Assessing an Abandoned Pyrite Cinder Deposit in Southeast Spain with Electrical Resistivity Tomography: A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Direct Current Method
2.3. Geochemistry Analysis
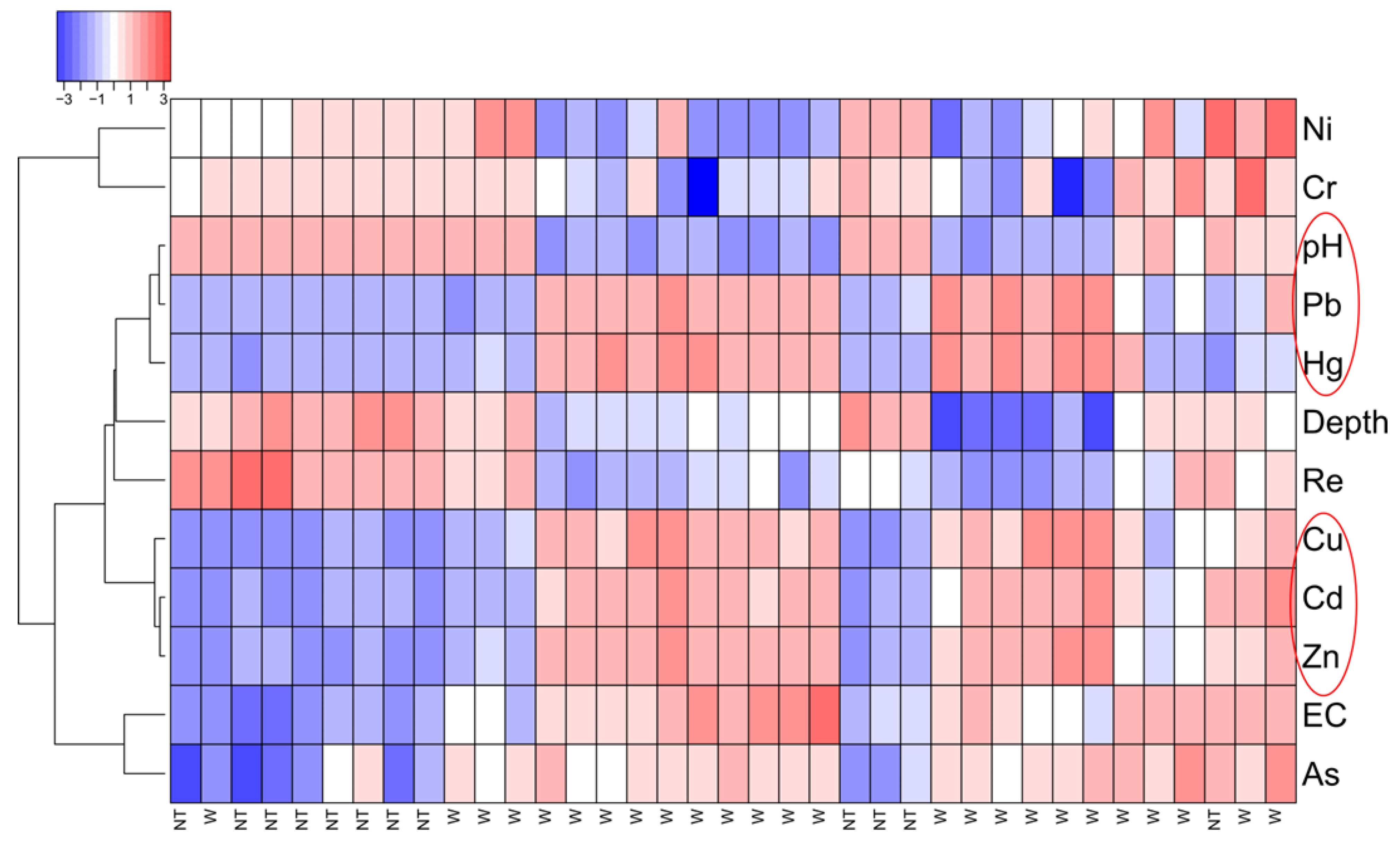
2.4. Statistical Analysis
3. Results and Discussion
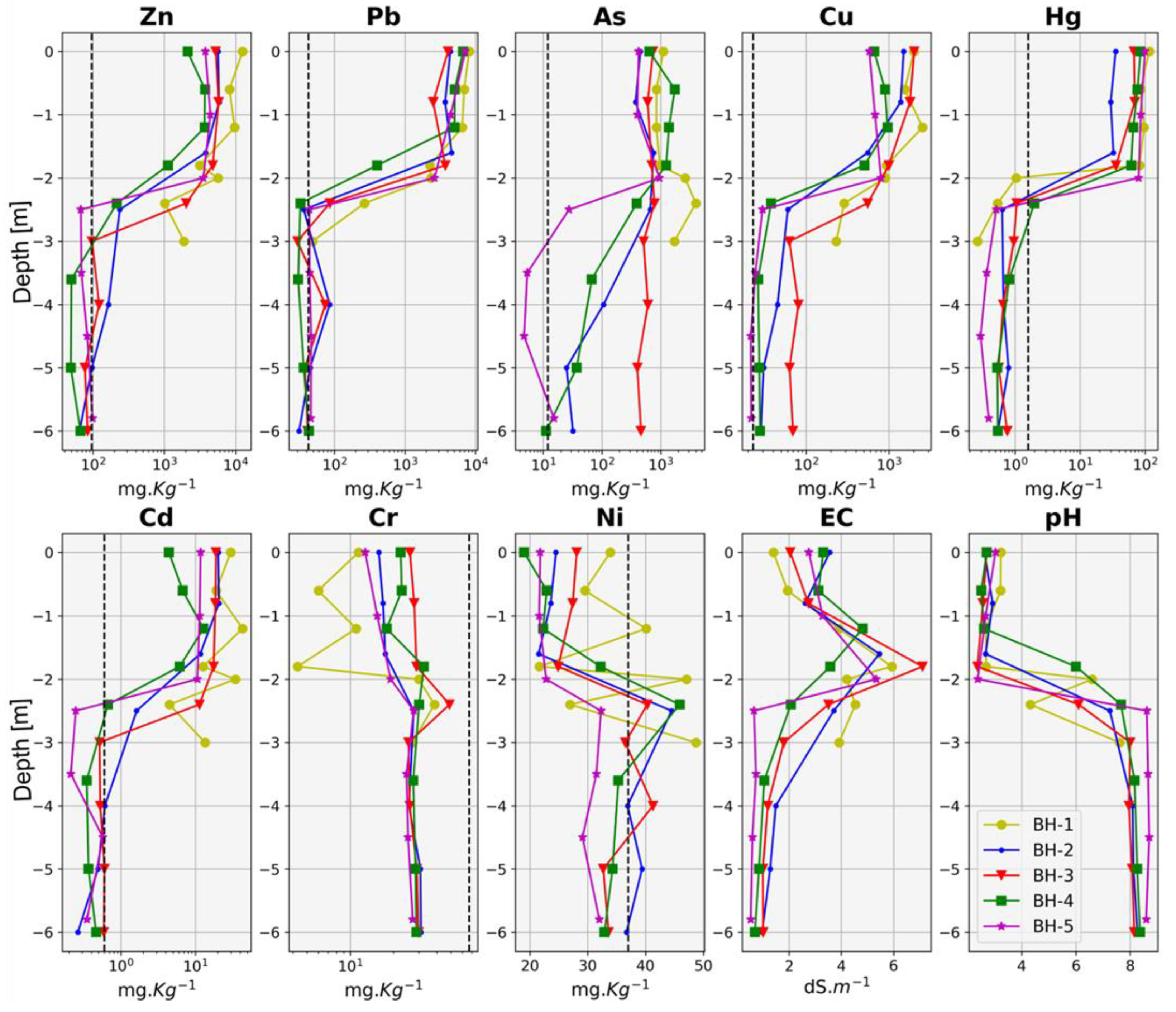
3.1. Geochemistry of the Pyrite Cinders
3.2. Electrical Resistivity Tomography and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alp, I.; Deveci, H.; Yazıcı, E.; Türk, T.; Süngün, Y. Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations. J. Hazard. Mater. 2009, 166, 144–149. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Hu, M.; Tao, L.; Qin, W.; Qiu, G. Role of pyrite in sulfuric acid leaching of chalcopyrite: An elimination of polysulfide by controlling redox potential. Hydrometallurgy 2016, 164, 159–165. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Spille, U.; Gabarrón, M.; Faz, Á.; Acosta, J.A. Evaluation of strategies for mitigating risks associated with metals in pyrite ash. J. Environ. Manag. 2018, 217, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bendz, D.; Tiberg, C.; Kleja, D.B. Mineralogical characterization and speciation of sulfur, zinc and lead in pyrite cinder from Bergvik, Sweden. Appl. Geochem. 2021, 131, 105010. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Han, Y.; Tang, Z.; Zhang, Y. Recovery of iron from pyrite cinder by suspension magnetization roasting-magnetic separation method: Process optimization and mechanism study. Sep. Purif. Technol. 2024, 332, 125652. [Google Scholar] [CrossRef]
- Fan, X.-H.; Deng, Q.; Gan, M.; Wang, H.-B. Effect of biochar as reductant on magnetizing-roasting behavior of pyrite cinder. J. Iron Steel Res. Int. 2015, 22, 371–376. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Selective adsorption mechanism of salicylic acid on pyrite surfaces and its application in flotation separation of chalcopyrite from pyrite. Sep. Purif. Technol. 2020, 240, 116650. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q.; Bai, X. Pyrogallic acid as depressant for flotation separation of pyrite from chalcopyrite under low-alkalinity conditions. Sep. Purif. Technol. 2021, 267, 118670. [Google Scholar] [CrossRef]
- Bai, X.; Liu, J.; Wen, S.; Lin, Y. Selective separation of chalcopyrite and pyrite using a novel organic depressant at low alkalinity. Miner. Eng. 2022, 185, 107677. [Google Scholar] [CrossRef]
- European Environment Agency. Progress in the Management of Contaminated Sites in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/progress-in-the-management-of (accessed on 1 September 2023).
