1. Introduction
Bentonites usually contain clay minerals (predominantly montmorillonite and secondarily kaolinite and illite), cristobalite, carbonates, zeolites, iron oxides and hydroxides, and relics of quartz and feldspar. They are widely used in their native forms, or after refinement, purification, or activation in many areas, because of high absorption rates displayed towards water or organic compounds, high cation exchange capacity and protein sedimentation properties [
1,
2,
3,
4]. The name bentonite was proposed for the first time for the cretaceous clays found in the formations from Benton, Wyoming, USA by Knight [
5]. Montmorillonite, its principal mineralogical component, was discovered in the type locality of Montmorillon, Vienne, Poitou-Charentes, France.
The main mineral component of bentonites is montmorillonite, a member of the smectite group, a hydrated di-octahedral alumino-silicate. Its general formula can be written as (Na,Ca)
0.33(Al,Mg)
2(Si
4O
10)(OH)
2·nH
2O. In contact with water, it can reversibly swell up to 15 times of its original volume, forming gelatinous colloidal suspensions or plastic films [
6]. Subordinate minerals can be cristobalite, tridymite, feldspar, halloysite, kaolinite [
7,
8]. The various forms of silica present in the bentonite deposits can be opal, cristobalite and quartz. Opaline silica is not considered to be harmful to humans, in contrast to cristobalite [
9].
In Romania, bentonitic clays appear in large quantities, with important deposits in the Transylvanian Basin (Răzoare, Valea Chioarului, Oraşu Nou, Ocna Mureş, Ciugud, Sănduleşti-Petreşti, Gurasada), or in western Romania’s Banat region (Brebu, Breaza), [
10].
Important bentonite clay mineral resources are available around Oraşu Nou in NE Romania [
11]. This deposit is situated at 30 km from the municipal cities of Baia Mare and Satu Mare, at the entrance of the Oaș region’s plateau. This area was explored with drillings revealing high quantities of bentonitic rocks. The bentonite bearing geological formations were studied 50 years ago [
12]. Some quarries were opened but nowadays the exploitation slowed to a halt, because of non-sustainable results. In this whole period, there was no complete description of physical, chemical, and structural properties of the minerals contained in the bentonite deposit, using modern physico-chemical methods. Therefore, no evaluation of their utility in a high-value refined, purified, or activated form exists, with modern nano- and microscale applications. Bentonite consists mainly of montmorillonite, with cristobalite as subordinate mineral, along with small amounts of quartz, feldspar, halloysite, kaolinite [
11]. From a mineralogical point of view, chemical alterations transformed the volcanic rocks in clay minerals, while the montmorillonite was the prevailing one [
13]. This mineral has formed through the break-down of feldspars, of the volcanic ash, and of the volcanic glass from the perlites.
Similar bentonite resources can be found in the Carpathian area such as those from the Hliník nad Hronom deposit in Slovakia [
7], but also in the world in Spain and in Turkey [
8,
14].
Bentonites in various refinement stages are used in over 30 industrial branches or in environmental protection [
3,
15,
16]. Its pharmaceutical applications are well known, for example under the commercial name “Smecta”, as an antidiarrheic, anti-flatulent, bowel protective against viruses and bacteria, and as a detoxifying agent [
10].
The resources of this region represent an important bentonite source. The possibility of open-pit extraction represents another important advantage. The properties and industrial applications of bentonites are a consequence of their mineralogy. The CEC capacity and the high plasticity index give them the possibility to be used in many fields [
17].
The main part of bentonites extracted on a world scale is used in foundries and as drilling-mud [
18]. Smaller quantities are used in special applications. According to Kuzvart [
19] and Patterson and Murray [
20], bentonites are used as ingredients in ceramics, in waterproofing or as sealant in civilian construction; petrochemistry, as a catalyst in raw petrol refinement and during the filtration of the products obtained after fractional distillation; to obtain white or Portland cement.
Meunier [
16] recommends using bentonites in the paper industry to obtain white glossy paper; in pharmaceutical and cosmetics industry in obtaining liniments, wet compresses, soaps, make-ups and powders, creams; in the food industry for the purification or as a rinsing agent for water, wine, beer, vegetable-oils.
