Sequential Extraction and Risk Assessment of Potentially Toxic Elements in River Sediments
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Justification of Study Area Selection
2.2. Reagents, Standard Reference Materials and Standards
2.3. Apparatus and Instrumentation
2.4. Sample Collection and Preservation
2.5. Sample Preparation
2.5.1. Microwave-Assisted Acid Digestion (Method A)
2.5.2. A Sequential Extraction Procedure (Method B)
2.6. Sample Analysis
2.7. Determination of Limit of Detection and Limit of Quantification
2.8. Analytical Data Quality Assurance/Quality Control
2.9. Statistical Analysis
3. Results and Discussion
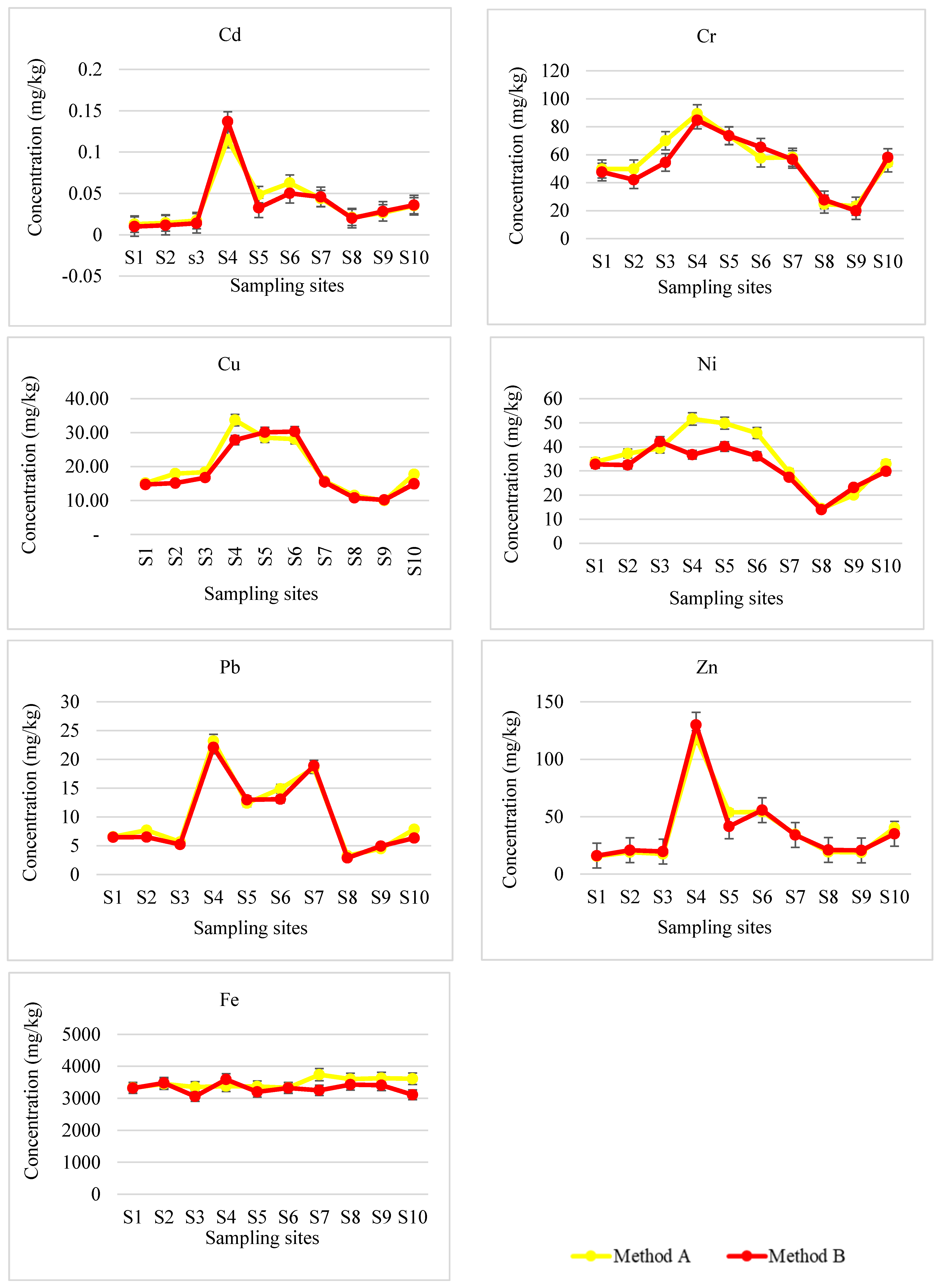
3.1. Performance of the Analytical Method
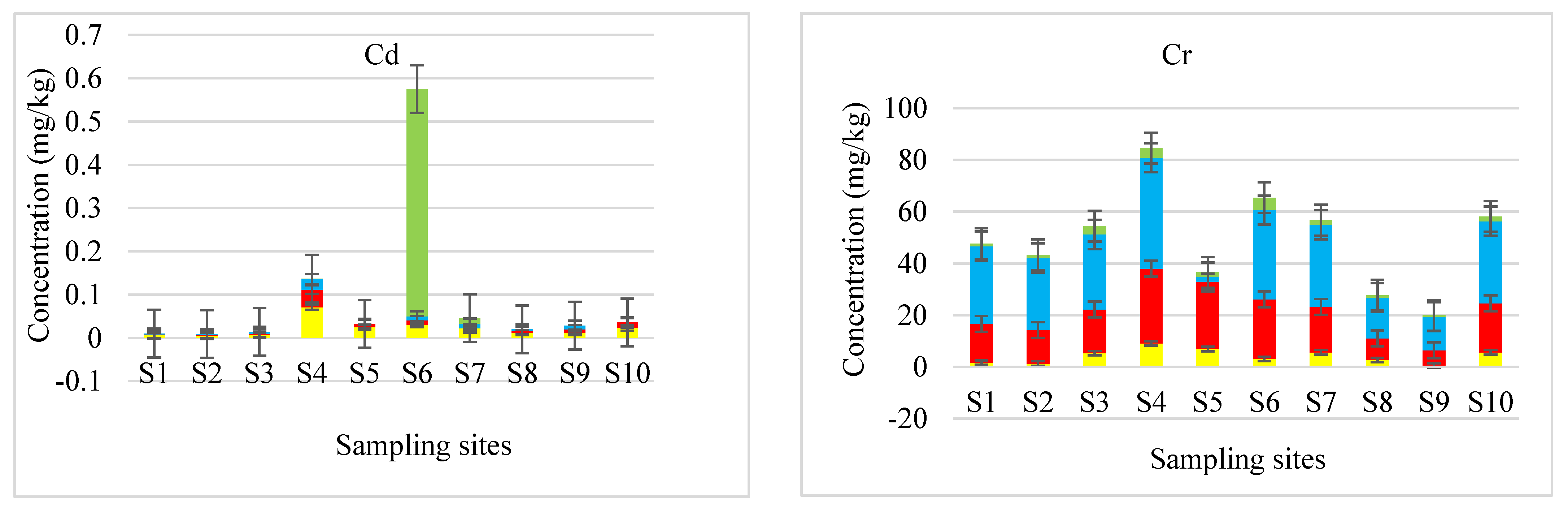
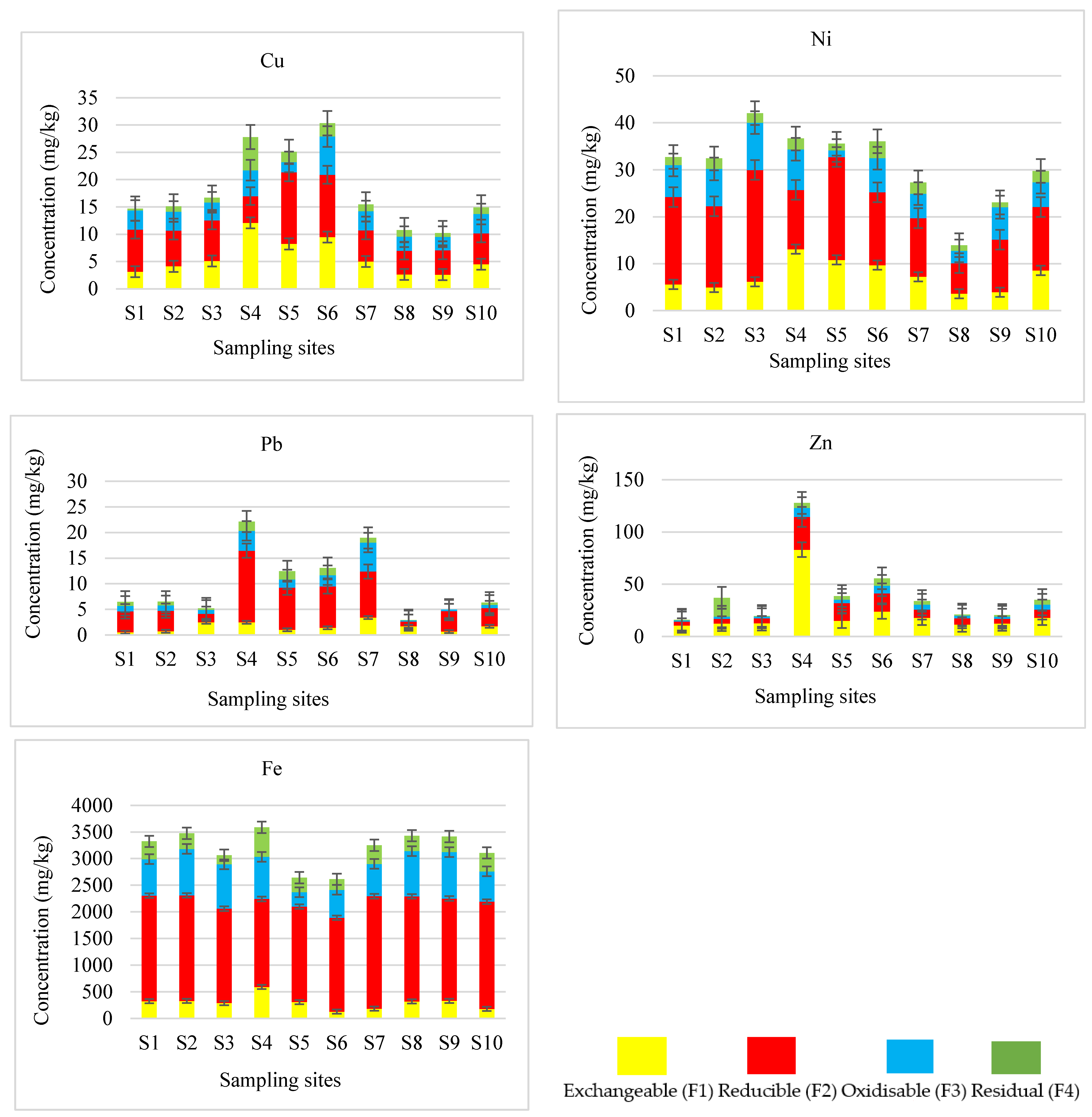
3.2. Fractionation of Potentially Toxic Elements in River Sediments
3.3. The Implications of Sequential Extracted Concentrations to the Environment
3.3.1. Determination of Contamination Factor
3.3.2. The Risk Assessment Code
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, C.-G.; Shi, J.-B.; He, B.; Liu, J.-F.; Liang, L.-N.; Jiang, G.-B. Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ. Int. 2004, 30, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Favas, P.J.C.; Rakshit, D.; Satpathy, K.K. Geochemical Speciation and Risk Assessment of Heavy Metals in Soils and Sediments, Environmental Risk Assessment of Soil Contamination, Maria C. Hernandez-Soriano, IntechOpen. 2014. Available online: https://www.intechopen.com/books/environmental-risk-assessment-of-soil-contamination/geochemical-speciation-andrisk-assessment-of-heavy-metals-in-soils-and-sediments (accessed on 29 April 2019).
- Vetrimurugan, E.; Jonathan, M.; Roy, P.D.; Shruti, V.; Ndwandwe, O. Bioavailable metals in tourist beaches of Richards Bay, Kwazulu-Natal, South Africa. Mar. Pollut. Bull. 2016, 105, 430–436. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, Y.; Gu, J.; Zhao, J. Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Mar. Pollut. Bull. 2013, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, L.; Liu, L.; Shi, W.; Meng, X. Comprehensive risk assessment of heavy metals in lake sediments from public parks in Shanghai. Ecotoxicol. Environ. Saf. 2014, 102, 129–135. [Google Scholar] [CrossRef]
