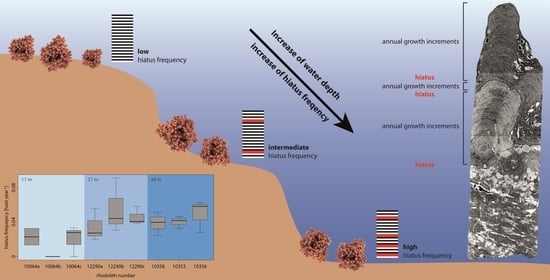
Growth Interruptions in Arctic Rhodoliths Correspond to Water Depth and Rhodolith Morphology
Abstract
:1. Introduction
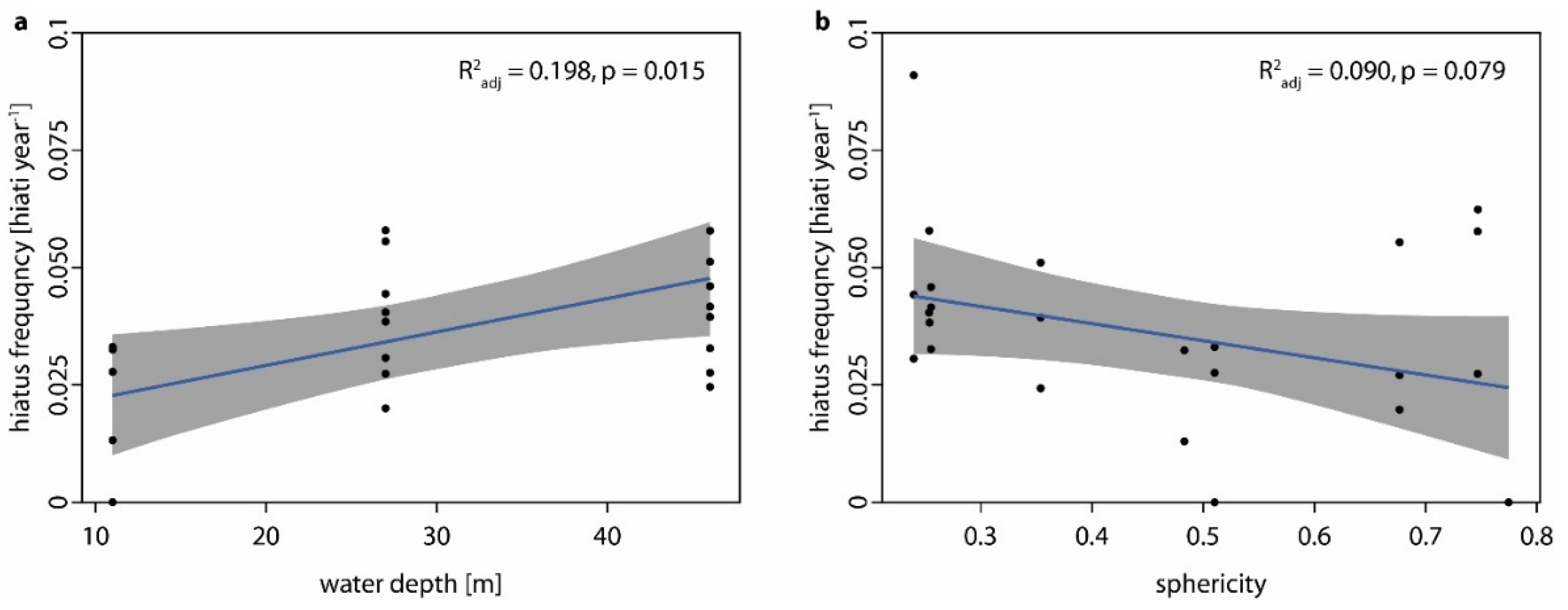
- Hiatus frequency increases with water depth as shallow-water specimens are turned more regularly by currents and by wave action (i.e., no thallus dieback between turning events), while the hydrodynamically induced turning of deep-water specimens is restricted to storm events so that chiefly biotic interactions cause movement (i.e., dieback between turning events). Additionally, the photosynthetically active radiation which is necessary for rhodolith growth decreases with water depth and the presence of detrimental fine sediments increases with water depth.
- Hiatus frequency decreases with sphericity, considering that (a) rounder rhodoliths are turned more easily, and because (b) the rounder the rhodolith, the less surface is in direct contact to the sediment when facing downward and the more rhodolith surface is reached by ambient light.
