Mineralogy, Fluid Inclusion, and C-O-Sr Isotope Geochemistry to Unravel the Evolution of the Magmatic-Hydrothermal System at the Igoudrane Silver-Rich Deposit (Imiter District, Eastern Anti-Atlas, Morocco)
Abstract
:1. Introduction
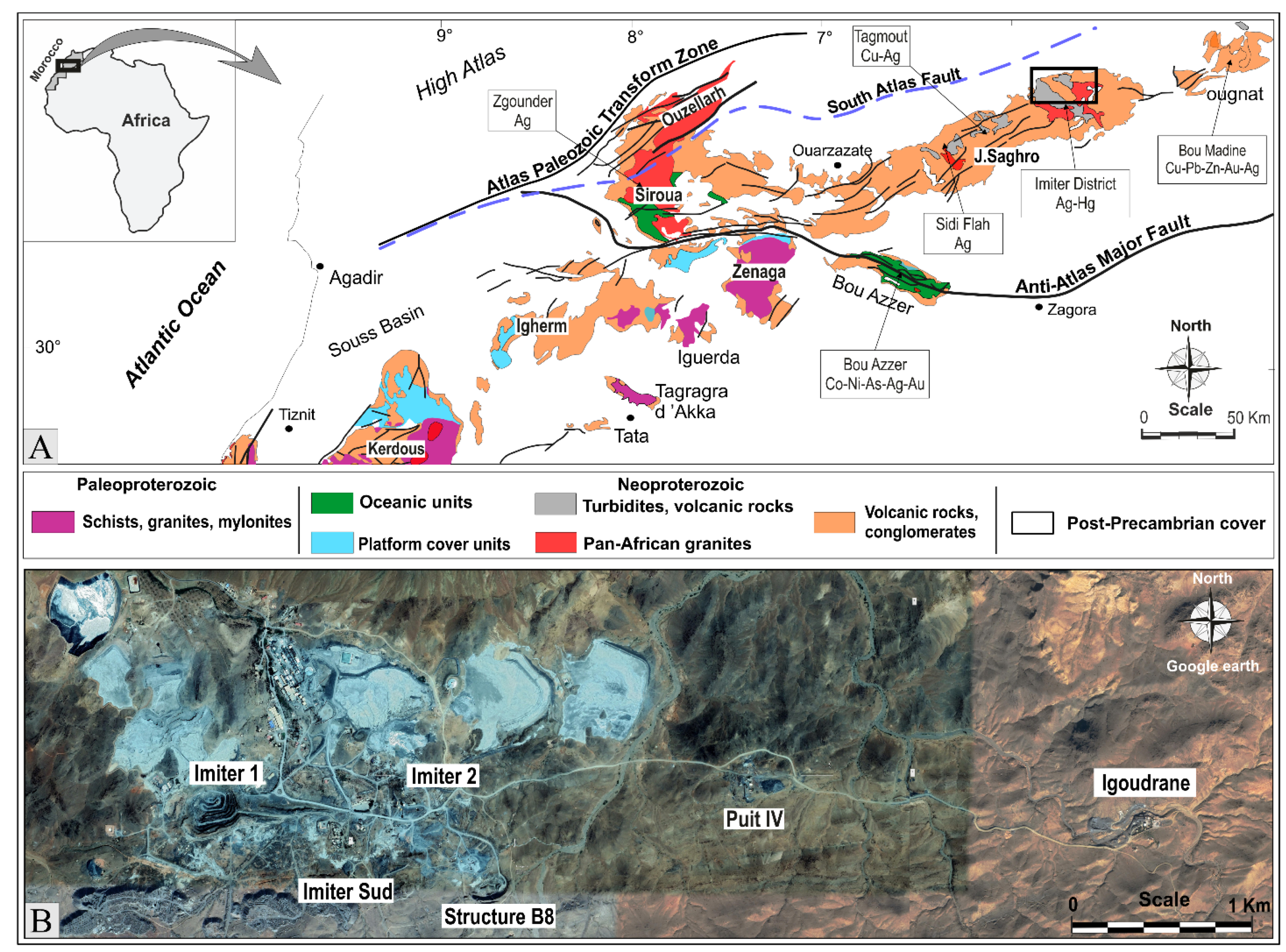
2. Regional Geological Setting
3. Local Geology Setting
3.1. Stratigraphy, Metamorphism, and Structural Geology
3.2. Igneous and Pyroclastic Rocks
3.2.1. Intrusive Rocks
3.2.2. Volcanic and Volcaniclastic Rocks
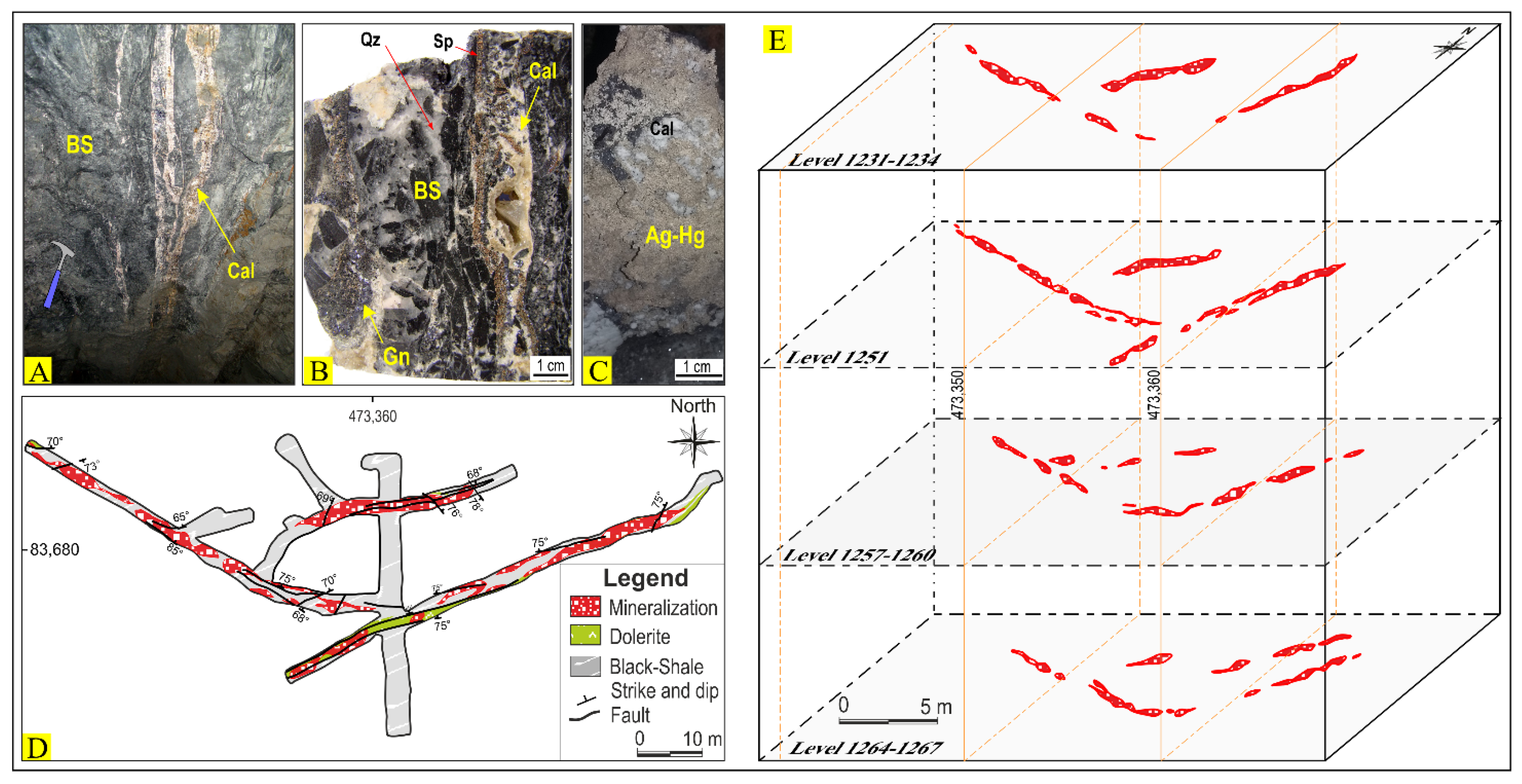
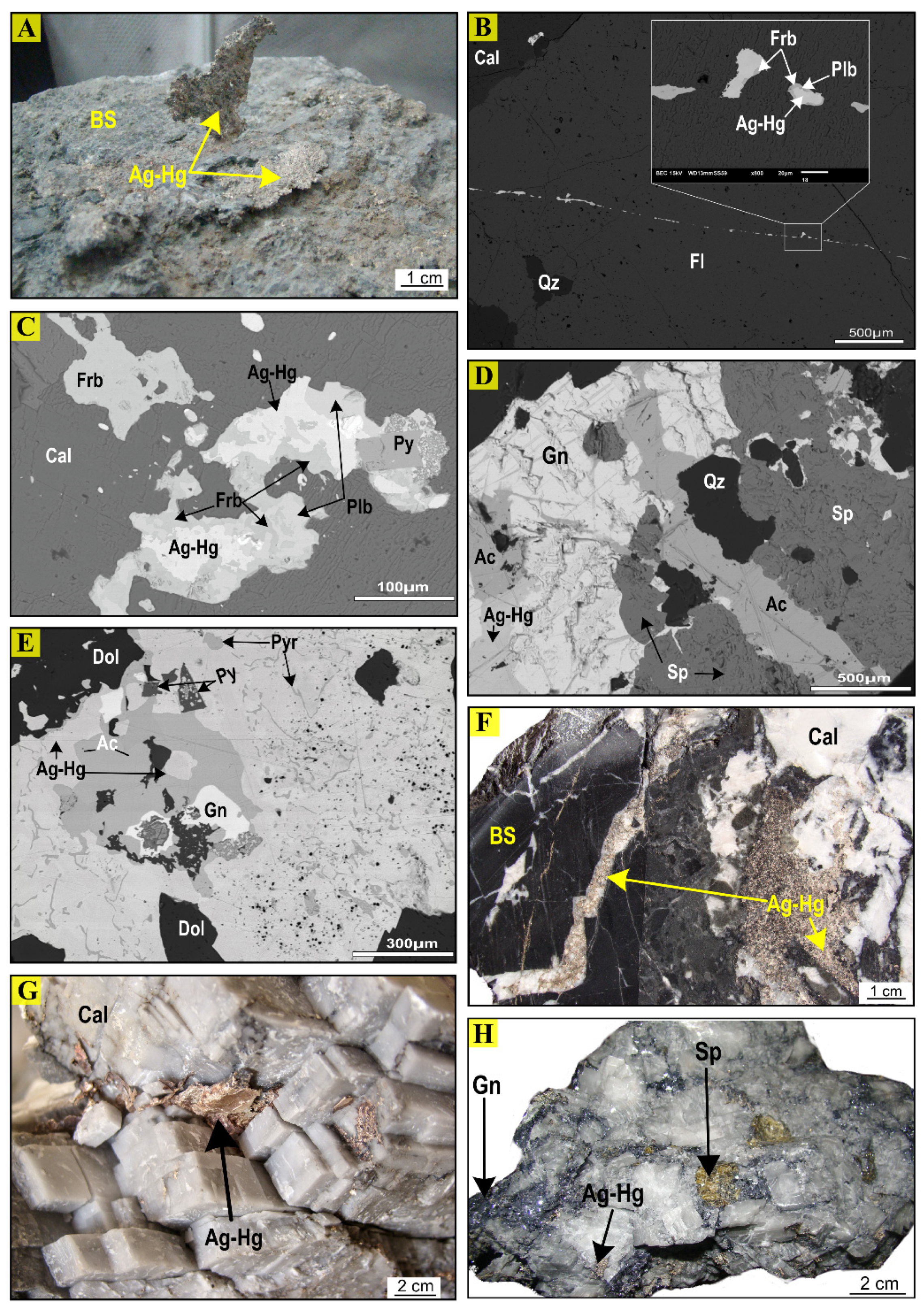
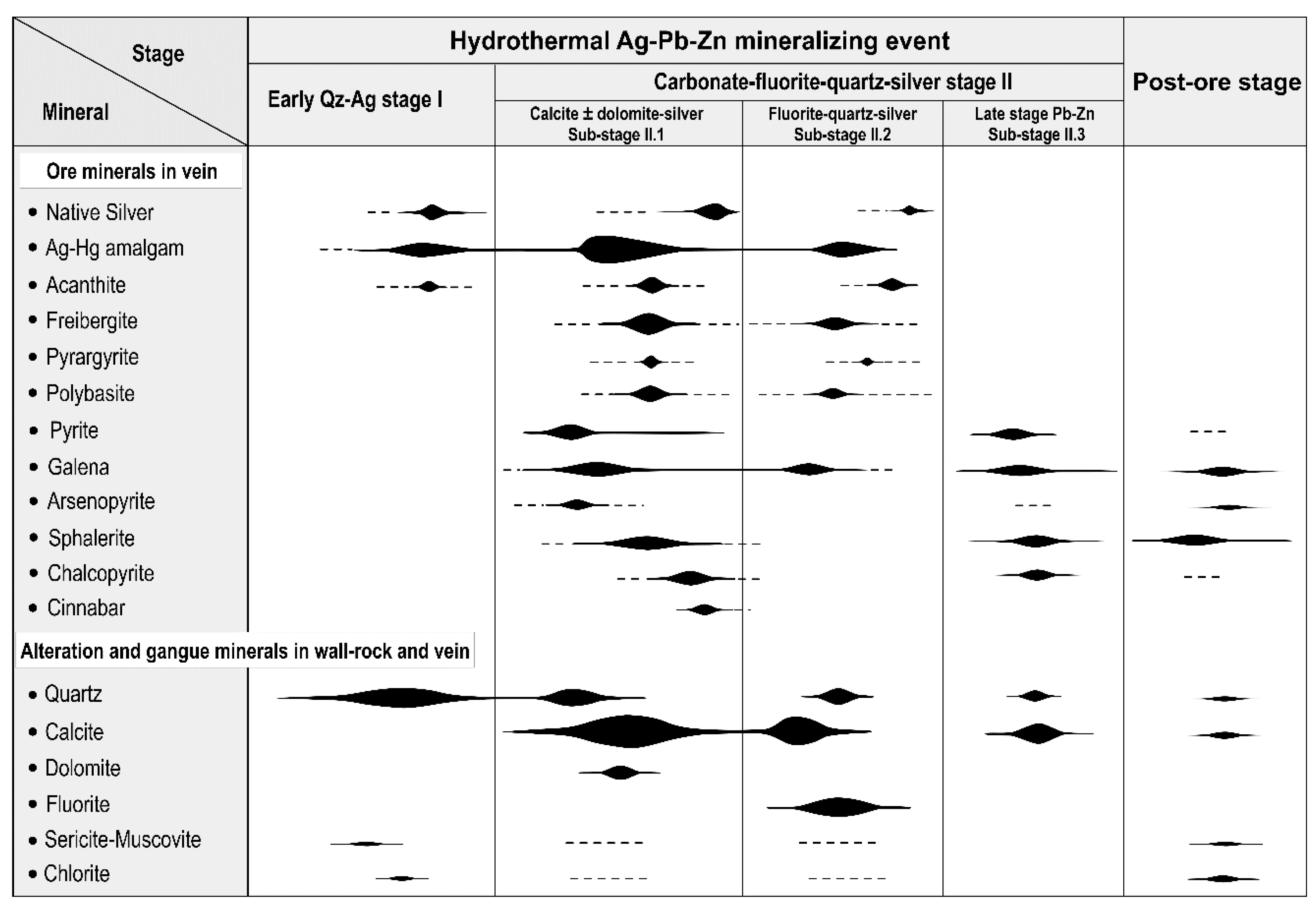
4. Mineralogy, Hydrothermal Alteration, Silver Mineralization, and Paragenesis
5. Sampling and Analytical Methods
