Recovery of Rare Earth Elements from Mining Tailings: A Case Study for Generating Wealth from Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Reagents
2.2. Analysis of Mineral Species
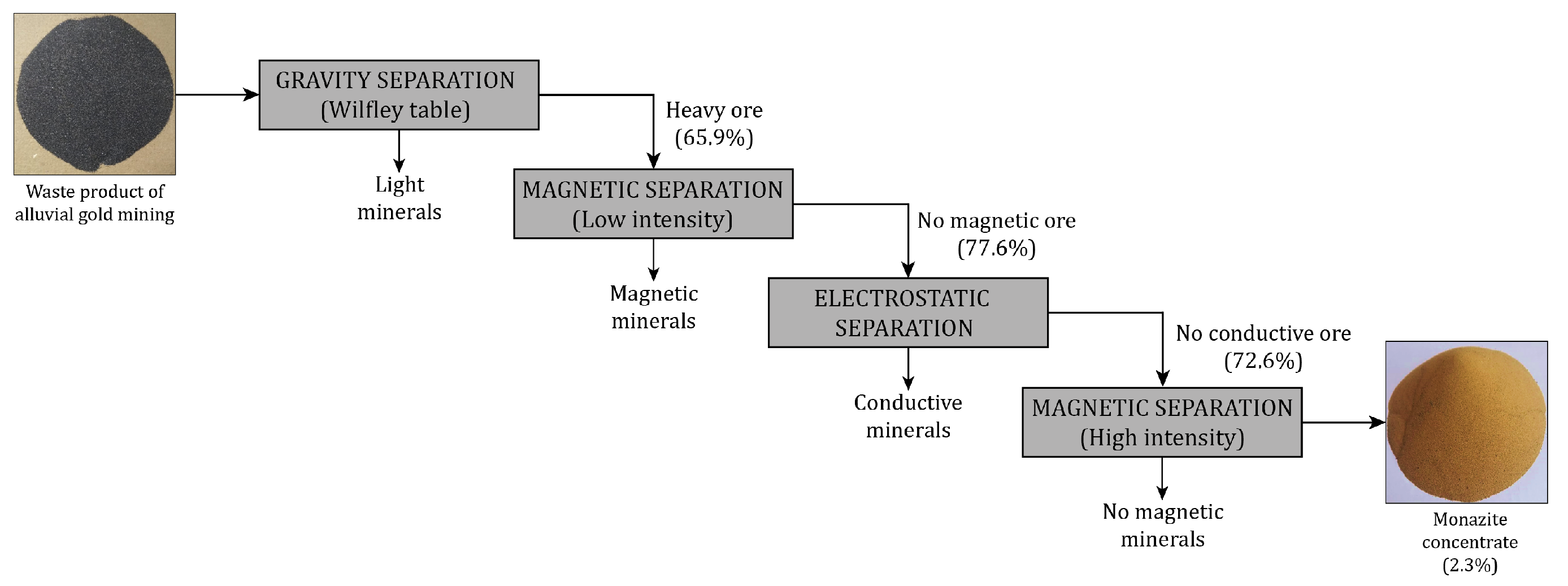
2.3. Obtaining Monazite Concentrate
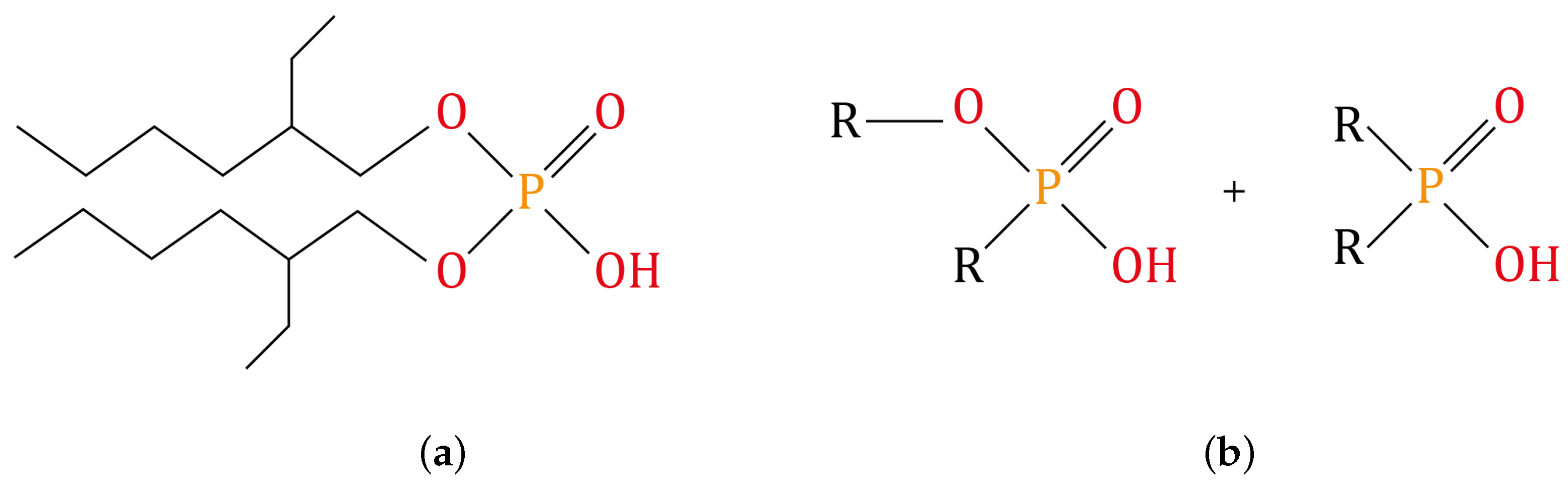
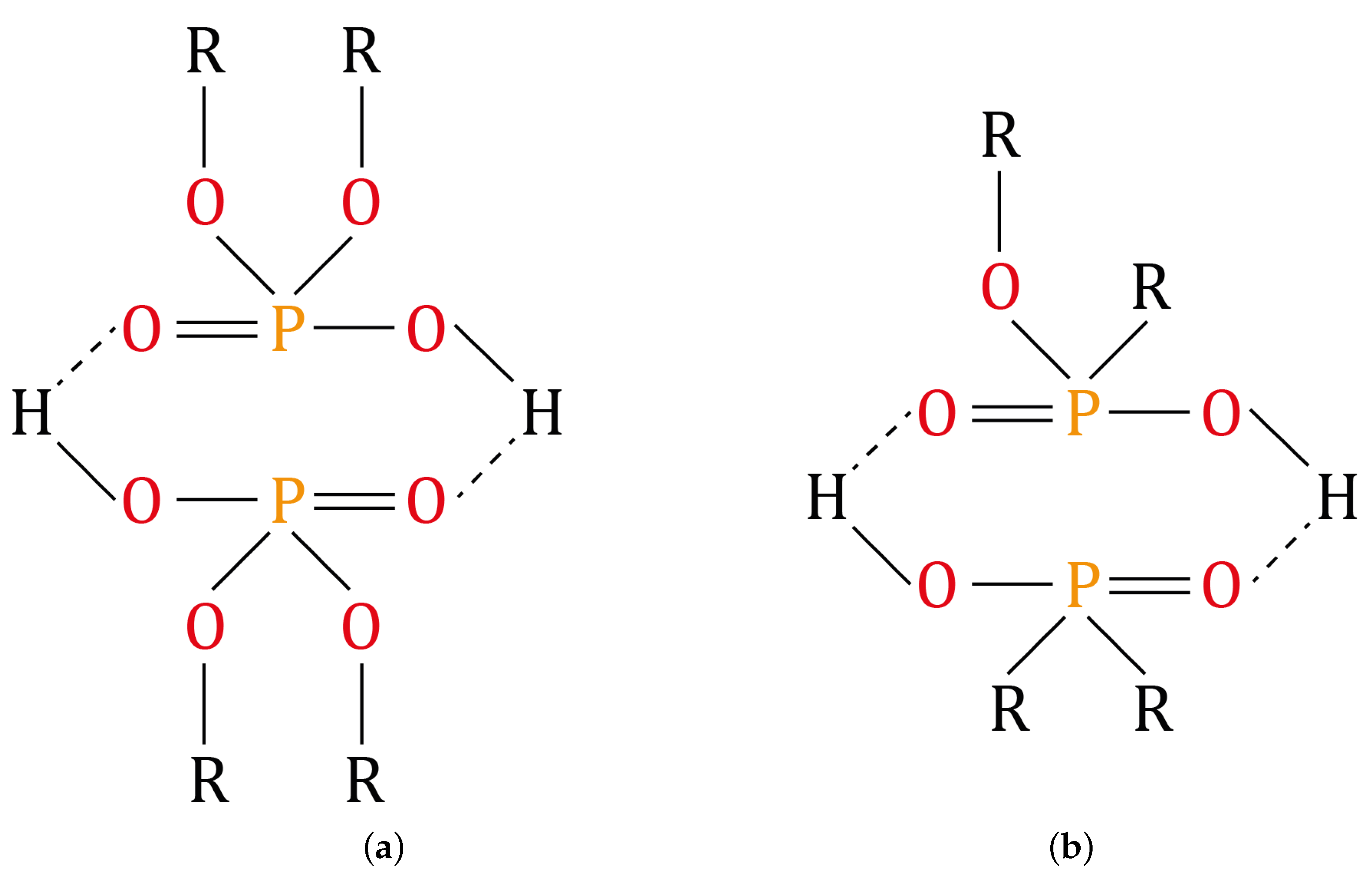
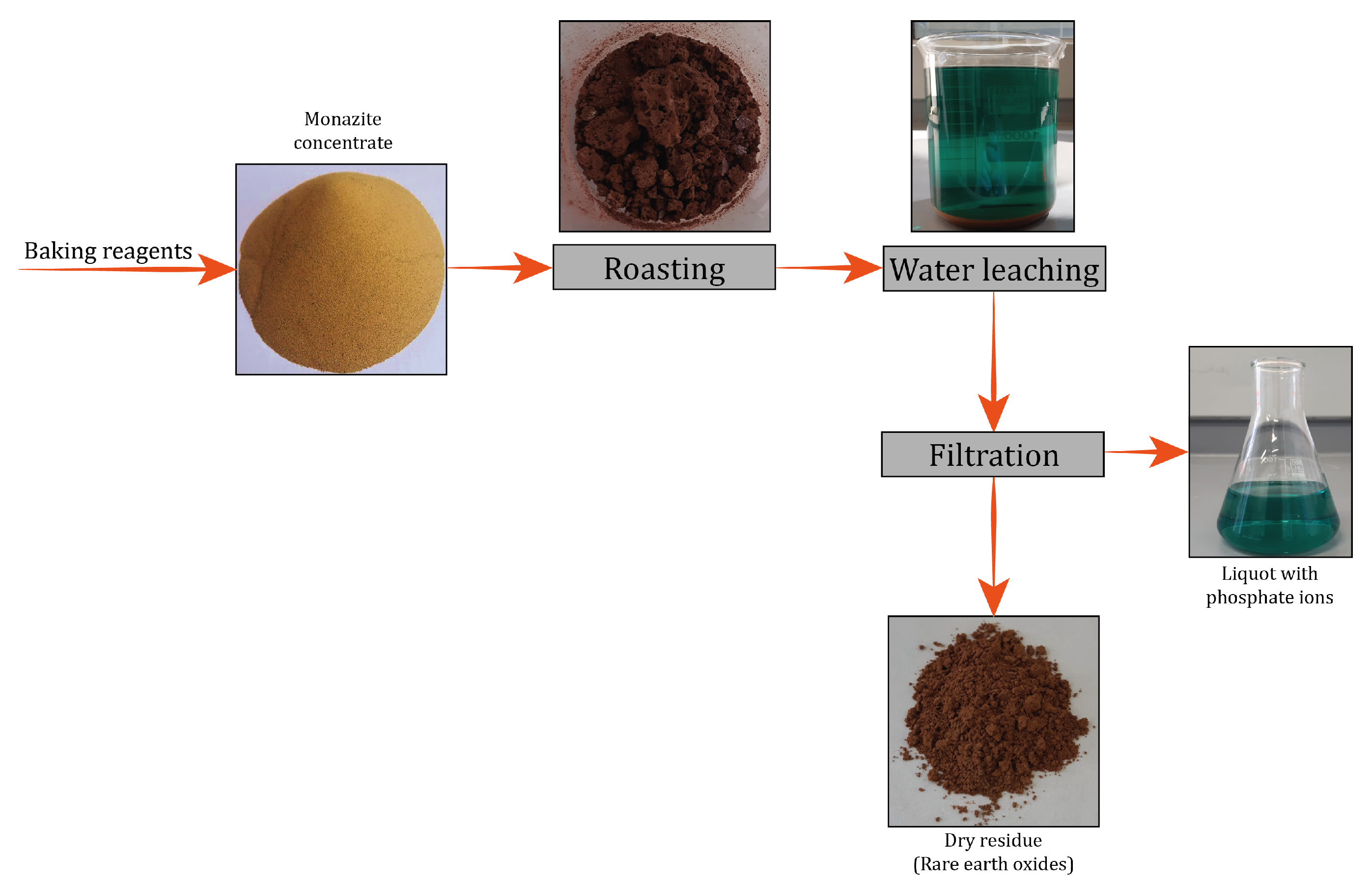
2.4. Dephosphorization of the Monazite Concentrate
2.5. Leaching of the Dephosphorized Product
2.6. Solvent Extraction (SX)
3. Results and Discussion
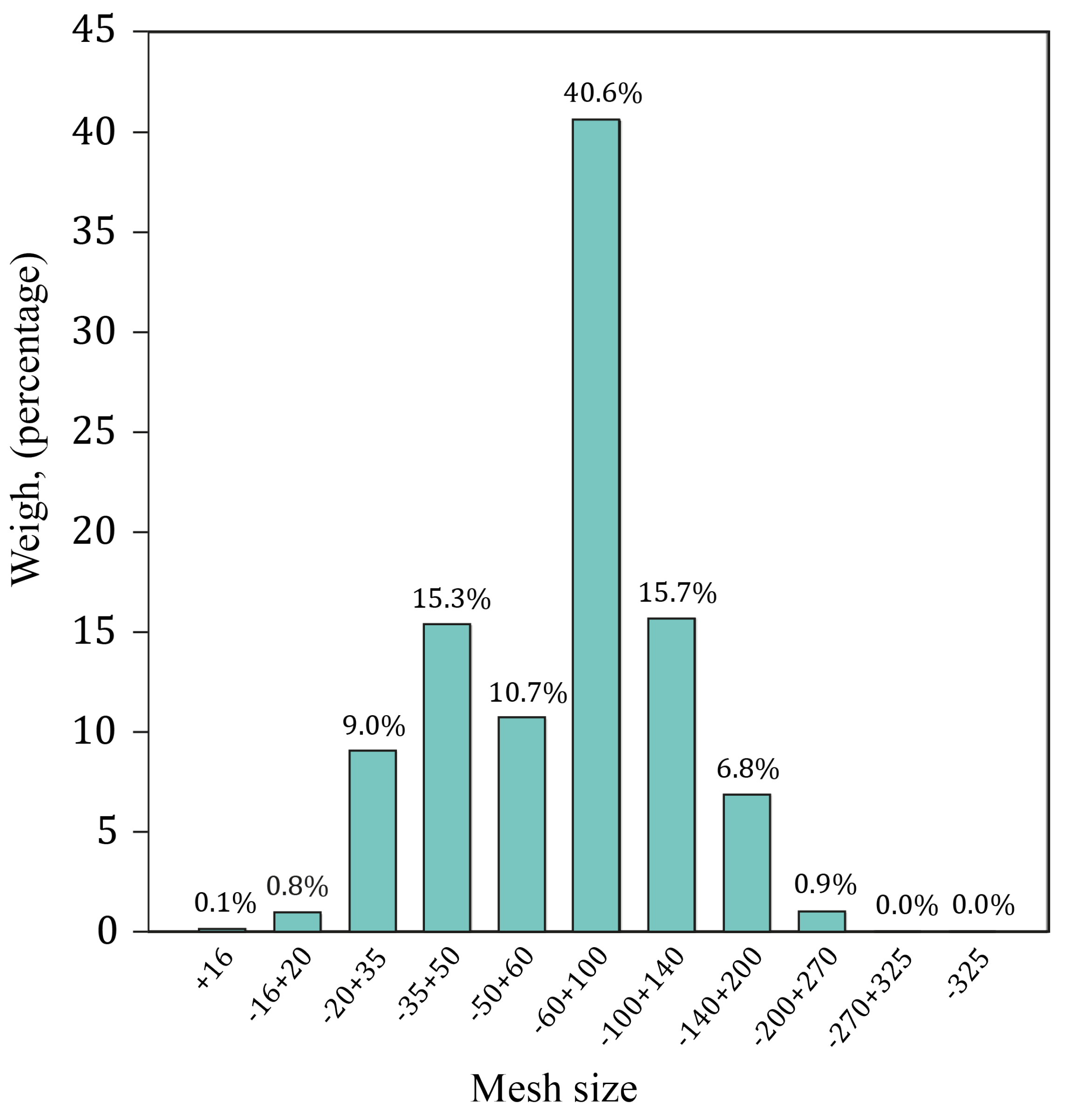
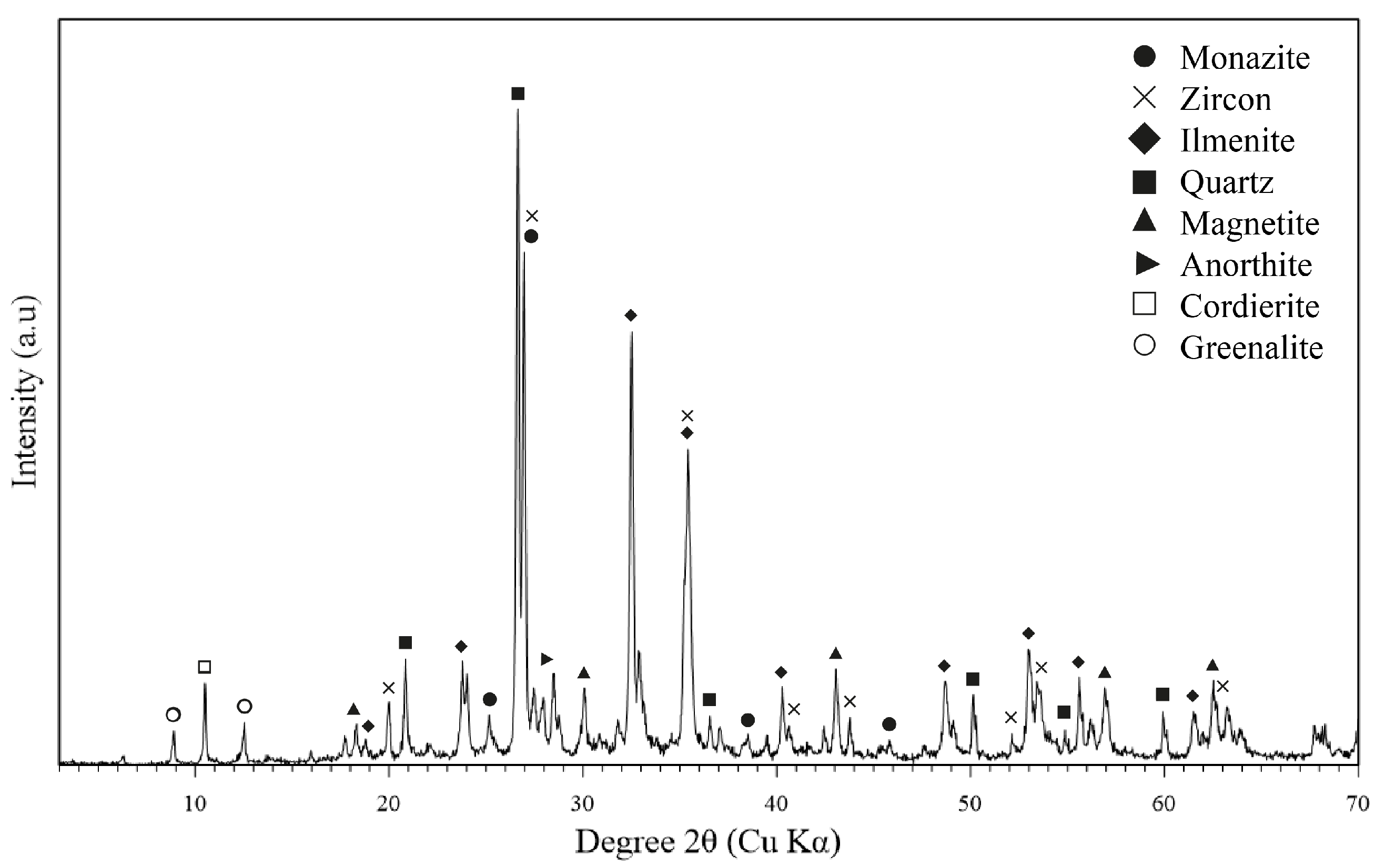
3.1. Alluvial Mining Waste Analysis
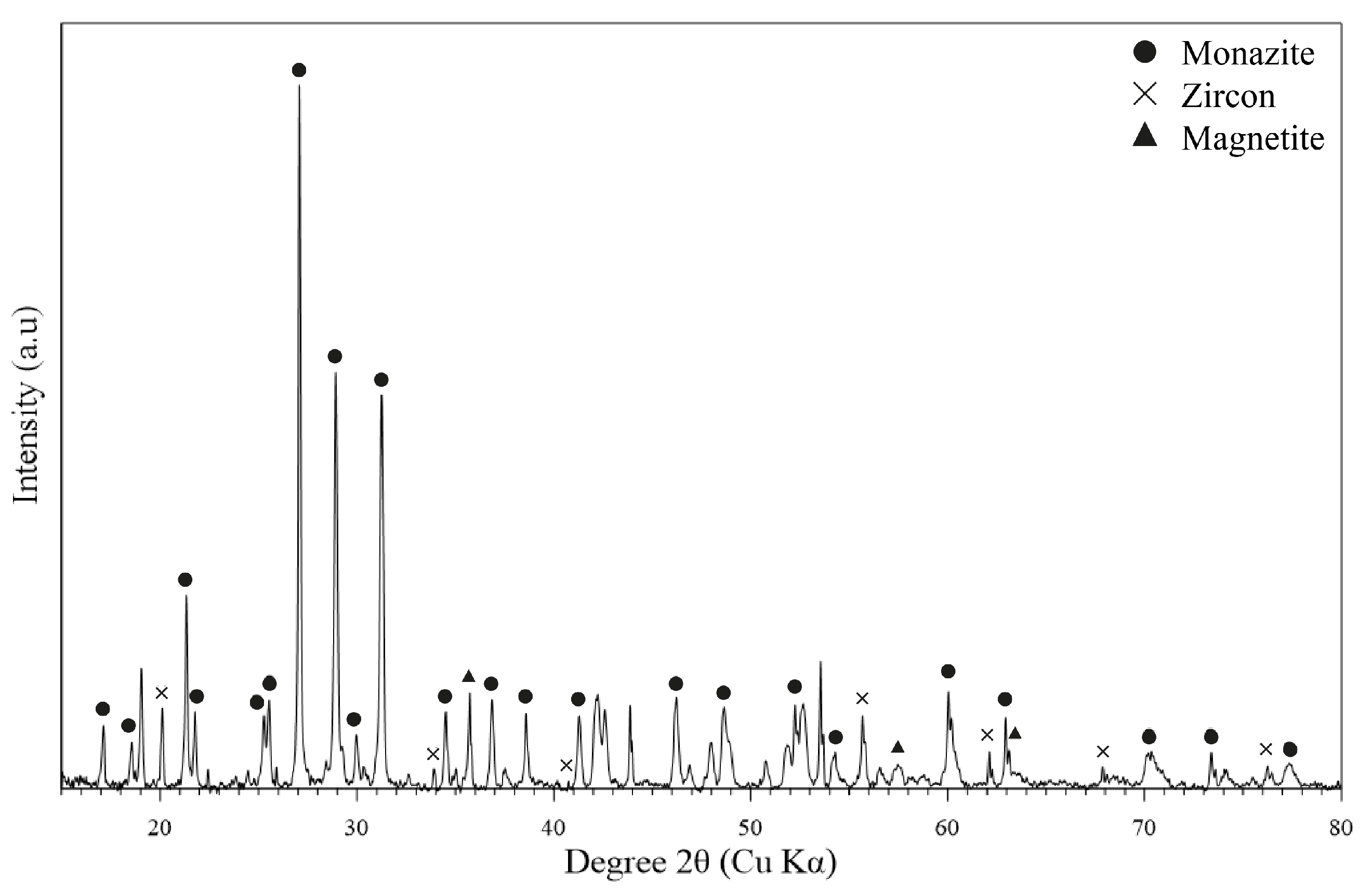
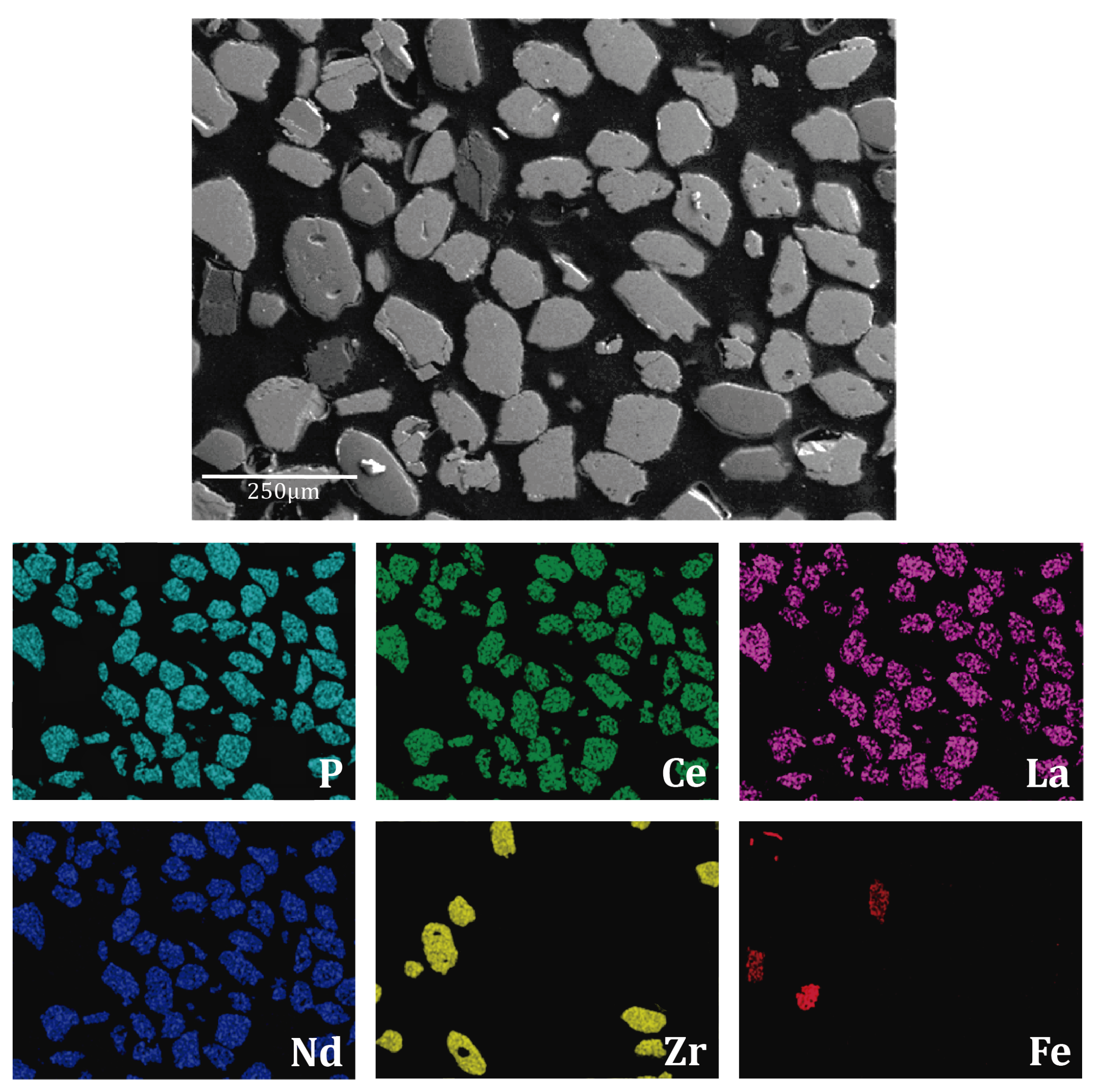
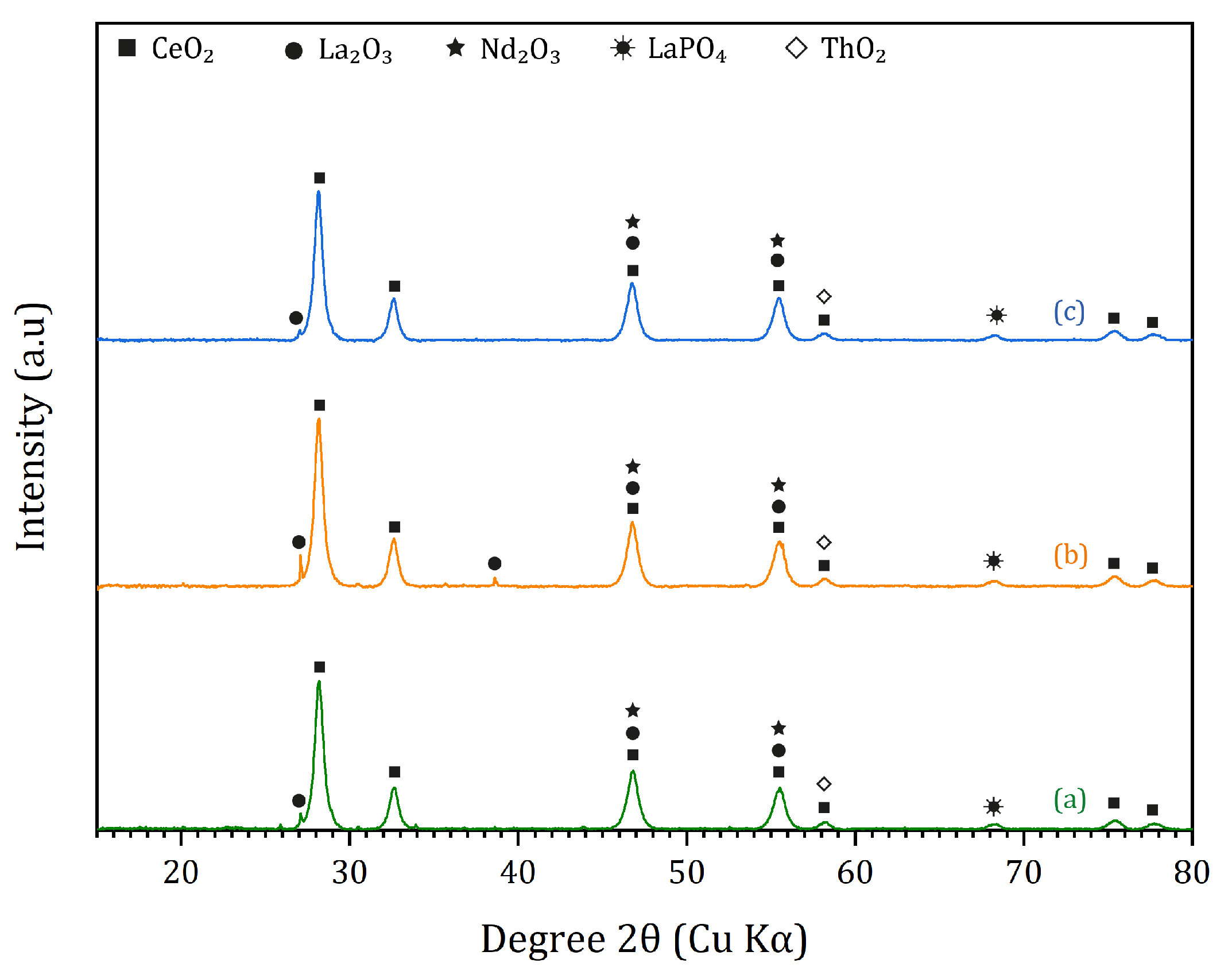
3.2. Monazite Concentrate
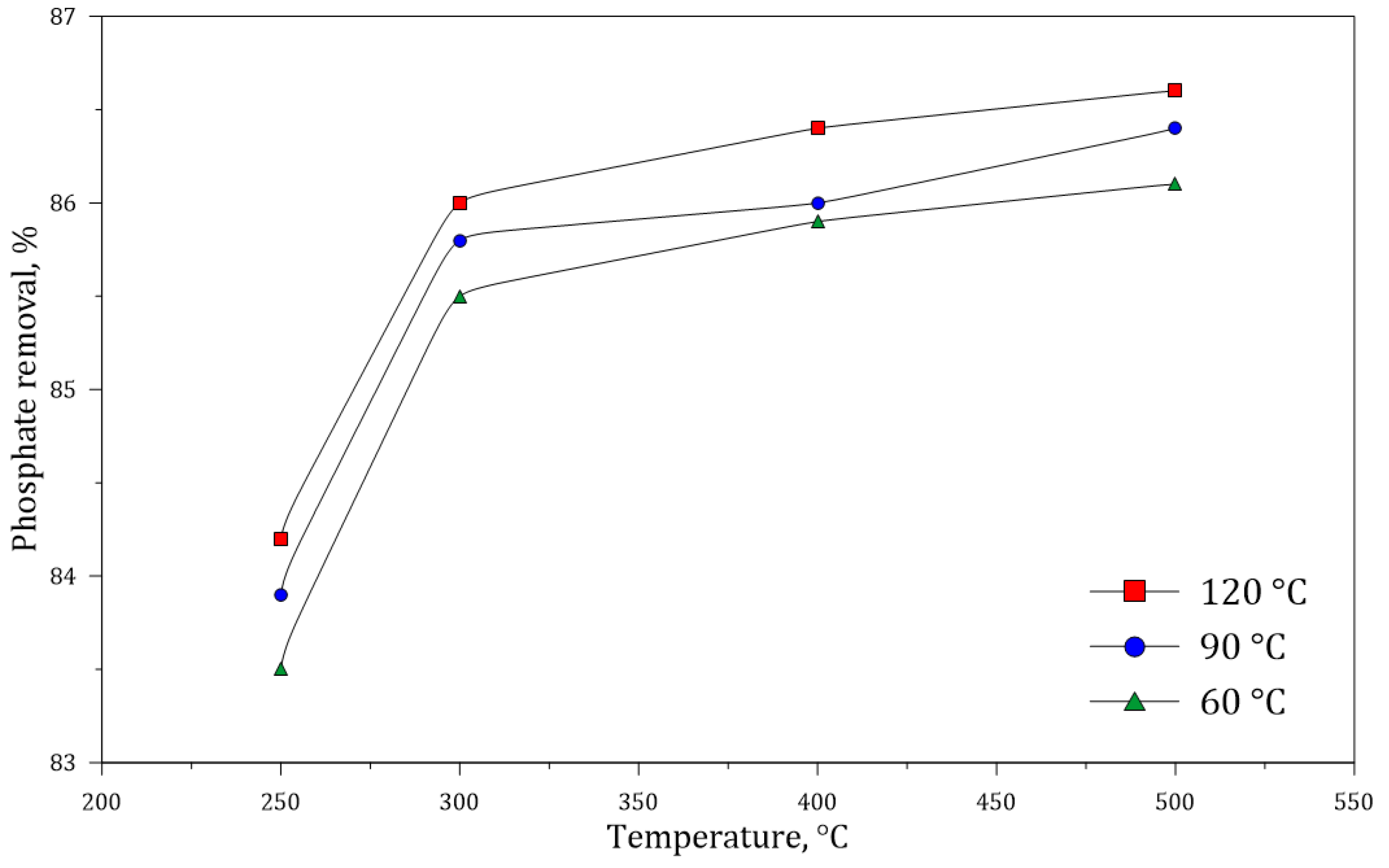
3.3. Dephosphorization of Monazite Concentrates
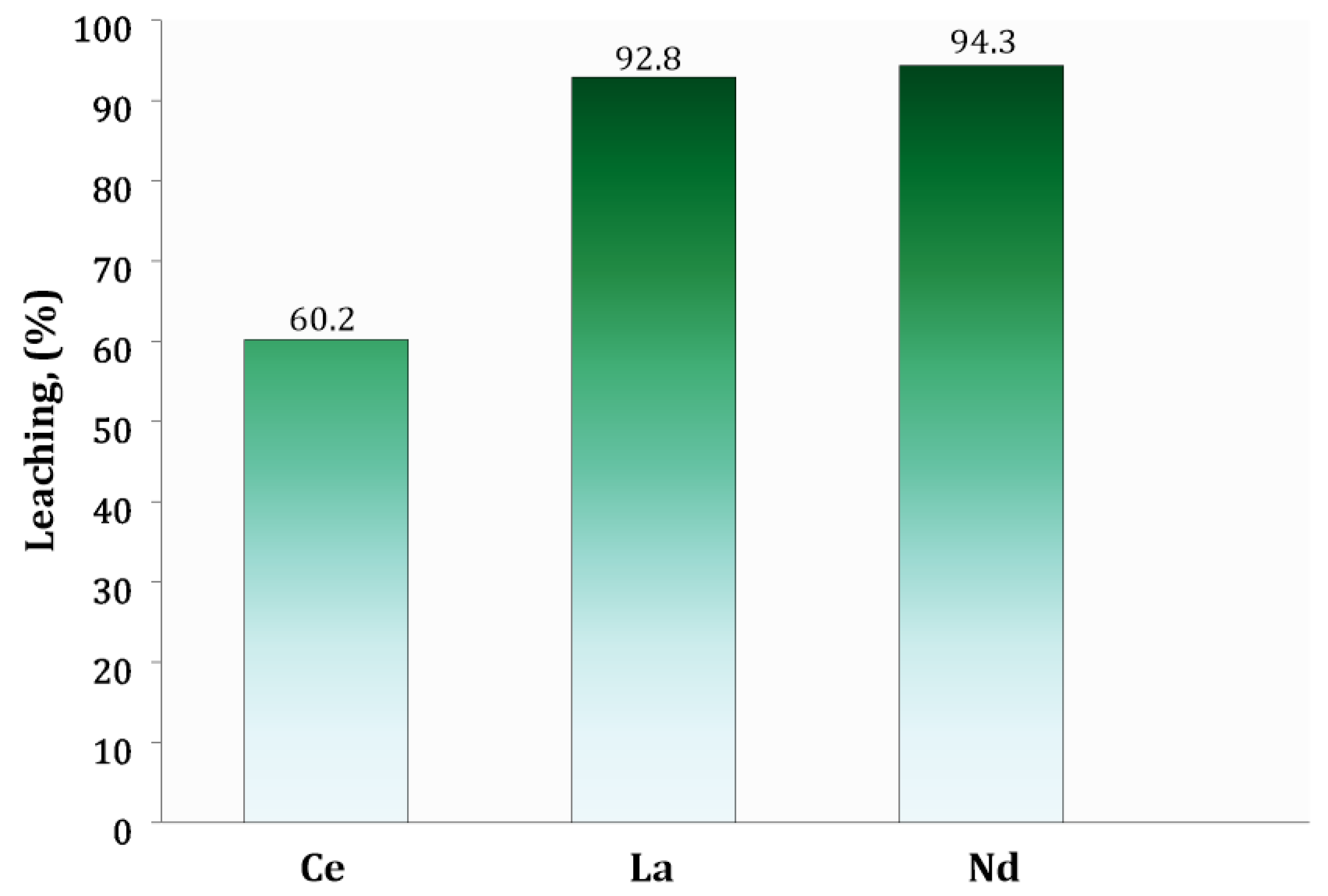
3.4. Leaching Process
3.5. Solvent Extraction
3.5.1. Effect of Contact Time
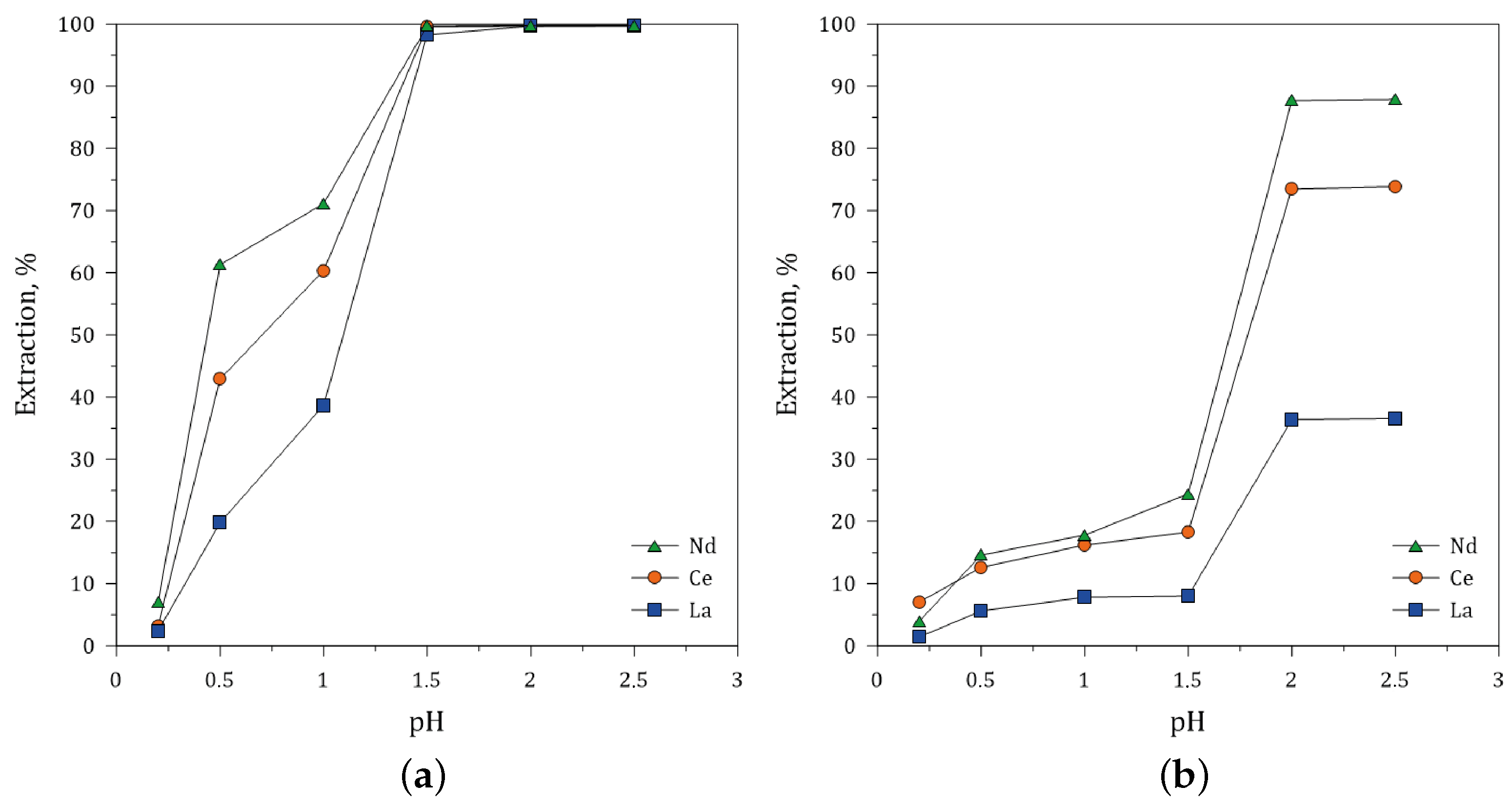
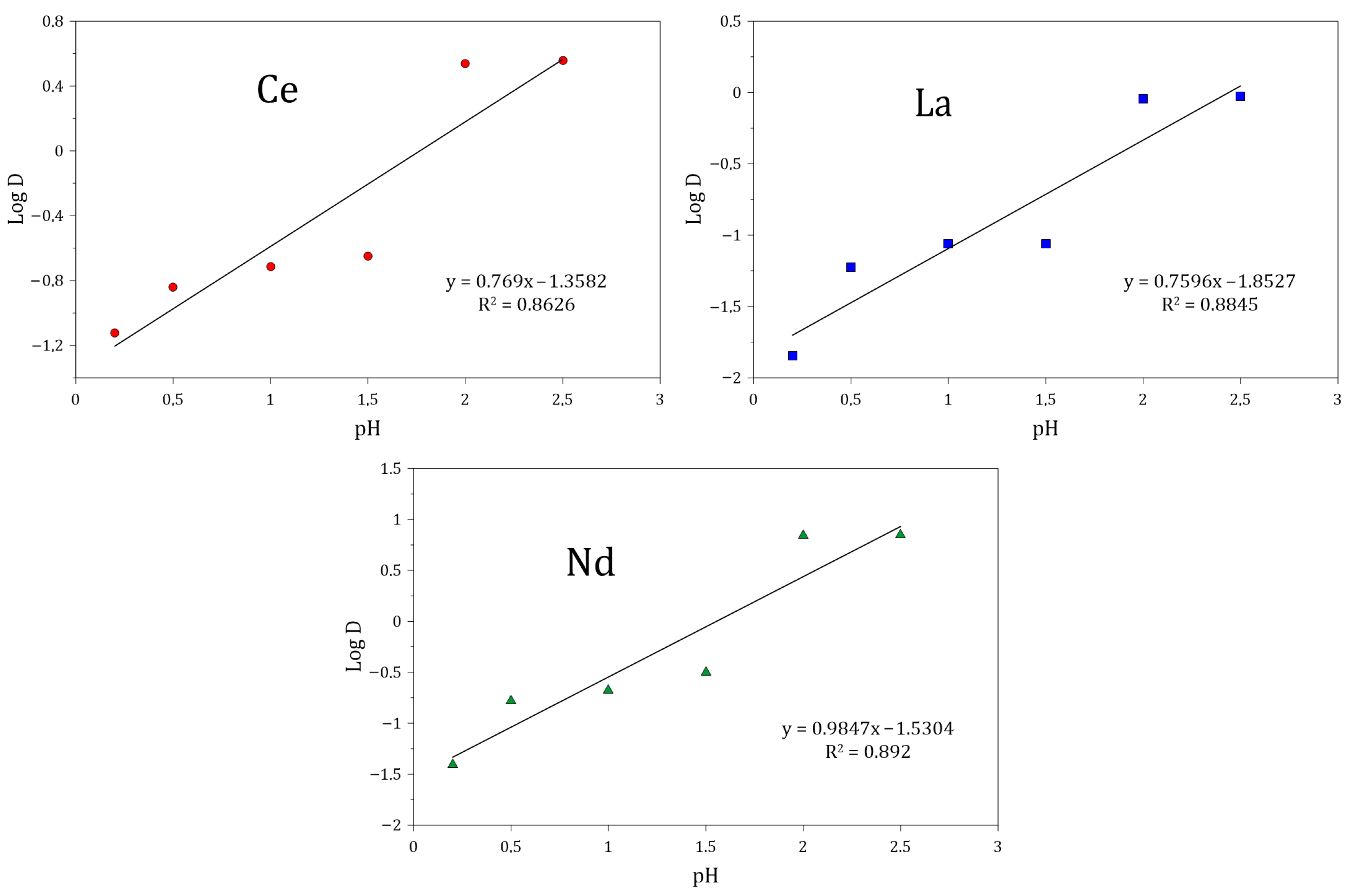
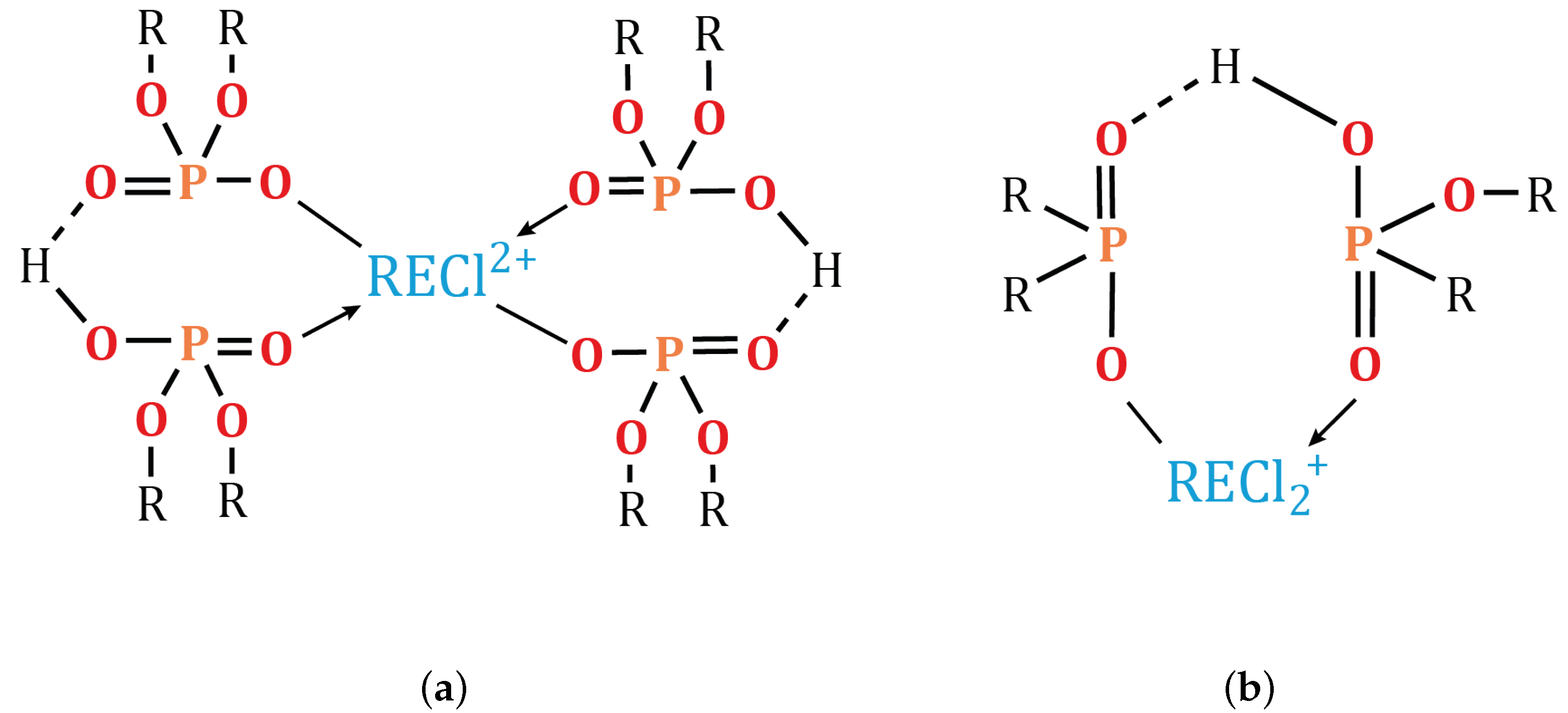
3.5.2. Effect of pH
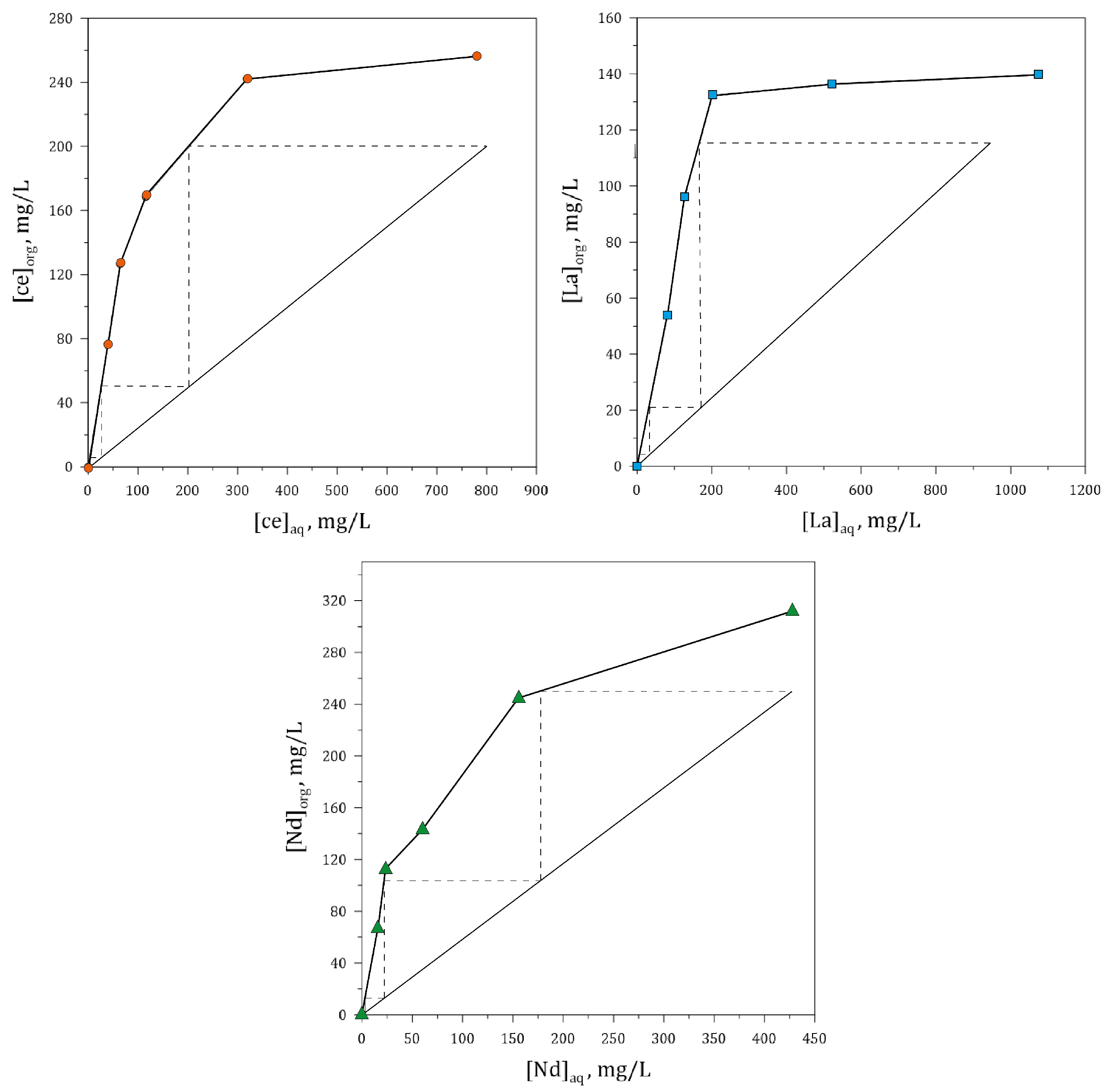
3.5.3. Effect of O/A Ratio
3.5.4. Separation Factor (SF)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rybak, J.; Gorbatyuk, S.M.; Kongar-Syuryun, C.; Khayrutdinov, A.M.; Tyulyaeva, Y.; Makarov, P.S. Utilization of Mineral Waste: A Method for Expanding the Mineral Resource Base of a Mining and Smelting Company. Metallurgist 2021, 64, 851–861. [Google Scholar] [CrossRef]
- Lucas, J.; Lucas, P.; Mercier, T.L.; Rollat, A.; Davenport, W. Rare Earths Science, Technology, Production and Use, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Suli, L.M.; Ibrahim, W.H.W.; Aziz, B.A.; Deraman, M.R.; Ismail, N.A. A Review of Rare Earth Mineral Processing Technology. Chem. Eng. Res. Bull 2017, 19, 20–35. [Google Scholar] [CrossRef] [Green Version]
