Preparation of Humic Acid from Weathered Coal by Mechanical Energy Activation and Its Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
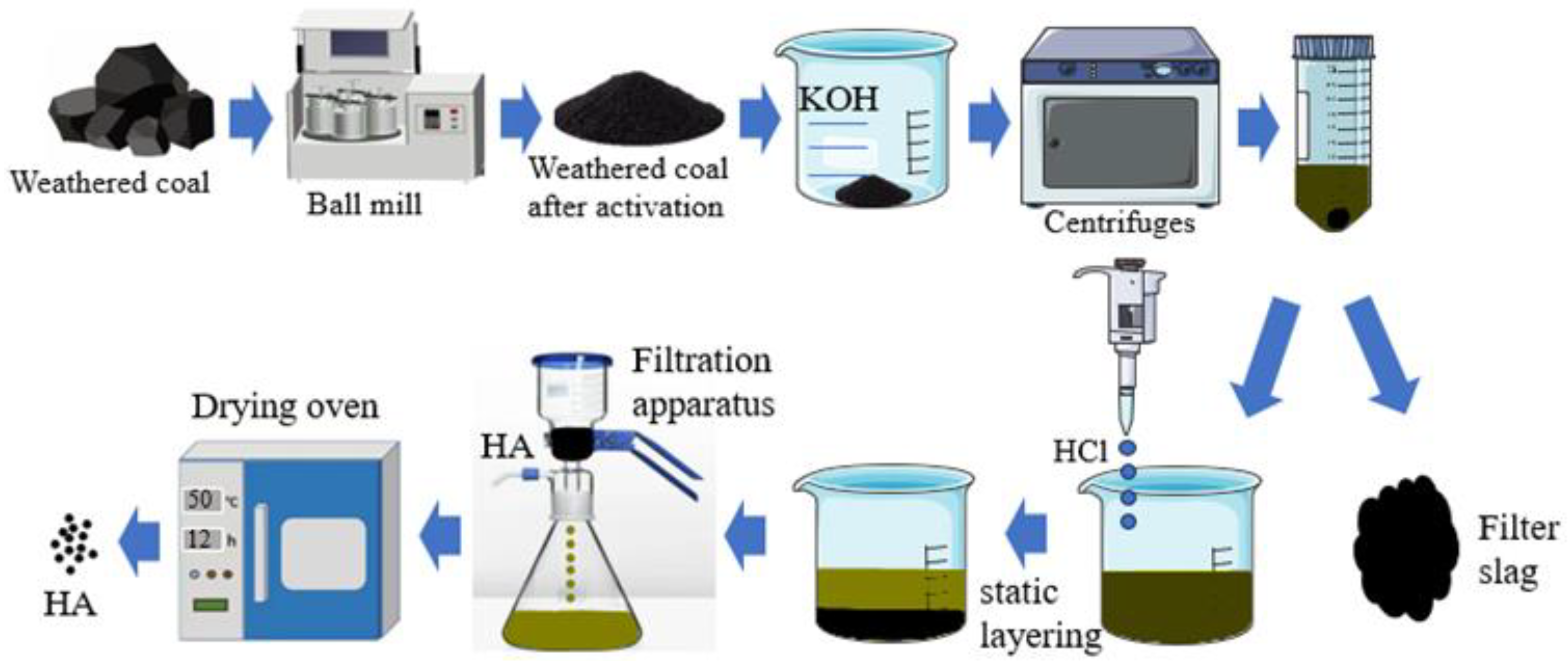
2.2. Mechanical-Energy-Activated Weathered Coal
2.3. HA Extraction
2.4. Characterization of the Properties of Weathered Coal and HA
3. Results and Discussion
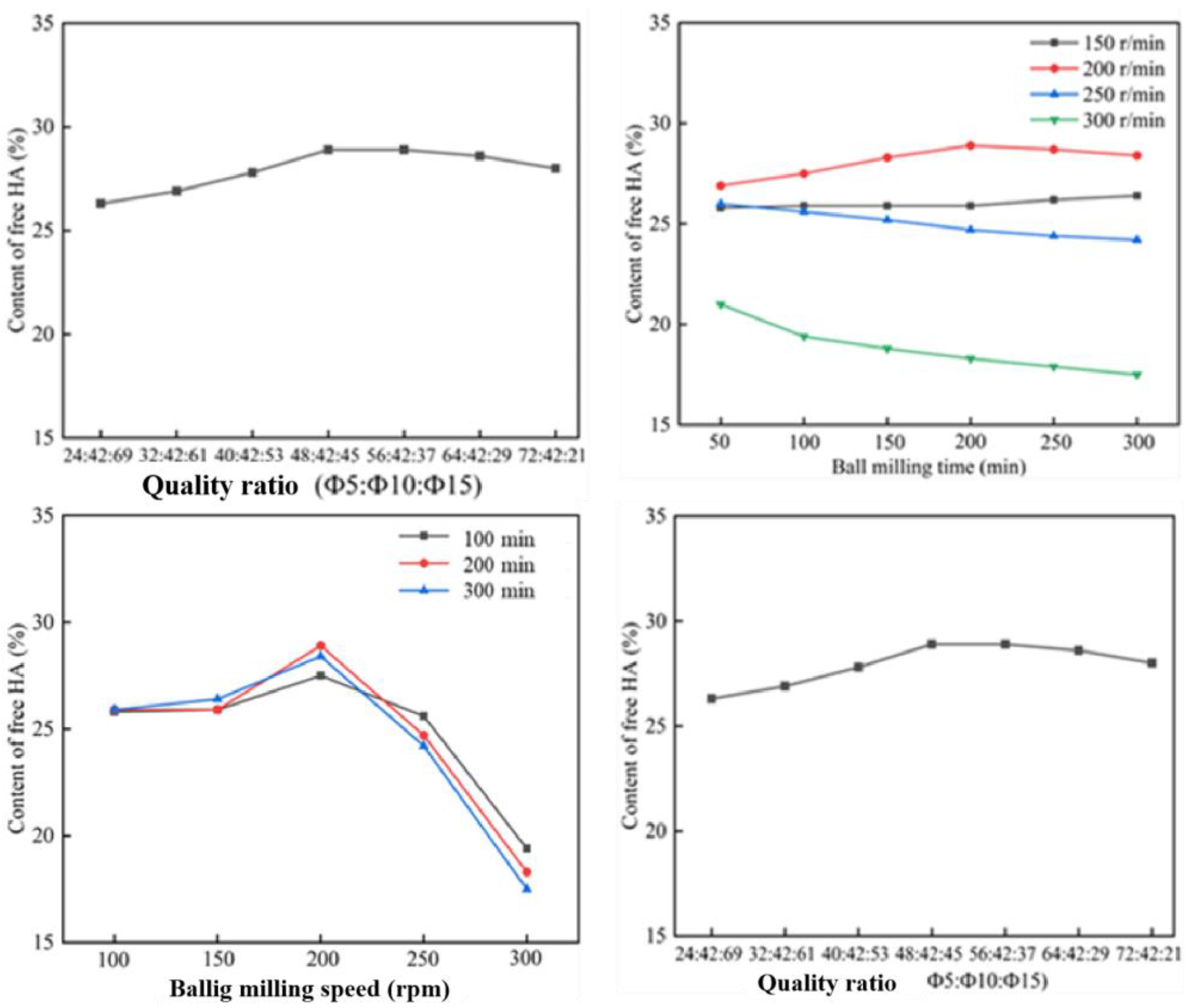
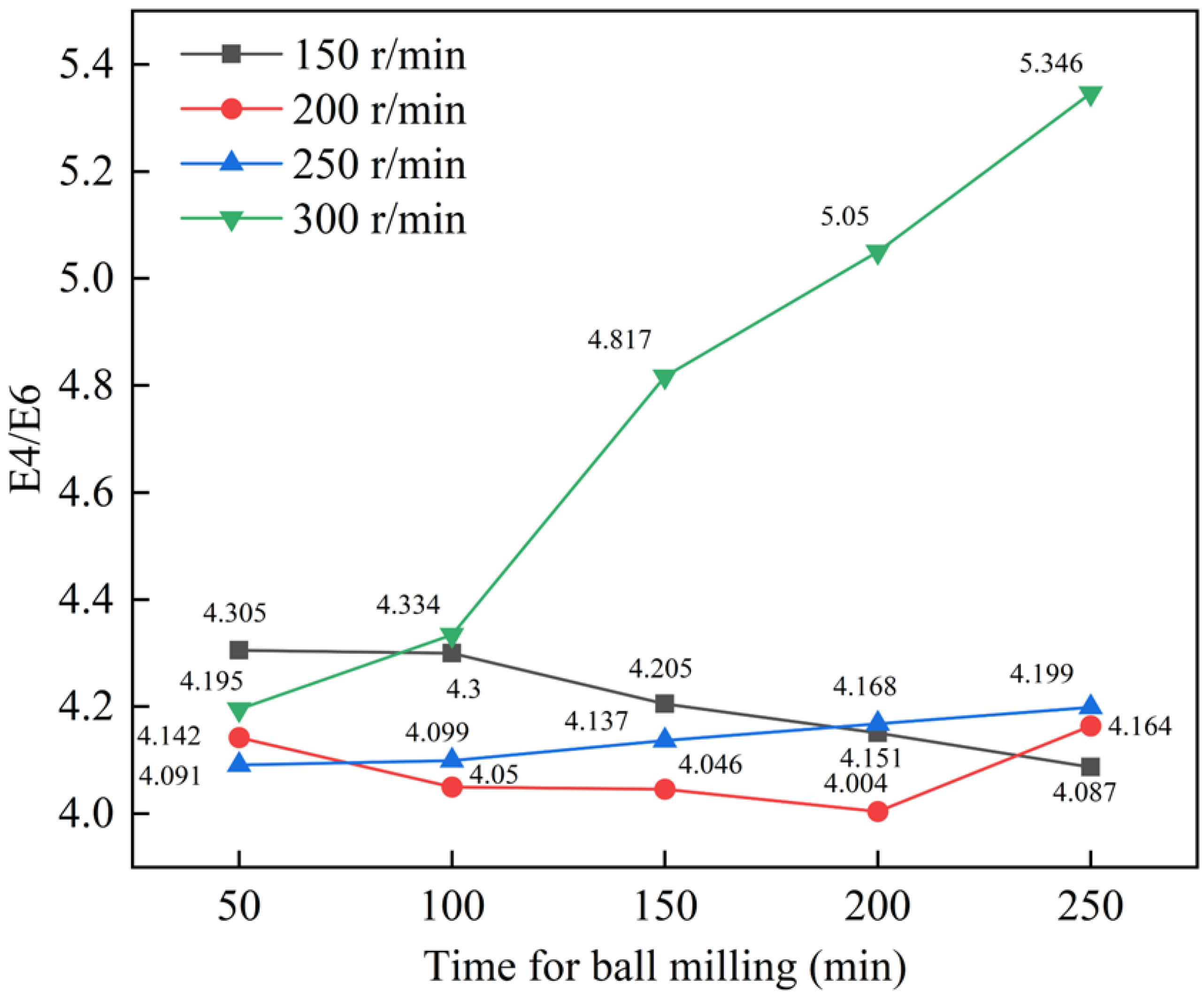
3.1. Effect of Mechanical Energy on Free HA Content in Weathering
3.2. Extraction of HA
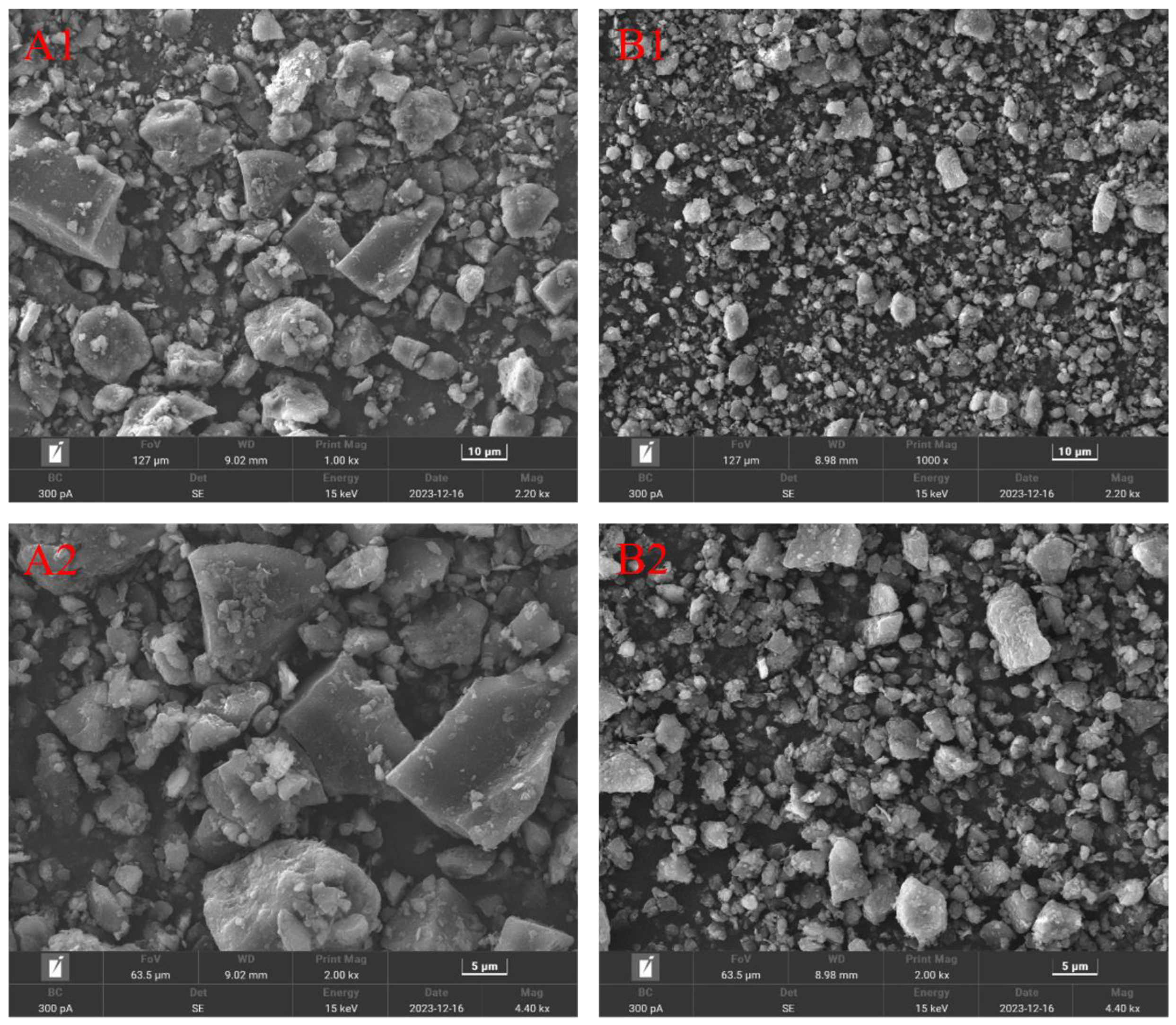
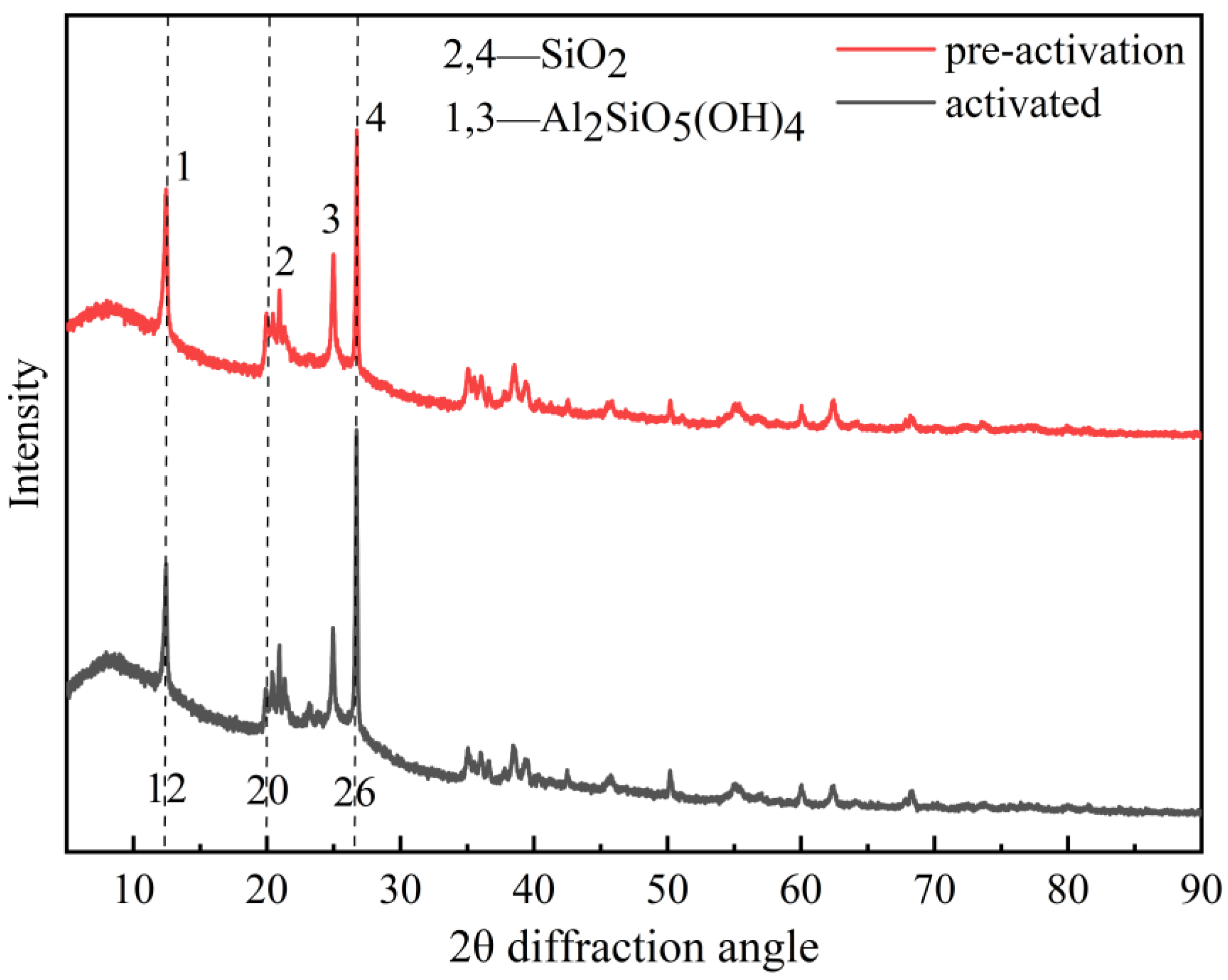
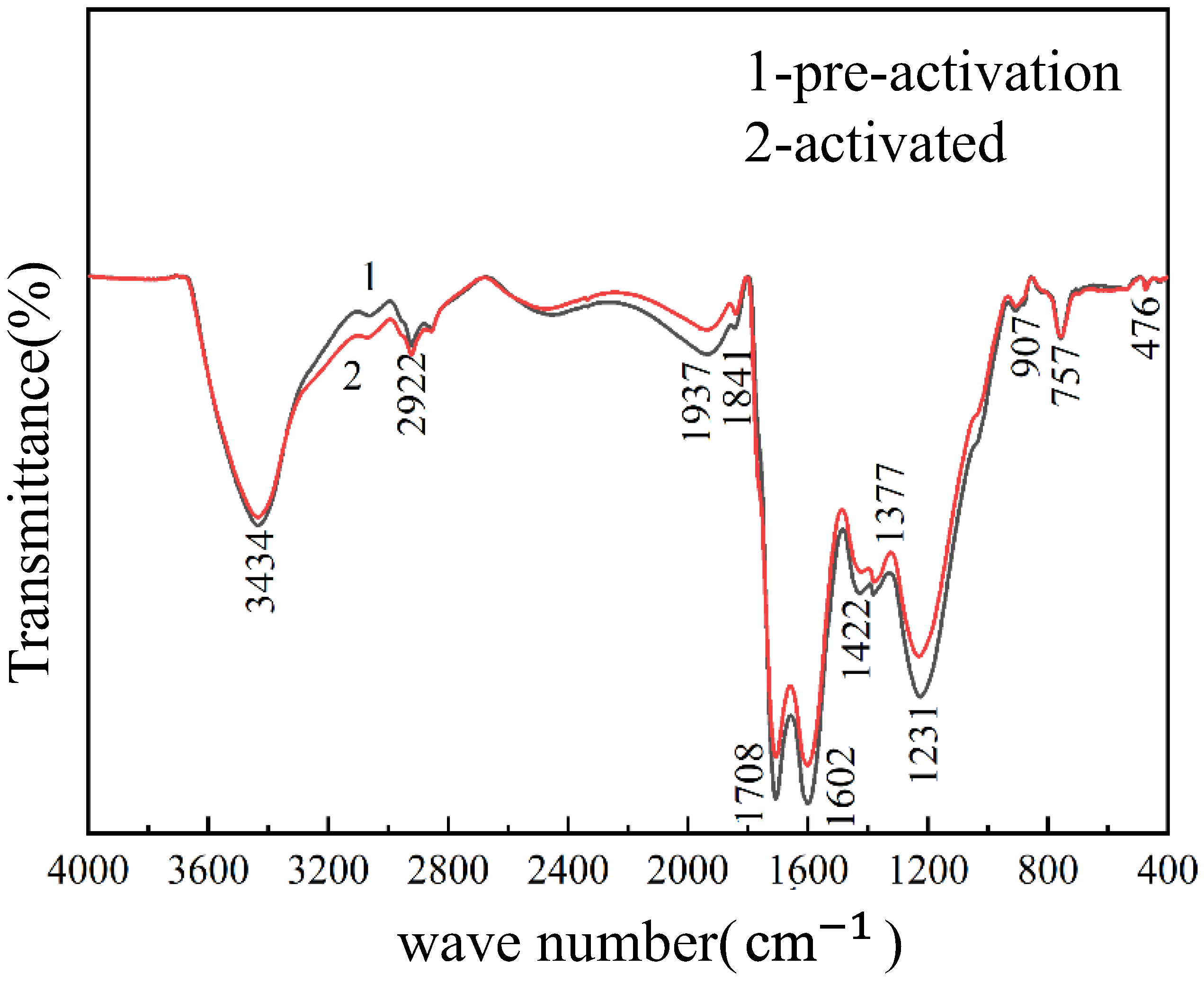
3.3. SEM, XRD, FT-IR Characterization and Other Test Results of Weathered Coal
3.4. Performance Analysis of HA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weisheng, D.; Yao, W.; Weijuan, G.; Guofu, W.; Muqing, Q. Effects of Fe(II) and humic acid on U(VI) mobilization onto oxidized carbon nanofibers derived from the pyrolysis of bacterial cellulose. Int. J. Biol. Macromol. 2024, 266, 131210. [Google Scholar]
- Yuxin, K.; Xing, Z.; Yuhang, R.; Xiaoli, Z.; Shaocheng, S.; Bing, K.; Ziye, Z.; Junqiang, W.; Baoshou, S. Remediation of polycyclic aromatic hydrocarbons polluted soil by biochar loaded humic acid activating persulfate: Performance, process and mechanisms. Bioresour. Technol. 2024, 399, 130633. [Google Scholar]
- Tan, K.H.; Giddens, J.E. Molecular weights and spectral characteristics of humic and fulvic acids. Geoderma 1972, 8, 221–229. [Google Scholar] [CrossRef]
