Abstract
While numerous studies have explored the mineralogical characteristics and purification techniques of high-purity quartz (HPQ), discussions on impurity control during various purification processes and their applications in photovoltaics, electronics, and optics remain limited. This review delves into the adverse effects of impurities such as aluminum, iron, and sodium in the manufacturing processes of these industries, emphasizing their critical role as these impurities can degrade material performance. This paper focuses on analyzing the types of impurities found in quartz and evaluates existing purification technologies such as acid washing, ultrasonic acid washing, chlorination roasting, and calcination quenching. It highlights the limitations of current technologies in processing quartz ore and discusses the advantages of different impurity types under various technological treatments. Moreover, it explores the environmental and economic impacts of these high-purity processes, underlining the necessity for more environmentally friendly and cost-effective purification techniques. The purpose of this review is to provide a comprehensive technical and strategic framework for the use of high-purity quartz in high-tech applications, supporting future research and industrial applications in this critical material field.
1. Introduction
High-purity quartz (HPQ) is a type of pristine quartz with minimal impurity levels, typically exceeding 99.995% SiO2 content. It is devoid of visually detectable inclusions or cloudiness and possesses minimal lattice-bound impurities, such as Al, Ti, and Fe [1,2]. The genesis of quartz deposits is influenced by multifarious factors, culminating in substantial disparities in quartz concentration, impurity mineral typologies, and their respective levels across geographies. Typically, indigenous quartz deposits harbor a plethora of impurity minerals, encompassing iron minerals (notably hematite and limonite), rutile, calcite, mica, feldspar varieties (including potassium and sodium feldspar), and clay minerals, among others [3]. During geotectonic processes, quartz crystals, with entrapped solid and fluid inclusions of metallic impurities, gases, and aqueous molecules, significantly enhance the impurity quotient of quartz silicates [4,5,6]. Diverse quartz deposits arise through processes ranging from volcanic activities to intricate geological transformations encompassing magma crystallization, differentiation, and assimilation.
Factors like the crustal depth, mineralization temperature, and contemporaneous pressure predominantly influence deposit types [7]. In this review, the term “quartz sand” (or “high-purity quartz sand”) refers specifically to quartz materials with grain sizes ranging from 0.063 to 2 mm. It should be noted that for the purpose of this review, “quartz sand” excludes larger, blocky, or lumpy quartz raw materials. To provide a clearer understanding, natural quartz crystals can be classified based on geological aspects:
- (1)
- Magmatic Quartz: Examples include alaskaite for Iota quartz, typically formed during the cooling of magma. These quartz crystals often have high purity but may contain trace metallic impurities [8,9].
- (2)
- Pegmatite Quartz: derived from pegmatites, these quartz crystals can be exceptionally pure, often used for high-end electronic and optical applications [10,11,12,13].
- (3)
- Hydrothermal Vein Quartz: formed from hydrothermal fluids, this type includes vein quartz with high SiO2 content but can have numerous gas–liquid inclusions and associated minerals like pyrite, hematite, mica, and feldspar [14].
- (4)
- Metamorphic Quartz: includes quartzites and metamorphogenic mobilizates formed under high-pressure and high-temperature conditions, often resulting in high-density, hard quartz with some impurities like iron minerals, feldspars, and clay minerals [15].
- (5)
- Sedimentary Quartz: Such as quartz sands and sedimentary quartzites, formed through erosion, transportation, sedimentation, and compaction processes. These deposits typically have a lower purity due to higher impurity levels of iron minerals and clays [16].
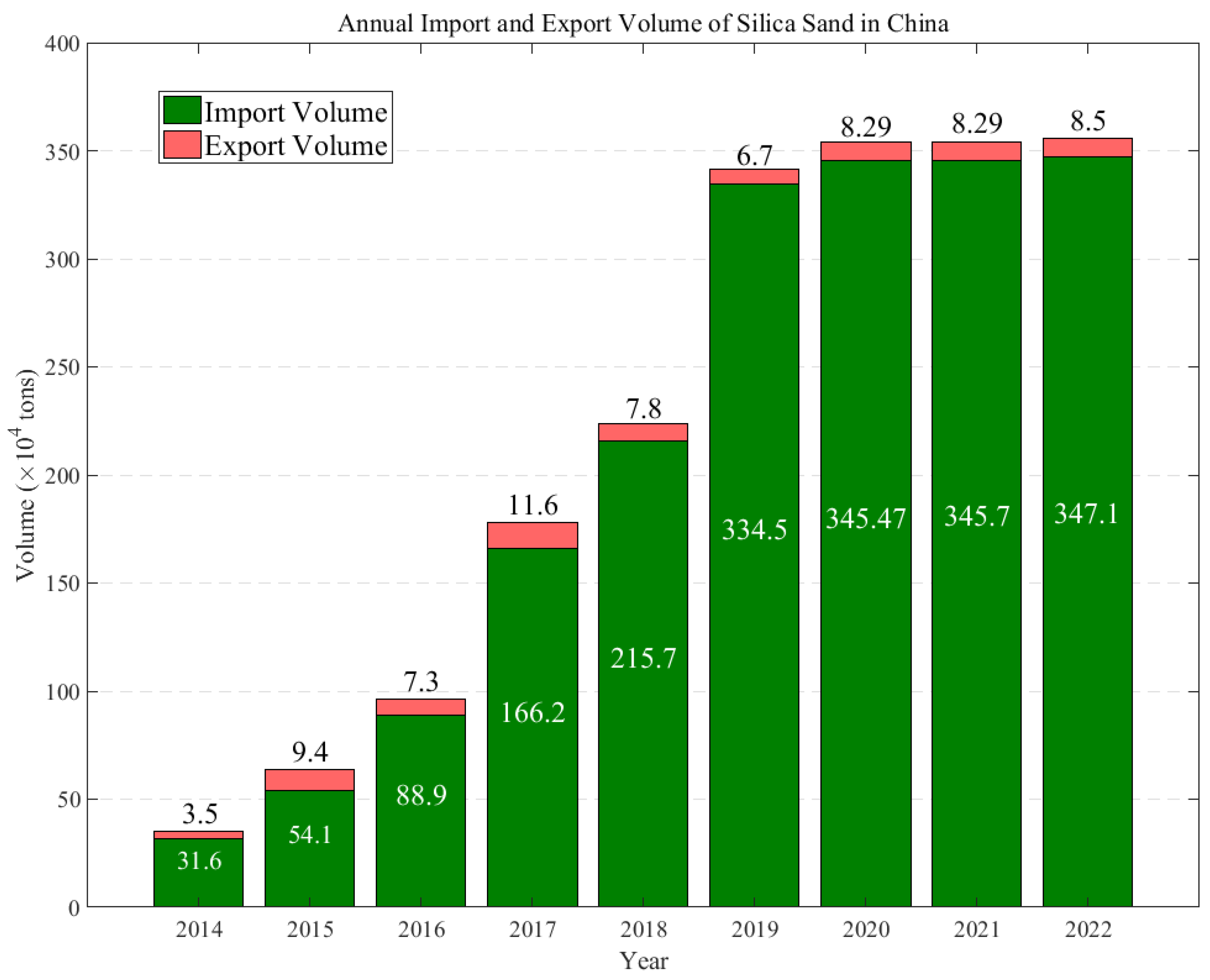
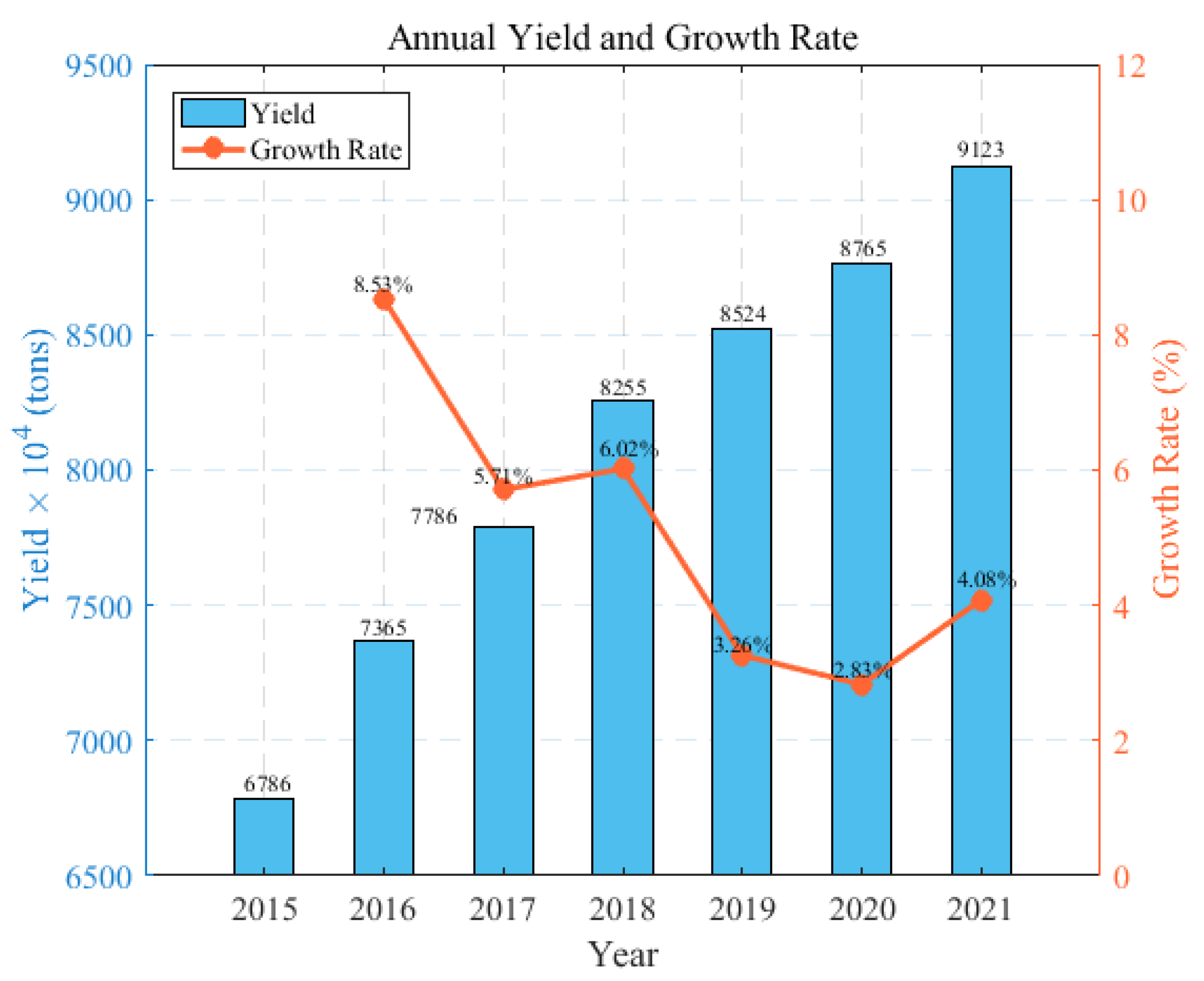
Currently, there is a noticeable technological gap in the production of quartz sand between China and the United States. In China, quartz sands derived from quartz ore often display suboptimal and inconsistent quality, characterized by variations in purity levels, particle size distribution, or contamination, which has led to a continuous dependency on imports to meet the demand for quartz sand. This dependency is illustrated in Figure 1. Figure 2 illustrates the trends in the annual production and growth rates of quartz sand in China between 2015 and 2021, highlighting the changes and demands within the industry, and presents the annual import and export volumes of quartz sand in China from 2014 to 2022. The data show a consistent increase in imports, highlighting China’s growing need for and reliance on foreign-sourced quartz sand. Although there is also an increase in exports, these remain significantly lower in volume when compared to imports, underlining the domestic challenges in producing quartz sand that meets high-quality standards.
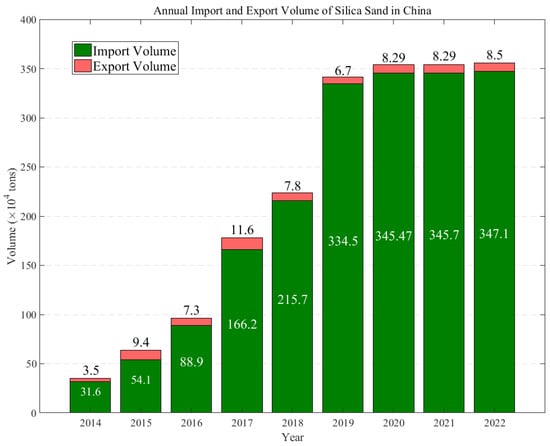
Figure 1.
Annual import and export volumes of quartz sand in China from 2014 to 2022. (Data from Guanjian Research Report network “China quartz sand industry status In-depth Analysis and investment trend research Report 2022–2029”).
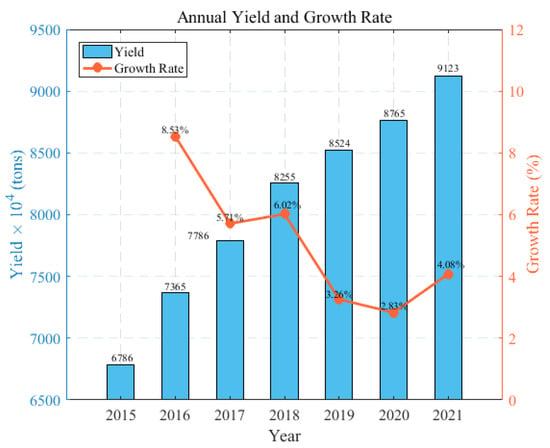
Figure 2.
Annual quartz sand production and growth rate in China from 2015 to 2021. (Data from Guanjian Research Report network “China quartz sand industry status In-depth Analysis and investment trend research Report 2022–2029”).
Some countries in the United States and Europe pioneered research on the substitution of quartz ore with crystal for the production of high-purity quartz sand. Through continuous development, these countries have matured their quartz sand refining processes, resulting in high-purity quartz sands characterized by excellent quality and stability. High-purity quartz sands produced by companies like Sibelco in the United States approach the standards of crystal sand in terms of metallic impurity and gas–liquid inclusion content [17]. For instance, the SiO2 content in their IOTA-8 quartz sand exceeds 99.999%, while the total metallic impurity content has been reduced to below 10 mg/kg, as indicated for some IOTA series quartz sands in Table 1. The high-purity quartz sands they produce find applications in various high-end industries, are considered international standards, and nearly monopolize the international market for high-purity quartz sands.

Table 1.
Metal impurities content in HPQ of IOTA and Chinese photovoltaic industry (mg/kg).
Based on the data presented in Table 1, it can be observed that the levels of metallic impurities in the IOTA series quartz sands have met the standards of high-purity quartz sands. Particularly, in the case of IOTA-Std, the major metallic impurity, Al, has been reduced to 14 mg/kg, addressing the challenging issue of Al contamination in quartz sands. This improvement is of significant importance in preventing crystallization issues in quartz glass materials under high-temperature conditions, while enhancing their thermal stability and transparency. Through refinement, IOTA-Std is suitable for use in the semiconductor industry and the manufacturing of high-temperature lamps. In the IOTA-4, IOTA-6, and IOTA-8 series of quartz sands, the Al content has been further reduced to below 8 mg/kg, further elevating the quality of high-purity quartz sands. Additionally, alkali metals also play a crucial role in determining the quality of quartz sands. Under high-temperature conditions, alkali metals can reduce the light transmittance of quartz glass, decrease its transparency, and potentially lead to deformation and crystallization issues, compromising the thermal stability of quartz glass. However, in IOTA-6 quartz sand, the total alkali metal impurity content has been reduced to below 1 mg/kg, mitigating the impact of alkali metals on quartz glass and improving its thermal stability. In IOTA-8 quartz sand, the total metallic impurity content has been decreased to below 10 mg/kg, while the SiO2 content reaches as high as 99.9995%. This high-purity quartz sand, used in the production of premium quartz crucibles and large-size silicon wafers, exhibits outstanding performance in terms of thermal stability and transparency [18].
High-purity quartz (HPQ) is a critical siliceous raw material widely used in the photovoltaic industry, semiconductors, large-scale integrated circuits, optical fibers, high-temperature lamp tubes, quartz crucibles, high-temperature glass, aerospace, and other high-tech fields [19]. It plays an essential role in the development of the high-tech silicon industry. High-grade quartz glass necessitates stringent control over metal impurities and gas–liquid inclusions within high-purity quartz sand to ensure optimal performance and durability [20]. The presence of these impurities can significantly deteriorate the glass’s integrity, affecting its transparency and resistance. Compared to high-purity quartz sand, ordinary quartz sand falls short in meeting the stringent production standards due to its higher impurity levels, which can drastically impair the performance and reliability of quartz glass products. Quartz glass is renowned for its outstanding properties, which include resistance to high temperatures, minimal thermal expansion, effective sound–light transmission, robust thermal shock resistance, low dielectric loss, and chemical stability, making it an invaluable material in various high-tech applications [21]. For instance, quartz glass exhibits remarkable resistance to acid corrosion—excluding interactions with hydrofluoric acid and hot phosphoric acid—being 30 times more resistant than standard industrial ceramics (such as alumina ceramics) and 150 times more resistant than typical stainless steel grades (such as 304 or 316 stainless steel), thereby offering significant advantages in environments requiring chemical inertness. In addition, it has good heat resistance and excellent thermal stability, which can be used for a long time at 1200 °C, higher than ordinary glass with a temperature resistance of 400 °C. Compared with regular glass, its thermal expansion coefficient is minimal, can withstand severe temperature changes, and has good thermal shock resistance [22].