- Zglinicki, K.; Malek, R.; Szamalek, K.; Wolkowicz, S. Mining Waste as a Potential Additional Source of HREE and U for the European Green Deal: A Case Study of Bangka Island (Indonesia). Minerals 2022, 12, 44. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, S. Separation of monazite from placer deposit by magnetic separation. Minerals 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. The Use of Mining Tailings as Analog of Rare Earth Elements Resources: Part 1–Characterization and Preliminary Separation. Miner. Process. Extr. Metall. Rev. 2021, 43, 701–715. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Guth, K.; Gaub, R.; Gutfleich, O.; Buchert, M.; Steenari, B.; Gerven, T.M.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Zanin, M.; Addai-Mensah, J.; Skinner, W. Recovery of rare earth elements minerals from iron oxide–silicate rich tailings—Part 1: Magnetic separation. Miner. Eng. 2019, 136, 50–61. [Google Scholar] [CrossRef]
- Lamus-Molina, A. Minerologia Aplicada al uso y Aprovechamiento de las Arenas Negras (El Bagre, Antioquia); Universidad Nacional de Colombia: Medellín, Colombia, 2005. [Google Scholar]
- Kerguelen, J. Caracterización y Aprovechamiento de Recursos Minerales en Colas de Terrazas Aluviales del Distrito Bagre-Nechí; Universidad Nacional de Colombia: Medellín, Colombia, 2016. [Google Scholar]
- Udayakumar, S.; Mohd-Noor, A.F.; Abdul-Hamid, S.A.; Rama-Putra, T.A.; Anderson, C.G. Chemical and Mineralogical Characterization of Malaysian Monazite Concentrate. Mining, Metall. Explor. 2020, 37, 415–431. [Google Scholar] [CrossRef]
