Analysis of Soil As Pollution and Investigation of Dominant Plants in Abandon Gold Mining Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Acquisition and Determine of Soil Data
2.3. Acquisition and Determination of Plant Data
2.4. Evaluation of Pollution Degree
2.4.1. Index of Geo-Accumulation
2.4.2. Potential Ecological Risk Index (PERI)
2.5. Calculation of Species Diversity Index
2.6. Bioaccumulation Factor (BCF)
2.7. Data Statistics and Analysis
3. Results
3.1. Descriptive Statistics
3.2. Evaluation of As Pollution Degree of Soil in Mining Area
3.2.1. Index of Geo-Accumulation
3.2.2. Potential Ecological Risk Assessment
3.3. Vegetation Composition and As Accumulation Characteristics
3.3.1. Vegetation Composition in the Mining Area
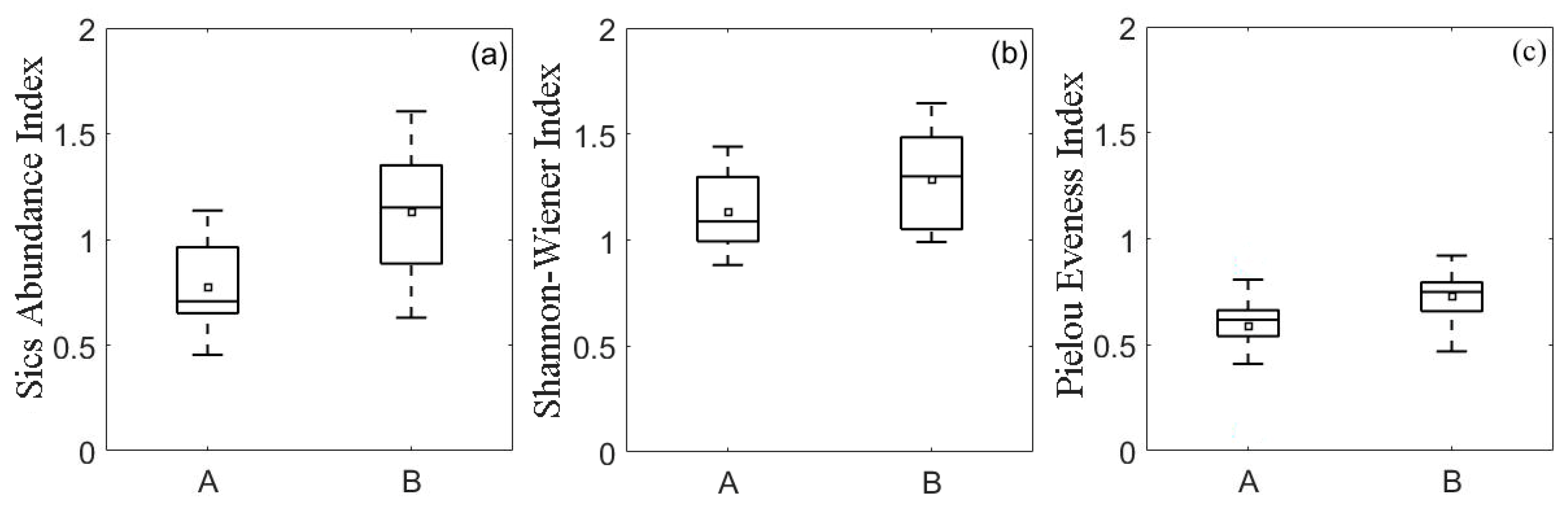
3.3.2. Correlation between Species Diversity Index and Soil As Pollution Degree
3.3.3. Enrichment Characteristics of As in Dominant Plants
4. Discussion
4.1. Potential Analysis of Phytoremediation
4.2. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.X.; Xing, Y.; Li, P.; Xia, J.C.; Liu, T.; Feng, X.B. Chemically-assisted phytoextraction from metal(loid)s-polluted soil at a typical Carlin-type gold mining area in southwest China. J. Clean. Prod. 2018, 189, 612–619. [Google Scholar] [CrossRef]
- Luo, G.F.; Han, Z.W.; Xiong, J.; He, Y.P.; Liao, J.H.; Wu, P. Heavy metal pollution and ecological risk assessment of tailings in the qinglong dachang antimony mine, China. Environ. Sci. Pollut. Res. 2021, 28, 33491–33504. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Liu, L.; Zheng, N.; Liu, H.; Wu, L.; Yue, W. Application of different indices for soil heavy metal pollution risk assessment comparison and uncertainty: A case study of a copper mine tailing site. Minerals 2022, 12, 1074. [Google Scholar] [CrossRef]
- Xiao, T.F.; Hong, B.; Yang, Z.H.; Yang, F. Hydrogeochemistry of arsenic and its environmental effects. Bull. Geol. Sci. Technol. 2001, 1, 71–76. (In Chinese) [Google Scholar]
- Liu, B.; Ai, S.; Zhang, W.; Huang, D.; Zhang, Y. Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Sci. Total Environ. 2017, 609, 822–829. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V. Invisible gold paragenesis and geochemistry in pyrite from orogenic and sediment-hosted gold deposits. Minerals 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Li, X.; Wang, M.; Liang, S. Spatial contamination and potential ecological risk assessment of heavy metals in farmland soil around nonferrous metal smeltery in north China. Minerals 2021, 11, 1357. [Google Scholar] [CrossRef]
- Bourliva, A.; Papadopoulou, L.; Aidona, E.; Giouri, S.K.; Vourlias, G. Characterization and geochemistry of technogenic magnetic particles (TMPs) in contaminated industrial soils: Assessing health risk via ingestion. Geoderma 2017, 295, 86–97. [Google Scholar] [CrossRef]