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179 Pt A, 108792. [Google Scholar] [CrossRef]
- ATSDR. Toxic Substances Portal. Available online: https://wwwn.cdc.gov/TSP/substances/SubstanceAZ.aspx?SST=A1 (accessed on 14 May 2024).
- Samouëlian, A.; Cousin, I.; Tabbagh, A.; Bruand, A.; Richard, G. Electrical resistivity survey in soil science: A review. Soil Tillage Res. 2005, 83, 173–193. [Google Scholar] [CrossRef]
- Loiseau, B.; Carrière, S.D.; Jougnot, D.; Singha, K.; Mary, B.; Delpierre, N.; Guérin, R.; Martin-StPaul, N.K. The geophysical toolbox applied to forest ecosystems—A review. Sci. Total. Environ. 2023, 899, 165503. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, T.; Bernstone, C.; Loke, M.H. A 3-D resistivity investigation of a contaminated site at Lernacken, Sweden. Geophysics 2002, 67, 1692–1700. [Google Scholar] [CrossRef]
- Cuong, L.P.; Van Tho, L.; Juzsakova, T.; Rédey, Á.; Hai, H. Imaging the movement of toxic pollutants with 2D electrical resistivity tomography (ERT) in the geological environment of the Hoa Khanh Industrial Park, Da Nang, Vietnam. Environ. Earth Sci. 2016, 75, 286. [Google Scholar] [CrossRef]
- Martín-Crespo, T.; Gómez-Ortiz, D.; Martín-Velázquez, S.; Martínez-Pagán, P.; José, C.d.I.-S.; Lillo, J.; Faz, Á. Abandoned Mine Tailings Affecting Riverbed Sediments in the Cartagena–La Union District, Mediterranean Coastal Area (Spain). Remote Sens. 2020, 12, 2042. [Google Scholar] [CrossRef]
- Martínez-Pagán, P.; Gómez-Ortiz, D.; Martín-Crespo, T.; Martín-Velázquez, S.; Martínez-Segura, M. Electrical Resistivity Imaging Applied to Tailings Ponds: An Overview. Mine Water Environ. 2021, 40, 285–297. [Google Scholar] [CrossRef]
- Martínez-Segura, M.A.; Vásconez-Maza, M.D.; García-Nieto, M.C. Volumetric characterisation of waste deposits generated during the production of fertiliser derived from phosphoric rock by using LiDAR and electrical resistivity tomography. Sci. Total. Environ. 2020, 716, 137076. [Google Scholar] [CrossRef] [PubMed]
- Dimech, A.; Cheng, L.; Chouteau, M.; Chambers, J.; Uhlemann, S.; Wilkinson, P.; Meldrum, P.; Mary, B.; Fabien-Ouellet, G.; Isabelle, A. A Review on Applications of Time-Lapse Electrical Resistivity Tomography Over the Last 30 Years: Perspectives for Mining Waste Monitoring. Surv. Geophys. 2022, 43, 1699–1759. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.A.; Netto, L.G.; Camarero, P.L.; Bertuluci, F.B.; Hartwig, M.E.; Domingos, R. Application of electrical resistivity tomography (ERT) in uranium mining earth dam. J. Geophys. Eng. 2022, 19, 1265–1279. [Google Scholar] [CrossRef]
- Sabor, K.; Jougnot, D.; Guerin, R.; Steck, B.; Henault, J.-M.; Apffel, L.; Vautrin, D. A data mining approach for improved interpretation of ERT inverted sections using the DBSCAN clustering algorithm. Geophys. J. Int. 2021, 225, 1304–1318. [Google Scholar] [CrossRef]
- Boon, D.P.; Chambers, J.E.; Hobbs, P.R.; Kirkham, M.; Merritt, A.J.; Dashwood, C.; Pennington, C.; Wilby, P.R. A combined geomorphological and geophysical approach to characterising relict landslide hazard on the Jurassic Escarpments of Great Britain. Geomorphology 2015, 248, 296–310. [Google Scholar] [CrossRef][Green Version]
