Chemical and Spectral Variations between Untreated and Heat-Treated Rubies from Mozambique and Madagascar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heat Treatment Experiments
2.3. Analytical Methods
3. Results
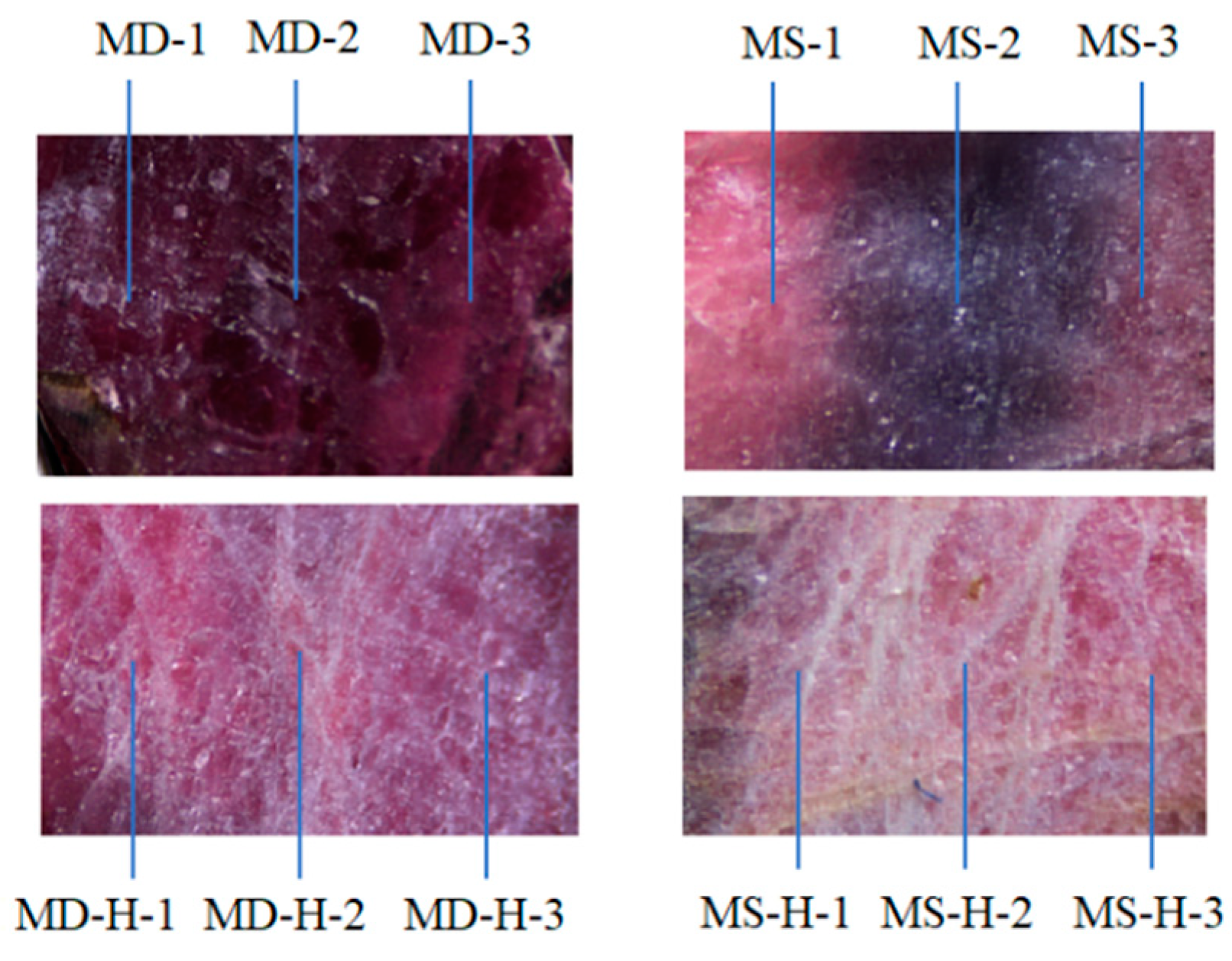
3.1. Microscopic Examination
3.2. Electron Microprobe Analysis
3.3. LA-ICP-MS Analysis
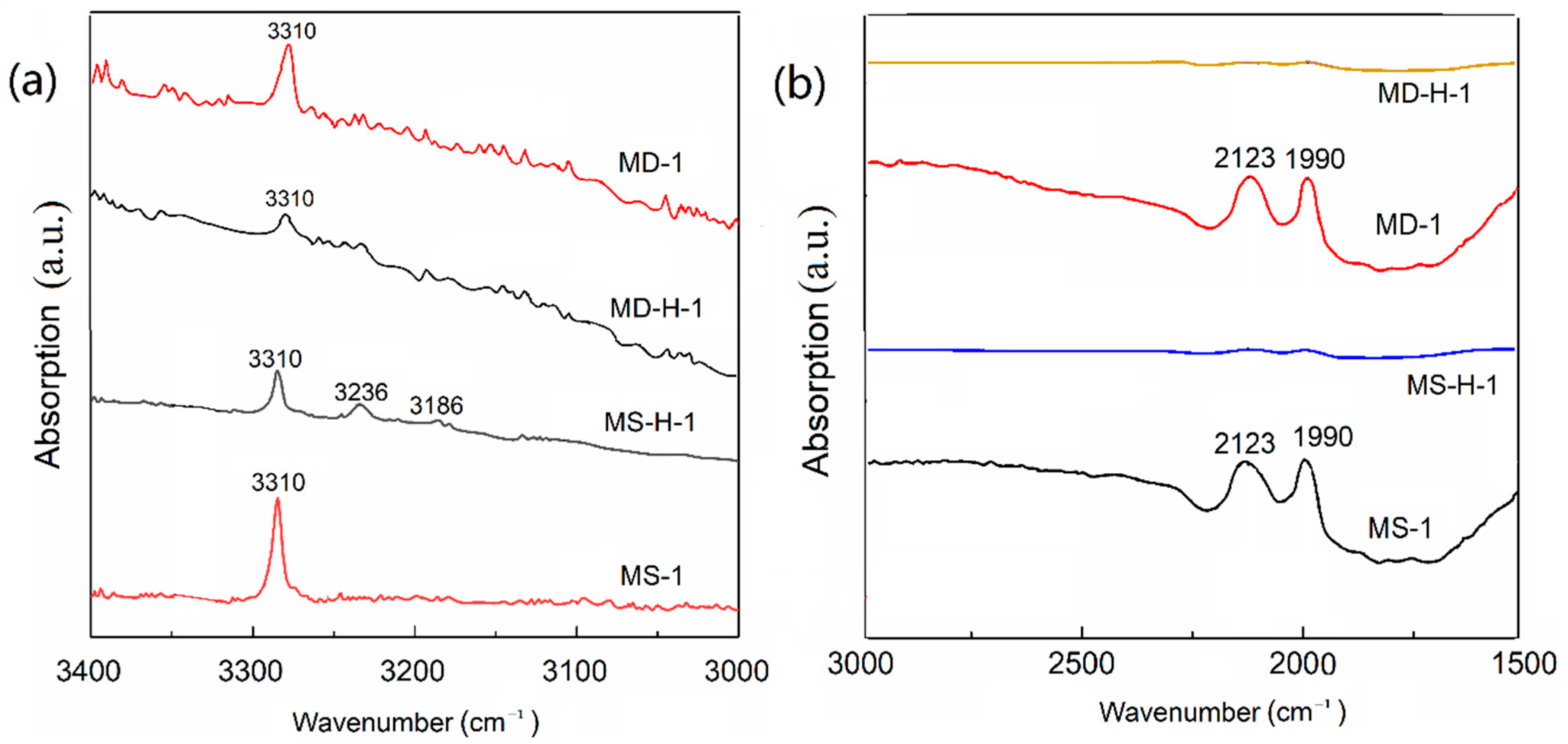
3.4. Infrared Spectra
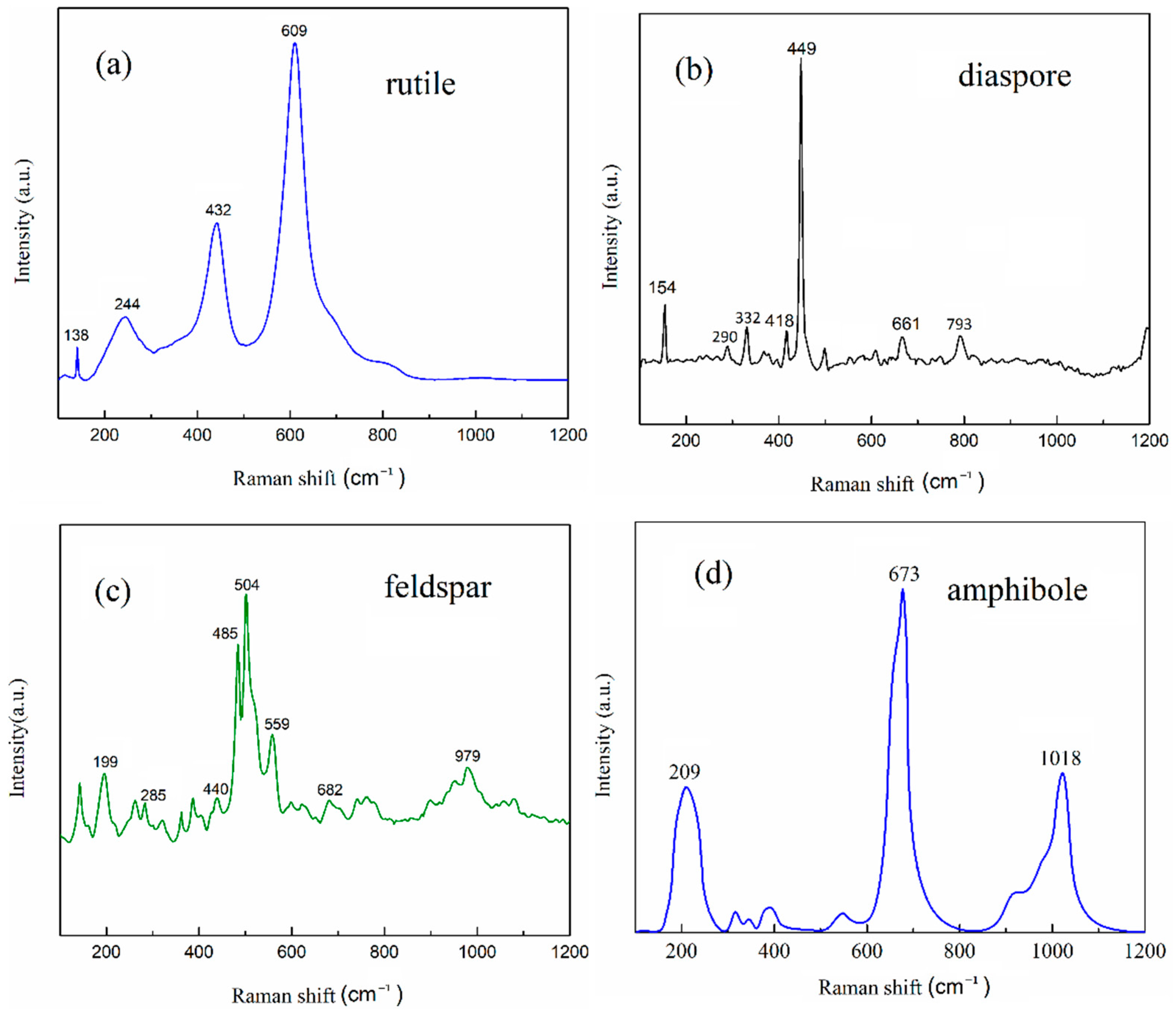
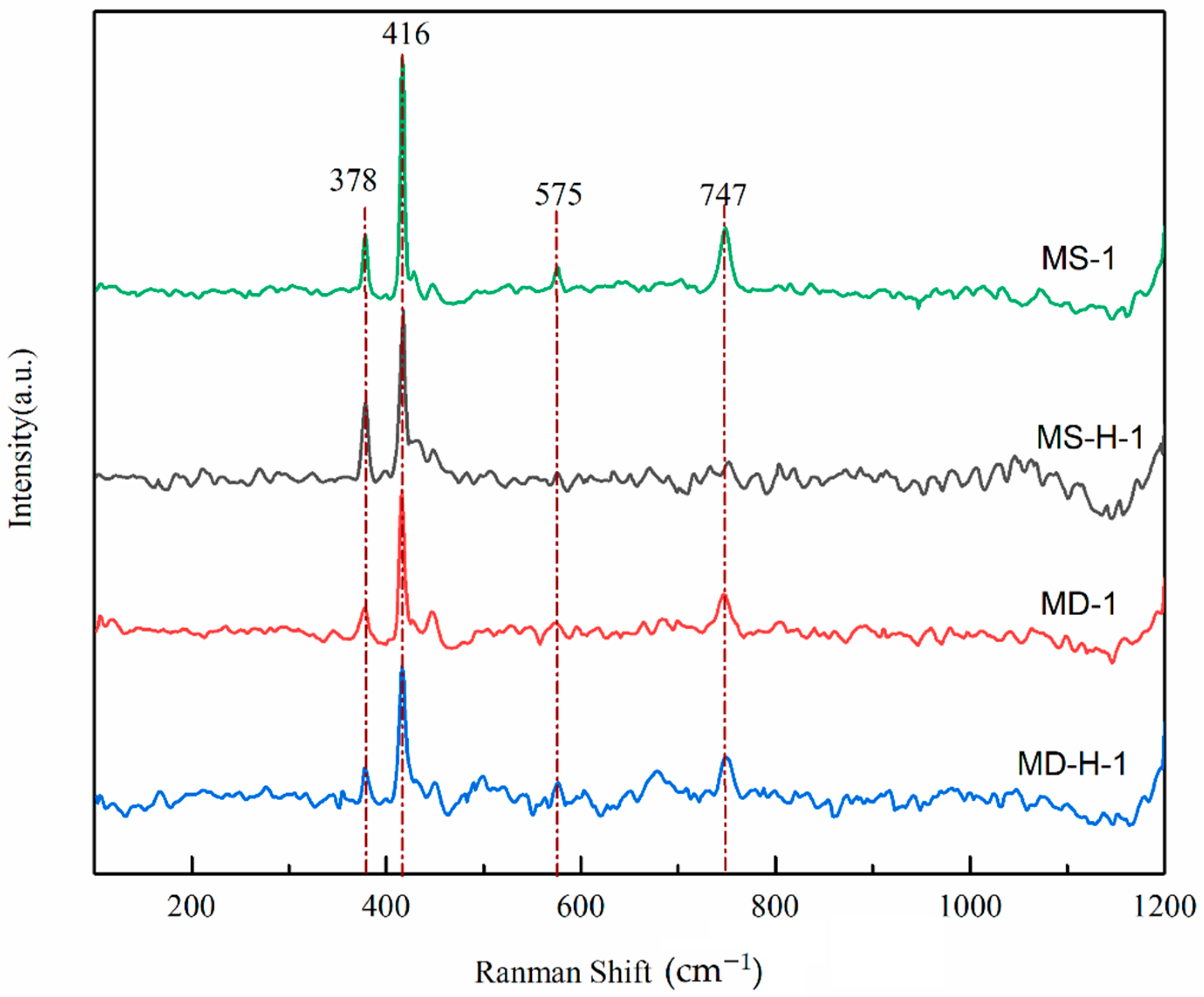
3.5. Raman Spectra
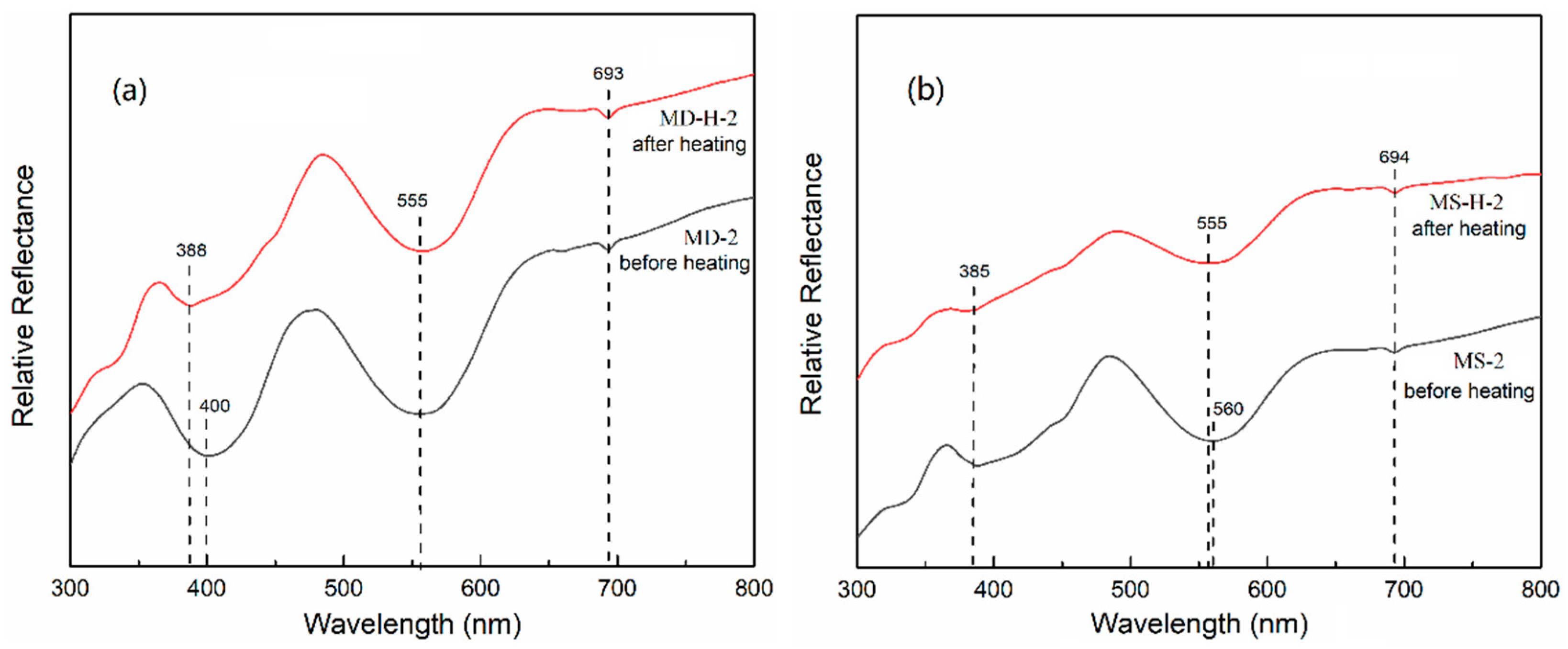
3.6. UV-Visible Spectra
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Zhu, Z.; Hui, L.; Zhang, Z.; Zhang, Y.; Li, G. Al2O3: Cr3+ microfibers by hydrothermal route: Luminescence properties. Mater. Res. Bull. 2012, 47, 2332–2335. [Google Scholar] [CrossRef]
- Achiwawanich, S.; Brack, N.; James, B.D.; Liesegang, J. Surface analysis of heat-treated Mong Hsu rubies. Appl. Surf. Sci. 2006, 252, 8646–8650. [Google Scholar] [CrossRef]
- McClure, S.F.; Smith, C.P.; Wang, W.; Hall, M. Identification and durability of lead glass-filled rubies. Gems Gemol. 2006, 42, 22–34. [Google Scholar] [CrossRef]
- Tengchaisri, T.; Bootkul, D.; Intarasiri, S.; Tippawan, U.; Kuznetsov, A.Y. Coloration changes in natural ruby induced by oxygen ion implants correlated with cathodoluminescence data. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 502, 29–36. [Google Scholar] [CrossRef]
- Achiwawanich, S.; James, B.D.; Liesegang, J. XPS and ToF-SIMS analysis of natural rubies and sapphires heated in an inert (N2) atmosphere. Appl. Surf. Sci. 2007, 253, 6883–6891. [Google Scholar] [CrossRef]