- Liang, J.H.; Liu, P.P.; Chen, Z.; Sun, G.X.; Li, H. Rapid evaluation of arsenic contamination in paddy soils using field portable X-ray fluorescence spectrometry. J. Environ. Sci. 2018, 64, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Xue, N.D.; Han, Z.G. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years-science direct. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2020, 278, 123805. [Google Scholar] [CrossRef]
- Zheng, Y. Global solutions to a silent poison. Science 2020, 368, 818–819. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, I.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Cui, X.Q.; Zhang, J.W.; Wang, X.T.; Pan, M.H.; Lin, Q.; Khan, K.Y.; Yan, B.B.; Li, T.Q.; He, Z.L.; Yang, X.E.; et al. A review on the thermal treatment of heavy metal hyperaccumulator: Fates of heavy metals and generation of products. J. Hazard. Mater. 2021, 405, 123832. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Shi, X.; You, X.G.; Liu, J.L. Relationship of the heavy metal distribution in sediments to vegetion diversity and macroinvertebrate communities in riparian wetlands. Acta Ecol. Sin. 2021, 41, 2001–2010. (In Chinese) [Google Scholar]
- Chen, T.B.; Wei, C.Y.; Huang, Z.C.; Huang, Q.F.; Lu, Q.G.; Fan, Z.L. Arsenic hyperaccumulator plant Pteris vittata L. and its arsenic accumulation characteristics Chin. Sci. Bull. 2002, 47, 207–210. (In Chinese) [Google Scholar] [CrossRef]
- Meharg, A.A. Variation in arsenic accumulation: Hyperaccumulation in ferns and their allies. New Phytol. 2003, 157, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Chen, T.B.; Huang, Z.C.; Zhang, X.Q. Cretan Brake (Pteris cretica L.): An arsenic-accumulating plant. Acta Ecol. Sin. 2002, 22, 777–778. (In Chinese) [Google Scholar]
- Yan, H.L.; Gao, Y.W.; Wu, L.L.; Wang, L.Y.; Zhang, T.; Dai, C.H.; Xu, W.X.; Feng, L.; Ma, M.; Zhu, Y.G.; et al. Potential use of the Pteris vittata arsenic hyperaccumulation-regulation network for phytoremediation. J. Hazard. Mater. 2019, 368, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Ma, L.Q.; Santos, J.A.G. Three new arsenic hyperaccumulating ferns. Sci. Total Environ. 2006, 364, 24–31. [Google Scholar] [CrossRef]
- Wang, H.J.; Cui, S.P.; Wu, D.M.; Yang, X.Y.; Wang, H.B.; Wang, Z.Z. Effects of kinetin on arsenic speciation and antioxidative enzymes in fronds of the arsenic hyperaccumulator Pteris cretica var. nervosa and non-hyperaccumulator Pteris ensiformis. Environ. Exp. Bot. 2021, 191, 104622. [Google Scholar] [CrossRef]
- Zhang, N.M.; Xia, Y.S.; Chen, B.D.; Zhang, X.; Bao, L.; Lu, W.H.; Xue, X.R.; Qin, T.F.; Zhong, X.; Wang, X.Y.; et al. Mycorrhiza and Iron Chemical Remediation of Arsenic Contaminated Soil; Science Press: Beijing, China, 2019. [Google Scholar]
