Abstract
There is a controversy involved in the models of the formation of serpulid tube microstructures, which either have been formed in similar ways to molluscan structures or in an alternative, unique serpulid way. A scanning electron microscope (SEM) study of the tube microstructure of Crucigera zygophora has been performed. A new serpulid tube microstructure, an aggregative SIOP, has been discovered in C. zygophora, herein termed ASIOP. During the first phase of crystallization, the sparsely located nuclei of the ASIOP structure formed, and in the second phase of crystal growth, the nucleation of spherultic sectors took place on the surface of preformed nuclei. The ASIOP structure differs from SIOP by more sparsely located crystallisation centres (nuclei) and the slower formation (i.e., crystallisation) of basic units. The formation of the ASIOP structure cannot be fully explained by the classical carbonate slurry model. Future comparative studies should show whether molluscan crossed spindle-like structures and serpulid SIOP structures are structural analogues.
1. Introduction
Polychaete annelids of three groups—serpulids, cirratulids, and sabellids—are known to construct calcareous tubes [,,]. The earliest serpulids and sabellid tubeworms appeared in the Permian [], and cirratulids in the Oligocene []. Among all annelid tubeworms, only serpulids build exclusively calcareous tubes. Serpulid tubes are composed of calcite, aragonite, or a mixture of both CaCO3 minerals. The calcium for the tubes of serpulid worms is derived, at least in part, from the surrounding sea water. One would expect to observe differences in the microstructure and mineral composition of serpulid tubes between marine, brackish, and freshwater species. However, observations by Vinn et al. [] did not identify any differences in mineralogy or tube microstructure between marine, brackish, and freshwater serpulid species. Serpulid tubes show diverse micostructural fabrics, from simple to complex, whereas some are unique to animals []. Most serpulid species have single-layered tubes, though many have two-layered tubes, and some species even have multiple tube layers [,,,,,]. Vinn et al. [] found that about 34% of serpulid species have tube walls with complex structures containing more than one distinct layer. Among the most complex tubes are those of C. zygophora, F. sabiuraensis, H. dianthus, and S. caribensis that have three-layered tubes, and C. websteri and P. kraussii that have four-layered tubes. In complex tubes with a multilayered structure, each layer is usually composed of a different type of microstructure, though in a few species, such as B. langerhansi, the crystals of the external and internal tube layers have similar architecture and differ only by their dimensions. In fresh water serpulid M. cavatica, both the inner and outer tube layers have an IOP structure and differ from each other only by the quantity of homogeneous carbonate cement. The spherulitic prismatic, regularly ridged prismatic, and simple prismatic structures always form the outer or the inner tube layer in multilayered serpulid species. Serpulid biomineralization shows a variable extent of control over crystal orientation, and four major categories of serpulid tube microstructures have been established: (1) isotropic structures lacking orientation, such as homogeneous structures and irregularly oriented structures; (2) semi-oriented structures; (3) a simple type of oriented structures, such as spherulitic prismatic, regularly ridged prismatic, and simple prismatic structures, where the orientation of crystals can involve epitaxy; and (4) a complex type of oriented structures, such as LF, SLF, and OF structures, where the orientation of crystals cannot be achieved by the epitaxial growth. In the case of simple oriented structures, the orientation of the structural elements is achieved by epitaxial growth of the crystal in the direction of its longitudinal axis. However, in LF and SLF structures, crystals have different but constant orientations in adjacent growth increments. In the case of OF structure, the crystals have almost uniform orientation in all growth increments, but their longitudinal axis is not continuous through the successive growth increments. Vinn et al. [] suggested that the orientation of crystals in complex oriented structures is very likely completely controlled by the organic matrix. The majority of the serpulid species have tubes that lack an oriented microstructure, though some have tubes with a semi-oriented microstructure. Vinn et al. [] found that only 23% of serpulid species are capable of building tubes with oriented microstructures of a simple type, where epitaxial growth can explain the orientation of crystals. Somewhat surprisingly, 25% of serpulid species can mineralize oriented microstructures, where the crystal orientation is supposed to result from a complex control mechanism that is capable of detecting or ‘memorizing’ the orientation of crystals in the previously deposited growth increment. Such complex oriented serpulid tube structures are morphologically similar to LF structures in molluscs and likely evolved for a similar purpose to strengthen the mechanical properties of the material []. The complex oriented structures of serpulids have material properties that are mechanically superior, as compared with tube structures that are lacking orientation or with simple types of oriented prismatic structures. Vinn et al. [] that serpulid structures lacking orientation might be plesiomorphic. It is possible that complex oriented structures evolved in serpulids as an adaptation to combine fast tube growth with mechanical strength []. The diverse tube microstructures of serpulids contrast with the monotonous architecture of sabellid and cirratulid tubes [].
