Abstract
In this study, raw smectite (Sm), from the Guarapuava–Parana–Brasil region, was saturated with copper ions (Cu-Sm) by ion exchange and the samples Sm and Cu-Sm were used in crystal violet (CV) adsorption and applied as an antimicrobial and antifungal hybrid pigment. Samples (Sm and Cu-Sm) were used to remove crystal violet (CV) dye from aqueous media, simulating wastewater. Samples after use as adsorbents were characterized and named smectite/adsorbed dye (Sm/Dye) and copper smectite/adsorbed dye (Cu-Sm/Dye); and they were applied as hybrid pigments with antimicrobial action. The Sm and Cu-Sm were characterized by X-ray diffractometry (XRD), energy dispersive X-ray fluorescence (EDXRF), vibrational spectroscopy (FTIR), Zeta potential (ζ), scanning electron microscopy (SEM), and colorimetry (CIE L*a*b*), enabling the identification of the presence of intercalated copper ions and on the smectite surface. The adsorption assays were carried out to evaluate the effects of initial dye concentration and contact time. Tests for application as a hybrid pigment showed good compatibility with commercial white paint being applied on plaster blocks and later photoaging and chemical stability tests were performed in acid and basic environments, both were discussed by colorimetry (CIE L*a*b*), thus being able to relate it to the color variation (∆E). The samples (Sm, Cu-Sm, Sm/Dye, and Cu-Sm/Dye) were dispersed in white paint at 10% and 20% (% w/w) to evaluate the ability to inhibit different microorganisms. The modification with copper ions promoted an increase in the adsorptive capacity relative to the raw smectite and provided antibacterial and antifungal action to the hybrid pigment against Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, and Candida albicans. The Cu-Sm and Cu-Sm/Dye samples showed excellent results against all studied microorganisms and reveal successful materials that can be used in environments that require microbiological protection.
1. Introduction
One of the main problems confronted by industries, e.g., plastic, paper, leather, rubber, cosmetics, pharmaceutical, and textile, that use dyes in their products is the difficulty of efficient treatment and the control of the number of effluents after the dyeing of the products [1]. Around 10% to 15% of the dyes are discharged into the environment as wastewater during the coloring process; these dyes can often be harmful to the global environment and living organisms [1,2].
Synthetic dyes are considered the most difficult to remove due to their complex aromatic structures, which makes them more stable in the aquatic environment and problematic to be biodegraded, promoting a high load of organic pollution, such as toxicity (cytotoxicity, mutagenicity, and genotoxicity), turbidity, and aesthetic problems [3]. One of the most industrially used cationic organic dyes is crystal violet or gentian violet, with broad utility in disinfection, biological dye, dermatological agent, and bacteriostatic agent, as well as being used as a purple dye in textile dyeing and paper printing, although it is known to be carcinogenic, mutagenic, and recalcitrant [4,5]. Considering that the color is the main contaminant to be recognized in wastewater, merely less than 1 ppm for CV dyes is highly visible and undesirable [6].
The elimination from wastewater dyes may be performed using different methods, including the process of coagulation, precipitation, flocculation, adsorption processes, etc. [4,6,7]. Where each of the alternatives presents its costs, removal capacity, and by-products generated, each adsorption stands out in terms of low cost, simplicity, and easy operation [7,8]. In the search for alternatives and economically viable adsorbent materials, the clays are naturally abundant materials, present in various types of soils, low-cost, have high sorption properties, and potential for ion exchange [9,10].
Bentonite is a clay that occurs naturally and contains predominantly the smectite phase (at least 50%), usually known as montmorillonite [11,12]. This clay type presents proprieties favorable to the adsorption of the cationic dye, preferred by the effective interaction due to the negatively charged surface of this clay [13,14]. The isomorphic substitution of cations between interlamellar space of montmorillonites may be carried out through the exchange of Na+, Ca2+, Mg2+, and Cu2+ [15], to add other functionalities to the resulting material [16]. Copper can be used in this cation exchange because it is an abundant, low-cost element, and its particles attracted huge interest, due to its wide antibacterial properties and its high potential to damage proteins and lipids of bacteria [17], making it a great candidate to replace silver as an antibacterial agent.
Recent studies demonstrated the stability of dyes in clay minerals, such as the Calvacanti et al. 2021 [18] study, whose study comprehended in preparation of a hybrid pigment based on carminic acid (CA) stabilized by three different clays: CA-saponite; CA-Al-pillared saponite; and hydrogels of saponite-CA or montmorillonite-CA covered by poly organosilane (POS) [18]. The results showed that the pigment coating with hydrogels provides an increase in the stability of the obtained pigments. Silva et al. 2020 [19] produced hybrid pigments from anthocyanin analogs and synthetic clay minerals in another study. The authors reported that hybrid pigment was prepared by the cation-exchange-mediated adsorption of flavylium cations (FL) on two synthetic clays, the mica-montmorillonite, and the laponite. The results demonstrate that the reported hybrid pigment improved the thermal stability, fluorescence, and attractive colors produced [19].
However, it is important to emphasize that is not commonly found in studies of the adsorption of organic dye into clays with the main goal of pigments, much less to produce a hybrid pigment with antibacterial antifungal properties. Recently, Alorabi et al., 2021, investigated natural clay as a low-cost adsorbent for crystal violet dye removal and investigated its antimicrobial activity [20]. The results of the research demonstrated that the maximum adsorption capacities of both NCQ1 and NCQ3 adsorbents (clay samples collected from different parts of the Albaha region, Saudi Arabia) were found to be 206.73 mg g−1 and 203.66 mg g−1, respectively. The antimicrobial study concluded that the samples NCQ1 and NCQ5 with high percentages for iron and aluminum showed significant activity against the tested microbial strains [20]. Presently, Chwastowski et al., 2023, [21] investigated the bioremediation of crystal violet by waste beet pulp shreds and assessed of antimicrobial properties of the obtained product. The research study showed that the equilibrium adsorption turned out to be the Langmuir isotherm model with an adsorption capacity equaling 28.07 mg g−1. For the antimicrobial test, the minimal growth showed that the final product inhibits the growth of Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Staphylococcus aureus. Considering that Escherichia coli (MIC = 500 ppm) and Pseudomonas aeruginosa (MIC = 300 ppm) were more resistant to BPSCV (beet pulp shreds with adsorbed crystal violet) than Candida albicans and Staphylococcus aureus (MIC = 100 ppm) [21]. Even with the report of some recent studies describing the study of adsorption of crystal violet and investigation of its final product as an antibacterial agent, it is not possible to find studies performed on this final product as a hybrid pigment as an antibacterial as an antifungal agent. This fact brings great relevancy to this research study, that is, using an abundant natural clay from Guarapuava, using it as a potential adsorbent of a carcinogen and toxic dye such as crystal violet and reusing this final product as a hybrid pigment with antimicrobial and antifungal properties.
In this study, the raw smectite (Sm) was modified by isomorphic substitution of Cu2+ ions and was studied for application as an adsorbent of the dye violet crystal, a potential contaminant of industrial wastewater. However, the contaminant can still harm the environment because it is only adsorbed on the clay surface if there is no appropriate application for this material. Reusing the adsorbed dye as a functional antibacterial hybrid pigment was possible. The antibacterial property was explored against Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus), bacteria that cause several infectious diseases [22,23]. The pigments Cu-Sm and Cu-Sm/Dye with adsorbed dye showed antibacterial action and may be dispersed in commercial paint, it can be applied indoors and outdoors in possible locations where there is a higher number of bacterial proliferations such as hospitals, schools, nursing homes, and daycare centers to reduce the risk of infections.
In the current study, raw smectite (Sm) and smectite modified with copper (Cu-Sm) were previously characterized by XRD, EDXR, FTIR-ATR mode, Potential Zeta, and SEM. After that, the sample was used as an adsorbent of violet crystal (VC) dye, and its adsorption parameters such isotherm model (Langmuir and Freundlich), and kinetics was investigated. The new pigments, that is, the samples of smectites (Sm and Cu-Sm) were then investigated as antibacterial and antifungal pigments. The pigments were also performed its colorimetric analysis of the powders and the painted blocks, before and after the photoaging and chemical stability tests
2. Materials and Methods
2.1. Materials
The Brazilian smectite was provided by MLS Engineering and was used as received. All the chemicals used were of analytical grade. Copper sulfate (CuSO4·5H2O; P.A., Biotec), sodium hydroxide (NaOH; P.A., NEON), and hydrochloric acid (HCl, 37%, NEON). The dye used was crystal violet (C25H30N3Cl, 407.98 g/mol), according to the specifications of the manufacturer (Farmax) as a hydroalcoholic solution containing 1% of the dye.
2.2. Mineralogical Analysis
To identify the phases, present in the raw smectite (Sm), a fraction (<2 μm) was separated according to the following methodology: 6.0 g of Sm was classified using a granulometric stainless steel sieve (212 μm and 65 mesh, Berbel brand). Then, the Sm was dispersed in 3 L of deionized water (2% w/v). The suspension was kept under mechanical agitation for 24 h, after which it was kept at rest for 2 h. Then, the upper fraction was transferred to a 1 L beaker and kept at rest for another 1 h. Thus, 100 mL of the suspension, the upper part, was removed and filtered under vacuum using a funnel with porous plate (number 1) and nylon filtration membrane (pore size of 0.45 µm, Kasvi brand). The raw smectite and micrometer fraction were dried in an oven (at 60 °C) for 24 h. Mineralogical analysis was performed by X-ray diffraction and X-ray fluorescence.
2.3. Saturation of Raw Smectite with Copper Ions
The Sm was saturated by preparing a suspension in a 2% ratio (w/v) using 1000 mL of distilled water, and it was kept under constant agitation in a magnetic stirrer for 6 h. After that time, 200 mL of an aqueous solution of copper sulfate (0.3 mol L−1) was added. The mixture had its pH adjusted close to 5 with NaOH (2.0 mol L−1), it is known that pH ≥ 6 occurs the precipitation of copper hydroxide, which undertakes the adsorption results [24]. The reaction was kept under stirring for another 20 h for copper ions intercalation. The sample was vacuum filtered using a porous plate funnel (number 2) and washed with distilled water to remove excess soluble. Afterward, it was dried at room temperature, macerated, and sieved (60 mesh). The solid obtained was labeled Cu-Sm.
