Structural Characterisation of Zeolites Derived from Lithium Extraction: Insights into Channel- and Cage-Type Frameworks
Abstract
1. Introduction
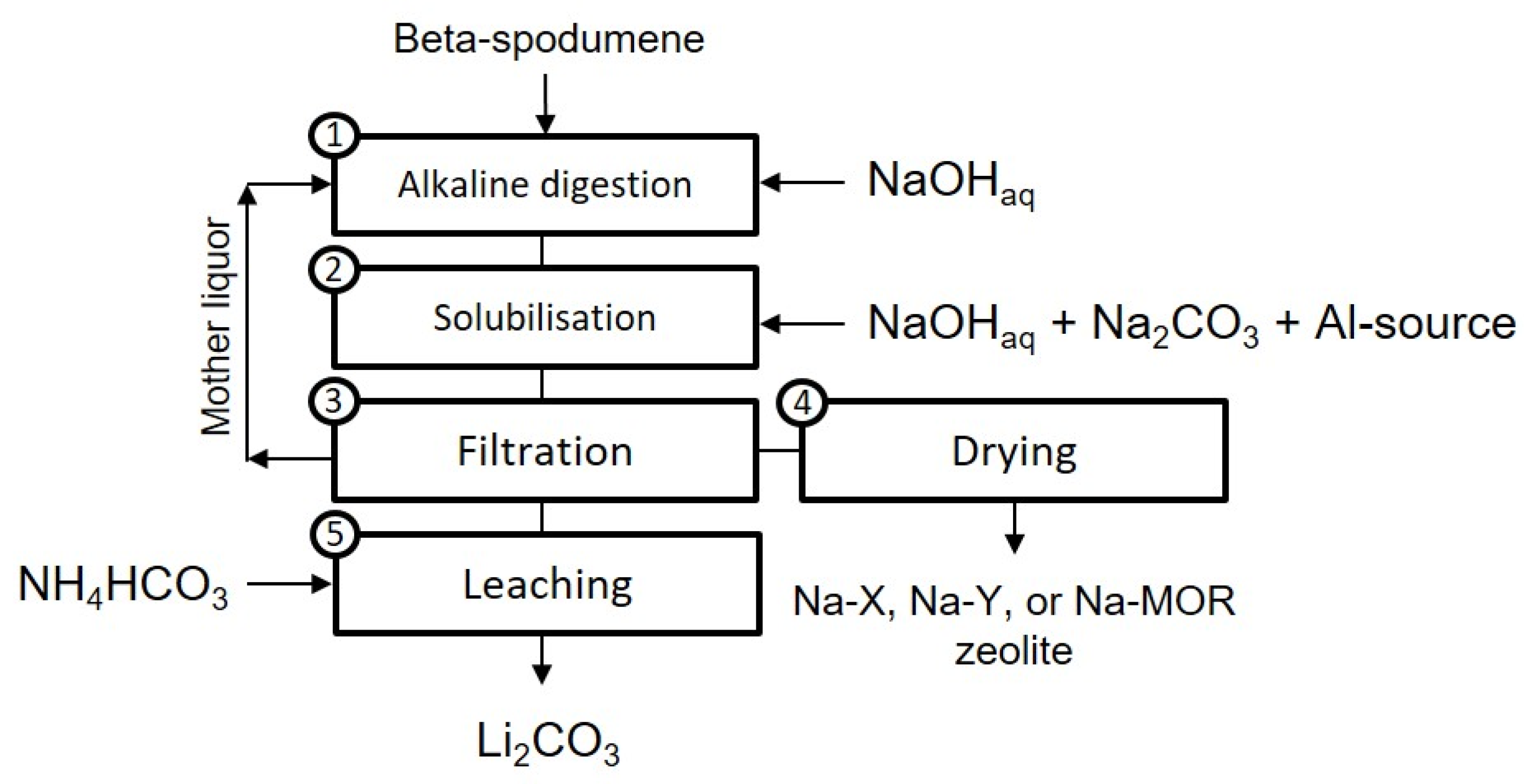
2. Materials and Methods
3. Results and Discussion
3.1. Structural Framework
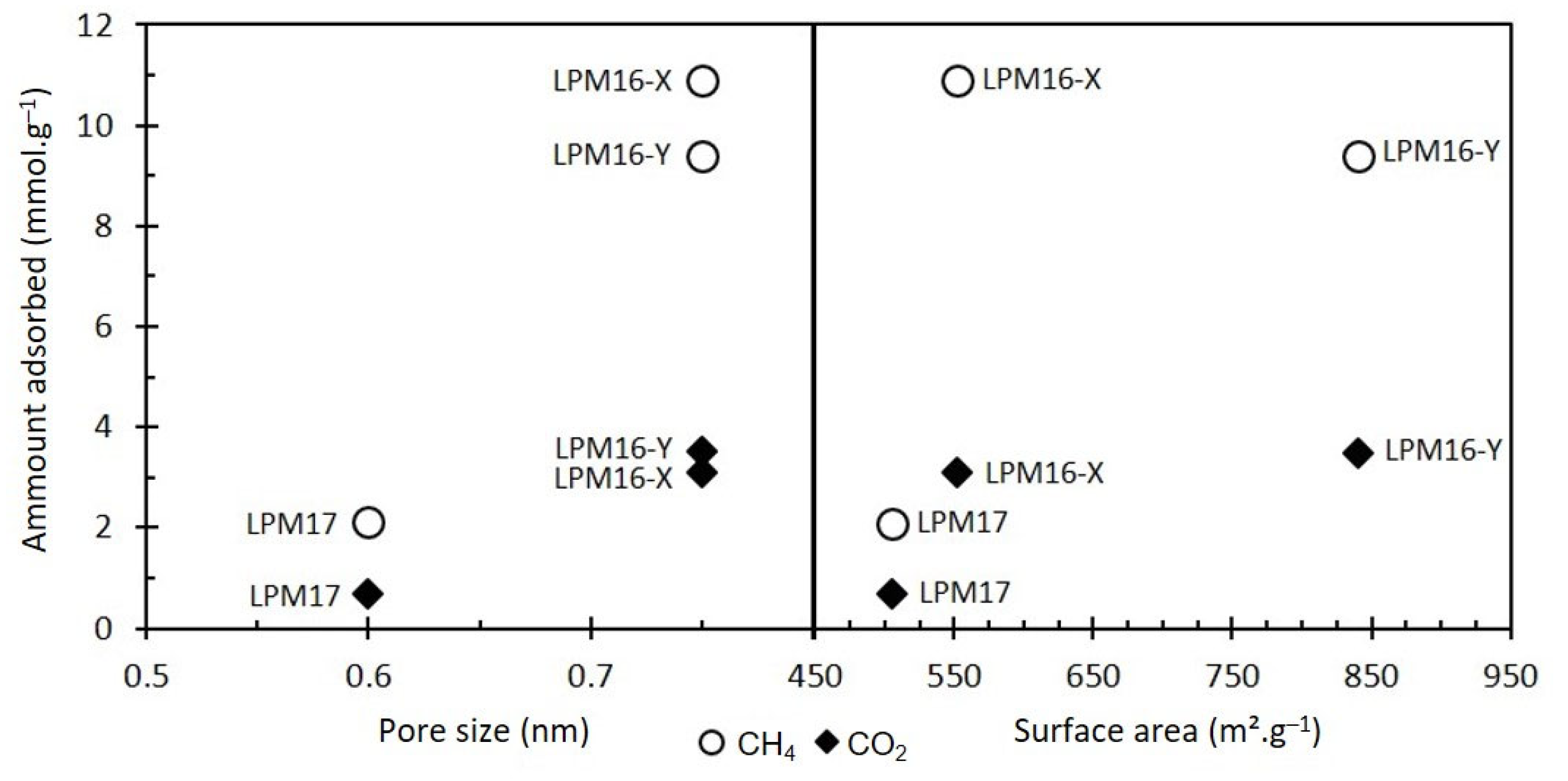
3.2. Physicochemical Properties
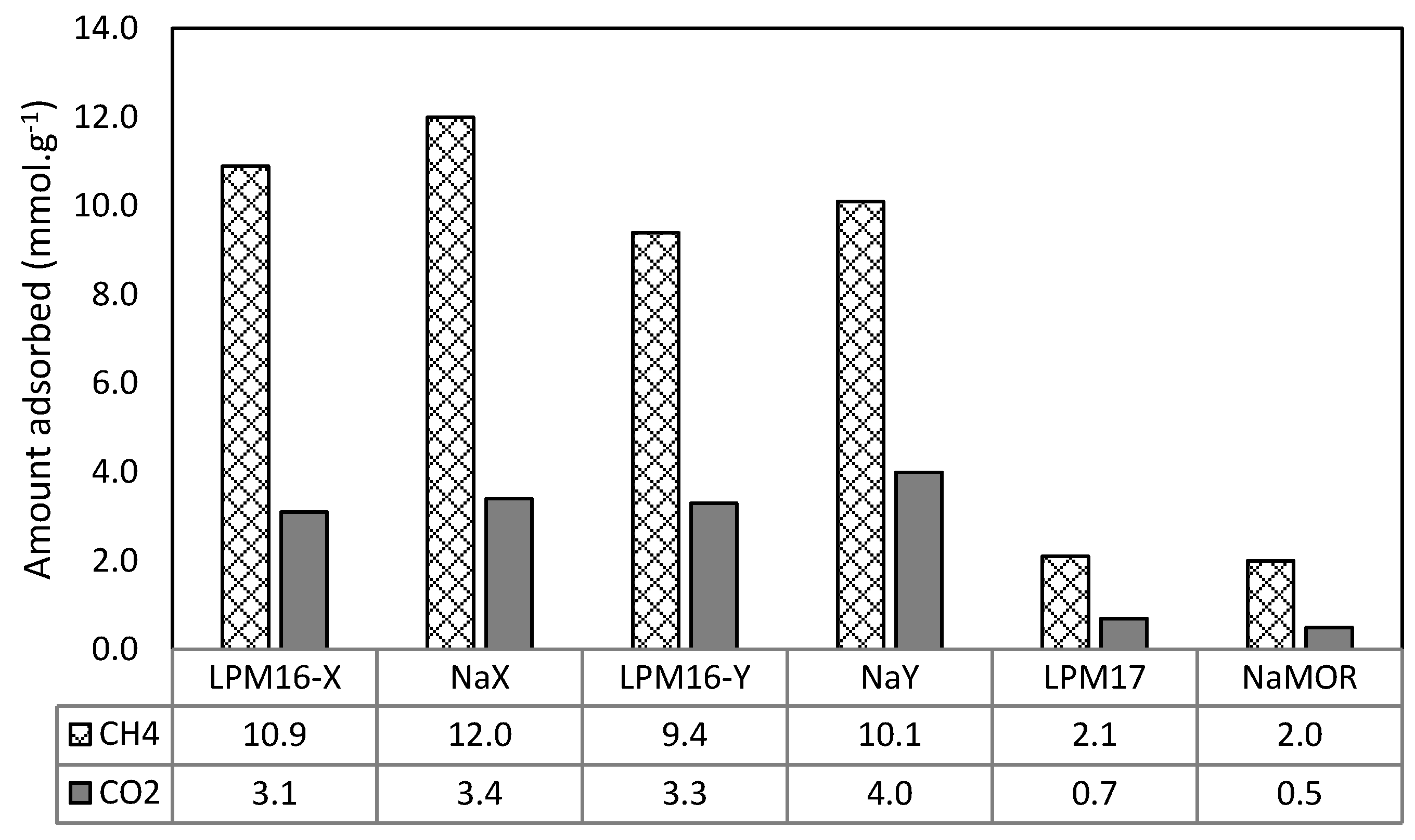
3.3. Gas Adsorption Capacity
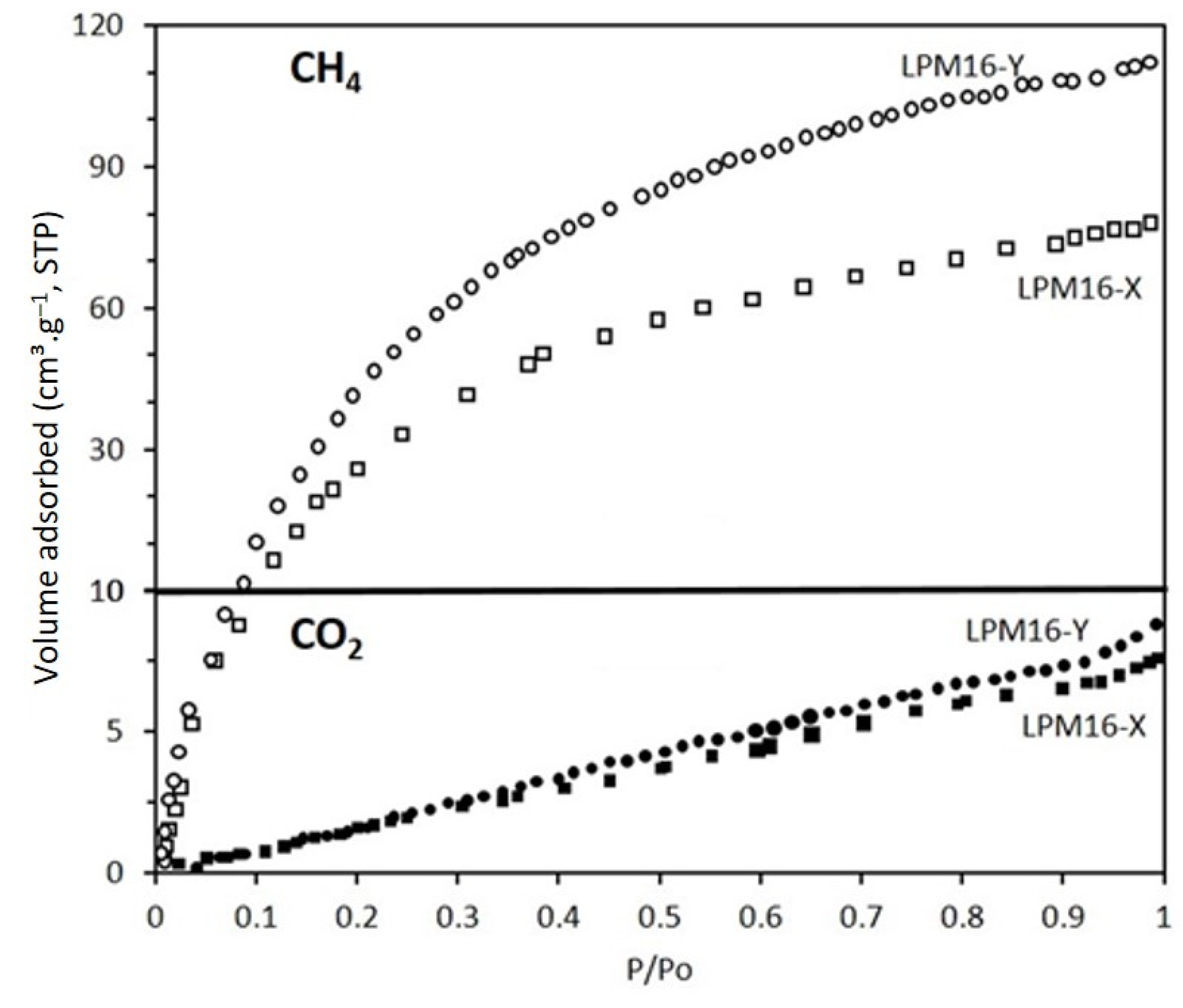
3.4. Adsorption Isotherms
3.5. Adsorption Kinetics
3.6. Adsorbate–Adsorbent Interaction
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Najafi, A.M.; Soltanali, S.; Ghassabzadeh, H. Enhancing the CO2, CH4, and N2 adsorption and kinetic performance on FAU zeolites for CO2 capture from flue gas by metal incorporation technique. Chem. Eng. J. 2023, 468, 143719. [Google Scholar] [CrossRef]
- Moradi, H.; Azizpour, H.; Bahmanyar, H.; Rezamandi, N.; Zahedi, P. Effect of Si/Al Ratio in the Faujasite Structure on Adsorption of Methane and Nitrogen: A Molecular Dynamics Study. Chem. Eng. Technol. 2021, 44, 1221–1226. [Google Scholar] [CrossRef]
