Inhibitory Effects of Polysaccharides on the Dolomitization Reaction of Calcite at 200 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Temperature Dolomite Synthesis Experiments
2.2. XRD and SEM Analyses
2.3. Transmission Electron Microscopy (TEM)
3. Results and Discussion
3.1. Reaction Pathway
3.2. Evolution of pH
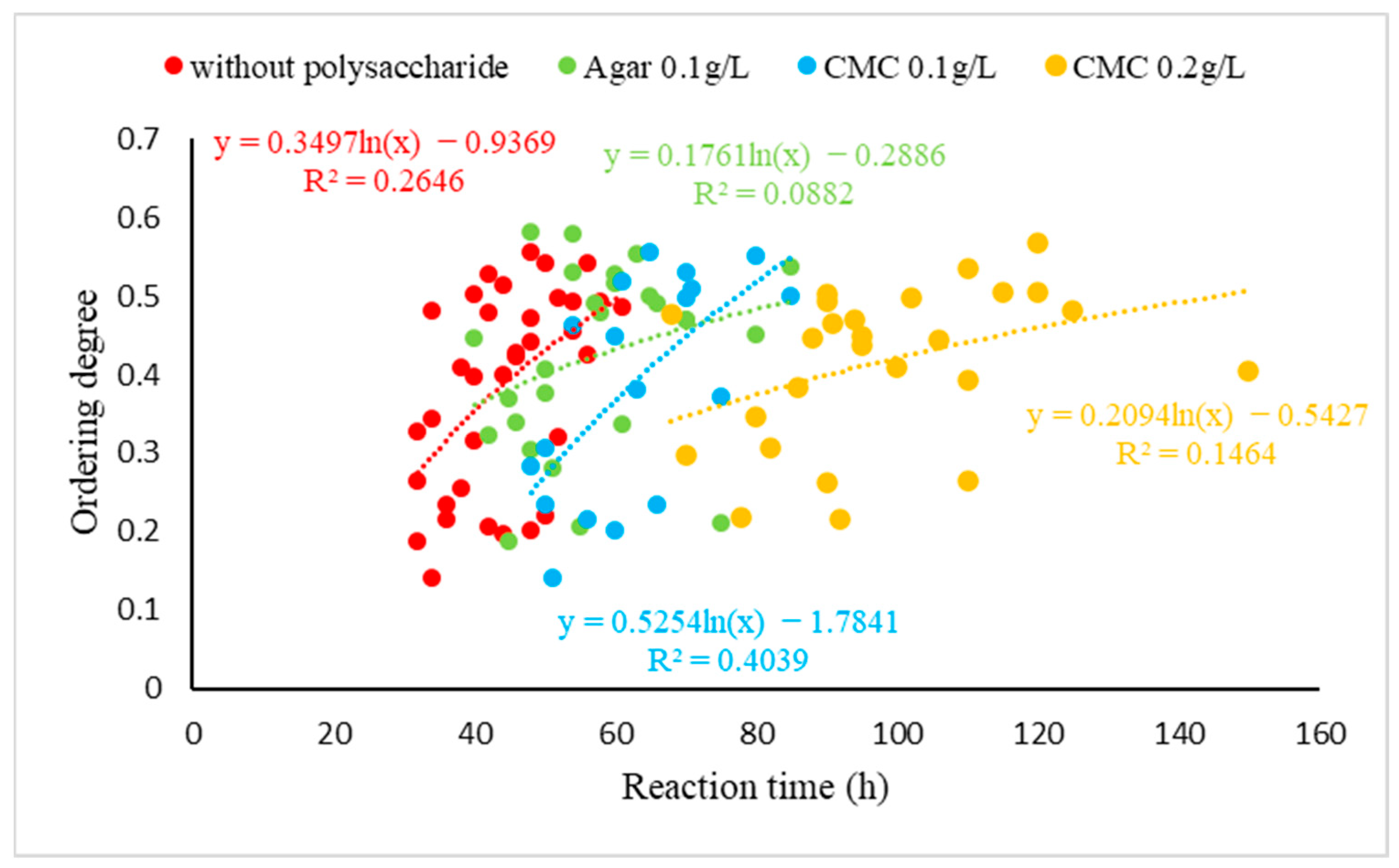
3.3. Stoichiometry and Cation Ordering
3.4. Polysaccharides’ Impact on Calcite Dolomitization
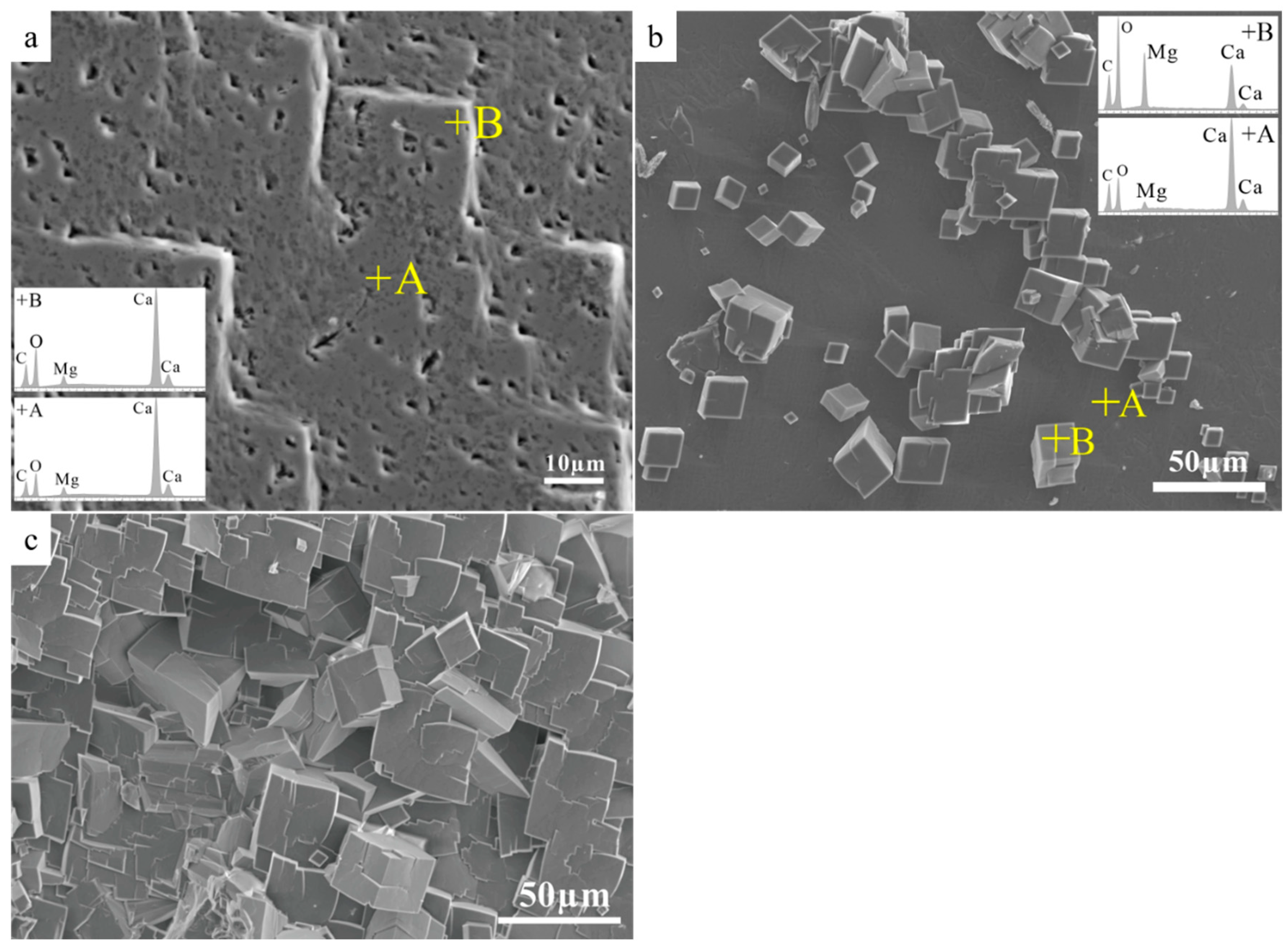
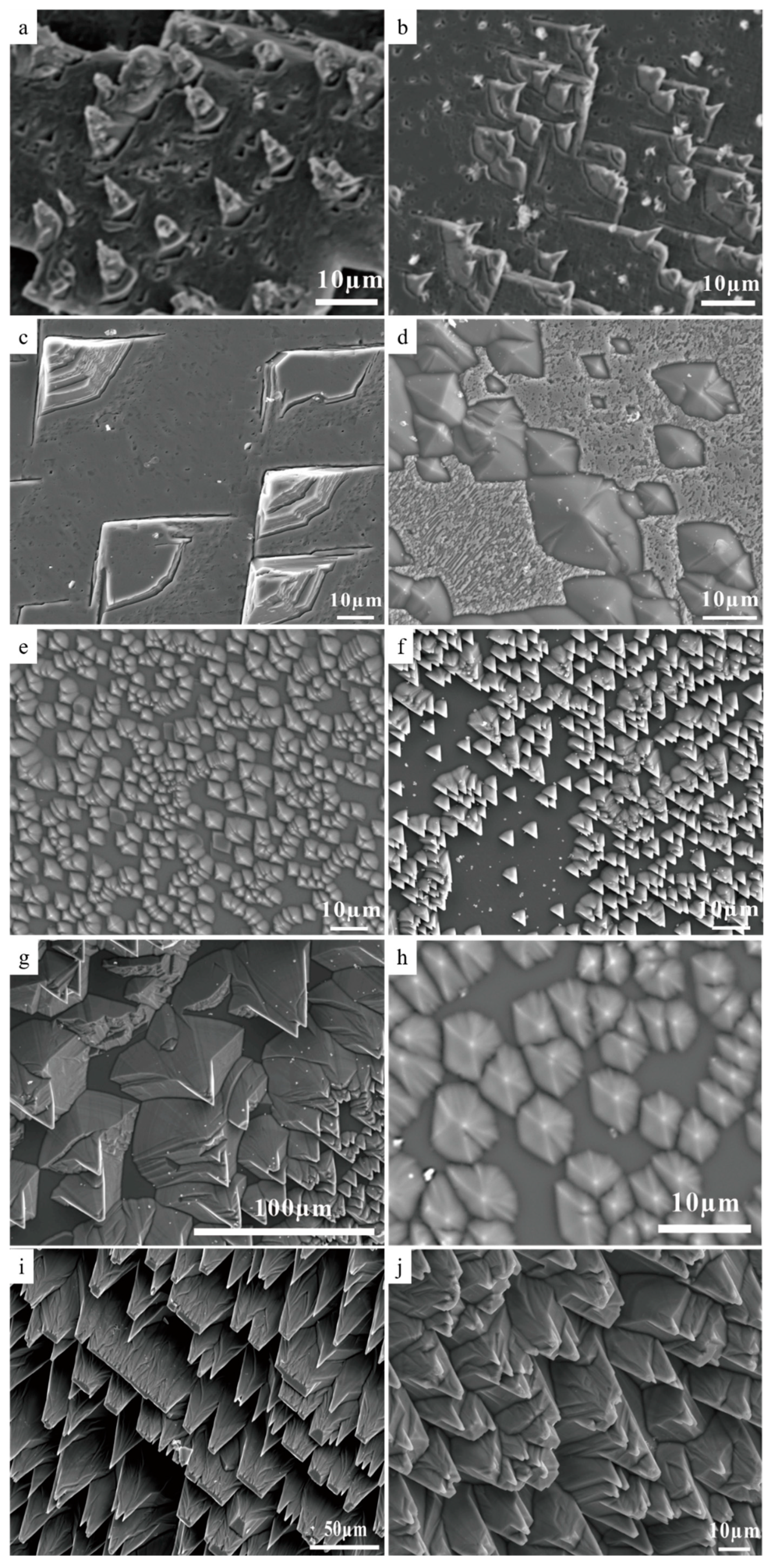
3.5. SEM Studies of Cleaved Calcite Crystal Reaction Products
3.6. Calcite Dissolution Inhibition by Surface Site Inhibition
3.7. HRTEM Observation of Antiphase Boundaries (APBs) in Dolomite
4. Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, Nucleation and Growth of Dolomite in the Laboratory and Sedimentary Environment: A Review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Budd, D.A. Cenozoic Dolomites of Carbonate Islands: Their Attributes and Origin. Earth Sci. Rev. 1997, 42, 1–47. [Google Scholar] [CrossRef]
- Blake, D.F.; Peacor, D.R.; Wilkinson, B.H. The Sequence and Mechanism of Low-Temperature Dolomite Formation; Calcian Dolomites in a Pennsylvanian Echinoderm. J. Sediment. Res. 1982, 52, 59–70. [Google Scholar] [CrossRef]
- Goldsmith, J.R.; Graf, D.L. Structural and Compositional Variations in Some Natural Dolomites. J. Geol. 1958, 66, 678–693. [Google Scholar] [CrossRef]
- Warren, J. Dolomite: Occurrence, Evolution and Economically Important Associations. Earth Sci. Rev. 2000, 52, 1–81. [Google Scholar] [CrossRef]
- Holland, H.D.; Zimmermann, H. The Dolomite Problem Revisited1. Int. Geol. Rev. 2000, 42, 481–490. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and Models of Dolomitization: A Critical Reappraisal. Geol. Soc. Lond. Spec. Publ. 2004, 235, 7–63. [Google Scholar] [CrossRef]
- McKenzie, J.A.; Vasconcelos, C. Dolomite Mountains and the Origin of the Dolomite Rock of Which They Mainly Consist: Historical Developments and New Perspectives. Sedimentology 2009, 56, 205–219. [Google Scholar] [CrossRef]
- Petrash, D.A.; Bialik, O.M.; Bontognali, T.R.R.; Vasconcelos, C.; Roberts, J.A.; McKenzie, J.A.; Konhauser, K.O. Microbially Catalyzed Dolomite Formation: From near-Surface to Burial. Earth Sci. Rev. 2017, 171, 558–582. [Google Scholar] [CrossRef]
- Eriksson, P.G.; Banerjee, S.; Octavian, C.; Corcoran, P.; Eriksson, K.; Hiatt, E.; Laflamme, M.; Lenhardt, N.; Long, D.; Miall, A.; et al. Secular Changes in Sedimentation Systems and Sequence Stratigraphy. Gondwana Res. 2012, 24, 468–489. [Google Scholar] [CrossRef]
- Pina, C.M.; Pimentel, C.; Crespo, Á. Dolomite Cation Order in the Geological Record. Chem Geol 2020, 547, 119667. [Google Scholar] [CrossRef]
