Characterization of a Nickel Sulfide Concentrate and Its Implications on Pentlandite Beneficiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
2.3. Laser Diffraction Measurement
2.4. ICP-OES Analysis
2.5. Quantitative X-ray Diffraction (QXRD) Analysis
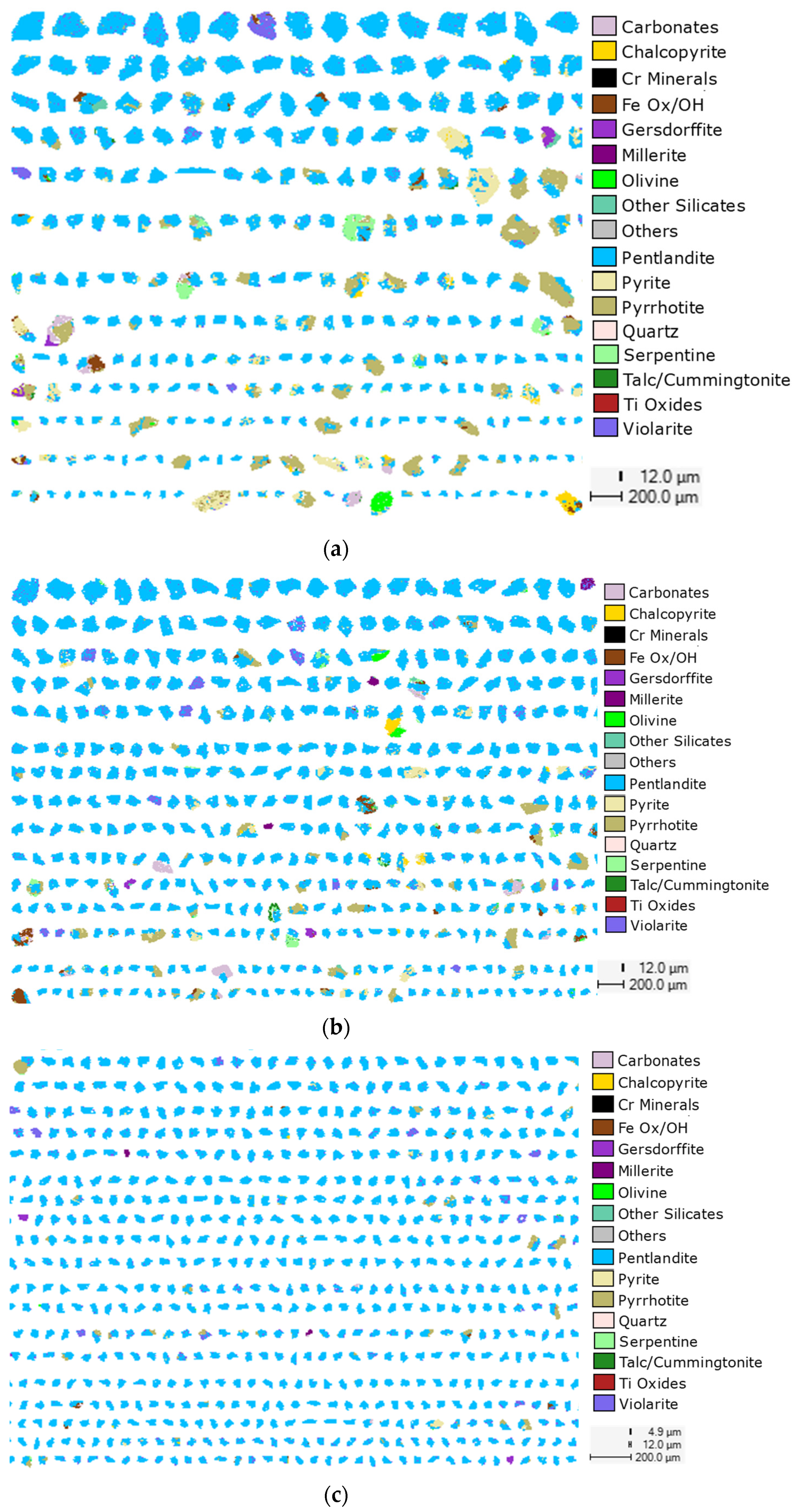
2.6. QEMSCAN Analysis
3. Results
3.1. Size Analysis
3.2. Chemical Composition
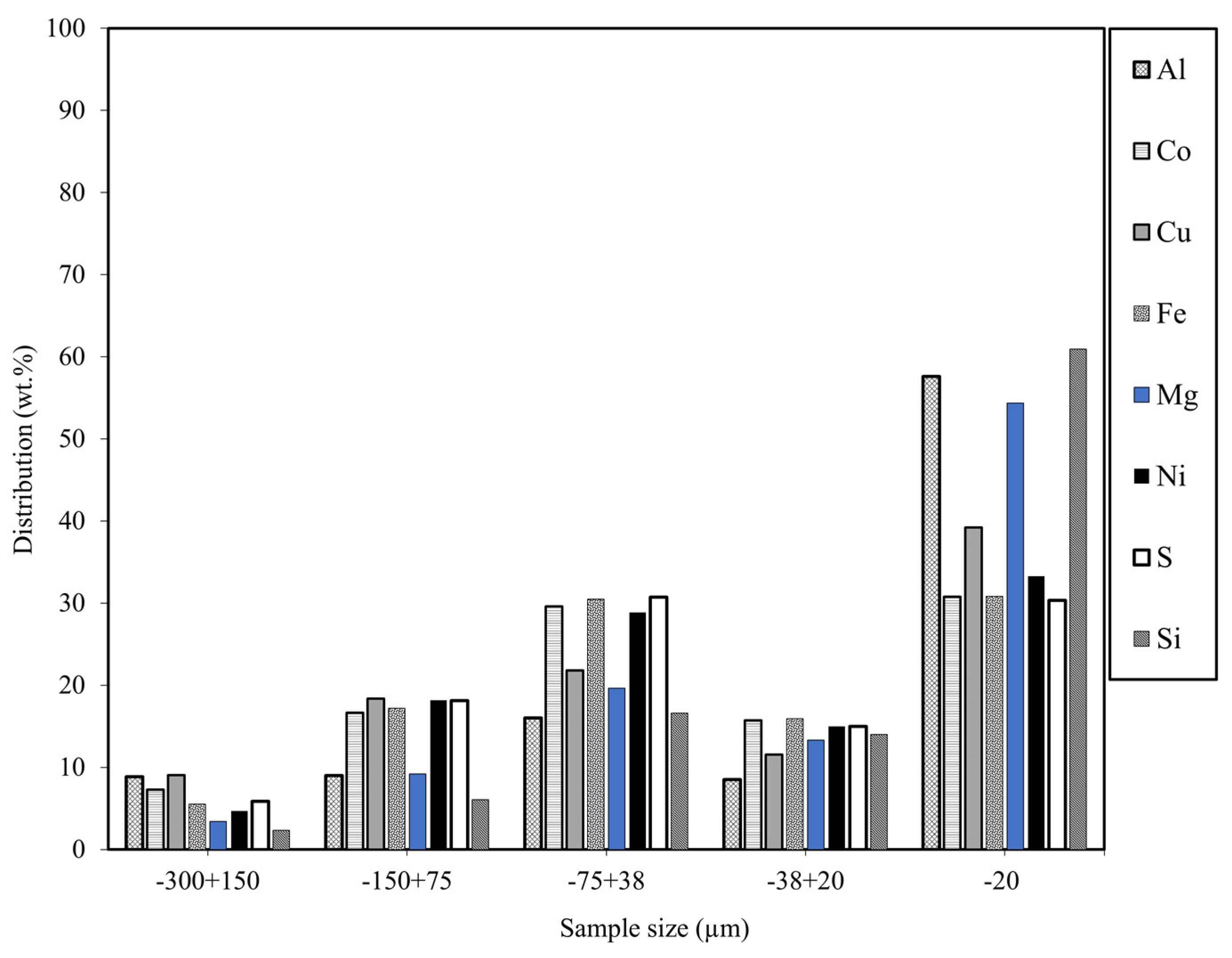
Bulk Elemental Composition
3.3. Mineralogical Composition
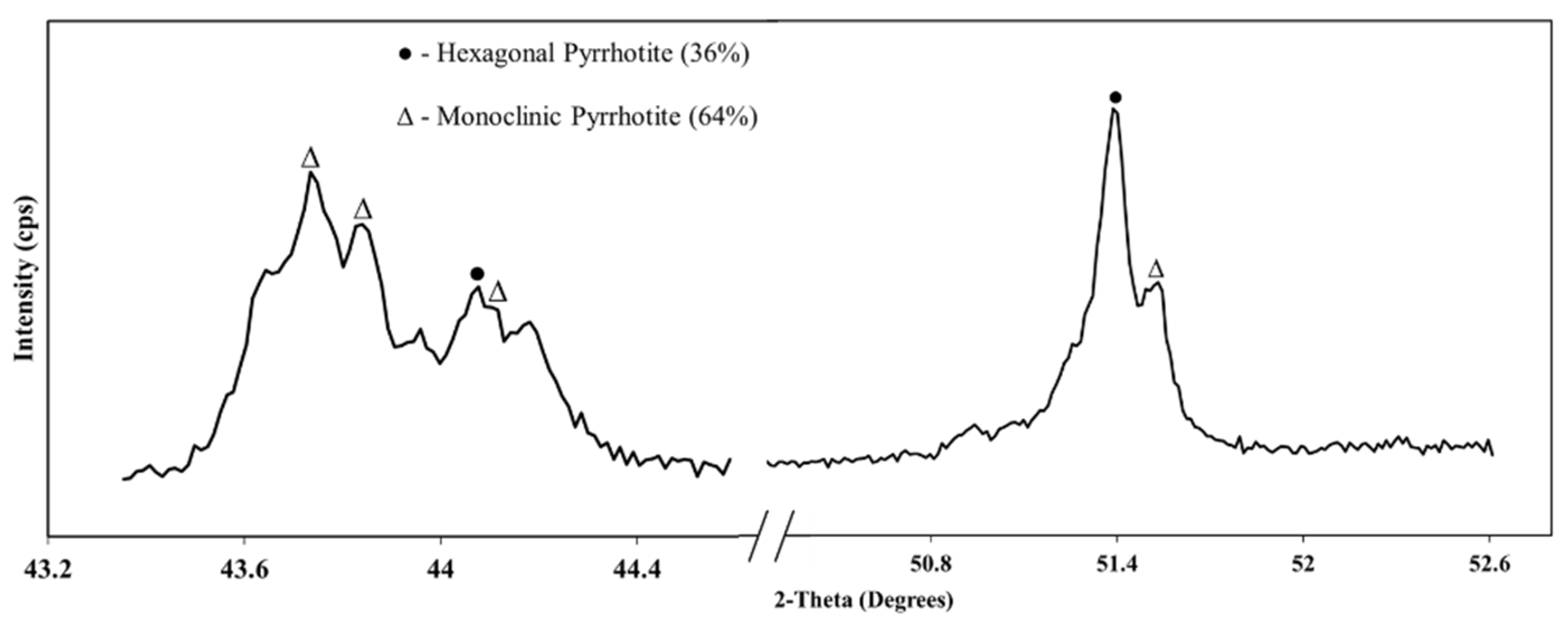
3.3.1. QXRD Analysis
3.3.2. Distribution of Nickel and Gangue Minerals
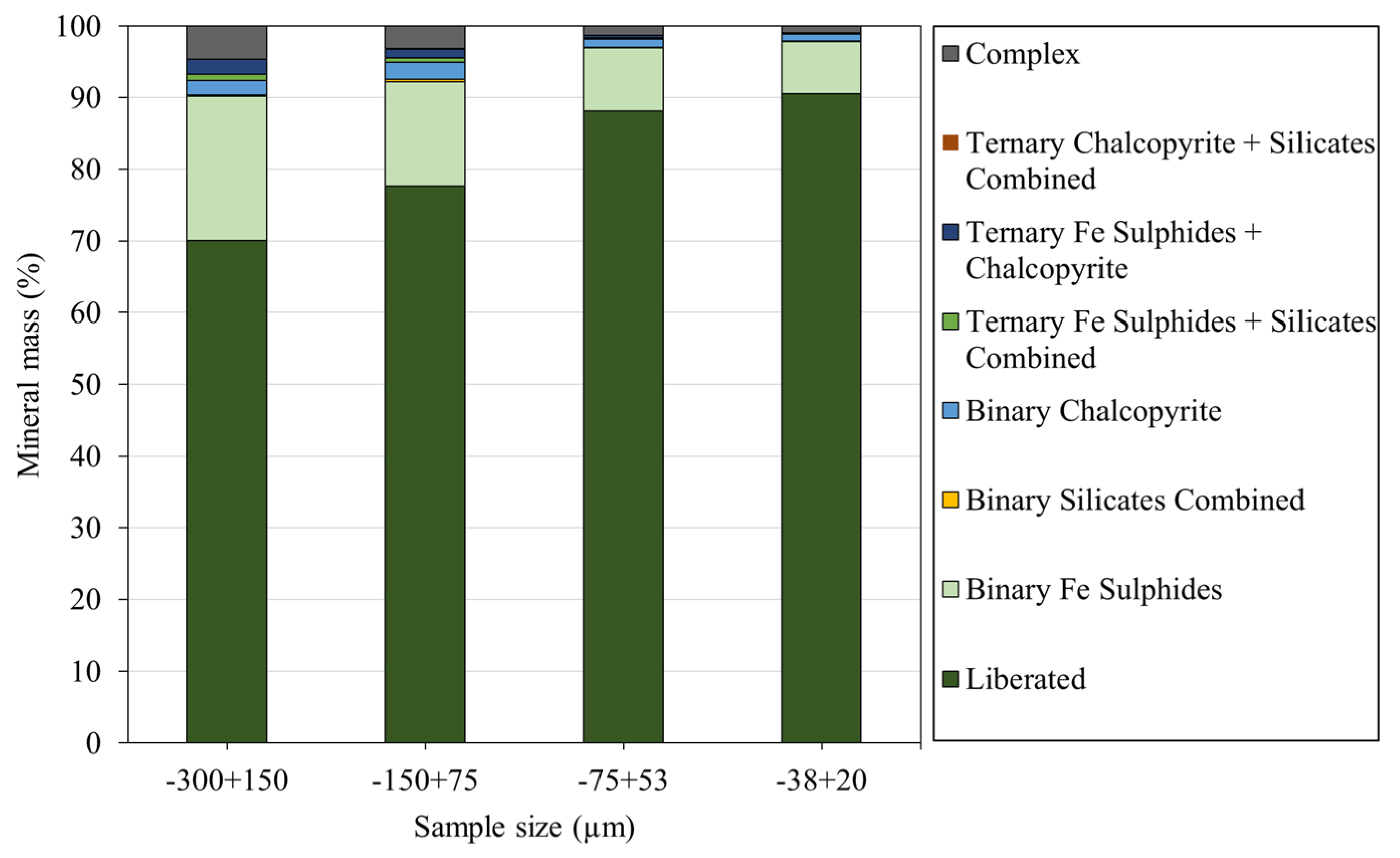
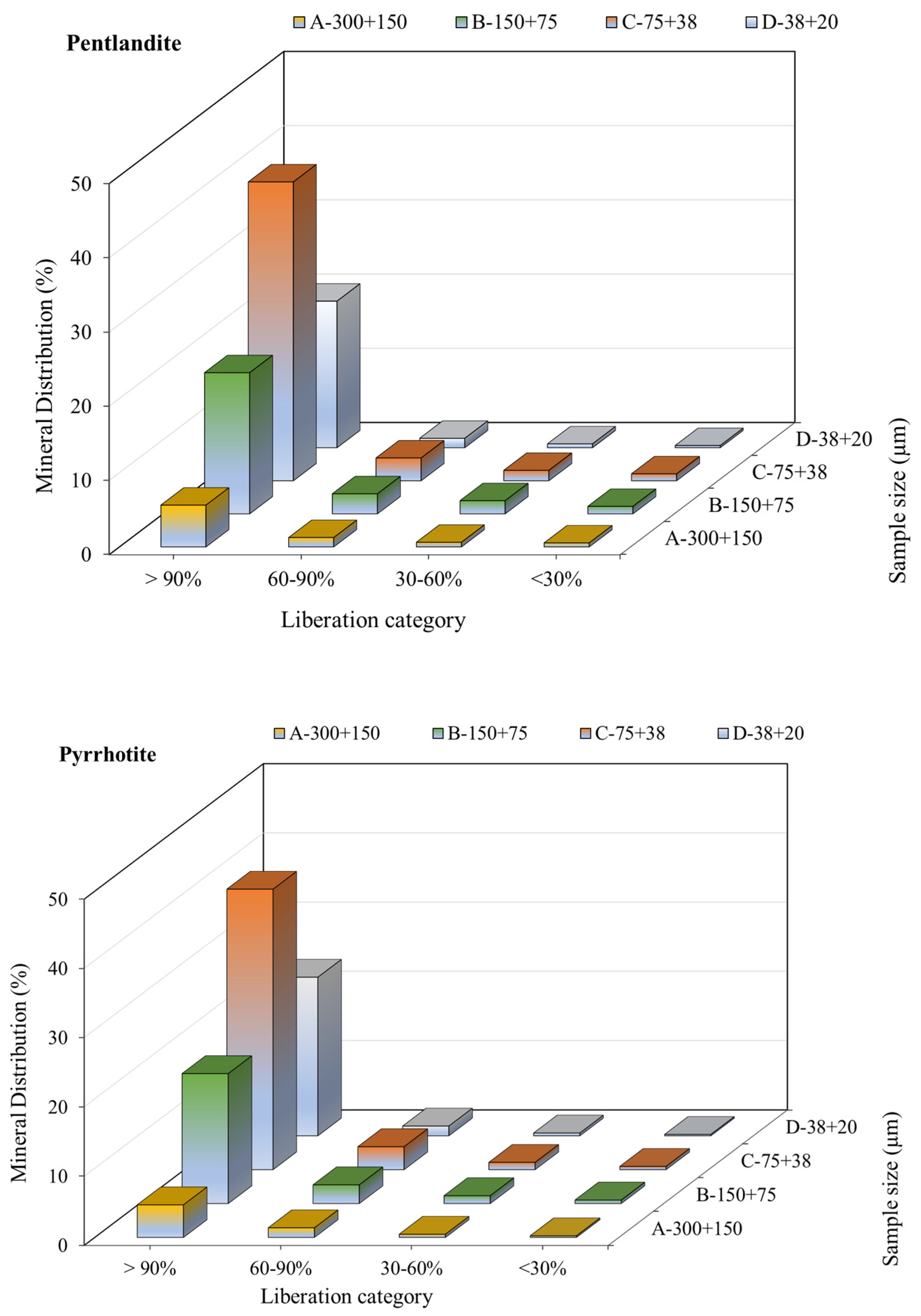
3.3.3. Mineral Liberation and Locking Characteristics
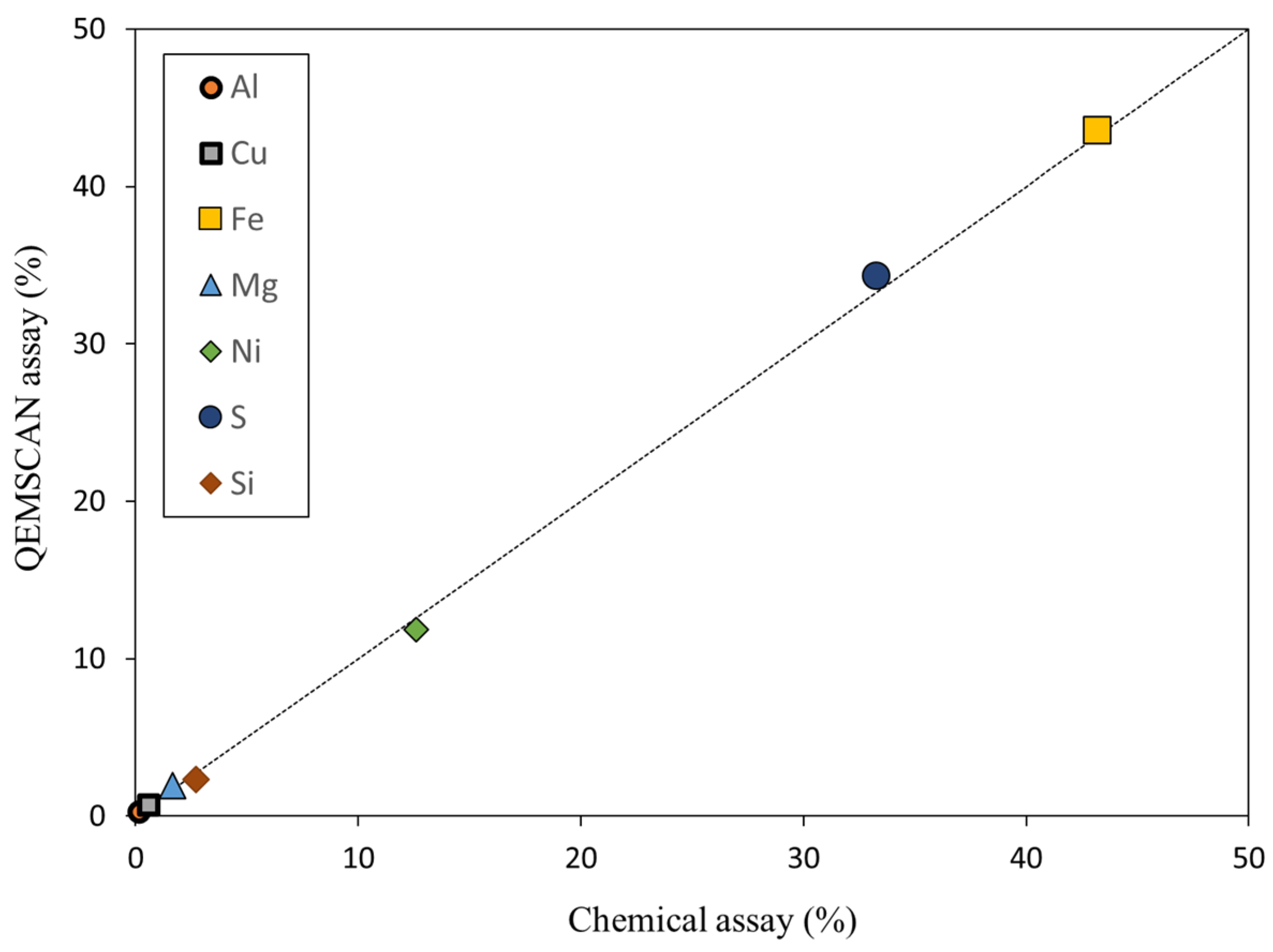
4. QEMSCAN Data Validation
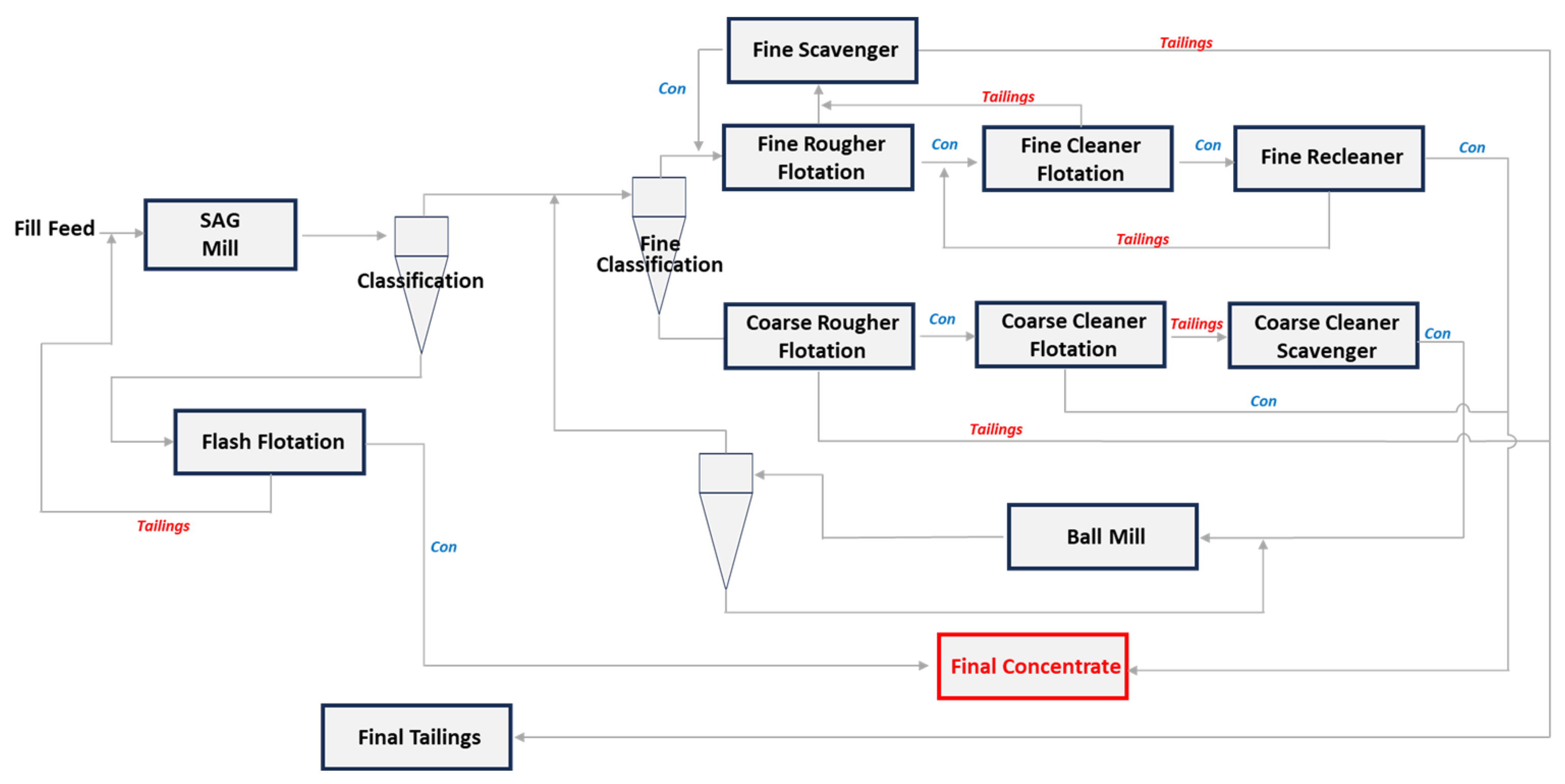
5. Possible Beneficiation Approaches
5.1. Gravity Separation
5.2. Magnetic Separation
5.3. Froth Flotation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michele, M. US Geological Survey, Mineral Commodity Summaries; January 2022; National Minerals Information Center: Reston, VA, USA, 2022. [Google Scholar]
- Dulfer, H.; Milligan, P.R.; Coghlan, R.; Czarnota, K.; Highet, L.M.; Champion, D.C.; Skirrow, R.G. Potential for Intrusion-Hosted Ni-Cu-PGE Sulfide Deposits in Australia: A Continental-Scale Analysis of Mineral System Prospectivity; Geoscience Australia: Canberra, Australia, 2016. [Google Scholar]
- Williams, N.C. Mass and magnetic properties for 3D geological and geophysical modelling of the southern Agnew–Wiluna Greenstone Belt and Leinster nickel deposits, Western Australia. Aust. J. Earth Sci. 2009, 56, 1111–1142. [Google Scholar] [CrossRef]
- Cahill, J.; Lee, M. Ground control at Leinster nickel operations. J. S. Afr. Inst. Min. Metall. 2006, 106, 471–478. [Google Scholar]