The main goal of this review is to delve into the control of impurities during the purification of high-purity quartz (HPQ) and its application in critical industries such as photovoltaics, electronics, and optics. This study places particular emphasis on the adverse effects of impurities such as aluminum, iron, and sodium, which can degrade material performance in high-end applications. It evaluates existing purification technologies, such as acid leaching, ultrasonic acid washing, chlorination roasting, and calcination water quenching, highlighting the limitations of current approaches in processing quartz minerals. This review discusses the advantages of different impurity types under various technological processes and explores the environmental and economic impacts of these high-purity treatments. The purpose of this review is to provide a comprehensive technical and strategic framework for the use of high-purity quartz in high-tech applications, supporting future research and industrial applications in this critical material field.
2. Application Fields
2.1. Photovoltaic Industry
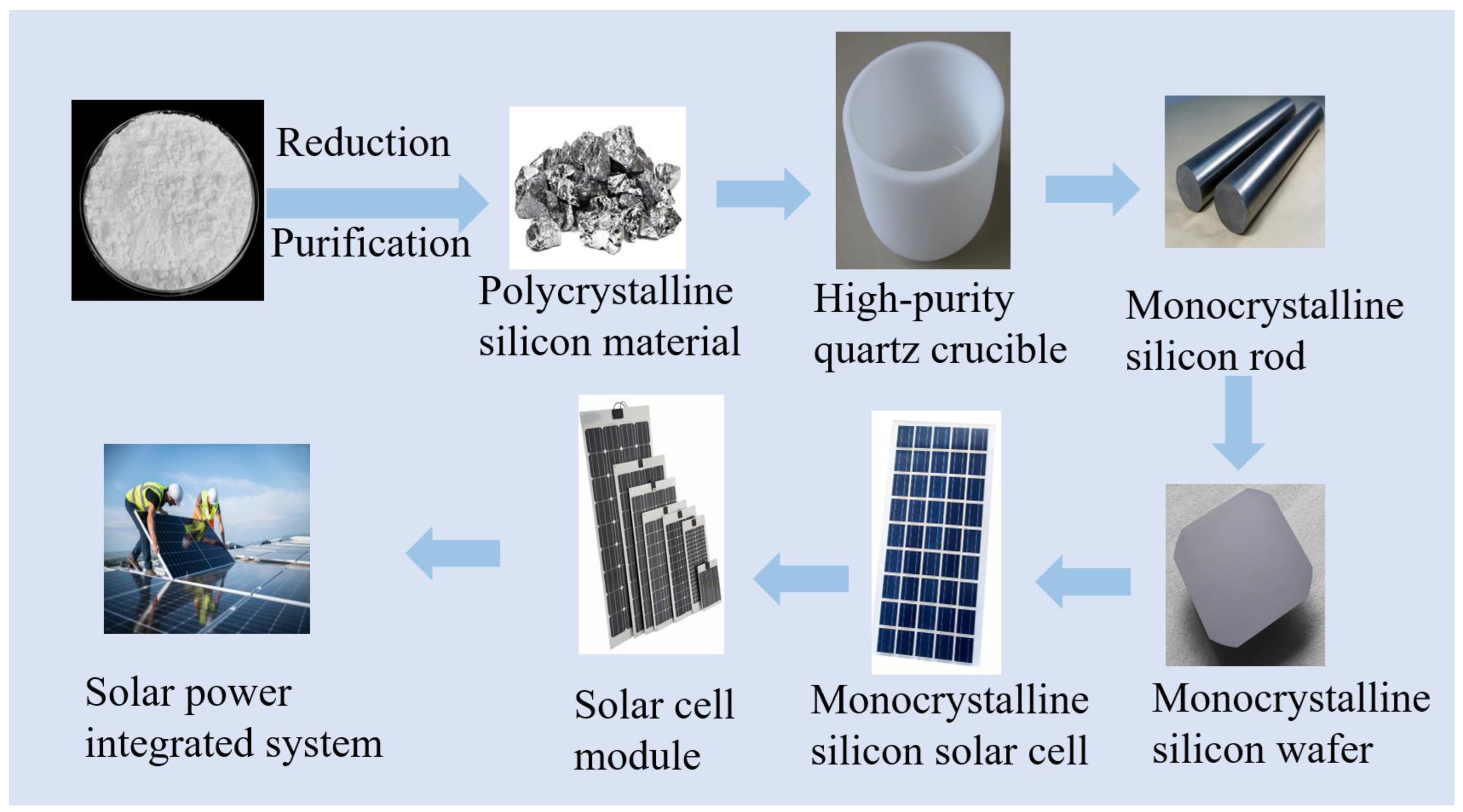
Silicon holds significant value in the photovoltaic industry and is a crucial material in the production of solar cells. Solar cells are devices that convert sunlight into electricity, primarily by utilizing semiconductor materials to harness the generated electron–hole pairs through the photovoltaic process [23]. High-purity crystalline silicon, a semiconductor material, plays a pivotal role in solar cell manufacturing, serving as the foundation for both N-type and P-type silicon wafers used in solar cell fabrication [24]. The production of high-purity crystalline silicon for the photovoltaic industry involves a series of meticulous processes, including raw material selection, refining, crystal growth, and wafer slicing. Among these, crystal growth stands out as a critical step in the production of high-purity crystalline silicon. Typically, methods such as the Czochralski (CZ) or Float Zone (FZ) technique are employed to grow single-crystal silicon rods [25]. Figure 3 illustrates the process flow of photovoltaic cells manufactured using high-purity quartz.
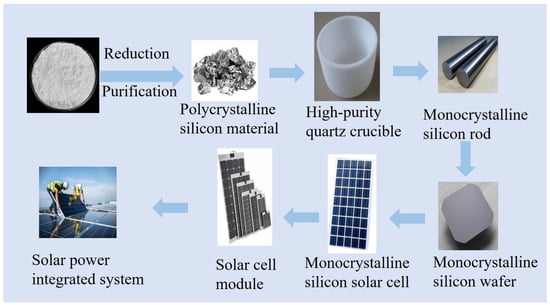
Figure 3.
Flowchart of photovoltaic cell production using high purity quartz.
With the exponential growth of China’s burgeoning new energy industry, the photovoltaic sector has experienced continuous expansion. This burgeoning sector has seen an escalating need for significant quantities of crystalline silicon materials, with silicon materials comprising as much as 80% of the photovoltaic industry in China. During the production of photovoltaic cells, stringent requirements are imposed regarding the metallic impurity content in quartz sand, particularly in relation to crystalline silicon materials [26]. In the photovoltaic sector, the impurity levels in quartz sand must be rigorously controlled to exceedingly low standards. Particularly, the content of iron (Fe) must be less than 5 ppm because iron impurities absorb ultraviolet light and cause the recombination of photogenerated carriers (electrons and holes), thus reducing the efficiency of photovoltaic devices. Alkali metals such as sodium (Na) and potassium (K), as well as phosphorus (P), even in trace amounts, can migrate during high-temperature processes, impacting the performance and lifespan of photovoltaic components. Additionally, impurities like calcium (Ca) and phosphorus (P) can affect the stability of quartz materials under high temperatures, which is crucial for ensuring the long-term reliability of photovoltaic devices. The total impurity content must be kept below 300 ppm to meet the stringent manufacturing standards for photovoltaics, where such controls are particularly critical. To address these demands, the utilization of high-purity quartz sand as a foundational raw material for quartz crucibles has emerged as an ideal choice, primarily due to its outstanding heat-resistant properties [27]. It is noteworthy that both quartz crucibles and crystalline silicon materials fall within the category of silica-based materials. Consequently, during the high-temperature refining process of silicon crystals, quartz crucibles effectively serve to prevent contamination of the silicon crystals. This wide-ranging application of quartz crucibles within the photovoltaic industry, where they are employed for silicon crystal refinement and in the production processes of silicon wafers and silicon rods, has positioned them as vital consumables and integral process materials within the photovoltaic sector [28]. Solar energy, characterized as a sustainable and environmentally friendly energy source, is projected to witness a notable upsurge in utilization within the context of the burgeoning new energy landscape. In accordance with forecasts, it is anticipated that by 2040, solar power generation will represent a substantial share, potentially accounting for up to 20% of the total electricity generation. In light of this trajectory, high-purity quartz sand, serving as a foundational material in the solar photovoltaic industry, is poised to experience further amplification in market demand as the photovoltaic sector continues its rapid expansion [29].
2.2. Electronics Technology Industry
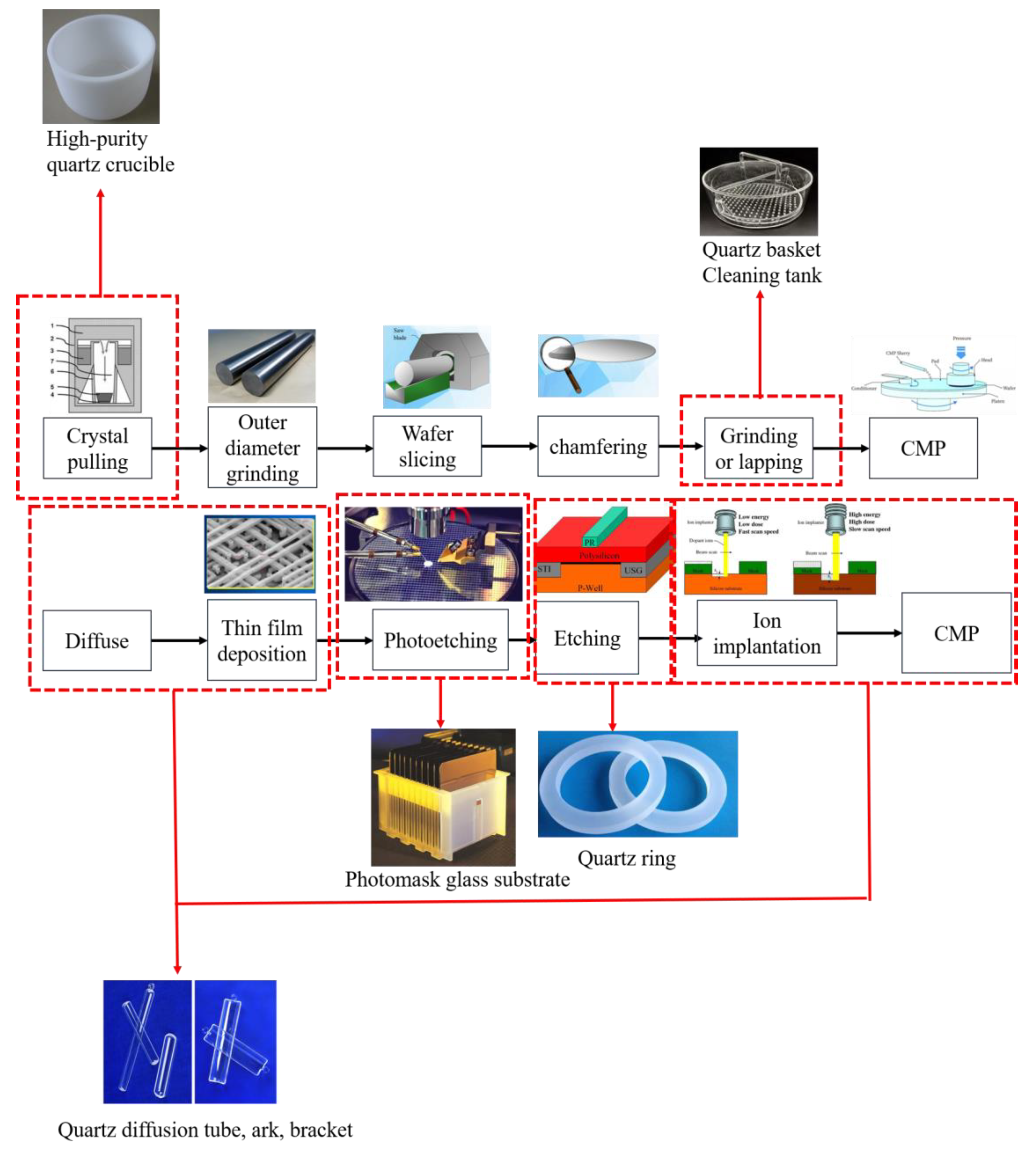
Historically, the supply of high-purity quartz has been dependent on imports from abroad. However, due to significant advancements in quartz production technology, which have been spurred by targeted research within the industry, domestic manufacturing capabilities have seen substantial improvements in recent years [30]. As a result, the domestic market not only fulfills its own demands but also produces a surplus, enabling exports to international markets. Yet, with the ongoing rapid advancements in the integrated circuit (IC) industry, marked by an increase in component integration, the specifications for high-purity quartz sand have become more stringent. These specifications include enhanced radiopurity, higher levels of chemical purity, and specific particle shapes, elevating the challenges faced in producing this crucial material. Figure 4 illustrates the application of high-purity quartz at different stages of semiconductor fabrication.
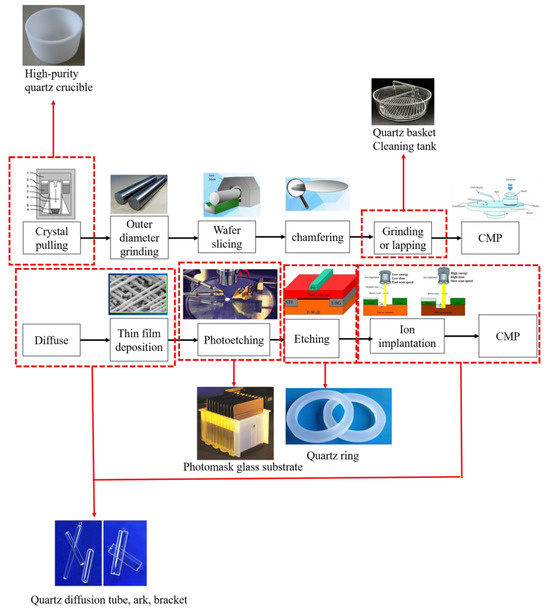
Figure 4.
High-purity quartz used in various stages of semiconductor preparation.
In the semiconductor manufacturing domain, the use of quartz crucibles or high-purity quartz components with elevated impurity levels can lead to several detrimental effects on the production and functionality of semiconductor devices. Impurities such as aluminum, boron, and phosphorus, even in minuscule quantities, can act as unintentional dopants, altering the electrical properties of silicon wafers. These alterations can result in undesirable variations in doping concentrations, leading to inconsistencies in the electrical performance of semiconductors across a wafer. Furthermore, thermal impurities such as calcium and magnesium can compromise the structural integrity of the quartz used in crucibles. During high-temperature processes typical in semiconductor fabrication, these impurities can lead to the formation of microcracks in quartz crucibles, exposing the silicon to potential contamination during the crystal growth process. Such structural defects not only shorten the service life of the crucibles but also increase the risk of producing flawed silicon ingots, which can result in substantial yield losses. Therefore, maintaining the exceptional purity of high-purity quartz is crucial to prevent contamination. Similarly, its ability to withstand high temperatures, including resistance to thermal shock and stability, is vital for enduring the harsh conditions of the Czochralski process and subsequent rapid thermal treatments during wafer processing. These processes involve high-temperature gradients and rapid heat transfer rates that can easily damage materials without sufficient thermal stability. Moreover, in cleanroom environments for wafer processing, the ability of high-purity quartz to act as an effective barrier against impurity contamination is indispensable for maintaining the integrity of semiconductors, as any extraneous particles can result in chip failures. Thus, the properties of high-purity quartz are essential for ensuring the high standards required for semiconductor manufacturing, directly influencing the efficiency and reliability of the semiconductors produced [31].
