Synthesis of Geopolymers Incorporating Mechanically Activated Fly Ash Blended with Alkaline Earth Carbonates: A Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Activation
2.3. Reactivity Test
3. Results and Discussion
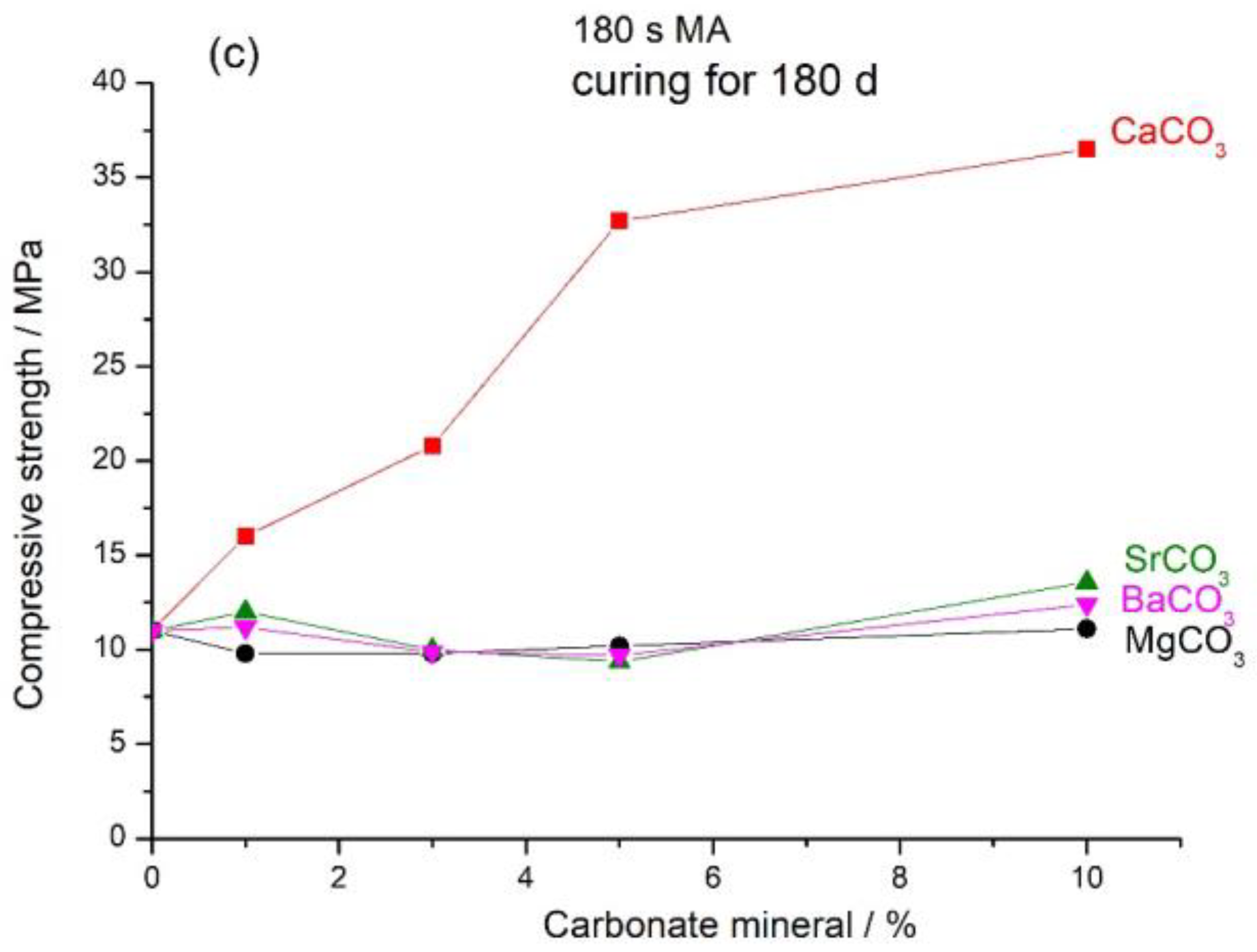
3.1. Mechanical Properties
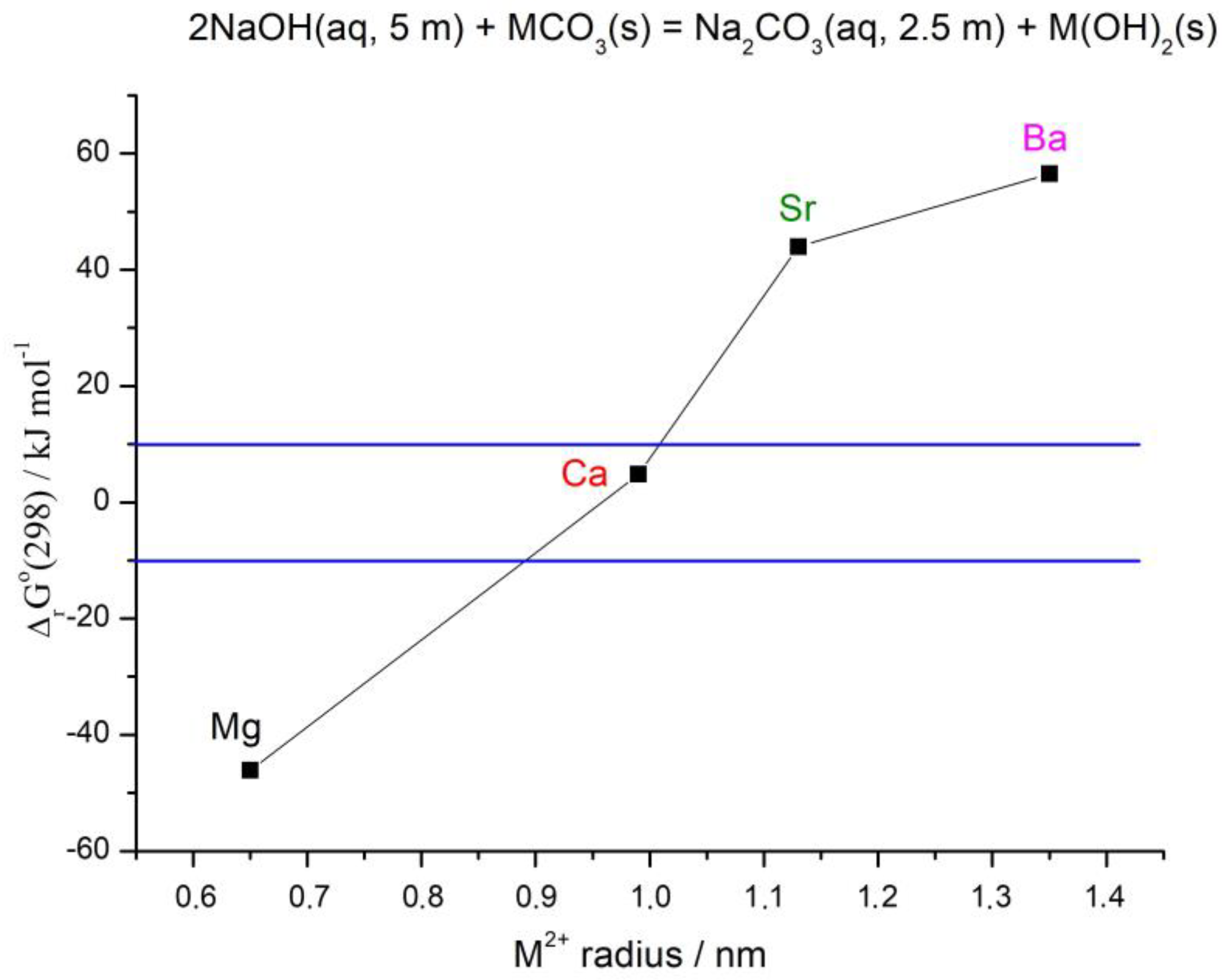