In general, the tube microstructures of modern serpulid species are relatively well studied [,,,], but much less is known about the tube microstructures of fossil polychaete tubeworms, and many major discoveries are still expected to be made [,,,,,,,,]. Similarly to the microstructures, the mineralogy of modern serpulids is, in general, well studied. Historically, serpulids have been envisioned as having a small number of simple skeletal microstrustructures and as lacking the complex microstructures that are characteristic of brachiopods, bryozoans, and molluscs []. They were believed to have inferior biomineralization mechanisms as compared to molluscs, arthropods, and bryozoans []. However, the discovery of complex microstructures in serpulids has cast doubt on the traditional models of their tube formation (i.e., carbonate slurry model) [,,,,,] and opened debate about the possibility of more complex biomineralization mechanisms in serpulids [].
The aim of the present paper is to describe a new isotropic skeletal microstructure in serpulids, compare it to the known serpulid structures, and discuss the possibility of matrix controlled biomineralization in serpulids.
2. Materials and Methods
Two specimens of Cruzigera zygophora (V.Pol.3287) from the collections of Naturalis Biodiversity Center were studied. The studied specimens originate from Canoe Bay, Alaska, USA. The specimens were cut using a small electrical saw, oriented, and mounted in epoxy resin for machine grinding. We have prepared five- to ten-millimetre-long longitudinal sections and single cross sections of C. zygophora tubes. The tube sections were polished with a polishing machine and etched in a 1% solution of acetic acid for 5 min. The sample was mounted on an aluminium stub and gold sputtered prior to SEM study with a Hitachi S-4300 Field Emission Scanning Electron Microscope equipped with an Inca EDX system at 2 kV. The fossils were scanned in a back-scattered electron (BSE) imaging mode. All SEM images were digitally acquired. The SEM study was carried out at the Swedish Museum of Natural History.
3. Results
C. zygophora has chevron-shaped growth lamellae, and the discovered structure occurs in the internal part of the tube wall (Figure 1).
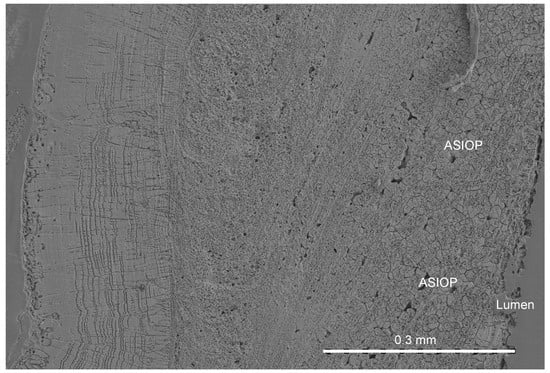
Figure 1.
The location of the ASIOP structure in the longitudinal section of the C. zygophora tube.
The new structure is formed by relatively large aggregations of spherultic sectors. The dimeter of aggragations is 12–24 µm (Figure 2a–d). The multiple sectors of spherulites are grown on the small nuclei (Figure 2c and Figure 3) in every direction. The nuclei are themselves slightly spherulitic, and there can be one to three nuclei per single aggregation. The diameter of nuclei is 3.3–4.5 µm. The nuclei are sparsely located and irregularly oriented within each growth increment of the ASIOP structure (Figure 2 and Figure 4). The cross sections of nuclei are circular to somewhat angular. The substructure of the nuclei is composed of elongated, slender crystallites subparallel to the longitudinal axis of nuclei. The external surface of the aggregates is angular; both concave and convex sides occur. The structure occurs only in the inner tube layer of Crucigera zygophora. The structure closely resembles the SIOP structure (Figure 5a,b) in the morphology of its building block-sectors of spherulites []. On the other hand, the irregularly oriented, slightly spherulitic rod-like nuclei of the ASIOP structure resemble the basic structural units of the SIOP structure. However, the basic units of the SIOP structure are single sectors of shperulites, whereas the ASIOP is composed of aggregations of spherulitic sectors. The aggregations of spherulitic sectors in ASIOP are much larger than the single spherultic sectors in the SIOP structure. The elemental composition of the tube shown by EDX analyses revealed a magnesium content of Mg = 11.2 wt% which corresponds to a high-Mg calcite. The lack of a considerable amount of Sr also indicates the calcitic composition of the structure.