- Amer, T.A.; El-Sheikh, E.M.; Hassanin, M.A.; Fathy, W.M. Processing of Monazite Mineral Concentrate for Selective Recovery of Uranium. Chem. Afr. 2019, 2, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Erberg, C.; Petranicova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Zglinicki, K.; Szamałek, K.; Szamalek, K.; Wolkowicz, S. Critical Minerals from Post-Processing Tailing. A Case Study from Bangka Island, Indonesia. Minerals 2021, 11, 352. [Google Scholar] [CrossRef]
- Dehaine, Q.; Filippov, L.O.; Joussemet, R. Rare earths (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-products of kaolin production—Part 2: Gravity processing of micaceous residues. Miner. Eng. 2017, 100, 200–210. [Google Scholar] [CrossRef]
- Norgate, T.; Jahanshahi, S. Low grade ores - Smelt, leach or concentrate? Miner. Eng. 2010, 23, 65–73. [Google Scholar] [CrossRef]
- Berry, L.; Agarwal, V.; Galvin, J.; Safarzadeh, M.S. Decomposition of monazite concentrate in sulphuric acid. Can. Metall. Q. 2018, 57, 422–433. [Google Scholar] [CrossRef]
- Udayakumar, S.; Rezan, S.A.; Noor, A.F.M.; Putra, T.A.R.; Ibrahim, I.; Baharun, N. The dephosphorization behaviour of malaysian monazite concentrates. In AIP Conference Proceedings, Penang, Malaysia, 31 October–1 November 2019. [Google Scholar]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. Crystal chemistry of the monazite and xenotime structures. Am. Miner. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, M.K.; Yoo, K.; Panda, R.; Lee, J.Y.; Kumar, J.R.; Pathak, D.D. Advanced process to dephosphorize monazite for effective leaching of rare earth metals (REMs). Hydrometallurgy 2019, 187, 203–2011. [Google Scholar] [CrossRef]
- Clavier, N.; Podor, R.; Dacheux, N. Crystal chemistry of the monazite structure. J. Eur. Ceram. Soc. 2011, 31, 941–976. [Google Scholar] [CrossRef]
