Effect of Bulk Nanobubbles on the Flocculation and Filtration Characteristics of Kaolin Using Cationic Polyacrylamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.2.1. Preparation of Bulk NBs
2.2.2. Flocculation and Dewatering Tests
2.3. Analytical Methods
2.3.1. Zeta Potential Measurement
2.3.2. Polarizing Microscopy Observation
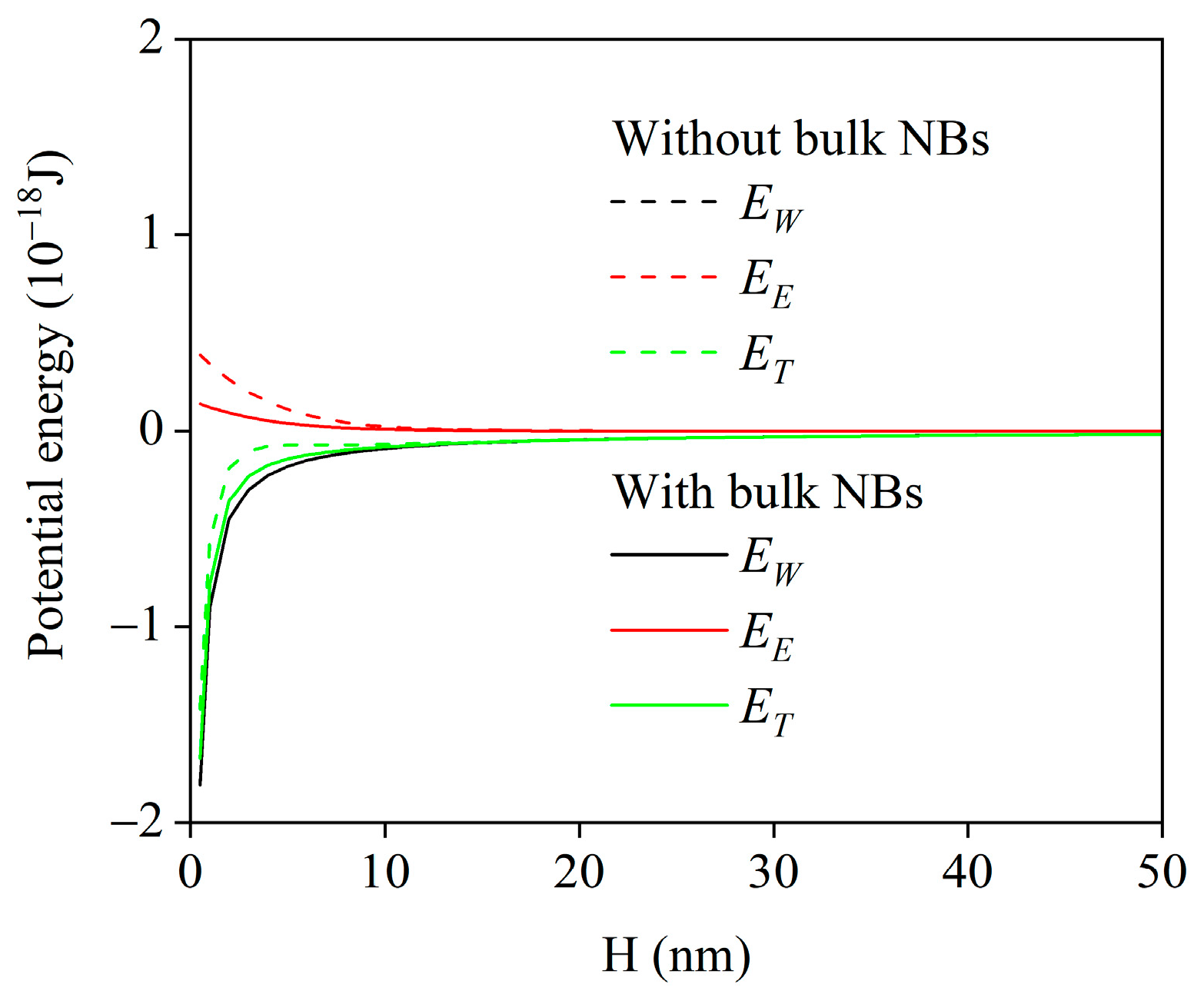
2.3.3. DLVO Theory Calculation Process
3. Results
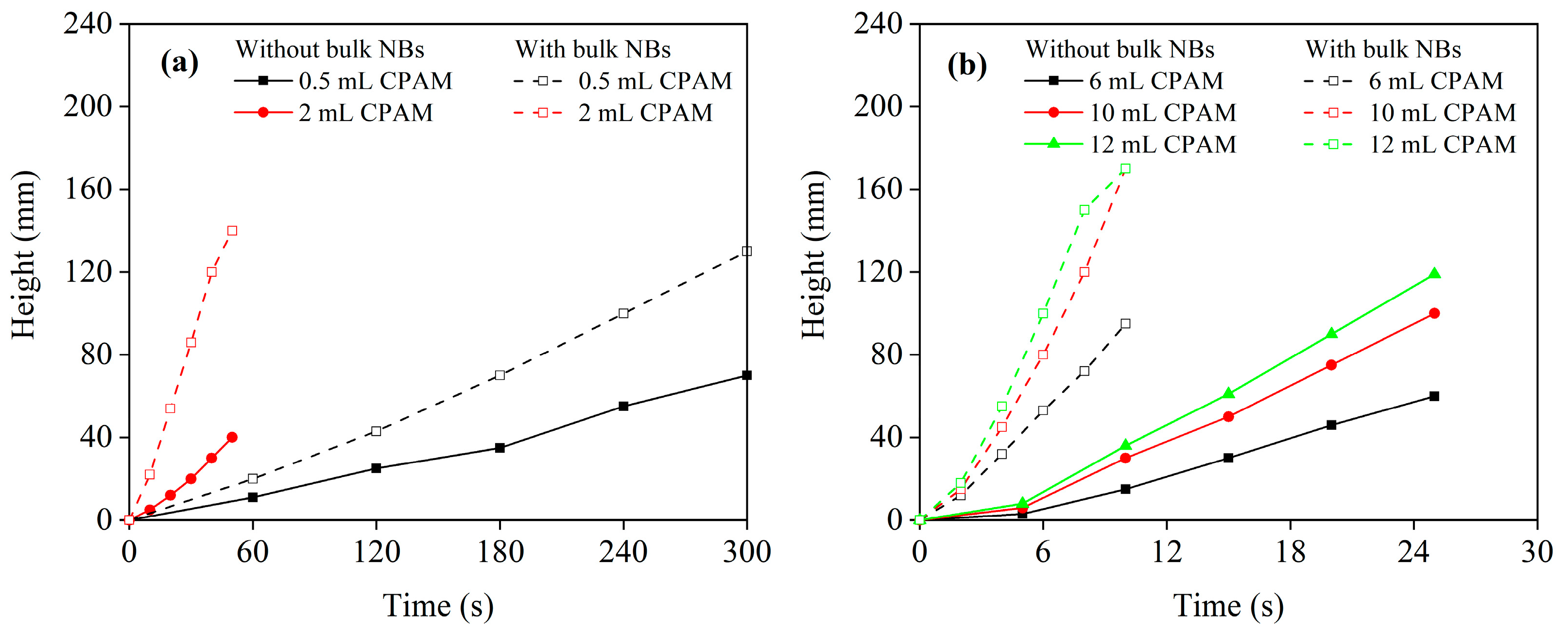
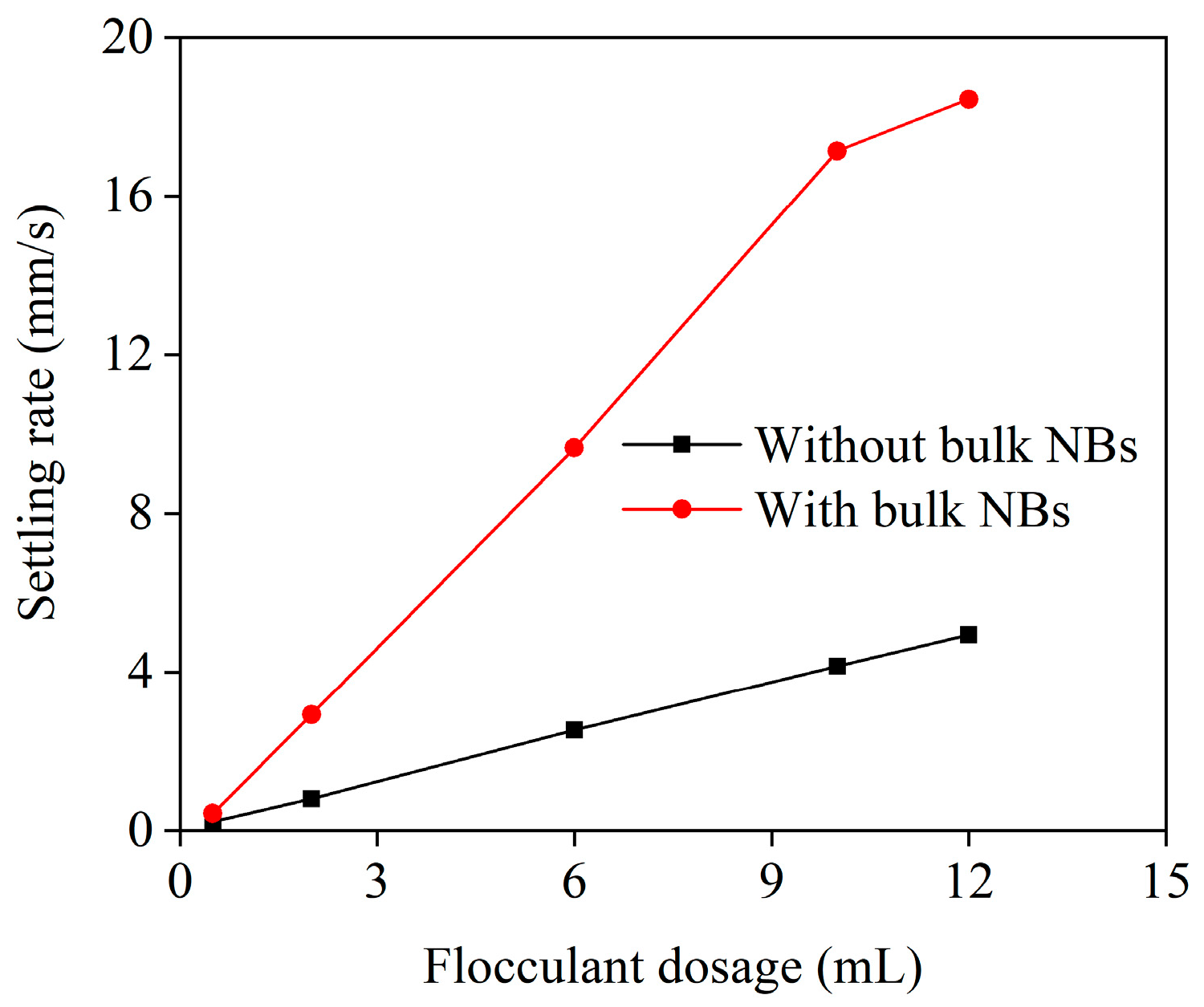
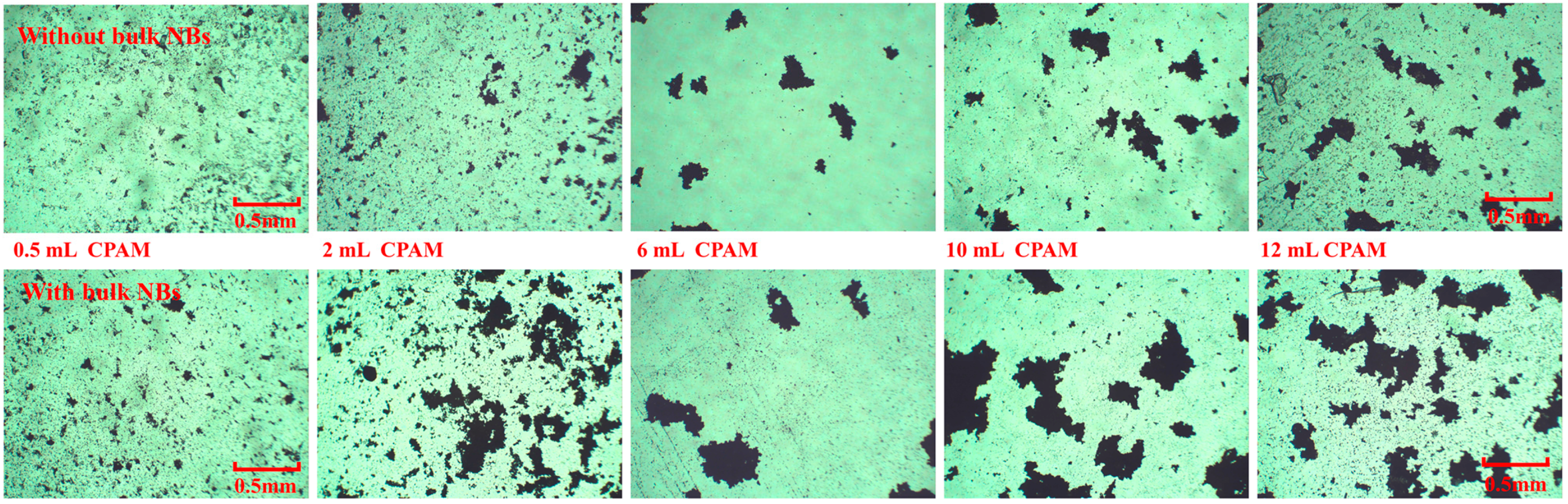
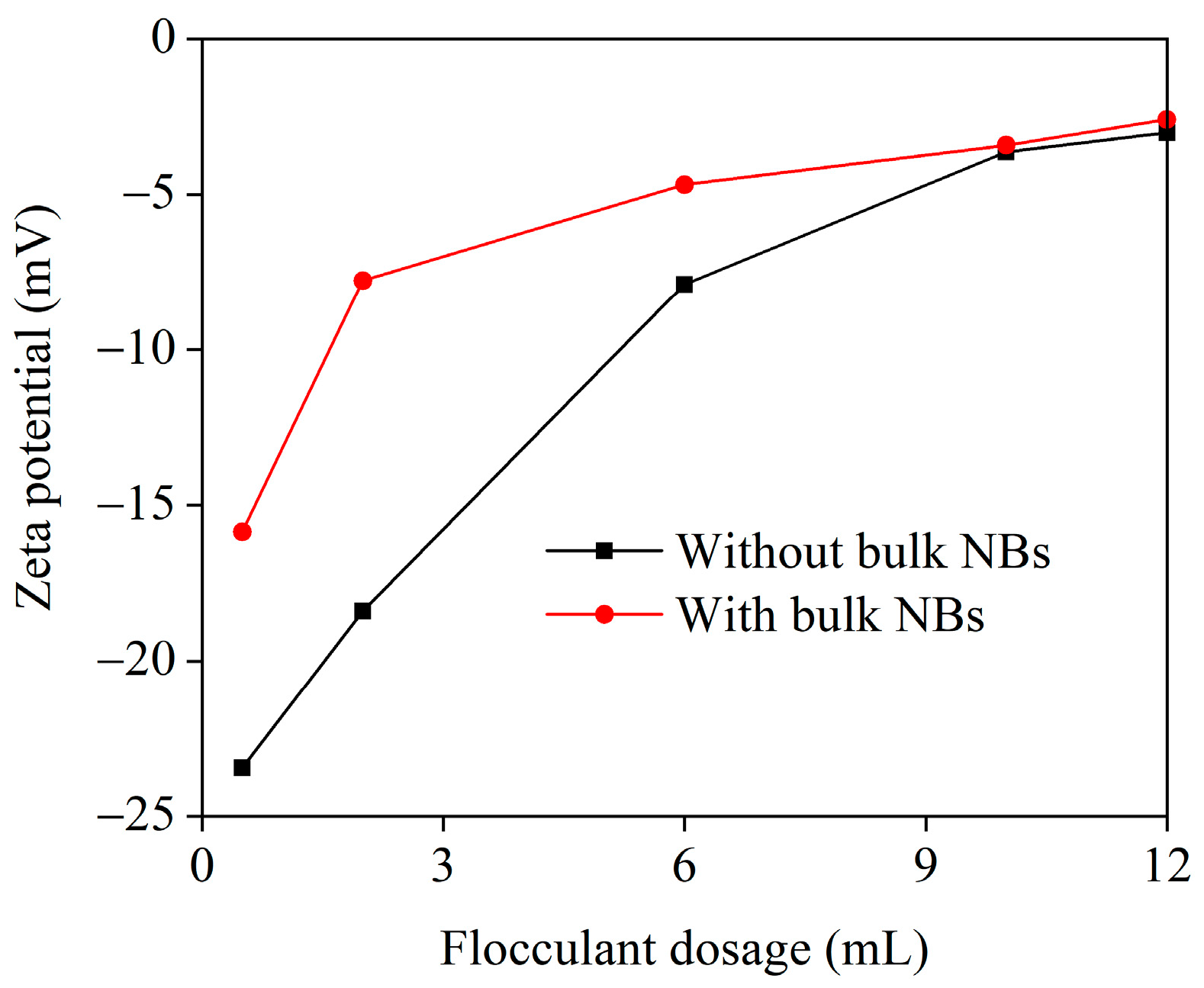
3.1. Flocculation Performance
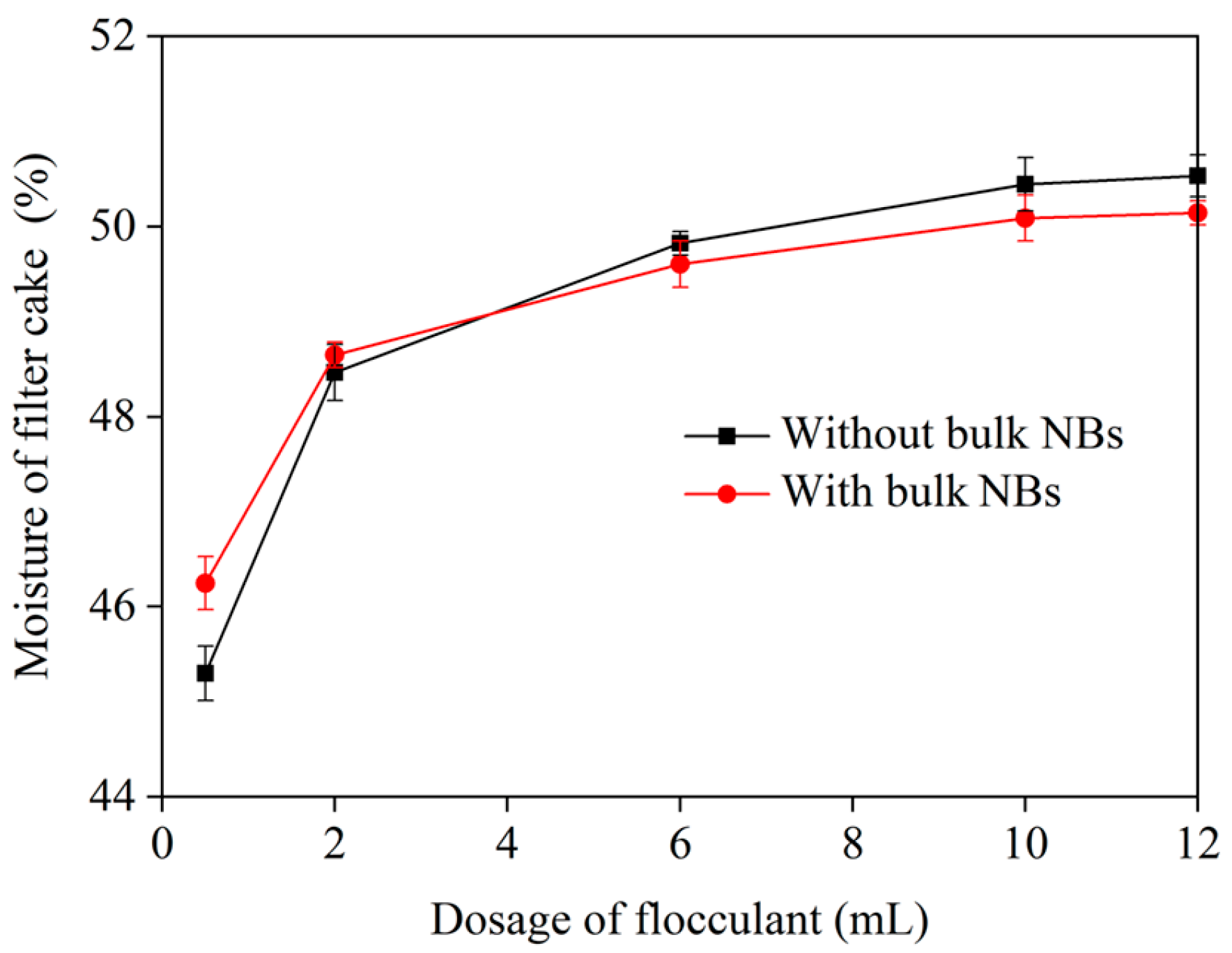
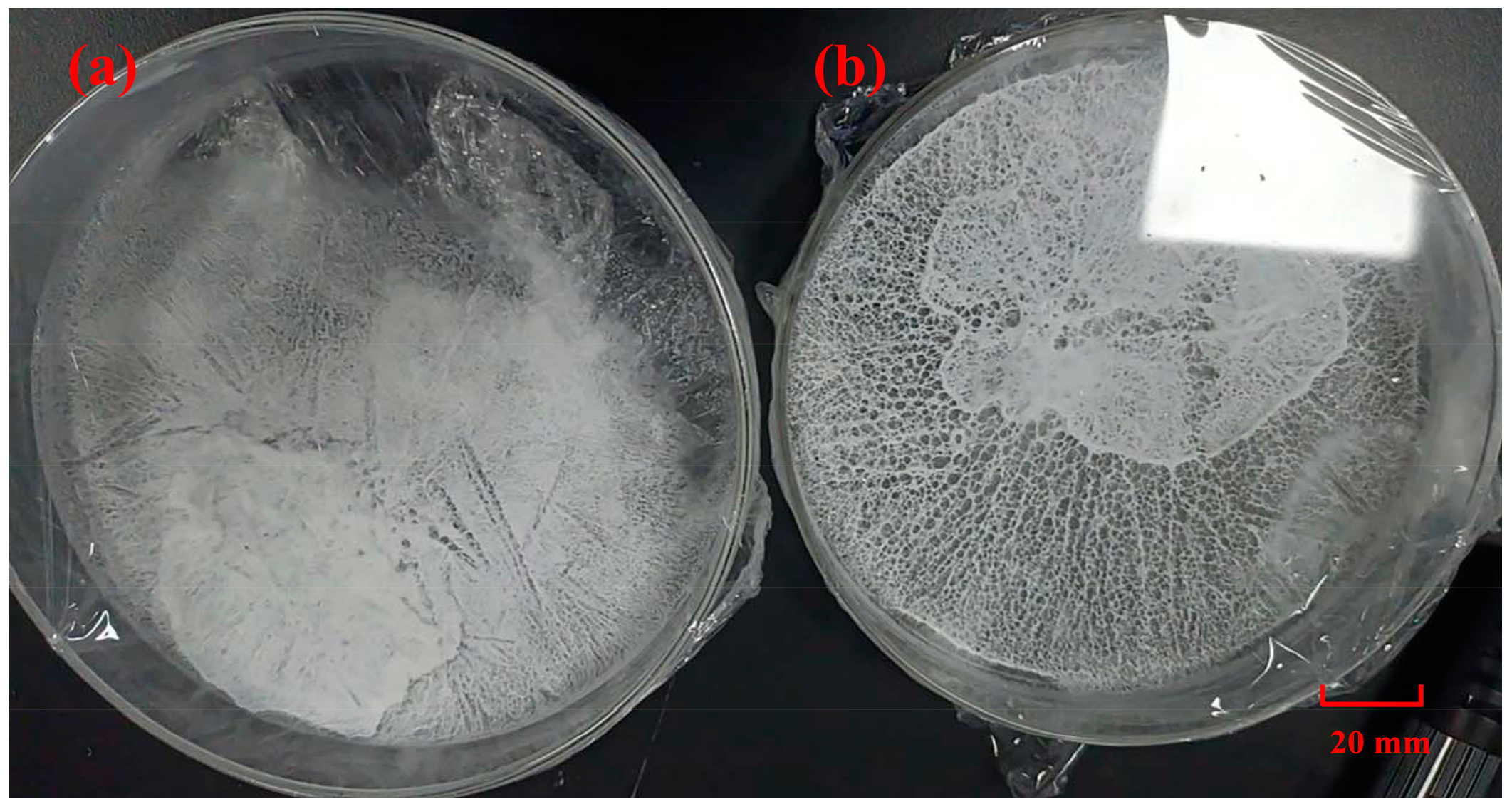
3.2. Dewatering Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Ramos, J.J.; Leiva, W.H.; Castillo, C.N.; Ihle, C.F.; Fawell, P.D.; Jeldres, R.I. Seawater flocculation of clay-based mining tailings: Impact of calcium and magnesium precipitation. Miner. Eng. 2020, 154, 106417. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Z.; Guo, K.; Fei, J.; Dong, Z.; Xiong, H. Geopolymeric flocculation-solidification of tail slurry of shield tunnelling spoil after sand separation. Constr. Build. Mater. 2023, 374, 130954. [Google Scholar] [CrossRef]
- Liu, D.; Edraki, M.; Fawell, P.; Berry, L. Improved water recovery: A review of clay-rich tailings and saline water interactions. Powder Technol. 2020, 364, 604–621. [Google Scholar] [CrossRef]
- Lagaly, G.; Ziesmer, S. Colloid chemistry of clay minerals: The coagulation of montmorillonite dispersions. Adv. Colloid Interface Sci. 2003, 100–102, 105–128. [Google Scholar] [CrossRef]
- Lee, K.E.; Teng, T.T.; Morad, N.; Poh, B.T.; Hong, Y.F. Flocculation of kaolin in water using novel calcium chloride-polyacrylamide (CaCl2-PAM) hybrid polymer. Sep. Purif. Technol. 2010, 75, 346–351. [Google Scholar] [CrossRef]
- Agmo Hernández, V. An overview of surface forces and the DLVO theory. ChemTexts 2023, 9, 10. [Google Scholar] [CrossRef]
