Assessment of Soil Contamination by Gas Cloud Generated from Chemical Fire Using Metabolic Profiling and Associated Bacterial Communities
Abstract
:1. Introduction
2. Materials and Methods
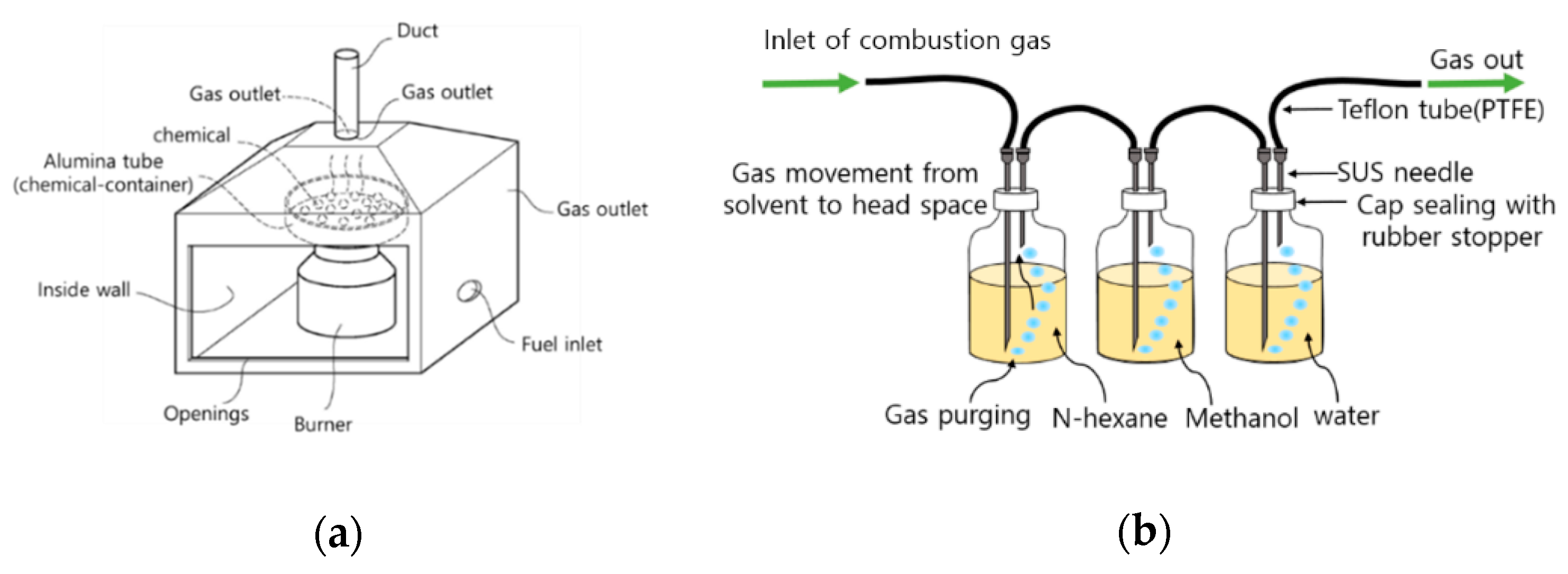
2.1. Identification of Combustion Products in Gas Cloud and Pollutants Remained in the Soil under Laboratory Condition
2.2. Extraction of Metabolites in Contaminated Soils (Field)
2.2.1. Soil Sampling and Metabolite Extraction in Field Soil (Chemical Accident Site)
2.2.2. Bacterial Community Identification (Field)
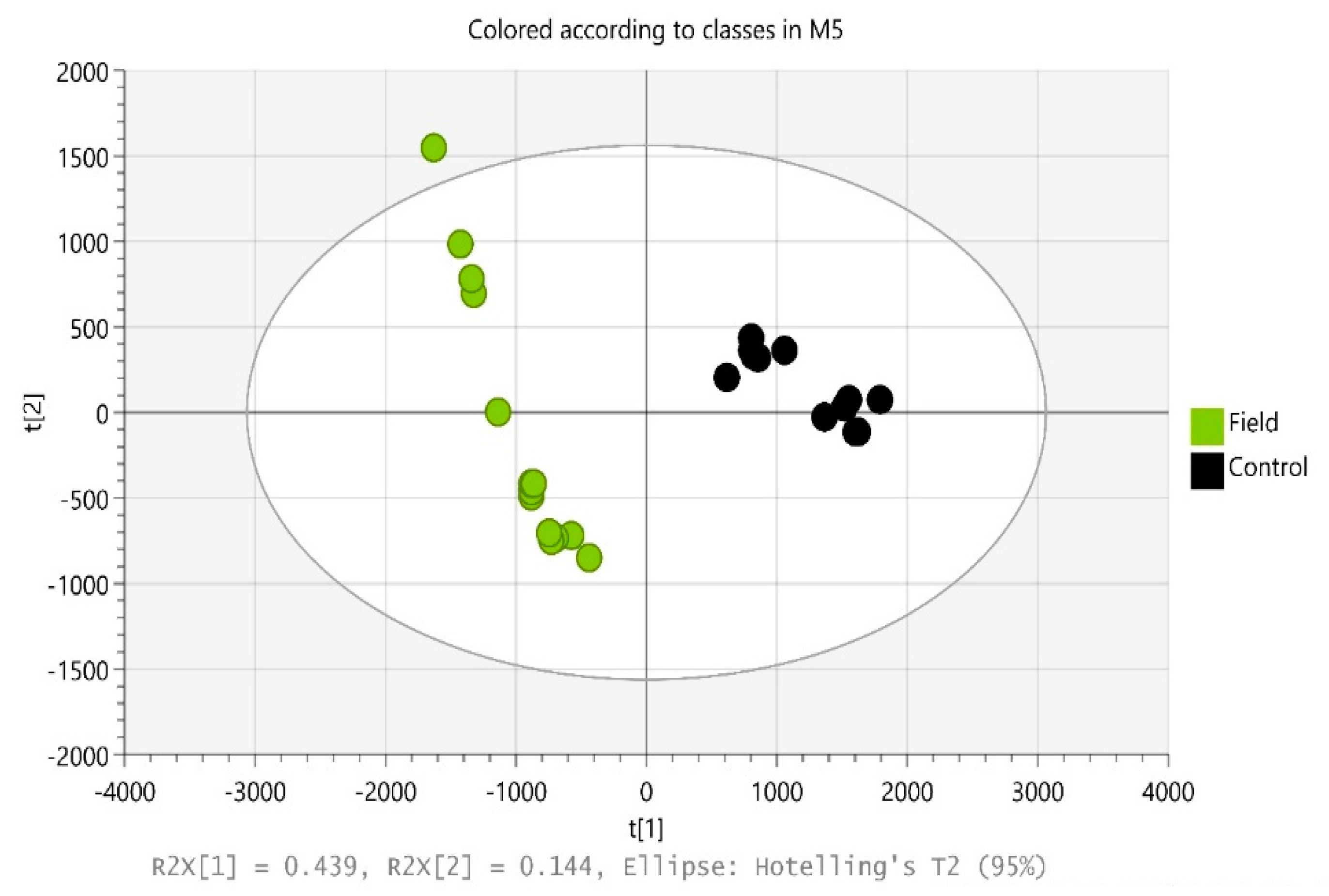
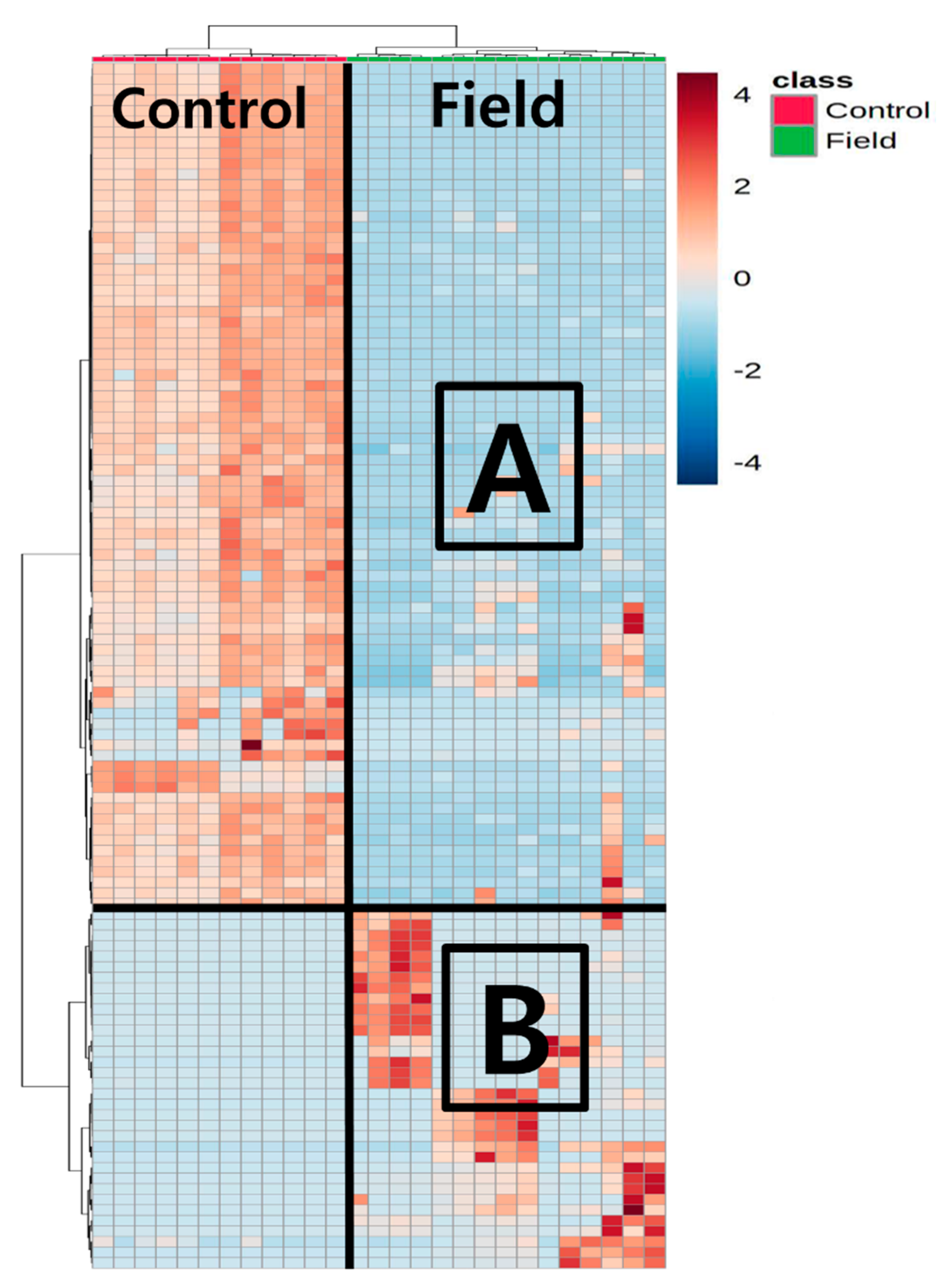
2.2.3. Statistical Analysis
3. Results and Discussion
3.1. Identification of Combustion Products in Gas Cloud Generated from Toluene Combustion
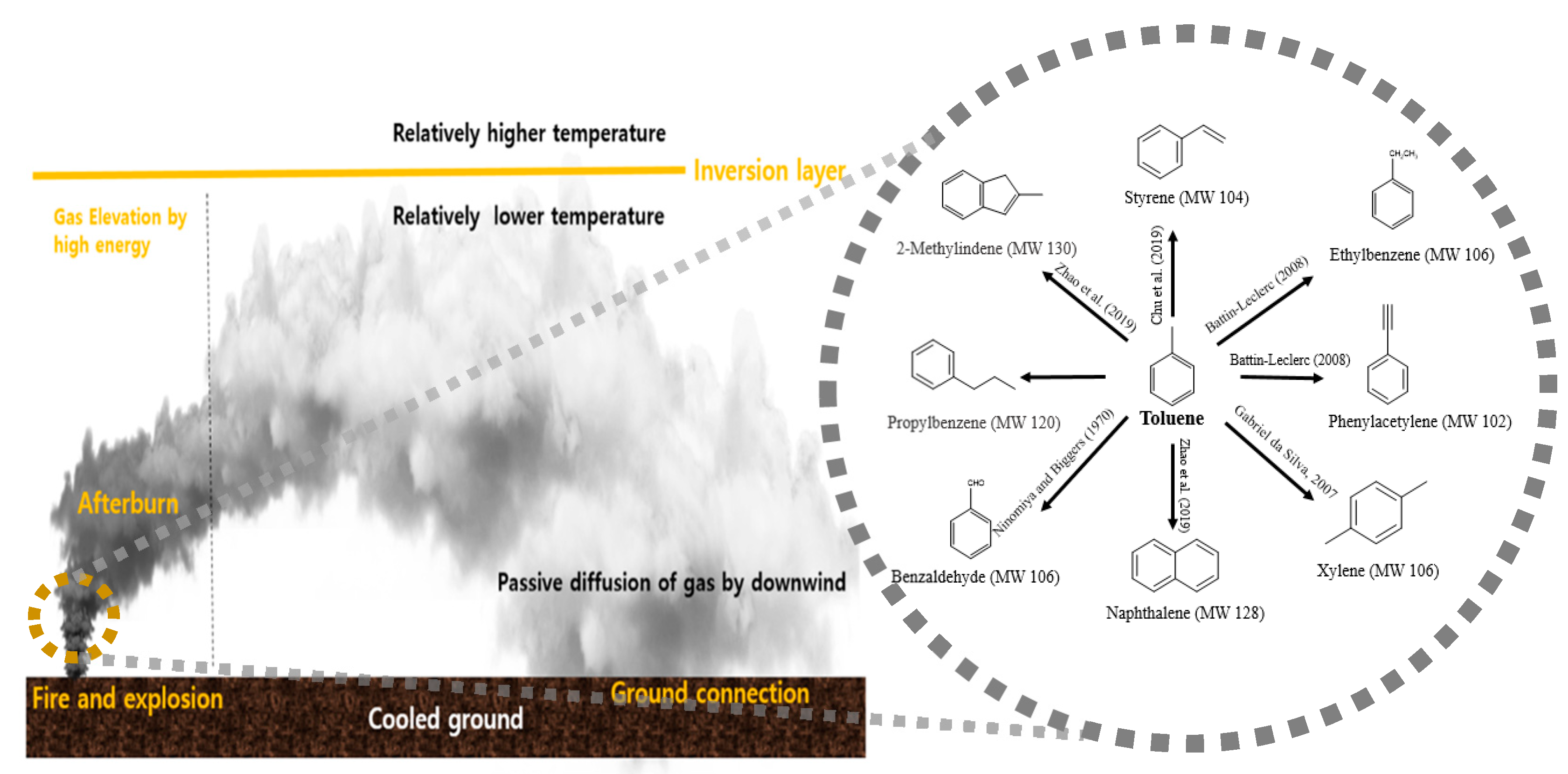
3.2. Overview of Metabolites Discovered in Filed Soil (Contaminated Site)
3.2.1. Propose the Relationship between Toluene Combustion Products and Metabolites in Toluene Affected Soils
3.2.2. Secondary Metabolites, Antibiotic, and Therapeutic Materials in the Chemical Accident Site
3.2.3. Metabolite for Protecting Cell Membrane in Bacteria
3.2.4. Secondary Metabolites of Plant Surviving from Contaminated Soil
3.3. Microbial Community Analysis in Field Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Jung, S.; Woo, J.; Kang, C. Analysis of severe industrial accidents caused by hazardous chemicals in South Korea from January 2008 to June 2018. Saf. Sci. 2020, 124, 104580. [Google Scholar] [CrossRef]
- National Institute of Chemical Safety, Integraed Information System of Chemical (National Institute of Chemical Safety). Available online: https://icis.me.go.kr/search/searchType2.do (accessed on 1 February 2021).
- Kang, I.-S.; Xi, J.; Hu, H.-Y. Photolysis and photooxidation of typical gaseous VOCs by UV Irradiation: Removal performance and mechanisms. Front. Environ. Sci. Eng. 2018, 12, 8. [Google Scholar] [CrossRef]
- Agency, E.E. Temperature Inversion Traps Pollution at Ground Level. European Environment Agency. Available online: https://www.eea.europa.eu/media/infographics/temperature-inversion-traps-pollution-at/view (accessed on 1 February 2021).
- Yang, S.; Jeon, K.; Kang, D.; Han, C. Accident analysis of the Gumi hydrogen fluoride gas leak using CFD and comparison with post-accidental environmental impacts. J. Loss Prev. Process Ind. 2017, 48, 207–215. [Google Scholar] [CrossRef]
