Trace Element Composition of Pyrite from Selected Black Shale and Chert Exposures in the Central Belt of Peninsular Malaysia: Implications for Mineral Exploration
Abstract
1. Introduction
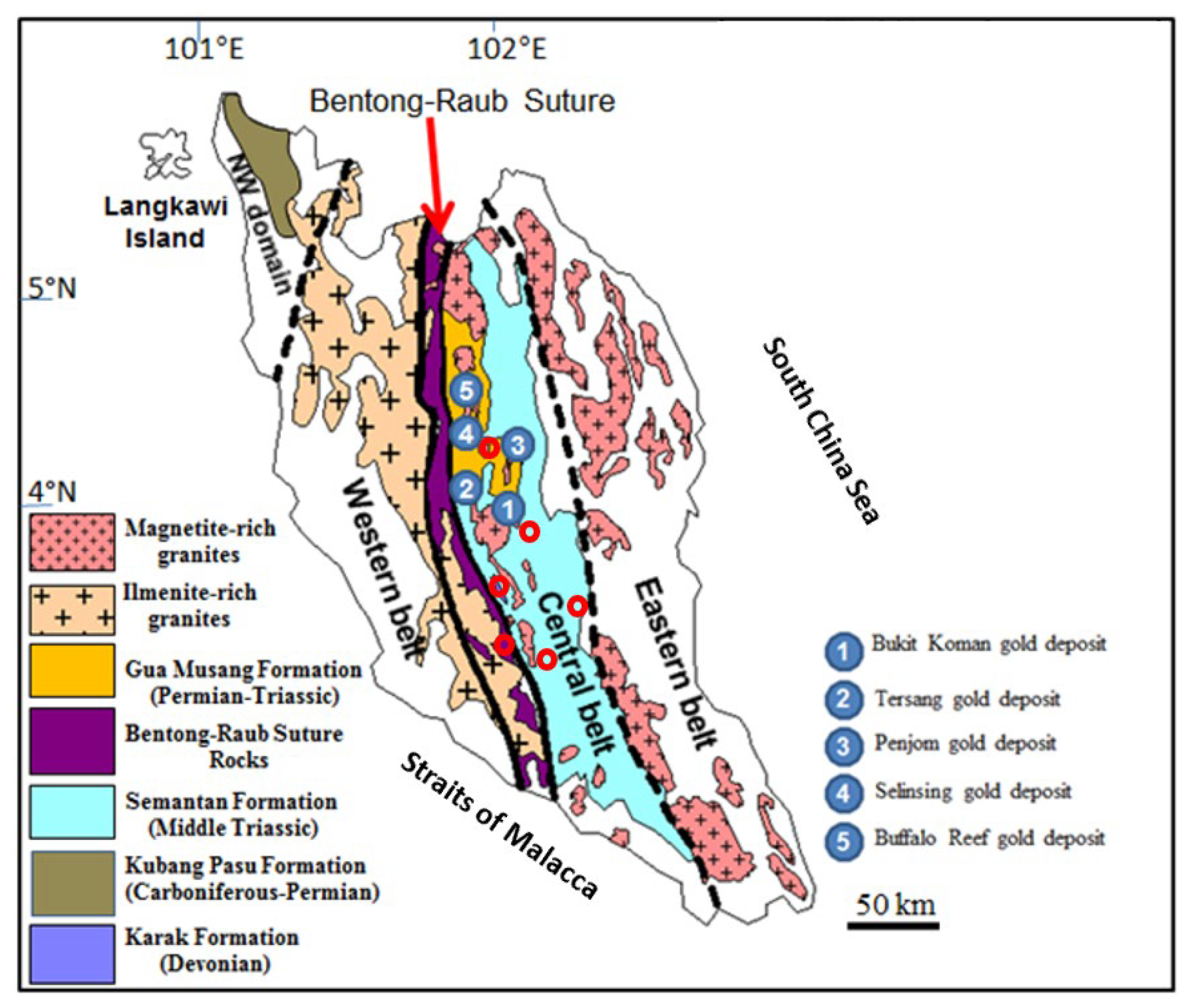
2. Geological Setting
3. Materials and Methods
3.1. LA ICP-MS Method
3.2. EPMA Method
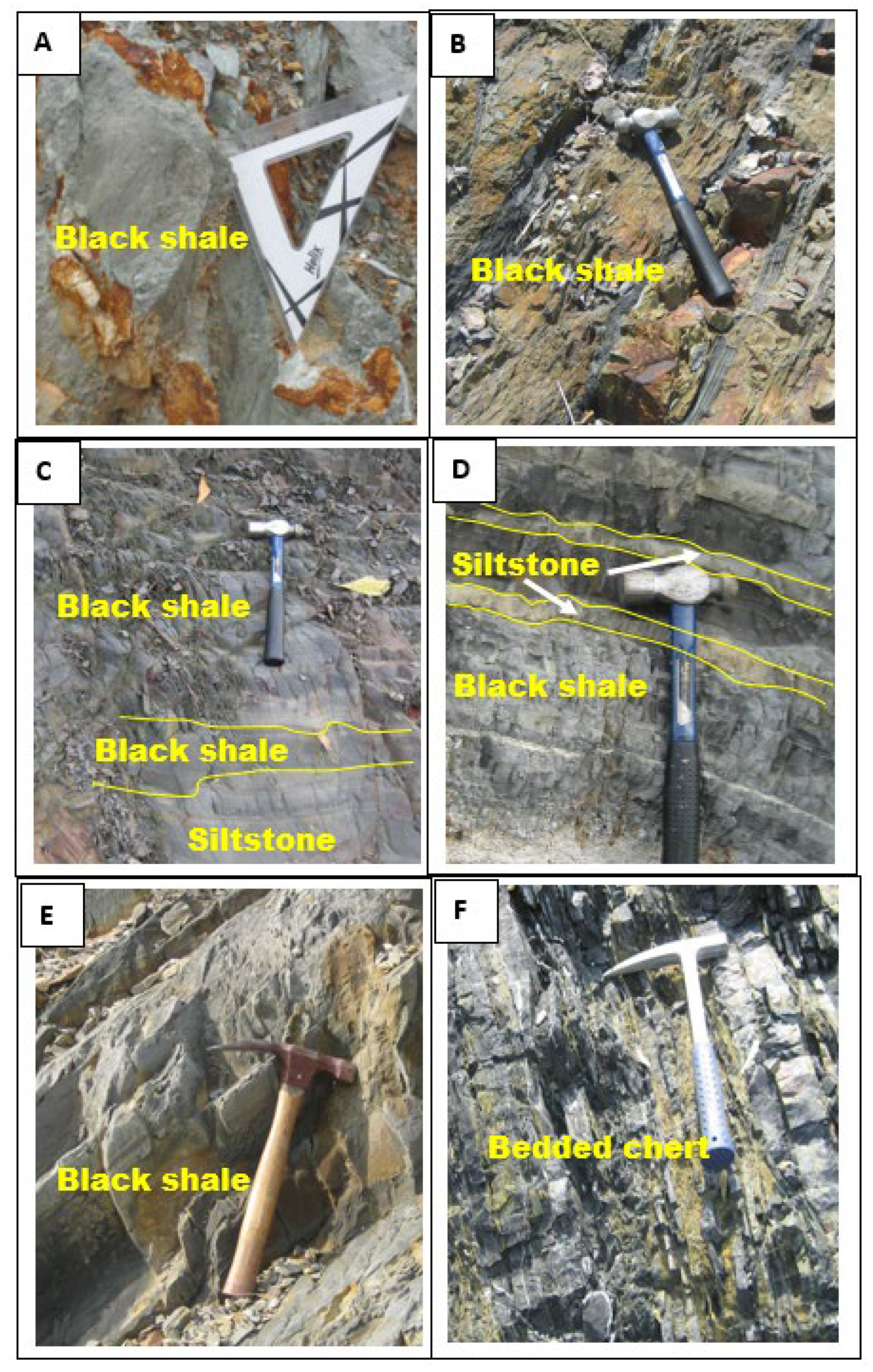
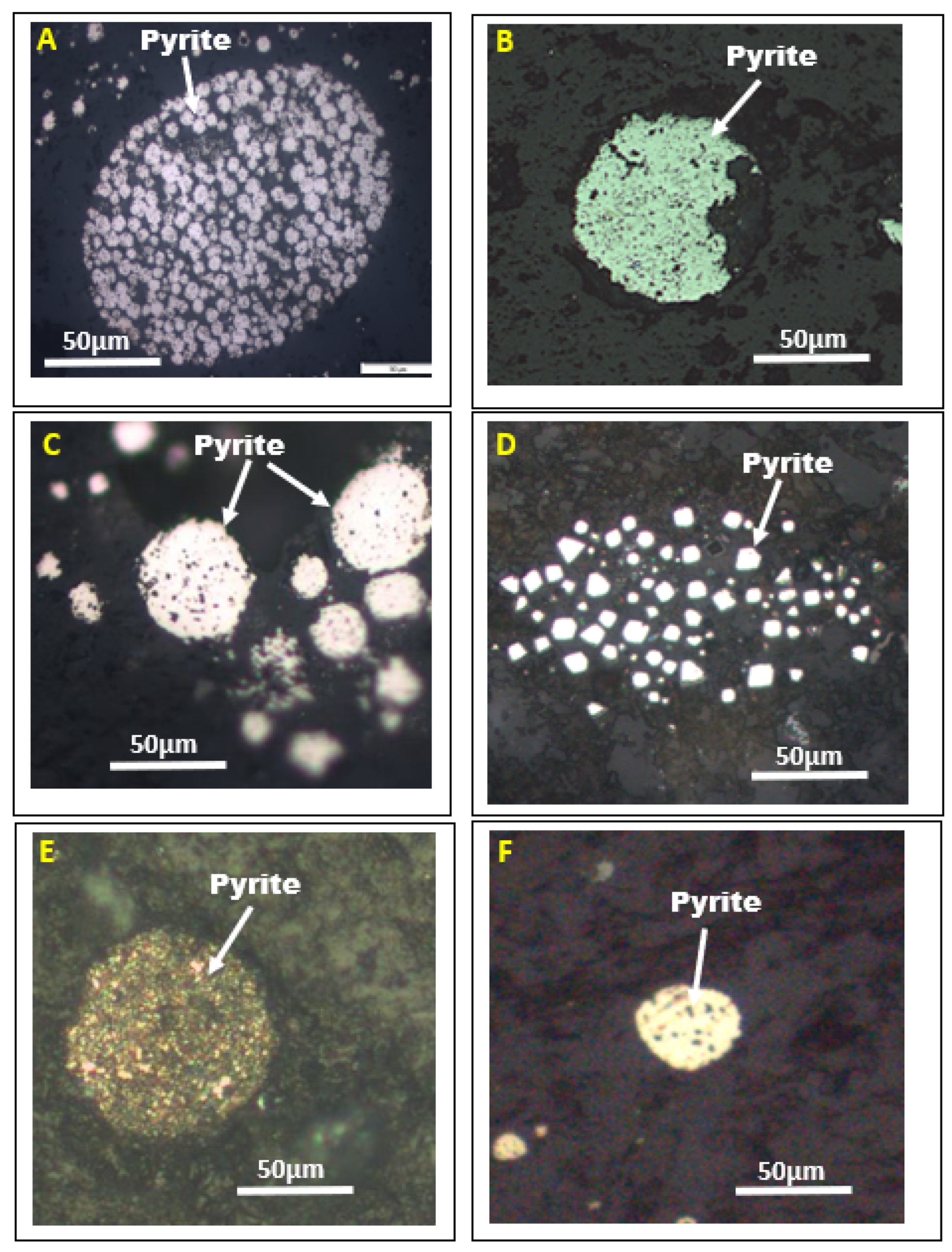
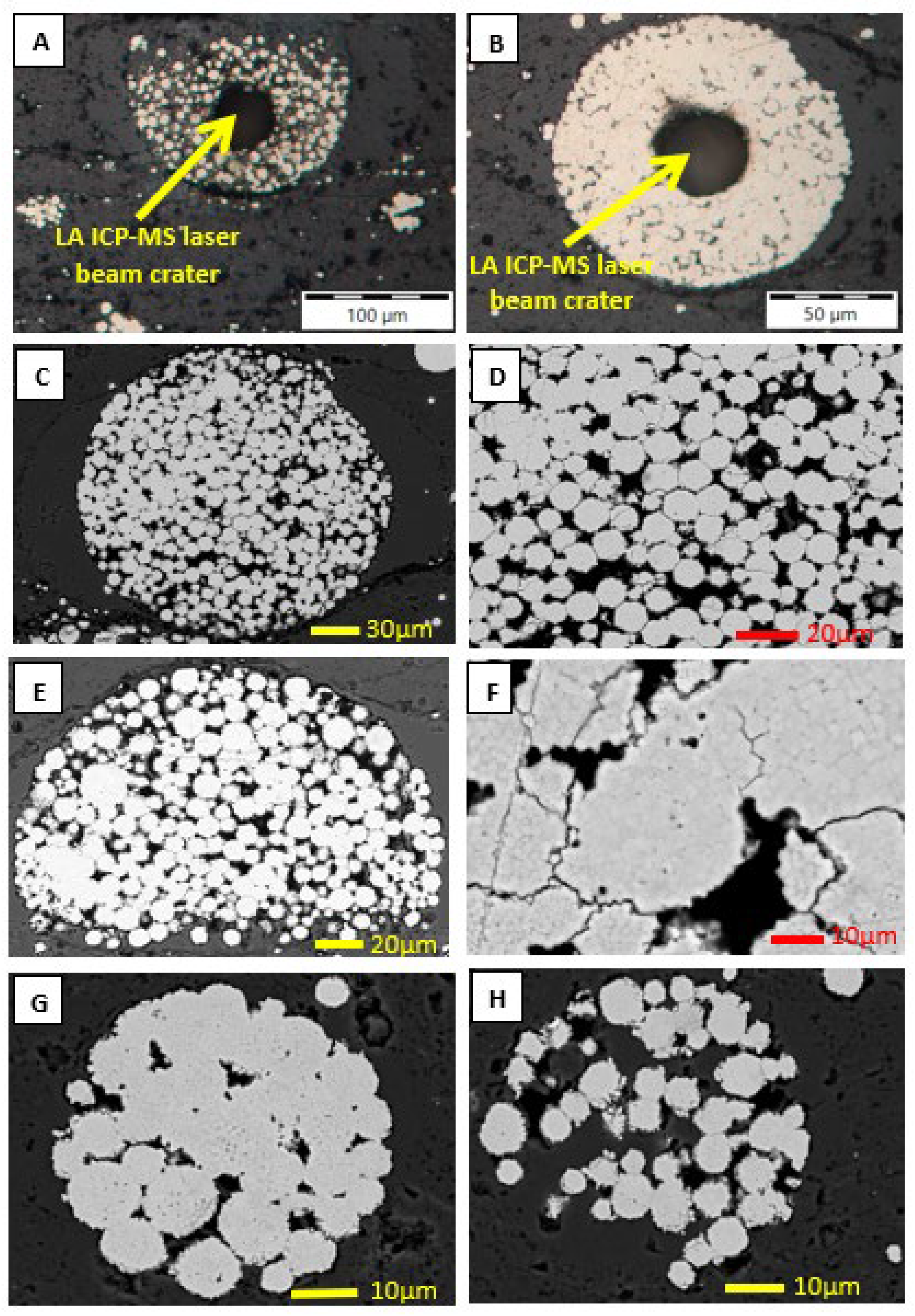
4. Texture of Pyrite
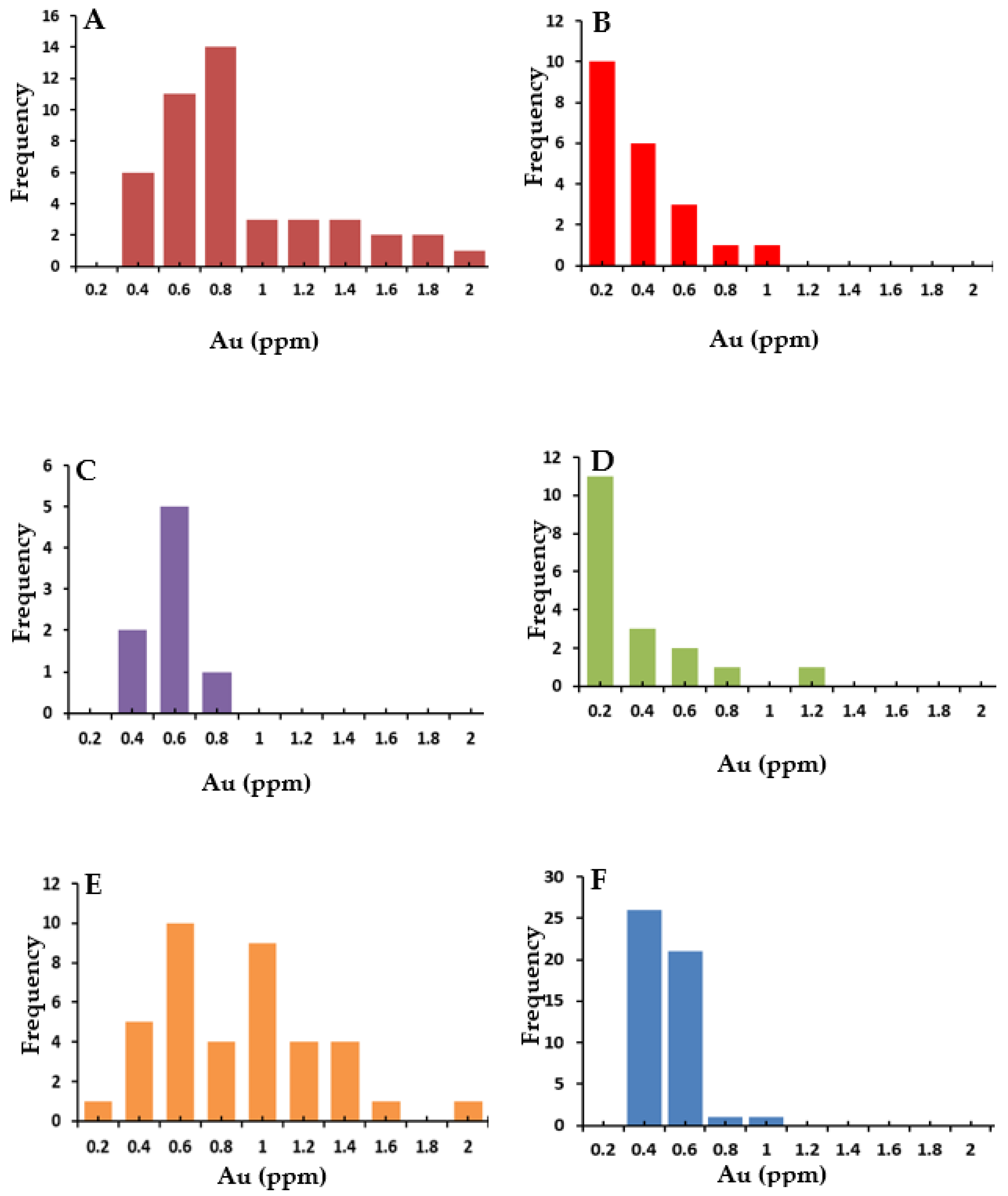
5. Results
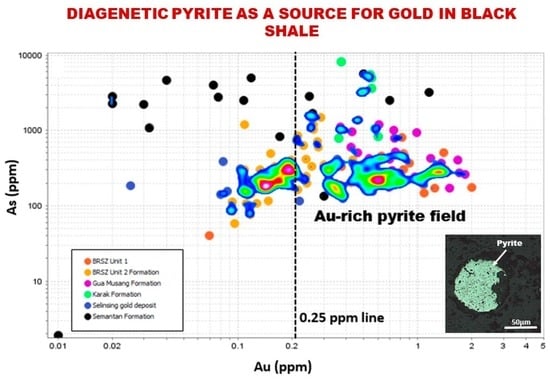
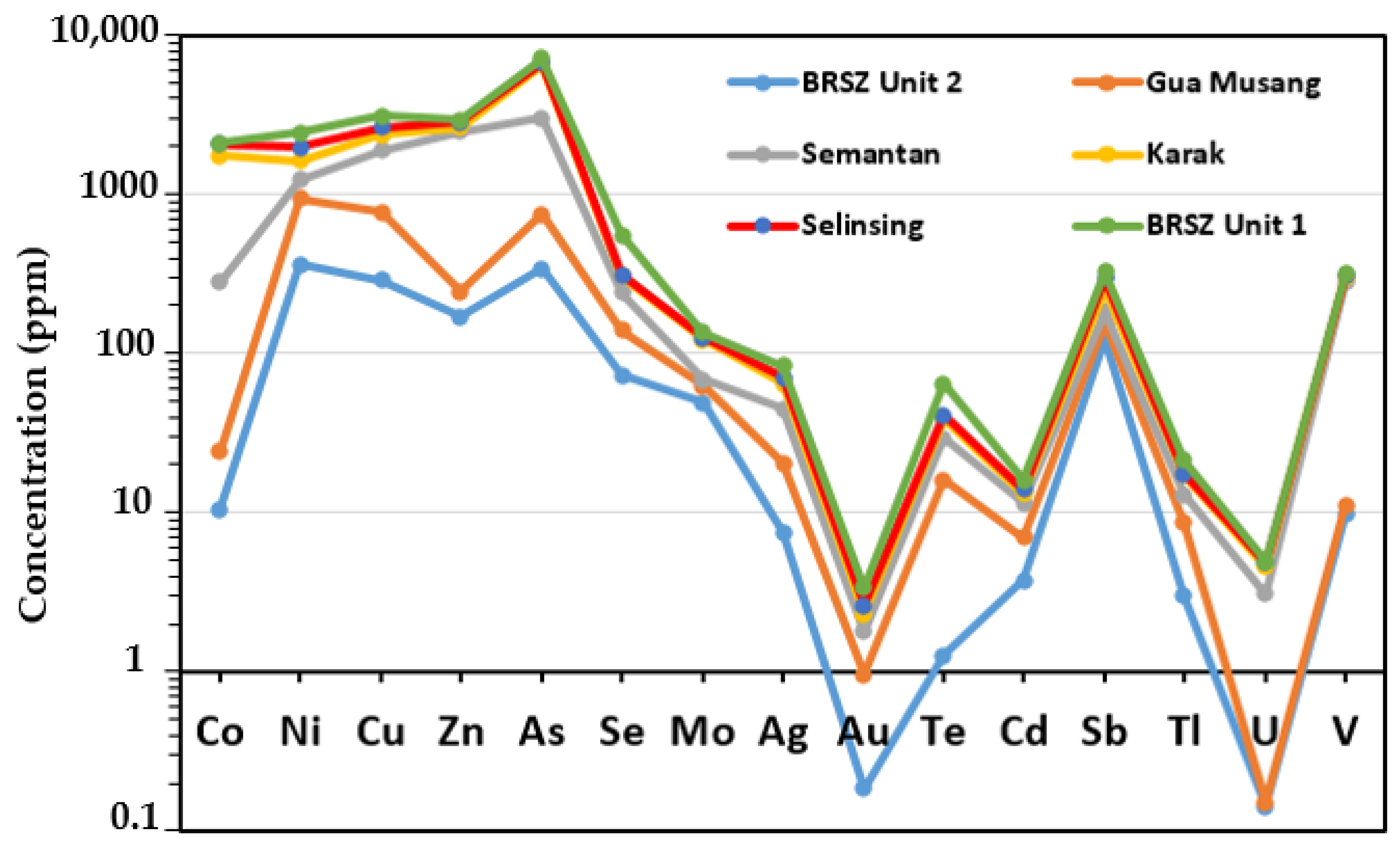
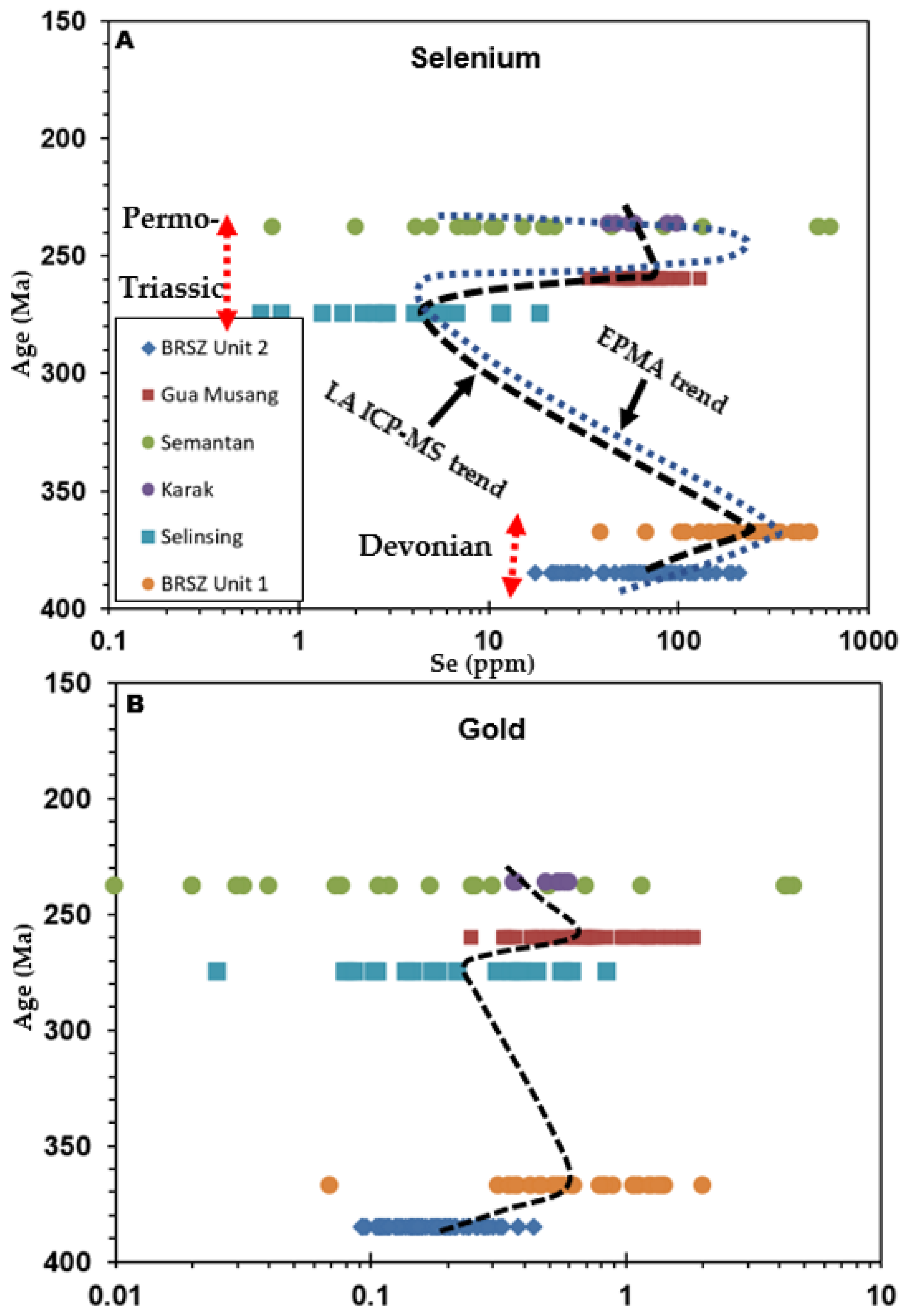
6. Discussion
6.1. Origin of Pyrite
6.2. Relationship among Metals and Control on Depositional Processes
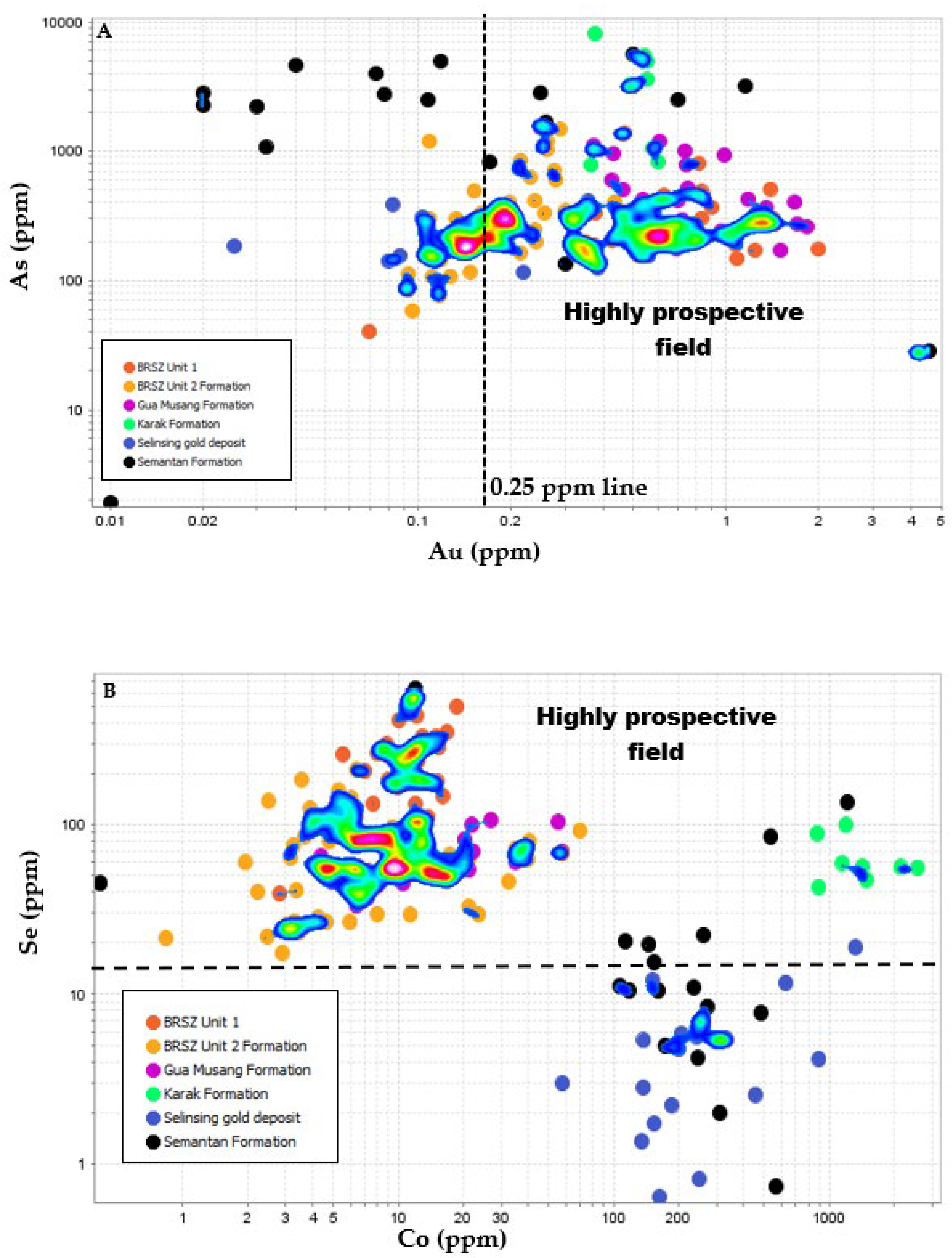
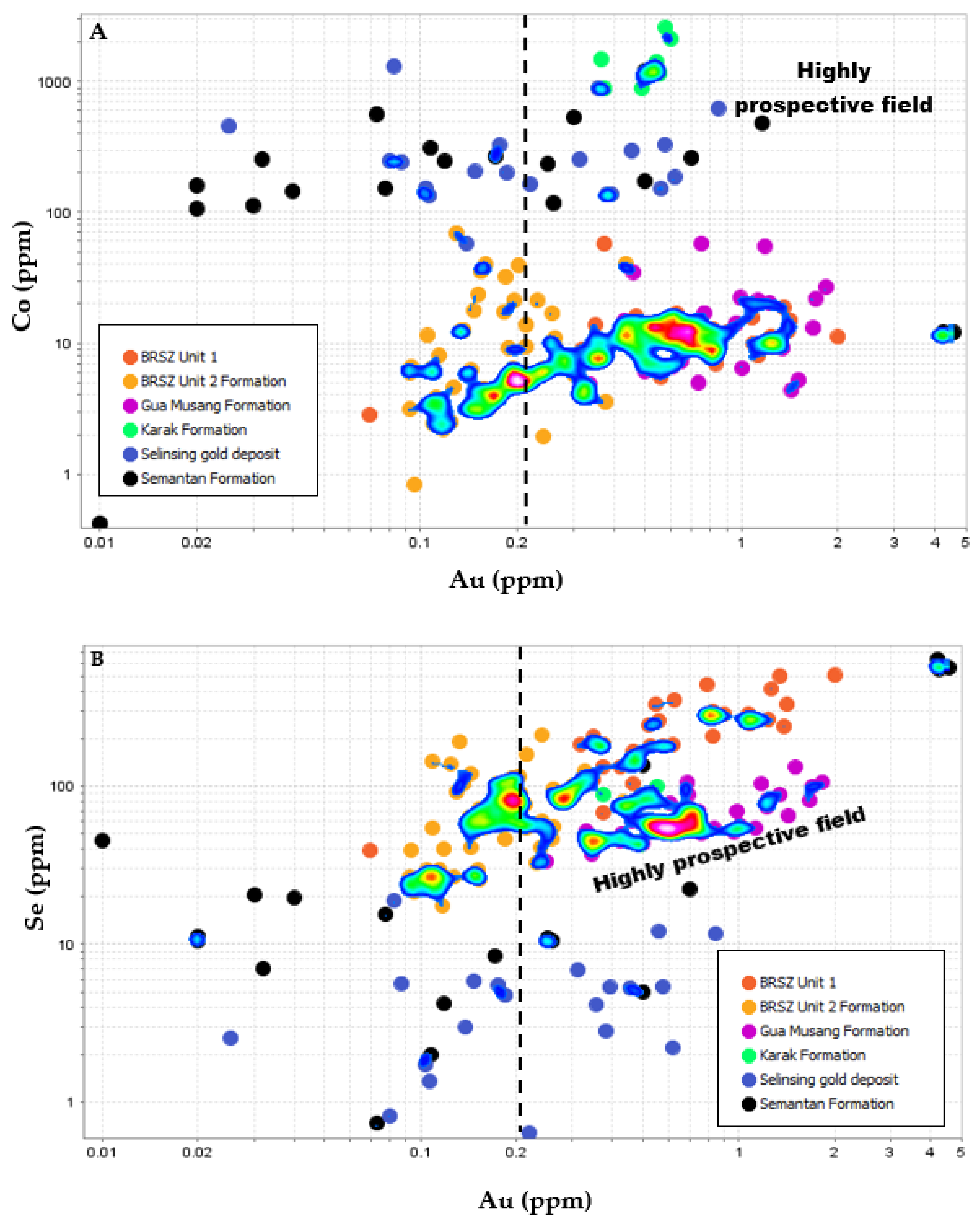
6.3. Exploration Implications
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoieb, M.A.; Gebretsadik, H.T.; Rahmani, O.; Ismail, M.S.; Ibad, S.M. Geochemical characteristics of the Silurian-Devonian Kroh black shales, Peninsular Malaysia: An implication for hydrocarbon exploration. J. Geochem. Explor. 2022, 232, 106891. [Google Scholar] [CrossRef]
- Rickard, D. Sulfidic sediments and sedimentary rocks: Developments in Sedimentology. Sulfidic Sediments Sediment. Rocks 2012, 65, 801. [Google Scholar]
- Huerta-diaz, M.A.; Morse, J.W. Pyritization of trace-metals in anoxic marine sediments. Geochim. Cosmochim. Acta 1992, 56, 2681–2702. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Gregory, D.; Meffre, S.; Large, R.R. Comparison of metal enrichment in pyrite framboids from a metal-enriched and metal-poor estuary. Am. Mineral. 2014, 99, 633–644. [Google Scholar] [CrossRef]