- Guinea, A.; Bicknell, J.; Cox, N.; Swan, H.; Simmons, N. Characterization of legacy landfills with electrical resistivity tomography; a comparative study. J. Appl. Geophys. 2022, 203, 104716. [Google Scholar] [CrossRef]
- Cheng, Q.; Tao, M.; Chen, X.; Binley, A. Evaluation of electrical resistivity tomography (ERT) for mapping the soil–rock interface in karstic environments. Environ. Earth Sci. 2019, 78, 439. [Google Scholar] [CrossRef]
- Evangelista, L.; de Silva, F.; D’onofrio, A.; Di Fiore, V.; Silvestri, F.; di Santolo, A.S.; Cavuoto, G.; Punzo, M.; Tarallo, D. Application of ERT and GPR geophysical testing to the subsoil characterization of cultural heritage sites in Napoli (Italy). Measurement 2017, 104, 326–335. [Google Scholar] [CrossRef]
- Gabarrón, M.; Faz, A.; Acosta, J. Use of multivariable and redundancy analysis to assess the behavior of metals and arsenic in urban soil and road dust affected by metallic mining as a base for risk assessment. J. Environ. Manag. 2018, 206, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Vásconez-Maza, M.D.; Bueso, M.C.; Faz, A.; Acosta, J.; Martínez-Segura, M.A. Assessing the behaviour of heavy metals in abandoned phosphogypsum deposits combining electrical resistivity tomography and multivariate analysis. J. Environ. Manag. 2021, 278 Pt 1, 111517. [Google Scholar] [CrossRef] [PubMed]
- AEMET. Predicción Por Municipios. San Javier (Murcia). Available online: https://www.aemet.es/es/eltiempo/prediccion/municipios/san-javier-id30035 (accessed on 15 May 2024).
- IGME. Geological Map of Cartagena, Spain. Available online: http://info.igme.es/cartografiadigital/datos/mgd50/memorias/Memoria977.pdf (accessed on 15 May 2024).
- Ayuntamiento de Cartagena. Available online: https://www.cartagena.es/barrios_diputaciones.asp (accessed on 15 May 2024).
- Everett, M.E. Near-Surface Applied Geophysics; Cambridge University Press (CUP): Cambridge, UK, 2013. [Google Scholar]
- Loke, M.H.; Chambers, J.E.; Rucker, D.F.; Kuras, O.; Wilkinson, P.B. Recent developments in the direct-current geoelectrical imaging method. J. Appl. Geophys. 2013, 95, 135–156. [Google Scholar] [CrossRef]
- Reynolds, J.M. An Introduction to Applied and Environmental Geophysics, 2nd ed.; John Wiley & Son: Hoboken, NJ, USA, 2011. [Google Scholar]
- IRIS-Instruments. Available online: http://www.iris-instruments.com/er-product.html#electrical-resistivity (accessed on 28 August 2023).
- Loke, M.H. Tutorial: 2-D and 3-D Electrical Imaging Surveys. Available online: https://sites.ualberta.ca/~unsworth/UA-classes/223/loke_course_notes.pdf (accessed on 1 January 2020).
- Vasconez-Maza, M.D.; Thiesson, J.; Guerin, R.; Delarue, F.; Mendieta, A.; Jougnot, D. Optimisation of an ERT acquisition for soil-plant interaction in presence of biochar. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 23–28 April 2023. [Google Scholar]
- U.S. EPA. EPA Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. Available online: https://www.epa.gov/esam/us-epa-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-and-oils (accessed on 24 March 2020).