6. Analytical Results
6.1. Host-Rock Geochemistry
6.2. Silver- and Base Metal-Bearing Mineral Chemistry
6.3. Trace Element and REE+Y Compositions of Hydrothermal Calcite and Fluorite
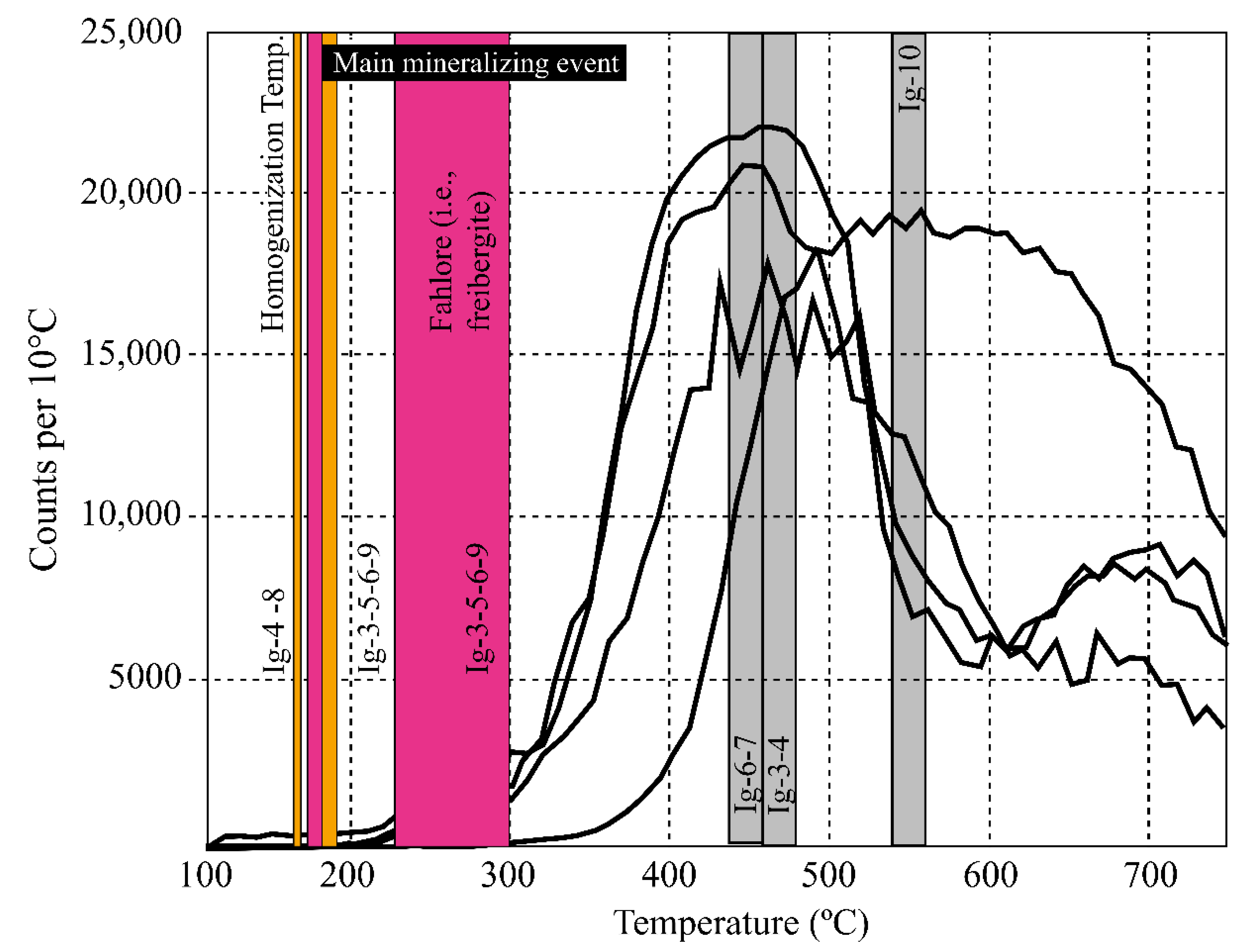
6.4. Fluid Inclusion Microthermometry
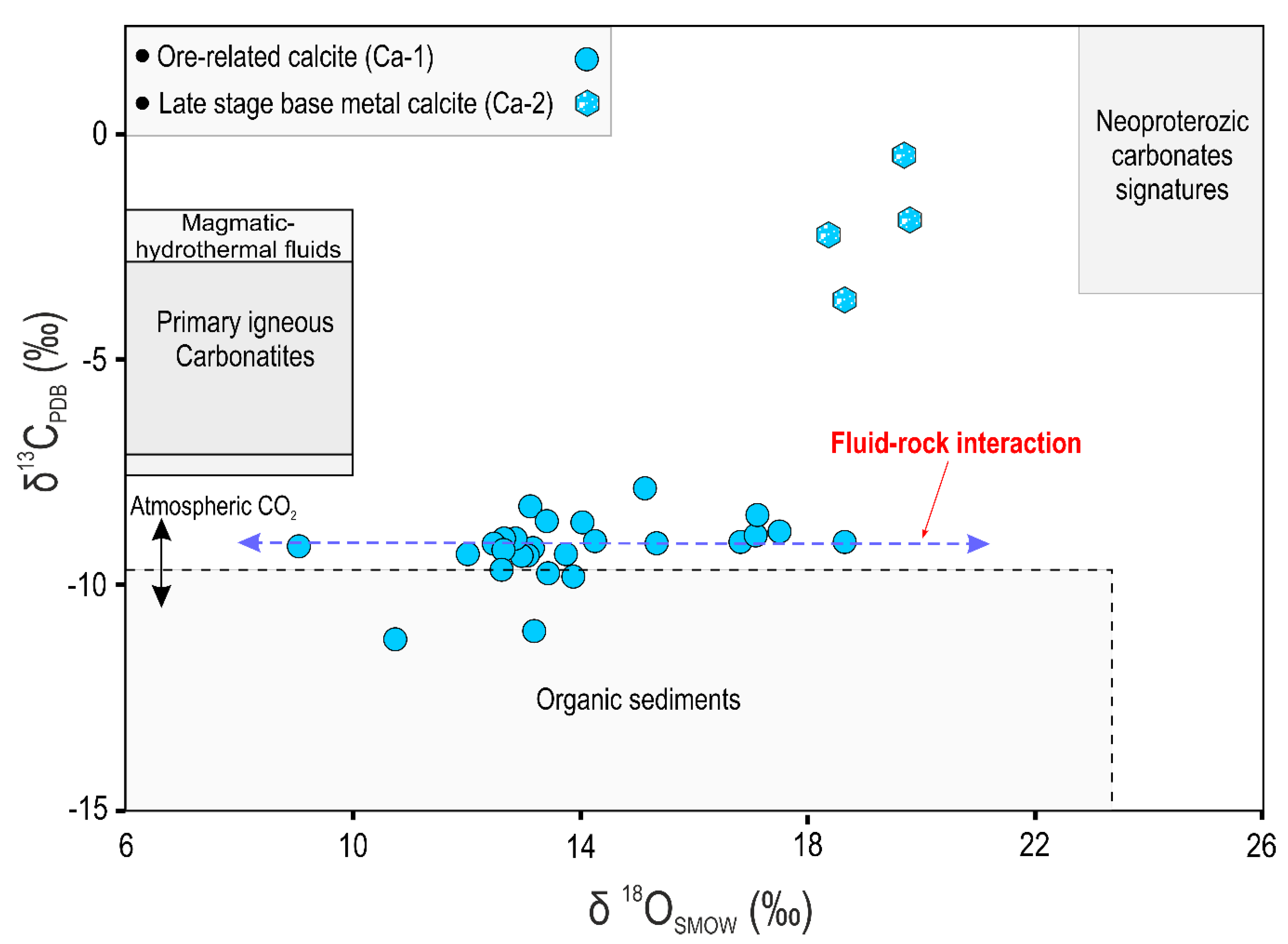
6.5. Carbon and Oxygen Isotopes
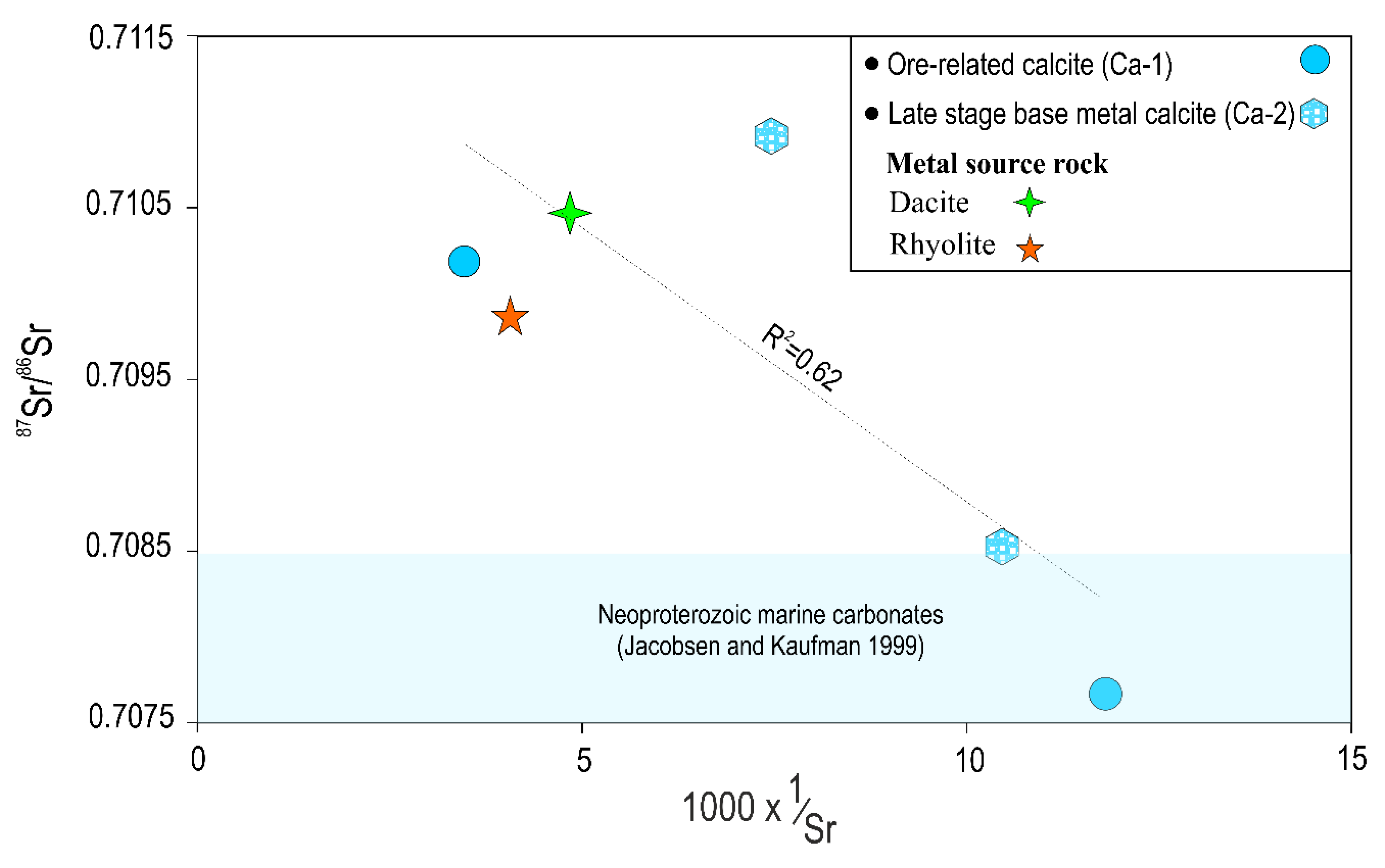
6.6. Strontium Isotopes
7. Discussion
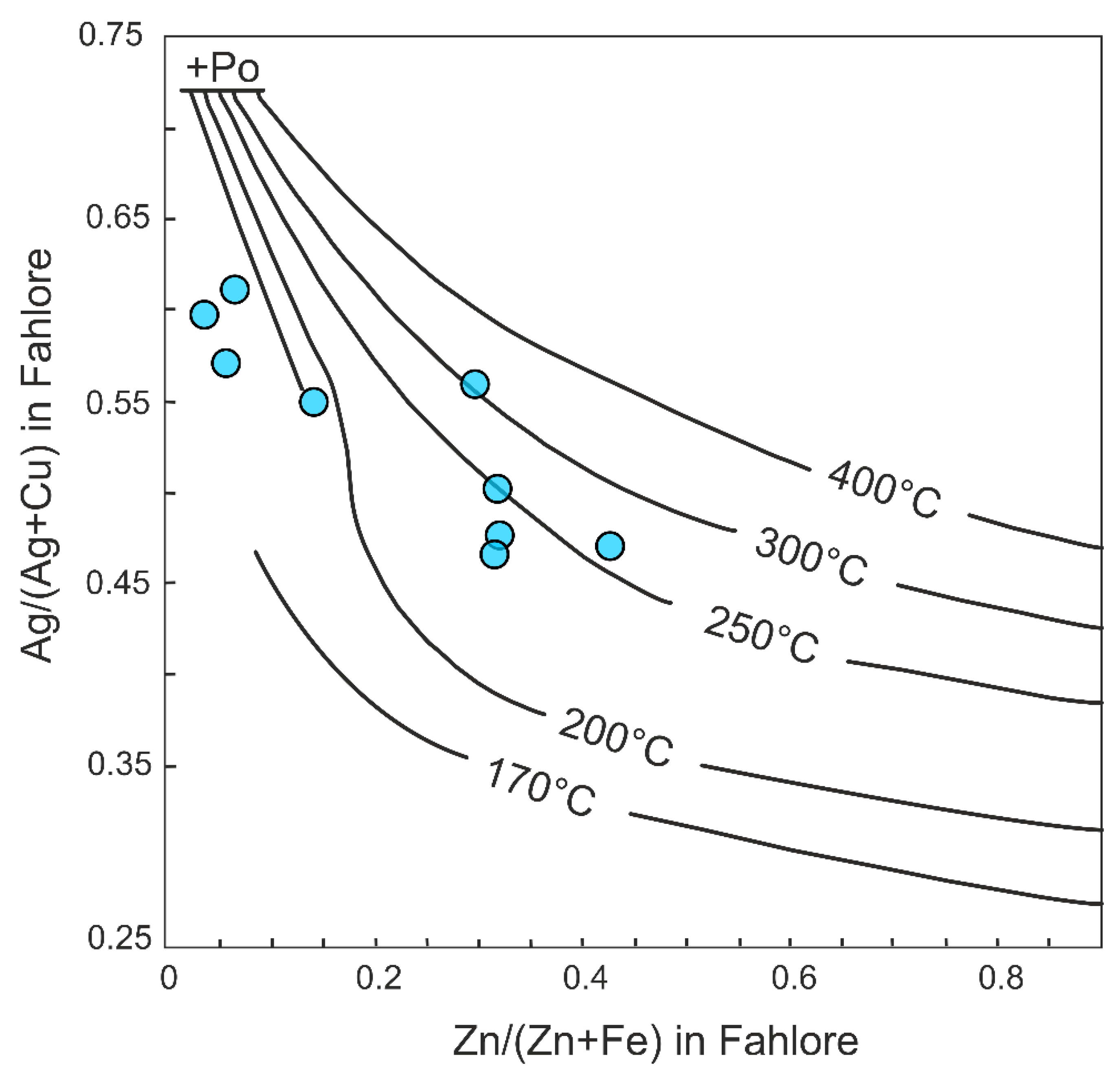
7.1. Physicochemical Conditions and Sources of the Ore Fluids
7.2. Evolution of the Ore-Forming Fluid and Ore Depositional Processes
7.3. Geochemical Signature of Fluorite as a Proxy for Fluid Source and Evolution of the Mineralizing Fluids
8. Concluding Remarks
- 1.