- Swain, S.; Pradhan, S.K.; Jeevitha, M.; Acharya, P.; Debata, M.; Dash, T.; Nayak, B.B.; Mishra, B.K. Microwave heat treatment of natural ruby and its characterization. Appl. Phys. A 2016, 122, 224. [Google Scholar] [CrossRef]
- Wanthanachaisaeng, B.; Sripoonjan, T.; Lhuaamporn, T.; Nilhud, N.; Toaree, S.; Leelawatanasuk, T. Alteration of inclusion in heated Mozambique ruby. In Proceedings of the 5th GIT International Gem and Jewelry Conference (GIT 2016), Pattaya, Thailand, 9–13 November 2016. [Google Scholar]
- Pardieu, V.; Saeseaw, S.; Detroyat, S.; Raynaud, V.; Sangsawong, S.; Bhusrisom, T.; Engniwat, S.; Muyal, J. “Low Temperature” Heat Treatment of Mozambique Ruby—Results Report; GIA: Carlsbad, CA, USA, 16 April 2015; pp. 1–34. [Google Scholar]
- Peretti, A.; Schmetzer, K.; Bernhardt, H.J.; Mouawad, F. Rubies from Mong Hsu. Gems Gemol. 1995, 31, 2–26. [Google Scholar] [CrossRef]
- Emmett, J.L.; Scarratt, K.; McClure, S.F.; Moses, T.; Douthit, T.R.; Hughes, R.; Novak, S.; Shigley, J.E.; Wang, W.; Bordelon, O.; et al. Beryllium Diffusion of ruby and sapphire. Gems Gemol. 2003, 39, 1–52. [Google Scholar] [CrossRef]
- Chulapakorn, T.; Intarasiri, S.; Bootkul, D.; Singkarat, S. Identification of deposit types of natural corundum by PIXE. Nucl. Instrum. Methods Phys. Res. B 2014, 331, 108–112. [Google Scholar] [CrossRef]
- Palke, A.C.; Saeseaw, S.; Renfro, N.D.; Sun, Z.; McClure, S.F. Geographic Origin Determination of Ruby. Gems Gemol. 2019, 55, 580–612. [Google Scholar] [CrossRef]