- BAM. Homepage—Trace Elements in Contaminated Soil. Available online: https://rrr.bam.de/RRR/Content/EN/Downloads/RM-Certificates/RM-cert-environment/bam_u110de.html (accessed on 29 August 2023).
- Real Decreto 9/2005, de 14 de Enero, Por El Que SE Establece la Relación de Actividades Potencialmente Contaminantes Del Suelo Y Los Criterios Y Estándares Para la Declaración de Suelos Contaminados. 2005. Available online: https://www.boe.es/eli/es/rd/2005/01/14/9 (accessed on 1 September 2023).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 20 July 2023).
- Engle, S.; Whalen, S.; Joshi, A.; Pollard, K.S. Unboxing cluster heatmaps. BMC Bioinform. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Blowes, D.W.; Ptacek, C.J.; Jambor, J.L.; Weisener, C.G.; Paktunc, D.; Gould, W.D.; Johnson, D.B. The geochemistry of acid mine drainage. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Maza, M.D.V.; Martínez-Segura, M.A.; Bueso, M.C.; Faz, Á.; García-Nieto, M.C.; Gabarrón, M.; Acosta, J.A. Predicting spatial distribution of heavy metals in an abandoned phosphogypsum pond combining geochemistry, electrical resistivity tomography and statistical methods. J. Hazard. Mater. 2019, 374, 392–400. [Google Scholar] [CrossRef]