- Berner, Z.A.; Puchelt, H.; Nöltner, T.; Kramar, U. Pyrite geochemistry in the Toarcian Posidonia Shale of southwest Germany: Evidence for contrasting trace-element patterns of diagenetic and syngenetic pyrites. Sedimentology 2013, 60, 548–573. [Google Scholar] [CrossRef]
- Gregory, D.; Large, R.R.; Halpin, J.A.; Baturina, E.L.; Lyons, T.W.; Wu, S.; Sack, P.J.; Chappaz, A.; Maslennikov, V.V.; Bull, S.W. Trace element content of background sedimentary pyrite in black shales. Econ. Geol. 2015, 110, 1389–1410. [Google Scholar] [CrossRef]
- Large, R.R.; Halpin, J.A.; Danyushevsky, L.V.; Maslennikov, V.V.; Bull, S.W.; Long, J.A.; Gregory, D.D.; Elena Lounejeva Lyons, T.W.; Sack, P.J.; McGoldrick, P.J.; et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean–atmosphere evolution. Earth Planet. Sci. Lett. 2014, 389, 209–220. [Google Scholar] [CrossRef]
- Large, R.R.; Gregory, D.D.; Steadman, J.A.; Tomkins, A.G.; Lounejeva, E.; Danyushevsky, L.V.; Halpin, J.A.; Maslennikov, V.; Sack, P.J.; Mukherjee, I.; et al. Gold in the oceans through time. Earth Planet. Sci. Lett. 2015, 428, 139–150. [Google Scholar] [CrossRef]
- Large, R.R.; Bull, S.W.; Maslennikov, V.V. A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Soc. Econ. Geol. 2011, 106, 331–358. [Google Scholar] [CrossRef]
- Makoundi, C.; Zaw, K.; Large, R.R.; Meffre, S.; Lai, C.K.; Hoe, T.G. Geology, geochemistry and metallogenesis of the Selinsing gold deposit, Central Malaysia. Gondwana Res. 2014, 26, 241–261. [Google Scholar] [CrossRef]