- Bacon, J.R.; Davidson, C. Is there a future for sequential chemical extraction? Analyst 2007, 133, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Hanay, Ö.; Hasar, H.; Kocer, N.N.; Aslan, S. Evaluation for agricultural usage with speciation of heavy metals in a municipal sewage sludge. Bull. Environ. Contam. Toxicol. 2008, 81, 42–46. [Google Scholar] [CrossRef]
- Nemati, K.; Abu Bakar, N.K.; Sobhanzadeh, E.; Abas, M.R. A modification of the BCR sequential extraction procedure to investigate the potential mobility of copper and zinc in shrimp aquaculture sludge. Microchem. J. 2009, 92, 165–169. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, A.N.K.; Mhd Abas, R.B.; Sobhanzadeh, E.; Hin Low, K.H. Comparison of unmodified and modified BCR sequential extraction schemes for the fractionation of heavy metals in shrimp aquaculture sludge from Selangor, Malaysia. Environ. Monit. Assess. 2011, 176, 313–320. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Andrade, M.; Lago-Vila, M.; Rodríguez-Seijo, A.; Vega, F. Sequential extraction of heavy metals in soils from a copper mine: Distribution in geochemical fractions. Geoderma 2014, 230–231, 108–118. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Ashton, P.J. State of the nation report: An overview of the current status of water quality and eutrophication in South African Rivers and Reservoirs. Counc. Sci. Ind. Res. 2008, 3, 1–7. [Google Scholar]
- Bologo, V.; Maree, J.; Carlsson, F. Application of magnesium hydroxide and barium hydroxide for the removal of metals and sulphate from mine water. Water SA 2012, 38, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Fatoki, O.; Lujiza, N.; Ogunfowokan, A.; Fatoki, O.; Lujiza, N.; Ogunfowokan, A. Trace metal pollution in Umtata River. Water SA 2002, 28, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Letsoalo, M.R.; Godeto, T.W.; Magadzu, T.; Ambushe, A.A. Selective speciation of inorganic arsenic in water using nanocomposite based solid-phase extraction followed