- Najafi, A.M.; Khorasheh, F.; Soltanali, S.; Ghassabzadeh, H. Equilibrium and Kinetic Insights into the Comprehensive Investigation of CO2, CH4, and N2 Adsorption on Cation-Exchanged X and Y Faujasite Zeolites. Langmuir 2023, 39, 15535–15546. [Google Scholar] [CrossRef] [PubMed]
- Kenvin, J.; Mitchell, S.; Sterling, M.; Warringham, R.; Keller, T.C.; Crivelli, P.; Jagiello, J.; Pérez-Ramírez, J. Quantifying the Complex Pore Architecture of Hierarchical Faujasite Zeolites and the Impact on Diffusion. Adv. Funct. Mater. 2016, 26, 5621–5630. [Google Scholar] [CrossRef]
- Garcia, G.; Cabrera, S.; Hedlund, J.; Mouzon, J. Selective synthesis of FAU-type zeolites. J. Cryst. Growth 2018, 489, 36–41. [Google Scholar] [CrossRef]
- Ferdov, S. Conventional synthesis of layer-like zeolites with faujasite (FAU) structure and their pathway of crystallization. Microporous Mesoporous Mater. 2020, 303, 110263. [Google Scholar] [CrossRef]
- Wolfgang, L. Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef]
- Cairon, O. Impacts of composition and post-treatment on the Brønsted acidity of steam-treated faujasite: Insights from FTIR spectroscopy. ChemPhysChem 2013, 14, 244–251. [Google Scholar] [CrossRef]