- Given, R.K.; Wilkinson, B.H. Dolomite Abundance and Stratigraphic Age; Constraints on Rates and Mechanisms of Phanerozoic Dolostone Formation. J. Sediment. Res. 1987, 57, 1068–1078. [Google Scholar] [CrossRef]
- Balci, N.; Demirel, C.; Akcer Ön, S.; Gültekin, A.H.; Kurt, M.A. Evaluating Abiotic and Microbial Factors on Carbonate Precipitation in Lake Acigöl, a Hypersaline Lake in Southwestern Turkey. Quat. Int. 2018, 486, 116–128. [Google Scholar] [CrossRef]
- Last, F.M.; Last, W.M.; Halden, N.M. Carbonate Microbialites and Hardgrounds from Manito Lake, an Alkaline, Hypersaline Lake in the Northern Great Plains of Canada. Sediment. Geol. 2010, 225, 34–49. [Google Scholar] [CrossRef]
- Last, F.M.; Last, W.M.; Halden, N.M. Modern and Late Holocene Dolomite Formation: Manito Lake, Saskatchewan, Canada. Sediment. Geol. 2012, 281, 222–237. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A. Microbial Mediation of Modern Dolomite Precipitation and Diagenesis under Anoxic Conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sediment. Res. 1997, 67, 378–390. [Google Scholar] [CrossRef]
- Diloreto, Z.A.; Garg, S.; Bontognali, T.R.R.; Dittrich, M. Modern Dolomite Formation Caused by Seasonal Cycling of Oxygenic Phototrophs and Anoxygenic Phototrophs in a Hypersaline Sabkha. Sci. Rep. 2021, 11, 4170. [Google Scholar] [CrossRef] [PubMed]
- Areias, C.; Barbosa, C.F.; Cruz, A.P.S.; McKenzie, J.A.; Ariztegui, D.; Eglinton, T.; Haghipour, N.; Vasconcelos, C.; Sánchez-Román, M. Organic Matter Diagenesis and Precipitation of Mg-Rich Carbonate and Dolomite in Modern Hypersaline Lagoons Linked to Climate Changes. Geochim. Cosmochim. Acta 2022, 337, 14–32. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.E.; Słowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I.D. Carbonate and Silicate Biomineralization in a Hypersaline Microbial Mat (Mesaieed Sabkha, Qatar): Roles of Bacteria, Extracellular Polymeric Substances and Viruses. Sedimentology 2018, 65, 1213–1245. [Google Scholar] [CrossRef]
- Samylina, O.S.; Zaytseva, L. V Characterization of Modern Dolomite Stromatolites from Hypersaline Petukhovskoe Soda Lake, Russia. Lethaia 2019, 52, 1–13. [Google Scholar] [CrossRef]
- Bontognali, T.; Vasconcelos, C.; Warthmann, R.; Bernasconi, S.; Dupraz, C.; Strohmenger, C.; McKenzie, J. Dolomite Formation within Microbial Mats in the Coastal Sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 2010, 57, 824–844. [Google Scholar] [CrossRef]
- Mauger, C.; Compton, J. Formation of Modern Dolomite in Hypersaline Pans of the Western Cape, South Africa. Sedimentology 2011, 58, 1678–1692. [Google Scholar] [CrossRef]