- Rhodolith weight and volume do not significantly affect hiatus frequency since both hydrodynamics and biotic interactions (such as for instance rhodolith-moving echinoids [12]) are able to move rhodoliths of all the size classes we have studied.
2. Materials and Methods
2.1. Rhodolith Sampling
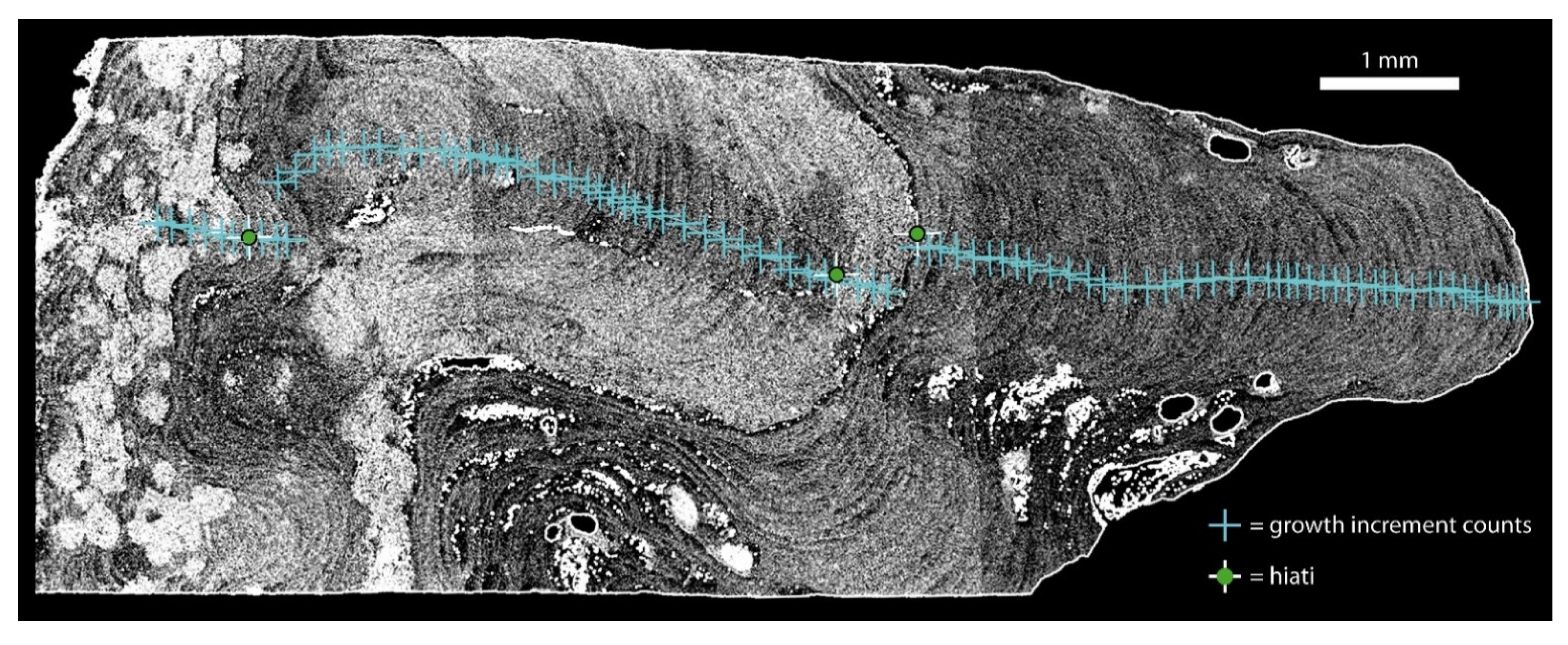
2.2. Protuberance Sampling and Examination
2.3. Statistical Analyses
3. Results
3.1. Rhodolith and Protuberance Data
3.2. Statistical Analyses
4. Discussion
4.1. The Environmental Tolerances of Lithothamnion glaciale
4.2. The Influence of Water Depth on Hiatus Frequency
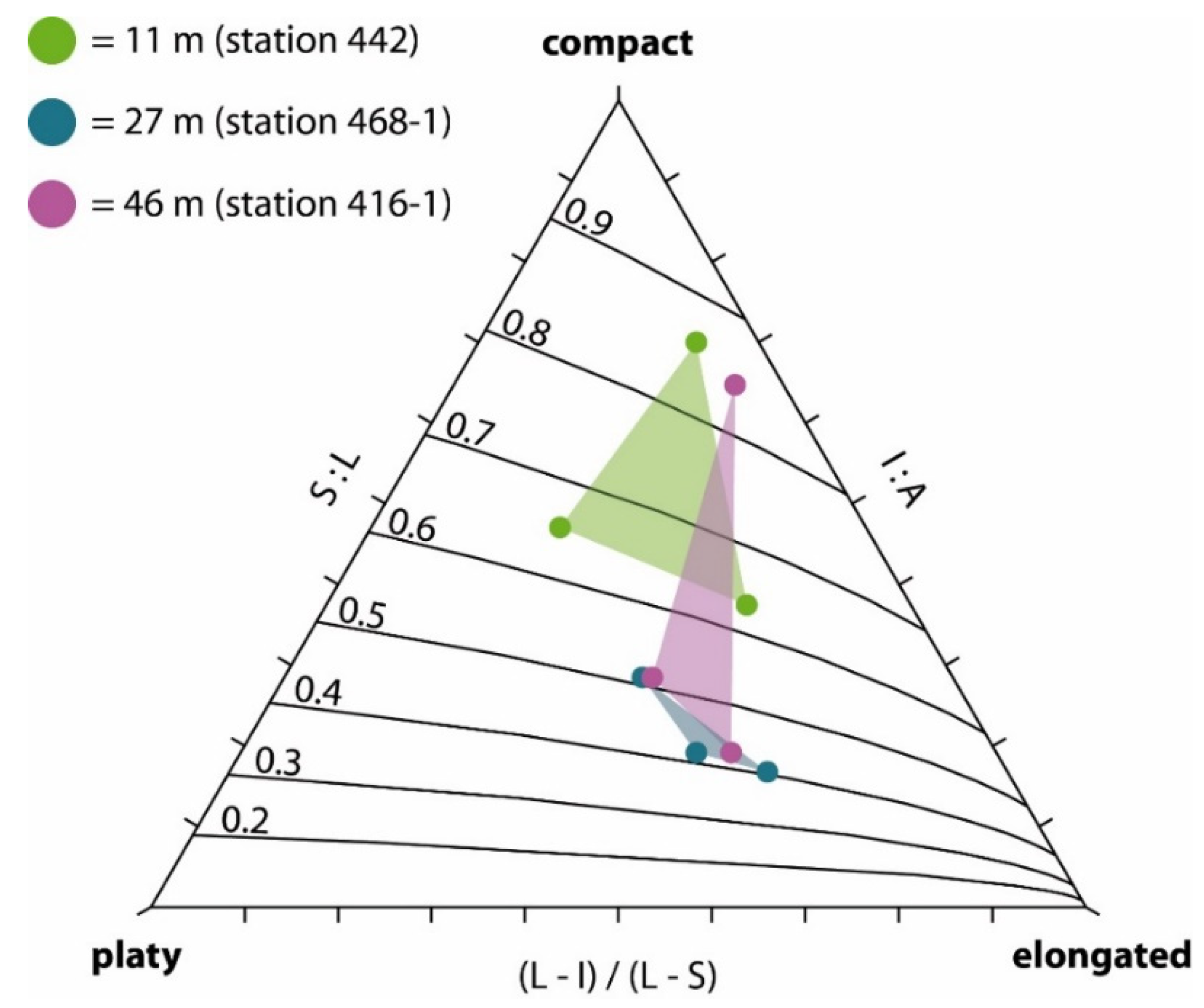
4.3. Morphological Aspects Influencing Hiatus Frequency
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teichert, S.; Freiwald, A. Polar coralline algal CaCO3-production rates correspond to intensity and duration of the solar radiation. Biogeosciences 2014, 11, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Teichert, S.; Woelkerling, W.J.; Rüggeberg, A.; Wisshak, M.; Piepenburg, D.; Meyerhöfer, M.; Form, A.; Büdenbender, J.; Freiwald, A. Rhodolith beds (Corallinales, Rhodophyta) and their physical and biological environment at 80°31′N in Nordkappbukta (Nordaustlandet, Svalbard Archipelago, Norway). Phycologia 2012, 51, 371–390. [Google Scholar] [CrossRef]
- Teichert, S.; Woelkerling, W.J.; Rüggeberg, A.; Wisshak, M.; Piepenburg, D.; Meyerhöfer, M.; Form, A.; Freiwald, A. Arctic rhodolith beds and their environmental controls. Facies 2014, 60, 15–37. [Google Scholar] [CrossRef]
- Kjellman, F.R. The algae of the Arctic Sea. In Kongliga Svenska Vetenskaps-Akademiens Handlingar; P.A. Norstedt & söner: Stockholm, Sweden, 1883; Volume 20, pp. 1–350. [Google Scholar]