2.3. Glass Industry
The glass industry is a significant application area for quartz materials, which impart many outstanding properties to silicate glass and its products, such as exceptional transparency, high mechanical strength, and reliable chemical stability [14]. The SiO2 content in the raw materials plays a crucial role in determining the performance of the final silicate glass products [32]. Moreover, the purity and transparency of high-purity quartz have a paramount impact on the performance and stability of optical devices. Therefore, in advanced optical applications, premium-quality quartz crystals find widespread use in demanding optical instruments such as high-definition and high-transmittance optical lenses, high-precision microscopy equipment, quasi-molecular laser optical devices, and filters, among others [33]. In the manufacturing process of high-quality glass and optical devices, even trace amounts of impurities in quartz can significantly compromise the material’s integrity and functionality. Impurities such as aluminum (Al), iron (Fe), and titanium (Ti) are particularly influential. Aluminum can introduce unwanted coloration and decrease the thermal resistance of the glass, which is critical for applications involving high thermal stresses, such as in certain optical fibers and glass used in high-temperature environments. Iron, even in minimal concentrations, can severely affect the optical clarity of glass by imparting a greenish tint, which is detrimental for applications requiring high light transmittance, such as in solar panels and precision optical instruments. Additionally, iron impurities can increase the photosensitivity of glass, leading to degradation when exposed to ultra-violet light. Titanium, while useful in specific contexts for its refractive properties, can cause problems when present as an impurity in quartz used for optical applications. It can lead to the scattering of light within the glass, reducing the overall transparency and effectiveness of optical lenses and filters. Moreover, the presence of titanium can also affect the viscosity of the glass melt, complicating the manufacturing process. Other impurities, such as calcium (Ca) and magnesium (Mg), though generally less harmful, can still play a role in the quality of glass. These elements can influence the melting temperature and viscosity of the glass, affecting the energy efficiency of the glass production process and the quality of the final product. Calcium and magnesium can also promote devitrification, where the glass loses its amorphous structure and begins to crystallize, thereby reducing the mechanical strength and transparency of the glass. To mitigate these issues, stringent control of impurity levels is essential. This includes the implementation of advanced purification techniques such as flotation, acid leaching, and magnetic separation to reduce the presence of detrimental impurities to acceptable levels (Sources: https://www.iqsdirectory.com/articles/glass-cutting/quartz-glass.html (accessed on 22 May 2024), and https://www.pgo-online.com/intl/faq/optical-quartz-glass-faq.html (accessed on 22 May 2024)).
2.4. Chemical Industry
In the chemical industry, the application requirements of quartz sand differ somewhat from those of typical glass, ceramics, and refractory materials [34]. Nevertheless, quartz sand is essential in the production of products such as water glass, silica gel, and sodium silicate. Additionally, quartz sand plays a significant role as an industrial filler and is widely used in organic polymer materials such as plastics, rubber, coatings, paints, and adhesives to enhance their physical and chemical properties [35]. For instance, incorporating quartz sand into plastics can improve their compressive and tensile strength.
Quartz plays a predominant role as a glassy component in ceramic glazes, imparting a range of outstanding physical and chemical properties to ceramic glaze surfaces, such as strength, glossiness, wear resistance, hardness, and chemical resistance [36]. Moreover, an increased SiO2 content in the glaze can elevate its melting temperature and viscosity [37]. Fused quartz ceramics, utilizing fused quartz or quartz glass as raw materials, are produced through processes involving raw material crushing, grading, ball milling for slurry preparation, and then casting or slip casting, followed by sintering [38]. Fused quartz ceramics exhibit numerous excellent properties, including a low thermal expansion coefficient, exceptional thermal shock stability, good electrical insulation performance, and resistance to acid and alkali corrosion [39]. Since their inception, fused quartz ceramics have gained widespread promotion and application globally. Initially, they were employed primarily as low-grade refractory materials in some traditional fields such as metallurgy and the glass industry. However, with the relentless efforts of researchers, the performance of fused quartz ceramics has experienced rapid improvements and enhancements. Today, they find extensive use in high-tech sectors, including the atomic energy, aerospace, and defense industries [40].
3. Impurity Types and Analysis Techniques
3.1. Impurity Types
3.1.1. Atomic Defects (Point Defects)
Atomic defects, or zero-dimensional point defects, include vacancies and substituting elements at silicon lattice positions or interstitial sites. These defects can significantly affect the quality of quartz. Common substituting elements are aluminum (Al), iron (Fe), boron (B), and titanium (Ti), which replace silicon (Si) within the quartz lattice, forming new tetrahedral structures [41,42].
3.1.2. Line Defects
Line defects, or one-dimensional defects, such as dislocations, can occur due to stress during the quartz crystal growth process. These defects can propagate and affect the mechanical properties of the crystal.
3.1.3. Plane Defects
Two-dimensional defects include grain boundaries and small-angle grain boundaries. These defects occur where different crystalline orientations meet, impacting the crystal’s overall integrity and purity.
3.1.4. Volume Defects
Three-dimensional defects, such as inclusions of minerals, gases, and liquids, are common in natural quartz. These inclusions can significantly impact the purity of high-purity quartz products.
3.1.5. Fluid Inclusions
Fluid inclusions are commonly found in minerals and rocks, with their abundance typically ranging from approximately 102 to 109 per cubic centimeter and diameters generally not exceeding 50 μm. The types, sizes, and contents of these fluid inclusions have a significant impact on the quality of high-purity quartz and therefore require in-depth research and control. Based on the nature of the fluid within these inclusions, they can be categorized as pure gases, pure liquids, gas–liquid hybrids, or three-phase inclusions [43]. During their formation, these inclusions capture supersaturated solutions, and as the temperature decreases, these solutions crystallize into minerals such as halite, sylvite, and various silicate minerals. Consequently, fluid inclusions contain various impurities, including Na, K, and Ca, making them a major source of impurities in high-purity quartz products [44].
3.1.6. Silicate Melt Inclusions
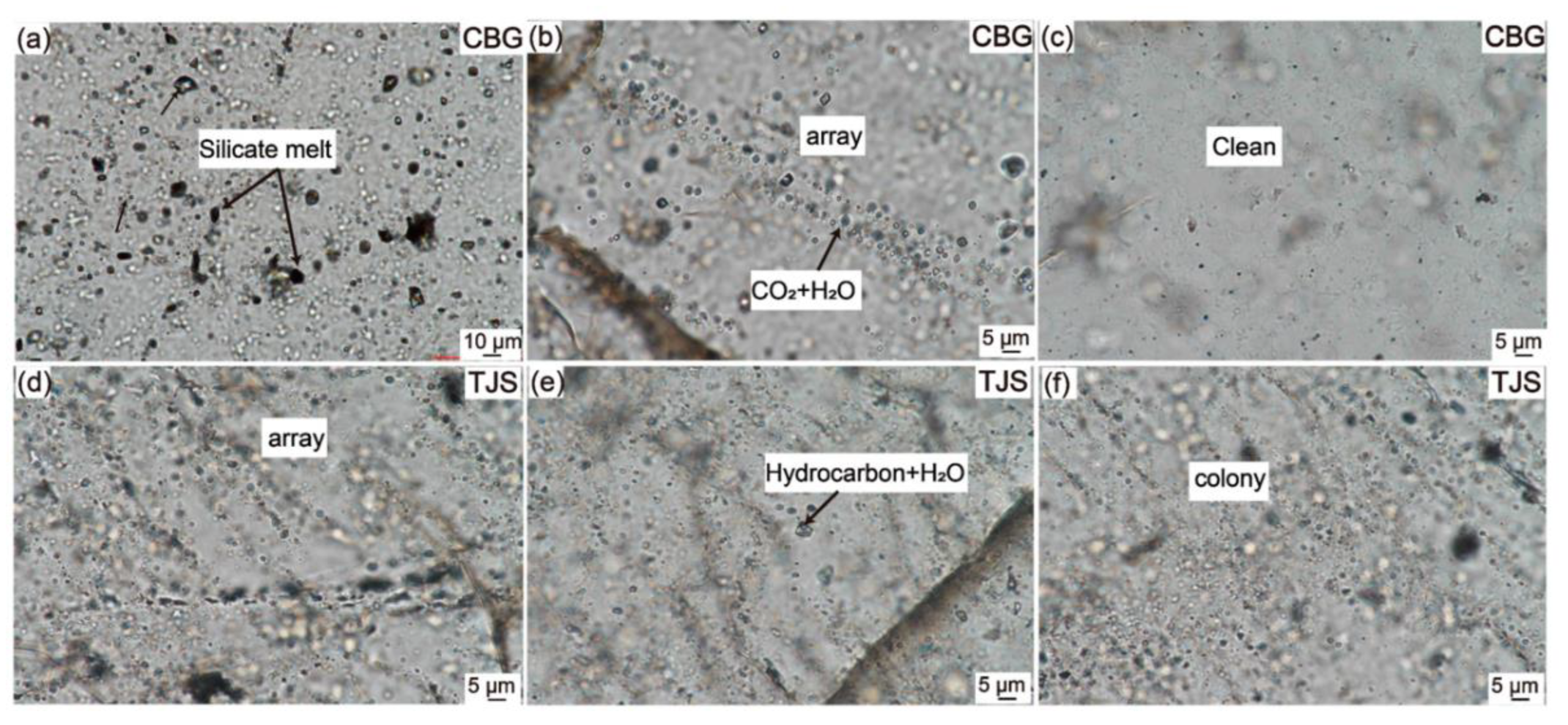
Silicate melt inclusions, on the other hand, are minute bubbles formed within quartz minerals found in igneous and granitic rocks [45]. They exhibit either a glassy or microcrystalline state and are relatively rare. These inclusions typically exist in crystalline form and are often intermingled or concealed within fluid inclusions, making it challenging to differentiate them from the surrounding rock, such as granite and granodiorite [46]. Figure 5 offers a visual representation of the fluid and silicate melt inclusions present in CBG (Chibougamau Quartz Vein in Canada) and TJS (Tianjingshan Quartz Vein in China) raw vein quartz. The chemical composition of silicate melt inclusions is similar to that of silicate melts, primarily consisting of elements such as Si, Al, Fe, Ca, Na, and K. Additionally, they may contain elements like F, Cl, B, P, Li, Cs, and Rb, with concentrations sometimes reaching several percentage points [47]. Therefore, silicate melt inclusions are a significant source of impurities in the production of high-purity quartz sand from granitic rocks. Despite extensive research on reducing the fluid inclusion content in quartz over the years, challenges remain, especially in eliminating gas-rich phases and tiny-sized fluid inclusions [48]. Hence, selecting quartz with minimal or no fluid inclusions is crucial for the preparation of high-purity quartz.
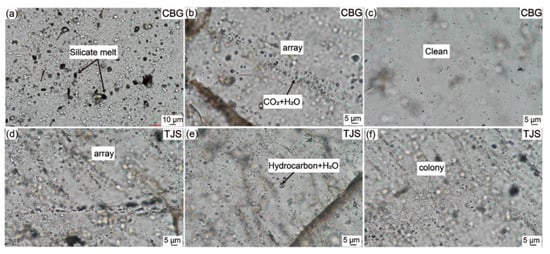
Figure 5.
Photomicrographs (TPM) of the silicate melt and fluid inclusions in CBG and TJS raw vein quartz. (a) Silicate melt inclusions in CBG quartz. (b) Fluid inclusions composed of CO2 and H2O and array in CBG quartz. (c) No inclusions in some areas in CBG quartz. (d) Array of some fluid inclusions in TJS quartz. (e) Fluid inclusions composed of hydrocarbon and H2O in TJS quartz. (f) The colony spherical fluid inclusions in TJS quartz [49]. CBG (Chibougamau Quartz Vein in Canada), TJS (Tianjingshan Quartz Vein in China).
3.1.7. Quartz Impurity Type
We have meticulously documented the occurrence patterns and various forms of impurity elements in quartz, as presented in Table 2. This study delves deeply into the complex interactions of these impurity elements within the crystalline structure of quartz and categorizes them into two primary categories: lattice substitution impurities and charge-compensating impurities.

Table 2.
Occurrence state and existence form of different impurity elements in quartz.
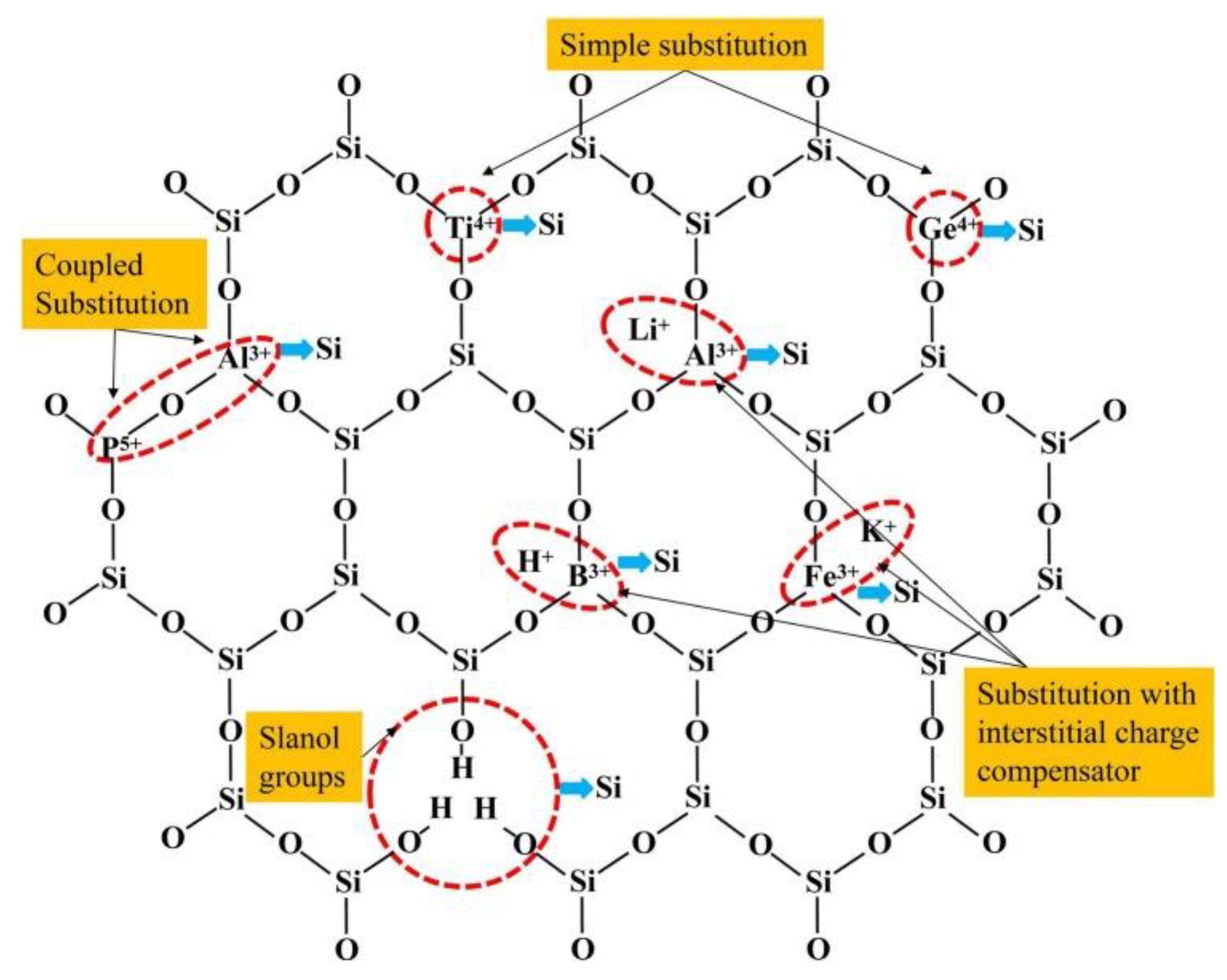
Lattice substitution impurities involve elements like Al3+ (aluminum), Fe3+ (iron), B3+ (boron), Ti4+ (titanium), and Ge4+ (germanium) [50]. These impurities substitute Si4+ (silicon) within the quartz lattice, leading to the creation of new tetrahedral formations. Among these, aluminum (Al3+) substitution is notably prevalent in natural quartz, exerting a substantial influence on its quality. Furthermore, recent research has demonstrated that B3+ can also replace Si4+ and form boron tetrahedra within the quartz structure [51]. As illustrated in Figure 6, the distribution of these impurity ions within the lattice of vein quartz showcases the variety and positioning of these elements. Charge-compensating impurities come into play to maintain electrical neutrality within the quartz lattice [52]. They encompass monovalent ions such as Li+ (lithium), Na+ (sodium), K+ (potassium), and H+ (hydrogen), as well as occasionally trivalent P5+ (phosphorus). These ions infiltrate interstitial positions, effectively counterbalancing charge discrepancies stemming from lattice substitutions. Their relatively large ionic radii (ranging from 0.078 nm to 0.178 nm) confine them to the interstitial positions, facilitating diffusion both into and out of the quartz lattice [53]. Additionally, the presence of hydrogen (H) can lead to the formation of water molecules within the quartz lattice, impacting its melting temperature and overall quality.