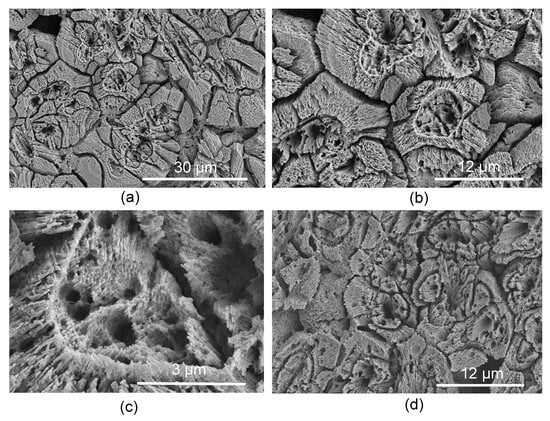
Figure 2.
(a–c) Transverse section through ASIOP structure. (c) Close up of the nucleus. (d) Longitudinal section through ASIOP structure.
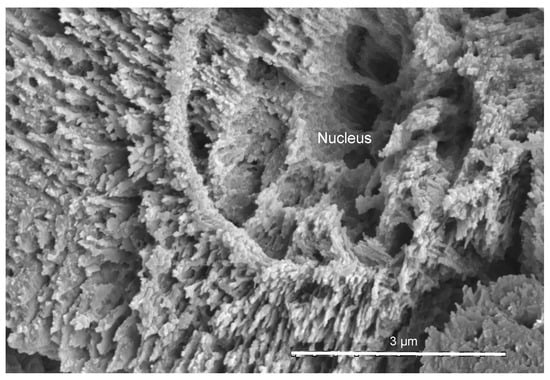
Figure 3.
The detailed view of the nucleus in transverse section.
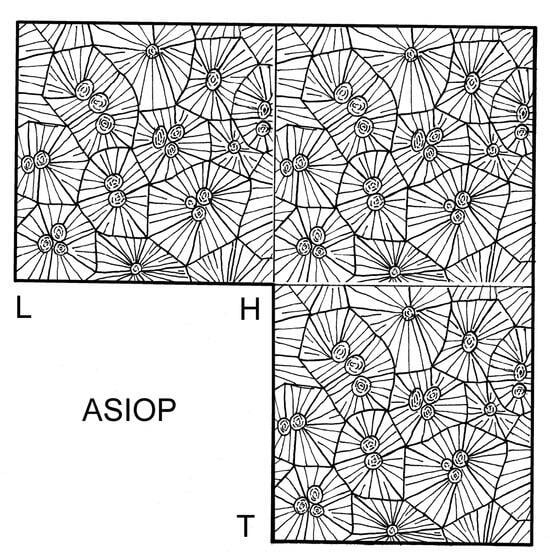
Figure 4.
Schematic line drawing of ASIOP structure. L—longitudinal section, H—horizontal section, T—transverse section.
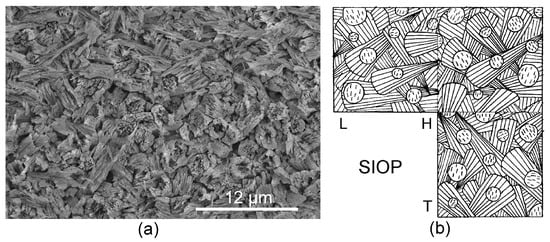
Figure 5.
(a) Transverse section through SIOP structure in Vermiliopsis infundibulum (Serpulidae). (b) Schematic line drawing of SIOP structure.