- Teichert, S. Hollow rhodoliths increase Svalbard′s shelf biodiversity. Sci. Rep. 2014, 4, 6972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, S.; Halfar, J.; Zack, T.; Hetzinger, S.; Blicher, M.; Juul-Pedersen, T. Comparison of climate signals obtained from encrusting and free-living rhodolith coralline algae. Chem. Geol. 2018, 476, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Teichert, S.; Voigt, N.; Wisshak, M. Do skeletal Mg/Ca ratios of Arctic rhodoliths reflect atmospheric CO2 concentrations? Polar Biol. 2020, 43, 2059–2069. [Google Scholar] [CrossRef]
- Freiwald, A.; Henrich, R. Reefal coralline algal build-ups within the Arctic Circle: Morphology and sedimentary dynamics under extreme environmental seasonality. Sedimentology 1994, 41, 963–984. [Google Scholar] [CrossRef]
- Blake, C.; Maggs, C.A. Comparative growth rates and internal banding periodicity of maerl species (Corallinales, Rhodophyta) from northern Europe. Phycologica 2003, 42, 606–612. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Hoey, T.B.; Nienow, P.; Fallick, A.E.; Claverie, T. Reconstructing Greenland ice sheet runoff using coralline algae. Geology 2012, 40, 1095–1098. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Law, A. Temperature controls on coralline algal skeletal growth. J. Phycol. 2010, 46, 331–335. [Google Scholar] [CrossRef]
- Wisshak, M.; Neumann, H.; Rüggeberg, A.; Büscher, J.; Linke, P.; Raddatz, J. Epibenthos dynamics and environmental fluctuations in two contrasting Polar carbonate factories (Mosselbukta and Bjørnøy-Banken, Svalbard). Front. Mar. Sci. 2019, 6, 667. [Google Scholar] [CrossRef]
- Bosence, D.W.J. The occurrence and ecology of recent Rhodoliths—A review. In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 225–242. [Google Scholar]
- Wilson, S.; Blake, C.; Berges, J.A.; Maggs, C.A. Environmental tolerances of free-living coralline algae (maerl): Implications for European marine conservation. Biol. Conserv. 2004, 120, 279–289. [Google Scholar] [CrossRef]