- Mishchuk, N.A.; Marinin, A.I.; Marchenko, A.M. Coagulation, Sedimentation, and Consolidation of Aqueous Clay Dispersions. J. Water Chem. Technol. 2020, 42, 8–15. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Yang, X.; Jiang, F.; Wu, C.; Li, J.; Liu, K. Performance and mechanism of polyacrylamide stabilizers in coal water slurry. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 630, 127544. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Nazari, B.; Abdolalian, S.; Taghavijeloudar, M. An environmentally friendly approach for industrial wastewater treatment and bio-adsorption of heavy metals using Pistacia soft shell (PSS) through flocculation-adsorption process. Environ. Res. 2023, 235, 116595. [Google Scholar] [CrossRef]
- You, L.; Lu, F.; Li, D.; Qiao, Z.; Yin, Y. Preparation and flocculation properties of cationic starch/chitosan crosslinking-copolymer. J. Hazard. Mater. 2009, 172, 38–45. [Google Scholar] [CrossRef]
- Tian, D.; Xie, H.-Q. Synthesis and Flocculation Characteristics of Konjac Glucomannan-g-Polyacrylamide. Polym. Bull. 2008, 61, 277–285. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Q.; Shi, Y.; Cha, R. Preparation and Application of Cationic Modified Cellulose Fibrils as a Papermaking Additive. Int. J. Polym. Sci. 2016, 2016, 6978434. [Google Scholar] [CrossRef]
- Li, H.; Cai, T.; Yuan, B.; Li, R.; Yang, H.; Li, A. Flocculation of Both Kaolin and Hematite Suspensions Using the Starch-Based Flocculants and Their Floc Properties. Ind. Eng. Chem. Res. 2015, 54, 59–67. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chen, Y.-Z.; Yuan, S.-J.; Sheng, G.-P.; Yu, H.-Q. Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Res. 2009, 43, 5267–5275. [Google Scholar] [CrossRef]
- Shabanizadeh, H.; Taghavijeloudar, M. Potential of pomegranate seed powder as a novel natural flocculant for pulp and paper wastewater treatment: Characterization, comparison and combination with alum. Process Saf. Environ. Prot. 2023, 170, 1217–1227. [Google Scholar] [CrossRef]