2.4. Dye Adsorption
Adsorption tests were performed to investigate the Sm and Cu-Sm efficiency as adsorbents for removing dye from water. The parameters of contact time; and initial metal ion concentration were investigated. The adsorption experiments were carried out in conical flasks containing 40 mL of dye solution with an initial concentration ranging from 400 to 800 mg L−1. To this end, 100 mg of the adsorbent was added, and the solutions were kept under continuous shaking in a heating bath at 25 °C at fixed pH of 5.
The resulting solutions were centrifuged at 1200 rpm for 15 min, and dye concentration was then quantified using a UV-Vis spectrophotometer (Shimadzu UV-1800) at 582 nm. The adsorption isotherm experiments were carried out with 100 mg of the adsorbent in 40.0 mL of a 400 mg L−1 dye solution at 25 °C, varying the time between 1 and 30 min, and between 1 and 20 h, always at pH 7. The amount of adsorbed dye on the adsorbent and the percentage removal (%) was calculated by applying Equations (1) and (2), respectively:
where q is the amount of dye adsorbed by the adsorbent in mg g−1, Ci is the initial ion concentration in contact with the adsorbent (mg L−1), Cf is the dye concentration (mg L−1) after the batch adsorption process, m (g) is the mass of adsorbent, and V (L) is the volume of the dye solution.
2.5. Isotherms Models
Kinetic linearized equations pseudo-first-order and pseudo-second-order were used to study the adsorption process, the models are expressed according to Equations (3) and (4), respectively:
where K1 is the pseudo-first-order adsorption rate constant in min−1, K2 is the pseudo-second-order adsorption rate constant (g mg−1 min−1), qe and qt are the amounts adsorbed per gram of adsorbent at equilibrium in the time t, both in mg g−1. The parameters K1 and qe value can be determined by plotting ln (qe − qt) versus t.
The equilibrium isotherms were fitted to Langmuir and Freundlich, models following the linearized Equations (5) and (6), respectively:
where qe is the amount of solute adsorbed per gram of adsorbent at equilibrium (mg g−1); qmax is the maximum adsorption capacity (mg g−1), KL is adsorbate/adsorbent interaction constant (L mg−1), KF is the Freundlich adsorption capacity constant (mg g−1), Ce is the adsorbate concentration at equilibrium (mg L−1), and 1/n is the constant related to the heterogeneity of the surface.
2.6. Synthesis of the Hybrid Pigments
The pigments were obtained based on adsorption isotherms of Sm and Cu-Sm. For each sample, a suspension of 40.0 mL of the 400 mg L−1 dye solution and 100 mg of the solid were reacted at 25 °C for 4 h, at pH 7. The solids phase was recovered by centrifugation and dried at 70 °C for 24 h.
2.7. Characterization
The Zeta Potential (ζ) measurements were performed on ZETASIZER Malvern equipment, NANO ZS90 model, and the correlation between clay mass and solution volume was the same for all points on the curve and this value was 1 mg mL−1. The samples were characterized concerning their composition by energy dispersive X-ray fluorescence spectroscopy (EDXRF), the analyses were performed on a Shimadzu model EDX-7000, with an acquisition time of 60 s per analytical channel, with a vacuum atmosphere and a 10 mm collimator. The powder X-ray diffractogram was obtained on a Bruker X-ray diffractometer, model D2 Phaser, with Cu Kα radiation (λ = 1.5418 Å), with scan in 2θ from 2° to 50° and step rate of 0.2°/s, a power of 300 W, a voltage of 30 kV and a current of 10 mA. The degree of crystallinity (%) and average crystallite size (Å) were calculated using the EVA software, Bruker, linked to the D2 Phaser. Analysis of the phases present in the raw and fractionated smectite samples was performed using Match! Software [25]. Fourier transform infrared spectroscopy (FTIR) analyses were performed on a PerkinElmer Frontier spectrometer, in the 4000–650 cm−1 region, and 8 scans were performed with a resolution of 2 cm−1. The samples were analyzed in the Eco-ATR attenuated total reflectance acquisition mode, equipped with ZnSe ATR crystal.
2.8. Dispersion in White Paint and Colorimetric Measurements
For the dispersion of the pigment in white paint, the pigments (Sm/Dye, and Cu-Sm/Dye) were dispersed in commercial white paint, in the proportion of 5% (w/w), were and used to paint plaster specimens. The colorimetric measurements (CIE L*a*b*) were performed on pigments in powder form and as a pigment in white paint. In the CIELAB method, L* is the color lightness, ranging from black (L = 0) to white (L = 100), a* is the green (−)/red (+) axis, and b* is the blue (−)/yellow (+) axis [26]. Colorimetry data were obtained after the photostability and chemical stability test by a portable colorimeter and differences in colors between unexposed and exposed samples were calculated (∆E). The perception of the human eye admits values above 0.2–0.5 [27]. A portable colorimeter TEC60CP with an 8 mm opening was utilized for all data collection.
2.9. Photoaging Stability Test
Photoaging tests were simulated by exposure of the painting plaster specimens containing pigments ((Sm/Dye, and Cu-Sm/Dye)) dispersed in the white paint to white light irradiation for 24 h, 48 h, and 70 h using an LED lamp set to provide 55 Klux of illumination intensity. Thus, the fading test was applied to the pigments obtained before and after the adsorption of crystal violet; thus monitoring the total incident light dose, being 1320 Klux, 2640 Klux, and 3840 Klux, which corresponded to approximately 2.2, 4.4, and 6.4 years of exposure in a museum gallery illuminated at 200 Lux, i.e., 10 h of light exposure per day, 6 days per week, and 50 weeks per year [23,28,29].
2.10. Antimicrobial Activity Test
An initial assessment of the antimicrobial potential of the Sm, Cu-Sm, Sm/Dye, and Cu-Sm/Dye samples was conducted by the agar-diffusion disk test. The agar-diffusion disk test is a routinely used protocol in clinical laboratories to test for the most common fast-growing pathogens and certain fastidious bacteria [30]. The samples were dispersed in white paint at 10% and 20% (w/w) concentrations, and intensely homogenized. The pigmented paint with the samples was impregnated on filter paper, which was cut into 5 mm disks and used in the diffusion disk test.
Pathogenic Gram-positive strains Staphylococcus aureus (ATCC 25923), Listeria monocytogenes (ATCC 19111) and Gram-negative strains Escherichia coli (ATCC 25922), as well as yeast Candida albicans (ATCC 64548), were tested. Mueller–Hinton Agar (MH) and Sabouraud agar were used in the assays with bacteria and yeast, respectively. The initial assay to verify antimicrobial potential followed the protocols described by Laboratory Standard Institute (CLSI) [30,31,32]. Stock cultures of bacteria and yeast were activated (cultured) in Mueller-Hinton (37 °C, 24 h) and Sabouraud broths (28 °C, 24 h), respectively. The cell concentrations were then standardized according to the 0.5 MacFarland scale (≅ 1.5 × 108 CFU/mL), and the microorganisms were inoculated onto plates containing the culture medium with the aid of a sterile swab. The disks impregnated with samples were placed on the surface of the agar and the plates were kept for 1 h to allow diffusion, and then incubated at 37 °C for 24 h to bacteria and 48 h to yeast. The antibacterial potential was evaluated by measuring the halo of inhibition (mm). The negative control used was a disc, painted only with white paint. Analyses were performed in duplicate, and the results were expressed as mean and standard deviation. The zone of inhibition was considered the clear circular area around the antimicrobial disks where microorganisms cannot grow.
3. Results
3.1. Mineralogical Analysis by X-ray Diffraction
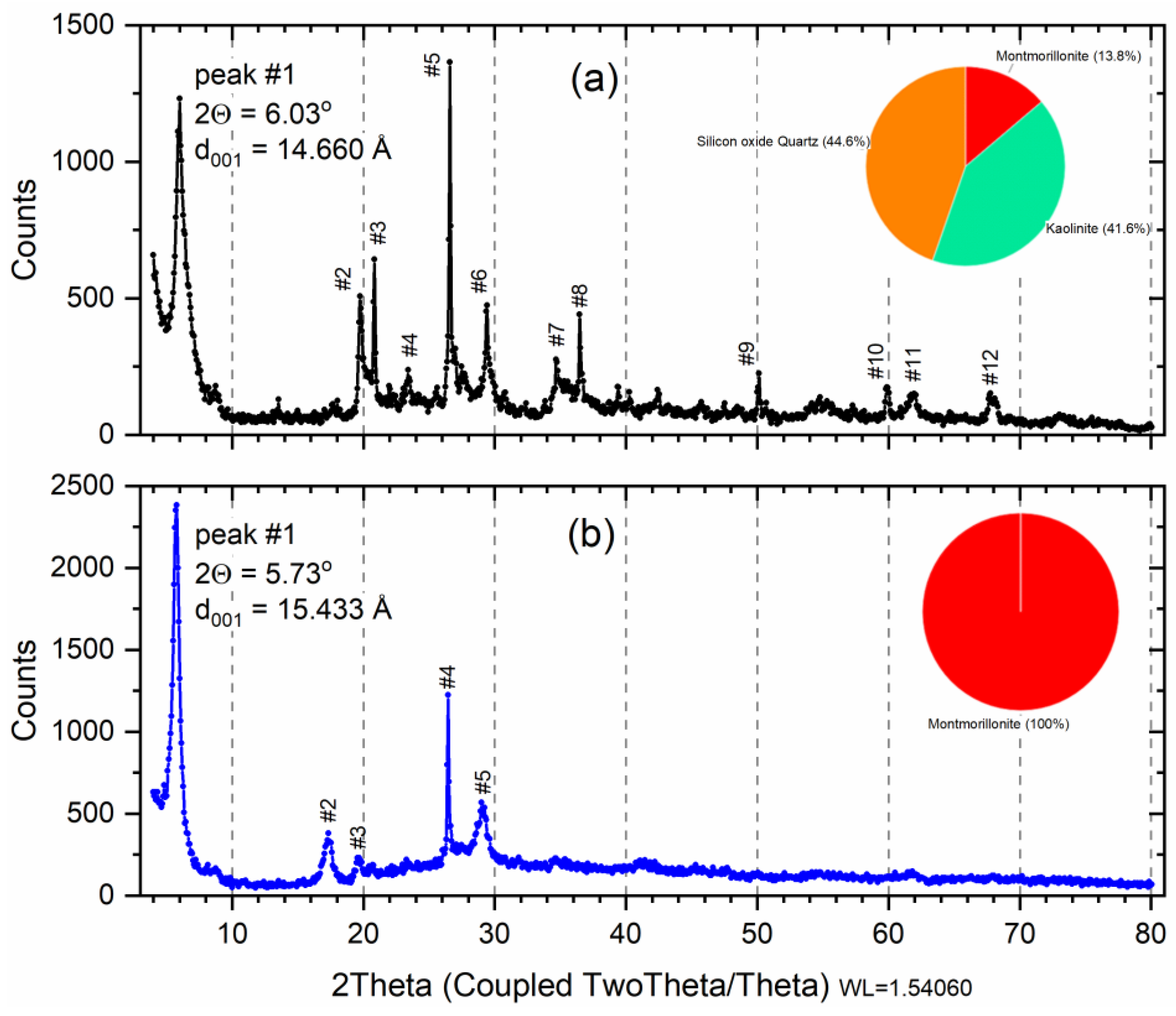
The X-ray diffraction profile for samples is shown in Figure 1. X-ray diffraction analysis on the raw initial material before the saturation (Figure 1a) indicates 13.8% of dioctahedral calcic montmorillonite (card 96-110-1055; Al2Ca0.5O12Si4) with 41.6% of Kaolinite (card 96-155-0599; Al2H4O9Si2) and 44.6% Quartz (card 96-500-0036; SiO2) denominated phases M, K, and Q, respectively (Table 1). The result of the composition analysis of each phase (Weight %) is shown in the pie chart (Figure 1a). The same profile of the diffractograms was characterized by bentonite clay as demonstrated in studies [33,34].