- Woelkerling, W.J. The Coralline Red Algae; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Halfar, J.; Williams, B.; Hetzinger, S.; Steneck, R.; Lebednik, P.A.; Winsborough, C.; Omar, A.; Chan, P.; Wanamaker, A.D., Jr. 225 years of Bering Sea climate and ecosystem dynamics revealed by coralline algal growth-increment widths. Geology 2011, 39, 579–582. [Google Scholar] [CrossRef]
- Hetzinger, S.; Halfar, J.; Zajacz, Z.; Wisshak, M. Early start of 20th-century Arctic sea-ice decline recorded in Svalbard coralline algae. Geology 2019. [Google Scholar] [CrossRef] [Green Version]
- Bosence, D. Ecological studies on two unattached coralline algae from western Ireland. Palaeontology 1976, 19, 365–395. [Google Scholar]
- Bosence, D.W.J. Coralline algae: Mineralization, taxonomy, and palaeoecology. In Calcareous Algae and Stromatolites; Riding, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 98–113. [Google Scholar]
- Marrack, E.C. The Relationship between Water Motion and Living Rhodolith Beds in the Southwestern Gulf of California, Mexico. PALAIOS 1999, 14, 159–171. [Google Scholar] [CrossRef]
- Quaranta, F.; Tomassetti, L.; Vannucci, G.; Brandano, M. Coralline algae as environmental indicators: A case study from the Attard member (Chattian, Malta). Geodiversitas 2012, 34, 151–166. [Google Scholar] [CrossRef]
- Lüning, K. Seaweeds. Their Environment, Biogeography and Ecophysiology; Wiley Interscience: London, UK, 1990; p. 544. [Google Scholar]
- Aguirre, J.; Riding, R.; Braga, J.C. Diversity of coralline red algae: Origination and extinction patterns from the Early Cretaceous to the Pleistocene. Paleobiology 2000, 26, 651–667. [Google Scholar] [CrossRef]