In agriculture, it enhances sandy soils, helping water retention, and, after drying-out, soil aeration; or it can be used as a feed supplement for animals [
21,
22]. Plant nutrition is also sustained by intermediation of base ion exchange, gradual fertilizer desorption and retention of pesticides [
23,
24]. Bentonites with a high montmorillonite content similar to the one found in Oraşu Nou can be used to immobilize fungicides or insecticides [
25]. Application of Ca-bentonite from Oraşu Nou as a soil amendment, can reduce soil water losses, significantly increase microbial biomass parameters, and crop yield [
26]. Its capacity to absorb pesticides was applied to detoxification of paraquat poisoning victims [
27].
The use of bentonites in environmental protection is reduced when compared to that of natural zeolites. Bentonites mixed with zeolites have been used to inactivate heavy metals in polluted soils, especially zinc [
28]. They can also be used in pollutant removal processes for industrial or civilian residual waters [
29,
30], for the purification of industrial gases, protection of waste storage areas [
31], including nuclear waste storages [
32,
33]. Bentonites from the Oraşul Nou deposit can be considered a potential buffer material for isolation of radioactive waste, where they can be used due to their low permeability and high CEC [
34]. Bentonite blocks serve as a sealing material around waste canisters to isolate them from the surrounding atmosphere [
35]. Recent research applied bentonite-based materials for the absorption of ammonium or heavy metal ions from wastewaters, especially for the retention of chromium [
36].
2. Geological Setting
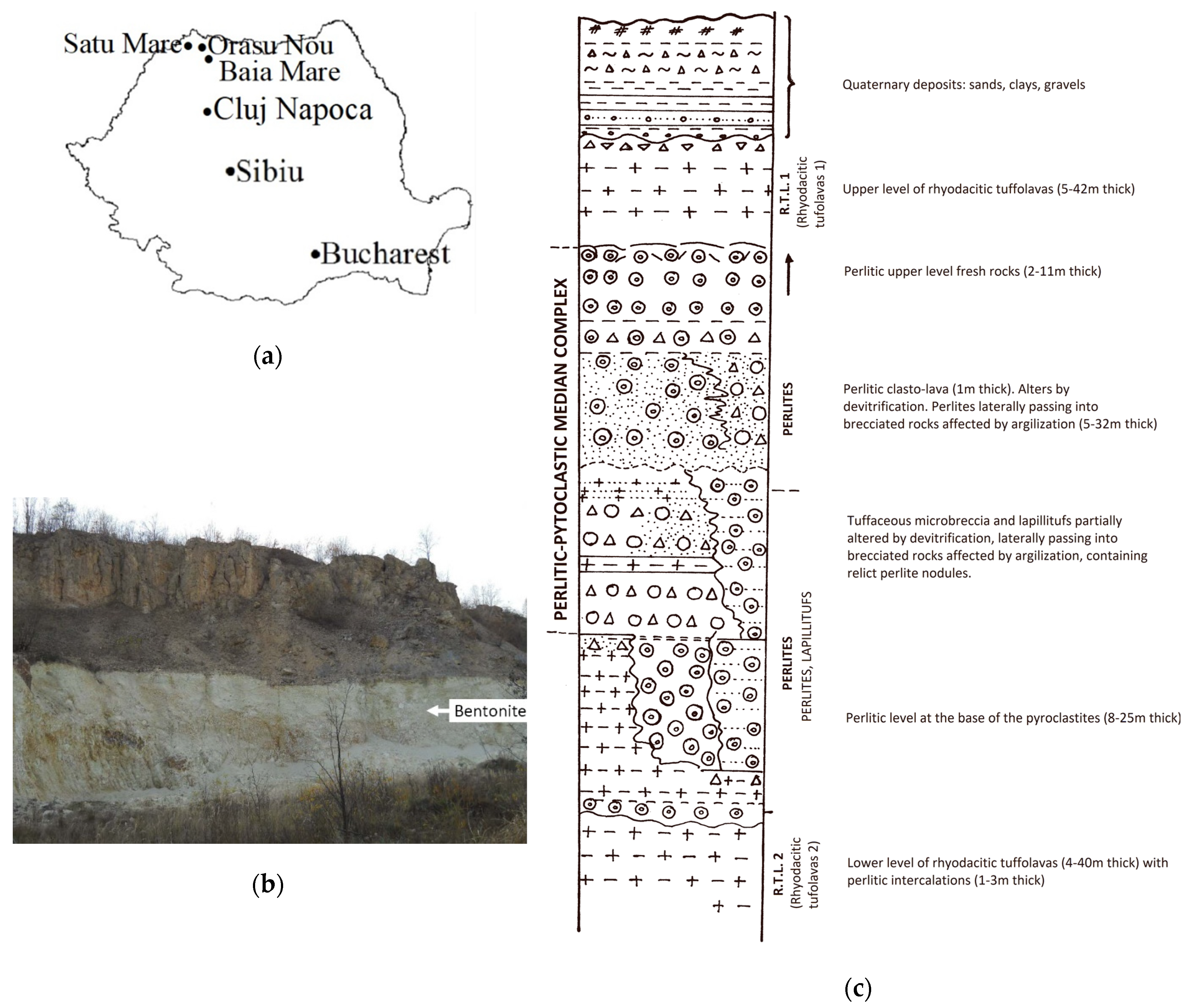