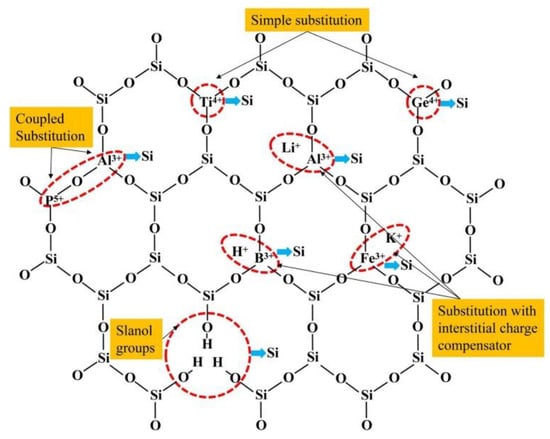
Figure 6.
Distribution of impurity ions in the lattice of vein quartz [54].
A meticulous comprehension of these impurity mechanisms is paramount for the precise evaluation of quartz quality and its suitability for diverse applications. This knowledge contributes significantly to the quality control and processing techniques in the critical quartz industry.
3.2. Analytical Technique
This section delves into the critical analysis of impurity elements, with a focus on Fe, Al, and other contaminants, and their role in determining high-purity quartz quality. Furthermore, we elucidate the performance benchmarks outlined in the key new materials application demonstration catalog (2019 Edition) of China, emphasizing the necessity for impurity levels, such as Fe, Mg, Cr, Ni, Cu, Mn, Ca, Al, Na, Li, K, and B, to remain below 6 ppm (6 × 10−6). Notably, Al and Fe stand as the most pernicious impurities, underscoring their minimal tolerance in high-purity quartz. The paramount objective in high-purity quartz processing is the exclusion of Al, Fe, B, Ca, Mg, K, Na, and other impurities, with the cumulative impurity content not surpassing 50 ppm.
We present various micro-scale analytical techniques for studying the mineral composition and trace elements in quartz raw materials, essential for selecting suitable feedstock and guiding further research. When it comes to the analysis of mineral components and trace elements in high-purity quartz raw materials, several commonly used testing methods and techniques provide comprehensive insights:
- (1)
- Electron Probe Micro-Analysis (EPMA): EPMA is a suitable in situ microbeam analysis technique for analyzing trace elements in quartz, especially for elements like Ti and Al at low levels [55]. However, its usefulness for high-purity quartz is limited as the element contents are often too low for accurate measurement.
- (2)
- Secondary Ion Mass Spectrometry (SIMS): SIMS offers extremely high sensitivity and analytical precision, capable of measuring many elements at low ppm levels, including hydrogen (H) in quartz [56]. However, its spatial resolution is relatively lower compared to EPMA. Additionally, there is a lack of high-quality matrix-matched external standard reference samples for SIMS, resulting in longer analysis times and higher costs.
- (3)
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): ICP-MS has low detection limits and sufficiently high precision. It allows for the simultaneous analysis of multiple elements and offers a relatively high analysis speed and cost-effectiveness [57]. Nevertheless, ICP-MS is susceptible to interferences from different elements, fractionation effects, and memory effects, with a relatively lower spatial resolution.
- (4)
- X-ray Diffraction Data Fitting and Refinement Analysis: the fitting and refinement of X-ray diffraction data of quartz raw materials can calculate cell parameters, allowing for an assessment of the degree of lattice substitution [58].
- (5)
- LA-ICP-MS Image Scanning Analysis: LA-ICP-MS is used for local chemical analysis and image scanning, identifying areas of element enrichment and planar distribution within minerals [59].
- (6)
- Infrared Absorption Spectroscopy Analysis: Infrared spectroscopy absorption peaks can reflect the extent of lattice substitution [60]. It can be used to detect microclusters and structural water molecules in quartz with low lattice impurity content [61].
- (7)
- Cathodoluminescence (CL) Feature Analysis: CL provides sensitive information on crystal type, lattice defects, orderliness, microstructure, impurity elements, physicochemical conditions of crystal growth, and various external influencing factors. In general, instruments in use are optical microscopes equipped with an electron gun (OM-CL), scanning electron microscopes (SEM-CL), and electron microprobes [62]. Important applications of CL imaging are the identification of minerals and the visualization of their real structure and crystal chemistry (internal textures, zonal growth, etc.). In addition, CL spectroscopy is an effective method for the spatially resolved analysis of point defects (lattice defects or incorporated trace elements) responsible for the luminescence signal in solids. The combination of CL imaging and CL spectroscopy is the outstanding means of understanding the information that may be derived from CL images, to characterize the defect structure of minerals and materials, or to detect the distribution of certain trace elements in quartz (e.g., Al3+, Ti4+, Li+) not detectable by other analytical methods [63].
Through these analytical methods, highly sensitive and detailed in situ elemental data can be obtained [64]. This knowledge, indispensable for the production of high-purity quartz materials, not only informs processing techniques but also ensures high standards of quality control in this critical industry.
In conclusion, the amalgamation of advanced analytical techniques, as delineated in this section, empowers researchers and industry professionals to gain a nuanced understanding of impurity distribution and mineral composition in quartz raw materials. These analytical methods are essential for the production of high-purity quartz materials, ensuring not only the refinement of processing techniques but also upholding the highest standards of quality control in this critical industry.
4. Processing Methods and Techniques
4.1. Acid Leaching Process
The quartz purification process comprises a series of intricate steps, including calcination, water quenching, grinding, screening, sorting, magnetic separation [65], flotation [66], acid leaching, ultrasonic treatment, chlorination roasting, and more [67,68]. Each method is meticulously selected based on the specific characteristics of impurities, with the ultimate objective of achieving effective purification [67,69]. While the comprehensive utilization of these methods augments quartz purity, it concurrently exposes inherent limitations as the process scales up.
For instance, the incorporation of hydrofluoric acid (HF) during the acid leaching process serves as an efficient means of eradicating impurities; however, it carries a concomitant reduction in quartz recovery rates [70]. The sizing of screened quartz particles can diminish to the micron level, thereby posing potential challenges related to dust pollution [71]. Hence, in the pursuit of environmental sustainability and green purification practices, the enhancement of quartz purity has emerged as an increasingly emphasized concern [72].
Calcination and water quenching are employed with the primary intent of disintegrating inclusions, thereby facilitating subsequent purification processes [73]. Screening endeavors to ameliorate size-related effects, as a plethora of studies have demonstrated the pronounced influence of quartz particle size distribution on the efficiency of purification techniques, particularly flotation. The ideal particle size range for quartz typically encompasses a few micrometers to several hundred micrometers. Magnetic separation capitalizes on the disparate magnetic properties between iron-bearing impurities and quartz, effecting a successful separation. Notably, iron impurities within the native quartz ore often manifest in the form of hematite, thereby warranting the contemplation of robust magnetic separation methods. In recent years, certain researchers have harnessed superconducting high-gradient magnetic separation (S-HGMS) for iron removal, yielding momentous outcomes [74].
Acid leaching emerges as a formidable method for addressing impurities such as iron, aluminum, and inclusions [75,76]. It is frequently harnessed to expunge thin-film iron from quartz particle surfaces and iron impurities nestled within quartz particles, in addition to other deleterious impurities [77]. Given that quartz is generally impervious to acids (with the exception of HF), the hallmark feature of acid leaching lies in the dissolution of other impurity minerals. Commonly employed acids encompass sulfuric acid, hydrochloric acid, nitric acid, hydrofluoric acid, and oxalic acid, among others [78]. Typically, the introduction of a reducing agent, such as ferrous sulfate, augments the leaching of impurity minerals [79].
Shao et al. [80] employed a mixed solution of 4 mol/L HCl, 1 mol/L HNO3, and 0.25 mol/L HF for the purification of 99.98% quartz. The acid leaching was carried out at 200 °C for 5 h, resulting in the final quartz with a purity of 99.994%. Li et al. [74] utilized the process of superconducting high-gradient magnetic separation (S-HGMS) coupled with fluoride-free mixed acid leaching to prepare quartz from high-silicon iron ore tailings. The optimal leaching conditions were a solid-to-liquid ratio of 1:4, leaching temperature of 80 °C, reaction time of 10 h, and a mixed acid solution of HNO3, HCl, and H2SO4 in a molar concentration ratio of 1:4:1. Under these conditions, the SiO2 purity reached a maximum value of 99.92%.
Zhang et al. [81] used phosphoric acid as the leaching agent for quartz sand under the conditions of 80 °C temperature, 10% solid-to-liquid ratio, quartz sand with a particle size of less than 100 mesh, and leaching for 120 min. It is worth noting that the addition of organic acids had unexpected effects on impurities like Al and Fe. Li et al. [82] leached quartz with a mixture of HCl and H2C2O4 under ultrasound at 80 °C, increasing the removal rate of Al to 53%, resulting in quartz samples with an Al content of 17.7 ppm. Aysenur Tuncuk et al. [83] applied a full factorial design and analysis of variance to evaluate the sulfuric acid leaching process. In the presence of reducing agents (oxalic acid, citric acid, and glucose), the best leaching conditions (slurry concentration of 20%, H2SO4 concentration of 0.5 M, oxalic acid concentration of 10 g/L, temperature of 90 °C, and leaching time of 120 min) led to the removal of 98.9% of Fe2O3. In the presence of oxalic acid, the final quartz product had an Fe2O3 content of 1 ppm. Compared to traditional stirring methods, this approach significantly accelerated the iron leaching process, reduced the leaching acid concentration, and improved the removal efficiency.
A substantial body of research underscores the existence of a synergistic effect engendered by the deployment of mixed acids and organic acids, proving markedly more efficacious in expelling diverse impurity metal ions compared to single acids. Mixed acids, in particular, exhibit superlative removal efficiency concerning lattice impurity ions. Acid leaching fundamentally represents a solid–liquid chemical reaction transpiring at the solid–liquid interface, epitomized by Equation (1).
aA(s) + bB(l) = cC(l) + dD(s)
In this equation, the liquid-phase reactant B typically signifies an acid, A denotes impurities or quartz mineral particles, while C and D designate products in the solid–liquid phase. Conventionally, this process is delineated into three sequential stages:
- (1)
- Adsorption of acid at the interface and cracks of quartz particles.
- (2)
- Chemical reactions between impurities, quartz minerals, and adsorbed acid at the interface.
- (3)
- Dissolution and diffusion of reaction products into the slurry.
The kinetics of these three stages hinge on an array of factors, inclusive of acid type, concentration, reaction temperature, particle dimensions, and agitation intensity [84]. For instance, the reaction of hematite on the surface of quartz particles with hydrochloric acid and sulfuric acid finds representation in Equations (2) and (3).
Fe (OH)3 + 3HCl → FeCl3 + 3H2O
2Fe (OH)3 + 3H2SO4 → Fe2(SO4)3 + 6H2O
The inclusion of hydrofluoric acid (HF) expedites the reaction rate of impurities such as mica and feldspar with quartz. However, in processes entailing HF, reactions transpire between HF and quartz, resulting in elevated quartz purity albeit at the cost of reduced quartz recovery rates. These reactions are delineated in Equations (4)–(6).
NaAlSi3O8 + 22HF → NaF + AlF3↓ + 3H2SiF6 + 8H2O
KAlSi3O8 + 22HF → KF + AlF3↓ + 3H2SiF6 + 8H2O
4SiO2 + 18HF → 3H2SiF6 + H2SiO3 + 5H2O
Moreover, oxalic acid, characterized by the chemical formula HOOC-COOH, serves as a dicarboxylic acid frequently enlisted for leaching quartz silicates [85]. In contrast to hydrochloric acid and sulfuric acid, oxalic acid possesses moderate potency and can expedite the dissolution of the iron oxide film on quartz particle surfaces, thereby engendering the formation of more stable chelate ions. This phenomenon arises from the fact that iron ions in oxalic acid harbor unbound electron pairs from iron, thereby permitting hybridization with d2sp3 and culminating in the creation of more robust inner chelate salts. Additionally, oxygen anions in oxalic acid evince augmented electronegativity in comparison to oxygen anions in quartz, thereby expediting the capture of surface-bound impurities. The chemical reactions between iron elements and oxalic acid are elucidated in Equations (7)–(9).
Fe3+ + [C2O4]2− → [Fe(C2O4)]+
[Fe(C2O4)]+ + [C2O4]2− → [Fe(C2O4)2]−
[Fe(C2O4)2]− + [C2O4]2− → [Fe(C2O4)3]3−
Furthermore, phosphoric acid (H3PO4) exhibits substantial chelating prowess concerning iron ions during the H+ ionization process. Even under analogous conditions, the aptitude of H3PO4 to expel iron impurities from quartz transcends that of H2C2O4. The chemical reactions between iron elements and phosphoric acid are elucidated in Equation (10).
8H+ + 4 PO43− +Fe2O3 → [Fe(PO4)2]3− + [Fe(HPO4)2]− + 3H2O
Li et al. [86] used a strategy combining superconducting high-gradient magnetic separation (S-HGMS) with acid leaching techniques. Employing a mixed acid ratio of 5 mol/L HCl and 1 mol/L HF, at a leaching temperature of 353.15 K, and a duration of 12 h, they achieved remarkable removal rates for Fe, Al, Mg, and Ca, recorded at 95.20%, 85.60%, 97.99%, and 97.19%, respectively. The study also incorporated an in-depth investigation into the leaching kinetics of metallic impurities. The results indicated that the dissolution behavior of these impurities was predominantly governed by the nucleophilic action of HF, aptly described by the shrinking core model. Notably, the dissolution behavior exhibited significant variance across different impurities, offering a nuanced perspective into the mechanisms underpinning the removal of impurities during the mixed acid leaching process.
Further augmenting their methodology, Li et al. [87] implemented an ultrasound-assisted non-fluoride acid leaching technique. Utilizing a mixed acid composition of 4 mol/L HCl and 2 mol/L H2C2O4, and maintaining the reaction temperature at 80 °C, they conducted three leaching cycles. This approach led to the removal efficiencies of Al, Ca, Fe, and Mg surpassing 97%. These findings robustly validate the synergistic effect of mixed acids in the elimination of various metallic impurities, particularly highlighting the efficacy of combining organic acids with inorganic acids.
It is imperative to underscore that mixed acids and organic acids, as substantiated by a plethora of studies, exhibit a conspicuously more potent synergistic effect in the elimination of dissimilar impurity metal ions, particularly to the benefit of lattice impurity ions [88,89,90].
4.2. Calcination–Water Quenching Acid Leaching Process
The calcination–water quenching process plays a pivotal role in the purification of quartz, as it influences the efficiency of subsequent steps like magnetic separation, flotation, and acid leaching. This process involves heating quartz to a high temperature, followed by rapid cooling, inducing thermal stress that generates microcracks within the quartz structure. These cracks facilitate the exposure and removal of impurities embedded within the quartz matrix.
Ren et al. [91] compared the element contents of aluminum (Al) and iron (Fe) in the flotation concentrate, acid leaching concentrate, and final concentrate obtained through various process sequences involving calcination–water quenching. The analysis revealed that the positioning of the calcination–water quenching step significantly affects the removal of Al and Fe impurities.
The process enhances the purification results by making the quartz more receptive to subsequent treatments. In particular, the cracks induced by calcination–water quenching increase the specific surface area of quartz particles, thereby improving their interaction with leaching agents. This results in a more effective dissolution of impurities during the acid leaching stage. The findings indicate that the thermal stress generated during the quenching process not only exposes impurities but also modifies the physical structure of the quartz particles, making them more amenable to separation and chemical treatments.
Overall, the incorporation of the calcination–water quenching process into the quartz purification workflow significantly enhances the removal of metallic and non-metallic impurities, contributing to the production of high-purity quartz suitable for various industrial applications. Further detailed studies on the mechanism of this process are necessary to fully understand its implications and optimize its application in industrial settings.
4.3. Chloride Roasting Acid Leaching Process
Chloride roasting is a highly purified process that comes with high costs, limited processing capacity, and certain risks [92]. Currently, only the U.S.-based Sibelco corporation has successfully implemented this process in industrial applications. In China, some research institutions and companies have attempted it, achieving some success in laboratory tests, but it has not yet seen widespread adoption in large-scale industrial production. This process places high demands on raw materials, typically requiring traditional methods to purify quartz sand to achieve SiO2 purity levels of 99.99% with impurity levels below 100 × 10−6 to meet feedstock requirements.
During chloride roasting, impurities such as alkali metals and residual inclusions on the surface of quartz particles react with chlorine gas at high temperatures to form gaseous chlorides. Elements like Al and B have lower reactivity and can be carried away by the high-temperature gas stream, achieving deep purification. Typically, the main gas used in chloride roasting is Cl2, which can be used alone or in a mixture with HCl for calcination. Additionally, the introduction of high-purity nitrogen (N2) and argon (Ar) into the gas stream can reduce the burden of exhaust gas treatment. The equipment for chloride roasting usually needs to be designed according to specific requirements since there is currently no standardized equipment, and this process is continuously evolving and improving.