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Kumari, A.; Panda, R.; Lee, J.Y.; Thriveni, T.; Jha, M.K.; Pathak, D.D. Extraction of rare earth metals (REMs) from chloride medium by organo-metallic complexation using D2EHPA. Sep. Purif. Technol. 2019, 227, 115680. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, G.K. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Peelman, S.; Zhi, H.I.S.; Jilt, S.; Yang, Y. Leaching of Rare Earth Elements: Review of Past and Present Technologies In Rare Earths Industry Technological, Economic, and Environmental Implications; De Lima, I., Filho, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–334. [Google Scholar]
- Walawalkar, M.; Nichol, C.K.; Azimi, G. Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 2016, 166, 195–204. [Google Scholar] [CrossRef]
- Jorjani, E.; Shahbazi, M. He production of rare earth elements group via tributyl phosphate extraction and precipitation stripping using oxalic acid. Arab. J. Chem 2016, 9, S1532–S1539. [Google Scholar] [CrossRef]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Gupta, B.; Malik, P.; Deep, A. Solvent extraction and separation of tervalent Lanthanides and Yttrium using Cyanex 923. Solvent Extr. Ion Exch. 2003, 21, 239–258. [Google Scholar] [CrossRef]
- Hefiny, N.E.; Daoud, J.A. Extraction and separation of thorium(IV) and praseodymium (III) with CYANEX 301 and CYANEX 302 from nitrate medium. J. Radioanal. Nucl. Chem. 2004, 261, 357–363. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, J.; Meng, S.; Li, D. Synergistic extraction and separation of yttrium from heavy rare earths using mixture of sec-octylphenoxy acetic acid and bis(2,4,4-trimethylpentyl) phosphinic acid. Anal. Chim. Acta 2005, 533, 83–88. [Google Scholar] [CrossRef]
- Miranda, T.; Zinner, B. Separation of samarium and gadolinium solutions by solvent extraction. J. Alloys Compd. 1997, 249, 116–118. [Google Scholar] [CrossRef]