- Economic mineralization consists mainly of open-space fillings in veins, veinlet intergranular voids, stockwork, and breccias with silver-bearing sulfides (acanthite, argentite, polybasite), sulfosalts (argentiferous tetrahedrite of the freibergite-argentotennantite series, pyrargyrite, proustite), and Ag-Hg amalgams as the main ore minerals. Gangue minerals consist predominantly of calcite—and to a lesser extent fluorite, dolomite, and quartz.
- 2.
- Controls on the deposit are both stratigraphic and structural as most of the higher-grade orebodies are localized: (i) within the uppermost organic-rich black shale unit close to, and paralleling, the transcrustal Imiter Fault and its subsidiary satellites; and (ii) along the intersection of NW- and E-W-trending faults.
- 3.
- Mineralogy and textural relationships revealed a complex polyphase history, with at least three silver-bearing stages: (1) silver-quartz stage; (2) main silver-calcite ± fluorite stage; and (3) late carbonate-quartz base metal sulfide stage, of which the calcite-dominant and fluorite-dominant sub-stages are economically the most characteristic.
- 4.
- Silver-rich fluids are related to the involvement of an immiscible CO2-bearing F- and Cl-rich fluids that have evolved through magma degassing, fluid mixing, and subsequent cooling/dilution, along with interactions with the host rocks at decreasing temperatures. High concentration of fluorine and other complexing agents in this phase allowed trace elements, such as Ag and accompanying metals, to be transported in the hydrothermal solution. During the latest stage of mineralization, the hydrothermal system was invaded by meteoric fluid that entered and diluted the deep-seated magmatic brines.
- 5.
- Deposition of the silver-rich ± base metal-rich ores could have been achieved through three possible mechanisms, including: (1) drops in pressure in response to fluid immiscibility (i.e., boiling and subsequent degassing); (2) mixing and subsequent cooling/dilution; and (3) fluid–rock interactions along the transcrustal Imiter Fault and associated subsidiary strike-slip faults.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levresse, G. Contribution à l’établissement d’un modèle génétique des gisements d’Imiter (Ag-Hg), Bou Madine (Pb-Zn-Cu-Ag-Au) et Bou Azzer (Co-Ni-As-Au-Ag) dans l’Anti-Atlas marocain. Ph.D. Thesis, Institut National Polytechnique de Lorraine, Vandoeuvre-lès-Nancy, French, 2001. [Google Scholar]
- Cheilletz, A.; Levresse, G.; Gasquet, D.; Azizi-Samir, M.; Zyadi, R.; Archibald, D.A.; Farrar, E. The giant Imiter silver deposit: Neoproterozoic epithermal mineralization in the Anti-Atlas, Morocco. Miner. Deposita 2002, 37, 772–781. [Google Scholar] [CrossRef]
- Levresse, G.; Bouabdellah, M.; Cheilletz, A.; Gasquet, D.; Maacha, L.; Tritlla, J.; Banks, D.; Rachid, A.S.M. Degassing as the Main Ore-Forming Process at the Giant Imiter Ag–Hg Vein Deposit in the Anti-Atlas Mountains, Morocco. In Mineral Deposits of North Africa; Bouabdellah, M., Slack, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 85–106. [Google Scholar]
- Levresse, G.; Cheilletz, A.; Gasquet, D.; Reisberg, L.; Deloule, E.; Marty, B.; Kyser, K. Osmium, sulphur, and helium isotopic results from the giant Neoproterozoic epithermal Imiter silver deposit, Morocco: Evidence for a mantle source. Chem. Geol. 2004, 207, 59–79. [Google Scholar] [CrossRef]
- Essarraj, S.; Boiron, M.-C.; Cathelineau, M.; Tarantola, A.; Leisen, M.; Boulvais, P.; Maacha, L. Basinal Brines at the Origin of the Imiter Ag-Hg Deposit (Anti-Atlas, Morocco): Evidence from LA-ICP-MS Data on Fluid Inclusions, Halogen Signatures, and Stable Isotopes (H, C, O). Econ. Geol. 2016, 111, 1753–1781. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Slack, J.F. Mineral Deposits of North Africa; Bouabdellah, M., Slack, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; p. 594. [Google Scholar]
- Gasquet, D.; Ennih, N.; Liégeois, J.-P.; Soulaimani, A.; Michard, A. The Pan-African Belt. In Continental Evolution: The Geology of Morocco: Structure, Stratigraphy, and Tectonics of the Africa-Atlantic-Mediterranean Triple Junction; Michard, A., Saddiqi, O., Chalouan, A., Lamotte, D.F.d., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 33–64. [Google Scholar]
- Gasquet, D.; Levresse, G.; Cheilletz, A.; Azizi-Samir, M.R.; Mouttaqi, A. Contribution to a geodynamic reconstruction of the Anti-Atlas (Morocco) during Pan-African times with the emphasis on inversion tectonics and metallogenic activity at the Precambrian–Cambrian transition. Precambrian Res. 2005, 140, 157–182. [Google Scholar] [CrossRef]
- Thomas, R.J.; Fekkak, A.; Ennih, N.; Errami, E.; Loughlin, S.C.; Gresse, P.G.; Chevallier, L.P.; Liégeois, J.P. A new lithostratigraphic framework for the Anti-Atlas Orogen, Morocco. J. Afr. Earth Sci. 2004, 39, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Walsh, G.J.; Benziane, F.; Aleinikoff, J.N.; Harrison, R.W.; Yazidi, A.; Burton, W.C.; Quick, J.E.; Saadane, A. Neoproterozoic tectonic evolution of the Jebel Saghro and Bou Azzer—El Graara inliers, eastern and central Anti-Atlas, Morocco. Precambrian Res. 2012, 216-219, 23–62. [Google Scholar] [CrossRef]
- Michard, A.; Soulaimani, A.; Ouanaimi, H.; Raddi, Y.; Aït Brahim, L.; Rjimati, E.-C.; Baidder, L.; Saddiqi, O. Saghro Group in the Ougnat Massif (Morocco), an evidence for a continuous Cadomian basin along the northern West African Craton. Comptes Rendus Geosci. 2017, 349, 81–90. [Google Scholar] [CrossRef]
- Errami, E.; Linnemann, U.; Hofmann, M.; Gärtner, A.; Zieger, J.; Gärtner, J.; Mende, K.; El Kabouri, J.; Gasquet, D.; Ennih, N. From Pan-African Transpression to Cadomian Transtension at the West African Margin: New U–Pb zircon Ages from the Eastern Saghro Inlier (Anti-Atlas, Morocco). Geol. Soc. Lond. Spec. Publ. 2021, 503, 209–233. [Google Scholar]
- Liégeois, J.; Fekkak, A.; Bruguier, O.; Errami, E.; Ennih, N. The Lower Ediacaran (630–610 Ma) Saghro group: An orogenic transpressive basin development during the early metacratonic evolution of the Anti-Atlas (Morocco). In Proceedings of the IGCP485 4th Meeting, Algiers, Algeria, 2 September 2006. [Google Scholar]
- Abati, J.; Aghzer, A.M.; Gerdes, A.; Ennih, N. Detrital zircon ages of Neoproterozoic sequences of the Moroccan Anti-Atlas belt. Precambrian Res. 2010, 181, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Hefferan, K.; Soulaimani, A.; Samson, S.D.; Admou, H.; Inglis, J.; Saquaque, A.; Latifa, C.; Heywood, N. A reconsideration of Pan African orogenic cycle in the Anti-Atlas Mountains, Morocco. J. Afr. Earth Sci. 2014, 98, 34–46. [Google Scholar] [CrossRef]
- Linnemann, U.; Gerdes, A.; Hofmann, M.; Marko, L. The Cadomian Orogen: Neoproterozoic to Early Cambrian crustal growth and orogenic zoning along the periphery of the West African Craton—Constraints from U–Pb zircon ages and Hf isotopes (Schwarzburg Antiform, Germany). Precambrian Res. 2014, 244, 236–278. [Google Scholar] [CrossRef]
- Baidada, B.; Cousens, B.; Alansari, A.; Soulaimani, A.; Barbey, P.; Ilmen, S.; Ikenne, M. Geochemistry and Sm–Nd isotopic composition of the Imiter Pan-African granitoids (Saghro massif, eastern Anti-Atlas, Morocco): Geotectonic implications. J. Afr. Earth Sci. 2017, 127, 99–112. [Google Scholar] [CrossRef]
- El Boukhari, A.; Musumeci, G.; Algouti, A.B.; Cerrina Feroni, A.; Ghiselli, F.; Ottria, G.; Ouanaimi, H.; Pertusati, P.; Taj Eddine, K. Notice Explicative de la Carte Géologique du Maroc au 1/50.000, feuille Imi n’Ouzrou; Royaume Du Maroc Ministère De L’énergie Et Des Mines: Rabat, Morocco, 2007. [Google Scholar]
- Malusà, M.G.; Polino, R.; Feroni, A.C.; Ellero, A.; Ottria, G.; Baidder, L.; Musumeci, G. Post-Variscan tectonics in eastern anti-atlas (Morocco). Terra Nova 2007, 19, 481–489. [Google Scholar] [CrossRef]
- Massironi, M.; Moratti, G.; Algouti, A.H.; Benvenuti, M.; Dal Piaz, G.V.; Eddebbi, A.; El Boukhari, A.; Laftouhi, N.; Ouanaimi, H.; Schiavo, A.; et al. Carte géologique du Maroc au 1/50 000, feuille Boumalne. Notes Mém. Ser. Géol. Maroc 2007, 521, 1–80. [Google Scholar]
- Ouguir, H.; Macaudière, J.; Dagallier, G. Le protérozoïque supérieur d’imiter, Saghro oriental, Maroc: Un contexte géodynamique d’arrière-arc. J. Afr. Earth Sci. 1996, 22, 173–189. [Google Scholar] [CrossRef]
- Tuduri, J. Formation process, geometrical and chronological relationships of Au-Ag mineralization formed within Neoproterozoic volcanic context (Jbel Saghro, Anti-Atlas, Morocco). Consequences on the interactions between deformation, magmatism, volcanism and hydrothermalism. Ph.D. Thesis, Université d’Orléans, Orléans, France, 2005. [Google Scholar]