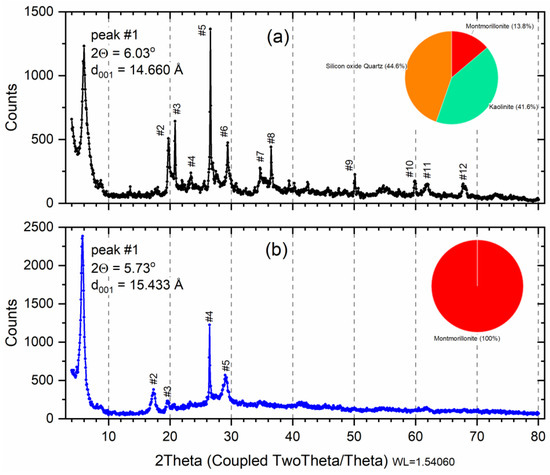
Figure 1.
X-ray diffraction of raw smectite (a); and montmorillonite phase (b). Insert: pie chart: Red—Montmorillonite; Orange—Quartz; Green—Kaolinite. Peak number (#) listed Table 1.

Table 1.
Match!® software indexed phase analysis for smectite (Sm) and montmorillonite (M) samples referring to the XRD of Figure 1.
Geologically, formed mainly from the chemical alteration of volcanic glass and pyroclastic or volcanoclastic rocks, bentonite clay rocks are composed basically of Na-montmorillonite. Through, the absence of this clay in natural occurrence, the term bentonites is usually used for 60–80% montmorillonite content of clay with similar properties [33]. Considering that the bentonite properties can vary due to the quantity and cation type of montmorillonite and minerals present, it is important to investigate the mineralogical quantification of clays [33].
In the indexing, 70 peaks were found for the sample Sm, but 12 peaks were highlighted (Figure 1a) and were properly identified according to phases M, K, or Q in Table 2. In Figure 1a, peak #1 is the most important because it indicates the montmorillonite phase, at 2θ = 6.02° referring to d = 14.66 Å, i.e., diffraction order (001) typical of the interlamellar distance of the 2:1 clays [35,36]. The Quartz phase (COD 9012600 SiO2) was characterized by the presence of reflection at 2θ = 22.049, 26.649°, and 50.210°, identified at peak #3, #5, and #9, respectively. The characteristic reflections that allowed Kaolinite phase (COD 1011045 Al2H4O9Si2) identification were 2θ = 19.892°, 26.502°, and 36.478°, identified at peak #2, #4, #8, respectively. Figure 1b shows an X-ray diffraction profile for a wet separated sample, indexing found only phase of montmorillonite with 100% (COD 9002779) for formula Al2Ca0.5O12Si4. Only five peaks were highlighted (Table 2), and peak #1 was shifted to 2θ = 5.73° (001), generating a d001 = 15.43 Å, which can be attributed to the retention of a greater amount of water since the samples were dried at 60 °C, characteristic of calcium montmorillonite [37].

Table 2.
The composition percentage for the sample Sm, M, and Sm-Cu was obtained by EDXRF.
The samples’ crystallite sizes (with k = 0.9 and k = 1.3) were calculated from X-ray line broadening using Scherrer’s equation [35]. The calculated values for the peak (001) were 210.3 Å (k = 0.9) and 303.3 (k = 1.3) for Sm, and 244.8 Å (k = 0.9) and 351.9 (k = 1.3).
3.2. Energy Dispersive X-ray Fluorescence (EDXRF)
Composition analysis was carried out to determine the chemical compositions of the Sm and M. Major quantities of SiO2 and Al2O3 are present in both clays (Sm and M), dominant constituents of all clay minerals [33]. The compositional analysis showed the presence of Ca, Fe, and K in the smectite (Table 1). The presence of these elements may be associated with mineral components of the sample, in addition to smectite and the form of interlayer cations. According to the CaO content (18.213%), the clay used in mays is calcium smectite (Ca-Sm).
The percentage of silicon and aluminum found was as expected, due to the presence of phyllosilicates, such as kaolinites and smectites [9]. It was noted that the silicon/aluminum ratio (~5:1) was slightly decreased after the copper insertion treatment performed. The Fe2O3 content present in the precursor clay may be related to isomorphic substitutions in the structure of the smectite clay mineral in the form of interlayer cations or the composition of other minerals [34], as crystalline phases such as goethite, maghemite, hematite, magnetite or other hydroxide source phases and iron oxides; however, these phases were not identified in X-ray diffraction (Figure 1).
The copper insertion in the clay caused a significant increase in the CuO content (34.69%). However, the high copper content observed cannot be attributed only to ion intercalation, since, in the sample diffractogram, it was possible to identify the formation of basic copper sulfate.
3.3. X-ray Diffractometry (XRD): Cu-Sm Sample
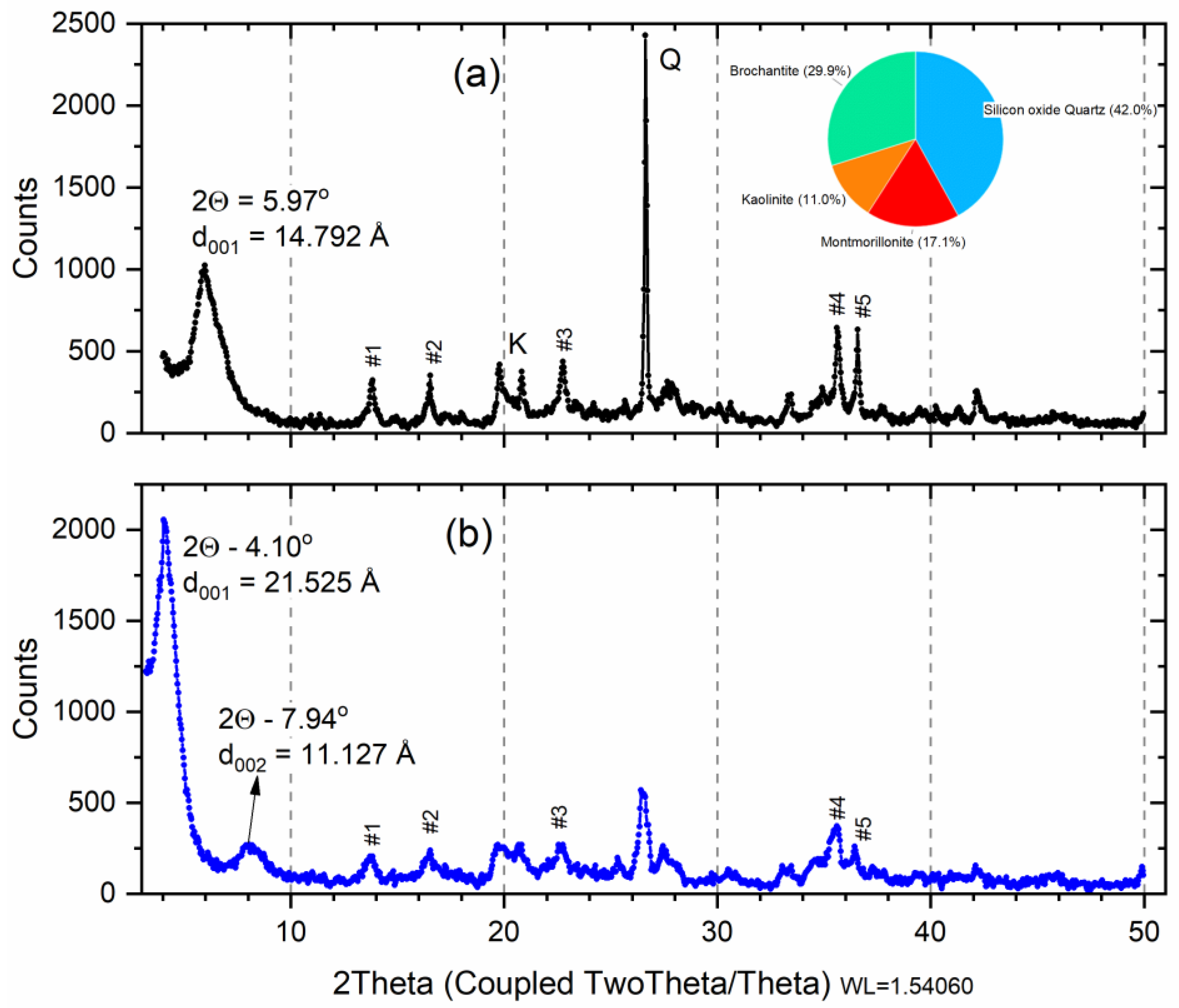
X-ray diffraction analysis on the raw smectite before the saturation indicates dioctahedral calcic montmorillonite (Ca-Sm) with an amount of kaolinite, quartz, and brochantite (Figure 2a). The X-ray diffraction analysis carried out on the sample after saturation (Cu-Sm, Figure 2a) indicates that the d-value of smectite increases to 14.79 Å (variation of 0.13 Å), this value corresponded to that found in the literature for intercalation of bivalent cations [34].