- Correa, M.M.J.; Silvas, F.P.C.; Alipradini, P.; Moraes, V.T.; Dreisinger, D.; Espinosa, D.C.R. Separation of copper from a leaching solution of printed circuit boards by using solvent extraction with D2EHPA. Braz. J. Chem. Eng. 2018, 35, 919–930. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhao, Z.; Dong, Y.; Sun, X. The novel extraction process based on CYANEX® 572 for separating heavy rare earths from ion-adsorbed deposit. Sep. Purif. Technol. 2015, 151, 303–308. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Li, L.; Li, J. Synthesis of organic phosphinic acids and studies on the relationship between their structure and extraction-separation performance of heavy rare earths from HNO3 solutions. Hydrometallurgy 2013, 137, 108–114. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Long, Z.; Zhang, Y.; Xue, X.; Zhu, Z. Synergistic extraction of rare earth by mixtures of 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester and di-(2-ethylhexyl) phosphoric acid from sulfuric acid medium. J. Rare Earths 2008, 26, 410–413. [Google Scholar] [CrossRef]
- Thakur, N.V. Separation of rare earths by solvent extraction. Miner. Process. Extr. Metall. Rev. 2000, 21, 277–306. [Google Scholar] [CrossRef]
- Moustafa, M.I.; Abdelfattah, N.A. Physical and chemical beneficiation of the egyptian beach monazite. Resour. Geol. 2010, 60, 288–299. [Google Scholar] [CrossRef]
- Panda, R.; Kumari, A.; Jha, M.K.; Hait, J.; Kumar, V.; Kumar, J.R.; Lee, J.Y. Leaching of rare earth metals (REMs) from Korean monazite concentrate. J. Ind. Eng. Chem. 2014, 20, 2035–2042. [Google Scholar] [CrossRef]
- Trinh, H.B.; Lee, J.C.; Srivastava, R.R.; Kim, S.; Ilyas, S. Eco-threat Minimization in HCl Leaching of PGMs from Spent Automobile Catalysts by Formic Acid Prereduction. ACS Sustain. Chem. Eng. 2017, 5, 7302–7309. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; Pietrelli, L.; Centofanti, M.; Piga, L.; Vegliò, F. Application of solvent extraction operation to recover rare earths from fluorescent lamps. J. Clean. Prod. 2018, 172, 2840–2852. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Review of Flotation and Physical Separation of Rare Earth Element Minerals. In Proceedings of the 4th UMaT Biennial International Mining and Mineral Conference, Tarkwah, Ghana, 3–6 August 2016. [Google Scholar]
- Kelly, E.G.; Spottiswood, D.J. Introduction to Mineral Processing, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Abo-Atia, T.; Wouters, W.; Monforte, G.; Spooren, J. Microwave chloride leaching of valuable elements from spent automotive catalysts: Understanding the role of hydrogen peroxide. Resour. Conserv. Recycl. 2021, 166, 105349. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Meng, Z.; Cui, J.; Zhao, W.; Li, L. Decomposition of bastnasite and monazite mixed rare earth minerals calcined by alkali liquid. J. Rare Earths 2012, 30, 155–158. [Google Scholar] [CrossRef]
- Chi, R.; Li, Z.; Peng, C.; Gao, H.; Xu, Z. Preparation of enriched cerium oxide from bastnasite with hydrochloric acid by two-step leaching. Metall. Mater. Trans. B 2006, 37, 155–160. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Huang, X.; Dong, J.; Long, Z.; Zhang, Y. Yttrium extraction from chloride solution with a synergistic system of 2-ethylhexyl phosphonic acid mono-(2-ethylhexyl) ester and bis(2,4,4- trimethylpentyl) phosphinic acid. Hydrometallurgy 2014, 147–148, 7–12. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, H.S.; Lee, M. Solvent extraction of Pr and Nd from chloride solutions using ternary extractant system of Cyanex 272, Alamine 336 and TBP. J. Ind. Eng. Chem. 2015, 31, 74–79. [Google Scholar] [CrossRef]
- Acharya, S.; Mishra, S.; Misra, P.K. Studies on extraction and separation of La(III) with DEHPA and PC88A in petrofin. Hydrometallurgy 2015, 156, 12–16. [Google Scholar] [CrossRef]