- Soulaimani, A.; Bouabdelli, M.; Piqué, A. L’extension continentale au Néo-Protérozoïque supérieur-Cambrien inférieur dans l’Anti-Atlas (Maroc); Bulletin de la Société Géologique de France: Paris, France, 2003. [Google Scholar]
- Benssaou, M.; Hamoumi, N. The Lower-Cambrian western Anti-Atlasic graben: Tectonic control of palaeogeography and sequential organisation. Comptes Rendus Géosci. 2003, 335, 297–305. [Google Scholar] [CrossRef]
- Buggisch, W.; Siegert, R. Paleogeography and facies of the ‘grès terminaux’ (uppermost Lower Cambrian, Anti-Atlas/Morocco). In The Atlas System of Morocco; Springer: Berlin/Heidelberg, Germany, 1988; pp. 107–121. [Google Scholar]
- Piqué, A.; Laville, E.; Chotin, P.; Chorowicz, J.; Rakotondraompiana, S.; Thouin, C. L’extension à Madagascar du Néogène à l’Actuel: Arguments structuraux et géophysiques. J. Afr. Earth Sci. 1999, 28, 975–983. [Google Scholar] [CrossRef]
- Soulaimani, A.; Michard, A.; Ouanaimi, H.; Baidder, L.; Raddi, Y.; Saddiqi, O.; Rjimati, E. Late Ediacaran–Cambrian structures and their reactivation during the Variscan and Alpine cycles in the Anti-Atlas (Morocco). J. Afr. Earth Sci. 2014, 98, 94–112. [Google Scholar] [CrossRef]
- Baidada, B.; Ikenne, M.; Barbey, P.; Soulaimani, A.; Cousens, B.; Haissen, F.; Ilmen, S.; Alansari, A. SHRIMP U–Pb zircon geochronology of the granitoids of the Imiter Inlier: Constraints on the Pan-African events in the Saghro massif, Anti-Atlas (Morocco). J. Afr. Earth Sci. 2019, 150, 799–810. [Google Scholar] [CrossRef]
- De Wall, H.; Kober, B.; Errami, E.; Ennih, N.; Greiling, R.O. Age de mise en place et contexte géologique des granitoïdes de la boutonnière d’Imiter (Saghro oriental, Anti-Atlas, Maroc): 2ème Colloque International. Marrakech Magmatisme Métamorphisme et Minéralisations Associées 2001, 10, 19. [Google Scholar]
- Tuduri, J.; Chauvet, A.; Barbanson, L.; Labriki, M.; Dubois, M.; Trapy, P.-H.; Lahfid, A.; Poujol, M.; Melleton, J.; Badra, L.; et al. Structural control, magmatic-hydrothermal evolution and formation of hornfels-hosted, intrusion-related gold deposits: Insight from the Thaghassa deposit in Eastern Anti-Atlas, Morocco. Ore Geol. Rev. 2018, 97, 171–198. [Google Scholar] [CrossRef] [Green Version]
- Bajja, A. Volcanisme syn à post orogénique du néoprotérozoïque de l’Anti-Atlas: Implications pétrogénétiques et géodynamiques. Ph.D. Thesis, Universite Chouaib Doukkali, El Jadida, Morocco, 1998. [Google Scholar]
- El Baghdadi, M.; El Boukhari, A.; Jouider, A.; Benyoucef, A.; Nadem, S. Calc-alkaline arc I-type granitoid associated with S-type granite in the Pan-African belt of eastern Anti-Atlas (Saghro and Ougnat, South Morocco). Gondwana Res. 2003, 6, 557–572. [Google Scholar] [CrossRef]
- Pouclet, A.; Aarab, A.; Fekkak, A.; Benharref, M. Geodynamic evolution of the northwestern Paleo-Gondwanan margin in the Moroccan Atlas at the Precambrian-Cambrian boundary. Spec. Pap.-Geol. Soc. Am. 2007, 423, 27–60. [Google Scholar]
- Soulaimani, A.; Ouanaimi, H.; Saddiqi, O.; Baidder, L.; Michard, A. The anti-atlas pan-african belt (Morocco): Overview and pending questions. Comptes Rendus Geosci. 2018, 350, 279–288. [Google Scholar] [CrossRef]
- Thomas, R.J.; Chevallier, L.P.; Gresse, P.G.; Harmer, R.E.; Eglington, B.M.; Armstrong, R.A.; de Beer, C.H.; Martini, J.E.J.; de Kock, G.S.; Macey, P.H.; et al. Precambrian evolution of the Sirwa Window, Anti-Atlas Orogen, Morocco. Precambrian Res. 2002, 118, 1–57. [Google Scholar] [CrossRef]
- Toummite, A.; Liegeois, J.P.; Gasquet, D.; Bruguier, O.; Beraaouz, E.H.; Ikenne, M. Field, geochemistry and Sr-Nd isotopes of the Pan-African granitoids from the Tifnoute Valley (Sirwa, Anti-Atlas, Morocco): A post-collisional event in a metacratonic setting. Mineral. Petrol 2013, 107, 739–763. [Google Scholar] [CrossRef]
- Michard, A.; Yazidi, A.; Benziane, F.; Hollard, H.; Willefert, S. Foreland thrusts and olistromes on the pre-Sahara margin of the Variscan orogen, Morocco. Geology 1982, 10, 253–256. [Google Scholar] [CrossRef]
- Hoepffner, C.; Soulaimani, A.; Piqué, A. The Moroccan Hercynides. J. Afr. Earth Sci. 2005, 43, 144–165. [Google Scholar] [CrossRef]
- Burkhard, M.; Caritg, S.; Helg, U.; Robert-Charrue, C.; Soulaimani, A. Tectonics of the Anti-Atlas of Morocco. Comptes Rendus Geosci. 2006, 338, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Marzoli, A.; Jourdan, F.; Puffer, J.H.; Cuppone, T.; Tanner, L.H.; Weems, R.E.; Bertrand, H.; Cirilli, S.; Bellieni, G.; De Min, A. Timing and duration of the Central Atlantic magmatic province in the Newark and Culpeper basins, eastern USA. Lithos 2011, 122, 175–188. [Google Scholar] [CrossRef]
- Bourdier, J.-L.; Hejja, Y.; Gaouzi, A.; El Basbas, A.; Ennaciri, A.; Zakir, A.; Baidder, L.; Maacha, L. Lithostratigraphie des formations édiacariennes NP3 de la boutonnière d’Imiter (Saghro, Anti-Atlas): Cycles volcano-plutoniques et implications sur l’âge de la faille d’Imiter. In Proceedings of the 11ème Colloque «Magmatisme, Métamorphisme et Minéralisations Associées» 3MA11El Jadida, El Jadida, Morocco, 23–24 April 2019; pp. 7–9. [Google Scholar]
- Hejja, Y.; Baidder, L.; Ibouh, H.; Bba, A.N.; Soulaimani, A.; Gaouzi, A.; Maacha, L. Fractures distribution and basement-cover interaction in a polytectonic domain: A case study from the Saghro Massif (Eastern Anti-Atlas, Morocco). J. Afr. Earth Sci. 2020, 162, 103694. [Google Scholar] [CrossRef]
- Tuduri, J.; Chauvet, A.; Ennaciri, A.; Barbanson, L. Modèle de formation du gisement d’argent d’Imiter (Anti-Atlas oriental, Maroc). Nouveaux apports de l’analyse structurale et minéralogique. Model of formation of the Imiter silver deposit (eastern Anti-Atlas, Morocco). New structural and mineralogical constraints. Comptes Rendus Geosci. 2006, 338, 253–261. [Google Scholar]
- Leistel, J.-M.; Qadrouci, A. Le gisement argentifère d’Imiter (Protérozöque supérieur de l’Anti-Atlas, Maroc). Contrôles des minéralisations, hypothèse génétique et perspectives pour l’exploration. Chronique de la recherche minière 1991, 502, 5–22. [Google Scholar]
- Gasquet, D.; Bouloton, J. Les filons de microdiorite des Jebilet centrales (Meseta marocaine): Pré-rifting permien. Abstract Réunion extraordinaire de la Société Géologique de France, Marrakech, Morocco 1995, 55. Available online: https://core.ac.uk/download/pdf/46813017.pdf (accessed on 1 January 2021).
- Youbi, N.; Bellon, H.; Marzin, A.; Piqué, A.; Cotten, J.; Cabanis, B. Du cycle orogénique hercynien au pré-rifting de l’Atlantique central au Maroc occidental: Les microdiorites des Jbilet sont-elles des marqueurs magmatiques de ce passage? Comptes Rendus de l’Académie des Sciences-Series IIA-Earth and Planetary Science 2001, 333, 295–302. [Google Scholar] [CrossRef]
- Dostal, J.; Keppie, J.D.; Hamilton, M.A.; Aarab, E.M.; Lefort, J.P.; Murphy, J.B. Crustal xenoliths in Triassic lamprophyre dykes in western Morocco: Tectonic implications for the Rheic Ocean suture. Geol. Mag. 2005, 142, 159–172. [Google Scholar] [CrossRef]
- Bouloton, J.; Gasquet, D.; Pin, C. Petrogenesis of the Early-Triassic quartz-monzodiorite dykes from Central Jebilet (Moroccan Meseta): Trace element and Nd-Sr isotope constraints on magma sources, and inferences on their geodynamic context. J. Afr. Earth Sci. 2019, 149, 451–464. [Google Scholar] [CrossRef]
- Ikenne, M.; Ennaciri, A.; Ouguir, H.; Cousens, B.; Ziyadi, R.; Mouhagir, M.; El-Gaouzi, A. Geochemical signature and geodynamic significance of an Ag-Hg mineralized dyke swarm in the neoproterozoic inlier of Imiter–Anti-Atlas (Morocco). Ofioliti 2007, 32, 109–118. [Google Scholar]
- Belkacim, S.; Gasquet, D.; Liégeois, J.-P.; Arai, S.; Gahlan, H.A.; Ahmed, H.; Ishida, Y.; Ikenne, M. The Ediacaran volcanic rocks and associated mafic dykes of the Ouarzazate Group (Anti-Atlas, Morocco): Clinopyroxene composition, whole-rock geochemistry and Sr-Nd isotopes constraints from the Ouzellarh-Siroua salient (Tifnoute valley). J. Afr. Earth Sci. 2017, 127, 113–135. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Slack, J.F. Geologic and Metallogenic Framework of North Africa. In Mineral Deposits of North Africa, Bouabdellah, M., Slack, J.F., Eds.; Springer International Publishing: Cham, Germany, 2016; pp. 3–81. [Google Scholar]