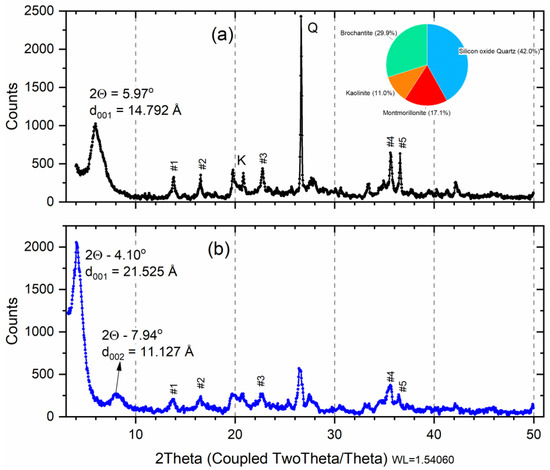
Figure 2.
X-ray diffraction of raw smectite (a) after saturation with copper ions (b). Insert: pie chart: Red—M; Blue—Q; Orange—K; and Green—Brochantite. Peak number (#) listed Table 1.
The percentage of silicon and aluminum found was as expected, due to the presence of phyllosilicates, such as kaolinites and smectites [9]. It was noted that the silicon/aluminum ratio (~5:1) was slightly decreased after the copper insertion treatment was performed. The Fe2O3 content present in the precursor clay may be related to isomorphic substitutions in the structure of the smectite clay mineral in the form of interlayer cations or the composition of other minerals [34], as crystalline phases such as goethite, maghemite, hematite, magnetite or other hydroxide source phases and iron oxides; however, these phases were not identified in X-ray diffraction (Figure 1).
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
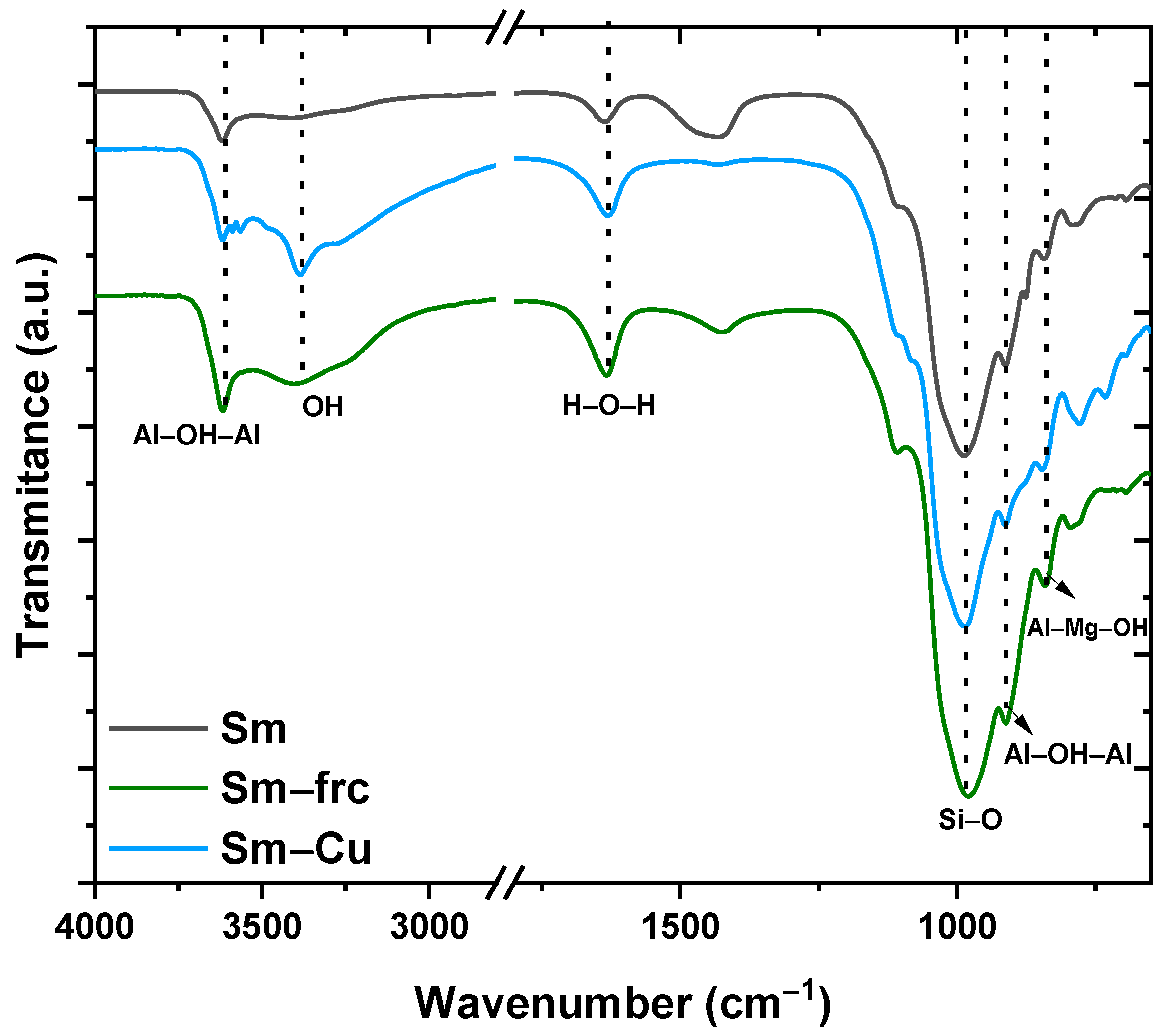
The infrared spectra of the samples Sm, M, and Sm-Cu are shown in Figure 3. The infrared spectrum of the Sm had a band at 3622 cm−1, it was attributed to Al2OH vibrational stretch, and it is characteristic of smectite with high aluminum content in octahedra [35]. The band at 3385 cm−1 was due to the water adsorbed on the clay surface, confirmed by the deformation band at 1642 cm−1, being a more evident band in the sample treated with copper, it may be related to high moisture content [38,39].

Figure 3.
Infrared spectra of natural smectite (Sm); fractionated smectite (M) and doped with copper ions (Cu-Sm).
In the range of 1300–400 cm−1, the bands present are related to Si-O stretching and bending, evidenced by Si-O stretching vibrations at 1108 cm−1 [35]. The bands at 910 and 840 cm−1 are related to the presence of Al2OH and AlMgOH groups, respectively, attributed to the partial substitution of Al by Mg in the octahedral sheet, originating OH deformations characteristic of smectites [38,39]. It was observed that the sample’s FTIR spectrum profile was very similar, showing there was no significant change in the raw smectite structure, corroborating the X-ray diffractometry (XRD) data, since the treatment consisted only of the process of exchange of cations.
3.5. Zeta Potential (ζ)
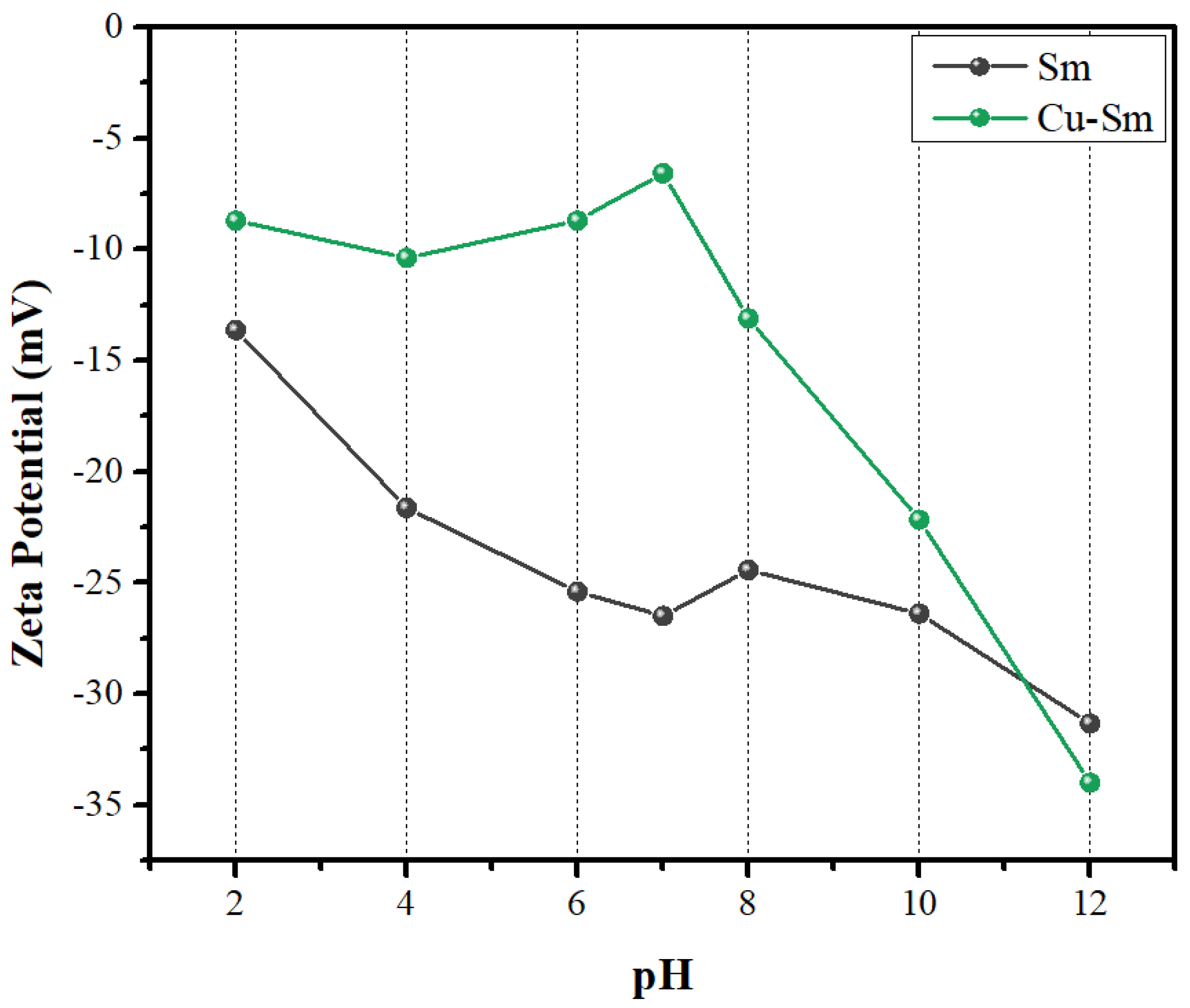
Smectite had zeta potential at a more negative magnitude (less than −15) (Figure 4), and the presence of alkaline cations (K+, Ca+) caused a decrease in the potential [40]. After Cu2+ intercalation, the Sm remained at a negative potential, but at a lower intensity, suggesting that the presence of the Cu2+ ion resulted in greater stability of the particles, because of the more positive potential. No isoelectric point was found in the worked pH range, and with increasing acidity, there was a decrease in potential magnitude [41].