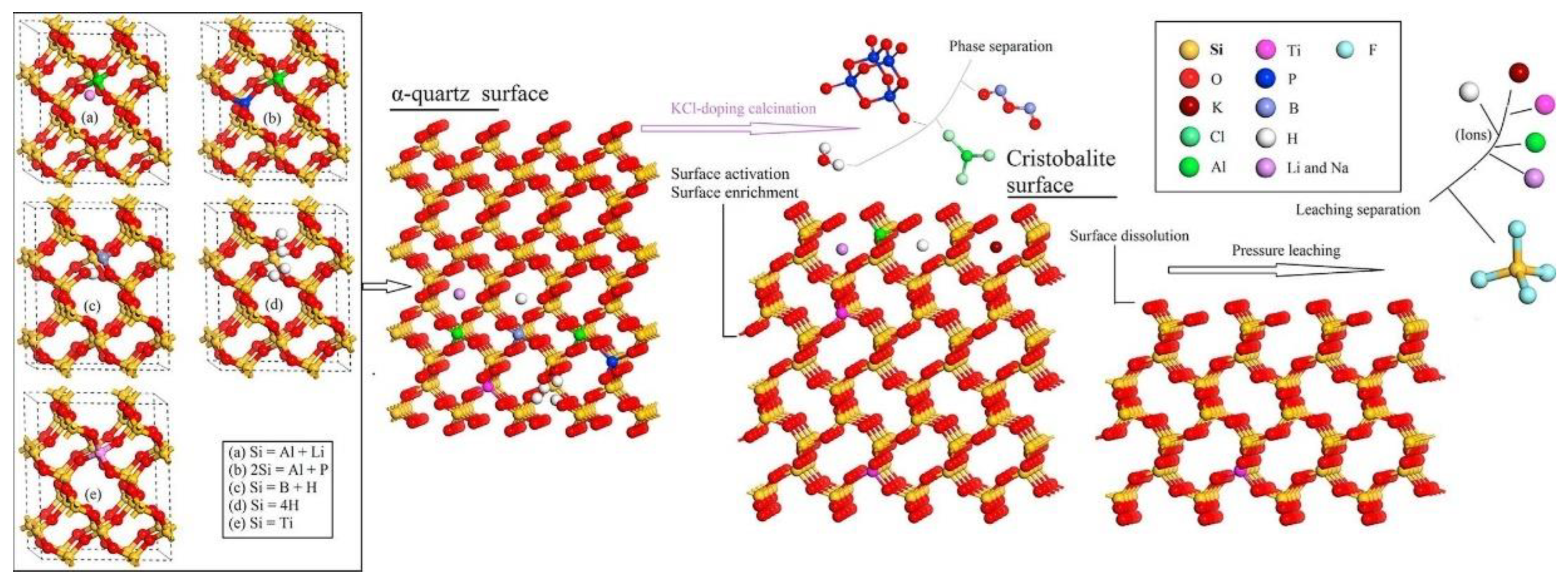
Chlorinating agents are divided into solid and gaseous chlorinating agents. Gaseous chlorinating agents include HCl and Cl2, while solid chlorinating agents encompass CaCl2, KCl, MgCl2, NaCl, and NH4Cl, among others. Lou et al. [93] conducted purification experiments on three quartz sand samples using a high-temperature chlorination process. The results show that dry HCl gas, Cl2, and a mixture of Cl2 and HCl all have a significant effect on removing metallic impurities like Na, Fe, and K. Dry HCl gas, in particular, demonstrated the best purification results. Under conditions of dry HCl gas at 300 g, a roasting temperature of 1000 °C, and a roasting time of 2 h, total impurities decreased from 37.81 ppmw to 25.87 ppmw. However, gaseous chlorinating agents come with demanding equipment requirements, and solid chlorinating agents provide an excellent alternative. Recent research indicates that some chlorinating agents have been used in these processes like NaCl chlorination roasting HF and HCl mixed acid leaching. Yan et al. [94] mixed quartz raw materials with 2 wt % NaCl, and then roasted at 820 °C for 2 h, and leached with 18% HCl and 2% HF at 50 °C for a certain duration. This process can reduce the iron content in quartz from 66.4 ppm to 0.8 ppm and the titanium content from 29.3 ppm to 5.5 ppm. Similarly, Lin et al. [95] employed 2 wt % KCl with quartz raw materials. They roasted the mixture at 900 °C for 45 h and conducted leaching with 3 mol/L H2SO4 and 0.5 mol/L HF in a sealed reactor at 200 °C for 4 h. This process decreased the iron content in quartz from 1.12 ppm to 0.143 ppm and the titanium content from 5.38 ppm to 4.18 ppm, reducing the total impurity content from 99.2 to 29.4 ppm. Lin also proposed the mechanism for separating trace elements bound in the crystal lattice during potassium chloride chlorination roasting and pressure leaching, as depicted in Figure 7. During roasting, α-quartz undergoes a phase transformation to beta quartz, releasing activated lattice elements. Due to a lower surface energy and the segregation of beta quartz, activated lattice elements accumulate on the surface of beta quartz. Non-metallic elements like H, B, and P, which are volatile oxides, can also be separated in beta quartz solid solutions. Some metal elements, especially Al, diffuse to the surface of the solid solution and then precipitate as low-boiling-point chlorides. Alkali metals like Li, Na, and K on the solid solution surface are removed via fluoride pressure leaching.
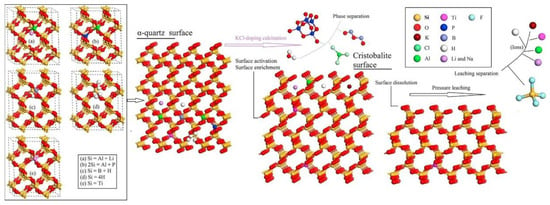
Figure 7.
Separation mechanism diagram of lattice-bound trace elements from quartz by KCl-doping calcination and pressure leaching [95].
While chloride roasting offers a promising avenue for high-purity material production, it is important to note that this process remains in the developmental stage, with several challenges to overcome before it can be widely adopted in industrial applications.
4.4. Microwave Thermal Treatment Acid Leaching
In recent years, the demand for high-purity silica, a critical component in applications like lighting and solar cell manufacturing, has surged, leading to the exploration of innovative purification methods. Conventional purification techniques often involve time-consuming thermal radiation and conduction heating processes, which can significantly delay the attainment of the necessary calcination temperature and thermal equilibrium. In contrast, microwave heating has emerged as a highly efficient and selective alternative [96]. This technology leverages the unique energy characteristics of microwaves, typically within the frequency range of 300 MHz to 300 GHz, enabling rapid and selective material heating [97]. The efficiency of microwave heating depends on the dielectric properties of the materials. Materials with higher dielectric constants are more susceptible to microwave heating, making it an attractive option for various mineral processing applications, especially in ore calcination and mineral purification, where selective heating can lead to enhanced energy efficiency.
A notable advantage of microwave heating is its ability to quickly reach thermal equilibrium due to the high penetration depth of microwaves. This rapid heating is attributed to the direct conversion of electromagnetic microwave energy into thermal energy, allowing materials to achieve thermal equilibrium in significantly less time compared to conventional methods. Additionally, microwave heating has proven effective in treating minerals with varying reactivity to microwaves. Certain minerals, such as magnetite, exhibit strong interactions with microwaves and are classified as highly reactive minerals, while others, like hematite, are considered moderately reactive, and silicon dioxide (silica) is generally considered non-reactive to microwaves.
The potential of microwave heating in mineral processing has been demonstrated in recent studies. A.J. Buttress et al. [98] introduced an environmentally friendly method for producing quartz powder from quartz pebbles initially containing impurities at a level of 158 ppm. This achievement was realized through a metallurgical upgrading process that involved microwave pretreatment, crushing, milling, high-intensity wet magnetic separation, and acid leaching. The outcome was a remarkable 80% reduction in residual impurities compared to untreated quartz pebbles. The selective heating of micro-fluidic inclusion sites containing impurities played a crucial role, causing their explosive decrepitation and facilitating subsequent impurity removal.
Building upon this success, Wangfeng Song et al. [99] explored the synergistic combination of microwave selective heating with NH4Cl as a chlorinating agent. Their approach involved low-grade quartz sand doped with NH4Cl, followed by microwave calcination and acid leaching. This method achieved a significant reduction in aluminum content from 738.8 ppmw to a mere 17.9 ppmw, alongside a decrease in total impurities from 1459 ppmw to 85.4 ppmw. These studies underscore the potential of microwave heating, especially when combined with suitable chlorinating agents, to enhance impurity removal and further elevate the purity of silica materials.
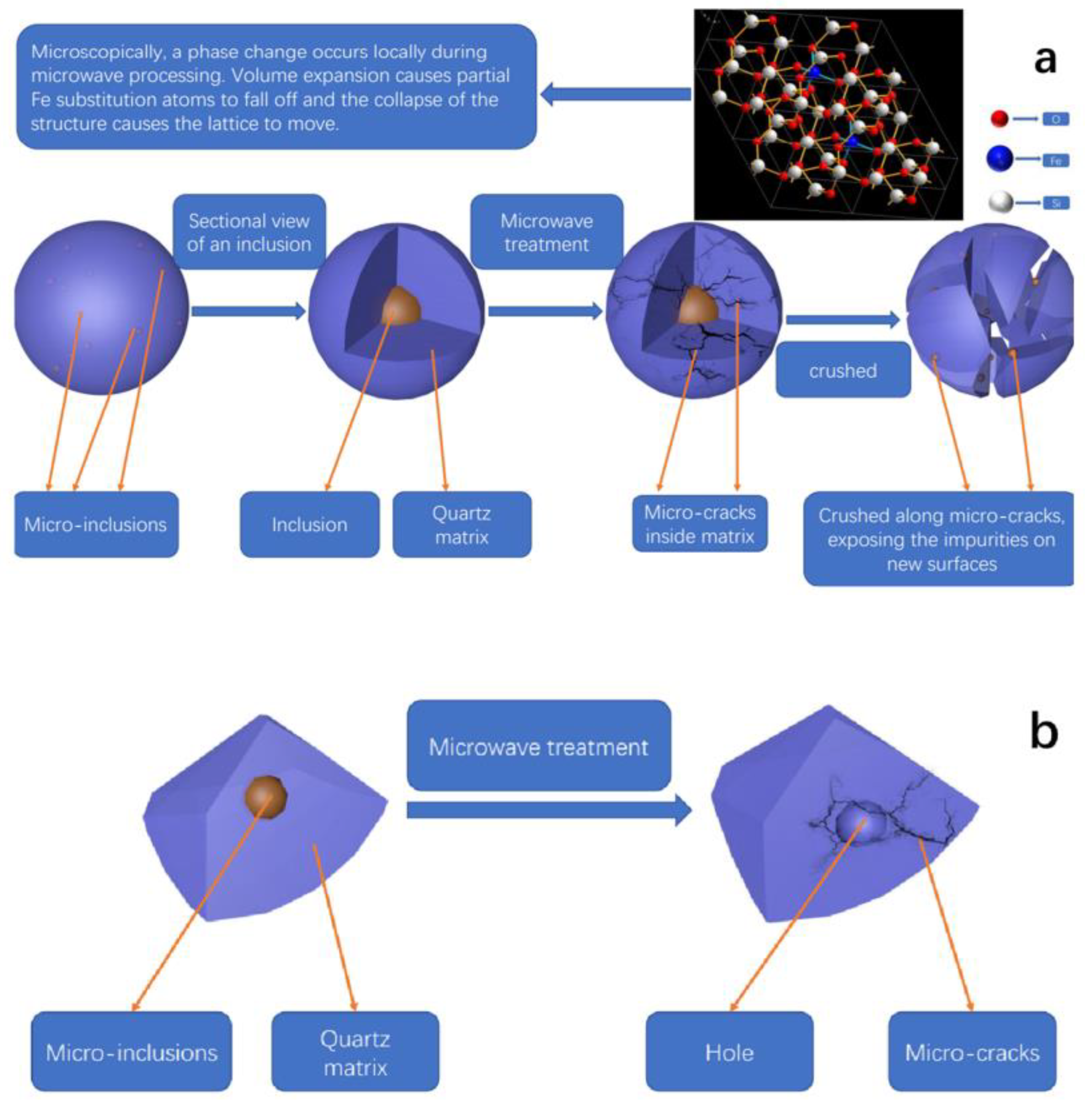
Li et al. [100] combined microwave heating with ultrasound-assisted acid leaching treatment, presenting a fast, energy-efficient, environmentally friendly, and straightforward method for purifying quartz. By leveraging the selective heating properties of microwaves, this unique technique induces micro-inclusions and cracks within the quartz matrix, as illustrated in the accompanying diagram. Figure 8 provides a schematic diagram illustrating the process of microwave treatment on quartz, detailing how the method targets and interacts with specific mineral components and impurities. Consequently, it enables effective impurity removal during subsequent processing steps while simultaneously limiting the diffusion of iron into the quartz matrix.
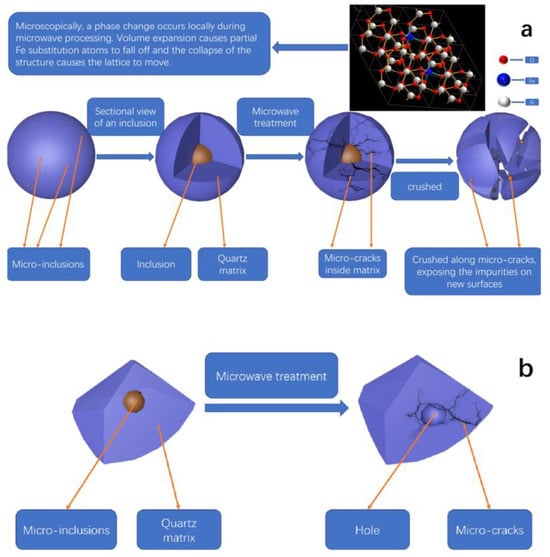
Figure 8.
The schematic diagram of microwave treatment [100]. (a) Process from micro-inclusions to the formation of micro-cracks and exposure of impurities after microwave treatment and crushing. (b) Formation of holes and micro-cracks in the quartz matrix due to microwave treatment.
However, it is crucial to acknowledge that the effectiveness of microwave heating depends on the dielectric properties of the involved materials. Materials with higher dielectric constants are more susceptible to the influence of microwave heating compared to those with lower dielectric constants. This inherent variability in dielectric properties plays a pivotal role in achieving selective heating through microwave technology. It is worth noting that different mineral compositions exhibit varying reactivity to microwaves. For instance, minerals like magnetite, characterized by strong interactions with microwaves, are classified as highly reactive minerals. In contrast, minerals like hematite are considered moderately reactive, while silicon dioxide (silica) is generally regarded as non-responsive to microwave energy. This intrinsic variability underscores the importance of tailoring microwave heating methods to suit specific mineral compositions.
These studies show that microwave chlorination remains a robust approach to significantly improve the efficiency and purity of silica purification. Its environmentally friendly, efficient, and controllable nature positions it as a promising avenue in the field of mineral processing and materials engineering. This advancement addresses the increasing demand for high-purity silica in various technological applications, including those critical to the future of clean energy and lighting technologies.