- Shabanizadeh, H.; Taghavijeloudar, M. A sustainable approach for industrial wastewater treatment using pomegranate seeds in flocculation-coagulation process: Optimization of COD and turbidity removal by response surface methodology (RSM). J. Water Process. Eng. 2023, 53, 103651. [Google Scholar] [CrossRef]
- Zhang, L.; Min, F.; Chen, J.; Wang, L.; Zhu, Y.; Ren, B. Study on flocculation performance and mechanism of cationic polyacrylamide on montmorillonite: Insights from experiments and molecular simulations. Chem. Phys. 2024, 579, 112190. [Google Scholar] [CrossRef]
- Zhou, S.; Bu, X.; Alheshibri, M.; Zhan, H.; Xie, G. Floc structure and dewatering performance of kaolin treated with cationic polyacrylamide degraded by hydrodynamic cavitation. Chem. Eng. Commun. 2022, 209, 798–807. [Google Scholar] [CrossRef]
- Taghavijeloudar, M.; Yaqoubnejad, P.; Ahangar, A.K.; Rezania, S. A rapid, efficient and eco-friendly approach for simultaneous biomass harvesting and bioproducts extraction from microalgae: Dual flocculation between cationic surfactants and bio-polymer. Sci. Total Environ. 2023, 854, 158717. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, M.; Li, A.; Yang, H. Flocculation and antimicrobial properties of a cationized starch. Water Res. 2017, 119, 57–66. [Google Scholar] [CrossRef]
- Ding, S.; Zou, H.; Zhou, S.; Bu, X.; Bilal, M.; Wang, X. The preparation of hydroxypropyl starch grafted acrylamide and its enhancement on flocculation of coal slime water. Energy Sources Part A-Recovery Util. Environ. Eff. 2022, 44, 7934–7948. [Google Scholar] [CrossRef]
- Ding, S.; Pan, F.; Zhou, S.; Bu, X.; Alheshibri, M. Ultrasonic-assisted flocculation and sedimentation of coal slime water using the Taguchi method. Energy Sources Part A-Recovery Util. Environ. Eff. 2023, 45, 10523–10536. [Google Scholar] [CrossRef]