- Puqing, G.; Sen, D. Study on Molecular Structure Model and Visualization of Humic Acids in Different Soils Based on Statistics. J. Soil Sci. Plant Nutr. 2022, 22, 2920–2932. [Google Scholar]
- Xie, Q.; Ma, X.; Ablat, H.; Nurmamat, X.; Jia, H.; Wang, F.; Zhao, Z. Effect of the Molecular Weight of Humic Acids on the Adsorption of As(V) on Goethite. Water Air Soil Pollut. 2024, 235, 153. [Google Scholar] [CrossRef]
- Aguilera, S.M.; Borie, G.; Peirano, P.; Rodriguez, M.; Grez, I.; Zunino, H. Chemical Characterization of Sewage Sludges in Chile and Their Potential Utilization as Amendment to Reclaim Soils for Forestation Purposes. J. Plant Nutr. 2007, 30, 1993–2003. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Jiang, H.; Hu, W. Direct observation of macromolecular structures of humic acid by AFM and SEM. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 121–125. [Google Scholar] [CrossRef]
- Shuiqin, Z.; Liang, Y.; Yanting, L.; Bingqiang, Z. Characteristics of Chinese Weathered Coal from Six Geographical Locations and Effects on Urease Activity Inhibition. Agronomy 2022, 12, 1531. [Google Scholar] [CrossRef]
- Quiroz-Urzola, H.; Castro-Suarez, J.R.; Colpas-Castillo, F. Extraction of Humic Acids from Different Origins: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1154, 012044. [Google Scholar] [CrossRef]