- Ivanov, K.S.; Maslennikov, V.V.; Artemyev, D.A.; Tseluiko, A.S. Highly Metalliferous Potential of Framboidal and Nodular Pyrite Varieties from the Oil-Bearing Jurassic Bazhenov Formation, Western Siberia. Minerals 2020, 10, 449. [Google Scholar] [CrossRef]
- Pašava, J.; Ackerman, L.; Žák, J. Multi-stage metal enrichment and formation of gold mineralization in black shales: The role of high heat flow in a rift setting. Min. Depos. 2023. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V.; Robert, F.; Danyushevsky, L.V.; Chang, Z. Multistage sedimentary and metamorphic origin of pyrite and gold in the Giant Sukhoi log deposit, Lena Gold Province, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Olivo, G.R.; Teagle, D.A.H.; Craw, D. Sulfide evolution during prograde metamorphism of the Otago and Alpine Schists, New Zealand. Can. Mineral. 2010, 48, 1267–1295. [Google Scholar] [CrossRef]
- Cave, B.J.; Stepanov, A.S.; Craw, D.; Large, R.R.; Halpin JA Thompson, J. Release of trace elements through the sub-greenschist facies breakdown of detrital rutile to metamorphic titanite in the Otago Schist, New Zealand. Can. Mineral. 2015, 53, 379–400. [Google Scholar] [CrossRef]
- Gregory, D. The Pyrite Trace Element Paleo-Ocean Chemistry Proxy (Elements in Geochemical Tracers in Earth System Science); Cambridge University Press: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Makoundi, C. Geology, Geochemistry and Metallogenesis of Selected Sediment-Hosted Gold Deposits in the Central Gold Belt, Peninsular Malaysia. Master’s Thesis, University of Tasmania, Tasmania, Australia, 2012; 212p. [Google Scholar]