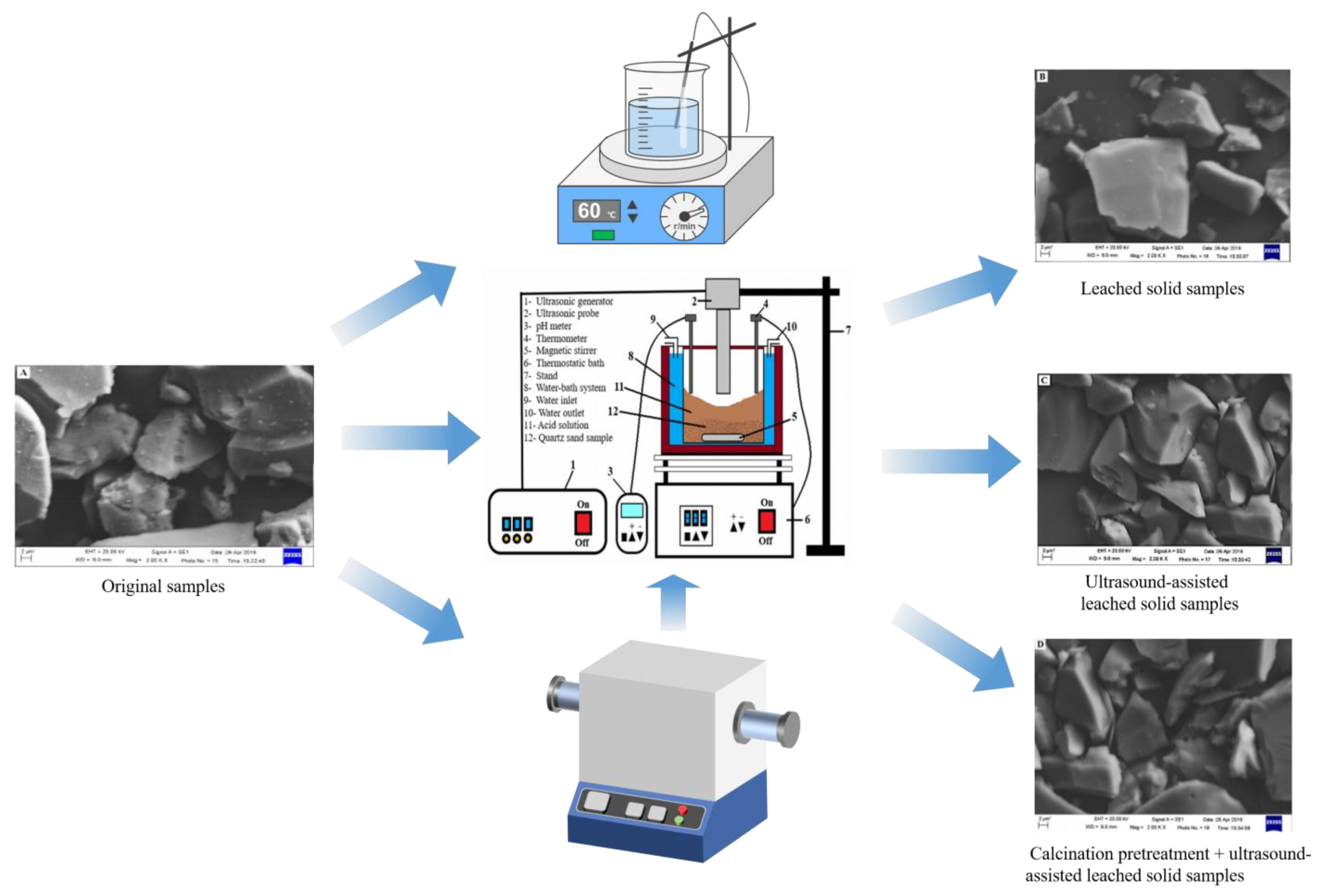
4.5. Ultrasonic-Assisted Acid Leaching
Ultrasonic waves are typically defined as sound waves with frequencies exceeding 20 kHz. At frequencies above 25 kHz, ultrasonic waves create microfields in liquids, crucial for enhancing ore processing efficiency [101,102]. Ultrasonically assisted ore beneficiation techniques significantly improve leaching rates and reduce leaching times. This enhancement is driven by the cavitation effect induced by ultrasonic waves, resulting in the formation of minute cracks on mineral surfaces, thereby boosting the dissolution efficiency of the employed chemical reagents [103,104].
Feihu Du et al. [105] employed oxalic acid with ultrasonic assistance for iron removal, achieving optimal conditions: a reaction temperature of 95 °C, stirring at 500 rpm, ultrasonic power of 150 W, acid concentration at 4 g/L, and a reaction time of 30 min, resulting in an impressive iron removal rate of 75.4%. However, in recent similar studies, Volkan Arslan et al. [106] proposed a process involving ultrasonically assisted acid leaching using a mixture of hydrochloric acid and oxalic acid. They employed the response surface methodology (RSM) to optimize and model the experimental parameters. By establishing a quadratic equation model, the study successfully described the relationship between the rate of iron removal and various experimental parameters. The accuracy of the model was verified through correlation coefficients. Furthermore, the study optimized the experimental conditions, including leaching temperature, leaching time, acid concentration, and ultrasonic power. Under these optimized conditions, an astounding iron removal rate of 95.08% was achieved. Simultaneously, the SiO2 content in the quartz concentrate significantly increased from 97.841% to 99.907%, while the Fe2O3 content decreased to as low as 0.011%.
In another study employing ultrasonically assisted acid leaching, Yang [107] introduced a composite process involving calcination pretreatment and ultrasonically assisted leaching, using a diluted mixture of hydrochloric acid and oxalic acid as the solvent. Under optimized experimental conditions, including calcination at 900 °C for 2 h, oxalic acid concentration of 10 g/L, hydrochloric acid concentration of 5%, liquid-to-solid ratio of 5, leaching temperature of 60 °C, ultrasonic power of 400 W, and a processing time of 30 min, the SiO2 content in the quartz concentrate was successfully increased from 97.6828% to 99.9047%. Simultaneously, the iron content significantly decreased from 0.0857% to 0.0223%. Figure 9 displays the SEM images before and after ultrasonication, revealing that nearly all the tiny particles attached or embedded on the quartz particle surface disappeared under the influence of ultrasonication. This phenomenon is attributed to the powerful pulse waves generated by ultrasonic waves, effectively dislodging mineral coatings from quartz sand into the liquid phase, making it easier for iron impurities to react with oxalic acid. However, the mechanism behind the ultrasonically assisted leaching of high-purity quartz impurities remains to be thoroughly investigated [107].
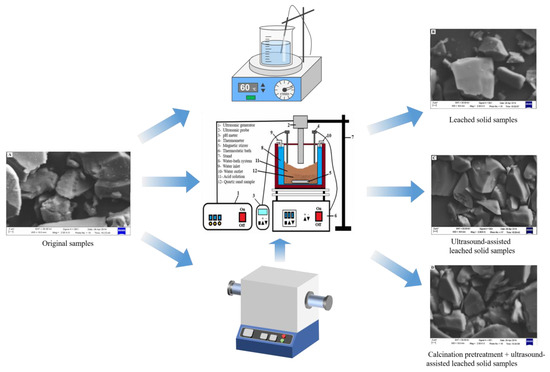
Figure 9.
SEM images of (A) original samples, (B) leached solid samples, (C) ultrasound-assisted leached solid samples, and (D) calcination pretreatment + ultrasound-assisted leached solid samples under different treatment methods [106,107]. Adapted and combined by Hailin Long.
Both ultrasonic and microwave techniques leverage energy to enhance chemical reactions but operate through different mechanisms. While ultrasonication relies on cavitation and acoustic streaming to create localized high-pressure zones, microwaves utilize dielectric heating to uniformly raise the temperature of the material. This can potentially lead to differences in impurity removal efficiency and energy consumption. Despite the notable removal of impurity minerals in quartz, such as inclusions and coatings, lattice impurities still prove challenging to eliminate completely. This process is currently at the experimental stage and requires further development before reaching industrial-scale implementation.
5. Discussion
To meet the growing demand for high-purity quartz, the careful evaluation and selection of purification methods tailored to specific process requirements are essential. Table 3 presents a comparative analysis of the merits and demerits of various techniques. The variability in raw materials often leads to a range of impurity types, which necessitates the customization of purification processes.

Table 3.
Comparison of quartz purification methods.
Table 4 provides a comprehensive overview of methods designed for treating distinct impurity categories. While the chlorination roasting process shows potential in producing high-purity quartz, its industrial application encounters several challenges. Significant challenges, such as cost factors, limited processing capacity, and safety concerns, hinder its widespread adoption [108]. To date, only the Sibelco Corporation in the U.S. has successfully implemented this technique. A comparison between gaseous chlorinating agents like HCl and Cl2 and solid counterparts such as NaCl and KCl merits attention, each carrying its unique advantages and disadvantages. The choice of chlorinating agent hinges on specific circumstances. Moreover, post-roasting acid leaching has significantly enhanced purification, lowering impurity levels further and demonstrating the synergistic effects of combining these two methods for various impurities. A profound understanding of chlorination roasting’s underlying mechanisms remains crucial. Existing research offers valuable insights but lacks depth. Future research should delve deeper into comprehensive mechanistic analyses and kinetics studies to enhance our understanding.

Table 4.
Methods of treating different impurity types.
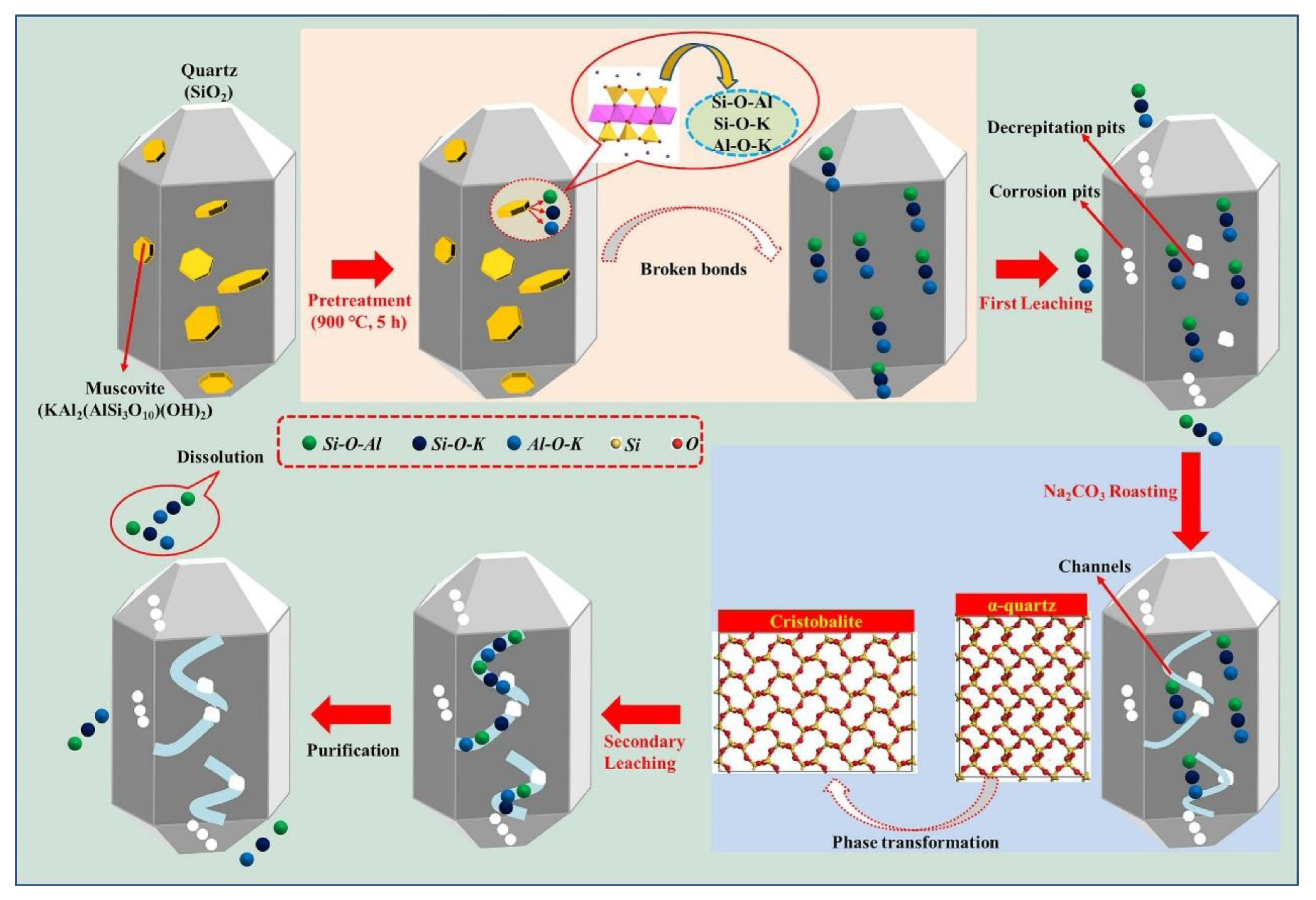
Li et al. [54] demonstrated that a combination of Na2CO3 roasting and H3PO4 hot-pressure leaching can purify vein quartz to achieve 99.995% SiO2. This process involves Na2CO3 roasting, which reduces the phase transition temperature and destroys the muscovite structure, followed by H3PO4 hot-pressure leaching to dissolve impurities. This innovative approach highlights the potential of alternative techniques in achieving the high purity levels required for industrial applications. Intriguingly, the lattice dynamics during Na2CO3 roasting facilitate a decline in the phase transition temperature from α-quartz to cristobalite, as illustrated in Figure 10. Crucially, this preparatory stage compromises the structural integrity of muscovite, augmenting the efficacy of the subsequent H3PO4 hot-pressure leaching process. This technique not only establishes a robust paradigm for the selective extirpation of muscovite impurities but also realizes a significant uptick in SiO2 purity. Harnessing a distinct raw material and pioneering processing, this innovative strategy enriches the current repertoire of quartz purification methodologies.
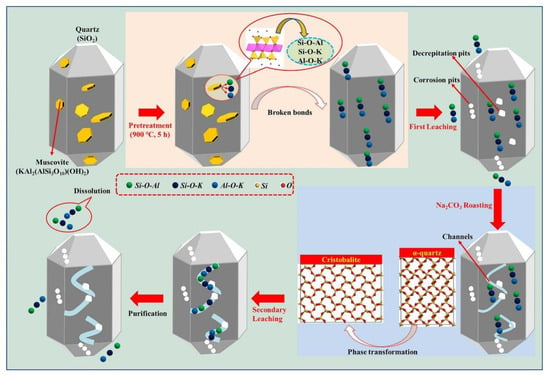
Figure 10.
Purification mechanisms via H3PO4 hot-pressure leaching and Na2CO3 roasting [54].
The environmental sustainability and safety implications of the chlorination roasting process warrant significant attention [109]. Efficient management of exhaust gases and solid waste is imperative, with ongoing efforts to improve eco-friendliness. Although equipment standardization is currently absent, future developments hold potential for meeting industrial demands. International collaboration and knowledge exchange within the chlorination roasting field can expedite its global application and development.
As underscored by recent studies, the judicious selection and management of chlorinating agents play a pivotal role in guaranteeing effective gas release and thorough impurity elimination [110,111]. This careful consideration not only bolsters purification efficacy but also contributes to the overall success of microwave-assisted chlorination processes. In summary, the chlorination roasting process is pivotal for achieving high-purity quartz. Addressing its challenges and refining its mechanisms is essential to meet the growing demand for high-purity quartz across various industries.
The ultrasonic-assisted acid leaching method offers notable benefits for the enhancement of quartz purity. By harnessing the power of ultrasonic waves, this technique considerably accelerates leaching rates, thereby reducing leaching time and optimizing production efficiency [87]. Furthermore, the selective heating attributes of ultrasound contribute to a decrease in energy consumption, positioning it as an environmentally conscientious option [112]. Despite its merits, this methodology faces certain hurdles, notably the elevated equipment expenses that might curtail its broader industrial uptake. Rigorous calibration of ultrasonic parameters is pivotal to achieve peak purification outcomes. Yet, the future prospects of this technique are undeniably bright, particularly for endeavors centered on the acquisition of supremely pure quartz.
Ultrasonic-assisted acid leaching necessitates in-depth research for a better understanding of its mechanisms and kinetics. Current research offers valuable insights but lacks comprehensive information. Future research directions may involve extensive mechanistic analysis and kinetics studies to enhance our understanding. In conclusion, the ultrasonic-assisted acid leaching process represents a potential high-purity quartz purification method with advantages in enhancing quartz purity and reducing energy consumption. While it presents certain challenges, ongoing research and development efforts have the potential to further improve its efficiency and sustainability, meeting the growing market demand for high-purity quartz.
6. Perspectives
With environmental regulations undergoing rapid transformation globally, the development of sustainable quartz purification processes has emerged as a pressing necessity. Recent advancements highlight the promise of cutting-edge waste acid treatment methodologies, such as solid-phase neutralization and membrane separation [113,114]. These approaches not only target a significant reduction in pollutant emissions but also advocate for the recycling of wastewater, thus markedly diminishing the dependence on primary freshwater sources. Beyond environmental considerations, technological innovations remain at the forefront. The upcoming decade is projected to unveil hybrid purification strategies that synergistically integrate the strengths of prevailing methods. For instance, combining acid leaching with ultrasonic-assisted acid leaching can significantly enhance impurity removal while reducing chemical usage and processing time.
Additionally, advancements in solid-phase extraction and selective adsorption using functionalized materials are expected to play a crucial role in the efficient separation of trace impurities. Exploring novel solvents and reagents that offer a higher selectivity and lower environmental impact will be critical in optimizing purification processes. Furthermore, there is substantial potential in probing alternative purification avenues, such as the use of ion-exchange resins and advanced oxidation processes. The pursuit of innovative materials or strategies custom-designed for the high-purity quartz domain is of paramount importance. Interdisciplinary research endeavors, amalgamating insights from materials science, chemical engineering, and nanotechnology, have the potential to catalyze revolutionary purification techniques. This includes developing nanomaterials with a high surface area and reactivity for more effective impurity adsorption and implementing green chemistry principles to design safer and more sustainable processes. These efforts will epitomize both operational excellence and environmental sustainability, ensuring the production of high-purity quartz meets the growing demands of advanced technological applications.