- Moussa, N.; Devarakonda, V. Prediction of Toxic Emissions from Chemical Fire and Explosion. Fire Saf. Sci. 2014, 11, 1457–1468. [Google Scholar] [CrossRef]
- Ahn, M.S.; Lee, H.E.; Cheon, K.S.; Joo, H.G.; Son, B.-S.; Ochang Chemical Safety Community. Feasibility Evaluation of Designated Quantities for Chemicals Requiring Preparation for Accidents in the Korean Chemical Accident Prevention System. Int. J. Environ. Res. Public Health 2020, 17, 1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, A.; Zhang, H.; Lei, Y. Surfactant flushing remediation of toluene contaminated soil: Optimization with response surface methodology and surfactant recovery by selective oxidation with sulfate radicals. Sep. Purif. Technol. 2013, 118, 612–619. [Google Scholar] [CrossRef]
- Fullerr, M.E. Effects of Toluene on Microbially-Mediated Processes Involved in the Soil Nitrogen Cycle. Microb. Ecol. 1995, 32, 171–184. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Lu, W.; Xu, B.; Ahmed, M.; Morozov, A.N.; Mebel, A.M.; Howlader, A.H.; Wnuk, S.F. Molecular mass growth through ring expansion in polycyclic aromatic hydrocarbons via radical-radical reactions. Nat. Commun. 2019, 10, 3689. [Google Scholar] [CrossRef]
- Yost, L.J.; Hartemink, A.E. How deep is the soil studied—An analysis of four soil science journals. Plant Soil 2020, 452, 5–18. [Google Scholar] [CrossRef]
- Kim, N.; Ahn, Y.; Jo, J.; Pyo, H.; Lee, J.; Choi, J. Soil assessment after chemical accidents using metabolic profiling and microbial community evaluation. Chemosphere 2021, 268, 129362. [Google Scholar] [CrossRef]
- Chu, T.C.; Buras, Z.J.; Smith, M.C.; Uwagwu, A.B.; Green, W.H. From benzene to naphthalene: Direct measurement of reactions and intermediates of phenyl radicals and acetylene. Phys. Chem. Chem. Phys. 2019, 21, 22248–22258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battin-Leclerc, F. Detailed chemical kinetic models for the low-temperature combustion of hydrocarbons with application to gasoline and diesel fuel surrogates. Prog. Energy Combust. Sci. 2008, 34, 440–498. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, W.; Li, T.; Li, W.; Yang, J.; Qi, F. Experimental and kinetic modeling investigation of rich premixed toluene flames doped with n-butanol. Phys. Chem. Chem. Phys. 2018, 20, 10628–10636. [Google Scholar] [CrossRef] [PubMed]