4. Discussion
4.1. Formation of the Structure
4.1.1. Discussion of the Process
The basic units of the nuclei and surrounding sectors of spherulites were formed successively in two phases and not simultaneously. During the first phase of crystallisation, the sparsely located nuclei formed. The direction of crystals in the nuclei is random and was controlled either by the organic matrix or by forces involved in the deposition of carbonate slurry. One could ask: if nuclei form in an organic matrix, where does the organic matrix go, and what is the mechanism for its disappearance? It is possible that the organic matrix occurred in a Ca, C, and O-rich fluid where it remained in place after the complete crystallisation of the mineral phase. Alternatively, prefabricated nuclei (i.e., granules of Neff) occurred in Ca, C, and O-rich fluid and were placed on the tube aperture by a serpulid collar. During the second phase, the nucleation of spherultic sectors took place on the surface of preformed nuclei. The surface of the nuclei was likely chemically active and initiated the nucleation of attached sectors of spherulites. This is supported by the occurrence of discontinuities between the nuclei and attached sectors of spherulites. Moreover, the orientation of crystallisation axis in the nuclei does not coincide with the crystallisation axis of the attached sectors of spherulites. Nevertheless, only the perpendicular to the surface of the nuclei orientation of the spherulitic sectors was predetermined by the chemical properties of the surface of the nuclei. The second phase of crystal growth lasted until the spherulitic sectors reached the growing edges of the opposite spherulitic sectors. The orientation of spherulitc sectors in the aggregations is rather random and was likely controlled by the organic matrix, as in the absence of such control, perfect spherulites would grow around the nuclei. The overall size of the aggregations is likely controlled by the spacing, that is, variability in distribution of the initial seed nuclei.
4.1.2. Serpulid Calcification Models and ASIOP Structure
The classical theory of serpulid biomineralization involves the so-called carbonate slurry model [,,,,]. According to the traditional view, calcareous granules, produced by the calcium-secreting glands, are used to build the tube in which the serpulid worm lives. There is a pair of exocrine glands within the subepithelial connective tissue of the ventro-lateral peristomium under the fold of the collar in serpulid Ficopomatus enigmaticus [,,,,]. The detailed histological and histochemical examinations of these glands in Spirobranchus triqueter were carried out by Hedley [,,]. There is also another glandular area in serpulids, namely the epithelium of the ventral shield surrounding the apertures of the calcium-secreting glands []. The function of calcium-secreting glands has been described in detail for several serpulid species [,,,,,]. The ideas derived from Neff’s studies [,] have been used as a standard’ model of serpulid tube formation. Neff [,] described the secretion of calcium carbonate in Spirobranchus americanus using observations made with the help of a transmission electron microscope (TEM). Neff’s observations suggest [,] that the calcium-secreting glands in S. americanus produce cubic or rhombohedral granules with average dimensions of 0.15–0.2 µm on a side. The secretory granules are composed of a fibrous organic matrix surrounding needle-like low magnesium calcite crystals. The calcareous granules are secreted in the form of carbonate slurry. After secretion, carbonate slurry that contains granules is shaped and plastered onto the tube aperture by the undersurface of the worm’s collar. The structures resulting from such a process must lack orientation. The early SEM studies of serpulid tube microstructures confirmed the later opinion and cast no doubt on the classical carbonate slurry model []. However, in addition to numerous isotropic structures [], serpulids possess diverse fabrics of oriented prismatic structures [,], which cannot be explained by the traditional carbonate slurry model. In addition to the common spherulitic prismatic structure and the more rare simple prismatic and regularly ridged prismatic structures, serpulids also produce structures that resemble molluscan lamella-fibriallar (LF) structure []. Weedon [] argued that complex oriented tube microstructures, such as the LF structure in Spirobranchus triqueter, are formed during a controlled moulding of the carbonate slurry in forward and backward applications by the worm’s collar. The latter idea was recently supported by Buckman and Harries [], who suggested that “the difference in orientation observed between unordered and ordered fabrics can be explained by the application (or lack of) of rotational force created by serpulid collar”. However, Weedon [] also admitted that spherulitic prismatic structures in serpulids cannot be explained by a carbonate slurry model. Similarly, the ASIOP structure clearly cannot be fully explained by the traditional carbonate slurry model. The sectors of spherulites attached to elongate nuclei clearly form after the deposition of the material. However, the elongate nuclei themselves are either derived from carbonate slurry or, alternatively, nucleated after the secretion of carbonate-rich organics. Thus, at least partially ASIOP structure is formed similarly to the majority of invertebrates, including molluscs. Future studies should show whether the nuclei of the ASIOP structure have something to do with the carbonate slurry or not. The diameters of nuclei are about 20× larger than the dimensions of secretory granules in S. americanus, speaking against the possibility of nuclei being the calcareous granules. Nevertheless, one cannot rule out the possibility that the nuclei are the result of the incremental growth of calcareous granules, though no growth increments have been observed in the sections of nuclei. Alternatively, the secretory granules in C. zygophora could be much larger than those in S. americanus.