- Jaafar, B.A. Geology and mineral resources of the Karak and Temerloh areas, Pahang. Geol. Soc. Malays. Mem. 1976, 15, 138. [Google Scholar]
- Metcalfe, I. The Bentong-Raub Suture Zone. J. Asian Earth Sci. 2000, 18, 691–712. [Google Scholar] [CrossRef]
- Makoundi, C. Geochemistry of Black Shales, Sandstones; Chert in Malaysia: Insights into Gold Source Rocks. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2016. [Google Scholar]
- Makoundi, C.; Zaw, K.; Endut, Z. U-Pb Dating, Lead Isotopes, and Trace Element Composition of Pyrite Hosted in Black Shale and Magmatic Rocks, Malaysia: Implications for Orogenic Gold Mineralization and Exploration. Minerals 2023, 13, 221. [Google Scholar] [CrossRef]
- Wan, F.; Wan, H.; Heru Sigit, P. Type deposits of Primary Gold Mineralisation in the Central Belt, Peninsular Malaysia. Bul. Persat. Geol. Malays. 2002, 45, 111–116. [Google Scholar]
- Basir, J. Chert blocks in Bentong-Raub Suture Zone: A heritage of Palaeo-Tethys. Bull. Geol. Soc. Malays. 2013, 59, 85–91. [Google Scholar]
- Mustaffa, K.; Hadi, A.; Rahman, A. A fivefold stratigraphic and tectonic subdivision of Peninsular Malaysia. In Dynamic Stratigraphy and Tectonics of Peninsular Malaysia—Problems and Issues; a thematic publication; Geological Society of Malaysia: Kuala Lumpur, Malaysia, 1999; pp. 38–63. [Google Scholar]
- Makoundi, C. Facies analysis of the Triassic Jelai Formation in the Central Basin of Peninsular Malaysia: Implications on Paleogeography and Tectonics. Master’s Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2004; 140p. [Google Scholar]