- Sripoonjan, T.; Wanthanachaisaeng, B.; Leelawatanasuk, T. Phase transformation of epigenetic iron staining: Indication of low-temperature heat treatment in Mozambique ruby. J. Gemol. 2016, 35, 156–161. [Google Scholar] [CrossRef]
- Monarumit, N.; Lhuaamporn, T.; Satitkune, S.; Wongkokua, W. Effect of Beryllium Heat Treatment in Synthetic Ruby. J. Appl. Spectrosc. 2019, 86, 486–492. [Google Scholar] [CrossRef]
- Stone-Sundberg, J.; Thomas, T.; Sun, Z.; Guan, Y.; Cole, Z.; Equall, R.; Emmett, J.L. Accurate Reporting of Key Trace Elements in Ruby and Sapphire Using Matrix-Matched Standards. Gems Gemol. 2017, 53, 438–451. [Google Scholar] [CrossRef]
- Giuliani, G.; Groat, L.A.; Fallick, A.E.; Pignatelli, I.; Pardieu, V. Ruby Deposits: A review and geological classification. Minerals 2020, 10, 597. [Google Scholar] [CrossRef]
- Wu, G.H.; Zhou, H.Y.; Zhang, H.S.; Ling, H.F.; Ma, W.; Zhao, H.Q.; Chen, J.L.; Liu, J.H. New index of ferromanganese crusts reflecting oceanic environmental oxidation. Sci. China Ser. D Earth Sci. 2007, 50, 371–384. [Google Scholar] [CrossRef]
- Calligaro, T.; Poirot, J.P.; Querre, P. Trace element finger printing of jewelry rubies by external beam PIXE. Beam Interact. Mater. At. 1999, 150, 628–634. [Google Scholar]
- Yang, T.; Sun, X.; Shi, G.; Li, D.; Zhou, H. The genetic linkage between the Yuanjiang marble-hosted ruby deposit and Cenozoic tectonic evolution of the Ailao Shan-Red River shear zone (Southwest China). J. Asian Earth Sci. 2019, 177, 38–47. [Google Scholar] [CrossRef]
- Giuliani, G.; Groat, L.A. Geology of corundum and emerald gem deposits: A review. Gems Gemol. 2019, 55, 464–489. [Google Scholar] [CrossRef]