- Ramadan, R.S.; Dawood, Y.H.; Yehia, M.M.; Gad, A. Environmental and health impact of current uranium mining activities in southwestern Sinai, Egypt. Environ. Earth Sci. 2022, 81, 213. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Q.; Wang, G.; Xie, S.; Wang, E. Experimental study on the effects of ultrasonic excitation on pore structure of water-bearing coal. Int. J. Rock Mech. Min. Sci. 2023, 170, 105548. [Google Scholar] [CrossRef]
- Li, H.; Ding, S.; Yuan, J. Extraction of Humic Acids from Lignite and Its Use as a Biochar Activator. ACS Omega 2023, 8, 12206–12216. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Cegarra, J.; Abad, M. A comparison between alkaline and decomplexing reagents to extract humic acids from low rank coals. Fuel Process. Technol. 1996, 48, 51–60. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Lago, W.S.R.; Aymes-Chodur, C.; Ahoussou, A.P.; Yagoubi, N. Physico-chemical ageing of ethylene–norbornene copolymers: A review. J. Mater. Sci. 2017, 52, 6879–6904. [Google Scholar] [CrossRef]
- Mi, T.; Zhang, X.; Liu, P.; Gao, W.; Li, J.; Xu, N.; Yuan, C.; Cui, B. Ultrasonication effects on physicochemical properties of biopolymer-based films: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 63, 5044–5062. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.; Mingelgrin, U. Mechanochemistry: A review of surface reactions and environmental applications. Appl. Clay Sci. 2012, 67–68, 141–150. [Google Scholar] [CrossRef]
- Nerea, G.-D.-A.; Emilio, J.-P.; Joan Josep, R.; Carlos, M.-M. A review of surface topographical modification strategies of 3Y-TZP: Effect in the physicochemical properties, microstructure, mechanical reliability, and biological response. J. Eur. Ceram. Soc. 2023, 43, 2977–3004. [Google Scholar]