- Cairon, O. CO adsorption on N2-precovered NaY faujasite: A FTIR analysis of the resulting adsorbed species. ChemPhysChem 2013, 14, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Cychosz, K.A.; Melinte, G.; El Siblani, H.; Gilson, J.-P.; Thommes, M.; Fernandez, C.; Mintova, S.; Ersen, O.; Valtchev, V. Opening the Cages of Faujasite-Type Zeolite. J. Am. Chem. Soc. 2017, 139, 17273–17276. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamizhdurai, P.; Mangesh, V.L.; Ragupathi, C.; Krishnan, P.S.; Ramesh, A. Recent advances in the synthesis and applications of mordenite zeolite—Review. RSC Adv. 2020, 11, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Erdos, M.; Moultos, O.A.; Shang, R.; Vlugt, T.J.H.; Heijman, S.G.J.; Rietveld, L.C. The adsorption mechanisms of organic micropollutants on high-silica zeolites causing S-shaped adsorption isotherms: An experimental and Monte Carlo simulation study. Chem. Eng. J. 2020, 389, 123968. [Google Scholar] [CrossRef]
- Derouane, E.G.; Chang, C.D. Confinement effects in the adsorption of simple bases by zeolites. Microporous Mesoporous Mater. 2000, 35–36, 425–433. [Google Scholar] [CrossRef]