Figure 4.
Zeta potential measures as a function of pH for Sm and Cu-Sm.
The negative potential across the entire pH range showed a better interaction with cationic dyes, justifying the nature of the dye used in this study [42]. Crystal violet dye had a pKa equal to 0.8 and it was ionized at a pH range of 4–10, that is, it existed as a cationic specie at pH 7 [43]. The pH (pH ~ 7) of the adsorption assay in this study was not adjusted and was the appropriate condition for the adsorption of CV into Sm and Cu-Sm. As observed in the zeta potential result (Figure 5), the entire pH range investigated was negative and can attract the positively charged ions of CV dye. An adsorption study evaluating the effect of the pH will not bring relevant results; in acid pH, the smectite samples were not stable and in basic pH, the deprotonation of the dye will occur [43].
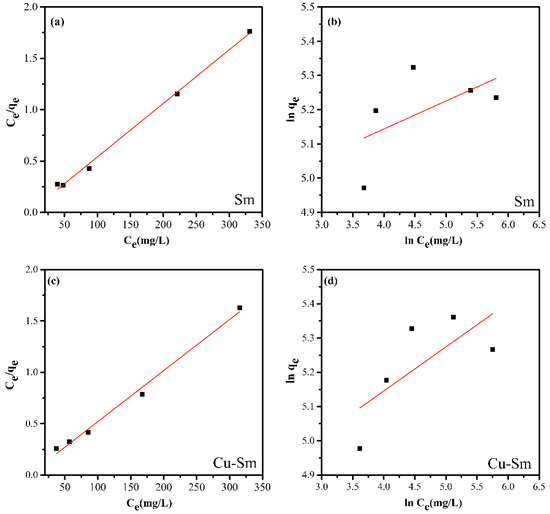
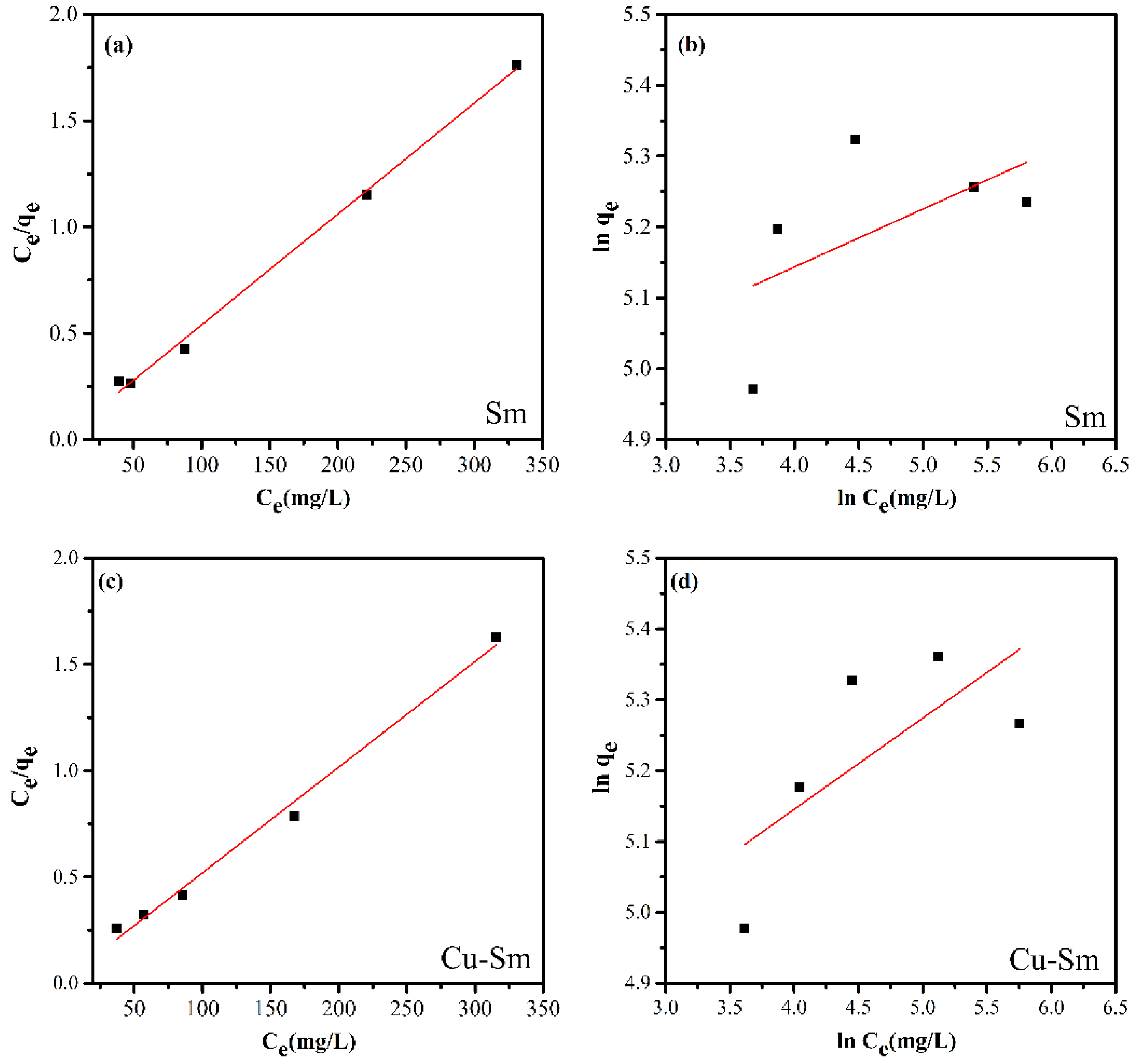
Figure 5.
The linear experimental fitting model of the Langmuir (a,c) and Freundlich (b,d) adsorption isotherm for the adsorption of violet crystal onto Sm and Cu-Sm at 298 K.
3.6. Adsorption Isotherms
Based on the linear correlation coefficients (R2) (Table 3) obtained from the linearization of isotherms (Figure 5), it is possible to state that the model that best fits the experimental data was the Langmuir isotherm model. Thus, it was suggested that the adsorption occurred in a monolayer, in the presence of a defined number of interaction sites of equal energy, and that the dye molecules did not interact with each other. The highest adsorptive capacity was Cu-Sm with a qmax value of 201.207 mg g−1.

Table 3.
Parameters of Langmuir and Freundlich isotherms for adsorption of violet crystal onto Sm and Cu-Sm at 298 K.
The smectite’s adsorption efficiency (Table 4) was higher at lower concentrations (400 mg L−1), corroborating the isothermal data, as these suggested a defined number of active sites, and each site interacted with just one dye molecule forming a monolayer. Therefore, the presence of a greater number of molecules resulted in a decrease in removal efficiency (%). However, if we consider the mass (mg) of dye removed, the decrease in efficiency of the increase in concentration did not reflect the effective amount removed. For example, for the highest concentration (800 mg L−1), the efficiency was 60%; but the mass removed was 480 mg, that is, an amount greater than the lowest concentration (400 mg L−1). This occurred due to the possibility of interaction between the crystal violet molecules, leading to the formation of bilayers.

Table 4.
Percentage of crystal violet dye removal by adsorbents.
Correlation coefficients, R12 and R22, the pseudo-first-order and pseudo-second-order rate parameters, K1 and K2, and sorption capacity qe1 and qe2 for the pseudo-first-order and pseudo-second-order equations, respectively, are demonstrated in Table 5. As observed, results for the pseudo-second-order reaction mechanism showed good compliance of the adsorption capacity (qe) data with the equation, and the regression coefficients for the linear plots were higher than 0.99. According to the fit coefficient (R2) (Table 5), the pseudo-second-order model was better suited to describe the adsorption kinetics of the crystal violet dye in the samples. The theoretical adsorption capacity (qe) values were close to those obtained experimentally.

Table 5.
Kinetic parameters for adsorption of violet crystal onto Sm and Cu-Sm at 298 K.
Table 6 presents comparatively the adsorption capacity (qmax) of some adsorbents concerning the crystal violet dye. The best performance in the literature was for olive leaves powder with a qmax of 133 mg g−1. Considering the smectite sample, its performance was 43.7% superior to olive; and considering Sm-Cu, the dye removal capacity was 51% higher than olive. It should be noted that raw smectite has a low cost per ton and can be reused as a hybrid pigment, as discussed below.

Table 6.
Comparison of adsorption capacities (qmax) of CV using different adsorbents.