- Teh, M.; Pheeney, J. Australian Resource Reviews: Nickel 2020; Geoscience Australia: Canberra, Australia, 2021. [Google Scholar]
- Legrand, D.L.; Bancroft, G.M.; Nesbitt, H.W. Surface characterization of pentlandite, (Fe,Ni)9S8, by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 1997, 51, 217–228. [Google Scholar] [CrossRef]
- Senior, G.; Thomas, S. Development and implementation of a new flowsheet for the flotation of a low grade nickel ore. Int. J. Miner. Process. 2005, 78, 49–61. [Google Scholar] [CrossRef]
- Tian, H.; Pan, J.; Zhu, D.; Yang, C.; Guo, Z.; Xue, Y. Improved beneficiation of nickel and iron from a low-grade saprolite laterite by addition of limonitic laterite ore and CaCO3. J. Mater. Res. Technol. 2020, 9, 2578–2589. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Dreisinger, D.B.; Espinosa, D.C.R. A Review of Nickel, Copper, and Cobalt Recovery by Chelating Ion Exchange Resins from Mining Processes and Mining Tailings. Min. Metall. Explor. 2019, 36, 199–213. [Google Scholar] [CrossRef]
- Ayedzi, L.D.; Abaka-Wood, G.B.; Zanin, M.; Skinner, W. Electrokinetic study of pentlandite and quartz for froth flotation separation. In Proceedings of the 18th Procemin-Geomet 2022, Virtual, 5–7 October 2022; pp. 184–193. [Google Scholar]
- Grammatikopoulos, T.; Mercer, W.; Gunning, C.; Prout, S. Quantitative characterization of the REE minerals by QEMSCAN from the Nechalacho Heavy Rare Earth Deposit, Thor Lake Project, NWT, Canada. SGS Miner. Serv. Tech. Pap. 2011, 7, 1–11. [Google Scholar]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. The Use of Mining Tailings as Analog of Rare Earth Elements Resources: Part 1—Characterization and Preliminary Separation. Miner. Process. Extr. Metall. Rev. 2022, 43, 701–715. [Google Scholar] [CrossRef]