- Cartier, L.E. Ruby and sapphire from Marosely Madagascar. J. Gemol. 2009, 31, 171–179. [Google Scholar] [CrossRef]
- Saeseaw, S.; Kongsomart, B.; Atikarnsakul, U.; Khowpong, C.; Vertriest, W.; Soonthorntantikul, W. Update on “Low-Temperature” Heat Treatment of Mozambican Ruby: A Focus on Inclusions and FTIR Spectroscopy; GIA: Carlsbad, CA, USA, 30 April 2018; pp. 1–47. [Google Scholar]
- Pardiu, V.; Thanachakaphad, J. Rubies reportedly from Mozambique. Gems Gemol. 2012, 48, 149–150. [Google Scholar]
- Beran, A.; Rossman, G.R. OH in naturally occurring corundum. Eur. J. Miner. 2006, 18, 441–447. [Google Scholar] [CrossRef]
- Sinha, J.K.; Mishra, P.K. Spectroscopic and microstructural studies of ruby gemstones of Sinapalli, Odisha. J. Geol. Soc. India 2015, 86, 657–662. [Google Scholar] [CrossRef]
- Hara, Y.; Nicol, M. Raman spectra and the structure of rutile at high pressures. Phys. Status Solidi 2010, 94, 317–322. [Google Scholar] [CrossRef]
- Yang, T.; Sun, X.; Shi, G.; Liu, Y. LA-ICP-MS U–Pb Dating of Cenozoic Rutile Inclusions in the Yuanjiang Marble-Hosted Ruby Deposit, Ailao Shan Complex, Southwest China. Minerals 2021, 11, 433. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. Comparison of Raman spectra in characterizing gibbsite, bayerite, diaspore and boehmite. J. Raman Spectrosc. 2001, 32, 745–750. [Google Scholar] [CrossRef]
- Mernagh, T.P. Use of the laser Raman microprobe for discrimination amongst feldspar minerals. J. Raman Spectrosc. 2010, 22, 453–457. [Google Scholar] [CrossRef]
- Chopelas, A. Single crystal Raman spectra of forsterite, fayalite, and monticellite. Am. Mineral. 1991, 76, 1101–1109. [Google Scholar]
