1. Introduction
Cyanide leaching residues produced from the gold mining industry in China are one of the main hazardous wastes, which occupy many lands and generate potentially serious impacts on local environments [
1,
2,
3]. Generally, valuable iron minerals are found in significant amounts in such residues, but their enrichment and recovery are still economically unviable and with low efficiency, due to their ultrafine distributions in the residues [
4,
5]. This outcome mainly results from the requirement for ultrafine grinding of gold ores, to improve the gold and silver leaching efficiency with total-slime cyanidation technology. Another situation encountered in the exploration of gold ores lies in the fact that a certain portion of gold particles are associated with magnetite and limonite minerals, in which the limonite grains are sensitive to the grinding process and, therefore, are very easy to be lost into tailings [
6,
7].
In the past decade, the recovery of iron values from various tailings and leaching residues has become stringent, as the demand for iron materials has been steadily growing. For instance, a reduced roasting-sulfuric acid leaching process was proposed to recover ultrafine iron and gold minerals from a cyanide leaching residue, which contained 29.55% iron and 5.60 g/t gold, and a leaching ratio of 91.26% for iron and 71.43% for gold was, respectively, achieved under the optimized conditions [
8]. Additionally, a heating leaching process with sulfuric acid was also attempted to recover hematite from a cyanide leaching residue and produced an iron leaching ratio reaching as high as 93.33% [
9]. Another technical route of roasting–magnetic separation was proposed to process a cyanide leaching residue, in which hematite is the main iron mineral, and a magnetite concentrate assaying 61.78% iron grade with 60.67% iron recovery was produced [
10]. A similar process was used to recover iron from a high-sulfur cyanide tailing; under strong reduction conditions, a reduced iron product with 90.68% Fe and 92.71% iron recovery was obtained [
11]. Liu et al. proposed a process of chlorination roasting–carbothermic reduction–magnetic separation for the treatment of an Au-bearing cyanide residue and produced an iron concentrate containing 82.17% Fe, with a total Fe recovery of 79.68% [
1]. These processes containing leaching or roasting operations are effective for iron extraction from low grade tailings and residues, but they are complex and include metallurgy treatment such that they become inevitably involved with high energy consumption and low economic viability. In fact, until today, few industrial applications of these processes were reported. Therefore, the effective enrichment of iron minerals prior to metallurgy is commonly required for removing the large amounts of gangue minerals in the residue, due to the technological and economic constraints of direct metallurgy.
Pulsating high-gradient magnetic separation (PHGMS) is one of the most effective methods for enrichment and recovery of ultrafine and weakly magnetic iron minerals, mainly due to its advantages of large processing capacity, low operation cost, high applicability, and environmental friendliness over other separation methods [
2,
12,
13]. Particularly in recent years, superlarge SLon-5000 PHGMS separators with sufficiently high background magnetic induction were successfully developed, and their operating power consumption for per ton ore reached as low as 0.15 kWh. Moreover, the water used in the PHGMS process may be totally reused through gravity settling process, and therefore, it provides strong possibilities for economically and greenly recovering ultrafine iron values from tailings and leaching residues [
14].
In this paper, an attempt was made to evaluate the feasibility of iron recovery from a cyanide leaching residue, which was produced from a superlarge gold mining company in Yunnan Province, using magnetic separation. Detailedly, the occurrence features of iron minerals in the residue were determined by chemical composition analysis, iron phase analysis, and mineral liberation analysis (MLA). Based on these analyses, a technological route of low-intensity magnetic separation (LIMS) and PHGMS was proposed, and the effects of their key operating parameters on the separation performance were elucidated for enrichment and recovery of ultrafine magnetite and limonite from the residue, respectively. Moreover, the magnetic capture of iron minerals was investigated using the size-by-size and chemical analyses of the iron concentrates. The results would provide important technological support for the comprehensive utilization of the residue in industry, increasing the economic and environmental benefits for the company.
2. Experimental
2.1. Description of Cyanide Leaching Residue
The cyanide leaching residue was obtained from the Heqing Beiya Mining Co., Ltd., Dali, China. For this investigation, the residue was naturally air-dried and then gently broken up into powders using a roller. The chemical compositions and iron phase analyses with a deviation less than 0.10% were conducted for the residues, and the results are illustrated in
Table 1 and
Table 2, respectively.