- Su, Y.; Du, H.; Huo, Y.; Xu, Y.; Wang, J.; Wang, L.; Zhao, S.; Xiong, S. Characterization of cationic starch flocculants synthesized by dry process with ball milling activating method. Int. J. Biol. Macromol. 2016, 87, 34–40. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, H.; Yuan, B.; Huang, M.; Yang, H.; Li, A.; Bai, J.; Cheng, R. Synthesis of amphoteric starch-based grafting flocculants for flocculation of both positively and negatively charged colloidal contaminants from water. Chem. Eng. J. 2014, 244, 209–217. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shi, J.; Deng, J.; Sun, H.; Wu, J.; Ye, Z. Flocculation and dewatering of the Kaolin slurry treated by single- and dual-polymer flocculants. Chemosphere 2023, 328, 138445. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, M.; Kus, A.; Gierczycki, A.T. Influence of ultrasonic conditioning of flocculant on the aggregation process in a tank with turbine mixer. Chem. Eng. Process. 2010, 49, 205–211. [Google Scholar] [CrossRef]
- Chistyakova, G.V.; Koksharov, S.A.; Vladimirova, T.V. Dependence of the solubility of atmospheric oxygen in weakly alkaline aqueous solutions on surfactant concentration. Russ. J. Phys. Chem. A 2012, 86, 1753–1755. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Bu, X.; Wang, M.; An, B.; Shao, H.; Ni, C.; Peng, Y.; Xie, G. A novel flotation technique combining carrier flotation and cavitation bubbles to enhance separation efficiency of ultra-fine particles. Ultrason. Sonochem. 2020, 64, 105005. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, L.; Yang, H.; Gui, X.; Schönherr, H.; Kappl, M.; Cao, Y.; Xing, Y. Recent advances for understanding the role of nanobubbles in particles flotation. Adv. Colloid Interface Sci. 2021, 291, 102403. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Bu, X.; Zhou, S.; Tian, X.; Ni, C.; Nazari, S.; Alheshibri, M. Effect of aging time, airflow rate, and nonionic surfactants on the surface tension of bulk nanobubbles water. J. Mol. Liq. 2022, 359, 119274. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Nazari, S.; Bu, X.; Hassanzadeh, A.; Ni, C.; He, Y.; Xie, G. An Assessment of the Role of Combined Bulk Micro- and Nano-Bubbles in Quartz Flotation. Minerals 2022, 12, 944. [Google Scholar] [CrossRef]