- Nateewattana, J.; Trichaiyaporn, S.; Saeouy, M.; Nateewattana, J.; Thavornyutikarnm, P.; Choonluchanon, S.; Pengchai, P. Phytoremediation of arsenic in submerged soil by wetland plants. Int. J. Phytorem. 2011, 13, 35–46. [Google Scholar] [CrossRef]
- Rodriguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef]
- Zou, X.L.; Zhou, Y. Arsenic (As) uptake, accumulation and translation by willows in wetlands. J. Jiangxi Univ. Sci. Technol. 2004, 35, 7–12. (In Chinese) [Google Scholar]
- Bali, A.S.; Sidhu, G.P.S. Arsenic acquisition, toxicity and tolerance in plants-from physiology to remediation: A review. Chemosphere 2021, 283, 131050. [Google Scholar] [CrossRef]
- Guarino, G.; Zuzolo, D.; Marziano, M.; Baiamonte, G.; Morra, L.; Benotti, D.; Gresia, D.; Stacu, E.R.; Cicchella, D.; Sciarrillo, R. Identification of native-metal tolerant plant species in situ: Environmental implications and functional traits. Sci. Total Environ. 2019, 650, 3156–3167. [Google Scholar] [CrossRef]
- Xi, C.F.; Zhu, K.G.; Zhou, M.Z.; Du, G.H.; Li, X.R.; Zhang, S.Y.; Yang, B.Q.; Hou, C.Q.; Tang, J.C.; Zhou, C.H. Soil of China; Chinese Agriculture Press: Beijing, China, 1998. [Google Scholar]
- GB 15618-2018; Soil Environmental Quality–Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018. Available online: http://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/t20180703_446029.shtml (accessed on 6 June 2018).
- GB 36600-2018; Soil Environmental Quality–Risk Control Standard for Soil Contamination of a Development Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/t20180703_446027.shtml (accessed on 22 June 2018).
- HJ/T 166-2004; The Technical Specification for Soil Environmental Monitoring. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2004. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/200412/t20041209_63367.shtml (accessed on 9 December 2004).
- HJ 680-2013; Soil and Sediment—Determination of Mercury, Arsenic, Selenium, Bismuth, Antimony—Microwave Dissolution/Atomic Fluorescence Spectrometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2013. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201312/t20131203_264304.shtml (accessed on 21 November 2013).
- HJ 962-2018; Soil—Determination of pH—Potentiometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2019. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/jcffbz/201808/t20180815_451430.shtml (accessed on 1 January 2019).
- DB61T902.5-2013; Determination of Heavy Mental in Plant Extract-Determination of Arsenic. Inspection and Quarantine of Shaanxi Province: Xi’an, China, 2013. Available online: http://219.144.196.28/std/db_details.asp?id=133 (accessed on 1 May 2014).