by inductively coupled plasma-mass spectrometry detection. J. Environ. Sci. Health Part A 2019, 54, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Polokwane Municipality Report. Integrated Development Planning (IDP) Report for Polokwane Municipality (2012–2013); Polokwane Municipal Council: Polowane, South Africa, 2012. [Google Scholar]
- Rauret, G.; López Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P.H. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediments and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.L.A.; Alonso, E.V.; Cordero, M.T.S.; Pavon, J.M.C.; De Torres, A.G. Fractionation of heavy metals in sediments by using microwave assisted sequential extraction procedure and determination by inductively coupled plasma mass Spectroscopy. Microchem. J. 2011, 98, 234–239. [Google Scholar] [CrossRef]
- Melaku, S.; Wondimu, T.; Dams, R.; Moens, L. Multi-element analysis of Tinishu river sediments, ethiopia by ICP-MS after microwave assisted digestion. Can. J. Anal. Sci. Spectrosc. 2005, 50, 31–40. [Google Scholar]
- Ciceri, E.; Giussani, B.; Pozzi, A.; Dossi, C.; Recchia, S. Problems in the application of the three-step BCR sequential extraction to low amounts of sediments: An alternative validated route. Talanta 2008, 76, 621–626. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Contract Laboratory Program for National Functional Guidelines for Inorganic Data Review; United States EPA: Washington, DC, USA, 2010. [Google Scholar]
- Saleem, M.; Iqbal, J.; Shah, M.H. Geochemical speciation, anthropogenic contamination, risk assessment and source identification of selected metals in freshwater sediments—A case study from Mangla Lake, Pakistan. Environ. Nanotechnol. Monit. Manag. 2015, 4, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Marmolejo-Rodríguez, A.J.; Prego, R.; Meyer-Willerer, A.; Shumilin, E.; Cobelo-Garcia, A. Total and labile metals in surface sediments of the tropical river-estuary system of Marabasco (Pacific coast of Mexico): Influence of an iron mine. Mar. Pollut. Bull. 2007, 55, 459–468. [Google Scholar] [CrossRef]