- Gabriel da Silva, C.-C.C.; Bozzelli, J.W. Toluene combustion: Reaction paths, thermochemical properties, and kinetic analysis for the methylphenyl radical + O2 reaction. J. Phys. Chem. 2007, 35, 8663–8676. [Google Scholar] [CrossRef]
- Ninomiya, J.S.; Biggers, B. Effects of toluene content in fuel on aromatic emissions in the exhaust. J. Air Pollut. Control 1970, 20, 609–611. [Google Scholar] [CrossRef] [Green Version]
- Levitin, J. A Verification of a New Coastal Area Dispersion Model. In Air Pollution Modeling and Its Application XIII; Springer: Boston, MA, USA, 2000; pp. 589–596. [Google Scholar]
- Anda, A.; Illes, B. Impact of Simulated Airborne Soot on Maize Growth and Development. J. Environ. Prot. 2012, 3, 773–781. [Google Scholar]
- Ruiz, J.; Bilbao, R.; Murillo, M.B. Adsorption of Different VOC onto Soil Minerals from Gas Phase Influence of Mineral Type of VOC and Air Humidity. Environ. Sci. Technol. 1998, 32, 1079–1084. [Google Scholar] [CrossRef]
- Park, W.P.; Chang, K.M.; Koo, B.J.; Hyun, H.N. Cation Exchange Capacity in Korean Soils Determined by the Copper(II) Acetate Spectrophotometry Method. Korean J. Soil Sci. Fertil. 2017, 50, 653–662. [Google Scholar]
- Breus, I.P.; Mishchenko, A.A. Sorption of volatile organic contaminants by soils (a review). Eurasian Soil Sci. 2006, 39, 1271–1283. [Google Scholar] [CrossRef]
- Singh, R.; Lemire, J.; Mailloux, R.J.; Appanna, V.D. A novel strategy involved in [corrected] anti-oxidative defense: The conversion of NADH into NADPH by a metabolic network. PLoS ONE 2008, 3, e2682. [Google Scholar] [CrossRef]
- Iessi, E.; Marconi, M.; Manganelli, V.; Sorice, M.; Malorni, W.; Garofalo, T.; Matarrese, P. On the role of sphingolipids in cell survival and death. Int. Rev. Cell Mol. Biol. 2020, 351, 149–195. [Google Scholar]
- Kunz, T.C.; Kozjak-Pavlovic, V. Diverse Facets of Sphingolipid Involvement in Bacterial Infections. Front Cell Dev. Biol. 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Wang, Q.; Violi, A. Chemical pathways for the formation of benzofuran and dibenzofuran in combustion. Combust. Flame 2020, 212, 216–233. [Google Scholar] [CrossRef]