3.2. Thermodynamic Calculations
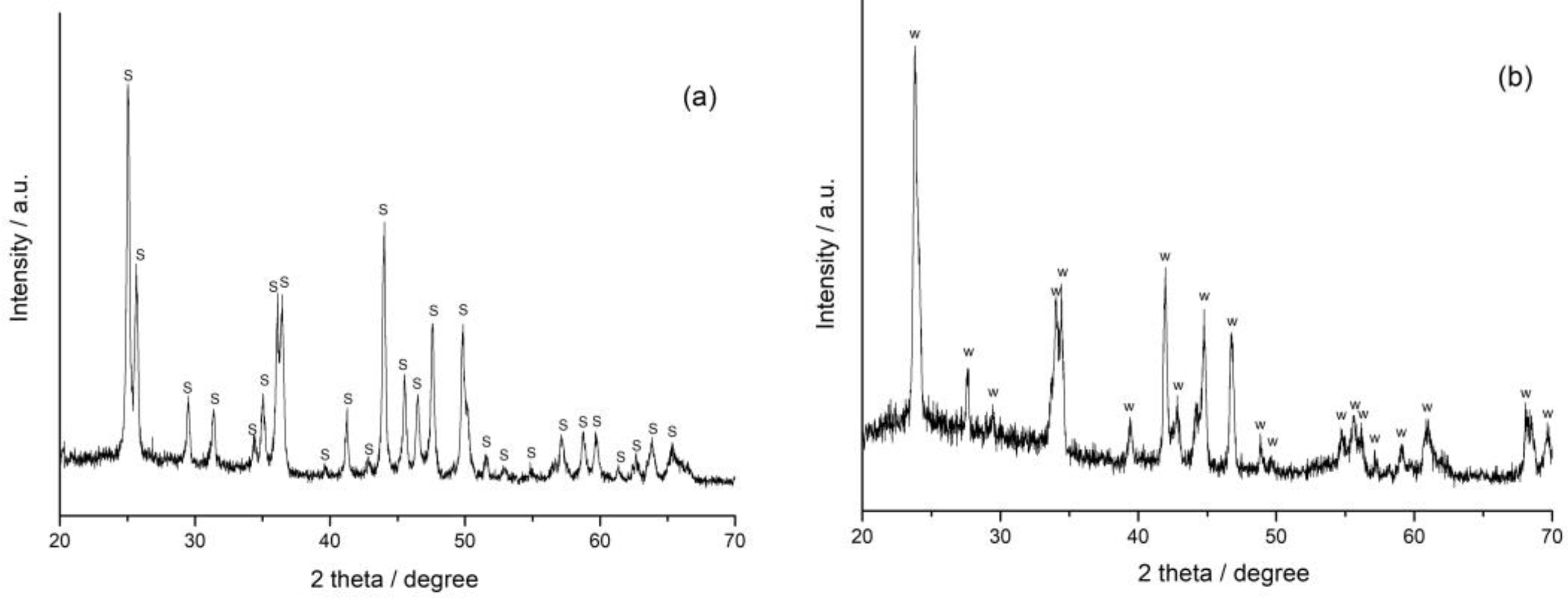