- Duuring, P.; Hassan, L.; Zelic, M.; Gessner, K. Geochemical and spectral footprint of metamorphosed and deformed VMS-style mineralization in the Quinns district, Yilgarn Craton, Western Australia. Econ. Geol. 2016, 111, 1411–1438. [Google Scholar] [CrossRef]
- Khoso, S.A.; Gao, Z.; Tian, M.; Hu, Y.; Sun, W. Adsorption and depression mechanism of an environmentally friendly reagent in differential flotation of Cu–Fe sulphides. J. Mater. Res. Technol. 2019, 8, 5422–5431. [Google Scholar] [CrossRef]
- Koulialias, D.; Kind, J.; Charilaou, M.; Weidler, P.; Löffler, J.; Gehring, A. Variable defect structures cause the magnetic low-temperature transition in natural monoclinic pyrrhotite. Geophys. J. Int. 2016, 204, 961–967. [Google Scholar] [CrossRef]
- Qiu, T.; Zhang, C.; Yang, L.; Wang, J.; Zhao, G.; Yan, H.; Wu, H.; Qiu, X.; Yang, B.; Liao, R. Effect of Electrochemical Interaction between Chalcopyrite and Hexagonal Pyrrhotite on Flotation Separation. Minerals 2023, 13, 1303. [Google Scholar] [CrossRef]
- Lu, P.; Chen, T.; Liu, H.; Li, P.; Peng, S.; Yang, Y. Green preparation of nanoporous pyrrhotite by thermal treatment of pyrite as an effective Hg (Ⅱ) adsorbent: Performance and mechanism. Minerals 2019, 9, 74. [Google Scholar] [CrossRef]
- Chareev, D.A.; Voronin, M.V.; Osadchii, E.G. Thermodynamic study of monoclinic pyrrhotite in equilibrium with pyrite in the Ag-Fe-S system by solid-state electrochemical cell technique. Am. Mineral. 2014, 99, 2031–2034. [Google Scholar] [CrossRef]
- Fosu, S.; Pring, A.; Skinner, W.; Zanin, M. Characterisation of coarse composite sphalerite particles with respect to flotation. Miner. Eng. 2015, 71, 105–112. [Google Scholar] [CrossRef]