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Li, K.; Peng, M.; Yang, Z.; Yang, K.; Liu, F.; Zhao, C.D.; Cheng, X.M.; Ma, H.H.; Guo, F.; Tang, S.Q.; et al. Trace metals pollution and health risks for planning area soils of 193 Chinese cities. Environ. Sci. 2020, 41, 1825–1837. (In Chinese) [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Xiao, R.; Guo, D.; Amjsd, A.L.; Mi, S.S.; Liu, T.; Ren, C.Y.; Li, R.H.; Zhang, Z.Q. Accumulation, ecological-health risks assessment, and source apportionment of heavy metals in paddy soils: A case study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Wu, K.M.; Lao, Z.L.; Wei, W.; Lin, Y.L.; Fan, H.B.; Hu, J.J. Assessment of trace metal contamination and ecological risk in the forest ecosystem of dexing mining area in northeast Jiangxi Province, China. Ecotoxicol. Environ. Saf. 2019, 167, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Joanna, B.K.; Ryszard, M.; Michał, G.; Tomasz, Z. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination-a review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [Green Version]
- Jaime, T.G.; Isabel, G.M.; Eduardo, S.; Manuel, A.B.; Catherine, T.; Elvira, A.D.; Anna, A.P.; Alexander, N. Advanced determination of the spatial gradient of human health risk and ecological risk from exposure to As, Cu, Pb, and Zn in soils near the ventanas industrial complex (Puchuncaví, Chile). Environ. Pollut. 2020, 258, 113388. [Google Scholar] [CrossRef]
- Amphalop, N.; Suwantarat, N.; Prueksasit, T.; Chokchai, Y.; Sarawut, S. Ecological risk assessment of arsenic, cadmium, copper, and lead contamination in soil in e-waste separating household area, Buriram Province, Thailand. Environ. Sci. Pollut. Res. 2020, 27, 44396–44411. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shi, Y.; Tian, H.J.; Li, H.; Zhang, L.; Niu, J.J.; Guo, G.L.; Zhang, C. Soil pollution characteristics and ecological risk assessment of As at a large-scale arsenic slag-contaminated site. Environ. Sci. 2018, 39, 5639–5646. [Google Scholar] [CrossRef]