- Jensen, N.K.; Rufford, T.E.; Watson, G.; Zhang, D.K.; Chan, K.I.; May, E.F. Screening Zeolites for Gas Separation Applications Involving Methane, Nitrogen, and Carbon Dioxide. J. Chem. Eng. Data 2011, 57, 106–113. [Google Scholar] [CrossRef]
- Ahn, H.; Moon, J.-H.; Hyun, S.-H.; Lee, C.-H. Diffusion mechanism of carbon dioxide in zeolite 4A and CaX pellets. Adsorption 2004, 10, 111–128. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.S.; Abney, M.B.; LeVan, M.D. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Grifoni, E.; Piccini, G.; Lercher, J.A.; Glezakou, V.-A.; Rousseau, R.; Parrinello, M. Confinement effects and acid strength in zeolites. Nat. Commun. 2021, 12, 2630. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Ilić, B.; Wettstein, S.G. A review of adsorbate and temperature-induced zeolite framework flexibility. Microporous Mesoporous Mater. 2017, 239, 221–234. [Google Scholar] [CrossRef]
- Klimeš, J.; Tew, D.P. Efficient and accurate description of adsorption in zeolites. J. Chem. Phys. 2019, 151, 234108. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, A.; Garbarino, G.; Riani, P.; Finocchio, E.; Bosio, B.; Ramírez, J.; Busca, G. Adsorption and separation of CO2 from N2-rich gas on zeolites: Na-X faujasite vs. Na-mordenite. J. CO2 Util. 2017, 19, 266–275. [Google Scholar] [CrossRef]
- Halasz, I.; Kim, S.; Marcus, B. Hydrophilic and hydrophobic adsorption on Y zeolites. Mol. Phys. 2002, 100, 3123–3132. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Bell, R.; Kuchta, B.; Poyet, T.; Llewellyn, P. Adsorption of non polar and quadrupolar gases in siliceous Faujasite: Molecular simulations and experiments. Adsorption 2005, 11, 331–336. [Google Scholar] [CrossRef]
- Nor Kamarudin, K.S.; Yuan, C.Y.; Hamdan, H.; Mat, H. Ftir Spectroscopy of Methane Adsorption on Zeolites. J. Chem. Nat. Resour. Engineneering 2008, 2, 31–39. [Google Scholar]



| Component | SiO2 | Al2O3 | Li2O | Fe2O3 | K2O | Others |
|---|---|---|---|---|---|---|
| Content (%) | 68.97 | 22.31 | 6.43 | 0.92 | 0.42 | <0.40 |
| By-Product | RC 1 (%) | Surface Area (m2/g) | Pore Volume (cm3/g) | APD 2 (nm) | ||
|---|---|---|---|---|---|---|
| BET | Micropore | Micropore | Meso/Macro | |||
| LPM16-X | 79 | 552.4 | 539.5 | 0.20 | 0.022 | 1.48 |
| LPM16-Y | 83 | 833.1 | 808.6 | 0.29 | 0.026 | 1.58 |