7. Conclusions
This review advances our understanding of high-purity quartz (HPQ) purification by exploring comprehensive methodologies that enhance impurity removal effectiveness and process sustainability. We have analyzed the behaviors of impurities like aluminum, iron, and sodium, which can undermine manufacturing processes in sectors such as photovoltaics, electronics, and optics. This study evaluates various purification technologies, including acid leaching, ultrasonic-assisted leaching, chlorination roasting, and calcination followed by water quenching, highlighting their strengths and environmental impacts. Despite advancements, continual technological evolution is necessary to meet stringent purity demands while adhering to environmental sustainability. Integrating eco-friendly practices within purification processes not only minimizes the ecological impacts but also aligns with sustainable development goals. The findings provide valuable insights into optimizing HPQ purification, offering a roadmap for achieving higher purity levels while maintaining environmental and economic sustainability.
Author Contributions
Conceptualization, H.L. and D.Z.; methodology, J.P.; software, S.L.; validation, Z.G., H.L. and S.L.; formal analysis, C.Y.; investigation, Z.G.; resources, Z.G.; data curation, H.L.; writing—original draft preparation, H.L.; writing—review and editing, H.L.; visualization, D.Z.; supervision, Z.G.; project administration, D.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation China (No. 52274343), China Baowu Low Carbon Metallurgy Innovation Foundation (BWLCF202102), and China Baowu Low Carbon Metallurgy Innovation Foundation (BWLCF202216).
Data Availability Statement
The data presented in this review are based on the comprehensive analyses and syntheses of the existing literature in the field. All sources of data are fully referenced in the manuscript. These sources include peer-reviewed journal articles, books, and other scientific publications, which are publicly accessible. No new empirical data were generated for this study. For further inquiries regarding the data sources, readers are encouraged to consult the original publications cited in the references section of this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Götze, J.; Möckel, R. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Li, J.; Wang, Y.; He, X.; Sun, Q.; Xiong, M.; Chen, Z.; Zeng, C.; Zheng, X.; Liang, C. A Facile and Universal Method to Purify Silica from Natural Sand. Green Process. Synth. 2022, 11, 907–914. [Google Scholar] [CrossRef]
- Du, X.; Liang, C.; Hou, D.L.; Sun, Z.M.; Zheng, S.L. Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz. Minerals 2019, 9, 140. [Google Scholar] [CrossRef]
- Bai, P.; Sharratt, P.; Yeo, T.Y.; Bu, J. A Facile Route to Preparation of High Purity Nanoporous Silica from Acid-Leached Residue of Serpentine. J. Nanosci. Nanotechnol. 2014, 14, 6915–6922. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wanvik, J.E.; Ihlen, P.M. Petrological and Chemical Characterisation of High-Purity Quartz Deposits with Examples from Norway. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möcke, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 71–118. [Google Scholar]
- Santos, M.F.M.D.; Fujiwara, E.; Schenkel, E.A.; Enzweiler, J.; Suzuki, C.K. Quartz Resources in the Serra De Santa Helena Formation, Brazil: A Geochemical and Technological Study. J. S. Am. Earth Sci. 2014, 56, 328–338. [Google Scholar] [CrossRef]
- Sigue, C.; Moundi, A.; Suh, C.E.; Dos Santos, M.F.M.; Fujiwara, E.; Suzuki, C.K.; Ndema-Mbongue, J.L. Assessment of Shear Zone-Derived Quartz from the Etam Area, Southwest Cameroon as Potential High-Purity Quartz Resource: Petrography, Geochemistry and Technological Studies. SN Appl. Sci. 2020, 2, 551. [Google Scholar] [CrossRef]
- Liu, K.C.; Guo, Y. Comparative Study of Mineralogical Characteristics of Natural and Synthetic Amethyst and Smoky Quartz. Crystals 2022, 12, 1735. [Google Scholar] [CrossRef]
- Korovkin, M.V.; Ananyeva, L.G.; Zherlitsyn, A.A.; Kondratiev, S.S.; Savinova, O.V.; Kurskaya, V.S. Assessment of Crystallinity Degree of Quartz Raw Materials. Bull. Tomsk Polytech. Univ.-Geo Assets Eng. 2023, 334, 59–67. [Google Scholar] [CrossRef]
- Nepomnyashchikh, A.I.; Fedorov, A.M.; Zhaboedov, A.P.; Volkova, M.G. High-Purity Quartzite from East Sayan. Russ. Geol. Geophys. 2023, 64, 1005–1014. [Google Scholar] [CrossRef]
- Fedorov, A.M.; Makrygina, V.; Mazukabzov, A.M.; Nepomnyashchikh, A.I.; Ayurzhanaeva, D.T.; Volkova, M.G. Resources of Quartz Raw Materials, Gargan Block, East Sayan Quartzite-Bearing Area. Georesury 2021, 23, 96–106. [Google Scholar] [CrossRef]
- Sawatzky, C.C.; Pe-Piper, G. Detrital Quartz Sources in the Scotian Basin, Eastern Canada, Using Hot-Cathode Cathodoluminescence: Availability of Coarse-Grained Sand for Reservoirs. Aapg Bull. 2013, 97, 1503–1520. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.C.; Shrivastava, S.; Csetenyi, L.J. Sulfuric Acid Resistance of Quartz Sandstone Aggregate Concrete. J. Mater. Civ. Eng. 2017, 29, 06017006. [Google Scholar] [CrossRef]
- Chen, L.H.; Li, Q.; Jiang, T. Comprehensive Utilization of Tailings in Quartz Vein-Hosted Gold Deposits. Minerals 2022, 12, 1481. [Google Scholar] [CrossRef]
- Götze, J. Chemistry, Textures and Physical Properties of Quartz—Geological Interpretation and Technical Application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Götze, J. Classification, Mineralogy and Industrial Potential of SiO2 Minerals and Rocks. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar]
- Grishin, Y.M.; Miao, L.; Borisov, L.A.; Serykh, N.M.; Kulagin, A.Y. Applications of Two Electric Arc Plasma Torches for the Beneficiation of Natural Quartz. Int. J. Miner. Metall. Mater. 2019, 26, 267–273. [Google Scholar] [CrossRef]
- Zobnin, N.N.; Torgovets, A.K.; Pikalova, I.A.; Yussupova, Y.S.; Atakishiyev, S.A. Influence of Thermal Stability of Quartz and the Particle Size Distribution of Burden Materials on the Process of Electrothermal Smelting of Metallurgical Silicon. Orient. J. Chem. 2018, 34, 1120–1125. [Google Scholar] [CrossRef]
- Prasetyo, A.B.; Handayani, M.; Sulistiyono, E.; Firdiyono, F.; Febriana, E.; Mayangsari, W.; Wahyuningsih, S.; Pramono, E.; Maksum, A.; Riastuti, R.; et al. Fabrication of High Purity Silica Precipitates from Quartz Sand toward Photovoltaic Application. J. Ceram. Process. Res. 2023, 24, 103–110. [Google Scholar]
- Korekina, M.A.; Savichev, A.N. Potential of Milky Quartz from the Larino Deposit in the Southern Urals in Production of High-Purity Quartz Concentrates. J. Min. Sci. 2023, 59, 157–166. [Google Scholar] [CrossRef]
- Zabezhailov, M.O.; Tomashuk, A.L.; Nikolin, I.V.; Plotnichenko, V.G.; Kryukova, E.B.; Koltashev, V.V. Mechanisms of Light Absorption in Gamma-Irradiated Blanks for Optical Fibers Based on High-Purity Quartz Glass. Tech. Phys. Lett. 2005, 31, 498–499. [Google Scholar] [CrossRef]
- Nepomnyashchikh, A.I.; Volkova, M.G.; Zhaboedov, A.P.; Lesnikov, A.K.; Lesnikov, P.A.; Paklin, A.S.; Sizova, T.Y.; Spiridonov, A.M.; Fedorov, A.M.; Shalaev, A.A.; et al. Optical Glass Based on the East Sayan Mountain Quartzites. Glass Phys. Chem. 2018, 44, 130–136. [Google Scholar] [CrossRef]
- Chen, H.R.; Hu, D.L.; Zhang, J.B.; Yuan, S.; Wang, C.; Zhang, H.L.; Zhou, N.G.; Yang, D.R. Effects of Impurity Barrier Layer on the Red Zone at the Bottom of Cast Monocrystalline Si Ingot for Solar Cells. Sol. Rrl 2023, 7, 2300383. [Google Scholar] [CrossRef]
- Mukashev, B.N.; Betekbaev, A.A.; Kalygulov, D.A.; Pavlov, A.A.; Skakov, D.M. Study of Silicon Production Processes and Development of Solar-Cell Fabrication Technologies. Semiconductors 2015, 49, 1375–1382. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Lan, A.; Hsu, C.; Lan, C.W. Improvement of Multi-Crystalline Silicon Ingot Growth by Using Diffusion Barriers. J. Cryst. Growth 2014, 401, 727–731. [Google Scholar] [CrossRef]
- Fukuda, T.; Horioka, Y.; Suzuki, N.; Moriya, M.; Tanahashi, K.; Simayi, S.; Shirasawa, K.; Takato, H. Lifetime Improvement of Photovoltaic Silicon Crystals Grown by Czochralski Technique Using “Liquinert” Quartz Crucibles. J. Cryst. Growth 2016, 438, 76–80. [Google Scholar] [CrossRef]
- Peng, L.H.; Qin, S.; Gu, X.B. Effects of Melting Parameters and Quartz Purity on Silica Glass Crucible Produced by Arc Method. Eng. Res. Express 2020, 2, 015046. [Google Scholar] [CrossRef]
- Matsuo, H.; Ganesh, R.B.; Nakano, S.; Liu, L.J.; Kangawa, Y.; Arafune, K.; Ohshita, Y.; Yamaguchi, M.; Kakimoto, K. Thermodynamical Analysis of Oxygen Incorporation from a Quartz Crucible during Solidification of Multicrystalline Silicon for Solar Cell. J. Cryst. Growth 2008, 310, 4666–4671. [Google Scholar] [CrossRef]
- Guo, J.W.; Liu, X.M.; Yu, J.M.; Xu, C.F.; Wu, Y.F.; Pan, D.A.; Senthil, R.A. An Overview of the Comprehensive Utilization of Silicon-Based Solid Waste Related to Pv Industry. Resour. Conserv. Recycl. 2021, 169, 105450. [Google Scholar] [CrossRef]
- Xiao, J.L.; Dunham, S.; Liu, P.; Zhang, Y.W.; Kocabas, C.; Moh, L.; Huang, Y.G.; Hwang, K.C.; Lu, C.; Huang, W.; et al. Alignment Controlled Growth of Single-Walled Carbon Nanotubes on Quartz Substrates. Nano Lett. 2009, 9, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, G.; Marisa, D.S.; Søndenå, R.; Juel, M.; Autruffe, A.; Adamczyk, K.; Skarstad, H.V.; Ekstrøm, K.E.; Wiig, M.S.; You, C.C.; et al. Impurity Control in High Performance Multicrystalline Silicon. Phys. Status Solidi (A) 2017, 214, 1700319. [Google Scholar] [CrossRef]
- Nepomnyashchikh, A.I.; Shalaev, A.A.; Sizova, T.Y.; Paklin, A.S.; Sapozhnikov, A.N.; Pavlova, L.A. Onset Temperatures and Kinetics of Quartz Glass Crystallization. Crystallogr. Rep. 2018, 63, 290–294. [Google Scholar] [CrossRef]
- Hao, L.F.; Wang, Q.; Peng, P.; Cao, Z.X.; Jiao, W.C.; Yang, F.; Liu, W.B.; Wang, R.G.; He, X.D. Calibrating Conservative and Dissipative Response of Electrically-Driven Quartz Tuning Forks. Ultramicroscopy 2017, 174, 106–111. [Google Scholar] [CrossRef]
- Pivinskii, Y.E.; Dyakin, P.V.; Kolobov, A.Y. Research in the Field of Preparing Molded and Unmolded Refractories Based on High-Alumina Hcbs. Part 8. Effect of Firing Temperature on Properties of Materials Based on Mixed Hcbs Composition: Bauxite, Quartz Glass, and Reactive Alumina. Refract. Ind. Ceram. 2017, 57, 637–644. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.X.; Cholico-González, D.F.; Ortiz, N.L. Combination Formation in the Reinforcement of Metakaolin Geopolymers with Quartz Sand. Cem. Concr. Compos. 2017, 80, 115–122. [Google Scholar] [CrossRef]
- Mikhailenko, N.Y.; Rudkovskaya, N.V. Chemical Resistance of Zinc-Bearing Glass-Ceramic Glaze for Quartz Glass Ceramics. Glass Ceram. 2003, 60, 108–110. [Google Scholar] [CrossRef]
- Partyka, J.; Pasiut, K.; Jelen, P.; Michalek, J.; Kaczmarczyk, K.; Kozien, D. The Impact of Nano-Quartz on the Structure of Glass-Ceramic Glazes from the SiO2-Al2O3-Cao-Mgo-Na2O-K2O System. Ceram. Int. 2020, 46, 23888–23894. [Google Scholar] [CrossRef]
- Bu, J.L.; Wang, Z.F.; Dou, G.T.; Tian, Y.C.; Li, H.M. Study on Inhibiting Crystallization of Fused Quartz Ceramic Materials. Rare Met. Mater. Eng. 2007, 36, 357–360. [Google Scholar]
- Abdulagatov, I.M.; Emirov, S.N.; Tsomaeva, T.A.; Gairbekov, K.A.; Askerov, S.Y.; Magomedova, N.A. Thermal Conductivity of Fused Quartz and Quartz Ceramic at High Temperatures and High Pressures. J. Phys. Chem. Solids 2000, 61, 779–787. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hou, Q.L.; Tian, L.; Chen, P.F.; Xiao, J.K. Effect of In Situ Synthesis of Si2N2O on Microstructure and the Mechanical Properties of Fused Quartz Ceramics. Ceram. Int. 2020, 46, 8725–8729. [Google Scholar] [CrossRef]
- Boualem, A.; Leontie, L.; Lopera, S.A.G.; Hamzaoui, S. Synthesis and Characterization of Mesoporous Silica from Algerian River Sand for Solar Grade Silicon: Effect of Alkaline Concentration on the Porosity and Purity of Silica Powder. Silicon 2022, 14, 5231–5240. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and Mineral Chemistry of Quartz: A Review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Z.; Wang, C.; Hu, Y. Trace Element Concentrations and Mineralogy of Quartz Vein Deposits from Southeastern Hubei Province, China. Minerals 2022, 12, 814. [Google Scholar] [CrossRef]
- Dos Santos, M.; Fujiwara, E.; Schenkel, E.A.; Enzweiler, J.; Suzuki, C.K. Quartz Sand Resources in the Santa Maria Eterna Formation, Bahia, Brazil: A Geochemical and Morphological Study. J. S. Am. Earth Sci. 2015, 62, 176–185. [Google Scholar] [CrossRef]
- Farfan, G.A.; Rakovan, J.; Ackerson, M.R.; Andrews, B.J.; Post, J.E. The Origin of Trapiche-Like Inclusion Patterns in Quartz from Inner Mongolia, China. Am. Miner. 2021, 106, 1797–1808. [Google Scholar] [CrossRef]
- Lambrecht, G.; Diamond, L.W. Morphological Ripening of Fluid Inclusions and Coupled Zone-Refining in Quartz Crystals Revealed by Cathodoluminescence Imaging: Implications for Cl-Petrography, Fluid Inclusion Analysis and Trace-Element Geothermometry. Geochim. Cosmochim. Acta 2014, 141, 381–406. [Google Scholar] [CrossRef]
- Dal Martello, E.; Tranell, G.; Ostrovski, O.; Zhang, G.; Raaness, O.; Larsen, R.B.; Tang, K.; Koshy, P. Trace Elements in the Si Furnace. Part I: Behavior of Impurities in Quartz during Reduction. Metall. Mater. Trans. B 2013, 44, 233–243. [Google Scholar] [CrossRef]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Muggerud, A.M.F.; Erbe, A. In Situ High Temperature Spectroscopic Study of Liquid Inclusions and Hydroxyl Groups in High Purity Natural Quartz. Miner. Eng. 2021, 174, 107238. [Google Scholar] [CrossRef]
- Xia, M.; Sun, C.; Yang, X.; Chen, J. Assessment of Gold-Bearing Quartz Vein as a Potential High-Purity Quartz Resource: Evidence from Mineralogy, Geochemistry, and Technological Purification. Minerals 2023, 13, 261. [Google Scholar] [CrossRef]
- Dash, K.; Chandrasekaran, K.; Thangavel, S.; Dhaville, S.M.; Arunachalam, J. Determination of Trace Metallic Impurities in High-Purity Quartz by Ion Chromatography. J. Chromatogr. A 2004, 1022, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pathirage, S.S.; Hemalal, P.V.A.; Rohitha, L.P.S.; Ratnayake, N.P. Production of Industry-Specific Quartz Raw Material Using Sri Lankan Vein Quartz. Environ. Earth Sci. 2019, 78, 58. [Google Scholar] [CrossRef]