- Margoum, D.; Bouabdellah, M.; Klügel, A.; Banks, D.A.; Castorina, F.; Cuney, M.; Jébrak, M.; Bozkaya, G. Pangea rifting and onward pre-Central Atlantic opening as the main ore-forming processes for the genesis of the Aouli REE-rich fluorite–barite vein system, upper Moulouya district, Morocco. J. Afr. Earth Sci. 2015, 108, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Coplen, T.B. Reporting of stable carbon, hydrogen, and oxygen isotopic abundances. Ref. Intercomp. Mater. Stable Isot. Light Elem. IAEA-Tecdoc 1995, 825, 31–34. [Google Scholar] [CrossRef]
- Snoeck, C.; Lee-Thorp, J.; Schulting, R.; de Jong, J.; Debouge, W.; Mattielli, N. Calcined bone provides a reliable substrate for strontium isotope ratios as shown by an enrichment experiment. Rapid Commun. Mass Spectrom. 2015, 29, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Weis, D.; Kieffer, B.; Maerschalk, C.; Barling, J.; De Jong, J.; Williams, G.A.; Hanano, D.; Pretorius, W.; Mattielli, N.; Scoates, J.S. High-precision isotopic characterization of USGS reference materials by TIMS and MC-ICP-MS. Geochem. Geophys. 2006, 7. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef] [Green Version]
- Pearce, J.A. Geochemical fingerprinting of oceanic basalts with applications to ophiolite classification and the search for Archean oceanic crust. Lithos 2008, 100, 14–48. [Google Scholar] [CrossRef]
- Pearce, J.A. A user’s guide to basalt discrimination diagrams. Trace Elem. Geochem. Volcan. Rocks 1996, 12, 113. [Google Scholar]
- Maniar, P.D.; Piccoli, P.M. Tectonic discrimination of granitoids. Geol. Soc. Am. Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of eocene calc-alkaline volcanic rocks from the Kastamonu area, Northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Roedder, E. Volume 12: Fluid Inclusions; De Gruyter: Berlin, Gamany, 1984; p. 646. [Google Scholar]
- Steele-MacInnis, M.; Han, L.; Lowell, R.P.; Rimstidt, J.D.; Bodnar, R.J. The role of fluid phase immiscibility in quartz dissolution and precipitation in sub-seafloor hydrothermal systems. Earth Planet. Sci. Lett. 2012, 321, 139–151. [Google Scholar] [CrossRef]
- Oakes, J. Multiplying Inequalities: The Effects of Race, Social Class, and Tracking on Opportunities to Learn Mathematics and Science; ERIC: Santa Monica, CA, USA, 1990. [Google Scholar]
- Jenkyns, H.C.; Jones, C.E.; GrÖcke, D.R.; Hesselbo, S.P.; Parkinson, D.N. Chemostratigraphy of the Jurassic System: Applications, limitations and implications for palaeoceanography. J. Geol. Soc. 2002, 159, 351–378. [Google Scholar] [CrossRef]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.A.F.; Diener, A.; Ebneth, S.; Godderis, Y.; et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-F. Calculation of oxygen isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Zheng, Y.-F. Oxygen isotope fractionation in carbonate and sulfate minerals. Geochem. J. 1999, 33, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, S.B.; Kaufman, A.J. The Sr, C and O isotopic evolution of Neoproterozoic seawater. Chem. Geol. 1999, 161, 37–57. [Google Scholar] [CrossRef]
- Taylor Jr, H.P.; Frechen, J.; Degens, E.T. Oxygen and carbon isotope studies of carbonatites from the Laacher See District, West Germany and the Alnö District, Sweden. Geochim. Cosmochim. Acta 1967, 31, 407–430. [Google Scholar] [CrossRef]
- Keller, J.; Hoefs, J. Stable isotope characteristics of recent natrocarbonatites from Oldoinyo Lengai. In Carbonatite Volcanism; Springer: Berlin/Heidelberg, Germany, 1995; pp. 113–123. [Google Scholar]
- Lindgren, W. Mineral Deposits; McGraw-Hill Book Company, Incorporated: New York, NY, USA, 1933. [Google Scholar]
- Essarraj, S.; Boiron, M.-C.; Cathelineau, M.; Peiffert, C. Evaporitic brines and copper-sulphide ore genesis at Jbel Haïmer (Central Jebilet, Morocco). Ore Geol. Rev. 2020, 129, 103920. [Google Scholar] [CrossRef]
- Jochum, K.P.; Seufert, H.M.; Spettel, B.; Palme, H. The solar-system abundances of Nb, Ta, and Y, and the relative abundances of refractory lithophile elements in differentiated planetary bodies. Geochim. Cosmochim. Acta 1986, 50, 1173–1183. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A.; Dulski, P.; Hein, J.R. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim. Acta 1996, 60, 1709–1725. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Couëslan, C.; Yang, P. Calcite and dolomite in intrusive carbonatites. II. Trace-element variations. Mineral. Petrol. 2016, 110, 361–377. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Kamenetsky, V.S.; Sharygin, V.V.; Golovin, A.V. Trace-element partitioning in perovskite: Implications for the geochemistry of kimberlites and other mantle-derived undersaturated rocks. Chem. Geol. 2013, 353, 112–131. [Google Scholar] [CrossRef]
- Giggenbach, W. Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet. Sci. Lett. 1992, 113, 495–510. [Google Scholar] [CrossRef]
- Taylor, H.P. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Balabin, A.I.; Sack, R.O. Thermodynamics of (Zn, Fe) S sphalerite. A CVM approach with large basis clusters. Mineral. Mag. 2000, 64, 923–943. [Google Scholar] [CrossRef]
- Zhai, D.; Williams-Jones, A.E.; Liu, J.; Selby, D.; Voudouris, P.C.; Tombros, S.; Li, K.; Li, P.; Sun, H. The Genesis of the Giant Shuangjianzishan Epithermal Ag-Pb-Zn Deposit, Inner Mongolia, Northeastern China. Econ. Geol. 2020, 115, 101–128. [Google Scholar] [CrossRef]
- Sack, R.O.; Fredericks, R.; Hardy, L.S.; Ebel, D.S. Origin of high-Ag fahlores from the Galena mine, Wallace, Idaho, USA. Am. Mineral. 2005, 90, 1000–1007. [Google Scholar] [CrossRef]
- Deines, P. The carbon isotope geochemistry of mantle xenoliths. Earth-Sci. Rev. 2002, 58, 247–278. [Google Scholar] [CrossRef]
- Hoefs, J. Variations of stable isotope ratios in nature. In Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 93–227. [Google Scholar]
- Keppler, H.; Wyllie, P.J. Role of fluids in transport and fractionation of uranium and thorium in magmatic processes. Nature 1990, 348, 531–533. [Google Scholar] [CrossRef]
- Keppler, H.; Wyllie, P.J. Partitioning of Cu, Sn, Mo, W, U, and Th between melt and aqueous fluid in the systems haplogranite-H2O−HCl and haplogranite-H2O−HF. Contrib. Mineral. Petrol. 1991, 109, 139–150. [Google Scholar] [CrossRef]
- London, D.; Hervig, R.L.; Morgan, G.B. Melt-vapor solubilities and elemental partitioning in peraluminous granite-pegmatite systems: Experimental results with Macusani glass at 200 MPa. Contrib. Mineral. Petrol. 1988, 99, 360–373. [Google Scholar] [CrossRef]
- Webster, J.D.; Holloway, J.R.; Hervig, R.L. Partitioning of lithophile trace elements between H2O and H2O + CO2 fluids and topaz rhyolite melt. Econ. Geol. 1989, 84, 116–134. [Google Scholar] [CrossRef]
- Gagnon, J.E.; Samson, I.M.; Fryer, B.J.; Williams-Jones, A.E. Compositional heterogeneity in fluorite and the genesis of fluorite deposits: Insights from LA–ICP–MS analysis. Canad. Mineral. 2003, 41, 365–382. [Google Scholar] [CrossRef]
- Gabitov, R.I.; Sadekov, A.; Migdisov, A. REE incorporation into calcite individual crystals as one time spike addition. Minerals 2017, 7, 204. [Google Scholar] [CrossRef] [Green Version]
- Möller, P.; Bau, M.; Dulski, P.; Lüders, V. REE and yttrium fractionation in fluorite and their bearing on fluorite formation. In Proceedings of the 9th Quadrennial IAGOD Symposium, Beijing, China, 12–18 August 1994; pp. 575–592. [Google Scholar]
- Borisenko, A.; Borovikov, A.A.; Pavlova, G.G.; Kalinin, Y.A.; Nevolko, P.A.; Gushchina, L.V.; Lebedev, V.I.; Maacha, L.; Kostin, A.V. Formation conditions of Hg-silver deposition at the Imiter deposit (Anti-Atlas, Morocco). In Mineral Depostits for a High-Tech World, Proceedings of the 12th SGA Biennial Meeting 2013, Uppsala, Sweden, 12–15 August 2013; Jonsson, E., Ed.; Elanders Sverige AB: Uppsala, Sweden, 2013; Volume 3, pp. 1243–1246. [Google Scholar]

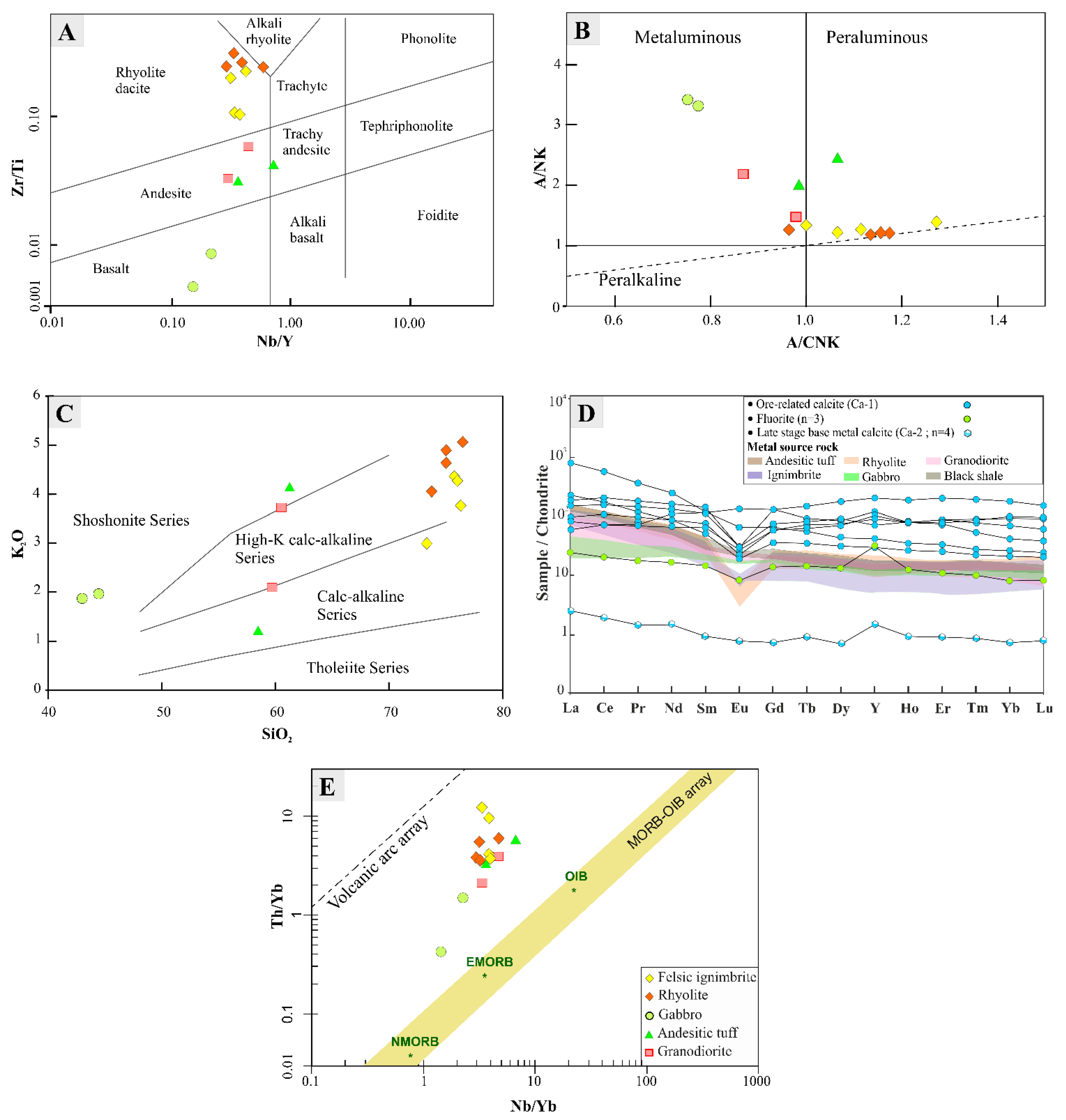



| Rock Type | Gabbro | Granodiorite | Felsic Ignimbrite | Rhyolite | Andesitic Tuff | Granodiorite | Black Shales | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. | RT1 | RT2 | RT4 | RT13 | RT14 | 19RT6 | 19RT7 | RT10 | RT12 | 19RT8 | 19RT5 | RT15 | RT16 | RT6 | BS |
| Major elements (wt.%) | |||||||||||||||
| SiO2 | 44.46 | 42.94 | 60.50 | 76.29 | 73.32 | 75.73 | 75.88 | 74.91 | 75.00 | 76.50 | 73.71 | 61.27 | 58.31 | 59.63 | 65.42 |
| Al2O3 | 16.22 | 16.53 | 16.32 | 11.58 | 13.21 | 12.57 | 12.66 | 12.24 | 12.57 | 12.97 | 12.96 | 15.85 | 12.53 | 15.48 | 15.42 |
| Fe2O3(T) | 15.10 | 15.36 | 5.37 | 1.19 | 1.56 | 1.73 | 1.44 | 1.57 | 1.48 | 0.95 | 1.62 | 3.83 | 6.87 | 7.25 | 5.32 |