- Rosen, M.R.; Coshell, L. A New Location of Holocene Dolomite Formation, Lake Hayward, Western Australia. Sedimentology 1992, 39, 161–166. [Google Scholar] [CrossRef]
- De Deckker, P.; Last, W.M. Modern Dolomite Deposition in Continental, Saline Lakes, Western Victoria, Australia. Geology 1988, 16, 29–32. [Google Scholar] [CrossRef]
- Angeletti, L.; Canese, S.; Franchi, F.; Montagna, P.; Reitner, J.; Walliser, E.O.; Taviani, M. The “Chimney Forest” of the Deep Montenegrin Margin, South-Eastern Adriatic Sea. Mar. Pet. Geol. 2015, 66, 542–554. [Google Scholar] [CrossRef]
- Bian, Y.; Feng, D.; Roberts, H.H.; Chen, D. Tracing the Evolution of Seep Fluids from Authigenic Carbonates: Green Canyon, Northern Gulf of Mexico. Mar. Pet. Geol. 2013, 44, 71–81. [Google Scholar] [CrossRef]
- Li, J.; Peng, X.; Bai, S.; Chen, Z.; Van Nostrand, J.D. Biogeochemical Processes Controlling Authigenic Carbonate Formation within the Sediment Column from the Okinawa Trough. Geochim. Cosmochim. Acta 2018, 222, 363–382. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, X.; Lin, Z.; Xu, L.; Gong, J.; Lu, H. Cold Seep Status Archived in Authigenic Carbonates: Mineralogical and Isotopic Evidence from Northern South China Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 95–105. [Google Scholar] [CrossRef]
- Magalhães, V.H.; Pinheiro, L.M.; Ivanov, M.K.; Kozlova, E.; Blinova, V.; Kolganova, J.; Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.M.; Kopf, A.J.; et al. Formation Processes of Methane-Derived Authigenic Carbonates from the Gulf of Cadiz. Sediment. Geol. 2012, 243–244, 155–168. [Google Scholar] [CrossRef]
- Naehr, T.H.; Eichhubl, P.; Orphan, V.J.; Hovland, M.; Paull, C.K.; Ussler, W.; Lorenson, T.D.; Greene, H.G. Authigenic Carbonate Formation at Hydrocarbon Seeps in Continental Margin Sediments: A Comparative Study. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1268–1291. [Google Scholar] [CrossRef]
- Stakes, D.S.; Orange, D.; Paduan, J.B.; Salamy, K.A.; Maher, N. Cold-Seeps and Authigenic Carbonate Formation in Monterey Bay, California. Mar. Geol. 1999, 159, 93–109. [Google Scholar] [CrossRef]
- Takeuchi, R.; Matsumoto, R.; Ogihara, S.; Machiyama, H. Methane-Induced Dolomite “Chimneys” on the Kuroshima Knoll, Ryukyu Islands, Japan. J. Geochem. Explor. 2007, 95, 16–28. [Google Scholar] [CrossRef]
- Cavagna, S.; Clari, P.; Martire, L. The Role of Bacteria in the Formation of Cold Seep Carbonates: Geological Evidence from Monferrato (Tertiary, NW Italy). Sediment. Geol. 1999, 126, 253–270. [Google Scholar] [CrossRef]
- Wang, S.; Yan, W.; Chen, Z.; Zhang, N.; Chen, H. Rare Earth Elements in Cold Seep Carbonates from the Southwestern Dongsha Area, Northern South China Sea. Mar. Pet. Geol. 2014, 57, 482–493. [Google Scholar] [CrossRef]