- Karampelas, S.; Wrle, M.; Hunger, K.; Lanz, H. Micro-Raman spectroscopy on two chalices from the Benedictine Abbey of Einsiedeln: Identification of gemstones. J. Raman Spectrosc. 2012, 43, 1833–1838. [Google Scholar] [CrossRef]
- Raghavan, S.; Imbrie, P.K.; Crossley, W.A. Spectral Analysis of R-lines and Vibronic Sidebands in the Emission Spectrum of Ruby Using Genetic Algorithms. Appl. Spectrosc. 2008, 62, 759. [Google Scholar] [CrossRef]
- Gaudry, E.; Cabaret, D.; Sainctavit, P.; Brouder, C.; Mauri, F.; Goulon, J.; Rogalev, A. Structural relaxations around Ti, Cr and Fe impurities in Al2O3; Probed by X-ray absorption near-edge structure combined with first-principles calculations. J. Phys. Condens. Matter 2005, 17, 5467–5480. [Google Scholar] [CrossRef][Green Version]
- Sorokina, E.S.; Litvinenko, A.K.; Hofmeister, W.; Häger, T.; Jacob, D.E.; Nasriddinov, Z.Z. Rubies and Sapphires from Snezhnoe, Tajikistan. Gems Gemol. 2015, 51, 160–175. [Google Scholar] [CrossRef]
- Garnier, V.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Dubessy, J.; Banks, D.; Vinh, H.Q.; Lhomme, T.; Maluski, H.; Pêcher, A.; et al. Marble hosted ruby deposits from Central and Southeast Asia: Towards a new genetic model. Ore Geol. Rev. 2008, 34, 169–191. [Google Scholar] [CrossRef]
- Groat, L.A.; Giuliani, G.; Stone-Sundberg, J.; Renfro, N.D.; Sun, Z. A review of analytical methods used in geographic origin determination of gemstones. Gems Gemol. 2019, 55, 512–535. [Google Scholar] [CrossRef]
- Majumdar, A.S.; Mathew, G. Distinct Ruby Suite at Sardapur, Orissa: A spectroscopic investigation. J. Geol. Soc. India 2012, 80, 715–722. [Google Scholar] [CrossRef]