| MnO | 0.18 | 0.23 | 0.10 | 0.04 | 0.05 | 0.04 | 0.03 | 0.01 | 0.03 | 0.06 | 0.07 | 0.08 | 0.11 | 0.13 | 0.18 |
| MgO | 6.86 | 8.10 | 2.63 | 0.28 | 0.20 | 0.50 | 0.52 | 0.18 | 0.36 | 0.39 | 0.37 | 1.80 | 6.97 | 3.82 | 2.57 |
| CaO | 8.83 | 9.40 | 2.69 | 1.60 | 0.80 | 0.37 | 0.64 | 0.11 | 0.19 | 0.29 | 1.68 | 4.41 | 3.63 | 5.89 | 0.94 |
| Na2O | 1.66 | 1.72 | 4.65 | 2.79 | 4.68 | 2.75 | 3.37 | 2.99 | 3.48 | 3.16 | 3.63 | 2.15 | 2.34 | 2.94 | 2.48 |
| K2O | 1.99 | 1.87 | 3.72 | 3.77 | 2.99 | 4.33 | 4.28 | 4.90 | 4.62 | 5.06 | 4.06 | 4.12 | 1.18 | 2.09 | 2.74 |
| TiO2 | 1.41 | 1.33 | 0.58 | 0.12 | 0.14 | 0.11 | 0.10 | 0.10 | 0.10 | 0.05 | 0.12 | 0.57 | 0.58 | 0.89 | 0.77 |
| P2O5 | 0.09 | 0.10 | 0.12 | 0.01 | 0.04 | 0.04 | 0.01 | <0.01 | <0.01 | 0.01 | 0.02 | 0.11 | 0.11 | 0.20 | 0.29 |
| LOI | 2.32 | 2.28 | 4.12 | 2.31 | 1.42 | 1.52 | 1.31 | 1.78 | 1.06 | 1.15 | 2.07 | 6.33 | 6.85 | 1.89 | 2.98 |
| Total | 99.12 | 99.85 | 100.79 | 99.98 | 98.41 | 99.69 | 100.23 | 98.79 | 98.89 | 100.58 | 100.31 | 100.52 | 99.47 | 100.21 | 99.11 |
| Trace elements (ppm) | |||||||||||||||
| As | 13.00 | 12.00 | 12.00 | <5 | 5.00 | <5 | <5 | 44.00 | <5 | <5 | <5 | <5 | <5 | 11.00 | 315.33 |
| B | 17.00 | 16.00 | 31.00 | 5.00 | <2 | 46.00 | <2 | ||||||||
| Ag | 0.60 | <0.5 | 0.70 | 0.50 | <0.5 | 0.80 | 1.20 | <0.5 | 0.60 | 0.50 | 0.80 | <0.5 | 0.60 | 0.50 | 0.90 |
| Ba | 439.00 | 633.00 | 916.00 | 802.00 | 757.00 | 2135.00 | 304.00 | 366.00 | 369.00 | 1240.00 | 494.00 | 843.00 | 385.00 | 871.00 | 1002.67 |
| Be | <1 | <1 | 1.00 | 1.00 | 2.00 | 3.00 | 2.00 | 1.00 | 2.00 | <1 | 3.00 | 3.00 | 1.00 | 1.00 | 2.33 |
| Bi | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
| Cl | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | ||||||||
| Cr | <20 | 30.00 | 70.00 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 230.00 | 750.00 | 70.00 | 90.00 |
| Cs | 1.40 | 2.80 | 1.50 | 2.30 | 1.20 | 4.80 | 2.00 | 2.70 | 2.30 | 1.60 | 2.70 | 6.10 | 0.50 | 1.60 | 2.27 |
| Co | 46.00 | 56.00 | 12.00 | 1.00 | 1.00 | 1.00 | 1.00 | <1 | 1.00 | <1 | 1.00 | 10.00 | 34.00 | 20.00 | 15.67 |
| Cu | 40.00 | <10 | 20.00 | 50.00 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 20.00 | <10 | 30.00 | 40.00 |
| F | <0.01 | 0.01 | <0.01 | 0.04 | 0.03 | 0.01 | 0.02 | ||||||||
| Ga | 16.00 | 16.00 | 15.00 | 13.00 | 12.00 | 15.00 | 18.00 | 15.00 | 17.00 | 16.00 | 17.00 | 16.00 | 13.00 | 14.00 | 17.33 |
| Ge | 2.00 | 2.00 | 1.00 | 1.00 | <1 | 1.00 | 1.00 | <1 | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 1.00 | 1.33 |
| Hf | 2.10 | 1.10 | 4.50 | 2.10 | 2.60 | 5.00 | 5.10 | 4.20 | 4.50 | 3.10 | 4.80 | 3.60 | 2.60 | 4.40 | 3.90 |
| In | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Li | 7.10 | 9.50 | 13.20 | 14.30 | 84.80 | 33.30 | 40.50 | ||||||||
| Mo | <2 | <2 | <2 | <2 | <2 | <2 | 2.00 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | 3.00 |
| Nb | 4.00 | 2.00 | 8.00 | 3.00 | 5.00 | 12.00 | 12.00 | 10.00 | 10.00 | 7.00 | 10.00 | 8.00 | 5.00 | 7.00 | 11.33 |
| Ni | <20 | 20.00 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 30.00 | 210.00 | <20 | 43.33 |
| P | 0.02 | 0.01 | 0.01 | 0.01 | 0.04 | 0.08 | 0.07 | ||||||||
| Pb | 7.00 | <5 | 15.00 | 14.00 | 6.00 | 115.00 | <5 | 87.00 | 12.00 | 33.00 | 10.00 | 13.00 | 8.00 | 11.00 | 84.67 |
| Rb | 60.00 | 63.00 | 135.00 | 125.00 | 98.00 | 164.00 | 150.00 | 159.00 | 151.00 | 143.00 | 135.00 | 187.00 | 35.00 | 49.00 | 72.67 |
| S | 0.01 | 0.02 | <0.01 | <0.01 | <0.01 | 0.02 | 0.01 | 0.94 | <0.01 | 0.01 | 0.02 | <0.01 | <0.01 | 0.01 | 0.19 |
| Sn | 1.00 | 1.00 | 1.00 | 2.00 | 2.00 | 3.00 | 3.00 | 2.00 | 3.00 | 2.00 | 3.00 | 2.00 | 1.00 | 1.00 | 2.33 |
| Sb | 1.40 | 0.80 | 2.00 | 1.90 | 1.70 | 1.60 | 1.60 | 1.30 | 1.30 | 1.10 | 1.60 | 2.30 | 2.20 | 1.40 | 5.37 |
| Sc | 43.00 | 54.00 | 14.00 | 3.00 | 3.00 | 4.00 | 4.00 | 4.00 | 4.00 | 3.00 | 5.00 | 12.00 | 21.00 | 23.00 | 15.33 |
| Sr | 286.00 | 241.00 | 234.00 | 88.00 | 104.00 | 44.00 | 32.00 | 33.00 | 32.00 | 90.00 | 62.00 | 126.00 | 186.00 | 369.00 | 137.33 |
| Ta | 0.30 | 0.10 | 0.60 | 0.60 | 0.80 | 1.10 | 1.10 | 1.10 | 1.00 | 0.90 | 1.00 | 0.70 | 0.40 | 0.50 | 0.90 |
| Tl | 0.30 | 0.50 | 1.00 | 0.70 | 0.50 | 1.00 | 0.90 | 0.90 | 0.70 | 1.50 | 1.00 | 0.90 | 0.40 | 0.40 | 0.60 |
| V | 521.00 | 604.00 | 83.00 | 12.00 | 8.00 | 11.00 | <5 | <5 | <5 | <5 | 8.00 | 69.00 | 108.00 | 134.00 | 117.67 |
| W | <1 | <1 | <1 | <1 | <1 | 2.00 | 2.00 | 2.00 | 2.00 | <1 | 2.00 | 2.00 | 2.00 | <1 | 2.33 |
| Y | 17.00 | 14.00 | 16.00 | 8.00 | 13.00 | 29.00 | 31.00 | 17.00 | 35.00 | 21.00 | 32.00 | 11.00 | 14.00 | 20.00 | 24.33 |
| Zn | 70.00 | 70.00 | 90.00 | 30.00 | <30 | 60.00 | 30.00 | 110.00 | 40.00 | 80.00 | 50.00 | 60.00 | 110.00 | 80.00 | 360.00 |
| Zr | 76.00 | 31.00 | 202.00 | 78.00 | 91.00 | 151.00 | 152.00 | 141.00 | 140.00 | 89.00 | 142.00 | 144.00 | 107.00 | 174.00 | 160.00 |
| La | 11.20 | 4.80 | 30.50 | 17.00 | 38.10 | 31.50 | 33.10 | 24.10 | 38.70 | 34.30 | 33.00 | 22.50 | 20.30 | 23.00 | 34.23 |
| Ce | 24.60 | 12.40 | 58.80 | 30.90 | 68.60 | 66.90 | 69.30 | 51.00 | 71.20 | 53.10 | 70.60 | 41.10 | 38.70 | 48.30 | 69.77 |
| Pr | 3.18 | 1.89 | 6.29 | 3.20 | 6.68 | 7.86 | 8.18 | 5.69 | 9.18 | 6.90 | 8.38 | 4.44 | 4.31 | 5.59 | 7.70 |
| Nd | 14.20 | 10.10 | 24.30 | 11.20 | 21.70 | 29.40 | 30.30 | 19.40 | 34.30 | 23.60 | 31.50 | 14.80 | 15.90 | 21.60 | 28.80 |
| Sm | 3.80 | 2.80 | 4.30 | 2.20 | 3.50 | 5.90 | 6.10 | 3.70 | 7.00 | 4.30 | 6.80 | 3.10 | 3.30 | 4.30 | 5.77 |
| Eu | 1.07 | 0.94 | 1.02 | 0.47 | 0.63 | 0.37 | 0.36 | 0.17 | 0.33 | 0.48 | 0.42 | 0.78 | 0.96 | 0.96 | 1.35 |
| Gd | 4.10 | 3.50 | 3.60 | 1.70 | 2.60 | 5.10 | 5.30 | 2.70 | 5.90 | 3.50 | 5.60 | 2.70 | 2.80 | 4.40 | 5.13 |
| Tb | 0.60 | 0.50 | 0.50 | 0.30 | 0.40 | 0.90 | 0.90 | 0.50 | 1.00 | 0.60 | 1.00 | 0.40 | 0.50 | 0.70 | 0.80 |
| Dy | 3.90 | 3.40 | 3.20 | 1.50 | 2.40 | 5.10 | 5.30 | 3.30 | 5.80 | 3.10 | 5.60 | 2.30 | 2.90 | 4.00 | 4.83 |
| Ho | 0.80 | 0.60 | 0.60 | 0.30 | 0.50 | 1.00 | 1.00 | 0.60 | 1.10 | 0.60 | 1.10 | 0.50 | 0.60 | 0.80 | 0.90 |
| Er | 2.00 | 1.80 | 1.70 | 0.80 | 1.30 | 3.10 | 3.10 | 2.00 | 3.20 | 2.00 | 3.10 | 1.30 | 1.60 | 2.20 | 2.60 |
| Tm | 0.29 | 0.24 | 0.27 | 0.12 | 0.20 | 0.45 | 0.46 | 0.33 | 0.48 | 0.28 | 0.44 | 0.19 | 0.23 | 0.32 | 0.39 |
| Yb | 1.80 | 1.40 | 1.70 | 0.90 | 1.30 | 3.00 | 3.10 | 2.10 | 3.30 | 2.20 | 3.10 | 1.20 | 1.40 | 2.10 | 2.53 |
| Lu | 0.21 | 0.17 | 0.22 | 0.15 | 0.18 | 0.46 | 0.48 | 0.31 | 0.55 | 0.33 | 0.47 | 0.17 | 0.21 | 0.31 | 0.35 |
| ∑REY | 88.75 | 58.54 | 153.00 | 78.74 | 161.09 | 190.04 | 197.98 | 132.90 | 217.04 | 156.29 | 203.11 | 106.48 | 107.71 | 138.58 | 189.49 |
| Th | 2.70 | 0.60 | 6.70 | 10.70 | 12.10 | 11.50 | 12.50 | 12.50 | 12.60 | 12.20 | 11.00 | 6.80 | 4.70 | 4.50 | 9.27 |
| U | 1.10 | 0.70 | 3.50 | 4.10 | 4.90 | 5.10 | 5.30 | 5.00 | 6.00 | 5.60 | 5.00 | 4.70 | 2.50 | 2.10 | 2.43 |
| (La/Sm)CN | 1.90 | 1.11 | 4.58 | 4.99 | 7.03 | 3.45 | 3.50 | 4.20 | 3.57 | 5.15 | 3.13 | 4.69 | 3.97 | 3.45 | 3.83 |
| (La/Yb)CN | 4.46 | 2.46 | 12.87 | 13.55 | 21.02 | 7.53 | 7.66 | 8.23 | 8.41 | 11.18 | 7.64 | 13.45 | 10.40 | 7.86 | 9.69 |
| (La/Lu)CN | 5.72 | 3.03 | 14.86 | 12.15 | 22.68 | 7.34 | 7.39 | 8.33 | 7.54 | 11.14 | 7.52 | 14.18 | 10.36 | 7.95 | 10.48 |
| (Eu/Eu*)CN | 0.83 | 0.92 | 0.79 | 0.74 | 0.64 | 0.21 | 0.19 | 0.16 | 0.16 | 0.38 | 0.21 | 0.82 | 0.97 | 0.67 | 0.76 |
| No. | S | Cu | Ni | Co | Fe | As | Zn | Se | Ag | Cd | Sb | Hg | Sn | Pb | Bi | Total | Calculated Formula |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polybasite | On the basis of 29 a.p.f.u | ||||||||||||||||
| Po-1 | 14.03 | 4.12 | 0.01 | n.d | 0.03 | 0.29 | n.d | n.d | 71.13 | 0.56 | 9.53 | 0.25 | n.d | 0.12 | 0.05 | 100.11 | (Ag15.28Cu1.50Fe0.01Cd0.11Hg0.03Pb0.01Bi0.01)16.96(Sb1.81As0.09Ni0.01)1.91S10.14 |
| Po-2 | 12.63 | 3.88 | n.d | n.d | 0.06 | 0.27 | 0.68 | n.d | 74.79 | 0.46 | 8.81 | 0.08 | n.d | 0.23 | n.d | 101.90 | (Ag16.20Cu1.43Cd0.10Fe0.03Zn0.24Hg0.01Pb0.03)18.03(Sb1.69As0.08)1.77S9.20 |
| Po-3 | 14.15 | 4.37 | n.d | <0.01 | 0.09 | 0.22 | n.d | n.d | 72.70 | 0.26 | 10.03 | 0.25 | n.d | n.d | n.d | 102.07 | (Ag15.34Cu1.57Cd0.05Fe0.04Hg0.03)17.02(Sb1.81As0.09)1.94S10.04 |
| Po-4 | 13.99 | 5.78 | n.d | 0.03 | 0.03 | 0.10 | n.d | 0.01 | 70.25 | n.d | 10.33 | 0.06 | n.d | 0.26 | n.d | 100.83 | (Ag14.90Cu2.08Co0.01Fe0.01Cd0.11Hg0.01Pb0.03)17.04(Sb1.81As0.09)1.97S9.99 |
| Pyrargyrite | On the basis of 7 a.p.f.u | ||||||||||||||||
| Pr-1 | 16.87 | 0.03 | 0.05 | n.d | 0.01 | 1.11 | 0.95 | 0.05 | 62.04 | n.d | 20.19 | 0.02 | n.d | 0.30 | n.d | 101.61 | Ag3.10Pb0.01(Sb0.89As0.08)0.97S2.83 |