- Sun, W.; Jayaraman, S.; Chen, W.; Persson, K.A.; Ceder, G. Nucleation of Metastable Aragonite CaCO3 in Seawater. Proc. Natl. Acad. Sci. USA 2015, 112, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, P.A. An Oscillating Trend in Phanerozoic Non-Skeletal Carbonate Mineralogy. Nature 1983, 305, 19–22. [Google Scholar] [CrossRef]
- Hardie, L.A. Secular Variation in Seawater Chemistry: An Explanation for the Coupled Secular Variation in the Mineralogies of Marine Limestones and Potash Evaporites over the Past 600 m.y. Geology 1996, 24, 279–283. [Google Scholar] [CrossRef]
- Stanley, S.M.; Hardie, L.A. Secular Oscillations in the Carbonate Mineralogy of Reef-Building and Sediment-Producing Organisms Driven by Tectonically Forced Shifts in Seawater Chemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 144, 3–19. [Google Scholar] [CrossRef]
- Morse, J.W.; Wang, Q.; Tsio, M.Y. Influences of Temperature and Mg:Ca Ratio on CaCO3 Precipitates from Seawater. Geology 1997, 25, 85–87. [Google Scholar] [CrossRef]
- Hood, A.v.S.; Wallace, M.W.; Drysdale, R.N. Neoproterozoic Aragonite-Dolomite Seas? Widespread Marine Dolomite Precipitation in Cryogenian Reef Complexes. Geology 2011, 39, 871–874. [Google Scholar] [CrossRef]
- Van Smeerdijk Hood, A.; Wallace, M.W. Synsedimentary Diagenesis in a Cryogenian Reef Complex: Ubiquitous Marine Dolomite Precipitation. Sediment. Geol. 2012, 255–256, 56–71. [Google Scholar] [CrossRef]
- Lu, Y.; Paulmann, C.; Mihailova, B.; Malcherek, T.; Birgel, D.; López Correa, M.; Lin, Z.; Lu, L.; Milker, Y.; Peckmann, J. Fibrous Dolomite Formation at a Miocene Methane Seep May Reflect Neoproterozoic Aragonite-Dolomite Sea Conditions. Commun. Earth Environ. 2023, 4, 346. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Konishi, H.; Kemp, J.M.; Roden, E.E.; Shen, Z. Dissolved Sulfide-Catalyzed Precipitation of Disordered Dolomite: Implications for the Formation Mechanism of Sedimentary Dolomite. Geochim. Cosmochim. Acta 2012, 97, 148–165. [Google Scholar] [CrossRef]
- Lippmann, F. The Polymorphism Calcite-Aragonite. In Sedimentary Carbonate Minerals; Lippmann, F., Ed.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 97–147. ISBN 978-3-642-65474-9. [Google Scholar]
- Katz, A.K.; Glusker, J.P.; Markham, G.D.; Bock, C.W. Deprotonation of Water in the Presence of Carboxylate and Magnesium Ions. J. Phys. Chem. B 1998, 102, 6342–6350. [Google Scholar] [CrossRef]
- Xu, J.; Yan, C.; Zhang, F.; Konishi, H.; Xu, H.; Teng, H. Testing the Cation-Hydration Effect on the Crystallization of Ca-Mg-CO3 Systems. Proc. Natl. Acad. Sci. USA 2013, 110, 17750–17755. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Saldi, G.D.; Jordan, G.; Schott, J. On the Effect of Aqueous Barium on Magnesite Growth—A New Route for the Precipitation of the Ordered Anhydrous Mg-Bearing Double Carbonate Norsethite. Chem. Geol. 2017, 460, 93–105. [Google Scholar] [CrossRef]