- Rey, J.; Martínez, J.; Hidalgo, M.C.; Mendoza, R.; Sandoval, S. Assessment of Tailings Ponds by a Combination of Electrical (ERT and IP) and Hydrochemical Techniques (Linares, Southern Spain). Mine Water Environ. 2020, 40, 298–307. [Google Scholar] [CrossRef]
- Gabarrón, M.; Martínez-Pagán, P.; Martínez-Segura, M.A.; Bueso, M.C.; Martínez-Martínez, S.; Faz, Á.; Acosta, J.A. Electrical Resistivity Tomography as a Support Tool for Physicochemical Properties Assessment of Near-Surface Waste Materials in a Mining Tailing Pond (El Gorguel, SE Spain). Minerals 2020, 10, 559. [Google Scholar] [CrossRef]
- Martin-Crespo, T.; Gomez-Ortiz, D.; Velázquez, S.M.; Martínez-Pagán, P.; De Ignacio, C.; Lillo, J.; Faz, Á. Geoenvironmental characterization of unstable abandoned mine tailings combining geophysical and geochemical methods (Cartagena-La Union district, Spain). Eng. Geol. 2018, 232, 135–146. [Google Scholar] [CrossRef]
- Manrique, I.I.; Caterina, D.; Nguyen, F.; Hermans, T. Quantitative interpretation of geoelectric inverted data with a robust probabilistic approach. Geophysics 2023, 88, B73–B88. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 2013. [Google Scholar]
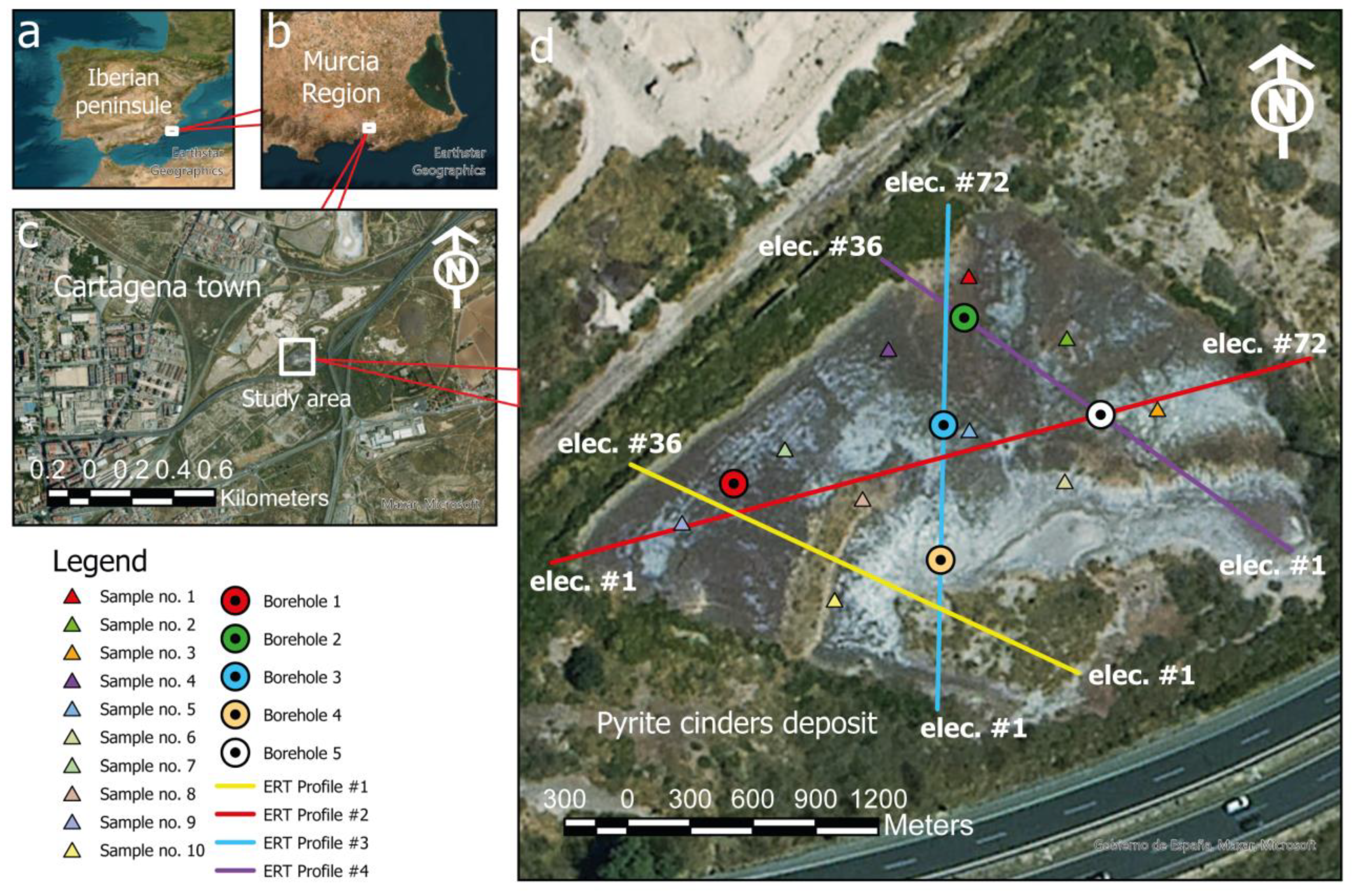
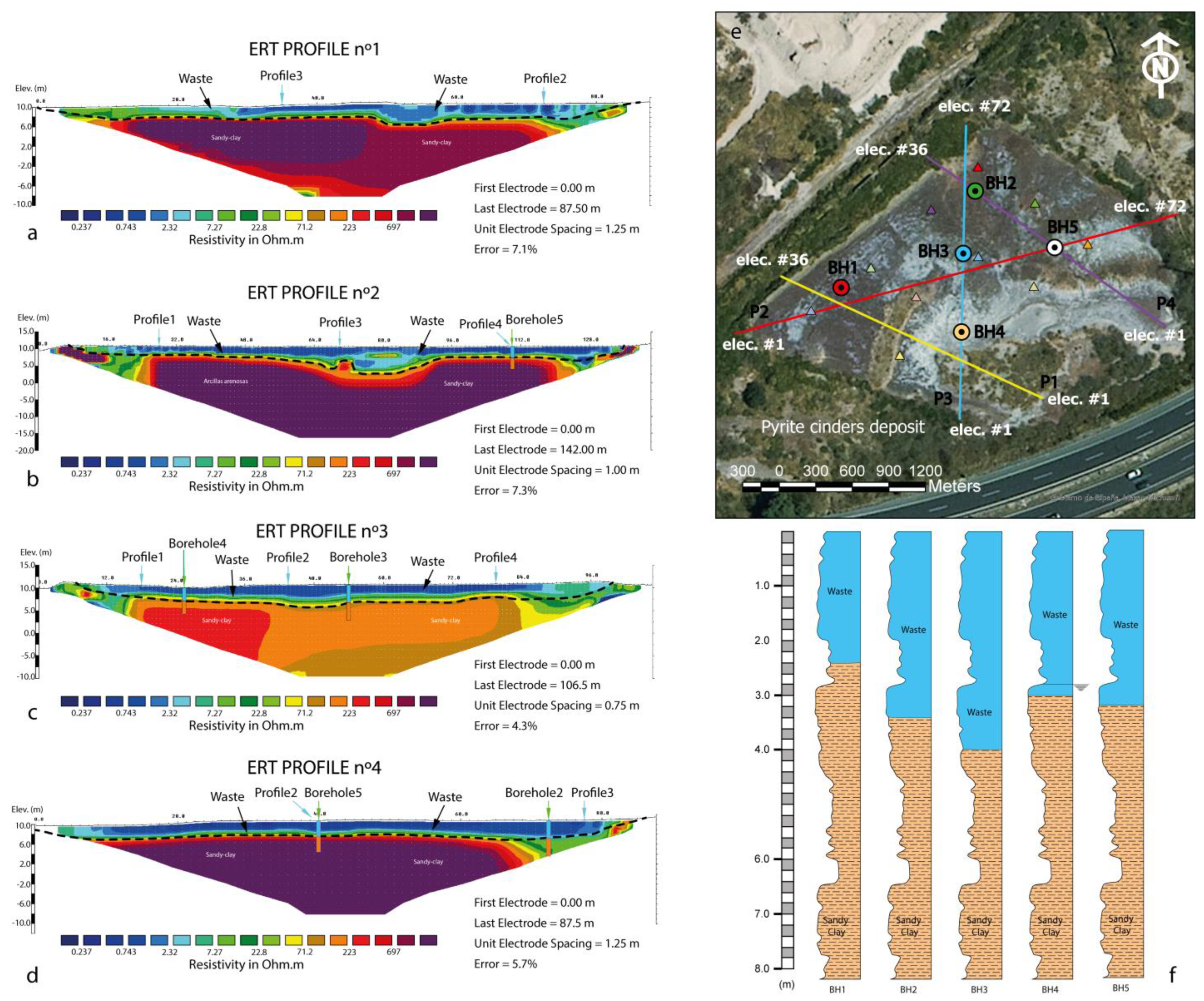
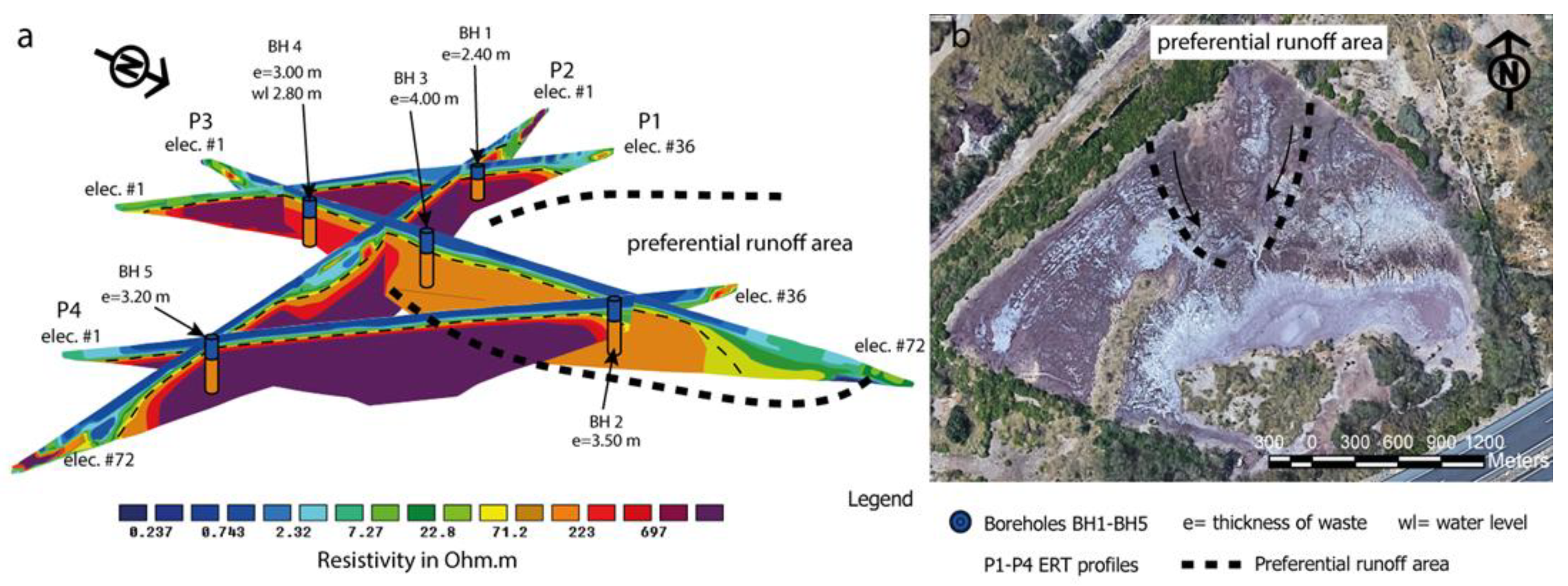
| #Elect | Sep (m) | Length (m) | |
|---|---|---|---|