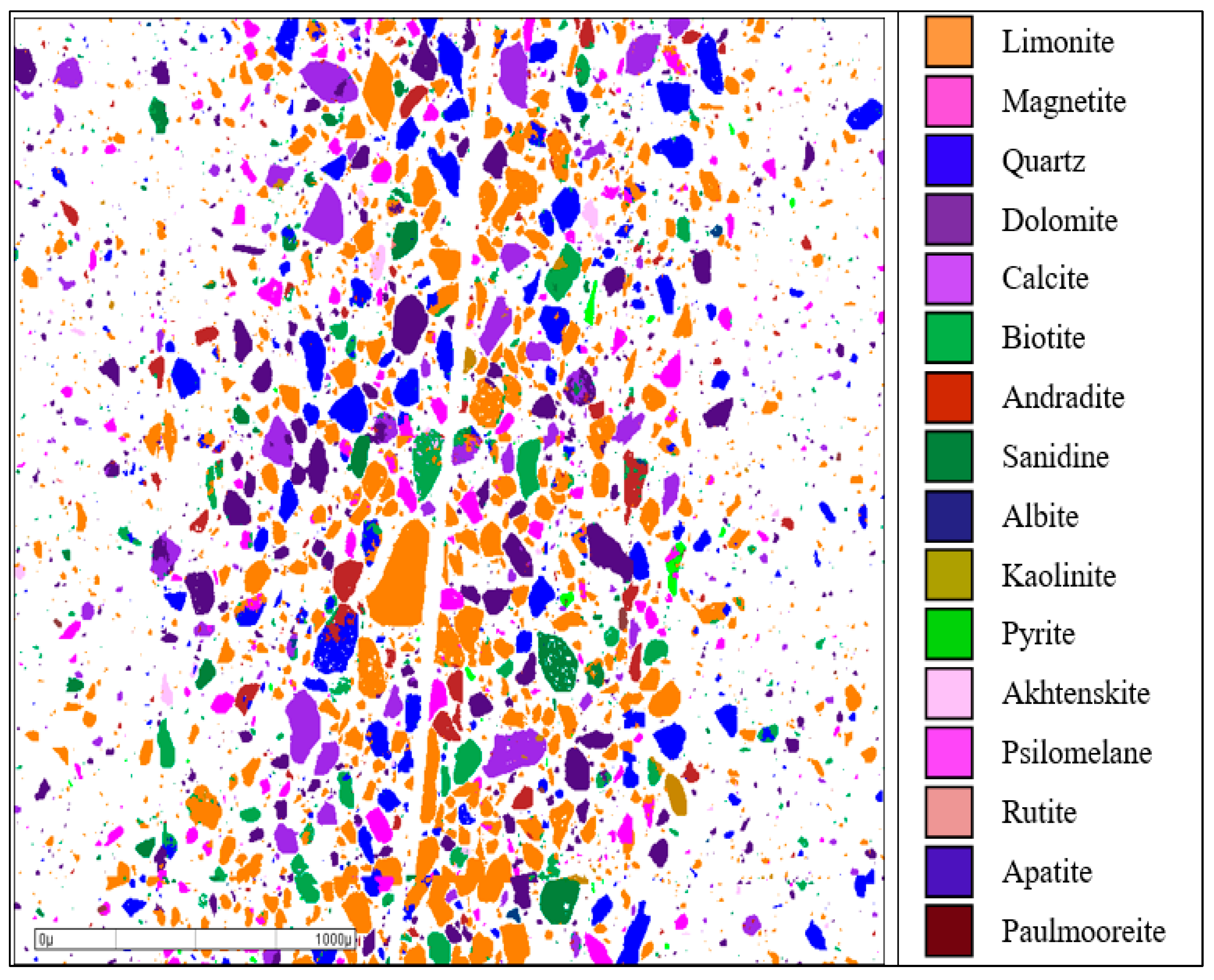
Table 1 shows that the residue contains 26.74% iron, which is mainly distributed in magnetite and limonite particles, as shown in
Table 2. The iron grades of magnetite and limonite in the residue are, respectively, 7.04% and 18.56%, with their iron distributions relative to total iron reaching 26.33% and 69.41%, respectively. The non-magnetic gangues in the residue are mainly composed of quartz, carbonate dolomite, calcite, and a small amount of feldspar and biotite. It is worth noting that the Cu, Pb, and Zn elements with very low grades are mainly dispersed in iron and manganese oxides, coronadite, and sphalerite, respectively; they are defined as unrecoverable minerals using current separation techniques.