- Edahbi, M.; Benzaazoua, M.; Plante, B.; Doire, S.; Kormos, L. Mineralogical characterization using QEMSCAN® and leaching potential study of REE within silicate ores: A case study of the Matamec project, Québec, Canada. J. Geochem. Explor. 2018, 185, 64–73. [Google Scholar] [CrossRef]
- Kirjavainen, V.; Heiskanen, K. Some factors that affect beneficiation of sulphide nickel–copper ores. Miner. Eng. 2007, 20, 629–633. [Google Scholar] [CrossRef]
- Lu, J.; Yuan, Z.; Li, M.; Zhao, X.; Tong, Z.; Li, L.; Qi, S. The role of sodium oleate (NaOL) in the magnetic separation of pentlandite from serpentine using magnetic coating. Powder Technol. 2019, 345, 492–500. [Google Scholar] [CrossRef]
- Yüce, A.E.; Bulut, G.; Boylu, F.; Önal, G. Process Flowsheet Development for Beneficiation of Nickel Ore. Miner. Process. Extr. Metall. Rev. 2007, 29, 57–67. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J. Wills’ mineral processing technology: An introduction to the practical aspects of ore treatment and mineral recovery. In Technology & Engineering; Butterworth-Heinemann: Oxford, UK, 2015; p. 512. [Google Scholar]
- Abaka-Wood, G.B.; Quast, K.; Zanin, M.; Addai-Mensah, J.; Skinner, W. A study of the feasibility of upgrading rare earth elements minerals from iron-oxide-silicate rich tailings using Knelson concentrator and Wilfley shaking table. Powder Technol. 2019, 344, 897–913. [Google Scholar] [CrossRef]
- Bulatovic, S. Flotation of Nickel and Nickel–Copper Ores. In Handbook of Flotation Reagents; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2007; pp. 401–441. [Google Scholar]