3.7. Adsorption Mechanism
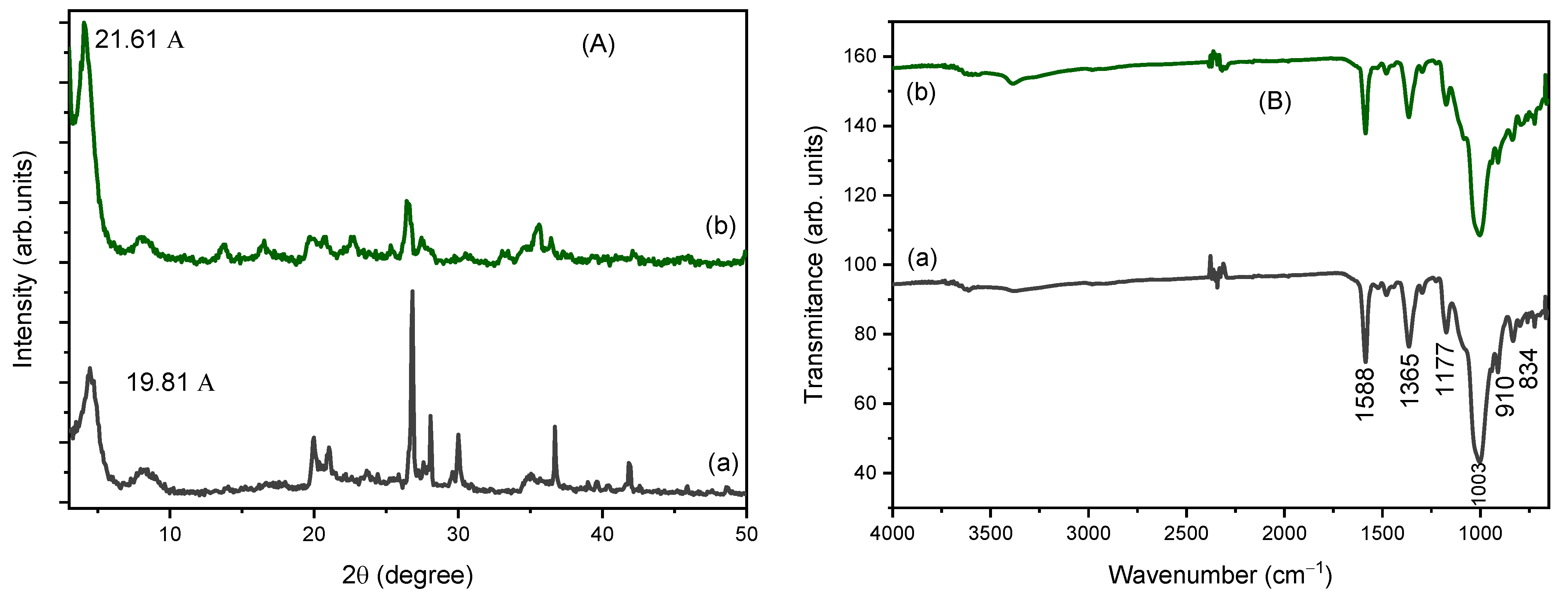
To investigate the mechanism of adsorption of CV dye into Sm and Cu-Sm adsorbents, the XRF, FTIR, isotherm, and kinetic studies were considered. The main interpretation of how CV structure is adsorbed on both Sm and Cu-Sm adsorbents is based on ion exchange. As observed in XRF results obtained from samples after adsorption assay (Figure 6A), an increase in the interlayer space of the clays occurred, as observed in the displacement of the peak (001) to lower (2θ) degree. The interlayer space for sample Sm was d001 = 14.98 Å. After the sorption of the dye (Sm/Dye), the interlayer space became d001 = 19.81 Å. The same result was observed for the Cu-Sm sample; the d001 before adsorption was 14.78 Å, and after the CV adsorption became 21.61 Å. The increase in the interlayer space observed suggests that the adsorption of CV dye occurred in the interlayer space of the clay.

Figure 6.
X-ray diffraction (A), and Infrared spectra (B) of the samples: Cu-Sm/Dye (a) and Sm/Dye (b).
FTIR spectra (Figure 6B) obtained for the sample after the CV adsorption showed a band around 1588 cm−1 attributed to stretching vibrations of the bond C=C. The other two bands observed at 1363 cm−1 and 1177 cm−1 were attributed to the C-N aromatic bond, these are the typical bands of the CV dye. The peak at 994 cm−1 was shifted to major frequency at 1003 cm−1 for the both Sm/Dye and Cu-Sm/Dye samples; this fact can be explained due the ion exchange of CV dye with K+, Na+, and Ca2+ on the surface of the clay or in the interlayer space [20]. From the equilibrium adsorption study, it was observed that the adsorption process turned out to be the Langmuir isotherm model. This demonstrated that the adsorption occurred in a monolayer, in the presence of a defined number of interaction sites of equal energy, and that the dye molecules did not interact with each other. The adsorption process occurred as chemical bonds, as demonstrated in the nonlinear kinetic studies with a better fit to pseudo-second order model kinetics.
3.8. Hybrid Pigments—Color Properties
The colorimetric parameters obtained for the prepared pigments prepared at pH 7 are shown in Table 7. The different values of L*, a*, and b* obtained for samples (Sm and Cu-Sm) justified the change in the sample’s colors after intercalation with copper ions and dye adsorption. The precursor clay obtained positive values for a*(red) and b*(yellow), this coloration was possibly caused by the presence of Fe3+ ions, identified by EDXRF. Copper insertion in the Sm structure caused a greenish tone, with negative a* and b* chromatic parameters (green and blue, respectively). This color was characteristic of compounds with Cu2+ ions and brochantite compounds evidenced by X-ray diffraction.

Table 7.
Colorimetric parameters of pigments in powder and dispersed in paint.
The crystal violet dye sorption resulted in dark violet tones for the Sm before and after copper saturation, with low luminosity values (L*) and within a red/blue color quadrant, as they presented positive a* and negative b* values. The total color difference (∆E) was calculated for the pigments in powder form and after dispersion in paint, all variations were very strong. Regarding saturation (C*), Sm and Cu-Sm showed less saturated color after dispersion in paint, the opposite occurred with pigments after dye sorption, as the chromatic parameters increased in dispersed form, accentuating the color.
3.9. Hybrid Pigments—Stability Test
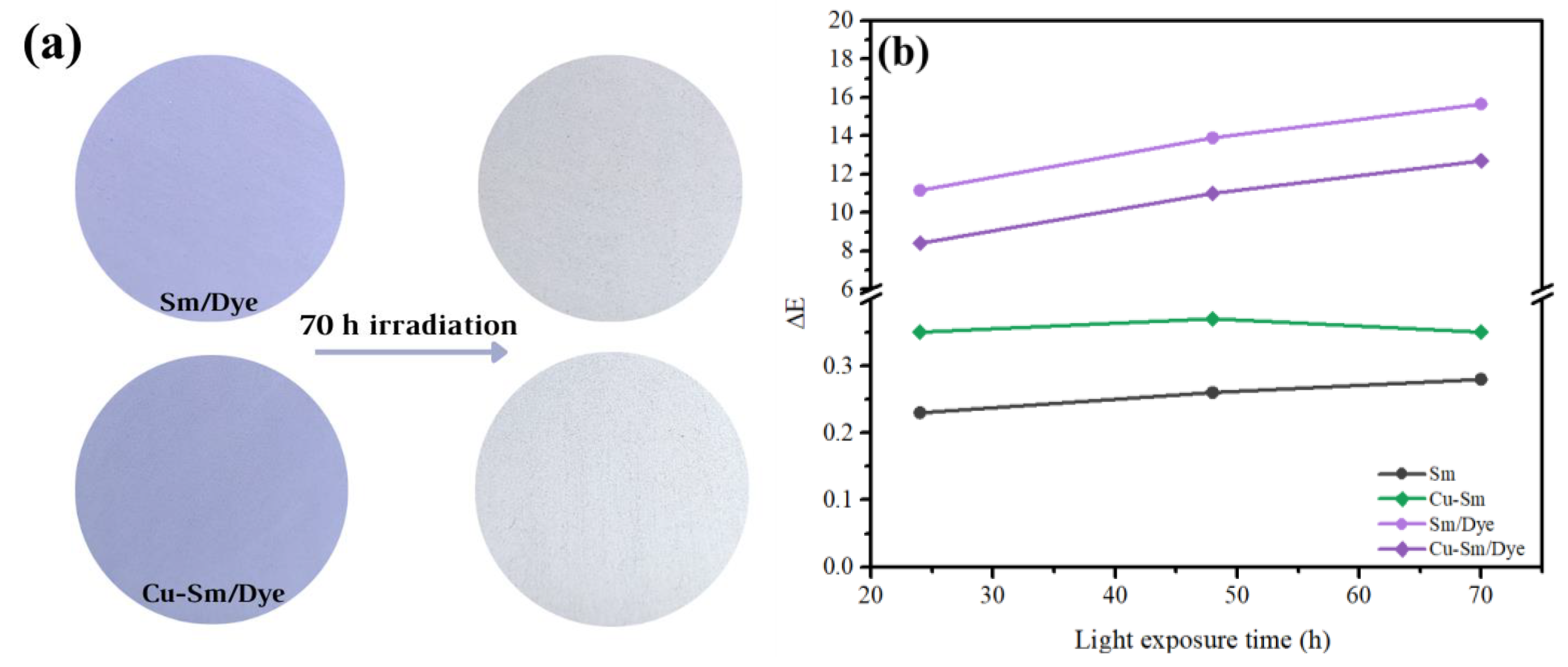
The color of the new pigments (Sm/Dye and Cu-Sm/Dye) dispersed in water-based paint was evaluated under light exposure after 70 h (Figure 7a). Measurements of L*a*b* parameters were carried out at different light irradiation times. This test simulated the exposure of pigments to an ambient with light radiation.

Figure 7.
(a) Digital color of the pigments in water-based paint before (left) and after (right) light-induced aging for 70 h and (b) color differences (∆E*) the water-based paint samples exposed to light-induced aging.
The color difference (∆E) obtained from the values of the L*, a*, and b* parameters for the new pigments before and after light exposure is shown in Figure 7b. The Sm/Dye showed a ΔE value of 11.15, while Cu-Sm/Dye presented a ΔE value of 8.4 after the test for 24 h, indicating that the color change of both pigments could be regarded as a very strong difference (ΔE > 0.5) [44], and this difference increased over time of exposure. In other words, discoloration occurred in all samples and was more pronounced in the Sm/Dye. The results suggested the saturation of Sm with copper denotes higher stability for the obtained pigment (Cu-Sm/Dye). Even though both pigments Sm/Dye and Cu-Sm/Dye presented a very strong difference in color before and after light exposure, the main objective of this research study still was to use a natural and abundant clay of the region and to use it as a potential adsorbent of a carcinogenic and very toxic dye (VC) to use this new material as a new hybrid pigment.