| Profile 1 | 36 | 2.5 | 87.5 |
| Profile 2 | 72 | 2.0 | 142 |
| Profile 3 | 72 | 1.5 | 106.5 |
| Profile 4 | 36 | 2.5 | 87.5 |
| Sample | Cd | As | Cr | Cu | Ni | Pb | Zn | Hg | EC | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| mg·kg−1 | dS·m−1 | |||||||||
| S1 | 18.7 | 2518.0 | 8.5 | 1708.0 | 14.2 | 6720.0 | 7307.0 | 24.0 | 2.9 | 4.0 |
| S2 | 7.3 | 7137.0 | 8.4 | 564.0 | 3.8 | 5577.0 | 1839.0 | 31.0 | 5.0 | 3.1 |
| S3 | 5.3 | 7354.0 | 5.1 | 449.0 | 3.8 | 8768.0 | 1733.0 | 150.0 | 4.9 | 2.8 |
| S4 | 5.5 | 6859.0 | 7.0 | 345.0 | 4.9 | 6264.0 | 2045.0 | 43.0 | 5.4 | 3.4 |
| S5 | 4.1 | 4356.0 | 5.0 | 720.0 | 3.2 | 5399.0 | 1839.0 | 95.0 | 3.0 | 3.5 |
| S6 | 7.0 | 21,423.0 | 7.9 | 527.0 | 23.6 | 10,352.0 | 1849.0 | 87.0 | 5.4 | 2.7 |
| S7 | 14.7 | 3132.0 | 6.3 | 852.0 | 17.3 | 6380.0 | 7945.0 | 16.0 | 6.9 | 5.9 |
| S8 | 1.5 | 2231.0 | 3.2 | 419.0 | 2.3 | 8841.0 | 1002.0 | 108.0 | 1.7 | 3.4 |
| S9 | 4.4 | 6002.0 | 9.9 | 205.0 | 14.2 | 2996.0 | 1445.0 | 13.0 | 3.1 | 7.0 |
| S10 | 1.2 | 4535.0 | 5.1 | 678.0 | 5.7 | 8878.0 | 1409.0 | 49.0 | 1.1 | 3.4 |
| Mean | 7.0 | 6554.7 | 6.6 | 646.7 | 9.3 | 7017.5 | 2841.3 | 61.6 | 3.9 | 3.9 |
| SD | 5.3 | 5273.1 | 2.0 | 396.6 | 7.0 | 2071.2 | 2413.1 | 43.6 | 1.7 | 1.3 |