The residue sample was classified into several fractions using a series of sieves (+20 μm) and elutriation methods (−20 μm), and the iron grades in each fraction were determined by a chemical method, as shown in
Table 3. It shows that the weight and iron distribution for −30 μm fractions are 60.44% and 58.47%, respectively, indicating that the residue is seriously muddied, and most iron minerals occur as ultrafine grains. This is attributed to the use of the total-slime cyanide leaching method, for gold and silver in the upstream process.
2.2. Liberation Analysis of Magnetite and Limonite in Residue
Approximately 30,000 particles in the residue were automatically observed and counted to representatively reflect the liberation characteristics of target minerals, i.e., magnetite and limonite in the residue through a Mineral Liberation Analysis method (MLA650, FEI Company, Hillsboro, OR, USA). Based on this analysis, an appropriate separation technology for the recovery of ultrafine magnetite and limonite from the residue was determined.
2.3. Descriptions for Magnetic Separations
A wet drum LIMS separator (Φ 400 × 600, Wuhan Prospecting Machinery Factory, Wuhan, China) was used for the recovery of magnetite from the residue; in this separator, the highest magnetic induction of 0.35 T is achievable on the drum surface. When this magnetic separator was being operated, a direct current flow was generated through the energizing coils, resulting in a magnetic field on the drum surface. Firstly, the separating zone of the separator was filled with fresh water. Then, a slurry containing 500 g residue at 25% solid weight was evenly fed into the separator within 60 s. Magnetite particles were attracted from the slurry onto the drum surface and transferred to the concentrate flushing zone by the rotating drum, while weakly magnetic and non-magnetic particles exited from the separating zone to become tailings. The lifted magnetite particles were flushed down by flushing beams to obtain a magnetite concentrate.
The tailings produced from the above LIMS process were further separated through a cyclic pilot-scale PHGMS separator (SLon-100, SLon Magnetic Separator Ltd., Ganzhou, China) to recover limonite particles. This separator has a maximum background magnetic induction of 1.8 T, and for this investigation, a rod matrix was adopted. The separation principle and operation procedure of this separator were described previously [
15,
16].
The produced magnetite and limonite concentrates were filtered, dried, and weighed, with their iron grades determined by chemical analysis. The theoretical iron recovery in the concentrates was calculated by Formula (1).
where
ε (%) is the iron recovery,
γ (%) is the weight of the concentrate, and
α (%) and
β (%) are the iron grades of residue and concentrate, respectively.
4. Conclusions
The cyanide leaching residue contained 26.74% Fe, with iron mainly distributed in the forms of magnetite and limonite. The iron grades from magnetite and limonite in the residue were 7.04% Fe and 18.56%, respectively, reaching 26.33% and 69.41%, respectively, of the total iron. The residue had a proportion of full liberation particles reaching 67.40% for magnetite and 73.00% for limonite, respectively, with particle sizes ranging from 9.6 µm to 75.0 µm. The gangue minerals in the residue were mainly carbonated dolomite and calcite, with a small amount of quartz, feldspar, biotite, and clay minerals.
With a LIMS roughing (0.30 T)–cleaning (0.15 T) process under the optimum conditions, a magnetite concentrate assaying 64.05% Fe at 9.65% mass weight and 23.11% iron recovery was produced from the residue, with magnetite recovery reaching as high as 85.59%. From the tailings of this LIMS process, a qualified limonite concentrate assaying 50.94% Fe at 21.97% mass weight and 41.85% iron recovery was produced through a PHGMS roughing (1.2 T)–cleaning (0.8 T) process under the optimized conditions, with limonite recovery, reaching 54.33%. This LIMS–PHGMS process reached an effective recovery for ultrafine (below 30.00 µm) iron minerals from the residue, which is quantified as 51.46% fraction recovery in the magnetite and limonite concentrates. This research work provides a valuable reference for the comprehensive utilization of iron values from such residues.