- Sorokina, E.S.; Hofmeister, W.; Häger, T.; Mertz-Kraus, R.; Buhre, S.; Saul, J.M. Morphological and chemical evolution of corundum (ruby and sapphire): Crystal ontogeny reconstructed by EPMA, LA-ICPMS, and Cr3+ Raman mapping. Am. Mineral. 2016, 101, 2716–2722. [Google Scholar] [CrossRef]
- Garnier, V.; Ohnenstetter, D.; Giuliani, G.; Blanc, P.; Schwarz, D. Trace-element contents and cathodoluminescence of “Trapiche” rubies from Mong Hsu (Myanmar): Geological significance. Miner. Petrol. 2002, 76, 179–193. [Google Scholar] [CrossRef]
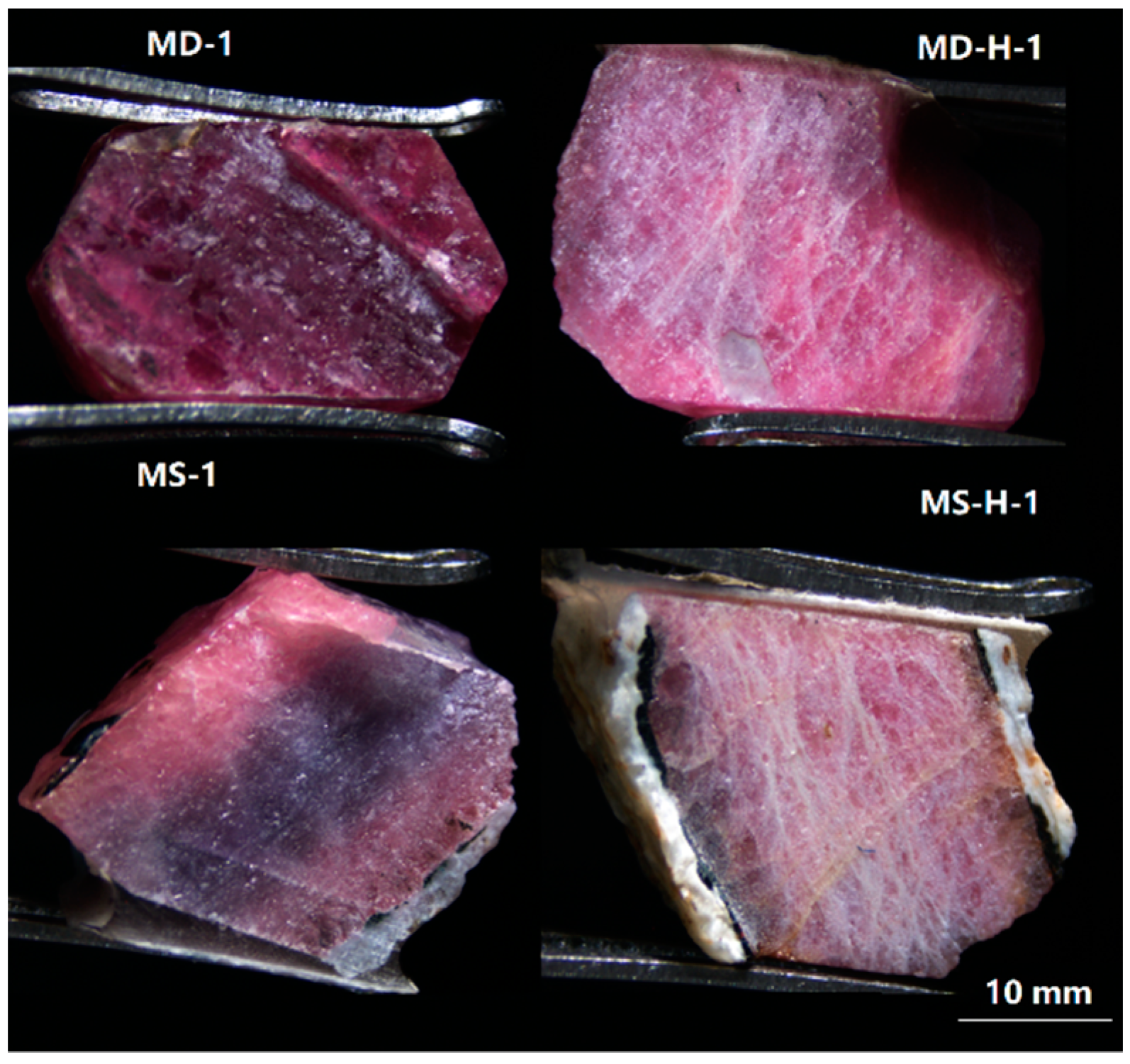
| Label | Color | Transparency | Weight (ct) | Specific Gravity (SG) | Appearance |
|---|---|---|---|---|---|
| MD-1 | pink | slightly transparent | 15.27 | 3.98 | crack |
| MD-2 | pink | slightly transparent | 11.26 | 3.97 | cleavage, crack |
| MD-3 | pink | slightly transparent | 11.26 | 3.98 | cleavage, crack |
| MD-4 | pink | slightly transparent | 11.26 | 3.97 | cleavage, crack |
| MD-5 | pink | slightly transparent | 11.26 | 3.98 | cleavage, crack |
| MD-6 | pink | slightly transparent | 11.26 | 3.97 | cleavage, crack |
| MS-1 | purple red | opaque | 18.54 | 3.97 | cleavage |
| MS-2 | purple red | opaque | 14.35 | 3.98 | cleavage |
| MS-3 | purple red | opaque | 10.02 | 3.96 | cleavage |
| MS-4 | purple red | opaque | 8.14 | 3.97 | yellow |
| MS-5 | purple red | opaque | 12.38 | 3.98 | cleavage |
| MS-6 | purple red | opaque | 11.21 | 3.96 | cleavage |
| MS-7 | purple red | opaque | 16.54 | 3.98 | crack |
| MS-8 | purple red | opaque | 13.75 | 3.98 | crack |