- Wang, J.; Guo, G.-J.; Han, Y.; Hou, Q.; Geng, M.; Zhang, Z. Mechanolysis mechanisms of the fused aromatic rings of anthracite coal under shear stress. Fuel 2019, 253, 1247–1255. [Google Scholar] [CrossRef]
- Ponomarenko, A.T.; Tameev, A.R.; Shevchenko, V.G. Action of Mechanical Forces on Polymerization and Polymers. Polymers 2022, 14, 604. [Google Scholar] [CrossRef]
- Watanabe, T.; Isobe, T.; Senna, M. Mechanisms of Incipient Chemical Reaction between Ca(OH)2 and SiO2 under Moderate Mechanical Stressing: II. Examination of a Radical Mechanism by an EPR Study. J. Solid State Chem. 1996, 122, 291–296. [Google Scholar] [CrossRef]
- Kadja, G.T.M.; Suprianti, T.R.; Ilmi, M.M.; Khalil, M.; Mukti, R.R.; Subagjo. Sequential mechanochemical and recrystallization methods for synthesizing hierarchically porous ZSM-5 zeolites. Microporous Mesoporous Mater. 2020, 308, 110550. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Ciesielczuk, J.; Kruszewski, Ł.; Misz-Kennan, M.; Blake, D.R.; Stracher, G.; Moszumańska, I. Gaseous compounds and efflorescences generated in self-heating coal-waste dumps—A case study from the Upper and Lower Silesian Coal Basins (Poland). Int. J. Coal Geol. 2013, 116–117, 247–261. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Song, T.; Sun, Q.; Kong, G.; Wang, F. Microstructure of Coal Gangue and Precipitation of Heavy Metal Elements. J. Spectrosc. 2017, 2017, 3128549. [Google Scholar] [CrossRef]