- O′Connell, L.G.; James, N.P.; Harvey, A.S.; Luick, J.; Bone, Y.; Shepherd, S.A. Reevaluation of the Inferred Relationship between Living Rhodolith Morphologies, Their Movement, and Water Energy: Implications for Interpreting Paleoceanographic Conditions. Palaios 2021, 35, 543–556. [Google Scholar] [CrossRef]
- Wisshak, M.; Bartholomä, A.; Beuck, L.; Büscher, J.; Form, A.; Freiwald, A.; Halfar, J.; Hetzinger, S.; van Heugten, B.; Hissmann, K.; et al. Habitat Characteristics and Carbonate Cycling of Macrophyte-Supported Polar Carbonate Factories (Svalbard)—Cruise No. MSM55—11 June–29 June 2016—Reykjavik (Iceland)—Longyearbyen (Norway); MARIA S. MERIAN-Berichte: Bremen, Germany, 2017; p. 58. [Google Scholar]
- Sneed, E.D.; Folk, R.L. Pebbles in the lower Colorado river, Texas. A study in particle morphogenesis. J. Geol. 1958, 66, 114–150. [Google Scholar] [CrossRef]
- Graham, D.J.; Midgley, N.G. Graphical representation of particle shape using triangular diagrams: An Excel spreadsheet method. Earth Surf. Process. Landf. 2000, 25, 1473–1477. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Gantt, E. Pigmentation and photoacclimation. In Biology of the Red Algae; Cole, K.M., Sheath, R.G., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 203–219. [Google Scholar]
- Bilan, M.I.; Usov, A.I. Polysaccharides of calcareous algae and their effect on the calcification process. Russ. J. Bioorganic Chem. 2001, 27, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Rudels, B.; Meyer, R.; Fahrbach, E.; Ivanov, V.V.; Østerhus, S.; Quadfasel, D.; Schauer, U.; Tverberg, V.; Woodgate, R.A. Water mass distribution in Fram Strait and over the Yermak Plateau in summer 1997. Ann. Geophys. 2000, 18, 687–705. [Google Scholar] [CrossRef]
- Cottier, F.; Tverberg, V.; Inall, M.; Svendsen, H.; Nilsen, F.; Griffiths, C. Water mass modification in an Arctic fjord through cross-shelf exchange: The seasonal hydrography of Kongsfjorden, Svalbard. J. Geophys. Res. 2005, 110. [Google Scholar] [CrossRef] [Green Version]