| Label | Al2O3 | Na2O | Cr2O3 | SiO2 | TiO2 | MgO | FeO | MnO | NiO |
|---|---|---|---|---|---|---|---|---|---|
| MD-1 | 99.489 | 0.010 | 0.156 | 0.021 | 1.078 | 0.007 | 0.664 | 0.012 | 0.041 |
| MD-2 | 99.424 | 0.033 | 0.174 | 0.032 | 0.559 | 0.011 | 0.567 | 0.019 | 0.010 |
| MD-3 | 98.809 | 0.079 | 0.241 | 0.015 | 1.675 | 0.009 | 0.551 | 0.021 | 0.029 |
| Average | 99.241 | 0.041 | 0.190 | 0.023 | 1.104 | 0.009 | 0.594 | 0.017 | 0.027 |
| MD-H-1 | 98.308 | 0.019 | 0.271 | 0.005 | 0.559 | 0.012 | 0.616 | 0.016 | 0.062 |
| MD-H-2 | 99.275 | 0.020 | 0.179 | 0.002 | 0.24 | 0.013 | 0.420 | 0.010 | 0.024 |
| MD-H-3 | 98.632 | 0.016 | 0.376 | 0.010 | 1.397 | 0.014 | 0.481 | 0.019 | 0.029 |
| Average | 98.738 | 0.018 | 0.275 | 0.006 | 0.732 | 0.013 | 0.506 | 0.015 | 0.038 |
| MS-1 | 98.925 | 0.026 | 0.080 | 0.012 | 1.665 | 0.006 | 0.364 | 0.009 | 0.017 |
| MS-2 | 98.782 | 0.012 | 0.102 | 0.031 | 1.678 | 0.012 | 0.392 | 0.022 | 0.019 |
| MS-3 | 98.216 | 0.024 | 0.093 | 0.021 | 2.477 | 0.016 | 0.301 | 0.021 | 0.018 |
| Average | 98.641 | 0.021 | 0.092 | 0.021 | 1.940 | 0.011 | 0.352 | 0.017 | 0.018 |
| MS-H-1 | 99.201 | 0.031 | 0.117 | 0.017 | 1.397 | 0.021 | 0.349 | 0.019 | 0.021 |
| MS-H-2 | 98.956 | 0.021 | 0.132 | 0.029 | 1.518 | 0.009 | 0.382 | 0.015 | 0.025 |
| MS-H-3 | 99.251 | 0.012 | 0.121 | 0.004 | 1.199 | 0.017 | 0.247 | 0.019 | 0.140 |
| Average | 99.136 | 0.021 | 0.123 | 0.017 | 1.371 | 0.016 | 0.326 | 0.018 | 0.062 |
| Label | Cr (ppm) | Fe (ppm) | Ti (ppm) | Mg (ppm) | Ga (ppm) | V (ppm) |
|---|---|---|---|---|---|---|
| MD-1 | 842 | 3230 | 71.8 | 46.1 | 89.7 | 15.4 |
| MD-2 | 880 | 3250 | 66.1 | 44.9 | 91.1 | 16.9 |