- Shao, H.Q.; Zhang, Y.W.; Qu, C.; Li, W.H.; Zhao, Y.J.; Cai, H.M.; Wu, C.D.; Liu, J.M.; Liu, M. Analysis of heavy metal contamination in the soil and enrichment capabilities of terrestrial plants around a typical vanadium smelter area. Chin. J. Eng. 2020, 42, 302–312. (In Chinese) [Google Scholar]
- Ma, L.; Komar, K.; Tu, C.; Zhang, W.H.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 411, 438. [Google Scholar] [CrossRef]
- Wei, S.H.; Zhou, Q.X.; Wang, X.; Zhang, K.S.; Guo, G.L. A newly discovered cadmium hyperaccumulator plant Solanum nigrum L. Chin. Sci. Bull. 2004, 49, 2568–2573. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Lu, J.Z.; Lu, H.W.; Li, J.; Liu, J.; Feng, S.S.; Guan, Y.L. Multi-criteria decision analysis of optimal planting for enhancing phytoremediation of trace heavy metals in mining sites under interval residual contaminant concentrations. Environ. Pollut. 2019, 255, 113255. [Google Scholar] [CrossRef]
- Fu, S.; Wei, C.Y.; Li, L.H. Characterizing the accumulation of various heavy metals in native plants growing around an old antimony mine. Hum. Ecol. Risk Assess. Int. J. 2014, 22, 882–898. [Google Scholar] [CrossRef]
- Wang, X.; Xia, J.B.; Cao, X.B. Physiological and ecological characteristics of Periploca sepium Bunge under drought stress on shell sand in the Yellow River Delta of China. Sci. Rep. 2020, 10, 9567. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.M.; Zhang, X.P. Perip selocapium bge—A muli-purpose native tree. Mod. Agric. Sci. 2008, 12, 43–55. (In Chinese) [Google Scholar]
- Dedic, J.; Djokic, J.; Galjak, J.; Milentijevic, G.; Lazarevic, D.; Šarkočević, Ž.; Lekic, M. An experimental investigation of the environmental risk of a metallurgical waste deposit. Minerals 2022, 12, 661. [Google Scholar] [CrossRef]