- Ikotun, B.D.; Adams, F.V.; Ikotun, A.G. Application of three xanthates collectors on the recovery of nickel and pentlandite in a low-grade nickel sulfide ore using optimum flotation parameters. Part. Sci. Technol. 2017, 35, 462–471. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Magnetic separation of monazite from mixed minerals. In Chemeca 2016: Chemical Engineering-Regeneration, Recovery and Reinvention; Engineers Australia: Melbourne, VIC, Australia, 2016. [Google Scholar]
- Abaka-Wood, G.; Addai-Mensah, J.; Skinner, W. Review of flotation and physical separation of rare earth element minerals. In Proceedings of the 4th UMaT Biennial International Mining and Mineral Conference, Tarkwa, Ghana, 3–6 August 2016. [Google Scholar]
- Oberteuffer, J. Magnetic separation: A review of principles, devices, and applications. IEEE Trans. Magn. 1974, 10, 223–238. [Google Scholar] [CrossRef]
- Becker, M.; Villiers, J.d.; Bradshaw, D. The flotation of magnetic and non-magnetic pyrrhotite from selected nickel ore deposits. Miner. Eng. 2010, 23, 1045–1052. [Google Scholar] [CrossRef]
- Perring, C.S. A 3-D geological and structural synthesis of the Leinster area of the Agnew-Wiluna belt, Yilgarn craton, Western Australia, with special reference to the volcanological setting of komatiite-associated nickel sulfide deposits. Econ. Geol. 2015, 110, 469–503. [Google Scholar] [CrossRef]
- Kelebek, S.; Tukel, C. Separation of nickeliferous hexagonal pyrrhotite from pentlandite in Ni-Cu sulphide ores: Recovery by size performance. Miner. Eng. 2018, 125, 223–230. [Google Scholar] [CrossRef]
- Manouchehri, H.R. Pyrrhotite flotation and its selectivity against pentlandite in the beneficiation of nickeliferous ores: An electrochemistry perspective. Min. Metall. Explor. 2014, 31, 115–125. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, B. The effect of polyether on the separation of pentlandite and serpentine. J. Mater. Res. Technol. 2015, 4, 429–433. [Google Scholar] [CrossRef]