- Pimentel, C.; Pina, C.M. The Formation of the Dolomite-Analogue Norsethite: Reaction Pathway and Cation Ordering. Geochim. Cosmochim. Acta 2014, 142, 217–223. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Yao, Q.-Z.; Qian, F.-J.; Li, H.; Zhou, G.-T.; Fu, S.-Q. Formation Pathway of Norsethite Dominated by Solution Chemistry under Ambient Conditions. Am. Mineral. 2021, 106, 1306–1318. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Konishi, H.; Shelobolina, E.S.; Roden, E.E. Polysaccharide-Catalyzed Nucleation and Growth of Disordered Dolomite: A Potential Precursor of Sedimentary Dolomite. Am. Mineral. 2012, 97, 556–567. [Google Scholar] [CrossRef]
- Roberts, J.; Kenward, P.; Fowle, D.; Goldstein, R.; Gonzalez, L.; Moore, D. Surface Chemistry Allows for Abiotic Precipitation of Dolomite at Low Temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 14540–14545. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Shelobolina, E.S.; Konishi, H.; Converse, B.; Shen, Z.; Roden, E.E. The Catalytic Effect of Bound Extracellular Polymeric Substances Excreted by Anaerobic Microorganisms on Ca-Mg Carbonate Precipitation: Implications for the “Dolomite Problem”. Am. Mineral. 2015, 100, 483–494. [Google Scholar] [CrossRef]
- Shen, Z.; Szlufarska, I.; Brown, P.E.; Xu, H. Investigation of the Role of Polysaccharide in the Dolomite Growth at Low Temperature by Using Atomistic Simulations. Langmuir 2015, 31, 10435–10442. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.; Grujic, D.; Tiens, A.J. Microbial Mediation as a Possible Mechanism for Natural Dolomite Formation at Low Temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Wright, D.T. The Role of Sulphate-Reducing Bacteria and Cyanobacteria in Dolomite Formation in Distal Ephemeral Lakes of the Coorong Region, South Australia. Sediment. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Warthmann, R.; van Lith, Y.; Vasconcelos, C.; McKenzie, J.A.; Karpoff, A.M. Bacterially Induced Dolomite Precipitation in Anoxic Culture Experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; Mckenzie, J.A. Sulphate-Reducing Bacteria Induce Low-Temperature Ca-Dolomite and High Mg-Calcite Formation. Geobiology 2003, 1, 71–79. [Google Scholar] [CrossRef]