The geological formations from the Orașu Nou region (
Figure 1a) are of Neogene age and they consist of sedimentary and volcanic deposits [
12]. The volcanic formations are lava flows intercalated in sedimentary formations, that Sagatovici [
37] and Bârlea [
12] identified as being of Badenian age. Later, Rădan et al. [
38] established that they were of Pannonian age.
The bentonite resources are hosted in volcanic formation. In
Figure 1b. the volcanic rock deposits, with thicknesses up to 300 m, are represented by ignimbrite, perlite-facies rhyodacites and pyroclastic rocks from the Pannonian age. The bentonite deposits resulted from the alteration of the pyroclastic and perlite layers by hydrothermal-deuteric alteration.
The litho-stratigraphic column representation in
Figure 1.c shows that rhyodacite and tuffo-lava layers appear in ignimbritic facies in the lower (R.T.L. 2) and upper parts (R.T.L. 1). The median complex level is perlitic, consisting of lapilli and cinerite tuffs intercalated between two layers of rhyodacite perlites. The pyroclastic rock fragments are represented by hyaline rocks, volcanic rocks, metamorphic rocks, marls, and siltstones. The matrix of pyroclastic rocks is made of cinerite material. The rhyodacite perlites contain up to 95% volcanic glass as their main constituent and plagioclase felspars, sanidine, quartz and biotite. The median complex is pervasively substituted with montmorillonite and cristobalite forming the bentonite rocks, as can be seen in the outcrop in
Figure 1b. The deposits have lenticular shape 100–400 m long, 50–250 m wide, and 3–15 m thick. In these deposits, the bentonite rocks have a white color, a compact aspect and mainly a perlite structure.