- Bu, X.; Alheshibri, M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason. Sonochem. 2021, 76, 105629. [Google Scholar] [CrossRef]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S.J. A History of Nanobubbles. Langmuir 2016, 32, 11086–11100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Nazari, S.; Hassanzadeh, A.; Bu, X.; Ni, C.; Peng, Y.; Xie, G.; He, Y. The effect of preparation time and aeration rate on the properties of bulk micro-nanobubble water using hydrodynamic cavitation. Ultrason. Sonochem. 2022, 84, 105965. [Google Scholar] [CrossRef] [PubMed]
- Tao, D. Recent advances in fundamentals and applications of nanobubble enhanced froth flotation: A review. Miner. Eng. 2022, 183, 107554. [Google Scholar] [CrossRef]
- Diniz, P.H.V.; Azevedo, A.C.; Rubio, J. Filtration of fine mineral particles assisted by nanobubbles. Miner. Eng. 2023, 204, 108428. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Lei, W.; Yao, W.; Fan, R. Effect of Nanobubbles on the Slime Coating of Kaolinite in Coal Flotation. ACS Omega 2020, 5, 24773–24779. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zhang, M.; Zhang, Z.; Zhan, N.; Fan, R. Effect of bulk nanobubbles on the entrainment of kaolinite particles in flotation. Powder Technol. 2020, 362, 84–89. [Google Scholar] [CrossRef]
- Żbik, M.; Horn, R.G. Hydrophobic attraction may contribute to aqueous flocculation of clays. Colloid Surf. A-Physicochem. Eng. Asp. 2003, 222, 323–328. [Google Scholar] [CrossRef]
- Zhou, S.; Bu, X.; Wang, X.; Ni, C.; Ma, G.; Sun, Y.; Xie, G.; Bilal, M.; Alheshibri, M.; Hassanzadeh, A.; et al. Effects of surface roughness on the hydrophilic particles-air bubble attachment. J. Mater. Res. Technol.-JMRT 2022, 18, 3884–3893. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Harbottle, D.; Masliyah, J.; Xu, Z. Studying bubble–particle interactions by zeta potential distribution analysis. J. Colloid Interface Sci. 2015, 449, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yoon, R.