| MD-3 | 838 | 3178 | 65.0 | 43.5 | 80.3 | 16.0 |
| Average | 853 | 3219 | 67.6 | 44.8 | 87.0 | 16.1 |
| MD-H-1 | 1083 | 3080 | 38.0 | 24.5 | 87.8 | 14.7 |
| MD-H-2 | 1142 | 2894 | 36.0 | 23.5 | 80.3 | 14.0 |
| MD-H-3 | 1047 | 2992 | 36.5 | 24.2 | 81.5 | 13.8 |
| Average | 1090 | 2989 | 38.8 | 24.1 | 83.2 | 14.2 |
| MS-1 | 650 | 1768 | 42.1 | 25.4 | 38.4 | 2.4 |
| MS-2 | 640 | 1702 | 35.9 | 26.0 | 36.3 | 2.4 |
| MS-3 | 703 | 1680 | 40.9 | 37.6 | 37.2 | 2.6 |
| Average | 664 | 1717 | 39.6 | 29.7 | 37.3 | 2.5 |
| MS-H-1 | 684 | 1455 | 34.2 | 34.4 | 35.9 | 2.5 |
| MS-H-2 | 703 | 1500 | 30.5 | 38.8 | 33.5 | 2.4 |
| MS-H-3 | 767 | 1740 | 38.0 | 24.5 | 36.0 | 2.3 |
| Average | 718 | 1565 | 34.2 | 32.6 | 35.1 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Li, X.; Sun, L.; Qin, B. Chemical and Spectral Variations between Untreated and Heat-Treated Rubies from Mozambique and Madagascar. Minerals 2022, 12, 894. https://doi.org/10.3390/min12070894
Lu Q, Li X, Sun L, Qin B. Chemical and Spectral Variations between Untreated and Heat-Treated Rubies from Mozambique and Madagascar. Minerals. 2022; 12(7):894. https://doi.org/10.3390/min12070894
Chicago/Turabian StyleLu, Qi, Xinyi Li, Lihua Sun, and Binrong Qin. 2022. "Chemical and Spectral Variations between Untreated and Heat-Treated Rubies from Mozambique and Madagascar" Minerals 12, no. 7: 894. https://doi.org/10.3390/min12070894
APA StyleLu, Q., Li, X., Sun, L., & Qin, B. (2022). Chemical and Spectral Variations between Untreated and Heat-Treated Rubies from Mozambique and Madagascar. Minerals, 12(7), 894. https://doi.org/10.3390/min12070894