5. Conclusions
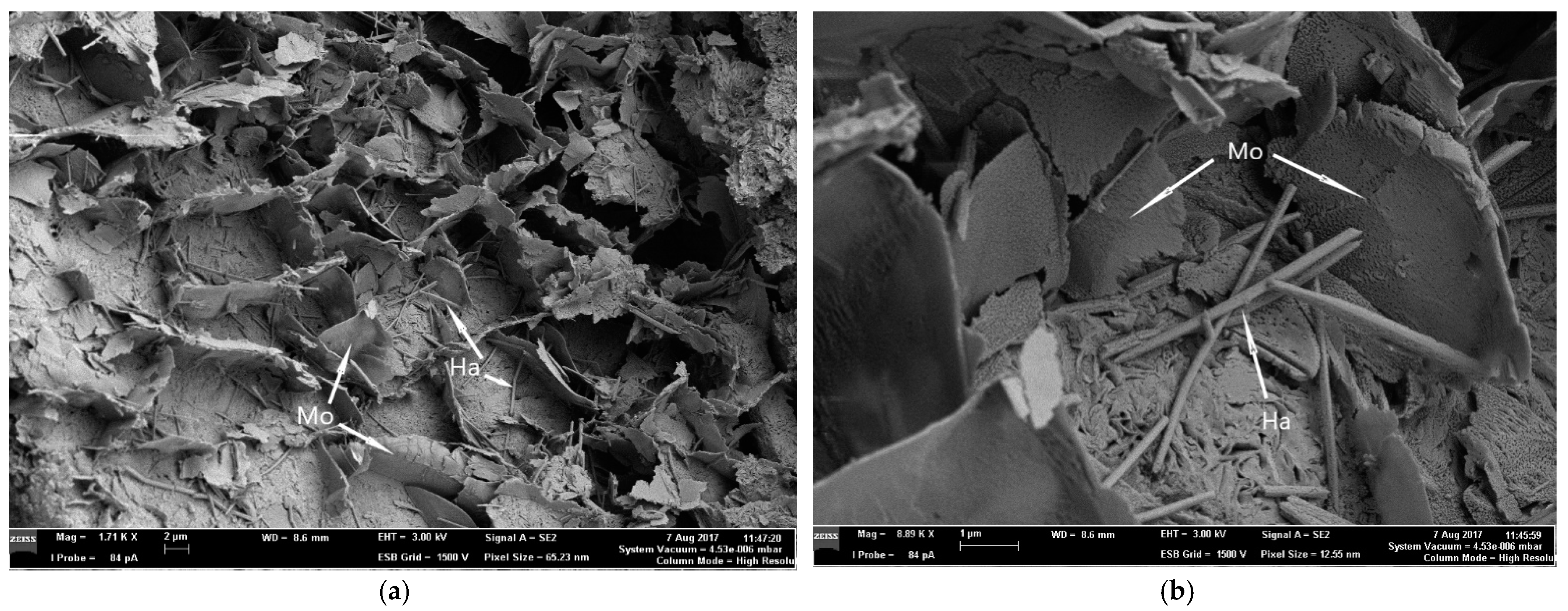
This is a general study using physico-chemical methods on the mineralogical composition and possible applications according to the determined physico-chemical properties of the bentonitized rocks and the white bentonite along its nano-/microstructured separate prepared by us, from the deposit near Orașu-Nou (Romania). The main mineral found in this bentonite deposit is montmorillonite. Beside it, small quantities of kaolinite, halloysite, illite, cristobalite, quartz, carbonates, and iron oxyhydroxides appear. This study revealed that the deposit formed after a hydrothermal-deuteric alteration of ignimbrite and pyroclastic volcanic rocks. The bentonitic material’s average pH is just below neutral, around 6.2, and its cation exchange capacity is averaged to be 45.89 cmol/kg. XRD, FT-IR and thermogravimetric studies of the hand-milled sample give characteristic results for montmorillonite, leading to a mainly Ca-type one and subordinately a Na-type. SEM imaging of the raw mineral proves the presence of lamellar montmorillonite and indicates possible small quantities of halloysite and other silica polymorphs. Halloysite was determined using XRD and from the SEM micrographs as nano-/microscale rod-like structures. Low temperature cristobalite can be identified in most of our XRD patterns, while its presence was conformed using thermogravimetric analysis. This mineral can be found in large quantities in the coarse fraction, mainly in the poorly bentonitized rocks, where lower montmorillonite concentrations were also determined. In relatively lower percentages it can be also found in the bentonites, but it is also present in low quantities in the finest fractions.
This work proves the presence of a high quality, easily and inexpensively refinable, high Ca-montmorillonite content bentonite deposit, especially when we are talking about the white-colored raw material. Because the immediate neighborhood of this deposit is also a region rich in this mineral, which, moreover, can be exploited using open-pit mining, real opportunities are opened for a feasible harness of this resource and its use in many industrial branches, agriculture, environmental protection, or in modern applications of nano-/microscale materials.