- Vatalis, K.I.; Charalampides, G.; Platias, S.; Benetis, N.P. Market Developments and Industrial Innovative Applications of High Purity Quartz Refines. Procedia Econ. Financ. 2014, 14, 624–633. [Google Scholar] [CrossRef]
- Rakov, L.T. Mechanisms of Isomorphic Substitution in Quartz. Geochem. Int. 2006, 44, 1004–1014. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Xia, Z.; Li, W.; Lei, S. Influences of Na2Co3 Roasting and H3Po4 Hot-Pressure Leaching on the Purification of Vein Quartz to Achieve High-Purity Quartz. Hydrometallurgy 2023, 218, 106065. [Google Scholar] [CrossRef]
- Lehmann, K.; Berger, A.; Götte, T.; Ramseyer, K.; Wiedenbeck, M. Growth Related Zonations in Authigenic and Hydrothermal Quartz Characterized by Sims-, Epma-, Sem-Cl- And Sem-Cc-Imaging. Mineral. Mag. 2009, 73, 633–643. [Google Scholar] [CrossRef]
- Deng, Y.J.; Wu, Q.H.; Li, Z.C.; Huang, X.; Rao, S.H.; Liang, Y.F.; Lu, H.L. Crystal Face Dependent Wettability of a-Quartz: Elucidation by Time-of-Flight Secondary Ion Mass Spectrometry Techniques Combined with Molecular Dynamics. J. Colloid Interface Sci. 2022, 607, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Konyshev, A.A.; Anosova, M.O.; Rusak, A.A.; Alekseev, I.A.; Yakushev, A.I.; Shapovalov, Y.B. Dikes of Quartz Porphyry and their Role in the Formation of the Salmi Batholith (South Karelia). Dokl. Earth Sci. 2020, 491, 127–130. [Google Scholar] [CrossRef]
- Zimin, M.D.; Zhaboedov, A.P.; Kolesnikov, S.S.; Nepomnyashchikh, A.I. Measurement of the Crystallinity Index of High-Purity Quartz at Various Stages of Separation and Study of its Structure by X-ray Diffraction and Electron Backscatter Diffraction. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2022, 16, 484–489. [Google Scholar] [CrossRef]
- Kiss, G.B.; Bendo, Z.; Garuti, G.; Zaccarini, F.; Király, E.; Molnár, F. Reconstruction of Hydrothermal Processes in the Cyprus Type Fe-Cu-Zn Deposits of the Italian Northern Apennines: Results of Combined Fluid Inclusion Microthermometry, Sem-Cl Imaging and Trace Element Analyses by La-Icp-Ms. Minerals 2021, 11, 165. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Pandey, O.P. Ftir and Tl Studies of Gamma Rays Irradiated Natural Quartz. J. Mater. Sci.-Mater. Electron. 2021, 32, 20767–20776. [Google Scholar] [CrossRef]
- Gawel, B.A.; Ulvensoen, A.; Lukaszuk, K.; Arstad, B.; Muggerud, A.; Erbe, A. Structural Evolution of Water and Hydroxyl Groups during Thermal, Mechanical and Chemical Treatment of High Purity Natural Quartz. RSC Adv. 2020, 10, 29018–29030. [Google Scholar] [CrossRef]
- Götze, J.; Kempe, U. A comparison of optical microscope-and scanning electron microscope-based cathodoluminescence (CL) imaging and spectroscopy applied to geosciences. Mineral. Mag. 2008, 72, 909–924. [Google Scholar] [CrossRef]
- Götze, J. Application of cathodoluminescence microscopy and spectroscopy in geosciences. Microsc. Microanal. 2012, 18, 1270–1284. [Google Scholar] [CrossRef]
- Whitmore, R.J.; Norton, K.P.; Ashworth, L.; Mackintosh, A.N. Sequential Quartz Purification of 125-63 Μm Material for in-Situ Cosmogenic Nuclide Analysis. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2022, 511, 105–112. [Google Scholar] [CrossRef]
- Hu, P.; Liang, L.; Peng, Y.; Yu, H.; Xie, G. Recovery of Microcrystalline Graphite from Quartz Using Magnetic Seeding. Minerals 2020, 10, 24. [Google Scholar] [CrossRef]
- Yin, W.; Wang, D.; Drelich, J.W.; Yang, B.; Li, D.; Zhu, Z.; Yao, J. Reverse Flotation Separation of Hematite from Quartz Assisted with Magnetic Seeding Aggregation. Miner. Eng. 2019, 139, 105873. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Wei, M.; Li, F.; Ban, B.; Li, J. Effect of Impurity Content Difference between Quartz Particles on Flotation Behavior and its Mechanism. Powder Technol. 2020, 375, 504–512. [Google Scholar] [CrossRef]
- Medjahed, S.; Kheloufi, A.; Bobocioiu, E.; Kefaifi, A.; Kerkar, F.; Lebbou, K. Quartz Ore Beneficiation by Reverse Flotation for Silicon Production. Silicon 2022, 14, 87–97. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zawala, J.; Drzymala, J.; Malysa, K. Influence of Hexylamine on Kinetics of Flotation and Bubble Attachment to the Quartz Surface. Sep. Sci. Technol. 2016, 51, 2681–2690. [Google Scholar] [CrossRef]
- Khalifa, M.; Ouertani, R.; Hajji, M.; Ezzaouia, H. Innovative Technology for the Production of High-Purity Sand Silica by Thermal Treatment and Acid Leaching Process. Hydrometallurgy 2019, 185, 204–209. [Google Scholar] [CrossRef]
- Dal Martello, E.; Bernardis, S.; Larsen, R.B.; Tranell, G.; Di Sabatino, M.; Arnberg, L. Electrical Fragmentation as a Novel Route for the Refinement of Quartz Raw Materials for Trace Mineral Impurities. Powder Technol. 2012, 224, 209–216. [Google Scholar] [CrossRef]
- Zhong, T.; Yu, W.; Shen, C.; Wu, X. Research on Preparation and Characterisation of High-Purity Silica Sands by Purification of Quartz Vein Ore from Dabie Mountain. Silicon 2022, 14, 4723–4729. [Google Scholar] [CrossRef]
- Pei, Z.; Lin, M.; Meng, Y.; Qiu, H.; Zhang, X.; Lei, S.; Li, Y.; Gerson, A.R. Efficient Separation of Trace Muscovite within the Surface/Interface of Quartz Grains from a Hydrothermal Deposit by Oxidizing Calcination and Catalytic Pressure Leaching. Min. Metall. Explor. 2019, 36, 313–325. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhao, X.; Pan, X.; Guo, P. Separation and Purification of High-Purity Quartz from High-Silicon Iron Ore Tailing: An Innovative Strategy for Comprehensive Utilization of Tailings Resources. Process Saf. Environ. Prot. 2023, 169, 142–148. [Google Scholar] [CrossRef]
- Lin, M.; Lei, S.M.; Pei, Z.Y.; Liu, Y.Y.; Xia, Z.J.; Xie, F.X. Application of Hydrometallurgy Techniques in Quartz Processing and Purification: A Review. Metall. Res. Technol. 2018, 115, 303. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wang, H.; Zhu, C.; Ren, B. A Review on Removal of Iron Impurities from Quartz Mineral. Minerals 2023, 13, 1128. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Li, X.; Zhang, Z. Iron Removal from Extremely Fine Quartz and its Kinetics. Sep. Purif. Technol. 2013, 108, 45–50. [Google Scholar] [CrossRef]
- Kefaifi, A.; Sahraoui, T.; Kheloufi, A.; Bobocioiu, E. Optimization of Quartz Sand Leaching Process Using Design Experiments Method (Doe). Silicon 2019, 11, 1481–1488. [Google Scholar] [CrossRef]
- Du, X.; Yang, D.; Zheng, S.; Sun, Z.; Li, C. Deep Insight into the Reductive Roasting Treatment on Iron Removing from Quartz. Adv. Powder Technol. 2021, 32, 4825–4832. [Google Scholar] [CrossRef]
- Shao, H.; Zang, F.; Ji, M.; Yu, M. Prepare and Mechanism of High Purity Quartz by Alkali Corrosion and Acid Leaching Process Using Vein Quartz. Silicon 2022, 14, 12475–12483. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, X.; Huang, H.; Zhou, L.; Xiong, T. High Efficiency Iron Removal from Quartz Sand Using Phosphoric Acid. Int. J. Miner. Process. 2012, 114–117, 30–34. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Shen, Q.; Zhang, Z.; Du, F. Further Purification of Industrial Quartz by Much Milder Conditions and a Harmless Method. Environ. Sci. Technol. 2010, 44, 7673–7677. [Google Scholar] [CrossRef]
- Tuncuk, A.; Akcil, A. Iron Removal in Production of Purified Quartz by Hydrometallurgical Process. Int. J. Miner. Process. 2016, 153, 44–50. [Google Scholar] [CrossRef]
- Long, H.L.; Tan, X.Z.; Ni, S.F.; Ma, A.Y.; Li, S.W.; Zhu, D.Q. Ammoniacal Leaching Behavior and Regularity of Zinc Ash. Int. J. Chem. React. Eng. 2023, 21, 895–906. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Wu, J.; Wu, D.; Wei, K.; Ma, W. Effect of Quartz Crystal Structure Transformations on the Removal of Iron Impurities. Hydrometallurgy 2021, 204, 105715. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Pan, X.; Zhao, X.; Guo, P.; Zhao, Z. Recovery and Preparation of High-Grade Silica from Iron Ore Tailings by S-Hgms Coupling with Acid Leaching Technology: Description of Separation Mechanism and Leaching Kinetics. Powder Technol. 2023, 424, 118523. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Pan, X.; Zhao, X.; Guo, P. Eco-Friendly Strategy for Preparation of High-Purity Silica from High-Silica Iots Using S-Hgms Coupling with Ultrasound-Assisted Fluorine-Free Acid Leaching Technology. J. Environ. Manag. 2023, 339, 117932. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.; Hajji, M.; Ezzaouia, H. Impurity Removal Process for High-Purity Silica Production by Acid Leaching. Epj Web Conf. 2012, 29, 00014. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, Y.; Jeong, S.; Chae, Y.; Ko, K. Acid Leaching Purification and Neutron Activation Analysis of High Purity Silicas. J. Radioanal. Nucl. Chem. 2009, 282, 629–633. [Google Scholar] [CrossRef]
- Tuncuk, A.; Akcil, A. Removal of Iron from Quartz Ore Using Different Acids: A Laboratory-Scale Reactor Study. Miner. Process Extr. Metall. Rev. 2014, 35, 217–228. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Z.; Liu, Y.; Gao, H.; Wu, F.; Song, Y. The Effects of Calcination-Water Quenching on Quartz Purification and its Mechanism. Min. Metall. Explor. 2023, 40, 2519–2527. [Google Scholar] [CrossRef]
- Long, H.; Li, H.; Ma, P.; Zhou, Z.; Xie, H.; Yin, S.; Wang, Y.; Zhang, L.; Li, S. Effectiveness of Thermal Treatment on Pb Recovery and Cl Removal from Sintering Dust. J. Hazard. Mater. 2021, 403, 123595. [Google Scholar] [CrossRef]
- Chenlin, L.; Guojun, Z.; Baohua, O.; Zhilong, L.; Junfeng, R.; Xueming, W.U.; Han, Z. Study on High Temperature Chlorination Purification of Quartz Sand. Ind. Miner. Process. 2020, 1, 16–19. [Google Scholar]
- Yong, Y.; Yi-Fei, L.U.; Cui-Hong, Z.; Wei-Chang, Z. A New Technology for Fe-and Ti-Removal from Quartz Sand. Multipurp. Util. Miner. Resour. 2009, 1, 16–19. [Google Scholar]
- Lin, M.; Pei, Z.; Li, Y.; Liu, Y.; Wei, Z.; Lei, S. Separation Mechanism of Lattice-Bound Trace Elements from Quartz by Kcl-Doping Calcination and Pressure Leaching. Miner. Eng. 2018, 125, 42–49. [Google Scholar] [CrossRef]
- Li, H.; Long, H.; Zhang, L.; Yin, S.; Li, S.; Zhu, F.; Xie, H. Effectiveness of Microwave-Assisted Thermal Treatment in the Extraction of Gold in Cyanide Tailings. J. Hazard. Mater. 2020, 384, 121456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Peng, J.; Srinivasakannan, C.; Li, S.; Yin, S. Microwave and Ultrasound Augmented Leaching of Complicated Zinc Oxide Ores in Ammonia and Ammonium Citrate Solutions. Metals 2017, 7, 216. [Google Scholar] [CrossRef]
- Buttress, A.J.; Rodriguez, J.M.; Ure, A.; Ferrari, R.S.; Dodds, C.; Kingman, S.W. Production of High Purity Silica by Microfluidic-Inclusion Fracture Using Microwave Pre-Treatment. Miner. Eng. 2019, 131, 407–419. [Google Scholar] [CrossRef]
- Song, W.; Jiang, X.; Chen, C.; Ban, B.; Wan, S.; Chen, J. Purification of Quartz Via Low-Temperature Microwave Chlorinated Calcination Combined with Acid Leaching and its Mechanism. Silicon 2023, 15, 971–981. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zuo, Q.; Li, J.; Ban, B.; Chen, J. Purification Mechanism of Quartz Sand by Combination of Microwave Heating and Ultrasound Assisted Acid Leaching Treatment. Silicon 2021, 13, 531–541. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, E.; Zhang, L.; Peng, J.; Zhou, J.; Srinivasakannan, C.; Yang, C. A Comparison of Ultrasound-Augmented and Conventional Leaching of Silver from Sintering Dust Using Acidic Thiourea. Ultrason. Sonochem. 2017, 34, 222–231. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Peng, J.; Li, J.; Lin, G.; Yu, X. Comparison of Ultrasonic-Assisted and Regular Leaching of Germanium from by-Product of Zinc Metallurgy. Ultrason. Sonochem. 2016, 31, 143–149. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Xie, H.; Yin, S.; Peng, J.; Li, S.; Yang, K.; Zhu, F. Ultrasound-Assisted Silver Leaching Process for Cleaner Production. JOM 2020, 72, 766–773. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 Magnetic Nanoparticles Synthesis from Tailings by Ultrasonic Chemical Co-Precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Li, X.; Zhang, Z. Improvement of Iron Removal from Silica Sand Using Ultrasound-Assisted Oxalic Acid. Ultrason. Sonochem. 2011, 18, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Arslan, V. The Modeling and Optimization of Iron Removal from Silica Sand Under Ultrasound-Assisted Leaching by Response Surface Methodology. Min. Metall. Explor. 2021, 38, 2229–2237. [Google Scholar] [CrossRef]
- Yang, C. Advanced Purification of Industrial Quartz Using Calcination Pretreatment Combined with Ultrasound-Assisted Leaching. Acta Geodyn. Geomater. 2018, 15, 187–195. [Google Scholar] [CrossRef]
- Jena, S.K.; Sahu, J.; Padhy, G.; Mohanty, S.; Dash, A. Chlorination Roasting-Coupled Water Leaching Process for Potash Recovery from Waste Mica Scrap Using Dry Marble Sludge Powder and Sodium Chloride. Int. J. Miner. Metall. Mater. 2020, 27, 1203–1215. [Google Scholar] [CrossRef]
- Long, H.; Chen, K.; Xu, C.; Li, H.; Xie, H.; Yin, S.; Wang, Y.; Zhang, L.; Li, S.; Ma, A. Efficient Recycling of Silver and Copper from Sintering Dust by Chlorination Roasting Process. Arab. J. Sci. Eng. 2021, 46, 6663–6672. [Google Scholar] [CrossRef]
- Li, H.Y.; Ma, A.Y.; Srinivasakannan, C.; Zhang, L.B.; Li, S.W.; Yin, S.H. Investigation on the Recovery of Gold and Silver from Cyanide Tailings Using Chlorination Roasting Process. J. Alloys Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Long, H.; Li, H.; Pei, J.; Srinivasakannan, C.; Yin, S.; Zhang, L.; Ma, A.; Li, S. Cleaner Recovery of Multiple Valuable Metals from Cyanide Tailings via Chlorination Roasting. Sep. Sci. Technol. 2021, 56, 2113–2123. [Google Scholar] [CrossRef]
- Li, X.X.; Li, T.H.; Gao, J.X.; Huang, H.Q.; Li, L.B.; Li, J.S. A Novel “Green” Solvent to Deeply Purify Quartz Sand with High Yields: A Case Study. J. Ind. Eng. Chem. 2016, 35, 383–387. [Google Scholar] [CrossRef]
- Botelho, A.B.; Tenório, J.; Espinosa, D. Separation of Critical Metals by Membrane Technology Under a Circular Economy Framework: A Review of the State-of-the-Art. Processes 2023, 11, 1256. [Google Scholar] [CrossRef]
- Kol Tsov, V.Y. Neutralization and Purification of Acidic Waste Waters Containing Hydrofluorosilicic Acid in the Production of Ultrapure Quartz. Glass Ceram. 2014, 70, 387–390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).