- Qianqian, S.; Chenyang, X.; Zengchao, G.; Diao, S. Extraction and characterization of humic acid with high bio-activity by mechanical catalytic treatment. Ind. Crops Prod. 2023, 206, 117623. [Google Scholar]
- Turčániová, L.; Kádárová, J.; Imrich, P.; Liptaj, T.; Vidlář, J.; Vašek, J.; Foldyna, F.; Sitek, J.; Baláž, P. Reactivity of mechanical activated coals for special utilization. J. Mater. Sci. 2004, 39, 5467–5470. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, F.; Cao, X.; Li, Y.; Xu, Y.; Weng, W.; Boulatov, R. Mechanochromism and Mechanical-Force-Triggered Cross-Linking from a Single Reactive Moiety Incorporated into Polymer Chains. Angew. Chem. Int. Ed. 2016, 55, 3040–3044. [Google Scholar] [CrossRef]
- Ederer, J.; Janoš, P.; Ecorchard, P.; Štengl, V.; Bělčická, Z.; Šťastný, M.; Pop-Georgievski, O.; Dohnal, V. Quantitative determination of acidic groups in functionalized graphene by direct titration. React. Funct. Polym. 2016, 103, 44–53. [Google Scholar] [CrossRef]
- Fatima, N.; Jamal, A.; Huang, Z.; Liaquat, R.; Ahmad, B.; Haider, R.; Ali, M.I.; Shoukat, T.; Alothman, Z.A.; Ouladsmane, M.; et al. Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples. Sustainability 2021, 13, 8969. [Google Scholar] [CrossRef]
- Li, S.; Tan, J.; Wang, Y.; Li, P.; Hu, D.; Shi, Q.; Yue, Y.; Li, F.; Han, Y. Extraction optimization and quality evaluation of humic acids from lignite using the cell-free filtrate of Penicillium ortum MJ51. RSC Adv. 2021, 12, 528–539. [Google Scholar] [CrossRef]