- Roberts, J.A.; Bennett, P.C.; González, L.A.; Macpherson, G.L.; Milliken, K.L. Microbial Precipitation of Dolomite in Methanogenic Groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Wright, D.T.; Wacey, D. Precipitation of Dolomite Using Sulphate-Reducing Bacteria from the Coorong Region, South Australia: Significance and Implications. Sedimentology 2005, 52, 987–1008. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A.; Zenobi, R.; Rivadeneyra, M.A. Aerobic Microbial Dolomite at the Nanometer Scale: Implications for the Geologic Record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; González, L.A.; Roberts, J.A. Precipitation of Low-Temperature Dolomite from an Anaerobic Microbial Consortium: The Role of Methanogenic Archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef]
- Bontognali, T.; Vasconcelos, C.; Warthmann, R.; Lundberg, R.; McKenzie, J. Dolomite-mediating Bacterium Isolated from the Sabkha of Abu Dhabi (UAE). Terra Nova 2012, 24, 248–254. [Google Scholar] [CrossRef]
- Alibrahim, A.; Al-Gharabally, D.; Mahmoud, H.; Dittrich, M. Proto-Dolomite Formation in Microbial Consortia Dominated by Halomonas Strains. Extremophiles 2019, 23, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Daye, M.; Higgins, J.; Bosak, T. Formation of Ordered Dolomite in Anaerobic Photosynthetic Biofilms. Geology 2019, 47, 509–512. [Google Scholar] [CrossRef]
- Deng, S.; Dong, H.; Lv, G.; Jiang, H.; Yu, B.; Bishop, M.E. Microbial Dolomite Precipitation Using Sulfate Reducing and Halophilic Bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Qiu, X.; Yao, Y.; Wang, H.; Shen, A.; Zhang, J. Halophilic Archaea Mediate the Formation of Proto-Dolomite in Solutions With Various Sulfate Concentrations and Salinities. Front. Microbiol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; McKenzie, J.A. Microbial Dolomite Precipitation under Aerobic Conditions: Results from Brejo Do Espinho Lagoon (Brazil) and Culture Experiments. In Perspectives in Carbonate Geology: A Tribute to the Career of Robert Nathan Ginsburg; International Association of Sedimentologists: Gent, Belgium, 2009; pp. 167–178. [Google Scholar]
- Zheng, T.; Qian, C. Influencing Factors and Formation Mechanism of CaCO3 Precipitation Induced by Microbial Carbonic Anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Shelobolina, E.S.; Konishi, H.; Roden, E.E. Precipitation of Low-Temperature Disordered Dolomite Induced by Extracellular Polymeric Substances of Methanogenic Archaea Methanosarcina Barkeri: Implications for Sedimentary Dolomite Formation. Am. Mineral. 2021, 106, 69–81. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, D.; Papineau, D.; Qiu, X.; Wang, H.; She, Z.; Zhao, L. Precipitation of High Mg-Calcite and Protodolomite Using Dead Biomass of Aerobic Halophilic Bacteria. J. Earth Sci. 2023, 34, 456–466. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; McKenzie, J.A.; Warthmann, R.J.; Vasconcelos, C. Microbially Influenced Formation of Mg-Calcite and Ca-Dolomite in the Presence of Exopolymeric Substances Produced by Sulphate-Reducing Bacteria. Terra Nova 2014, 26, 72–77. [Google Scholar] [CrossRef]