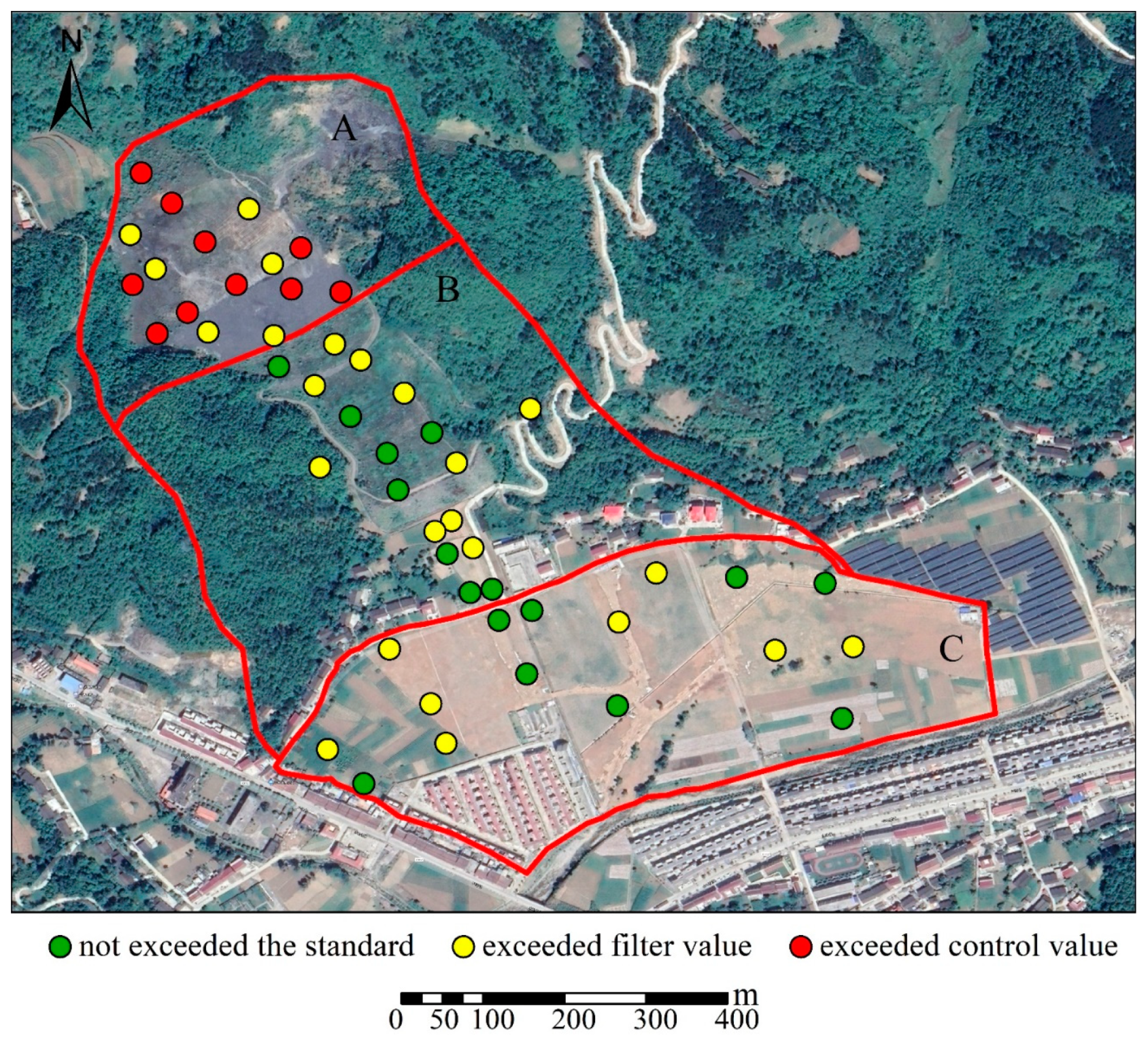

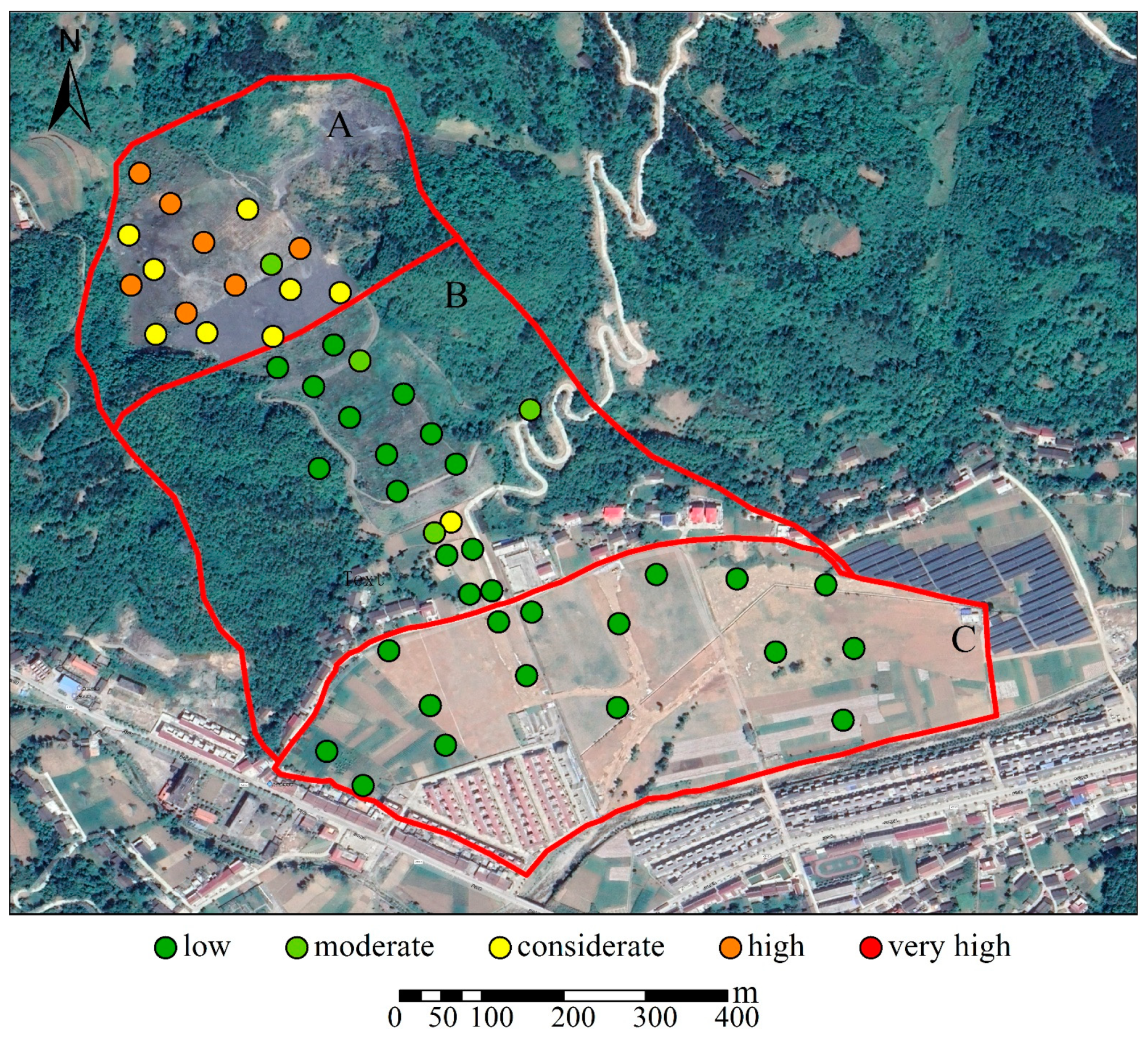

| Sampling Area | Types of Samples Collected |
|---|---|
| The pulp deposition area (A) | Soil samples, Periploca sepium Bunge, Robinia pseudoacacia L., Phragmites communis (Cav.) Trin. ex Steud., Setaria viridis (L.) Beauv., Imperata cylindrical (L.) Beauv., Erigeron annuus (L.) Pers. |
| The hillside (B) | Soil samples, Ailanthus altissima, Macleaya cordata (Willd.) R. Br., Periploca sepium Bunge, Medicago Sativa Linn, Erigeron annuus (L.) Pers., Aster subulatus Michx., Solanum nigrum L., Digitaria sanguinalis (L.) Scop., Polygonum L., Chenopodium album L. |
| The remediation field (C) | Soil samples. |
| Igeo | Pollution Degree | Ei | Pollution Degree |
|---|---|---|---|
| Igeo ≤ 0 | unpolluted | Ei < 40 | low |
| 0 < Igeo ≤ 1 | unpolluted to moderately polluted | 40 ≤ Ei < 80 | moderate |
| 1 < Igeo ≤ 2 | moderately polluted | 80 ≤ Ei < 160 | considerate |
| 2 < Igeo ≤3 | moderately to heavily polluted | 160 ≤Ei < 320 | high |
| 3 < Igeo ≤ 4 | heavily polluted | Ei ≥ 320 | very high |
| 4 < Igeo ≤ 5 | heavily to extremely polluted | ||
| 5 < Igeo ≤ 6 | extremely polluted |
| Site Type | Item | Max | Min | Mean | Coefficient of Variation | Risk Filter Value | Ratio | |
|---|---|---|---|---|---|---|---|---|
| Mining area | A | As (mg·kg−1) | 231.0 | 54.0 | 150.7 | 0.37 | 60 | 2.51 |
| pH | 8.9 | 8.0 | 8.4 | |||||
| B | As (mg·kg−1) | 100.0 | 13.3 | 35.2 | 0.71 | 25 | 1.41 | |
| pH | 8.6 | 7.5 | 8.2 | |||||
| C | As (mg·kg−1) | 41.5 | 16.1 | 25.9 | 0.28 | 25 | 1.03 | |
| pH | 8.4 | 7.5 | 8.1 | |||||
| Sampling Area | Igeo | ||
|---|---|---|---|
| Max | Min | Average | |
| A | 3.78 | 1.68 | 3.04 |
| B | 2.57 | −0.34 | 1.06 |
| C | 1.30 | −0.06 | 0.62 |
| Sampling Area | PERI | ||
|---|---|---|---|
| Max | Min | Average | |
| A | 191.07 | 48.21 | 134.55 |
| B | 89.29 | 11.88 | 31.44 |
| C | 37.05 | 14.38 | 23.11 |