- Song, M.; Wang, G.; Suo, Y.; Wu, Z.; Zhan, H.; Liu, W. Conversion of Weathered Coal into High Value-Added Humic Acid by Magnetically Recoverable Fe3O4/LaNiO3 Nanocatalysts under Solid-Phase Grinding Conditions. Catalysts 2022, 12, 392. [Google Scholar] [CrossRef]
- Dominik, N.; Kinga, M.; Magdalena, B.-G.; Marta, H.-M. Application of a modified method of humic acids extraction as an efficient process in the production of formulations for agricultural purposes. Pol. J. Chem. Technol. 2023, 25, 31–39. [Google Scholar]
- Ibarra, J.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Yan, J.; Lei, Z.; Li, Z.; Wang, Z.; Ren, S.; Kang, S.; Wang, X.; Shui, H. Molecular structure characterization of low-medium rank coals via XRD, solid state 13C NMR and FTIR spectroscopy. Fuel 2020, 268, 117038. [Google Scholar] [CrossRef]
- Zhu, G.; Ouyang, X.; Yang, Y.; Ruan, T.; Qiu, X. Selective cleavage of aryl ether bonds in dimeric lignin model compounds†. RSC Adv. 2016, 6, 17880–17887. [Google Scholar] [CrossRef]
- Li, M.; Yin, J.; Hu, L.; Chen, S.; Min, D.; Wang, S.; Luo, L. Effect of hydrogen peroxide bleaching on anionic groups and structures of sulfonated chemo-mechanical pulp fibers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124068. [Google Scholar] [CrossRef]
- Yang, G.; Lam, E.; Moores, A. Controlled Chitosan Molecular Weight Reduction by Mechanochemical and Aging-based Phosphoric Acid Hydrolysis. ChemRxiv 2023, 11, 7765–7774. [Google Scholar] [CrossRef]