- Kim, J.S.; Nagaphani-Kumar, B.; Lee, J.Y.; Lakshmi-Kantam, M.; Ramachandra-Reddy, B. Separation and Recovery of Light Rare-Earths from Chloride Solutions using Organophosphorus based Extractants. Sep. Sci. Technol. 2012, 47, 1644–1650. [Google Scholar] [CrossRef]





| Components | Percentage, %(w/w) |
|---|---|
| Si | 33.7 |
| Fe | 18.4 |
| Ce | 1.3 |
| La | 0.5 |
| Nd | 0.3 |
| Pr | 0.09 |
| Zr | 0.01 |
| Y | 0.07 |
| Th | 0.15 |
| U | 0.008 |
| 18.4 | |
| Others | Balance |
| Components | Percentage, %(w/w) |
|---|---|
| Ce | 21.3 |
| La | 9.7 |
| Nd | 8.7 |
| Pr | 1.3 |
| Zr | 0.5 |
| Y | 1.4 |
| Th | 3.5 |
| U | 0.3 |
| 26.1 | |
| Others | Balance |
| Components | Percentage, %(w/w) |
|---|---|
| Ce | 33.1 |
| La | 15.7 |
| Nd | 16.2 |
| Pr | 2.0 |
| Zr | 1.0 |
| Y | 2.2 |
| Th | 7.8 |
| U | 1.0 |
| 5.7 | |
| Others | Balance |
| Elemental Analysis | ||||
|---|---|---|---|---|
| Component | Units | Ce | La | Nd |
| Feed solid | % (w/w) | 23.3 | 10.7 | 10.5 |
| Pregnant leach liquor | mg/L | 3512.5 | 2477.0 | 2475.4 |
| Pregnant leach liquor | mol/L | 0.025 | 0.018 | 0.014 |
| Leaching efficiency | % | 60.2 | 92.8 | 94.3 |
| O/A Ratio | 0.5 | 1 | 2 | 3 | 5 |
|---|---|---|---|---|---|
| , (mL) | 360 | 300 | 180 | 150 | 100 |
| , (mL) | 180 | 300 | 360 | 450 | 500 |
| , (mg/L) | 1036.7 | 562.0 | 285.6 | 191.2 | 115.6 |
| , (mg/L) | 1214.3 | 658.3 | 334.5 | 224.0 | 135.4 |
| , (mg/L) | 740.0 | 401.1 | 203.9 | 136.5 | 82.5 |
| , (mg/L) | 780.5 | 319.8 | 116.8 | 64.5 | 39.5 |
| , | 0.3 | 0.8 | 1.4 | 2.0 | 1.9 |
| , (%) | 14.1 | 43.1 | 74.3 | 85.5 | 90.6 |
| , (mg/L) | 1074.6 | 522.0 | 202.2 | 127.8 | 81.7 |
| , | 0.1 | 0.3 | 0.7 | 0.8 | 0.7 |
| , (%) | 6.1 | 20.7 | 56.7 | 69.3 | 76.7 |
| , (mg/L) | 428.1 | 156.0 | 60.4 | 23.6 | 15.7 |
| , | 0.7 | 1.6 | 2.4 | 4.8 | 4.2 |