- Sivapragasam, S.; Grove, A. The Link between Purine Metabolism and Production of Antibiotics in Streptomyces. Antibiotics 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Chen, L.; Xiong, S.; Tong, Q. Enhanced acarbose production by Streptomyces M37 using a two-stage fermentation strategy. PLoS ONE 2017, 12, e0166985. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Liu, L.-L.; Feng, Z.-H.; Liu, Z.-Q.; Zheng, Y.-G. Optimization of media composition and culture conditions for acarbose production by Actinoplanes utahensis ZJB-08196. World J. Microbiol. Biotechnol. 2011, 27, 2759–2766. [Google Scholar] [CrossRef]
- Sun, Y.; Wilkinson, B.J.; Standiford, T.J.; Akinbi, H.T.; O’Riordan, M.X.D. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J. Bacteriol. 2012, 194, 5274–5284. [Google Scholar] [CrossRef] [Green Version]
- Murinova, S.; Dercova, K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef] [Green Version]
- Diefenbach, R.; Heipieper, H.J.; Keweloh, H. Conversion of cis Unsaturated Fatty Acids to trans, a Possible Mechanism for the Protection of Phenol-Degrading Pseudomonas putida P8 from Substrate Toxicity. Appl. Environ. Microbiol. 1992, 58, 1847–1852. [Google Scholar]
- Ghorbal, S.K.; Ghorbal, B.; Chatti, A.; Sethom, M.M.; Maalej, L.; Mihoub, M.; Kefacha, S.; Feki, M.; Hassen, A.L.A. Changes in membrane fatty acid composition of Pseudomonas aeruginosa in response to UV-C radiations. Curr. Microbiol. 2013, 67, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Kvesitadze, A.; Khatisashvili, G.; Sadunishvili, T.; Ramsden, J.J. Biochemical Mechanisms of Detoxification in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Shaw, P.D. Biosynthesis of nitro compounds. III. The enzymatic reduction of beta-nitroacrylic acid to beta-nitropropionic acid. Biochemistry 1967, 6, 2253–2260. [Google Scholar] [CrossRef]
- Merino, E.; Barrientos, A.; Rodríguez, J.; Naharro, G.; Luengo, J.M.; Olivera, E.R. Isolation of cholesterol- and deoxycholate-degrading bacteria from soil samples: Evidence of a common pathway. Applied Microbiol. Biotechnol. 2013, 97, 891–904. [Google Scholar] [CrossRef]
- Whitman, W.; Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.; Ludwig, W.; Suzuki, K.-I.; Parte, A. (Eds.) Bergey’s Manual of Systematic Bacteriology; Springer: New York, NY, USA, 2012; Volume 5. [Google Scholar]