- Kim, J.; Kimura, Y.; Puchala, B.; Yamazaki, T.; Becker, U.; Sun, W. Dissolution Enables Dolomite Crystal Growth near Ambient Conditions. Science (1979) 2023, 382, 915–920. [Google Scholar] [CrossRef]
- Hasegawa, M.; Konishi, H.; Kubota, Y. Inorganic Synthesis of Disordered Dolomite at Room Temperature. In Proceedings of the Goldschmidt, Lyon, France, 9–14 July 2023. [Google Scholar]
- Royse, C.F.; Wadell, J.S.; Petersen, L.E. X-Ray Determination of Calcite-Dolomite: An Evaluation. J. Sediment. Res. 1971, 41, 483–488. [Google Scholar] [CrossRef]
- Lumsden, D.N.; Chimahusky, J.S. Relationship between Dolomite Nonstoichiometry and Carbonate Facies Parameters. In Concepts and Models of Dolomitization; Zenger, D.H., Dunham, J.B., Ethington, R.L., Eds.; Special Publication; Society of Economic Paleontologists and Mineralogists: Claremore, OK, USA, 1980; Volume 28, pp. 123–137. [Google Scholar]
- Füchtbauer, H.; Goldschmidt, H. Beziehungen Zwischen Calciumgehalt Und Bildungsbedingungen Der Dolomite. Geol. Rundsch. 1966, 55, 29–40. [Google Scholar] [CrossRef]
- Sibley, D.F.; Nordeng, S.H.; Borkowski, M.L. Dolomitization Kinetics of Hydrothermal Bombs and Natural Settings. J. Sediment. Res. 1994, 64, 630–637. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Sibley, D.F. On the Evolution of Dolomite Stoichiometry and Cation Order during High-Temperature Synthesis Experiments: An Alternative Model for the Geochemical Evolution of Natural Dolomites. Sediment. Geol. 2011, 240, 30–40. [Google Scholar] [CrossRef]
- Kaczmarek, S.E.; Sibley, D.F. Direct Physical Evidence of Dolomite Recrystallization. Sedimentology 2014, 61, 1862–1882. [Google Scholar] [CrossRef]
- Li, J.; Chen, F.; Song, N.; Li, B.; Ma, Y. Investigation on the Influence of Additives on the Oriented Dissolution of Calcite. Soft Matter 2021, 17, 5025–5033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Qi, L.; Ma, Y. Investigation of the Influence of Cationic and Anionic Ions on the Oriented Dissolution of Calcite. CrystEngComm 2020, 22, 5316–5322. [Google Scholar] [CrossRef]
- Meng, R.; Ma, Y.; Long, X.; Qi, L. Calcite Microrod Arrays Fabricated via Anisotropic Dissolution of Calcite in the Presence of NH4I and (NH4)2SO4. CrystEngComm 2013, 15, 8867–8873. [Google Scholar] [CrossRef]
- Long, X.; Meng, R.; Wu, W.; Ma, Y.; Qi, L. Calcite Microneedle Arrays Produced by Inorganic Ion-Assisted Anisotropic Dissolution of Bulk Calcite Crystal. Chemistry 2014, 20, 4264–4272. [Google Scholar] [CrossRef]
- Gutjahr, A.; Dabringhaus, H.; Lacmann, R. Studies of the Growth and Dissolution Kinetics of the CaCO3 Polymorphs Calcite and Aragonite II. The Influence of Divalent Cation Additives on the Growth and Dissolution Rates. J. Cryst. Growth 1996, 158, 310–315. [Google Scholar] [CrossRef]