- Nazarbek, U.; Abdurazova, P.; Raiymbekov, Y. Extraction and Characterization of Humic Acid Based on Coal Mining Waste. Chem. Eng. Technol. 2022, 45, 1133–1140. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Goh, K.M. Infrared spectra of humic acids and related substances. Geochim. Cosmochim. Acta 1971, 35, 471–483. [Google Scholar] [CrossRef]


| Component | Moisture (Mad) | Ash Content (Ad) | Volatile Matter (Vdaf) | Fixed Carbon (FCad) | Total HA | Free HA |
|---|---|---|---|---|---|---|
| Content (%) | 6.10 | 43.56 | 45.51 | 32.36 | 31.9 | 25.8 |
| Element | N | C | H | S(%) | O | H/C | O/C | N/C | S/C |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 0.88 | 58.30 | 3.067 | 0.209 | 37.544 | 0.631 | 0.483 | 0.129 | 0.001 |
| Raw Materials | Extraction Methods | Extraction of HA | Reference |
|---|---|---|---|
| Lignite | Nitric acid oxidation | 57.8% | [29] |
| Lignite | Penicillium ortum MJ51 | 63.9% | [30] |
| Lignite | Hydrothermal treatment | 68% | [12] |
| Weathered coal | Fe3O4/LaNiO3 catalyst treatment | 48.5 | [31] |
| Peat | Ultrasound treatment | 60% | [32] |
| Weathered coal | Non-activated extraction | 67.3% | This study |
| Weathered coal | Activated by mechanical energy | 82.3% | This study |
| Weathered Coal | H/C | ||
|---|---|---|---|
| Pre-activation | 0.631 | 0.89 | 0.174 |
| Activated | 0.613 | 0.93 | 0.220 |
| Weathered Coal | Total Acidic Group Content (mmol/g) | Carboxylic Acid Content (μmol/g) | Phenol Hydroxyl Content (mmol/g) |
|---|---|---|---|
| Pre-activation | 2.89 | 282 | 2.61 |
| Activated | 3.23 | 454 | 2.77 |
| Element | Content (μg/mL) (before Activation) | Content (μg/mL) (after Activation) |
|---|---|---|