- Danyushevsky, L.V.; Robinson, P.; Gilbert, S.; Norman, M.; Large, R.R.; McGoldrick, P.; Shelley, J.M.G. Routine quantitative multi-element analysis of sulfide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Bull, S.; Scott, R.; Emsbo, P.; et al. Gold and Trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Allaz, J.M.; Williams, M.L.; Jercinovic, M.J.; Goemann, K.; Donovan, J. Multipoint Background Analysis: Gaining Precision and Accuracy in Microprobe Trace Element Analysis. Microsc. Microanal. 2019, 25, 30. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.J.; Snyder, D.A.; Rivers, M.L. An Improved Interference Correction for Trace Element Analysis. Microbeam Anal. 1993, 2, 223. [Google Scholar] [CrossRef]
- Armstrong, J.T. Quantitative analysis of silicates and oxide minerals: Comparison of Monte-Carlo, ZAF and Phi-Rho-Z procedures. Microbeam Anal. 1988, 1988, 239. [Google Scholar]
- Guy, B.M.; Beukes, N.J.; Gutzmer, J. Paleoenvironmental controls on the texture and chemical composition of pyrite from non-conglomeratic sedimentary rocks of the Mesoarchean Witwatersrand Supergroup, South Africa. S. Afr. J. Geol. 2010, 113, 195–228. [Google Scholar] [CrossRef]
- Vysotskiy, S.V.; Velivetskaya, T.A.; Ignatiev, A.V.; Slabunov, A.I.; Aseeva, A.V. Multiple Sulfur Isotope Evidence for Bacterial Sulfate Reduction and Sulfate Disproportionation Operated in Mesoarchaean Rocks of the Karelian Craton. Minerals 2022, 12, 1143. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The size distribution of framboidal pyrite in modern sediments: An indicator of redox conditions. Geochim. Et Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Makoundi, C.; Endut, Z.; Large, R.R.; Zaw, K.; Lounejeva, E.; Leman, M.S.; Mohamed, K.R.; Basori, M.B.I. Geochemistry of Pyritic Mudstones from the Singa Formation, Malaysia: Insights into Gold Potential, Source of Sulfur and Organic Matter. Geosciences 2021, 11, 279. [Google Scholar] [CrossRef]
- Bajwah, Z.U.; Seccombe, P.K.; Offler, R. Trace element distribution Co:Ni ratios and Genesis of the big cadia iron-copper deposit, new south wales, Australia. Min. Depos. 1987, 22, 292–300. [Google Scholar] [CrossRef]
- Rieger, P.; Magnall, J.M.; Gleeson, S.A.; Oelze, M. Pyrite chemistry records a multistage ore forming system at the Proterozoic George Fisher massive sulfide Zn-Pb-Ag deposit, Mount Isa, Australia. Front. Earth Sci. 2023, 11, 892759. [Google Scholar] [CrossRef]
- Rimmer, S.M. Geochemical paleoredox indicators in Devonian-Mississippian black shales, Central Appalachian Basin, USA. Chem. Geol. 2004, 206, 373–391. [Google Scholar] [CrossRef]
- Arthur, M.A.; Sageman, B.B. Marine black shales: A review of depositional mechanisms and environments of ancient deposits. Ann. Rev. Earth Planet. Sci. 1994, 22, 499–551. [Google Scholar] [CrossRef]
- Morford, J.L.; Emerson, S. The geochemistry of redox sensitive trace metals in sediments. Geochim. Cosmochim. Acta 1999, 63, 1735–1750. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, P.; Wang, G.-G.; Zhang, Y.-Q.; Sheng, Z.-L.; Zhang, S.-L.; Fan, M.-S.; Zhao, Z.-H. Hydrothermal evolution and gold precipitation mechanism of the Hekou gold deposit, North China Craton: Insights from pyrite texture, composition and in situ S isotope. Ore Geol. Rev. 2023, 156, 105387. [Google Scholar] [CrossRef]