- Shozi, M. Assessing the Distribution of Sedimentary Heavy Metals in the Msunduzi River Catchment, Kwazulu-Natal, South Africa. Master’s Thesis, University of KwaZulu-Natal, Durban, South Africa, 2015. [Google Scholar]
- Diaz-De Alba, M.; Galindo-Riano, M.D.; Casanueva-Marenco, M.J.; Garcia-Vargas, M.; Kosore, C.M. Assessment of the metal pollution, potential toxicity and speciation of sediments from Algeciras Bay (South of Spain) using chemometric tools. J. Hazard. Mater. 2011, 190, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Barba-Brioso, C.; Nieto, J.M.; Boski, T. Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (Guadiana saltmarshes, SW Iberian Peninsula): I. Surficial sediments. Sci. Total Environ. 2011, 409, 3666–3679. [Google Scholar] [CrossRef]
- Kong, M.; Dong, Z.; Chao, J.; Zhang, Y.; Yin, H. Bioavailability and ecological risk assessment of heavy metals in surface sediments of Lake Chaohu. China Environ. Sci. 2015, 35, 1223–1229. [Google Scholar]
- Li, Q.; Wu, Z.; Chu, B.; Zhang, N.; Cai, S.; Fang, J. Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ. Pollut. 2007, 149, 158–164. [Google Scholar] [CrossRef]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, Q.; Shao, S.; Zhang, N.; Shen, Q.; Liu, C. Heavy metal pollution, fractionation, and potential ecological risks in sediments from Lake Chaohu (Eastern China) and the surrounding rivers. Int. J. Environ. Res. Public Health 2015, 12, 14115–14131. [Google Scholar] [CrossRef] [Green Version]
- Passos, E.A.; Alves, J.C.; Santos, I.S.; Alves, J.P.; Garcia, C.A.B.; Costa, A.C.S. Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem. J. 2010, 96, 50–57. [Google Scholar] [CrossRef]
- Borgese, L.; Federici, S.; Zacco, A.; Gianoncelli, A.; Rizzo, L.; Smith, D.R.; Donna, F.; Lucchini, R.; Depero, L.E.; Bontempi, E. Metal fractionation in soils and assessment of environmental contamination in Vallecamonica, Italy. Environ. Sci. Pollut. Res. 2013, 20, 5067–5075. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Banerjee, D.K. Metal phase association of chromium in contaminated soils from an industrial area in Delhi. Chem. Speciat. Bioavailab. 2004, 16, 145–151. [Google Scholar] [CrossRef]