- O’Loughlin, E.J.; GSims, K.; Traina, S.J. Biodegradation of 2-methyl, 2-ethyl, and 2-hydroxypyridine by an Arthrobacter sp. isolated from subsurface sediment. Biodegradation 1999, 10, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fofana, B.; Somalraju, A.; Fillmore, S.; Zaidi, M.; Main, D.; Ghose, K. Comparative transcriptome expression analysis in susceptible and resistant potato (Solanum tuberosum) cultivars to common scab (Streptomyces scabies) revealed immune priming responses in the incompatible interaction. PLoS ONE 2020, 15, e0235018. [Google Scholar] [CrossRef] [PubMed]
- Sudtachat, N.; Ito, N.; Itakura, M.; Masuda, S.; Eda, S.; Mitsui, H.; Kawaharada, Y.; Minamisawa, K. Aerobic Vanillate Degradation and C1 Compound Metabolism in Bradyrhizobium japonicum. Applied Environ. Microbiol. 2009, 75, 5012. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, C.; Duraipandiyan, V.; Balakrishna, K.; Ignacimuthu, S. Petroleum and polycyclic aromatic hydrocarbons (PAHs) degradation and naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil. Bioresour. Technol. 2012, 112, 83–90. [Google Scholar]
- Bignell, D.R.D. Streptomyces scabies 87–22 Contains a Coronafacic Acid-Like Biosynthetic Cluster That Contributes to Plant–Microbe Interactions. Am. Phytopathol. Soc. 2010, 23, 161–175. [Google Scholar] [CrossRef] [Green Version]
- Nahar, N.; Alauddin, M.; Quilty, B. Toxic efects of toluene on the growth of activated sludge bacteria. World J. Microbiol. Biotechnol. 2000, 6, 307–311. [Google Scholar] [CrossRef]

| Combustion Product | Formula | Molecular Weight | tR (min) | Selected Ions (m/z) |
|---|---|---|---|---|
| Methylbenzene | C7H8 | 92.14 | 3.973 | 91,92,65 |
| Ethylbenzene | C8H10 | 106.17 | 7.030 | 106,91,51 |
| Ethynylbenzene | C8H6 | 102.13 | 7.869 | 102,76,50 |
| Benzaldehyde | C₇H₆O | 106.12 | 8.997 | 106,77,51 |
| 1-Phenyl-1-propyne | C9H8 | 116.16 | 9.926 | 116,115,63,89 |
| Naphthalene | C10H8 | 128.17 | 11.218 | 128,127,102 |
| 2-Methylindene | C10H10 | 130.19 | 13.154 | 130,129,115 |
| Field Soil | Texture (%) | pH | ORP | CEC | TOC | Mineral | ||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | (mV) | (cmol/kg) | (%) | |||
| 100 m | 68.57 | 15.42 | 16.01 | 7.27 | 113.4 | 21.64 | 1.05 | Kaolinite Halloysite Anorthite Quartz |
| 200 m | 69.42 | 15.24 | 15.34 | 6.67 | 137.8 | 29.87 | 1.05 | |
| 300 m | 69.56 | 13.95 | 16.49 | 6.14 | 179.0 | 23.40 | 0.39 | |
| 400 m | 57.78 | 28.54 | 13.68 | 6.97 | 106.9 | 20.76 | 0.62 | |
| 500 m | 76.36 | 11.00 | 12.64 | 8.29 | 44.1 | 23.43 | 1.25 | |
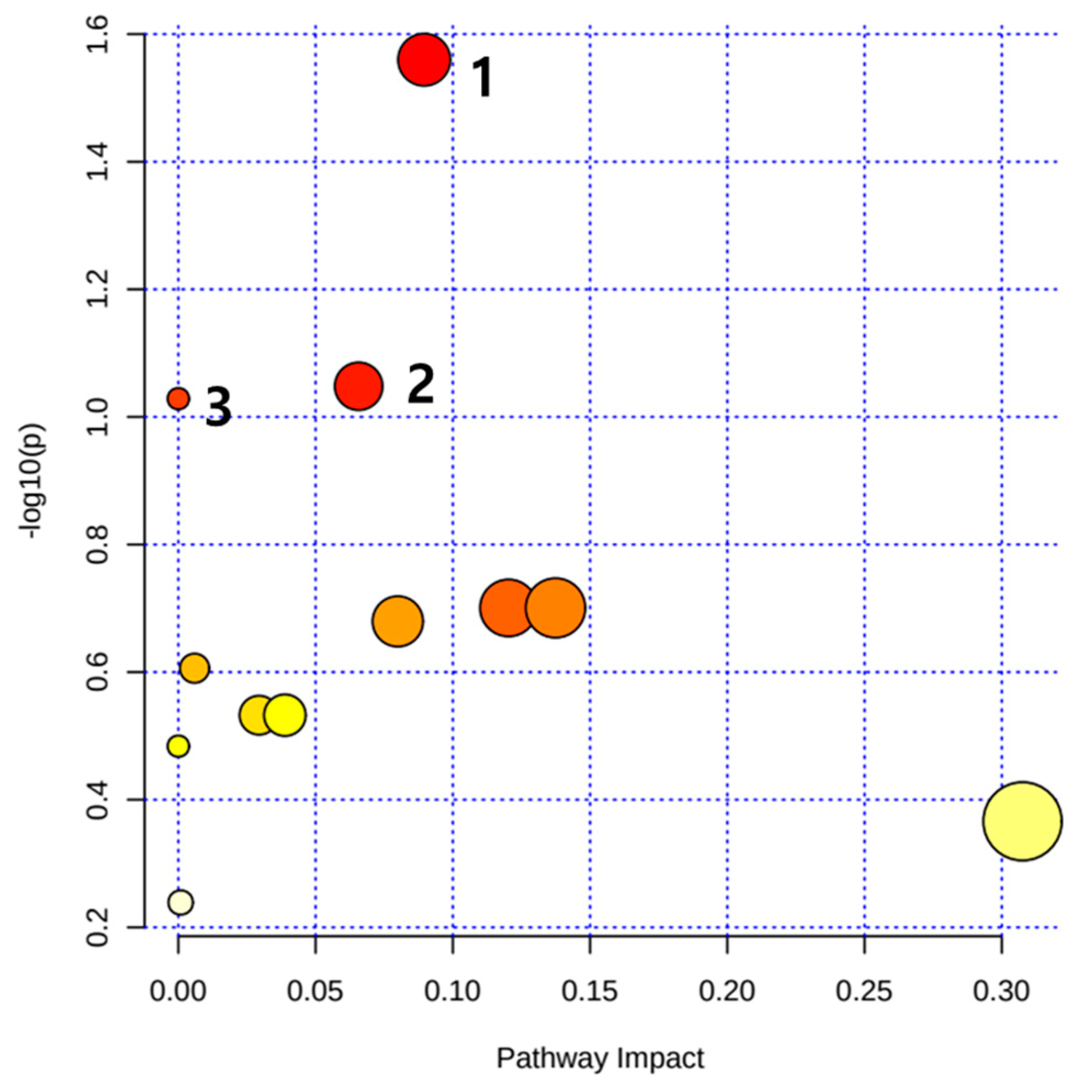