| , (%) | 26.7 | 61.1 | 82.6 | 93.5 | 95.5 |
| , (mg/L) | 962.8 | 491.2 | 249.3 | 166.4 | 103.4 |
| , | 0.08 | 0.14 | 0.15 | 0.15 | 0.12 |
| , (%) | 3.7 | 12.6 | 22.5 | 30.4 | 37.1 |
| , (mg/L) | 1176.0 | 321.4 | 313.8 | 207.9 | 126.0 |
| , | 0.03 | 0.06 | 0.07 | 0.08 | 0.08 |
| , (%) | 1.6 | 5.6 | 11.7 | 18.9 | 27.3 |
| , (mg/L) | 677.6 | 342.6 | 169.3 | 105.4 | 57.6 |
| , | 0.09 | 0.17 | 0.20 | 0.30 | 0.43 |
| , (%) | 4.4 | 14.6 | 29.0 | 47.0 | 68.4 |
| Separation Factor | ||||||
|---|---|---|---|---|---|---|
| Ce/La | Nd/Ce | Nd/La | ||||
| pH | D2EHPA | Cy 572 | D2EHPA | Cy 572 | D2EHPA | Cy 572 |
| 0.2 | 1.34 | 5.30 | 2.32 | 0.54 | 3.11 | 2.86 |
| 0.5 | 3.04 | 2.43 | 2.11 | 1.19 | 6.42 | 2.88 |
| 1.0 | 2.42 | 2.22 | 1.61 | 1.12 | 3.90 | 2.49 |
| 1.5 | 2.89 | 2.58 | 1.88 | 1.44 | 5.42 | 3.72 |
| 2.0 | 1.00 | 3.83 | 1.50 | 2.05 | 1.50 | 7.86 |
| 2.5 | 1.28 | 3.87 | 1.48 | 2.00 | 1.89 | 7.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverry-Vargas, L.; Ocampo-Carmona, L.M. Recovery of Rare Earth Elements from Mining Tailings: A Case Study for Generating Wealth from Waste. Minerals 2022, 12, 948. https://doi.org/10.3390/min12080948
Echeverry-Vargas L, Ocampo-Carmona LM. Recovery of Rare Earth Elements from Mining Tailings: A Case Study for Generating Wealth from Waste. Minerals. 2022; 12(8):948. https://doi.org/10.3390/min12080948
Chicago/Turabian StyleEcheverry-Vargas, Luver, and Luz Marina Ocampo-Carmona. 2022. "Recovery of Rare Earth Elements from Mining Tailings: A Case Study for Generating Wealth from Waste" Minerals 12, no. 8: 948. https://doi.org/10.3390/min12080948
APA StyleEcheverry-Vargas, L., & Ocampo-Carmona, L. M. (2022). Recovery of Rare Earth Elements from Mining Tailings: A Case Study for Generating Wealth from Waste. Minerals, 12(8), 948. https://doi.org/10.3390/min12080948