3.10. Hybrid Pigments—Antibacterial Properties
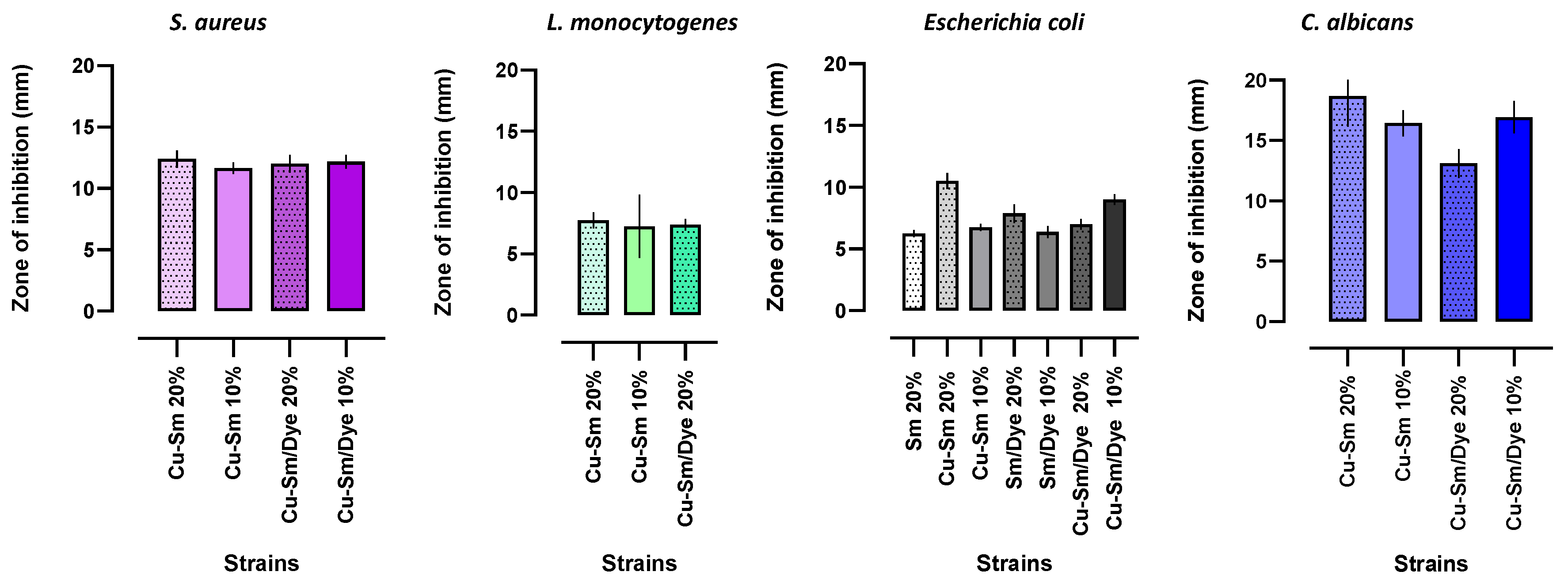
The diffusion disk method allowed an initial assessment of the antimicrobial potential of the samples. The antibacterial activity was tested by CLSI methodology against pathogenic Gram-positive S. aureus, L. monocytogenes, and Gram-negative bacteria E. coli. The yeast Candida albicans was also evaluated. The antimicrobial tests were performed for the samples before and after crystal violet and Sm adsorption. According to CLSI methodology, the inhibition zone around the sample measured the antimicrobial ability. Figure 8 shows the inhibition zone in millimeters (mm).

Figure 8.
Images of the inhibition halo of Cu-Sm and Cu-Sm/Dye dispersed in white paint (10% and 20%) by the diffusion method, duplicate tests. C: disk representing the control (white paint).
The raw Sm and Sm/Dye did not show antimicrobial activity against S. aureus, L. monocytogenes, and C. albicans, and showed a low potential against E. coli. Azmi et al. [47] reported the antibacterial activity of clays was not uniform due to differences in mineralogical and geochemical composition. The antibacterial activity of clay leachates was widely reported in the scientific literature and attributed to metal ion toxicity released from the clay mineral interlayer.
Crystal violet dye has known antibacterial properties [41]. Observing the results of Figure 9, a smectite sample containing crystal violet (Sm/Dye) showed antimicrobial potential against Gram-negative bacteria E. coli, but did not show antimicrobial activity against S. aureus, L. monocytogenes, and C. albicans. Cu-Sm samples in a percentage of 20% (% w/w) compared to Cu-Sm/Dye (20% w/w) showed better results against E. coli and C. albicans with inhibition zones of 10.5 ± 0.26 mm (tetracycline pattern: 22.0 ± 0.25 mm) and 18.6 ± 1.25 (fluconazole pattern: 22.0 ± 0.08 mm), respectively. These results suggest the potential of copper ions as an antimicrobial agent [48,49]. Comparing the inhibition halos obtained with the Cu-Sm sample (20%) against S. aureus and E. coli with the tetracycline antimicrobial pattern, an activity index (A.I) of 0.54 was observed (54% of the potential of the antimicrobial pattern) and 0.48 (48%), respectively, which demonstrated a high antimicrobial potential of the sample. The activity index relates the sample inhibition zone diameter in millimeters to the standard drug [23].

Figure 9.
Diameters of the inhibition zones (mm) produced by the samples Sm, Cu-Sm, Sm/Dye, and Cu-Sm/Dye dispersed in white paint at 10% and 20% (% m/m) on the tested microorganisms.
Previous literature reported greater susceptibility of these pathogens’ bacteria to the antibacterial action of copper [50,51]. Copper compounds are commonly used as antibacterial agents. This antibacterial property can be explained by the Cu2+ ions released continually from Cu-Sm in contact with bacteria [52,53] by a mechanism that, in general, is a combination of various types of interactions, such as membrane perforations, DNA damage, protein denaturation, among others [54]. According to Salah et al. [55], the mechanism of antimicrobial action of copper is complex, including the generation of reactive oxygen species (ROS), which is the main bactericidal mechanism. Reactive oxygen species can irreversibly damage cell membranes. Assays with the yeast also showed that the doping of the samples with copper ion promoted antimicrobial activity. The inhibition potential of cooper ion in smectite samples was observed in the results of inhibition against C. albicans, which was observed only for the samples Cu-Sm and Cu-Sm/Dye with statistically higher activity. In fungi, the antimicrobial action of copper involves cell membrane deterioration and the influx of copper ions [55].
Furthermore, the combination of Cu-Sm/Dye can be promising, especially when evaluating its antimicrobial potential against S. aureus and L. monocytogenes at a concentration of 20% compared to the Cu-Sm sample.
4. Conclusions
The smectite modification process with copper ions consisted of a simple execution step based on the characterizations performed by DRX, FTIR, and SEM. DRX results showed that the raw smectite contained more of other phases, such as kaolinite and quartz. After the mineralogical analysis, only the montmorillonite phase was observed. The sample Sm-Cu present change of the basal peak (001) indicates the intercalation of copper ions. The zeta potential study demonstrated that the choice of crystal violet dyer was due to the negative potential across the entire pH range showing a better interaction with cationic dye.
Regarding the adsorptive capacity of the samples, the kinetic data were better suited to the pseudo-second-order model and the best isothermal fit occurred for the Langmuir model. Thus, it described that dye/smectite interaction occurred in a monolayer or bilayer, from a defined number of interaction sites, and that each site interacted with only one dye molecule. Copper ions intercalation increased the smectite’s adsorptive capacity, and the characterizations evidenced the presence of the dye in the interlamellar space of the smectite.
After crystal violet dye sorption, the pigments showed characteristic violet coloration and demonstrated photostability. The best antibacterial activity was observed with the Cu-Sm sample. These results showed that cooper ions were responsible for this great inhibition. The Cu-Sm/Dye sample presented a good inhibition, resulting in a promising antibacterial material against Gram-positive strains Staphylococcus aureus (ATCC 25923), Listeria monocytogenes (ATCC 19111) and the Gram-negative strains Escherichia coli (ATCC 25922) and Candida Albicans (ATCC 64548). In general, it was found that the intercalation process with Cu2+ ions (colorants) increased the inhibition potential again bacteria and fungi and could be an alternative to produce inorganic hybrid pigments with good stability and saturated color.
Author Contributions
Conceptualization, M.L.M.R., N.B., J.O.P., D.F.L.H. and F.J.A.; validation, S.J., P.A., M.A.A.C. and F.J.A.; investigation, M.L.M.R., N.B., S.J., P.A., D.M., M.A.A.C. and F.J.A.; resources, M.L.M.R., S.J. and F.J.A.; writing—original draft preparation M.L.M.R., N.B., S.J. and F.J.A.; writing—review and editing, F.J.A.; supervision, F.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
M.L.M.R. appreciates CAPES (grant number 88887.628497/2021-00) for a graduate scholarship. F.J.A. is thankful for a CNPq Productivity grant (310815/2022-3) and the grants CNPq (427127/2018-1). S.J. is thankful for a CNPq PDJ post-doctorate grant (152230/2022-0).
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Marcio Liz for donating the raw clay.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noreen, S.; Bhatti, H.N.; Zuber, M.; Zahid, M.; Asgher, M. Removal of Actacid Orange-RL Dye Biocomposites: Modeling Studies. Pol. J. Environ. Stud. 2017, 26, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Saini, B.; Dey, A. Synthesis and Characterization of Copolymer Adsorbent for Crystal Violet Dye Removal from Water. Mater. Today Proc. 2022, 61, 342–350. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient Removal of Dyes Using Carboxymethyl Cellulose/Alginate/Polyvinyl Alcohol/Rice Husk Composite: Adsorption/Desorption, Kinetics, and Recycling Studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of Hazardous Dye Crystal Violet from Wastewater by Waste Materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of Toxic Crystal Violet Dye Using Coffee Husks: Equilibrium, Kinetics, and Thermodynamics Study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue on Low-Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, F.P.; Marinho, G.; Rodrigues, K. Corantes Têxteis: Uma Revisão. Holos 2013, 5, 98. [Google Scholar] [CrossRef]
- Klein, C.; Dutrow, B. Manual de Ciências Dos Minerais, 23rd ed.; weber Nowaczyk, D., Ed.; Bookman Editora: São Paulo, Brasil, 2012; ISBN 978-85-7780-963-9. [Google Scholar]
- De Freitas Melo, V.; Alleoni, L.R.F. Química e Mineralogia Do Solo: Conceitos Básicos e Aplicações; de Freitas Melo, V., Alleoni, L.R.F., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brasil, 2019; ISBN 9788586504266. [Google Scholar]
- Santos, S.S.G.; Franca, D.B.; Castellano, L.R.C.; Tigueiro, P.; Filho, E.C.S.; Santos, I.M.; Fonseca, M.G. Novel modified bentonites applied to the removal of an anionic azo-dye from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 5845, 124152. [Google Scholar] [CrossRef]
- Da Silva, J.C.S.; Franca, D.B.; Rodrigues, F.; Oliveira, D.M.; Trigueiro, P.; Filho, E.C.S.; Fonseca, M.G. What happens when chitosan meets bentonite under microwave-assisted conditions? Clay-based hybrid nanocomposites for dye adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125584. [Google Scholar] [CrossRef]
- Çelik, M.S. Electrokinetic Behavior of Clay Surfaces. Interface Sci. Technol. 2004, 1, 57–89. [Google Scholar] [CrossRef]
- Duarte-Neto, J.F.; Cartaxo, J.M.; Neves, G.A.; Menezes, R.R. Processos de Adsorção de Corantes Em Argilas Esmectíticas: Uma Revisão. Rev. Eletrônica Mater. Process. 2014, 9, 51–59. [Google Scholar]
- Zhou, C.; Tong, D.; Yu, W. Smectite Nanomaterials: Preparation, Properties, and Functional Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128145333. [Google Scholar]
- Silva, A.R.V.; Ferreira, H.C. Argilas Bentoníticas: Conceitos, Estruturas, Propriedades, Usos Industriais, Reservas, Produção e Produtores/Fornecedores Nacionais e Internacionais|PDF|Cerâmica|Adsorção. Available online: https://pt.scribd.com/document/361933187/Argilas-bentoniticas-conceitos-estruturas-propriedades-usos-industriais-reservas-producao-e-produtores-fornecedores-nacionais-e-internacionais (accessed on 8 August 2022).