- Wu, Y.F.; Evans, K.; Hu, S.Y.; Fougerouse, D.; Zhou, M.F.; Fisher, L.A.; Guagliardo, P.; Li, J.W. Decoupling of Au and As during rapid pyrite crystallization. Geology 2021, 49, 827–831. [Google Scholar] [CrossRef]
- Calvert, S.E.; Pedersen, T.F. Geochemistry of recent oxic and anoxic sediments: Implications for the geological record. Mar. Geol. 1993, 113, 67–88. [Google Scholar] [CrossRef]
- Wood, S.A. The role of humic substances in the transport and fixation of metals of economic interest (Au, Pt, Pd, U, V). Ore Geol. Rev. 1996, 11, 1–33. [Google Scholar] [CrossRef]
- Khatun, M.; Singh, S.; Chakravarti, R.; Venkatesh, A.S. Genetic constraints and possible mechanism of gold mineralization within the carbonaceous metasedimentary units of the Dalma volcano-sedimentary belt, North Singhbhum Mobile Belt, eastern India: Implications from pyrite geochemistry and carbon and sulfur isotope studies. Geol. J. 2020, 55, 5233–5250. [Google Scholar]
- Pitcairn, I.K.; Teagle, D.A.H.; Craw, D.; Olivo, G.K.; Kerrich, R.; Brewer, T.S. Sources of metals and fluids in orogenic gold deposits; Insights from the Otago and Alpine schists, New Zealand. Econ. Geol. 2006, 101, 1525–1546. [Google Scholar] [CrossRef]
- Large, R.R. Evidence for a Two-Stage Process in the Genesis of Sediment-Hosted Gold, Arsenic Deposits; Deb, M., Goldfarb, R., Eds.; Gold Metallogeny India and Beyond; Alpha Science International Ltd.: Oxford, UK, 2010; pp. 30–47. [Google Scholar]