| Freibergite | On the basis of 29 a.p.f.u | ||||||||||||||||
| Fb-1 | 21.18 | 15.10 | 0.02 | 0.01 | 4.30 | 1.53 | 0.84 | n.d | 31.57 | 0.21 | 25.71 | 0.14 | n.d | 0.46 | n.d | 101.07 | (Ag5.59Cu4.54)10.13(Zn0.25Fe1.47Pb0.01Sn0.01Cd0.04Hg0.01)1.81(As0.39Sb4.03Ni0.01)4.43S12.62 |
| Fb-2 | 22.43 | 18.17 | n.d | 0.03 | 3.30 | 1.21 | 2.84 | n.d | 27.56 | 0.11 | 24.97 | 0.29 | n.d | 0.17 | n.d | 101.07 | (Ag4.72Cu5.29)10.01(Zn0.80Fe1.09Co0.01Pb0.01Sn0.01Cd0.02Hg0.03)1.97(As0.30Sb3.79)4.09S12.93 |
| Fb-3 | 21.88 | 17.83 | n.d | n.d | 3.33 | 1.39 | 1.80 | 0.06 | 27.13 | n.d | 26.21 | 0.43 | n.d | 0.04 | n.d | 100.10 | (Ag4.74Cu5.29)10.03(Zn0.52Fe1.12Pb0.01Sn0.01Se0.01Hg0.04)1.70As0.35Sb4.06)4.41S12.86 |
| Fb-4 | 22.21 | 17.09 | 0.03 | n.d | 3.20 | 0.98 | 1.78 | n.d | 29.72 | n.d | 25.50 | 0.45 | n.d | 0.37 | n.d | 101.33 | (Ag5.16Cu5.04)10.20(Zn0.51Fe1.07Pb0.01Sn0.01Hg0.04)1.66(As0.25Sb3.92Ni0.01)4.18S12.97 |
| Fb-5 | 21.25 | 18.39 | 0.01 | <0.01 | 3.47 | 1.27 | 1.93 | 0.04 | 29.29 | 0.03 | 25.97 | 0.39 | n.d | 0.20 | n.d | 102.23 | (Ag5.08Cu5.42)10.50(Zn0.55Fe1.16Pb0.01Sn0.01Se0.01Hg0.04)1.78(As0.32Sb3.99)4.31S12.40 |
| Fb-6 | 20.17 | 14.84 | n.d | n.d | 2.50 | 1.01 | 1.27 | n.d | 32.10 | 0.11 | 24.59 | 2.10 | n.d | 1.12 | n.d | 99.81 | (Ag5.92Cu4.65)10.57(Zn0.39Fe0,89Pb0.01Sn0.01Cd0.02Hg0.21)1.61(As0.27Sb4,02)4.29S12.52 |
| Fb-7 | 19.99 | 13.24 | 0.02 | n.d | 4.94 | 0.67 | 0.32 | n.d | 34.67 | 0.20 | 25.71 | 0.55 | n.d | 0.21 | n.d | 100.51 | (Ag6.33Cu4.10)10.43(Zn0.10Fe1.74Pb0.01Sn0.01Cd0.03Hg0.05)1.95(As0.18Sb4.16Ni0.01)4.34S12.28 |
| Fb-8 | 19.62 | 13.46 | n.d | n.d | 3.76 | 1.07 | n.d | n.d | 32.00 | 0.04 | 24.16 | 2.43 | n.d | 3.75 | n.d | 100.29 | (Ag6.01Cu4.29)10.30(Fe1.36Pb0.01Sn0.01Cd0.01Hg0.25)1.98(As0.29Sb4.02)4.31S12.40 |
| Fb-9 | 20.97 | 13.82 | n.d | 0.01 | 4.70 | 1.06 | 0.10 | n.d | 33.97 | n.d | 25.69 | 0.04 | n.d | 0.58 | n.d | 100.94 | (Ag6.09Cu4.20)10.29(Zn0.03Fe1.63Pb0.01Sn0.01)1.72(As0.27Sb4.08)4.35S12.64 |
| Fb-10 | 21.03 | 14.87 | 0.02 | n.d | 5.04 | 1.19 | 0.20 | 0.06 | 33.59 | 0.08 | 24.99 | 0.10 | n.d | n.d | n.d | 101.17 | (Ag5.95Cu4.47)10.42(Zn0.06Fe1.72Pb0.01Sn0.01Cd0.01Hg0.01)1.82(As0.30Sb3.92Ni0.01)4.23S12.53 |
| Fb-11 | 20.21 | 14.79 | n.d | n.d | 4.44 | 1.36 | n.d | 0.07 | 32.57 | 0.11 | 24.96 | 0.24 | n.d | 0.54 | n.d | 99.29 | (Ag5.94Cu4.58)10.52(Fe1.57Pb0.01Sn0.01Se0.02Cd0.02Hg0.02)1.68(As0.36Sb4.03)4.39S12.41 |
| Fb-12 | 20.71 | 16.97 | n.d | n.d | 5.31 | 1.57 | n.d | 0.00 | 30.06 | n.d | 25.09 | 0.17 | n.d | 0.32 | n.d | 100.21 | (Ag5.33Cu5.11)10.44(Fe1.82Pb0.01Sn0.01Hg0.02)1.86(As0.40Sb3.94)4.34S12.35 |
| Silver amalgam | On the basis of 1 a.p.f.u | ||||||||||||||||
| S-1 | n.d | 0.08 | 0.01 | n.d | 0.02 | 0.05 | n.d | n.d | 77.11 | 0.26 | 0.33 | 23.03 | n.d | n.d | 0.01 | 100.9 | Ag0.85Hg0.14 |
| S-2 | n.d | n.d | n.d | 0.04 | 0.02 | n.d | n.d | 0.01 | 74.59 | n.d | 0.04 | 24.36 | n.d | 0.44 | 0.11 | 99.6 | Ag0.85Hg0.15 |
| S-3 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 74.53 | n.d | 0.03 | 24.21 | 0.01 | n.d | n.d | 98.78 | Ag0.85Hg0.15 |
| S-4 | n.d | n.d | 0.01 | 0.01 | 0.04 | 0.13 | n.d | n.d | 72.11 | 0.19 | 0.02 | 29.02 | n.d | n.d | n.d | 101.52 | Ag0.82Hg0.18 |
| S-5 | 0.02 | n.d | n.d | n.d | n.d | n.d | 0.11 | n.d | 93.76 | 0.33 | 0.26 | 5.64 | 0.10 | n.d | 0.10 | 100.31 | Ag0.96Hg0.03 |
| S-6 | 0.03 | 0.08 | n.d | n.d | 0.02 | n.d | n.d | n.d | 94.63 | 0.31 | 0.37 | 3.09 | 0.03 | 0.35 | n.d | 98.92 | Ag0.97Hg0.02 |
| S-7 | n.d | 0.02 | 0.02 | 0.01 | 0.03 | 0.14 | n.d | n.d | 83.46 | 0.15 | n.d | 16.11 | n.d | n.d | 0.08 | 100.01 | Ag0.90Hg0.09 |
| S-8 | n.d | 0.01 | n.d | 0.02 | n.d | n.d | n.d | 0.07 | 76.70 | 0.17 | 0.04 | 22.97 | 0.13 | n.d | n.d | 100.09 | Ag0.86Hg0.14 |
| S-9 | n.d | 0.02 | 0.02 | 0.02 | n.d | n.d | n.d | n.d | 78.28 | 0.31 | 0.12 | 21.59 | n.d | n.d | n.d | 100.35 | Ag0.87Hg0.13 |
| S-10 | n.d | 0.07 | n.d | n.d | 0.02 | n.d | 0.09 | n.d | 76.21 | n.d | n.d | 22.29 | 0.06 | n.d | 0.08 | 98.83 | Ag0.86Hg0.14 |
| S-11 | n.d | n.d | n.d | n.d | 0.01 | 0.07 | 0.00 | n.d | 77.61 | 0.26 | n.d | 23.02 | n.d | n.d | n.d | 100.98 | Ag0.86Hg0.14 |
| Acanthite | On the basis of 3 a.p.f.u | ||||||||||||||||
| Ac-1 | 13.49 | 0.07 | n.d | n.d | 0.04 | n.d | n.d | 0.04 | 85.06 | 0.01 | n.d | 0.02 | 0.06 | 0.42 | 0.02 | 99.22 | Ag1.95Pb0.01S1.04 |
| Ac-2 | 11.39 | 0.05 | <0.01 | n.d | 0.02 | 0.12 | 0.26 | 0.03 | 88.15 | n.d | 0.02 | 0.21 | n.d | 0.33 | 0.01 | 100.60 | Ag2.07Zn0.01S0.90 |
| Ac-3 | 6.65 | 0.01 | 0.01 | n.d | 0.21 | n.d | n.d | n.d | 91.89 | n.d | n.d | n.d | n.d | 0.52 | n.d | 99.30 | Ag2.40Fe0.01Pb0.01S0.58 |
| Chalcopyrite | On the basis of 4 a.p.f.u | ||||||||||||||||
| Cp-1 | 37.42 | 33.04 | 0.03 | n.d | 28.89 | n.d | n.d | 0.03 | 0.98 | 0.03 | n.d | 0.15 | 0.06 | 0.18 | n.d | 100.82 | (Cu0.94Ag0.02)0.96Fe0.93S2.11 |
| Cp-2 | 34.40 | 31.59 | 0.02 | n.d | 27.87 | 0.09 | n.d | n.d | 3.25 | n.d | 0.01 | n.d | 0.06 | 0.04 | n.d | 97.34 | (Cu0.95Ag0.06)1.01Fe0.95S2.04 |
| Cp-3 | 34.14 | 33.05 | n.d | n.d | 28.77 | n.d | n.d | 0.01 | n.d | 0.02 | n.d | 0.31 | n.d | n.d | 0.05 | 96.35 | Cu0.99Fe0.98S2.03 |
| Cp-4 | 34.51 | 33.11 | 0.02 | n.d | 28.82 | 0.24 | n.d | n.d | 0.03 | n.d | 0.20 | 0.17 | n.d | 0.22 | n.d | 97.31 | Cu0.98Fe0.97S2.03 |
| Cp-5 | 33.19 | 33.84 | <0.01 | n.d | 29.79 | n.d | n.d | 0.09 | 0.24 | 0.03 | 0.01 | n.d | n.d | 0.57 | n.d | 97.76 | Cu1.01Fe1.01S1.96 |
| Cp-6 | 33.75 | 33.39 | 0.01 | n.d | 29.83 | n.d | n.d | 0.09 | n.d | 0.02 | 0.10 | 0.04 | 0.03 | 0.14 | n.d | 97.40 | Cu0.99Fe1.01S1.99 |
| Galena | On the basis of 2 a.p.f.u | ||||||||||||||||
| Gn-1 | 13.44 | n.d | 0.04 | n.d | 0.04 | 0.02 | n.d | n.d | 0.45 | n.d | 0.16 | n.d | 0.24 | 85.70 | n.d | 100.09 | (Pb0.98Ag0.01)0.99S1.00 |
| Gn-2 | 13.78 | n.d | n.d | n.d | 0.05 | n.d | n.d | n.d | 1.99 | 0.44 | 0.04 | 0.11 | 0.09 | 84.09 | n.d | 100.58 | (Pb0.94Ag0.04Cd0.01)1.00S1.00 |
| Gn-3 | 13.02 | n.d | n.d | 0.04 | 0.04 | n.d | 0.27 | n.d | n.d | 0.21 | n.d | 0.10 | n.d | 87.09 | n.d | 100.77 | (Pb1.01Zn0.01)1.02S0.97 |
| Gn-4 | 13.81 | n.d | n.d | n.d | 0.07 | n.d | n.d | n.d | 0.52 | 0.21 | <0.01 | 0.03 | 0.07 | 87.06 | n.d | 101.77 | (Pb0.98Ag0.01)0.99S1.00 |
| Gn-5 | 14.04 | n.d | n.d | n.d | n.d | n.d | 0.07 | n.d | n.d | 0.00 | n.d | n.d | n.d | 87.17 | n.d | 101.28 | Pb0.98S1.02 |
| Gn-6 | 14.57 | 0.32 | <0.01 | n.d | n.d | n.d | n.d | 0.02 | n.d | 0.27 | 1.12 | n.d | 0.02 | 84.81 | n.d | 101.12 | (Pb0.93Cu0.01Cd0.01Sb0.02)0.97S1.03 |
| Pyrite | On the basis of 3 a.p.f.u | ||||||||||||||||
| Py-1 | 52.22 | 0.10 | n.d | n.d | 44.79 | n.d | n.d | 0.01 | 2.23 | n.d | 0.09 | 0.11 | n.d | 0.10 | n.d | 99.64 | Fe0.98Ag0.03S1.99 |
| Py-2 | 55.06 | 0.06 | 0.01 | n.d | 46.05 | 0.20 | n.d | 0.05 | 0.07 | n.d | 0.05 | n.d | n.d | 0.15 | n.d | 101.69 | Fe0.97S2.02 |
| Py-3 | 51.36 | 0.12 | <0.01 | n.d | 46.11 | 0.83 | n.d | 0.01 | n.d | 0.16 | 0.18 | 0.07 | n.d | 0.03 | n.d | 98.88 | Fe1.01As0.01S1.97 |
| Py-4 | 49.84 | 0.10 | 0.03 | n.d | 46.37 | 0.48 | n.d | <0.01 | n.d | n.d | 0.10 | 0.02 | <0.01 | 0.38 | n.d | 97.33 | Fe1.04As0.01S1.95 |
| Py-5 | 50.73 | 0.21 | n.d | n.d | 46.08 | 0.69 | n.d | n.d | n.d | n.d | 0.10 | 0.02 | 0.11 | 0.77 | n.d | 98.70 | Fe1.02As0.01S1.96 |
| Py-6 | 49.73 | 0.30 | 0.01 | n.d | 46.36 | 0.57 | n.d | 0.06 | n.d | 0.03 | 0.11 | 0.05 | 0.03 | 0.00 | n.d | 97.25 | Fe1.04As0.01Cu0.01S1.94 |
| Py-7 | 51.83 | 0.62 | 0.10 | n.d | 44.72 | 1.14 | 0.10 | 0.02 | 0.21 | 0.05 | 0.21 | n.d | 0.01 | 0.24 | n.d | 99.26 | Fe0.98As0.02Cu0.01S1.97 |
| Py-8 | 51.35 | 0.19 | n.d | n.d | 46.14 | 0.29 | n.d | n.d | 0.16 | n.d | 0.05 | n.d | 0.04 | 0.13 | n.d | 98.35 | Fe1.02S1.97 |
| Arsenopyrite | On the basis of 3 a.p.f.u | ||||||||||||||||
| Asp | 18.96 | n.d | 0.02 | n.d | 34.34 | 43.62 | n.d | 0.15 | 0.35 | n.d | n.d | n.d | 0.06 | 0.63 | 0.01 | 98.13 | As0.97Fe1.03Pb0.01S0.99 |
| Sphalerite | On the basis of 2 a.p.f.u | ||||||||||||||||
| Sp-1 | 29.31 | 0.03 | <0.01 | 0.02 | 1.13 | 0.15 | 66.72 | n.d | n.d | n.d | n.d | 0.15 | 0.07 | n.d | 0.04 | 97.63 | (Zn1.04Fe0.02)1.06S0.93 |
| Sp-2 | 31.46 | 0.01 | 0.01 | 0.01 | 1.35 | 0.22 | 65.94 | 0.03 | n.d | 0.06 | 0.06 | n.d | n.d | 0.14 | n.d | 99.29 | (Zn1.00Fe0.02)1.02S0.97 |
| Sp-3 | 32.79 | n.d | 0.04 | n.d | 0.97 | n.d | 66.30 | 0.03 | n.d | 0.27 | n.d | n.d | n.d | n.d | n.d | 100.41 | (Zn0.99Fe0.02)1.01S0.99 |
| Sp-4 | 34.03 | n.d | 0.01 | n.d | 3.89 | 0.21 | 61.28 | 0.12 | 0.27 | 0.22 | 0.07 | 0.00 | 0.03 | 0.08 | n.d | 100.21 | (Zn0.90Fe0.07)0.97S1.02 |
| Sp-5 | 33.11 | 0.40 | n.d | n.d | 0.22 | 0.32 | 65.78 | 0.04 | 0.57 | 0.34 | 0.40 | 0.12 | n.d | n.d | n.d | 101.29 | (Zn0.97Ag0.01Cu0.01)0.99S1.00 |