- Allahbakhsh, A.; Jarrahi, Z.; Farzi, G.; Shavandi, A. Three-Dimensional Nanoporous Cu-BTC/Graphene Oxide Nanocomposites with Engineered Antibacterial Properties Synthesized via a One-Pot Solvosonication Process. Mater. Chem. Phys. 2022, 277, 125502. [Google Scholar] [CrossRef]
- Calvacanti, G.R.S.; Rodrigues, F.; Zhuang, G.; Balme, S.; Janot, J.M.; Fonseca, M.G.; Jaber, M. Inorganic-organic hybrid pigments based on carminic acid and clay minerals. Dye. Pigm. 2021, 190, 109306. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Silva, K.M.; Silva, C.P.; Gonçalves, J.M.; Quina, F.H. Hybrid Pigments from Anthocyanin Analogues and Synthetic Clay Minerals. ACS Omega 2020, 5, 26592–26600. [Google Scholar] [CrossRef] [PubMed]
- Alorabi, A.Q.; Hassan, M.S.; Alam, M.M.; Zabin, S.A.; Alsenani, N.I.; Baghdadi, E. Natural Clay as a Low-Cost Adsorbent for Crystal Violet Dye Removal and Antimicrobial Activity. Nanomaterials 2021, 11, 2789. [Google Scholar] [CrossRef]
- Chwastowski, J.; Satoń, P.; Pięta, E.; Paluszkiewicz, C. Bioremediation of Crystal Violet by Organic Matter and Assessment of Antimicrobial Properties of the Obtained Product. Sustainability 2023, 15, 67. [Google Scholar] [CrossRef]
- Shams, S.; Ahmad, W.; Memon, A.H.; Shams, S.; Wei, Y.; Yuan, Q.; Liang, H. Cu/H3BTC MOF as a Potential Antibacterial Therapeutic Agent against Staphylococcus Aureus and Escherichia Coli. New J. Chem. 2020, 44, 17671–17678. [Google Scholar] [CrossRef]
- Trigueiro, P.; Rodrigues, F.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.G.; Fonseca, M.G.; Osajima, J.; Walter, P.; Jaber, M. When Anthraquinone Dyes Meet Pillared Montmorillonite: Stability or Fading upon Exposure to Light? Dye. Pigment. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Feng, N.C.; Guo, X.Y. Characterization of adsorptive capacity and mechanisms on adsorption of copper, lead, and zinc by modified orange peel. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar] [CrossRef]
- Match! Demonstration, version 3.10.1; Crystal Impact GbR: Bonn, Germany, 2022.
- Quindici, M. O Segredodas Cores; All Print: São Paulo, Brasil, 2013; ISBN 13-08586. [Google Scholar]
- Farbmetrische Bestimmung von Farbabständen Bei Körperfarben Nach Der CIELAB-Formel. Colorimetric Evaluation of Colour Differences of Surface Colours According to the CIELAB Formula. Available online: https://www.beuth.de/de/norm/din-6174/541403DIN6174:1979-01 (accessed on 5 June 2023).
- Zhuang, G.; Rodrigues, F.; Zhang, Z.; Fonseca, M.G.; Walter, P.; Jaber, M. Dressing Protective Clothing: Stabilizing Alizarin/Halloysite Hybrid Pigment and Beyond. Dye. Pigment. 2019, 166, 32–41. [Google Scholar] [CrossRef]
- Lima, L.C.B.; Silva, F.C.; Silva-Filho, E.C.; Fonseca, M.G.; Zhuang, G.; Jaber, M. Saponite-Anthocyanin Derivatives: The Role of Organoclays in Pigment Photostability. Appl. Clay Sci. 2020, 191, 105604. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Balaba, N.; Jaerger, S.; Horsth, D.F.L.; Primo, J.D.O.; Correa, J.D.S.; Bittencourt, C.; Zanette, C.M.; Anaissi, F.J. Polysaccharides as Green Fuels for the Synthesis of MgO: Characterization and Evaluation of Antimicrobial Activities. Molecules 2023, 28, 142. [Google Scholar] [CrossRef] [PubMed]
- Schons AB Appelt, P.; Correa, J.S.; Cunha, M.A.A.; Rodrigues, M.G.; Anaissi, F.J. Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action. Antibiotics 2023, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.E.V.; Angélica, R.S.; da Paz, S.P.A. Practical Mineralogical Quantification of Bentonites Supported for a PXRD Calibrated Hkl Model. Braz. J. Geol. 2021, 51, 1. [Google Scholar] [CrossRef]
- Fontaine, F.; Christidis, G.E.; Yans, J.; Hollanders, S.; Hoffman, A.; Fagel, N. Characterization and Origin of Two Fe-Rich Bentonites from Westerwald (Germany). Appl. Clay Sci. 2020, 187, 105444. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X Ray Diffraction, 3rd ed.; Hall, P., Ed.; Addison-Wesley Publishing: Boston, MA, USA, 2001; ISBN 0201610914, 9780201610918. [Google Scholar]
- Dos Santos, C.P.F.; Melo, D.M.A.; Melo, M.A.F.; VSobrinho, E.V. Caracterização e Usos de Argilas Bentonitas e Vermiculitas Para Adsorção de Cobre (II) Em Solução. Cerâmica 2002, 48, 178–182. [Google Scholar] [CrossRef]
- Chen, J.; Lu, J.; Su, L.; Ruan, H.; Zhao, Y.; Lee, C.; Cai, Z.; Wu, Z.; Jiang, Y. Enhanced Adsorption of Methyl Orange by Mongolian Montmorillonite after Aluminum Pillaring. Appl. Sci. 2022, 12, 3182. [Google Scholar] [CrossRef]
- Madejová, J. FTIR Techniques in Clay Mineral Studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Kaufhold, S.; Hein, M.; Dohrmann, R.; Ufer, K. Quantification of the Mineralogical Composition of Clays Using FTIR Spectroscopy. Vib. Spectrosc. 2012, 59, 29–39. [Google Scholar] [CrossRef]
- Şans, B.E.; Güven, O.; Esenli, F.; Çelik, M.S. Contribution of Cations and Layer Charges in the Smectite Structure on Zeta Potential of Ca-Bentonites. Appl. Clay Sci. 2017, 143, 415–421. [Google Scholar] [CrossRef]
- Darrow, M.M.; Guo, R.; Trainor, T.P. Zeta Potential of Cation-Treated Soils and Its Implication on Unfrozen Water Mobility. Cold Reg. Sci. Technol. 2020, 173, 103029. [Google Scholar] [CrossRef]
- Guida, I.I.S.; Falcão, S.S. Removal of Crystal Violet Textile Using Clay Maranhese of High Mounts as Adsorvent. Rev. Virtual De Quim. 2018, 10, 1087–1099. [Google Scholar] [CrossRef]
- Kumari, H.J.; Krishnamoorthy, P.; Arumigam, T.K.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from wastewater byadsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef]
- McLAREN, K. XIII—The Development of the CIE 1976 (L* a* b*) Uniform Colour Space and Colour-difference Formula. J. Soc. Dye. Colour. 2008, 92, 338–341. [Google Scholar] [CrossRef]
- Elsherif, K.M.; El-Dali, A.; Alkarewi, A.A.; Ewlad-Ahmed, A.M.; Treban, A. Adsorption of Crystal Violet Dye onto Olive Leaves Powder: Equilibrium and Kinetic Studies. Chem. Int. 2021, 7, 79–89. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Azmi, N.N.; Mahyudin, N.A.; Wan Omar, W.H.; Mahmud Ab Rashid, N.-K.; Ishak, C.F.; Abdullah, A.H.; Sharples, G.J. Antibacterial Activity of Clay Soils against Food-Borne Salmonella typhimurium and Staphylococcus aureus. Molecules 2022, 27, 170. [Google Scholar] [CrossRef]
- Abdullah; Hussain, T.; Faisal, S.; Rizwan, M.; Zaman, S.N.; Iqbal, M.; Iqbal, A.; Ali, Z. Green synthesis and characterization of copper and nickel hybrid nanomaterials: Investigation of their biological and photocatalytic potential for the removal of organic crystal violet dye. J. Saudi Chem. Soc. 2022, 26, 101486. [Google Scholar] [CrossRef]
- Ozkan, E.; Ozkan, F.T.; Allan, E.; Parkin, I.P. The use of zinc oxide nanoparticles to enhance the antibacterial properties of light-activated polydimethylsiloxane containing crystal violet. RSC Adv. 2015, 5, 8806–8813. [Google Scholar] [CrossRef]
- Chebout, O.; Trifa, C.; Bouacida, S.; Boudraa, M.; Imane, H.; Merzougui, M.; Mazouz, W.; Ouari, K.; Boudaren, C.; Merazig, H. Two new copper (II) complexes with sulfanilamide as ligand: Synthesis, structural, thermal analysis, electrochemical studies and antibacterial activity. J. Mol. Struct. 2022, 1248, 131446. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.T.; Kuo, T.R. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interf. Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramírez, I.E.; Arzate-Cardenas, M.A.; Mojarro-Olmos, A.; Romo-López, M.A. Synthesis, characterization, toxicological and antibacterial activity evaluation of Cu@ZnO nanocomposites. Ceram. Int. 2019, 45, 17476–17488. [Google Scholar] [CrossRef]
- Dong, W.; Lu, Y.; Wang, W.; Zhang, M.; Jing, Y.; Wang, A. A sustainable approach to fabricate new 1D and 2D nanomaterials from natural abundant palygorskite clay for antibacterial and adsorption. J. Chem. Eng. 2020, 382, 122984. [Google Scholar] [CrossRef]
- Bagchi, B.; Kar, S.; Dey, S.K.; Bhandary, S.; Roy, D.; Mukhopadhyay, T.K.; Das, S.; Nandy, P. In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids Surf. 2022, 108, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).