| No. | Metabolism | Total | Expected | Hits | Raw p | LOG(p) |
|---|---|---|---|---|---|---|
| 1 | Alanine, aspartate and glutamate metabolism | 22 | 0.268 | 2 | 0.0275 | 1.560 |
| 2 | Pyrimidine metabolism | 42 | 0.511 | 2 | 0.0895 | 1.048 |
| 3 | Nitrogen metabolism | 8 | 0.097 | 1 | 0.0936 | 1.029 |
| Upregulated Metabolites in Field Soil (Contaminated Soil) | |||||
|---|---|---|---|---|---|
| tR (min)_m/z | t.stat | p-Value | Mass Error (ppm) | Metabolite | Metabolism |
| 15.43_146.9797 | −4.3983 | 0.00060457 | −23.25573074 | 1,4-Dichlorobenzene | Derivative of benzene ring |
| 15.47_355.0695 | −2.2841 | 0.038491 | −9.933051337 | 2-Caffeoylisocitrate | |
| 15.47_265.9916 | −2.3449 | 0.034295 | −12.07499801 | 2-Hydroxy-beta-keto-L-tyrosyl-[pcp] | Novobiocin biosynthesis |
| 14.37_139.9872 | −2.7065 | 0.017037 | 58.77534925 | 3-Nitroacrylate | Beta-nitroacrylate reductase |
| 0.95_182.9844 | −2.533 | 0.023891 | −13.43082261 | 5-Chloro-1,2,4-trihydroxybenzene | Chlorocyclohexane and chlorobenzene degradation |
| 15.92_686.1990 | −2.2594 | 0.04031 | 14.99536091 | Acarbose | Acarbose and validamycin biosynthesis |
| 13.87_149.0227 | −2.6871 | 0.017691 | −11.97984821 | Hydroxyhydroquinone | -Chlorocyclohexane and chlorobenzene degradation -Benzoate degradation -Aminobenzoate degradation |
| 15.46_371.1010 | −2.2147 | 0.04387 | −2.081103867 | Camptothecin | -Biosynthesis of alkaloids derived from shikimate pathway |
| 14.60_141.9825 | −2.9159 | 0.01128 | 52.71728653 | Carbamoyl phosphate | Purine metabolism |
| 15.90_371.1008 | −2.5188 | 0.024559 | −11.12892128 | Ciprofloxacin | ABC transporters |
| 14.98_166.0264 | −2.2939 | 0.037783 | 0.529750536 | Delta1-Piperideine-6-L-carboxylate | Biosynthesis of plant secondary metabolites |
| 15.00_207.0162 | −2.4157 | 0.029955 | 21.61279894 | Dibenzofuran | Derivative of benzene ring |
| 13.83_279.1582 | −2.8061 | 0.014008 | 3.172566133 | Dibutyl phthalate | |
| 15.47_429.0878 | −2.1947 | 0.045551 | −3.571806454 | Diethylstilbestrol diphosphate | |
| 15.42_445.1191 | −2.3952 | 0.031151 | −13.87669464 | Granaticin | Biosynthesis of type II polyketide products |
| 0.98_158.9613 | −2.5656 | 0.022428 | −63.07337885 | Malonyl-[acyl-carrier protein] | Fatty acid biosynthesis |
| 15.97_663.4559 | −3.0953 | 0.0079051 | −72.40067762 | Mosesin 4 | |
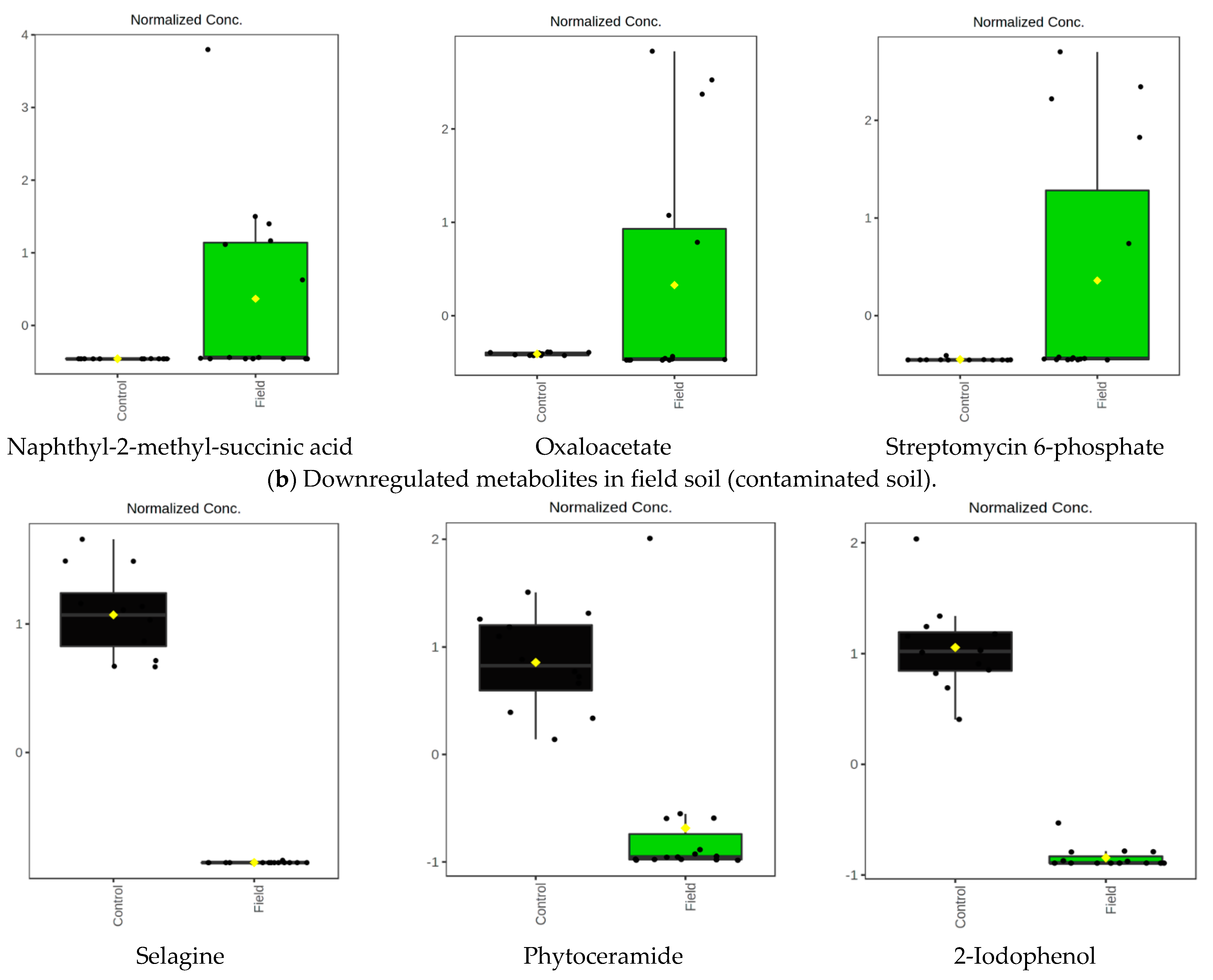