- Hanssen-Bauer, I.; Førland, E.; Hisdal, H.; Mayer, S.; Sandø, A.B.; Sorteberg, A. Climate in Svalbard 2100; Norwegian Environment Agency (Miljødirektoratet): Trondheim, Norway, 2019; p. 207. [Google Scholar]
- Onarheim, I.H.; Smedsrud, L.H.; Ingvaldsen, R.B.; Nilsen, F. Loss of sea ice during winter north of Svalbard. Tellus A Dyn. Meteorol. Oceanogr. 2014, 66, 23933. [Google Scholar] [CrossRef] [Green Version]
- Bromley, R.G. Comparative analysis of fossil and recent echinoid bioerosion. Palaeontology 1975, 18, 725–739. [Google Scholar]
- Lopes, R.P.; Pereira, J.C. Molluskan Grazing Traces (Ichnogenus Radulichnus Voigt, 1977) on a Pleistocene Bivalve from Southern Brazil, With the Proposal of a New Ichnospecies. Ichnos 2019, 26, 141–157. [Google Scholar] [CrossRef]
- Steneck, R.S. Herbivory and the evolution of nongeniculate coralline algae (Rhodophyta, Corallinales) in the North Atlantic and North Pacific. In Evolutionary Biogeography of the Marine Algae of the North Atlantic; Garbary, D.J., South, G.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume G22, pp. 107–129. [Google Scholar]
- Steneck, R.S. The ecology of coralline algal crusts: Convergent patterns and adaptive strategies. Annu. Rev. Ecol. Syst. 1986, 17, 273–303. [Google Scholar] [CrossRef]
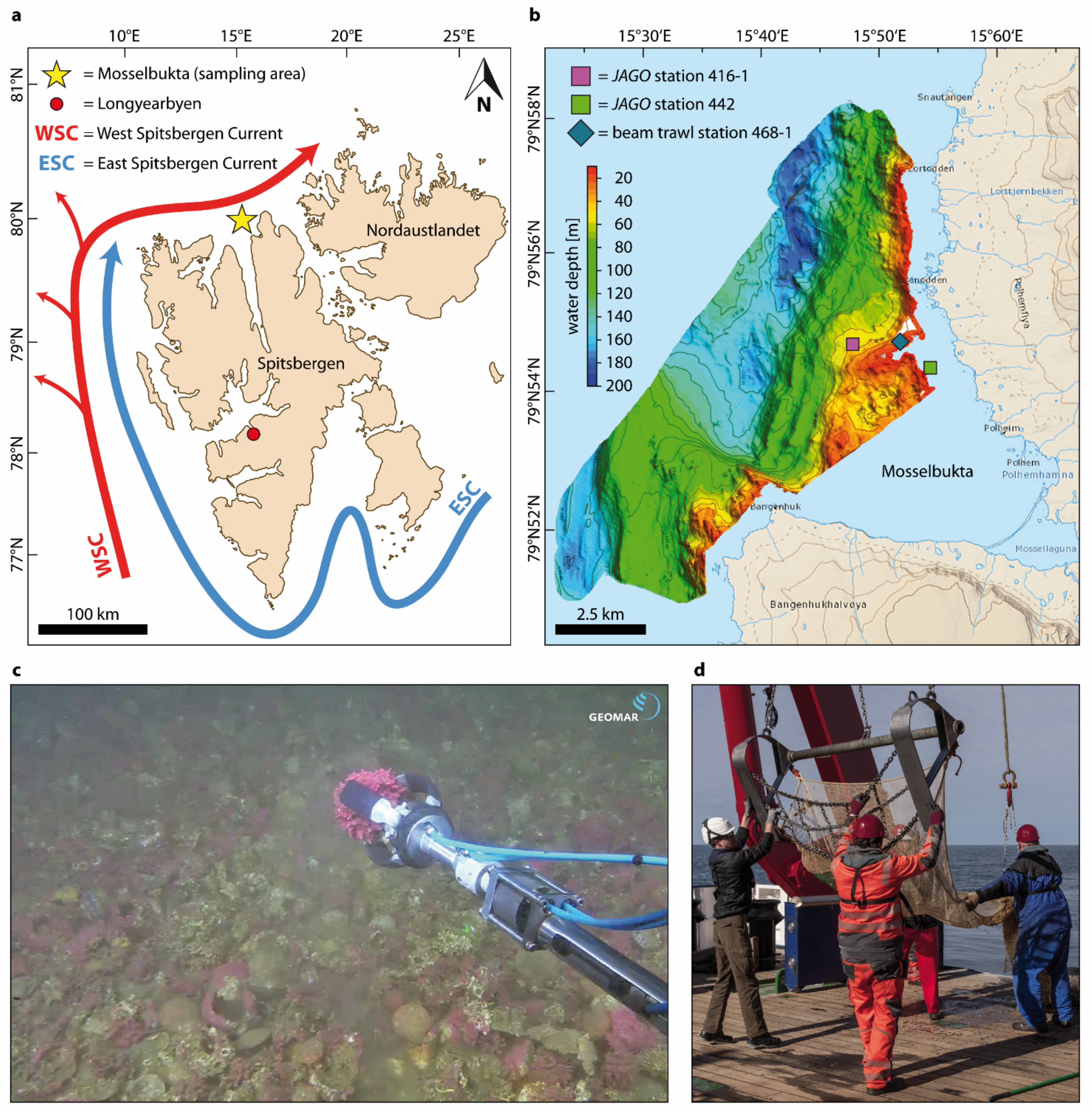
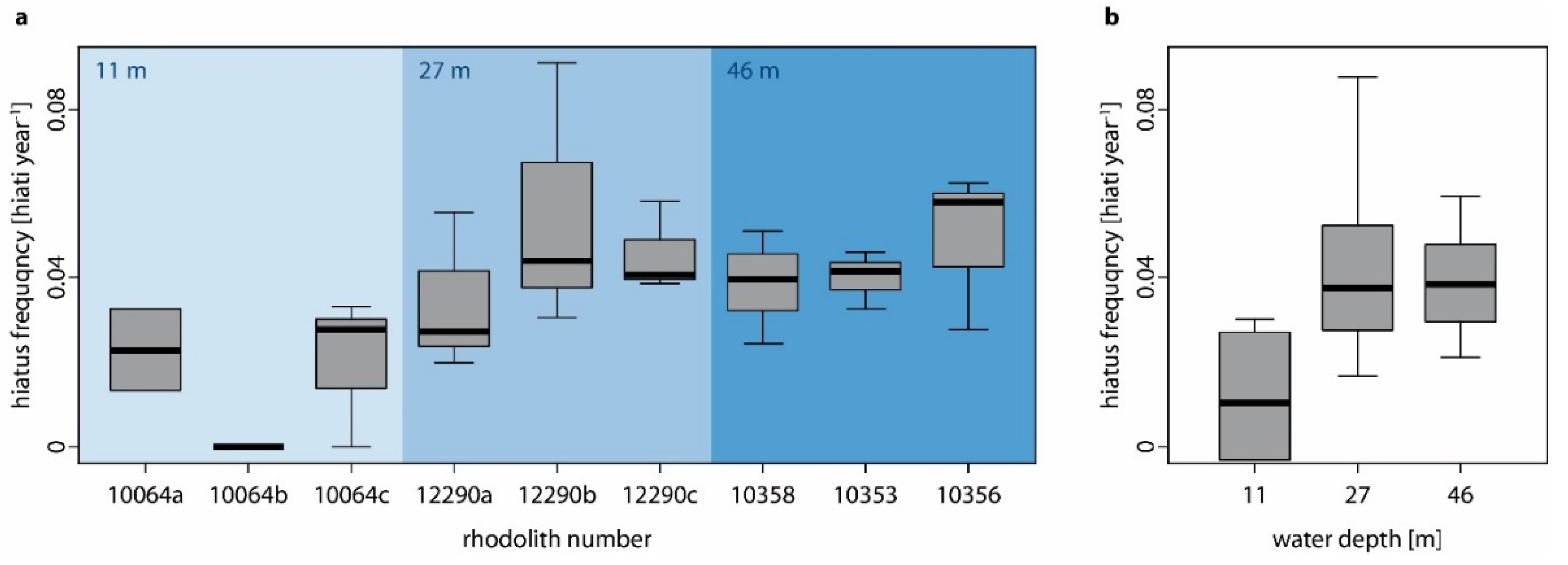

| Rhodolith Nr | Station | Water Depth [m] | Sampling Gear | Latitude | Longitude |
|---|---|---|---|---|---|
| 10064a | MSM55/442 | 11 | submersible | 79°54.85′ N | 15°54.85′ E |
| 10064b | MSM55/442 | 11 | submersible | 79°54.85′ N | 15°54.85′ E |
| 10064c | MSM55/442 | 11 | submersible | 79°54.85′ N | 15°54.85′ E |
| 12290a | MSM55/468-1 | 27 | beam trawl | 79°54.78′ N | 15°52.65′ E |
| 12290b | MSM55/468-1 | 27 | beam trawl | 79°54.78′ N | 15°52.65′ E |