- Arol, A.I.; Aydogan, A. Recovery enhancement of magnetite fines in magnetic separation. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 151–154. [Google Scholar] [CrossRef]
- Krasowska, M.; Malysa, K. Wetting films in attachment of the colliding bubble. Adv. Colloid Interface Sci. 2007, 134–135, 138–150. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors. Int. J. Miner. Process. 2017, 158, 55–62. [Google Scholar] [CrossRef]
- Ralston, J.; Fornasiero, D.; Mishchuk, N. The hydrophobic force in flotation-a critique. Colloids Surf. A Physicochem. Eng. Asp. 2001, 192, 39–51. [Google Scholar] [CrossRef]
- van Oss, C.J. The Water–Air Interface, in Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 149–160. [Google Scholar]
- Kelebek, S.; Tukel, C. The effect of sodium metabisulfite and triethylenetetramine system on pentlandite–pyrrhotite separation. Int. J. Miner. Process. 1999, 57, 135–152. [Google Scholar] [CrossRef]
- Wills, B.; Napier–Munn, T. Mineral Processing Technology; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2006; pp. 267–352. [Google Scholar]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of Fine Particles: A Review. Miner. Process. Extr. Metall. Rev. 2021, 42, 473–483. [Google Scholar] [CrossRef]
- Jameson, G.J. New directions in flotation machine design. Miner. Eng. 2010, 23, 835–841. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Safari, M.; Hoang, D.H.; Khoshdast, H.; Albijanic, B.; Kowalczuk, P.B. Technological assessments on recent developments in fine and coarse particle flotation systems. Miner. Eng. 2022, 180, 107509. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H. Surface nanobubbles and their roles in flotation of fine particles—A review. J. Ind. Eng. Chem. 2022, 106, 37–51. [Google Scholar] [CrossRef]