- Martin, J.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 178–206. [Google Scholar] [CrossRef]
- Wali, A.; Colinet, G.; Ksibi, M. Speciation of heavy metals by modified BCR sequential extraction in soils contaminated by phosphogypsum in Sfax, Tunisia. Environ. Res. Eng. Manag. 2015, 70, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Davutluoglu, O.I.; Seckin, G.; Ersu, C.B.; Yilmaz, T.; Sari, B. Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J. Environ. Manag. 2011, 92, 2250–2259. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Pratas, J.; Gomez, M.; Elisa, P.; Cala, V. Selective chemical extraction of heavy metals in tailings and soils contaminate by mining activity: Environmental implications. J. Geochem. Explor. 2011, 111, 160–171. [Google Scholar] [CrossRef]
- Tuzen, M. Determination of trace metals in the river Yesilirmak Sediments in Tokat, Turkey using sequential extraction procedure. Microchem. J. 2003, 74, 105–110. [Google Scholar] [CrossRef]
- Jain, C. Metal fractionation study on bed sediments of River Yamuna, India. Water Res. 2004, 38, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, Z.; Wai, O.; Li, Y. Chemical forms of Pb, Zn and Cu in the sediments profiles of the Peral River Estuary. Mar. Pollut. Bull. 2001, 42, 215–223. [Google Scholar] [CrossRef]
- Wong, C.S.; Wu, S.; Duzgoren-Aydin, N.S.; Aydin, A.; Wong, M.H. Trace metal contamination of sediments in an e-waste processing village in China. Environ. Pollut. 2007, 145, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Feng, C.; Yang, Y.; Niu, J.; Shen, Z. Risk assessment of sedimentary metals in the Yangtze Estuary: New evidence of the relationships between two typical index methods. J. Hazard. Mater. 2012, 241–242, 164–172. [Google Scholar] [CrossRef]
- Houben, D.; Sonnet, P. Zinc mineral weathering as affected by plant roots. Appl. Geochem. 2012, 27, 1587–1592. [Google Scholar] [CrossRef]
- Okonkwo, J.O.; Mothiba, M. Physico-chemical characteristics and pollution levels of heavy metals in the rivers in Thohoyandou, South Africa. J. Hydrol. 2005, 308, 122–127. [Google Scholar] [CrossRef]