| Sample No. | IG-09 | IG-22 | IG-46 | IG-16 | IG-22 | IG-48 | IG-213 | IG-100 (n = 4) | IF-120 (n = 3) |
|---|---|---|---|---|---|---|---|---|---|
| Mineral | Ore-Related Calcite (Ca-1) | Late StageCalcites (Ca-2) | Fluorites | ||||||
| Major elements (wt.%) | |||||||||
| CaO | 54.59 | 55.46 | 55.14 | 54.04 | 57.59 | 57.49 | 57.76 | 50.64 | 70.31 |
| MgO | 0.02 | 0.01 | 0.02 | 0.04 | 0.05 | 0.06 | 0.04 | 3.23 | 0.27 |
| FeO | 0.18 | 0.06 | 0.01 | 0.28 | 0.14 | 0.22 | 0.19 | 2.12 | 0.06 |
| MnO | 1.31 | 0.48 | 0.76 | 1.38 | 1.03 | 1.11 | 1.82 | 0.20 | 0.07 |
| Trace elements (ppm) | |||||||||
| Sr | 188.85 | 147.93 | 448.25 | 94.25 | 134.00 | 85.00 | 132.00 | 326.53 | 30.33 |
| Ba | 0.59 | 0.62 | 0.62 | 1.09 | 6.00 | 5.00 | 8.00 | 4.25 | 4.33 |
| La | 44.38 | 14.38 | 19.93 | 80.70 | 23.10 | 191.00 | 37.20 | 0.54 | 5.70 |
| Ce | 125.60 | 44.62 | 44.38 | 159.12 | 66.10 | 352.00 | 105.00 | 0.96 | 12.20 |
| Pr | 17.22 | 6.76 | 7.50 | 16.52 | 9.09 | 34.90 | 14.20 | 0.13 | 1.57 |
| Nd | 76.65 | 31.02 | 51.32 | 58.64 | 40.10 | 117.00 | 60.90 | 0.66 | 6.93 |
| Sm | 21.89 | 10.07 | 17.53 | 10.81 | 11.70 | 18.50 | 16.60 | 0.15 | 2.10 |
| Eu | 1.76 | 1.54 | 7.73 | 1.50 | 1.30 | 3.81 | 1.85 | 0.05 | 0.40 |
| Gd | 26.35 | 12.12 | 27.18 | 9.55 | 12.30 | 13.50 | 16.80 | 0.13 | 3.10 |
| Tb | 5.59 | 2.34 | 3.43 | 1.71 | 2.70 | 2.10 | 3.10 | 0.03 | 0.53 |
| Dy | 45.14 | 17.98 | 21.00 | 10.20 | 19.20 | 11.50 | 22.00 | 0.16 | 3.17 |
| Y | 323.46 | 162.62 | 187.02 | 60.65 | 143.00 | 67.00 | 112.00 | 2.12 | 49.33 |
| Ho | 10.68 | 4.43 | 4.59 | 1.95 | 4.40 | 2.00 | 4.50 | 0.05 | 0.70 |
| Er | 33.87 | 14.05 | 12.32 | 5.49 | 14.70 | 5.60 | 13.50 | 0.15 | 1.87 |
| Tm | 4.92 | 1.97 | 1.35 | 0.75 | 2.32 | 0.71 | 2.11 | 0.02 | 0.23 |
| Yb | 30.80 | 11.93 | 7.12 | 4.74 | 15.70 | 4.50 | 16.90 | 0.13 | 1.17 |
| Lu | 3.96 | 1.54 | 0.96 | 0.68 | 2.30 | 0.62 | 2.48 | 0.02 | 0.19 |
| ∑REY | 772.26 | 337.37 | 413.35 | 423.01 | 368.01 | 824.74 | 429.14 | 5.29 | 89.19 |
| Th | 0.01 | <0.01 | n.d | 0.03 | n.d | 0.20 | n.d | 0.01 | 0.10 |
| U | <0.01 | 0.01 | <0.01 | <0.01 | n.d | n.d | n.d | 0.32 | n.d |
| La/Lu | 11.22 | 9.31 | 20.67 | 118.10 | 10.04 | 308.06 | 15.00 | 26.84 | 30.00 |
| Y/Ho | 30.29 | 36.72 | 40.73 | 31.08 | 32.50 | 33.50 | 24.89 | 40.56 | 70.48 |
| Sm/Yb | 0.71 | 0.84 | 2.46 | 2.28 | 0.75 | 4.11 | 0.98 | 1.16 | 1.80 |
| (La/Lu)CN | 1.20 | 1.00 | 2.22 | 11.28 | 1.08 | 33.02 | 1.61 | 2.88 | 3.22 |
| (La/Sm)CN | 1.31 | 0.92 | 0.73 | 4.43 | 1.27 | 6.67 | 1.45 | 2.38 | 1.75 |
| (La/Yb)CN | 1.03 | 0.86 | 2.01 | 10.89 | 1.06 | 30.45 | 1.58 | 3.07 | 3.50 |
| (Gd/Lu)CN | 0.82 | 0.97 | 3.48 | 1.77 | 0.66 | 2.69 | 0.84 | 0.82 | 2.02 |
| (Tb/Yb)CN | 0.83 | 0.89 | 2.19 | 1.67 | 0.78 | 2.12 | 0.83 | 1.21 | 2.08 |
| (Eu/Eu*)CN | 0.22 | 0.43 | 1.08 | 0.45 | 0.33 | 0.74 | 0.34 | 1.01 | 0.48 |
| (Y/Y*)CN | 1.13 | 1.39 | 1.45 | 1.05 | 1.19 | 1.07 | 0.86 | 1.76 | 2.53 |
| (Ce/Ce*)CN | 1.11 | 1.11 | 0.89 | 1.07 | 1.12 | 1.06 | 1.12 | 0.88 | 1.00 |
| Sample No. | Stage/Sub-Stage | Mineral | Inclusion Type | Number | Tmgas (°C) | Tmhh (°C) | Tmice (°C) | Th (°C) | NaCl (wt % NaCleqv) | CaCl2 (wt % CaCl2eqv) | Total Salt (wt % NaCl + CaCl2 eqv) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ig-3 | II.1 | Calcite | Type 1 | 8 | [−64.9 to −58.4] | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 38 | ▪ | [−26.0 to −21.3] | [−18.0 to 12.0] | [218 to 265] | [9.7 to 15.2] | [4.6 to 9.6] | [15.0 to 25.3] | |||
| Ig-4 | II.1 | Calcite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 39 | ▪ | [−27.0 to −21.8] | [−17.9 to −6.0] | [150 to 218] | [6.1 to 17.8] | [1.2 to 10.3] | [9.5 to 20.3] | |||
| Ig-5 | II.1 | Calcite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 25 | ▪ | [−29.0 to −23.0] | [−16.5 to −11.0] | [159 to 193] | [9.9 to 14.9] | [4.5 to 5.9] | [14.9 to 28.0] | |||
| Ig-6 | II.1 | Calcite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 25 | ▪ | [−28.6 to −22.6] | [−20.8 to −13.6] | [173 to 187] | [5.5 to 17.4] | [4.0 to 18.2] | [17.2 to 25.6] | |||
| Ig-7 | II.1 | Calcite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 40 | ▪ | [−228.0 to 22.0] | [−19.8 to 19.0] | [163 to 191] | [8.0 to 19.1] | [2.3 to 12.8] | [12.9 to 21.7] | |||
| Ig-8 | II.1 | Calcite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 33 | ▪ | [−29.2 to −21.8] | [−19.2 to 12.5] | [166 to 225] | [6.2 to 18.2] | [1.7 to 12.4] | [16.3 to 21.2] | |||
| Ig-9 | II.1 | Calcite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 17 | ▪ | [−25.1 to −22.7] | [−19.9 to −13.0] | [170 to 189] | [11.8 to 14.9] | [3.3 to 9.2] | [16.7 to 21.5] | |||
| Ig-5 | I | Quartz | Type 1 | 7 | [−58.1 to 66.7] | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||
| Ig-5 | II.3 | Sphalerite | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 6 | ▪ | ▪ | [−6.8 to −3.6] | [135 to 152] | [5.9 to 10.2] | ▪ | [5.9 to 10.2] | |||
| Ig-5 | I | Quartz | Type 1 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Type 2 | 6 | ▪ | ▪ | [−5.0 to −2.1] | [195 to 218] | [3.5 to 7.9] | ▪ | [3.5 to 7.9] |
| Sample No. | Mineral/Stage | δ18OV-SMOW (‰) | δ13CV-PDB (‰) | 87Sr/86Sr ± 2σ | Calculated δ18Ofluid (‰) at 250 °C |
|---|---|---|---|---|---|
| Ig-1 | Calcite (Ca-1) | 12.7 | −9.4 | - | 5.2 |
| Ig-2 | Calcite (Ca-1) | 13.4 | −10.1 | - | 5.9 |
| Ig-3 | Calcite (Ca-1) | 12.6 | −10.0 | - | 5.1 |
| Ig-4 | Calcite (Ca-1) | 13.2 | −11.4 | - | 5.7 |
| Ig-5 | Calcite (Ca-1) | 13.8 | −10.1 | - | 6.3 |
| Ig-6 | Calcite (Ca-1) | 13.1 | −8.7 | - | 5.6 |
| Ig-7 | Calcite (Ca-1) | 13.0 | −9.8 | - | 5.5 |
| Ig-8 | Calcite (Ca-1) | 15.3 | −9.5 | 0.710198 ± 0.000016 | 7.8 |
| Ig-9 | Calcite (Ca-1) | 9.0 | −9.5 | - | 1.5 |
| Ig-10 | Calcite (Ca-1) | 14.3 | −9.4 | - | 6.8 |
| Ig-11 | Calcite (Ca-1) | 12.9 | −9.4 | 0.707657 ± 0.000016 | 5.4 |
| Ig-12 | Calcite (Ca-1) | 16.8 | −9.4 | - | 9.3 |
| Ig-13 | Calcite (Ca-1) | 12.0 | −9.7 | - | 4.5 |
| Ig-14 | Calcite (Ca-1) | 17.5 | −9.2 | - | 10.0 |
| Ig-15 | Calcite (Ca-1) | 10.7 | −11.6 | - | 3.2 |
| Ig-16 | Calcite (Ca-1) | 14.1 | −9.0 | - | 6.6 |
| Ig-17 | Calcite (Ca-1) | 17.1 | −9.3 | - | 9.6 |
| Ig-18 | Calcite (Ca-1) | 12.8 | −9.8 | - | 5.3 |
| Ig-19 | Calcite (Ca-1) | 15.1 | −8.3 | - | 7.6 |
| Ig-20 | Calcite (Ca-1) | 12.7 | −9.4 | - | 5.2 |
| Ig-21 | Calcite (Ca-1) | 13.4 | −9.0 | - | 5.9 |
| Ig-22 | Calcite (Ca-1) | 17.1 | −8.8 | - | 9.6 |
| Ig-23 | Calcite (Ca-1) | 12.5 | −9.5 | - | 5.0 |
| Ig-24 | Calcite (Ca-1) | 13.1 | −9.6 | - | 5.6 |
| Ig-25 | Calcite (Ca-1) | 18.7 | −9.4 | - | 11.2 |
| Ig-26 | Calcite (Ca-1) | 13.7 | −9.7 | - | 6.2 |
| Ig-27 | Calcite (Ca-1) | 12.6 | −9.7 | - | 5.1 |
| Ig-28 | Calcite (Ca-2) | 19.7 | −0.9 | - | 12.2 |
| Ig-29 | Calcite (Ca-2) | 18.4 | −2.6 | 0.708535 ± 0.000014 | 10.9 |
| Ig-30 | Calcite (Ca-2) | 19.8 | −2.3 | 0.710918 ± 0.000012 | 12.3 |
| Ig-31 | Calcite (Ca-2) | 18.7 | −4.1 | - | 11.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diallo, M.; Bouabdellah, M.; Levresse, G.; Yans, J.; Castorina, F.; Klügel, A.; Mouhagir, M.; El Mouden, S.; Maacha, L. Mineralogy, Fluid Inclusion, and C-O-Sr Isotope Geochemistry to Unravel the Evolution of the Magmatic-Hydrothermal System at the Igoudrane Silver-Rich Deposit (Imiter District, Eastern Anti-Atlas, Morocco). Minerals 2021, 11, 997. https://doi.org/10.3390/min11090997
Diallo M, Bouabdellah M, Levresse G, Yans J, Castorina F, Klügel A, Mouhagir M, El Mouden S, Maacha L. Mineralogy, Fluid Inclusion, and C-O-Sr Isotope Geochemistry to Unravel the Evolution of the Magmatic-Hydrothermal System at the Igoudrane Silver-Rich Deposit (Imiter District, Eastern Anti-Atlas, Morocco). Minerals. 2021; 11(9):997. https://doi.org/10.3390/min11090997
Chicago/Turabian StyleDiallo, Mamadoudjan, Mohammed Bouabdellah, Gilles Levresse, Johan Yans, Francesca Castorina, Andreas Klügel, Mohamed Mouhagir, Salim El Mouden, and Lhou Maacha. 2021. "Mineralogy, Fluid Inclusion, and C-O-Sr Isotope Geochemistry to Unravel the Evolution of the Magmatic-Hydrothermal System at the Igoudrane Silver-Rich Deposit (Imiter District, Eastern Anti-Atlas, Morocco)" Minerals 11, no. 9: 997. https://doi.org/10.3390/min11090997
APA StyleDiallo, M., Bouabdellah, M., Levresse, G., Yans, J., Castorina, F., Klügel, A., Mouhagir, M., El Mouden, S., & Maacha, L. (2021). Mineralogy, Fluid Inclusion, and C-O-Sr Isotope Geochemistry to Unravel the Evolution of the Magmatic-Hydrothermal System at the Igoudrane Silver-Rich Deposit (Imiter District, Eastern Anti-Atlas, Morocco). Minerals, 11(9), 997. https://doi.org/10.3390/min11090997