| Family | Formal Name | Growth Form |
|---|---|---|
| Leguminosae | Medicago L. | perennial herb |
| Robinia pseudoacacia L. | macrophanerophyte | |
| Lespedeza bicolor Turcz. | shrub | |
| Sophora davidii (Franch.) Skeels | shrub | |
| Melilotus officinalis (L.) Pall. | perennial herb | |
| Kummerowia striata (Thunb.) Schindl. | annual herb | |
| Medicago Sativa Linn | perennial herb | |
| Gramineae | Phragmites communis (Cav.) Trin. ex Steud. | perennial herb |
| Setaria viridis (L.) Beauv. | annual herb | |
| Imperata cylindrica (L.) Beauv. | perennial herb | |
| Digitaria sanguinalis (L.) Scop. | annual herb | |
| Arthraxon hispidus (Thunb.) Makino | annual herb | |
| Eriochloa villosa (Thunb.) Kunth | annual herb | |
| Compositae | Aster subulatus Michx. | annual herb |
| Erigeron annuus (L.) Pers. | annual herb | |
| Bidens pilosa L. | annual herb | |
| Artemisia carvifolia Buch.-Ham. ex Roxb. Hort. Beng. | annual herb | |
| Ageratina Adenophora (Spreng.) R.M. King et H.Rob. | perennial herb | |
| Sonchus arvensis L. | perennial herb | |
| Inula japonica Thunb. | perennial herb | |
| Artemisia argyi | perennial herb | |
| Solanaceae | Solanum nigrum L. | therophyte |
| Equisetaceae | Equisetum ramosissimum Desf | perennial herb |
| Asclepiadaceae | Periploca sepium Bunge | shrub |
| Simaroubaceae DC. | Ailanthus altissima | macrophanerophyte |
| Chenopodiaceae | Chenopodium album L. | perennial herb |
| Polygonaceae | Polygonum L. | perennial herb |
| Rubiaceae Juss. | Rubia cordifolia L. | perennial herb |
| Moraceae | Humulus scandens (Lour.) Merr. | perennial herb |
| Papaveraceae Juss. | Macleaya cordata (Willd.) R. Br. | perennial herb |
| Valerianaceae | Patrinia scabiosifolia Fisch. ex Trevir. | perennial herb |
| Labiatae | Mentha haplocalyx Briq. | perennial herb |
| Pteridaceae E. D. N. Kirchn. | Pteris vittata L., | Pteridophyta |
| Violaceae Batsch | Viola philippica | perennial herb |