- Cannell, A.; Blamey, N.; Brand, U.; Escapa, I.; Large, R.R. A revised sedimentary pyrite proxy for atmospheric oxygen in the Paleozoic: Evaluation for the Silurian-Devonian-Carboniferous period and the relationship of the results to the observed biosphere record. Earth-Sci. Rev. 2022, 231, 104062. [Google Scholar] [CrossRef]
- Frimmel, H.E. Episodic concentration of gold to ore grade through Earth’s history. Earth-Sci. Rev. 2018, 180, 148–158. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makoundi, C.; Zaw, K.; Endut, Z.; Zabidi, H. Trace Element Composition of Pyrite from Selected Black Shale and Chert Exposures in the Central Belt of Peninsular Malaysia: Implications for Mineral Exploration. Minerals 2023, 13, 829. https://doi.org/10.3390/min13060829
Makoundi C, Zaw K, Endut Z, Zabidi H. Trace Element Composition of Pyrite from Selected Black Shale and Chert Exposures in the Central Belt of Peninsular Malaysia: Implications for Mineral Exploration. Minerals. 2023; 13(6):829. https://doi.org/10.3390/min13060829
Chicago/Turabian StyleMakoundi, Charles, Khin Zaw, Zakaria Endut, and Hareyani Zabidi. 2023. "Trace Element Composition of Pyrite from Selected Black Shale and Chert Exposures in the Central Belt of Peninsular Malaysia: Implications for Mineral Exploration" Minerals 13, no. 6: 829. https://doi.org/10.3390/min13060829
APA StyleMakoundi, C., Zaw, K., Endut, Z., & Zabidi, H. (2023). Trace Element Composition of Pyrite from Selected Black Shale and Chert Exposures in the Central Belt of Peninsular Malaysia: Implications for Mineral Exploration. Minerals, 13(6), 829. https://doi.org/10.3390/min13060829