-H. Control of bubble ζ-potentials to improve the kinetics of bubble-particle interactions. Miner. Eng. 2020, 151, 106295. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Z.; Li, G.; Rowson, N.A. Attachment of solid particles to air bubbles in surfactant-free aqueous solutions. Chem. Eng. J. 2004, 59, 2639–2645. [Google Scholar] [CrossRef]
- Besra, L.; Sengupta, D.K.; Roy, S.K. Particle characteristics and their influence on dewatering of kaolin, calcite and quartz suspensions. Int. J. Miner. Process. 2000, 59, 89–112. [Google Scholar] [CrossRef]
- Gao, J.; Bu, X.; Zhou, S.; Wang, X.; Alheshibri, M.; Peng, Y.; Xie, G. Graphite flotation by β-cyclodextrin/kerosene Pickering emulsion as a novel collector. Miner. Eng. 2022, 178, 107412. [Google Scholar] [CrossRef]
- Wang, X.; Shaoqi, Z.; Bu, X.; Ni, C.; Xie, G.; Peng, Y. Investigation on interaction behavior between coarse and fine particles in the coal flotation using focused beam reflectance measurement (FBRM) and particle video microscope (PVM). Sep. Sci. Technol. 2021, 56, 1418–1430. [Google Scholar] [CrossRef]
- Ni, C.; Bu, X.; Xia, W.; Peng, Y.; Yu, H.; Xie, G. Observing slime-coating of fine minerals on the lump coal surface using particle vision and measurement. Powder Technol. 2018, 339, 434–439. [Google Scholar] [CrossRef]
- Lu, J.; Yuan, Z.; Liu, J.; Li, L.; Zhu, S. Effects of magnetite on magnetic coating behavior in pentlandite and serpentine system. Miner. Eng. 2015, 72, 115–120. [Google Scholar] [CrossRef]
- Dixon, D.V.; Soares, J.B.P. Molecular weight distribution effects of polyacrylamide flocculants on clay aggregate formation. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 649, 129487. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.-M.; Zhang, Q.-L. Dynamic experiments on flocculation and sedimentation of argillized ultrafine tailings using fly-ash-based magnetic coagulant. Trans. Nonferrous Met. Soc. China 2016, 26, 1975–1984. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, X.; Gan, K.; Chen, Y.; Xu, J.; Yang, J. Effect of zeta potential on properties of foamed colloidal suspension. J. Eur. Ceram. Soc. 2019, 39, 574–583. [Google Scholar] [CrossRef]