4.2. Evolution of Serpulid Microstructures and ASIOP Structure
The calcareous tubes of Serpulidae first appeared in the Permian []. The detailed studies of serpulid tube microstructures have just begun, and their evolution is still poorly known [,,,,]. Vinn [] suggested that plesiomorphic structures were presumably all isotropic. However, it is possible that biomineralization has already evolved in the common ancestor of serpulids and sabellids. The tube wall of modern sabellids is composed of two aragonitic layers with distinct microstructures []. The friable outer layer of sabellids is porous, and composed of mostly regularly shaped tiny spherulites, whereas the internal layer is much thicker and separated from the outer layer by multiple thin organic sheets. At the contact of the inner and outer layers, multiple centres with spherulitic structures originate. In sabellid Glomerula, bundles of needle-shaped crystallites forming primitive irregular prisms grow from these spherulitic centres towards the tube lumen [,]. Similarly to the prismatic structures of serpulids, the individual prisms are not encased by an organic sheet. The fossils of sabellids have single-layered tubes with a spherulitic prismatic structure [,]. The tube microstructure is known in the Lower Jurassic sabellids, but we expect it to represent a plesiomorphic condition as the calcareous tube microstructures in sabellids have not changed since the Early Jurassic []. One could hypothesise that the sabellid biomineralization also represents the plesiomorphic condition for the serpulid–sabellid clade.
The earliest serpulid tubes were likely aragonitic and had an irregularly oriented prismatic (IOP) structure []. The appearance of serpulids with IOP structure and chevron-shaped growth lamellae enabled them to grow much faster than their sabellid ancestors grew and, at the same time, expend less calcareous material. Alternatively, the first serpulids could have had tubes with a fine-grained homogeneous structure, which would have provided serpulids with similar advantages over their ancestors. The well-preserved tube wall of Lower Jurassic serpulid Serpula etalensis has an IOP structure []. The irregularly oriented aragonitic prismatic crystals forming the tube wall in S. etalenis are 0.5–1.0 μm in diameter and 3–6 μm in length. They show an oriented substructure of long rod-like crystals, which are 1.5–3.0 μm in length and 0.1–0.3 μm in diameter []. Unfortunately, the tube microstructure of the earliest fossil serpulids from the Permian of Sicily is not known [].
The SIOP structures are not known from fossil serpulids, but this is mostly because of the poor preservation potential of aragonitic tubes. All previously identified SIOP structures are aragonitic [,]. Vinn [] suggested that SIOP structures evolved directly from the ancestral IOP structure, as they differ only by the spherulitic growth of the basic units of the structure. Vinn [] hypothesised that the material properties of SIOP structures are advanced compared to ancestral IOP structures and allow serpulids to build mechanically stronger tubes. The advanced material properties of SIOP structures as compared to IOP structures are due to the intergrowth of basic structural elements in SIOP structures. The ASIOP structure is known only in single serpulid species and not in fossils. Based on the similarity of the nuclei of ASIOP structure to the basic units of the SIOP structure, we hypothesise that the ASIOP structure likely evolved from the SIOP structure via the addition of a second phase of crystal growth. The adaptive value of the ASIOP structure remains unclear, as it does not show any obvious advantage over the SIOP structure.
4.3. Similar Structures in the Other Invertebrates
Among all invertebrates, molluscan shell structures most closely resemble serpulid tube microstructures. There are several other previously published serpulid skeletal microstructures that resemble crossed structures of molluscs, such as an irregularly oriented platy (IOPL) structure in the outer tube layer of Neovermilia falcigera [,]. The IOPL structure is formed by slightly elongate crystal sheets that have a crossed orientation within each growth increment and closely resemble that of the molluscan intersected platy structure, described as a crossed structure characterised by two predominant dip directions of intersected plate-like crystallites []. SIOP structures were considered to be unique to serpulids [], but there are some molluscan structures that slightly resemble SIOP structures in serpulids. The SIOP and ASIOP structures, with its sectors of spherulites irregularly oriented within each growth increment, somewhat resemble spindle-like structures in molluscs [,]. However, second-order structural elements of the molluscan structure are more similar to spindles than to the sectors of spherulites. Thus, the biomieralization of these structures could be analogous, but the possible analogy is not certain and needs further study. The molluscan spindle-like structure is composed of random aggregations of elongated, spindle-like structural units that exhibit a parallel arrangement of laths []. In molluscs, the first-order unit dimensions are 5–50 μm along the long axis and 5–30 μm along the short axis. Both axes have variable size ratios. The second-order prisms have a dimeter of 0.2–0.4 μm in molluscs []. Future comparative studies of serpulid SIOP structures and molluscan structures composed of spindle-like elements should find out whether both types are structural analogues.