| 12290c | MSM55/468-1 | 27 | beam trawl | 79°54.78′ N | 15°52.65′ E |
| 10358 | MSM55/416-1 | 46 | submersible | 79°54.69′ N | 15°48.61′ E |
| 10353 | MSM55/416-1 | 46 | submersible | 79°54.69′ N | 15°48.61′ E |
| 10356 | MSM55/416-1 | 46 | submersible | 79°54.69′ N | 15°48.61′ E |
| Protuberance Nr | RhodolithNr | Water Depth [m] | Rhodolith Weight [g] | Rhodolith Volume [mm3] | Rhodolith Sphericity | Growth Increments [n] | Hiati [n] | Hiatus Frequency |
|---|---|---|---|---|---|---|---|---|
| 1 | 10064a | 11 | 34.5 | 12,421 | 0.616 | 75 | 1 | 0.0133 |
| 2 | 10064a | 11 | 34.5 | 12,421 | 0.616 | 92 | 3 | 0.0326 |
| 3 | 10064a | 11 | 34.5 | 12,421 | 0.616 | NA | NA | NA |
| 4 | 10064b | 11 | 148.3 | 68,779 | 0.844 | 90 | 0 | 0 |
| 5 | 10064b | 11 | 148.3 | 68,779 | 0.844 | NA | NA | NA |
| 6 | 10064b | 11 | 148.3 | 68,779 | 0.844 | 90 | 0 | 0 |
| 7 | 10064c | 11 | 361.1 | 140,111 | 0.638 | 63 | 0 | 0 |
| 8 | 10064c | 11 | 361.1 | 140,111 | 0.638 | 72 | 2 | 0.0278 |
| 9 | 10064c | 11 | 361.1 | 140,111 | 0.638 | 30 | 1 | 0.0333 |
| 10 | 12290a | 27 | 18.3 | 9245 | 0.771 | 73 | 2 | 0.0274 |
| 11 | 12290a | 27 | 18.3 | 9245 | 0.771 | 50 | 1 | 0.02 |
| 12 | 12290a | 27 | 18.3 | 9245 | 0.771 | 54 | 3 | 0.0556 |
| 13 | 12290b | 27 | 72.9 | 42,505 | 0.387 | 77 | 7 | 0.0909 |
| 14 | 12290b | 27 | 72.9 | 42,505 | 0.387 | 90 | 4 | 0.0444 |
| 15 | 12290b | 27 | 72.9 | 42,505 | 0.387 | 65 | 2 | 0.0308 |
| 16 | 12290c | 27 | 234 | 136,730 | 0.402 | 26 | 1 | 0.0385 |
| 17 | 12290c | 27 | 234 | 136,730 | 0.402 | 69 | 4 | 0.058 |
| 18 | 12290c | 27 | 234 | 136,730 | 0.402 | 74 | 3 | 0.0405 |
| 19 | 10358 | 46 | 75.7 | 32,941 | 0.5 | 122 | 3 | 0.0246 |
| 20 | 10358 | 46 | 75.7 | 32,941 | 0.5 | 39 | 2 | 0.0513 |
| 21 | 10358 | 46 | 75.7 | 32,941 | 0.5 | 101 | 4 | 0.0396 |
| 22 | 10353 | 46 | 135.4 | 64,353 | 0.402 | 122 | 4 | 0.0328 |