- Besra, L.; Sengupta, D.K.; Roy, S.K.; Ay, P. Flocculation and dewatering of kaolin suspensions in the presence of polyacrylamide and surfactants. Int. J. Miner. Process. 2002, 66, 203–232. [Google Scholar] [CrossRef]
- Besra, L.; Sengupta, D.K.; Roy, S.K.; Ay, P. Influence of polymer adsorption and conformation on flocculation and dewatering of kaolin suspension. Sep. Purif. Technol. 2004, 37, 231–246. [Google Scholar] [CrossRef]
- Besra, L.; Sengupta, D.K.; Roy, S.K.; Ay, P. Influence of surfactants on flocculation and dewatering of kaolin suspensions by cationic polyacrylamide (PAM-C) flocculant. Sep. Purif. Technol. 2003, 30, 251–264. [Google Scholar] [CrossRef]
- Shao, H.; Chang, J.; Lu, Z.; Grundy, J.S.; Xie, G.; Xu, Z.; Liu, Q. Probing Interaction of Divalent Cations with Illite Basal Surfaces by Atomic Force Microscopy. J. Phys. Chem. C 2020, 124, 2079–2087. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X. Chemical composition and surface property of kaolins. Miner. Eng. 2003, 16, 1279–1284. [Google Scholar] [CrossRef]
- Takahashi, M. ζ Potential of Microbubbles in Aqueous Solutions: Electrical Properties of the Gas−Water Interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Zhang, T.; Chen, Y.; Peng, Y.; Xie, G.; Wu, E. Comparison of mechanical flotation cell and cyclonic microbubble flotation column in terms of separation performance for fine graphite. Physicochem. Probl. Miner. Process. 2018, 54, 732–740. [Google Scholar]
- Joglekar, A.; May, A.T. Product excellence through design of experiments. Cereal Foods World 1987, 32, 857–868. [Google Scholar]