- Leonida, C. Floating New Ideas. Eng. Min. J. 2022, 223, 40–45. [Google Scholar]
- Jiang, K.; Dickinson, J.E.; Galvin, K.P. The kinetics of Fast Flotation using the Reflux Flotation Cell. Chem. Eng. Sci. 2019, 196, 463–477. [Google Scholar] [CrossRef]
- Wang, P.; Yvon, M.; Parkes, S.; Galvin, K.P. Enhancing nickel grade and recovery with counter-current washing of the concentrated bubbly-zone of a single stage REFLUX™ Flotation Cell. Miner. Eng. 2024, 206, 108506. [Google Scholar] [CrossRef]
- Parkes, S.; Wang, P.; Galvin, K.P. Investigating the System Flotation Kinetics of Fine Chalcopyrite in a REFLUX™ Flotation Cell using a Standardised Flotation Cell Reference Method. Miner. Eng. 2022, 178, 107411. [Google Scholar] [CrossRef]
- Boycott, A. Sedimentation of blood corpuscles. Nature 1920, 104, 532. [Google Scholar] [CrossRef]
- Chen, J.; Chimonyo, W.; Peng, Y. Flotation behaviour in reflux flotation cell—A critical review. Miner. Eng. 2022, 181, 107519. [Google Scholar] [CrossRef]
- Cole, M.J.; Galvin, K.P.; Dickinson, J.E. Maximizing recovery, grade and throughput in a single stage Reflux Flotation Cell. Miner. Eng. 2021, 163, 106761. [Google Scholar] [CrossRef]
- Jiang, K. Fast Flotation in a Reflux Flotation Cell. In Discipline of Chemical Engineering; The University of Newcastle: Callaghan, NSW, Australia, 2017. [Google Scholar]


| Sieve Size (µm) | Weight Retained (g) | Percentage Retained (%) | Cumulative Retained (%) | Cumulative Passing (%) |
|---|---|---|---|---|
| −300 + 150 | 11.8 | 5.3 | 5.3 | 100.0 |
| −150 + 75 | 36.0 | 16.2 | 21.5 | 94.7 |
| −75 + 38 | 64.0 | 28.8 | 50.2 | 78.5 |
| −38 + 20 | 34.0 | 15.3 | 65.5 | 49.8 |
| −20 | 76.8 | 34.5 | 100 | 34.5 |
| Total | 223 | 100 |