| 15.47_298.0540 | −2.5857 | 0.021568 | −4.498039682 | Naphthyl-2-methyl-succinic acid (Streptomyces scabiei) | Naphthalene degradation Degradation of aromatic compounds |
| 14.51_91.0081 | −2.9347 | 0.01087 | −60.60257231 | Oxalate | -Glycine, serine and -threonine metabolism Purine metabolism |
| 14.42_154.9893 | −2.2578 | 0.04045 | 37.38135766 | Oxaloacetate | -Citrate cycle (TCA cycle) -Alanine, aspartate and glutamate metabolism |
| 15.90_298.0540 | −2.3965 | 0.031075 | −29.87970305 | Paraoxon | Aminobenzoate degradation |
| 13.18_326.0701 | −2.7571 | 0.015427 | −3.307671162 | Rutecarpine | |
| 15.04_684.2011 | −2.519 | 0.024542 | 29.40706749 | Streptomycin 6-phosphate (Streptomyces scabiei) | Streptomycin biosynthesis |
| 14.89_281.0509 | −2.521 | 0.024455 | 9.005321238 | Thymidine;Deoxythymidine | Pyrimidine metabolism |
| Downregulated Metabolites in Field Soil (not Contaminated Soil) | |||||
| tR (min)_m/z | t.stat | p-Value | Mass Error (ppm) | Metabolite | Metabolism (KEGG) |
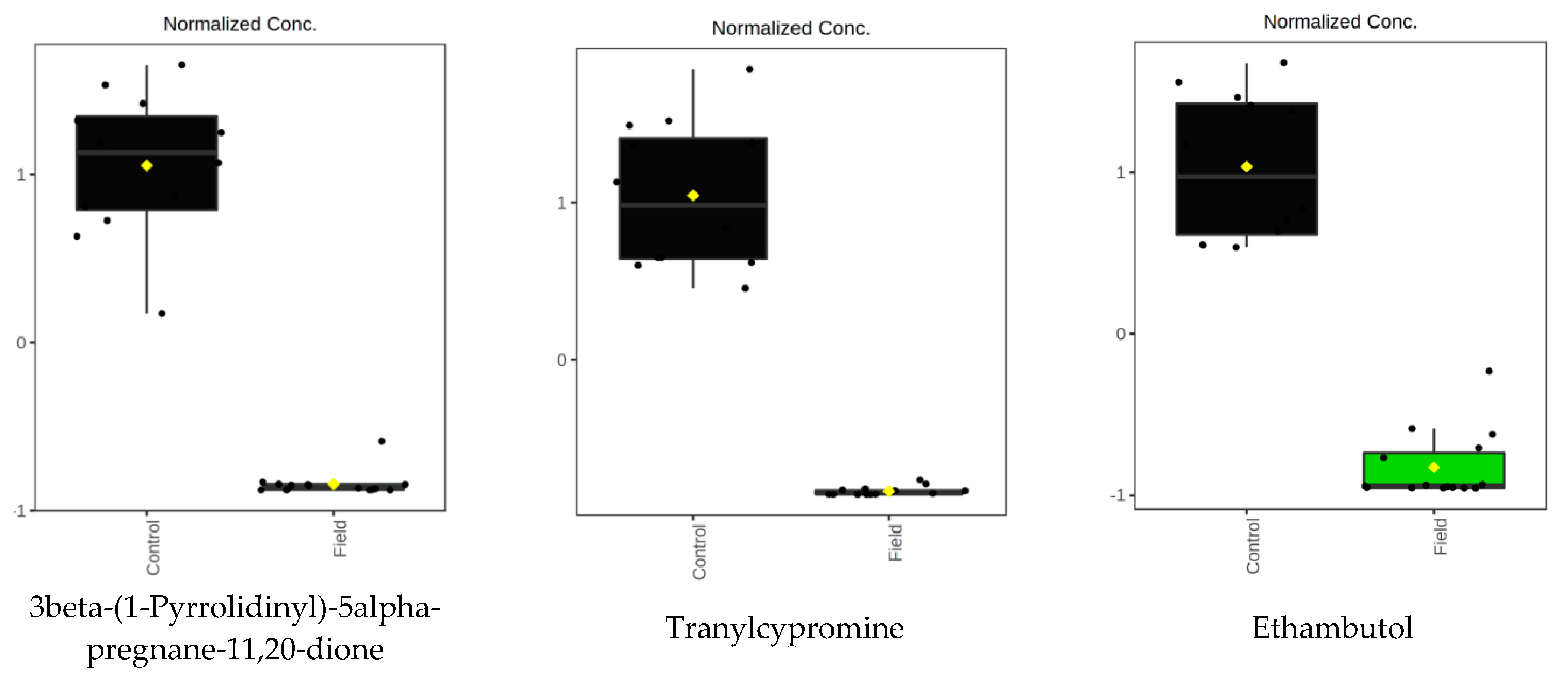
| 15.74_265.1299 | 19.888 | 5.65 × 10−10 | 4.653951133 | Selagine | |
| 15.58_242.9242 | 16.033 | 1.77 × 10−9 | 14.54408612 | 2-Iodophenol | |
| 14.85_424.2616 | 15.193 | 5.68 × 10−9 | −0.851091841 | 3beta-(1-Pyrrolidinyl)-5alpha-pregnane-11,20-dione | |
| 3.09_134.0937 | 14.018 | 2.18 × 10−8 | 20.32734589 | Tranylcypromine | |
| 15.43_227.1743 | 13.249 | 1.25 × 10−9 | −5.728148531 | Ethambutol | |
| 15.09_457.3471 | 13.097 | 2.71 × 10−9 | −6.252920748 | 6alpha-Hydroxycampestanol | Brassinosteroid biosynthesis |
| 11.18_317.2923 | 12.924 | 5.34 × 10−8 | −26.49916761 | 17-Propyl-5alpha-androst-2-en-17beta-ol | |
| 11.11_273.2666 | 11.969 | 0.000000119 | −32.65235568 | Taxa-4(5),11(12)-diene;Taxa-4,11-diene | Diterpenoid biosynthesis Biosynthesis of terpenoids and steroids |
| 12.62_336.2501 | 10.362 | 0.000000468 | 2.422853992 | (+)-Prosopinine | |
| 11.51_386.1729 | 10.236 | 0.000000134 | −0.610177392 | Dioncophylline C | |
| 13.58_440.3581 | 9.6318 | 0.000000131 | 16.37290739 | 3beta,4-Dimethyl-4-aza-5alpha-cholestane | |
| 11.58_459.489 | 9.3486 | 0.000000793 | 2.22584472 | Hentriacontane | |
| 7.19_285.2403 | 7.484 | 8.64 × 10−8 | 7.43701372 | 2-Methoxy-6Z-hexadecenoic acid | |
| 15.51_346.3066 | 6.6331 | 0.00000096 | −32.962628 | Phytoceramide | Sphingolipid metabolism |