| LPM17 | 100 | 505.1 | 452.9 | 0.17 | 0.070 | 2.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.L.d.; Nascimento, R.M.d.; Pergher, S.B.C. Structural Characterisation of Zeolites Derived from Lithium Extraction: Insights into Channel- and Cage-Type Frameworks. Minerals 2024, 14, 526. https://doi.org/10.3390/min14050526
Santos LLd, Nascimento RMd, Pergher SBC. Structural Characterisation of Zeolites Derived from Lithium Extraction: Insights into Channel- and Cage-Type Frameworks. Minerals. 2024; 14(5):526. https://doi.org/10.3390/min14050526
Chicago/Turabian StyleSantos, Leonardo Leandro dos, Rubens Maribondo do Nascimento, and Sibele Berenice Castellã Pergher. 2024. "Structural Characterisation of Zeolites Derived from Lithium Extraction: Insights into Channel- and Cage-Type Frameworks" Minerals 14, no. 5: 526. https://doi.org/10.3390/min14050526
APA StyleSantos, L. L. d., Nascimento, R. M. d., & Pergher, S. B. C. (2024). Structural Characterisation of Zeolites Derived from Lithium Extraction: Insights into Channel- and Cage-Type Frameworks. Minerals, 14(5), 526. https://doi.org/10.3390/min14050526