| Particle Size (µm) | Assays, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Al | Co | Cu | Fe | Mg | Ni | S | Si | |
| −300 + 150 | 0.3 | 0.4 | 1.0 | 43.2 | 1.7 | 11.1 | 34.4 | 1.3 |
| −150 + 75 | 0.1 | 0.3 | 0.6 | 44.0 | 1.5 | 14.1 | 34.9 | 1.1 |
| −75 + 38 | 0.1 | 0.3 | 0.4 | 43.9 | 1.8 | 12.6 | 33.3 | 1.7 |
| −38 + 20 | 0.1 | 0.3 | 0.4 | 43.2 | 2.3 | 12.3 | 30.6 | 2.7 |
| −20 | 0.3 | 0.3 | 0.6 | 37.0 | 4.2 | 12.1 | 27.4 | 5.2 |
| Head (Calc.) | 0.2 | 0.3 | 0.5 | 41.4 | 2.6 | 12.5 | 31.2 | 2.9 |
| Mineral Phase | Content (wt.%) |
|---|---|
| Pentlandite | 40 |
| Pyrrhotite | 44 |
| Talc | 6 |
| Magnetite | 5 |
| Violarite | 2 |
| Serpentine | 2 |
| Magnesite | 1 |
| Pyrite | <1 |
| Olivine | <1 |
| Quartz Total | <1 100 |
| Sample | −300 + 150 µm | −150 + 75 µm | −75 + 38 µm | −38 + 20 µm | |
|---|---|---|---|---|---|
| Mineral Mass % Liberated | NiFe Sulfides | 70 | 78 | 88 | 91 |
| Fe Sulfides | 69 | 81 | 89 | 92 | |
| Chalcopyrite | 36 | 50 | 59 | 72 | |
| Silicates Combined | 50 | 70 | 85 | 92 | |
| Oxides | 21 | 22 | 35 | 49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayedzi, L.D.; Zanin, M.; Skinner, W.; Abaka-Wood, G.B. Characterization of a Nickel Sulfide Concentrate and Its Implications on Pentlandite Beneficiation. Minerals 2024, 14, 414. https://doi.org/10.3390/min14040414
Ayedzi LD, Zanin M, Skinner W, Abaka-Wood GB. Characterization of a Nickel Sulfide Concentrate and Its Implications on Pentlandite Beneficiation. Minerals. 2024; 14(4):414. https://doi.org/10.3390/min14040414
Chicago/Turabian StyleAyedzi, Linda D., Massimiliano Zanin, William Skinner, and George B. Abaka-Wood. 2024. "Characterization of a Nickel Sulfide Concentrate and Its Implications on Pentlandite Beneficiation" Minerals 14, no. 4: 414. https://doi.org/10.3390/min14040414