5. Conclusions
Not all serpulid tube microstructures are known, and SEM studies often find new structures. Sometimes, these structures are unique to the invertebrates of serpulid polychaetes. A tube layer in Crucigera zygophora is composed of an ASIOP structure that is unique to serpulids. However, there are some molluscan structures that resemble SIOP structures in serpulids. During the first phase of crystallisation of the ASIOP structure, the sparsely located nuclei are formed. During the second phase of formation, nucleation in spherulitic sectors takes place on the surface of preformed nuclei. The ASIOP structure differs from SIOP by having more sparsely located crystallisation centres (nuclei) and slower formation (i.e., crystallisation) of basic units. The ASIOP structure likely evolved from an ancestral SIOP structure. The ASIOP structure is difficult to fully explain by the classical carbonate slurry model. The sectors of spherulites attached to elongate nuclei clearly were not secreted as carbonate slurry but formed after the deposition of the material on the aperture of the serpulid tube. The analogy of ASIOP structure with similar molluscan structures is possible.
Author Contributions
Conceptualization, O.V.; methodology, O.V.; writing—original draft preparation, O.V.; writing—review and editing, O.V., A.A.A., M.E.H. and S.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by King Saud University, Riyadh, Saudi Arabia, Researchers Supporting Project number RSP2024R140.
Data Availability Statement
The data presented in this study are available in current article.
Acknowledgments
We are grateful to Harry A. ten Hove for providing identified serpulid tubes for the study. We are also grateful to two anonymous reviewers for the constructive comments on the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Vinn, O.; Ten Hove, H.A.; Mutvei, H.; Kirsimäe, K. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zool. J. Linn. Soc. 2008, 154, 633–650. [Google Scholar] [CrossRef]
- Vinn, O.; Ten Hove, H.A.; Mutvei, H. On the tube ultrastructure and origin of calcification in sabellids (Annelida, Polychaeta). Palaeontology 2008, 51, 295–301. [Google Scholar] [CrossRef]
- Vinn, O. The ultrastructure of calcareous cirratulid (Polychaeta, Annelida) tubes. Est. J. Earth Sci. 2009, 58, 153–156. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Reitano, A.; Insacco, G. First record of sabellid and serpulid polychaetes from the Permian of Sicily. Acta Palaeontol. Pol. 2017, 62, 25–38. [Google Scholar] [CrossRef]
- Fischer, R.; Pernet, B.; Reitner, J. Organomineralization of cirratulid annelid tubes-fossil and recent examples. Facies 2000, 42, 35–50. [Google Scholar] [CrossRef]
- Bandel, K. The reconstruction of “Hyolithes kingi” as annelid worm from the Cambrian of Jordan. Mitt. Geol.-Paläontol. Inst. Univ. Hamburg 1986, 61, 35–101. [Google Scholar]
- Zibrowius, H.; ten Hove, H.A. Neovermilia falcigera (Roule, 1898), a deep- and cold-water serpulid polychaete common in the Mediterranean Plio-Pleistocene. Bull. Biol. Soc. 1987, 7, 259–271. [Google Scholar]
- Hove, H.A.T.; Smith, R.S. A re-description of Ditrupa gracillima Grube, 1878 (Polychaeta, Serpulidae) from the Indo-Pacific, with a discussion of the genus. Rec. Aust. Mus. 1990, 42, 101–118. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Mòllica, E. Serpula cavernicola Fassari and Mòllica, 1991 (Annelida Polychaeta): Diagnostic features of the tubes and new Mediterranean records. Mar. Life 2000, 10, 27–32. [Google Scholar]
- Sanfilippo, R. Additional notes on the little known serpulid Serpula israelitica Amoreux, 1976 and new Mediterranean records. In Atti III Giornate di Paleontologia Maggio; Società Paleontologica Italiana: Modena, Italy, 2003. [Google Scholar]