- Nasr, S.M.; Okbah, M.A.; El Haddad, H.S.; Soliman, N.F. Fractionation profile and mobility pattern of metals in sediments from the Mediterranean Coast, Libya. Environ. Monit. Assess. 2015, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nemati, K.; Abu Bakar, N.K.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Ikem, A.; Egiebor, N.O.; Nyavor, K. Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut. 2003, 149, 51–75. [Google Scholar] [CrossRef]
- Perin, G.; Craboleda, L.; Lucchese, M.; Cirillo, R.; Dotta, L.; Zanette, M.L.; Orio, A.A. Heavy metal speciation in the sediments of Northern Adriatic Sea- a new approach for environmental toxicity determination. In Heavy Metal in the Environment; Lakkas, T.D., Ed.; New Age International: Delhi, India, 1985; pp. 454–456. [Google Scholar]
- Mortazavi, S.; Attaeian, B.; Abdolkarimi, S. Risk assessment and environmental geochemistry of Pb, Cu and Fe in surface sediments (case study: Hashilan Wetland, Kermanshah, Iran). Ecopersia 2016, 4, 1411–1424. [Google Scholar] [CrossRef]
- Saeedi, M.; Li, L.Y.; Karbassi, A.; Zanjani, A.J. Sorbed metals fractionation and risk assessment of release in river sediment and particulate matter. Environ. Monit. Assess. 2012, 185, 1737–1754. [Google Scholar] [CrossRef] [PubMed]

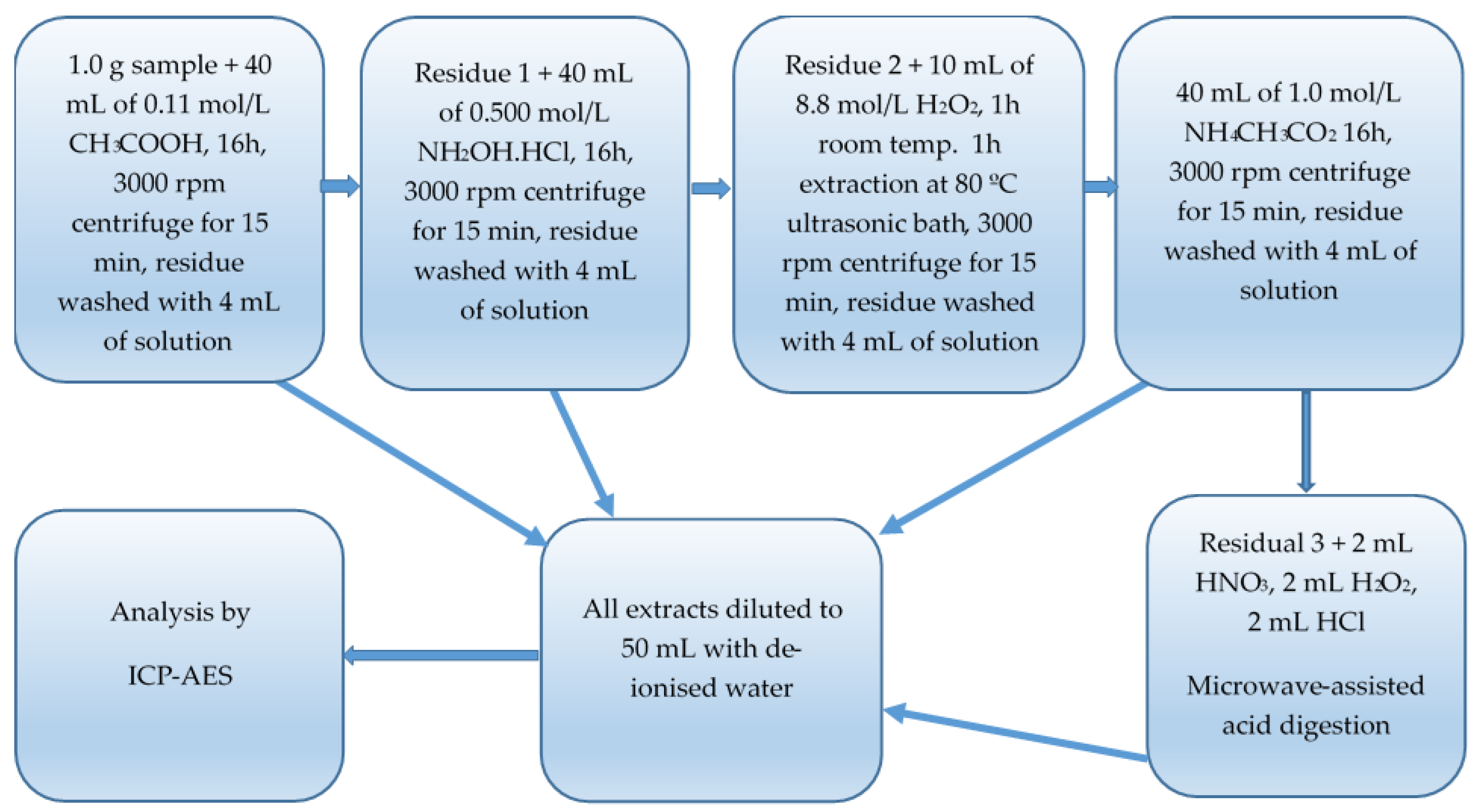
| ICP-MS Parameters | ICP-MS Operating Conditions |
|---|---|
| Lens voltage (V) | 10 |
| RF power (W) | 1150 |
| Flow rate of plasma gas (L·min−1) | 12 |
| Flow rate of nebuliser gas (L·min−1) | 0.95 |
| Flow rate of auxiliary gas (L·min−1) | 1.2 |
| ICP-AES Parameters | ICP-AES Operating Conditions |
| RF power (W) | 1200 |
| Flow rate of plasma gas (L·min−1) | 10 |
| Flow rate of nebulizer gas (L·min−1) | 0.7 |
| Flow rate of auxiliary gas (L·min−1) | 0.60 |
| View direction | Axial |
| View position | Low |
| F-AAS Parameters | F-AAS Operating Conditions |
| Flow rate of air (L·min−1) | 10.0 |
| Flow rate of acetylene (L·min−1) | 3.30 |
| Microwave-Assisted Digestion Procedure | BCR Sequential Extraction Procedure | |||||
|---|---|---|---|---|---|---|
| Element | LOD (ng·g−1) | LOQ (ng·g−1) | Stage 1 (mg·kg−1) | Stage 2 (mg·kg−1) | Stage 3 (mg·kg−1) | Residual (mg·kg−1) |