| NGR | 0.6 | 12.0 | 67.0 | 23.0 | 37.0 | 43.0 | 96.0 | 1.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásconez-Maza, M.D.; Martínez-Segura, M.A.; Martínez-Pagán, P.; Bueso, M.C.; Capa-Camacho, X.; Jabrane, O.; Faz, Á. Assessing an Abandoned Pyrite Cinder Deposit in Southeast Spain with Electrical Resistivity Tomography: A Case Study. Minerals 2024, 14, 652. https://doi.org/10.3390/min14070652
Vásconez-Maza MD, Martínez-Segura MA, Martínez-Pagán P, Bueso MC, Capa-Camacho X, Jabrane O, Faz Á. Assessing an Abandoned Pyrite Cinder Deposit in Southeast Spain with Electrical Resistivity Tomography: A Case Study. Minerals. 2024; 14(7):652. https://doi.org/10.3390/min14070652
Chicago/Turabian StyleVásconez-Maza, Marco D., Marcos A. Martínez-Segura, Pedro Martínez-Pagán, María C. Bueso, Ximena Capa-Camacho, Oussama Jabrane, and Ángel Faz. 2024. "Assessing an Abandoned Pyrite Cinder Deposit in Southeast Spain with Electrical Resistivity Tomography: A Case Study" Minerals 14, no. 7: 652. https://doi.org/10.3390/min14070652
APA StyleVásconez-Maza, M. D., Martínez-Segura, M. A., Martínez-Pagán, P., Bueso, M. C., Capa-Camacho, X., Jabrane, O., & Faz, Á. (2024). Assessing an Abandoned Pyrite Cinder Deposit in Southeast Spain with Electrical Resistivity Tomography: A Case Study. Minerals, 14(7), 652. https://doi.org/10.3390/min14070652