| Index | Correlation Coefficient |
|---|---|
| Sics Abundance Index | −0.498 * |
| Shannon-Wiener Index | −0.423 * |
| Pielou Evenness Index | −0.465 * |
| Area | Plant Species | Plant As Content (mg·kg−1) | Soil As Content (mg·kg−1) | BCF |
|---|---|---|---|---|
| A | Periploca sepium Bunge | 9.6 | 192.0 | 0.050 |
| Robinia pseudoacacia L. | 1.6 | 82.8 | 0.020 | |
| Phragmites communis (Cav.) Trin. ex Steud. | 7.2 | 126.0 | 0.036 | |
| Setaria viridis (L.) Beauv. | 4.9 | 105.0 | 0.047 | |
| Imperata cylindrica (L.) Beauv. | 1.1 | 135.0 | 0.008 | |
| Erigeron annuus (L.) Pers. | 15.9 | 149.0 | 0.107 | |
| B | Periploca sepium Bunge | 0.2 | 14.0 | 0.011 |
| Artemisia carvifolia Buch.-Ham. ex Roxb. Hort. Beng | 0.1 | 43.1 | 0.002 | |
| Erigeron annuus (L.) Pers. | 0.2 | 19.9 | 0.009 | |
| Aster subulatus Michx. | 0.1 | 38.5 | 0.002 | |
| Ailanthus altissima | 0.4 | 27.6 | 0.012 | |
| Macleaya cordata (Willd.) R. Br. | 0.2 | 21.0 | 0.010 | |
| Solanum nigrum L. | 0.3 | 19.8 | 0.013 | |
| Digitaria sanguinalis (L.) Scop. | 0.5 | 37.8 | 0.013 | |
| Chenopodium album L. | 0.8 | 31.9 | 0.024 | |
| Polygonum L. | 0.5 | 33.2 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Han, L.; Liu, Z.; Zhao, Y.; Zhai, Y.; Li, R.; Xia, L. Analysis of Soil As Pollution and Investigation of Dominant Plants in Abandon Gold Mining Area. Minerals 2022, 12, 1366. https://doi.org/10.3390/min12111366
Chen R, Han L, Liu Z, Zhao Y, Zhai Y, Li R, Xia L. Analysis of Soil As Pollution and Investigation of Dominant Plants in Abandon Gold Mining Area. Minerals. 2022; 12(11):1366. https://doi.org/10.3390/min12111366
Chicago/Turabian StyleChen, Rui, Lei Han, Zhao Liu, Yonghua Zhao, Yunmeng Zhai, Risheng Li, and Longfei Xia. 2022. "Analysis of Soil As Pollution and Investigation of Dominant Plants in Abandon Gold Mining Area" Minerals 12, no. 11: 1366. https://doi.org/10.3390/min12111366