| Cd | 0.030 | 0.10 | 0.008 | 0.038 | 0.0064 | 0.125 |
| Cr | 0.11 | 0.37 | 0.218 | 0.040 | 0.0064 | 0.041 |
| Cu | 0.48 | 1.60 | 0.008 | 0.013 | 0.0742 | 0.022 |
| Fe | 0.049 a | 0.16 a | 0.087 | 0.008 | 0.0573 | 0.005 |
| Ni | 0.62 | 2.08 | 0.017 | 0.004 | 0.0658 | 0.003 |
| Pb | 0.17 | 0.57 | 0.276 | 0.169 | 0.503 | 0.026 |
| Zn | 1.77 | 5.90 | 0.023 | 0.004 | 0.0806 | 0.041 |
| Element | BCR-280R | SRM 8704 | ||||
|---|---|---|---|---|---|---|
| Measured | Certified | Percentage Recovery | Measured | Certified | Percentage Recovery | |
| Mean ± SD (mg·kg−1) | Mean ± SD (mg·kg−1) | (%) | Mean ± SD (mg·kg−1) | Mean ± SD (mg·kg−1) | (%) | |
| Cd | 0.912 ± 0.002 | 0.85 ± 0.10 | 107 | 3.33 ± 0.023 | 2.94 ± 0.29 | 113 |
| Cr | 111 ± 14 | 126 ± 7.0 | 88 | na | ||
| Cu | 61.4 ± 0.63 | 53.0 ± 6.0 | 116 | NA | ||
| Fe | NA | 3.69 ᵇ | 3.97 ± 0.10 | 93 | ||
| Ni | 63.3 ± 2.6 | 69.0 ± 5.0 | 92 | 38.6 ± 10 | 42.9 ± 3.7 | 90 |
| Pb | NA | 210 ± 3.8 | 150 ± 17 | 140 | ||
| Zn | 208 ± 0.91 | 224 ± 25 | 93 | 402 ± 2.0 | 408 ± 15 | 98 |
| Fraction | Element | Certified Value | Measured Value | Percentage Recovery |
|---|---|---|---|---|
| Mean ± SD (mg·kg−1) | Mean ± SD (mg·kg−1) | (%) | ||
| F1 | Cd | 7.30 ± 0.40 | 7.13 ± 0.25 | 98 |
| Cr | 2.26 ± 0.16 | 2.69 ± 0.050 | 119 | |
| Cu | 49.3 ± 1.7 | 52.7 ± 3.0 | 107 | |
| Ni | 15.4 ± 0.9 | 13.2 ± 0.28 | 86 | |
| Pb | 3.18 ± 0.21 | 2.87 ± 0.092 | 90 | |
| Zn | 205 ± 6.0 | 223 ± 3.5 | 109 | |
| F2 | Cd | 3.77 ± 0.28 | 3.05 ± 0.36 | 81 |
| Cr | 45.7 ± 2.0 | 50.3 ± 1.0 | 110 | |
| Cu | 124 ± 3.0 | 126 ± 4.0 | 101 | |
| Ni | 26.6 ± 1.3 | 25.8 ± 2.3 | 97 | |
| Pb | 126 ± 3.0 | 107 ± 18 | 85 | |
| Zn | 114 ± 5.0 | 126 ± 9.3 | 111 | |
| F3 | Cd | 0.27 ± 0.06 | 0.218 ± 0.010 | 81 |
| Cr | 143 ± 7.0 | 110 ± 4.0 | 77 | |
| Cu | 55.0 ± 4.0 | 63.1 ± 2.1 | 115 | |
| Ni | 15.3 ± 0.9 | 18.2 ± 0.78 | 119 | |
| Pb | 9.30 ± 2.0 | 9.25 ± 0.49 | 99 | |
| Zn | 46.0 ± 4.0 | 46.5 ± 2.7 | 101 |
| Element | ICF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | Average | |
| Cd | 0.0802 | 0.0724 | 0.112 | 108 | 0.260 | 0.402 | 2.600 | 0.161 | 0.227 | 0.287 | 11.4 |
| Cr | 48.9 | 33.7 | 15.7 | 21.4 | 40.4 | 12.5 | 31.4 | 29.6 | 34.0 | 31.1 | 29.9 |
