Sediments as a Resource of Rare Earth Elements: An Overview of Recently Studied Sites" Minerals 11, no. 2: 142. https://doi.org/10.3390/min11020142
APA StyleMilinovic, J., Rodrigues, F. J. L., Barriga, F. J. A. S., & Murton, B. J. (2021). Ocean-Floor Sediments as a Resource of Rare Earth Elements: An Overview of Recently Studied Sites. Minerals, 11(2), 142. https://doi.org/10.3390/min11020142