- Parsiegla, K.I.; Katz, J.L. Calcite Growth Inhibition by Copper(II): II. Effect of Solution Composition. J. Cryst. Growth 2000, 213, 368–380. [Google Scholar] [CrossRef]
- Vinson, M.D.; Arvidson, R.S.; Luttge, A. Kinetic Inhibition of Calcite (104) Dissolution by Aqueous Manganese(II). J. Cryst. Growth 2007, 307, 116–125. [Google Scholar] [CrossRef]
- Olsen, R.; Leirvik, K.N.; Kvamme, B. Adsorption Characteristics of Glycols on Calcite and Hematite. AIChE J. 2019, 65, e16728. [Google Scholar] [CrossRef]
- Tang, H.; Xian, H.; He, H.; Wei, J.; Liu, H.; Zhu, J.; Zhu, R. Kinetics and Mechanisms of the Interaction between the Calcite (10.4) Surface and Cu2+-Bearing Solutions. Sci. Total Environ. 2019, 668, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Berelson, W.M.; Adkins, J.F.; Rollins, N.E.; Naviaux, J.D.; Pirbadian, S.; El-Naggar, M.Y.; Teng, H.H. An Atomic Force Microscopy Study of Calcite Dissolution in Seawater. Geochim. Cosmochim. Acta 2020, 283, 40–53. [Google Scholar] [CrossRef]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Cellulose at Temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Machel, H. Investigation of Burial Diagenesis in Carbonate Hydrocarbon Reservoir Rocks. Geosci. Can. 2005, 32, 103–128. [Google Scholar]
- Veetil, S.P.; Mucci, A.; Arakaki, T. Dolomite Dissolution Kinetics in Aqueous Solutions in the Presence of Organic and Inorganic Additives at 25 °C and PCO2~1 atm. Chem. Geol. 2018, 483, 98–110. [Google Scholar] [CrossRef]


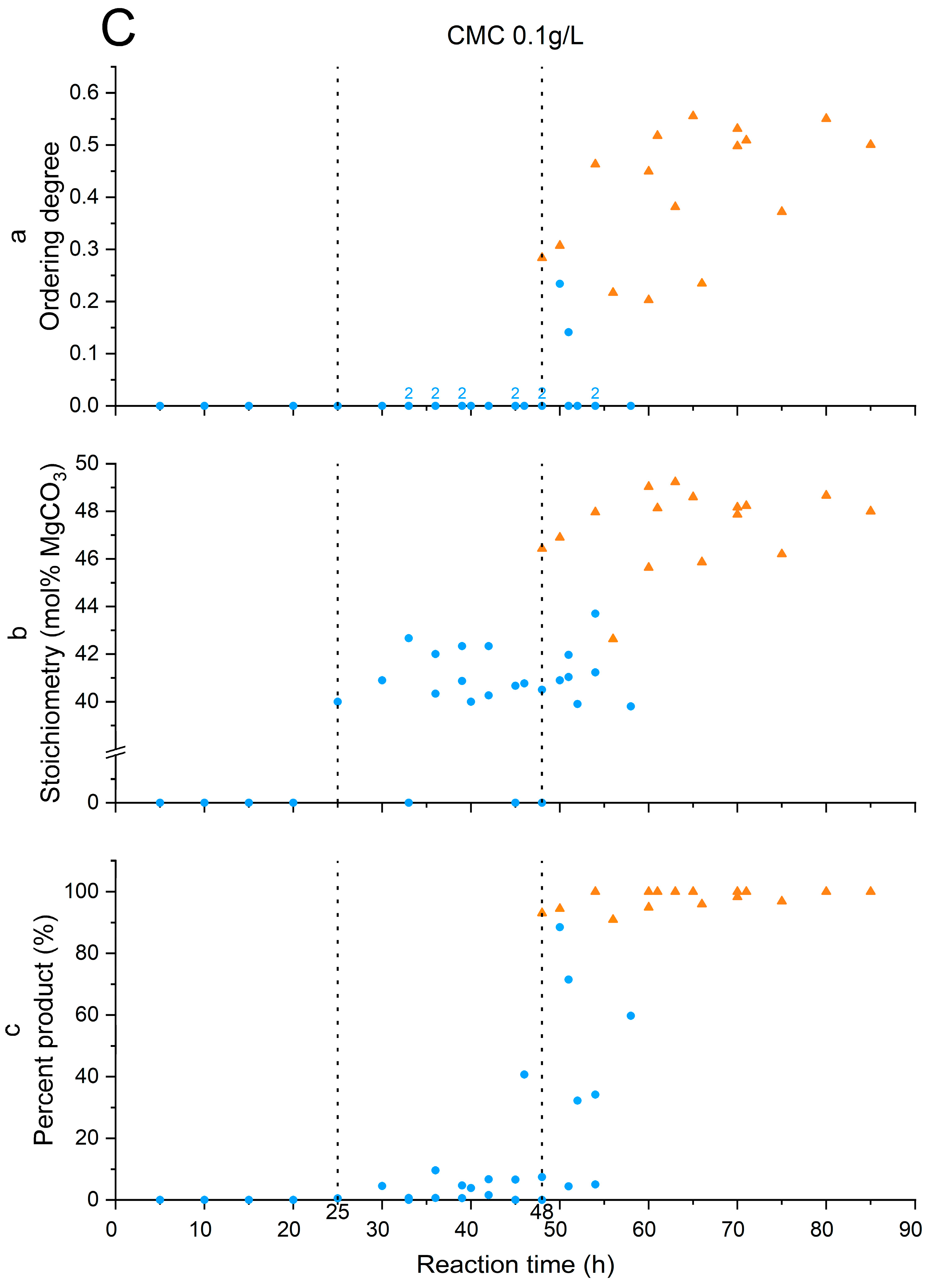
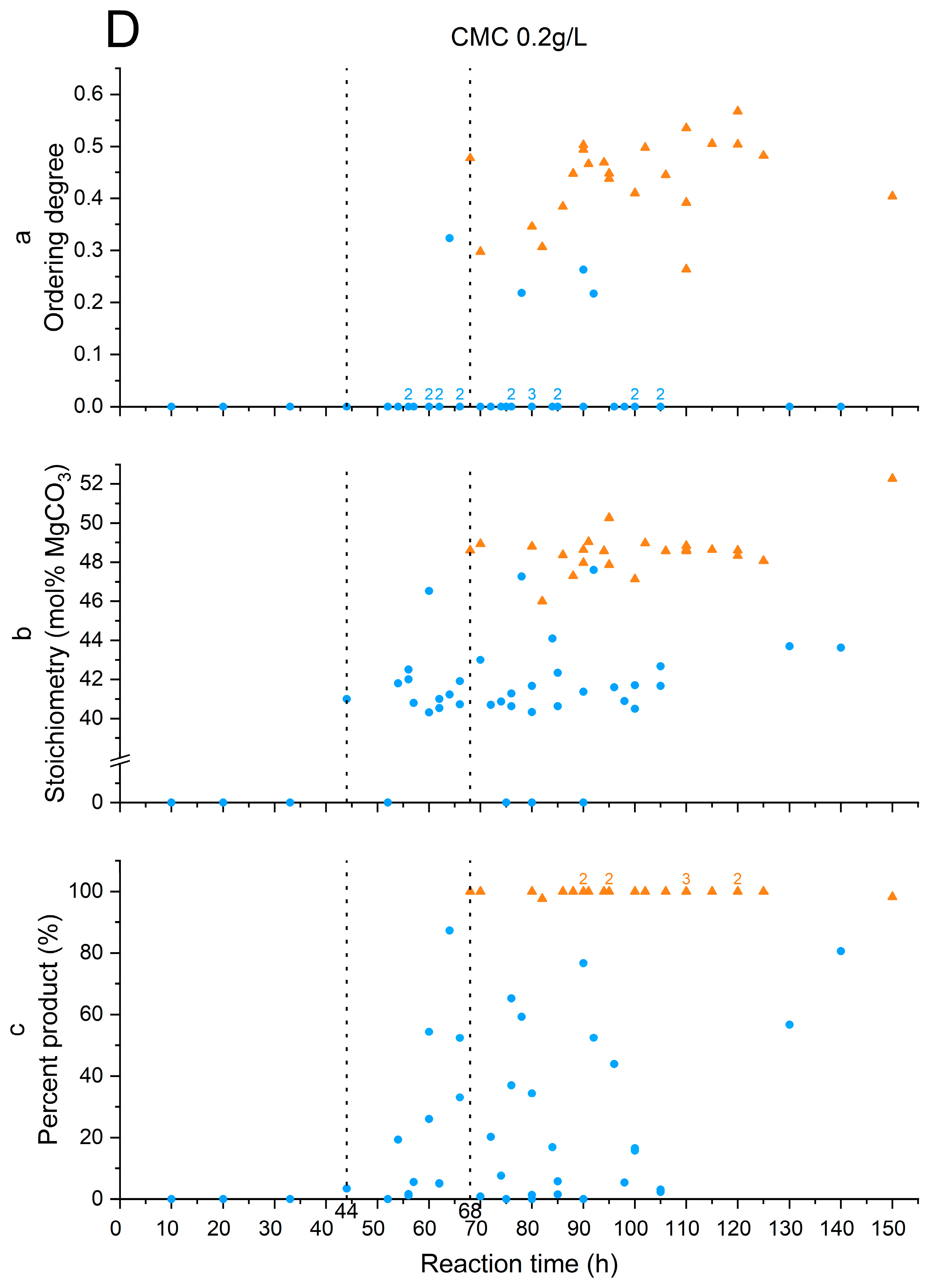
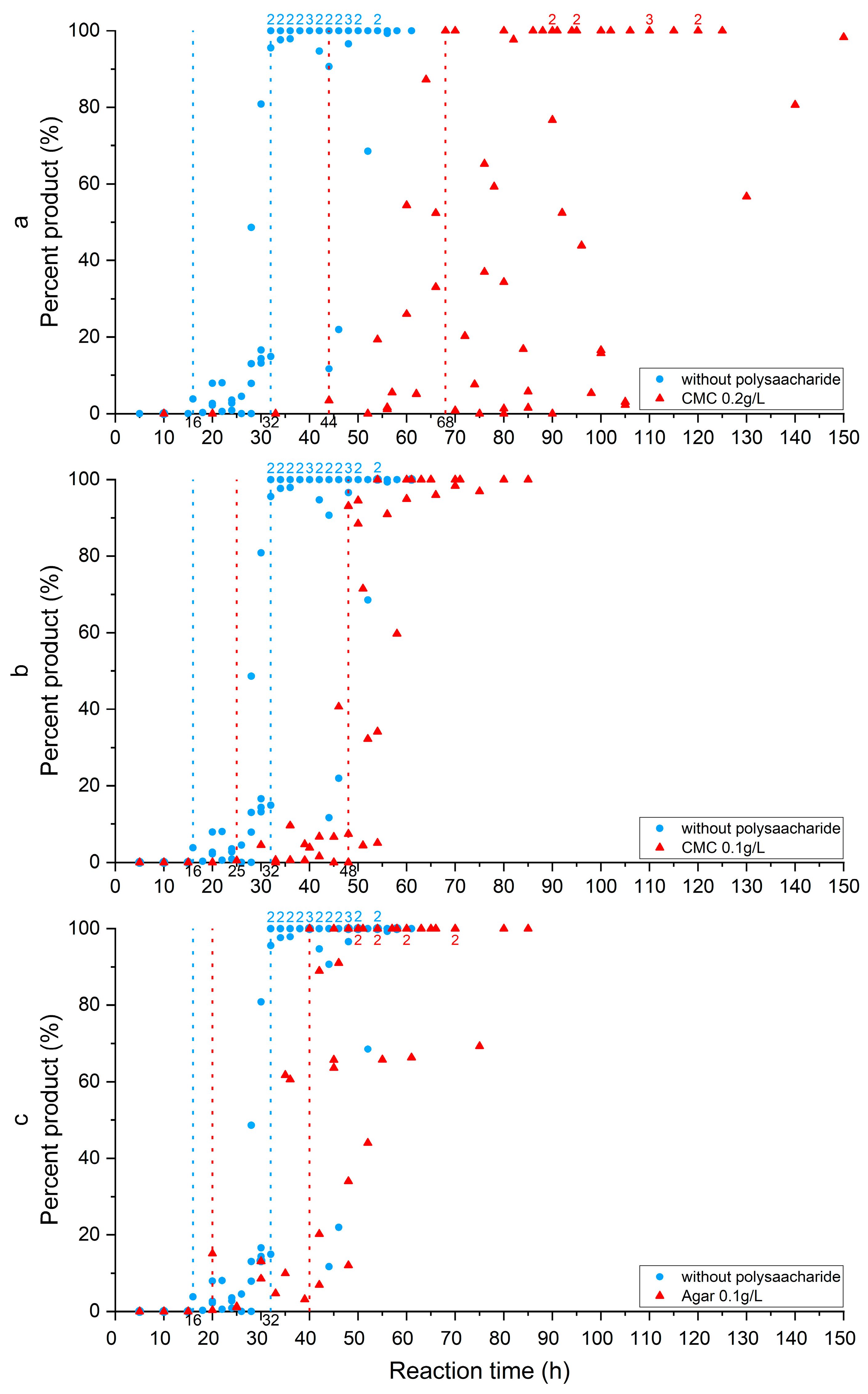

| Additive | Time of Induction Stage End (h) | Time of Replacement Stage End (h) |
|---|---|---|
| without polysaccharide | 16 | 32 |
| agar 0.1 g/L | 20 | 40 |
| CMC 0.1 g/L | 25 | 48 |
| CMC 0.2 g/L | 44 | 68 |
| Initial pH | Final pH | Heating Time (h) | |
|---|---|---|---|
| No.1 | 8.48 | 9.26 | 80 |
| No.2 | 8.50 | 8.68 | 100 |
| No.3 | 8.50 | 9.20 | 120 |
| No.4 | 8.48 | 8.78 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Konishi, H. Inhibitory Effects of Polysaccharides on the Dolomitization Reaction of Calcite at 200 °C. Minerals 2024, 14, 721. https://doi.org/10.3390/min14070721
Wei Y, Konishi H. Inhibitory Effects of Polysaccharides on the Dolomitization Reaction of Calcite at 200 °C. Minerals. 2024; 14(7):721. https://doi.org/10.3390/min14070721
Chicago/Turabian StyleWei, Yang, and Hiromi Konishi. 2024. "Inhibitory Effects of Polysaccharides on the Dolomitization Reaction of Calcite at 200 °C" Minerals 14, no. 7: 721. https://doi.org/10.3390/min14070721