| Cu | 42.4 | 15.0 | 18.2 | 3.6 | 15.0 | 11.5 | 11.7 | 7.97 | 14.6 | 11.2 | 15.1 |
| Fe | 8.98 | 10.8 | 16.9 | 5.5 | 10.8 | 12.1 | 8.3 | 10.9 | 10.7 | 7.9 | 10.3 |
| Ni | 18.1 | 13.5 | 20.0 | 14.8 | 27.2 | 9.10 | 10.4 | 10.6 | 21.8 | 11.4 | 15.7 |
| Pb | 7.05 | 8.27 | 16.5 | 11.3 | 7.02 | 8.35 | 19.9 | 188 | 190 | 13 | 46.9 |
| Zn | 26.6 | 1.06 | 30.4 | 26.5 | 11.3 | 7.08 | 9.05 | 21.9 | 12.2 | 7.0 | 15.3 |
| GCF | 152 | 82 | 118 | 191 | 112 | 61 | 93 | 269 | 283 | 82 | 144 |
| Element | RAC (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | Average | |
| Cd | 4.54 | 3.81 | 4.42 | 51.7 | 89.1 | 17.5 | 48.4 | 8.48 | 8.15 | 14.3 | 25.0 |
| Cr | 3.55 | 3.02 | 9.70 | 10.7 | 9.41 | 4.72 | 9.95 | 9.45 | 2.98 | 9.70 | 7.32 |
| Cu | 21.4 | 27.3 | 30.6 | 43.5 | 27.3 | 31.2 | 32.5 | 24.3 | 25.6 | 30.1 | 29.4 |
| Fe | 9.78 | 9.46 | 9.44 | 16.5 | 9.62 | 4.90 | 5.63 | 9.33 | 9.66 | 5.79 | 9.01 |
| Ni | 17.1 | 15.2 | 14.6 | 35.7 | 27.0 | 26.9 | 26.4 | 25.9 | 17.1 | 28.8 | 23.5 |
| Pb | 7.85 | 10.8 | 47.5 | 11.1 | 7.85 | 10.8 | 18.0 | 58.0 | 13.9 | 26.9 | 21.3 |
| Zn | 65.4 | 33.2 | 64.2 | 65.0 | 36.2 | 43.0 | 53.0 | 54.8 | 60.1 | 51.5 | 52.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matabane, D.L.; Godeto, T.W.; Mampa, R.M.; Ambushe, A.A. Sequential Extraction and Risk Assessment of Potentially Toxic Elements in River Sediments. Minerals 2021, 11, 874. https://doi.org/10.3390/min11080874
Matabane DL, Godeto TW, Mampa RM, Ambushe AA. Sequential Extraction and Risk Assessment of Potentially Toxic Elements in River Sediments. Minerals. 2021; 11(8):874. https://doi.org/10.3390/min11080874
Chicago/Turabian StyleMatabane, Dithobolong L., Taddese W. Godeto, Richard M. Mampa, and Abayneh A. Ambushe. 2021. "Sequential Extraction and Risk Assessment of Potentially Toxic Elements in River Sediments" Minerals 11, no. 8: 874. https://doi.org/10.3390/min11080874
APA StyleMatabane, D. L., Godeto, T. W., Mampa, R. M., & Ambushe, A. A. (2021). Sequential Extraction and Risk Assessment of Potentially Toxic Elements in River Sediments. Minerals, 11(8), 874. https://doi.org/10.3390/min11080874