- Wang, X.; Bu, X.; Ni, C.; Zhou, S.; Yang, X.; Zhang, J.; Alheshibri, M.; Peng, Y.; Xie, G. Effect of scrubbing medium’s particle size on scrubbing flotation performance and mineralogical characteristics of microcrystalline graphite. Miner. Eng. 2021, 163, 106766. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, Y.; Bu, X.; Xie, G. Effects of 20 kHz ultrasound on coal flotation: The roles of cavitation and acoustic radiation force. Fuel 2019, 256, 115938. [Google Scholar] [CrossRef]
- Jones, A.N.; Bridgeman, J. Investigating the characteristic strength of flocs formed from crude and purified Hibiscus extracts in water treatment. Water Res. 2016, 103, 21–29. [Google Scholar] [CrossRef]
- Amjad, H.; Khan, Z.; Tarabara, V.V. Fractal structure and permeability of membrane cake layers: Effect of coagulation–flocculation and settling as pretreatment steps. Sep. Purif. Technol. 2015, 143, 40–51. [Google Scholar] [CrossRef]
- Feng, Z.; Dong, X.; Chen, R. The relationship between permeability and pore structure of coal slime filter cake based on fractal characteristics. Coal Sci. Technol. 2023, 51, 312–322. [Google Scholar]
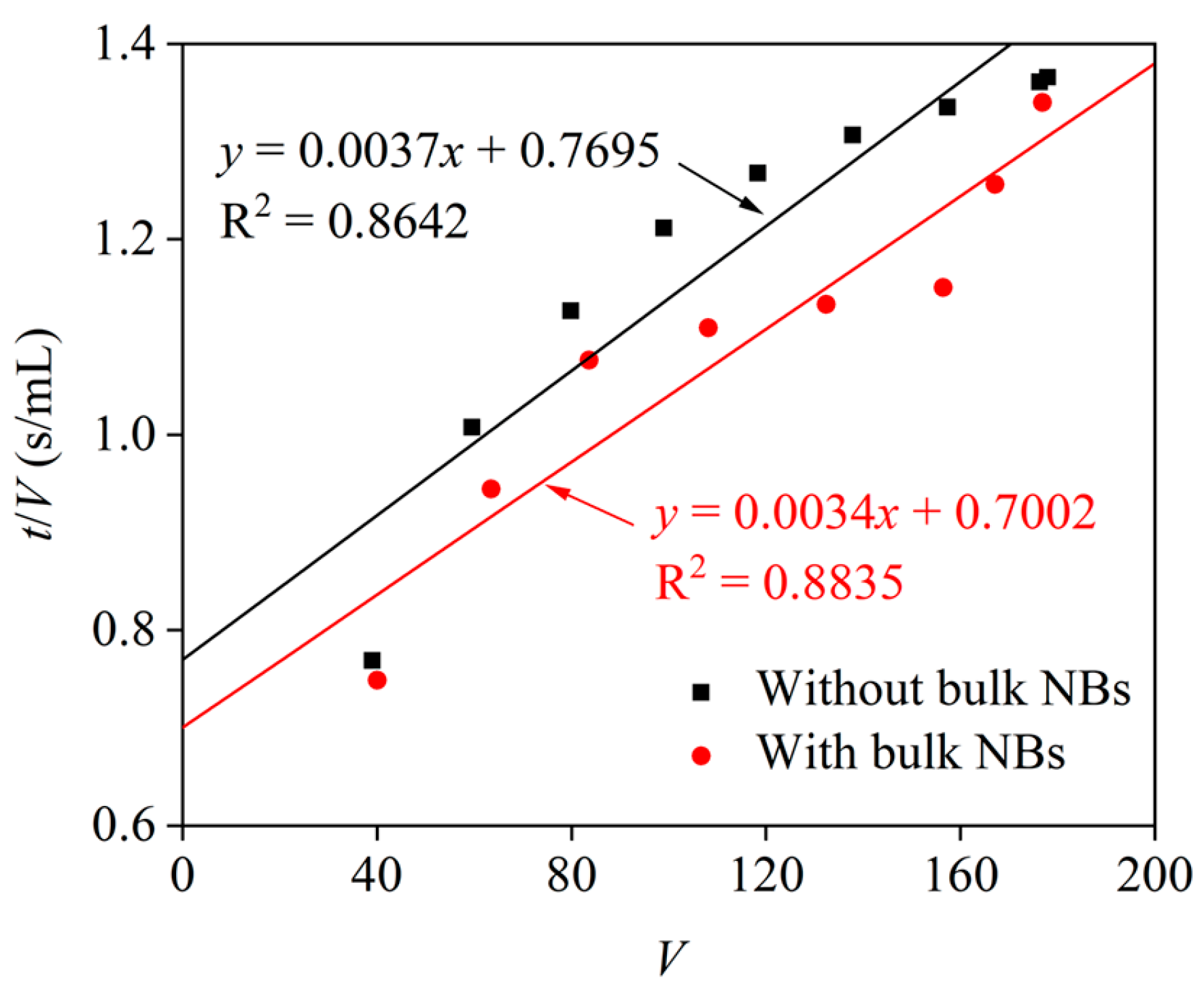
| Condition | Fitting Equation | α (m/kg) | Rm (m−1) | R2 |
|---|---|---|---|---|
| Without bulk NBs | y = 0.0037x + 0.7695 | 4.0 × 10−8 | 8.3 × 10−9 | 0.8642 |
| With bulk NBs | y = 0.0034x + 0.7002 | 3.6 × 10−8 | 7.6 × 10−9 | 0.8835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, G.; Bilal, M.; Sha, J.; Bu, X. Effect of Bulk Nanobubbles on the Flocculation and Filtration Characteristics of Kaolin Using Cationic Polyacrylamide. Minerals 2024, 14, 405. https://doi.org/10.3390/min14040405
Li Y, Ma G, Bilal M, Sha J, Bu X. Effect of Bulk Nanobubbles on the Flocculation and Filtration Characteristics of Kaolin Using Cationic Polyacrylamide. Minerals. 2024; 14(4):405. https://doi.org/10.3390/min14040405
Chicago/Turabian StyleLi, Yihong, Guangxi Ma, Muhammad Bilal, Jie Sha, and Xiangning Bu. 2024. "Effect of Bulk Nanobubbles on the Flocculation and Filtration Characteristics of Kaolin Using Cationic Polyacrylamide" Minerals 14, no. 4: 405. https://doi.org/10.3390/min14040405