| Mn | 0.0397 (2.10%) | 0.0367 (1.01%) |
| Cu | 0.0414 (0.13%) | 0.0398 (0.11%) |
| Cd | 0 (2.68%) | 0 (4.33%) |
| As | 0.0062 (3.54%) | 0.0064 (2.68%) |
| Mg | 0.0072 (1.75%) | 0.0036 (3.41%) |
| Ca | 0.0107 (0.81%) | 0.0088 (0.57%) |
| Cr | 0.0051 (2.41%) | 0.0062 (3.31%) |
| Al | 0.0074 (0.98%) | 0.0079 (2.64%) |
| Fe | 0.0186 (2.06%) | 0.0121 (3.11%) |
| Pb | 0.0069 (0.75%) | 0.0045 (1.03%) |
| Zn | 0.0088 (2.43%) | 0.0084 (1.36%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Xiao, R.; Condé, S.M.; Dong, C.; Xun, Y.; Guo, D.; Liu, H.; Liu, K.; Liang, M. Preparation of Humic Acid from Weathered Coal by Mechanical Energy Activation and Its Properties. Minerals 2024, 14, 648. https://doi.org/10.3390/min14070648
Feng X, Xiao R, Condé SM, Dong C, Xun Y, Guo D, Liu H, Liu K, Liang M. Preparation of Humic Acid from Weathered Coal by Mechanical Energy Activation and Its Properties. Minerals. 2024; 14(7):648. https://doi.org/10.3390/min14070648
Chicago/Turabian StyleFeng, Xiujuan, Rilong Xiao, Sékou Mohamed Condé, Chengliang Dong, Yanping Xun, Dalong Guo, Hui Liu, Kunpeng Liu, and Mingzhi Liang. 2024. "Preparation of Humic Acid from Weathered Coal by Mechanical Energy Activation and Its Properties" Minerals 14, no. 7: 648. https://doi.org/10.3390/min14070648
APA StyleFeng, X., Xiao, R., Condé, S. M., Dong, C., Xun, Y., Guo, D., Liu, H., Liu, K., & Liang, M. (2024). Preparation of Humic Acid from Weathered Coal by Mechanical Energy Activation and Its Properties. Minerals, 14(7), 648. https://doi.org/10.3390/min14070648