| 23 | 10353 | 46 | 135.4 | 64,353 | 0.402 | 87 | 4 | 0.046 |
| 24 | 10353 | 46 | 135.4 | 64,353 | 0.402 | 96 | 4 | 0.0417 |
| 25 | 10356 | 46 | 286.4 | 109,118 | 0.823 | 112 | 7 | 0.0625 |
| 26 | 10356 | 46 | 286.4 | 109,118 | 0.823 | 121 | 7 | 0.0579 |
| 27 | 10356 | 46 | 286.4 | 109,118 | 0.823 | 145 | 4 | 0.0276 |
| Water Depth [m] | Shapiro–Wilk | Levene’s | ANOVA | Kruskal–Wallis | ||||
|---|---|---|---|---|---|---|---|---|
| W | p | F | p | F | p | Chi-Squared | p | |
| 11 | 0.803 | 0.044 | 0.741 | 0.532 | NA | NA | 2.615 | 0.270 |
| 27 | 0.909 | 0.316 | 0.477 | 0.643 | 0.683 | 0.541 | NA | NA |
| 46 | 0.966 | 0.860 | 0.345 | 0.722 | 0.524 | 0.617 | NA | NA |
| Overall Model AIC = −197.18 R2adj = 0.24 p < 0.02 | AIC for Omitting Parameter | Estimate | p |
|---|---|---|---|
| water depth | −193.81 | 0.0006165 | <0.05 |
| sphericity | −196.73 | −0.0324994 | 0.1465 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlüter, M.; Pyko, I.; Wisshak, M.; Schulbert, C.; Teichert, S. Growth Interruptions in Arctic Rhodoliths Correspond to Water Depth and Rhodolith Morphology. Minerals 2021, 11, 538. https://doi.org/10.3390/min11050538
Schlüter M, Pyko I, Wisshak M, Schulbert C, Teichert S. Growth Interruptions in Arctic Rhodoliths Correspond to Water Depth and Rhodolith Morphology. Minerals. 2021; 11(5):538. https://doi.org/10.3390/min11050538
Chicago/Turabian StyleSchlüter, Moritz, Ines Pyko, Max Wisshak, Christian Schulbert, and Sebastian Teichert. 2021. "Growth Interruptions in Arctic Rhodoliths Correspond to Water Depth and Rhodolith Morphology" Minerals 11, no. 5: 538. https://doi.org/10.3390/min11050538
APA StyleSchlüter, M., Pyko, I., Wisshak, M., Schulbert, C., & Teichert, S. (2021). Growth Interruptions in Arctic Rhodoliths Correspond to Water Depth and Rhodolith Morphology. Minerals, 11(5), 538. https://doi.org/10.3390/min11050538