| 15.83_909.5309 | 6.4734 | 0.0000143 | 21.06185413 | Zn-Bacterio-chlorophyll a | Porphyrin and chlorophyll metabolism |
| 14.87_445.191 | 4.5605 | 0.00014874 | 1.879348973 | Blasticidin S | |
| 12.21_414.2045 | 3.0239 | 0.0070218 | −0.922343749 | Icaceine | |
| 14.63_573.4865 | 2.7964 | 0.01731 | −2.037897249 | 1-O-(1Z-Tetradecenyl)-2-(9Z-octadecenoyl)-sn-glycerol | |
| 14.88_903.5644 | 2.7956 | 0.017375 | 38.74803849 | Bacterio-pheophytins | Porphyrin and chlorophyll metabolism |
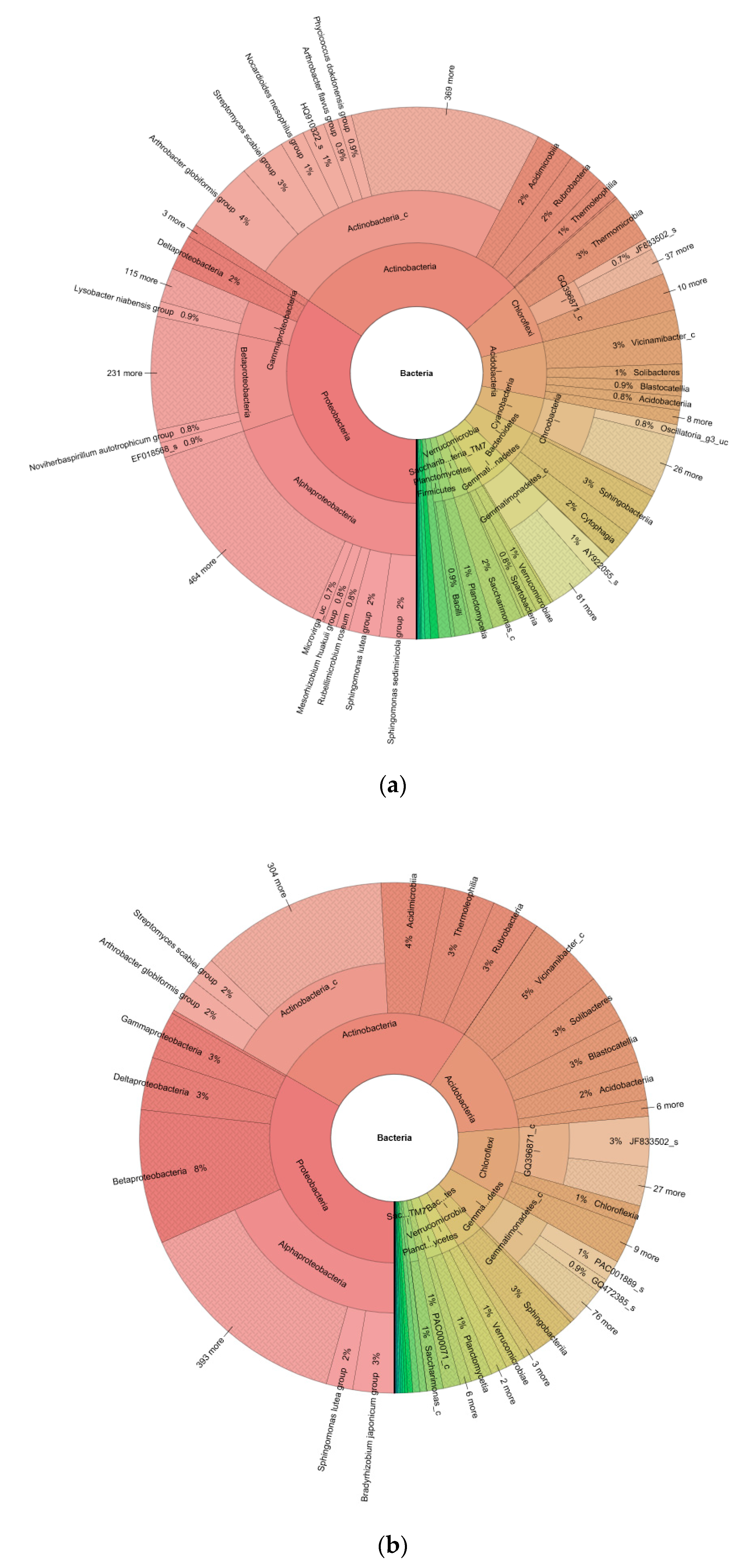
| Phylum | Control | Field |
|---|---|---|
| Proteobacteria | 34 | 33 |
| Actinobacteria | 29 | 26 |
| Chloroflexi | 8 | 9 |
| Acidobacteria | 7 | 14 |
| Cyanobacteria | 5 | <1 |
| Species | Control | Field |
| Arthrobacter globiformis group | 3825 | 933 |
| Streptomyces scabiei group | 2424 | 722 |
| Sphingomonas lutea group | 1644 | 714 |
| Sphingomonas sediminicola group | 1967 | 266 |
| Nocardioides mesophilus group | 1292 | 116 |
| Lysobacter niabensis group | 814 | 6 |
| Bradyrhizobium japonicum group | 319 | 1112 |
| Mycobacterium farcinogenes group | 54 | 238 |
| Hamadaea flava | 56 | 140 |
| Sphingomonas pruni group | 47 | 118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.; Ahn, Y.; Pandi, K.; Pyo, H.; Kim, N.; Yun, S.-T.; Kim, M.; Lee, J.; Choi, J. Assessment of Soil Contamination by Gas Cloud Generated from Chemical Fire Using Metabolic Profiling and Associated Bacterial Communities. Minerals 2021, 11, 372. https://doi.org/10.3390/min11040372
Jo J, Ahn Y, Pandi K, Pyo H, Kim N, Yun S-T, Kim M, Lee J, Choi J. Assessment of Soil Contamination by Gas Cloud Generated from Chemical Fire Using Metabolic Profiling and Associated Bacterial Communities. Minerals. 2021; 11(4):372. https://doi.org/10.3390/min11040372
Chicago/Turabian StyleJo, Jungman, Yongtae Ahn, Kalimuthu Pandi, Heesoo Pyo, Naeun Kim, Seong-Taek Yun, Minseok Kim, Jeongae Lee, and Jaeyoung Choi. 2021. "Assessment of Soil Contamination by Gas Cloud Generated from Chemical Fire Using Metabolic Profiling and Associated Bacterial Communities" Minerals 11, no. 4: 372. https://doi.org/10.3390/min11040372
APA StyleJo, J., Ahn, Y., Pandi, K., Pyo, H., Kim, N., Yun, S.-T., Kim, M., Lee, J., & Choi, J. (2021). Assessment of Soil Contamination by Gas Cloud Generated from Chemical Fire Using Metabolic Profiling and Associated Bacterial Communities. Minerals, 11(4), 372. https://doi.org/10.3390/min11040372