- Nishi, E. On the internal structure of calcified tube walls in Serpulidae and Spirorbidae (Annelida, Polychaeta). Mar. Foul. 1993, 10, 17–20. [Google Scholar] [CrossRef]
- Sanfilippo, R. Spirorbid Polychaetes as boreal guests in the Mediterranean Pleistocene. Riv. Ital. Paleontol. Strat. 1998, 104, 279–286. [Google Scholar]
- Sanfilippo, R. Ditrupa brevis n.sp., a new serpulid from the Mediterranean Neogene with comments on the ecology of the genus. Riv. Ital. Paleontol. Strat. 1999, 105, 455–464. [Google Scholar]
- Weedon, M.J. Tube microstructure of Recent and Jurassic serpulid polychaetes and the question of the Palaeozoic ‘spirorbids’. Acta Palaeontol. Pol. 1994, 39, 1–15. [Google Scholar]
- Senowbari-Daryan, B. Barbafera carnica Senowbari-Daryan, 1980: A Triassic Worm-tube. Facies 1997, 36, 57–68. [Google Scholar] [CrossRef]
- Vinn, O.; Mutvei, H.; Ten Hove, H.A.; Kirsimäe, K. Unique Mg-calcite skeletal ultrastructure in the tube of the serpulid polychaete Ditrupa. Neues Jahrb. Geol. Paläontol. 2008, 248, 79–89. [Google Scholar] [CrossRef]
- Buckman, J.O. The tube of Ditrupa bartonensis (Annelida, Serpulidae), from the Eocene of southern England: Observations on microstructure and its significance. Palaeontol. Electr. 2020, 23, a37. [Google Scholar] [CrossRef]
- Hedley, R.H. Studies on serpulid tube formation. I. The secretion of the calcareous and organic components of the tube by Pomatoceros triqueter. Quart. J. Microsc. Sci. 1956, 97, 411–419. [Google Scholar] [CrossRef]
- Hedley, R.H. Studies on serpulid tube formation. II. The calcium-secreting glands in the peristomium of Spirorbis, Hydroides and Serpula. Quart. J. Microsc. Sci. 1956, 97, 421–427. [Google Scholar] [CrossRef]
- Hedley, R.H. Tube formation by Pomatoceros triqueter (Polychaeta). J. Mar. Biol. Assoc. U. K. 1958, 37, 315–322. [Google Scholar] [CrossRef]
- Neff, J.M. Ultrastructural studies of the secretion of calcium carbonate by the serpulid polychaete worm, Pomatoceras caerulus. Zeit. Zellforsch. 1971, 120, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.M. Ultrastructure of calcium phosphate containing cells in the serpulid Pomatoceros caeruleus. Calc. Tiss. Res. 1971, 7, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Buckman, J.O.; Harries, D.B. Reef forming Serpula vermicularis from Scotland and Ireland: Tube structure, composition and implications. Zool. Anz. 2020, 288, 53–65. [Google Scholar] [CrossRef]
- Vinn, O. On the unique isotropic aragonitic tube microstructure of some serpulids (Polychaeta, Annelida). J. Morph. 2013, 274, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Kočí, T.; Milàn, J.; Jäger, M. Neovermilia gundstrupensis sp. nov. (Polychaeta, Serpulidae) from the Selandian (middle Paleocene) of Fyn, Denmark. Bull. Geol. Soc. Denmark. 2023, 72, 135–151. [Google Scholar] [CrossRef]
- Słowiński, J.; Banasik, K.; Vinn, O. Insights into the mineral composition and ultrastructure of the Jurassic sabellid tubes (Annelida, Polychaeta): The evolution of sabellid calcification and its palaeoecological implications. Lethaia 2023, 56, 1–12. [Google Scholar] [CrossRef]
- Vinn, O.; Jäger, M.; Kirsimäe, K. Microscopic evidence of serpulid affinities of the problematic fossil tube “Serpula” etalensis from the Lower Jurassic of Germany. Lethaia 2008, 41, 417–421. [Google Scholar] [CrossRef]
- Vinn, O.; Alkahtane, A.A.; El Hedeny, M.; Al Farraj, S. Discovery of rod-type crossed lamellar structure in Spiraserpula (Serpulidae, Polychaeta) from the Middle Miocene of Austria. Neues Jahrb. Geol. Paläont. Abh. 2023, 310, 91–97. [Google Scholar] [CrossRef]
- Nishida, K.; Nakashima, R.; Majima, R.; Hikida, Y. Ontogenetic Changes in Shell Microstructures in the Cold Seep-Associated Bivalve, Conchocele bisecta (Bivalvia: Thyasiridae). Paleontol. Res. 2011, 15, 193–212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).