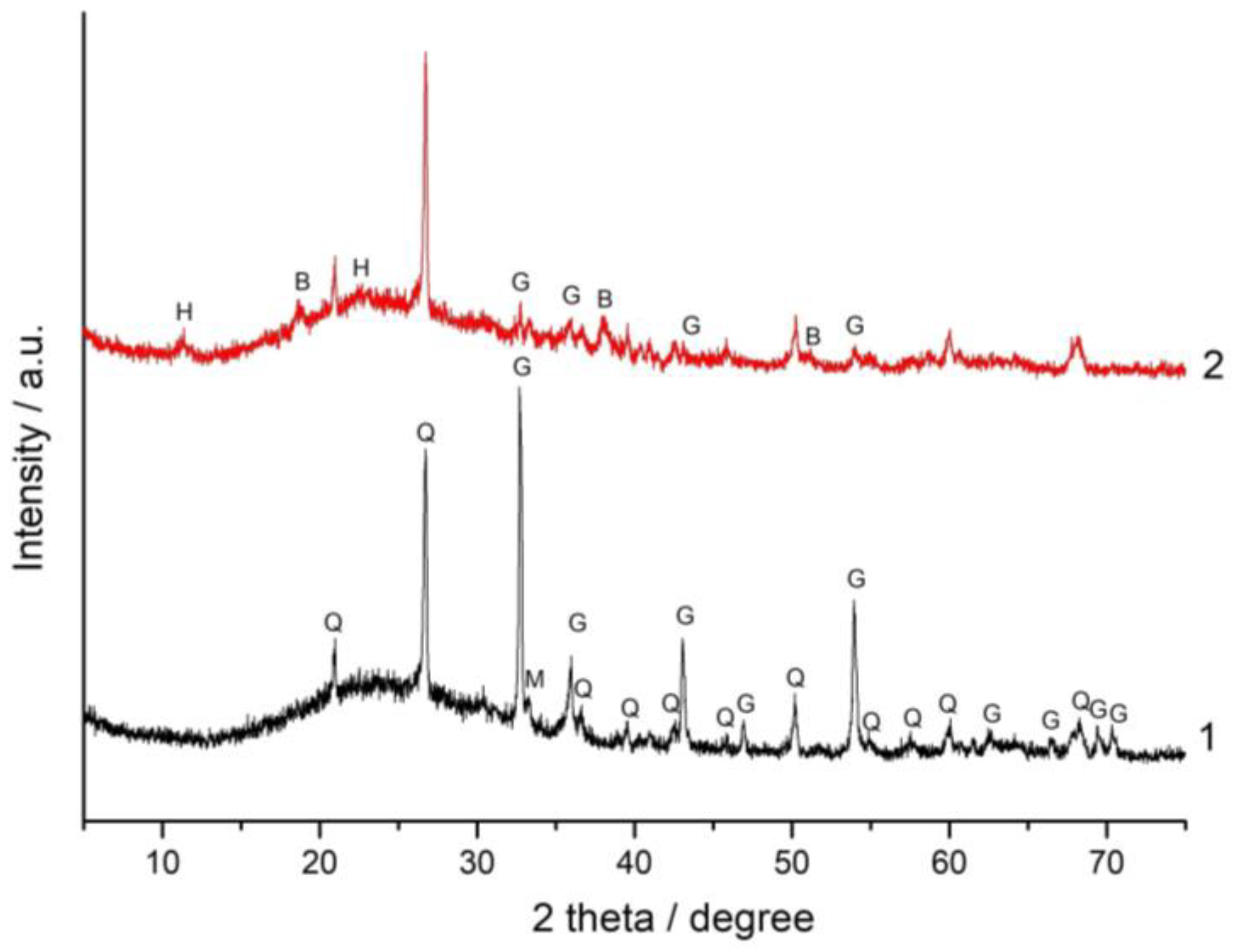
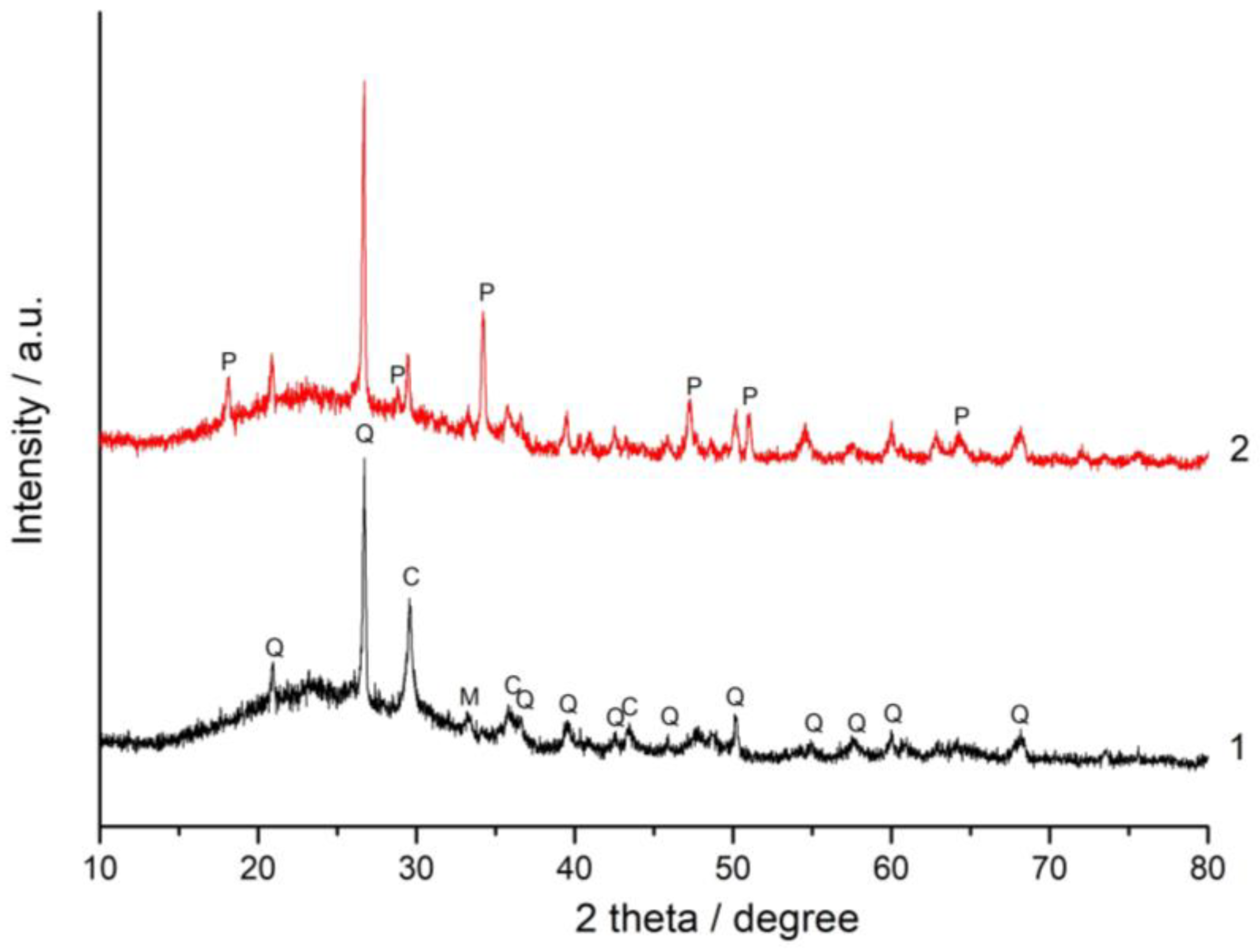
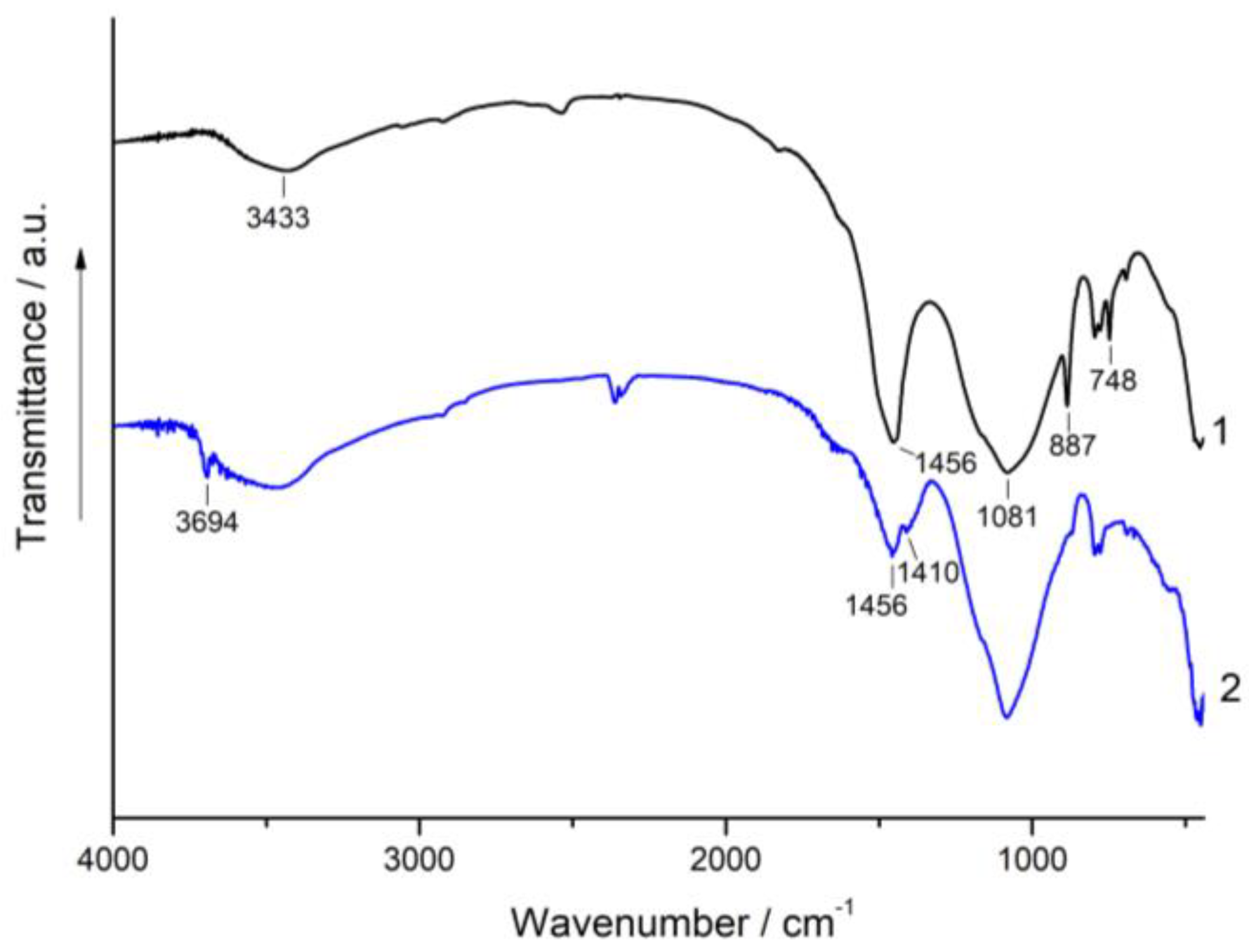
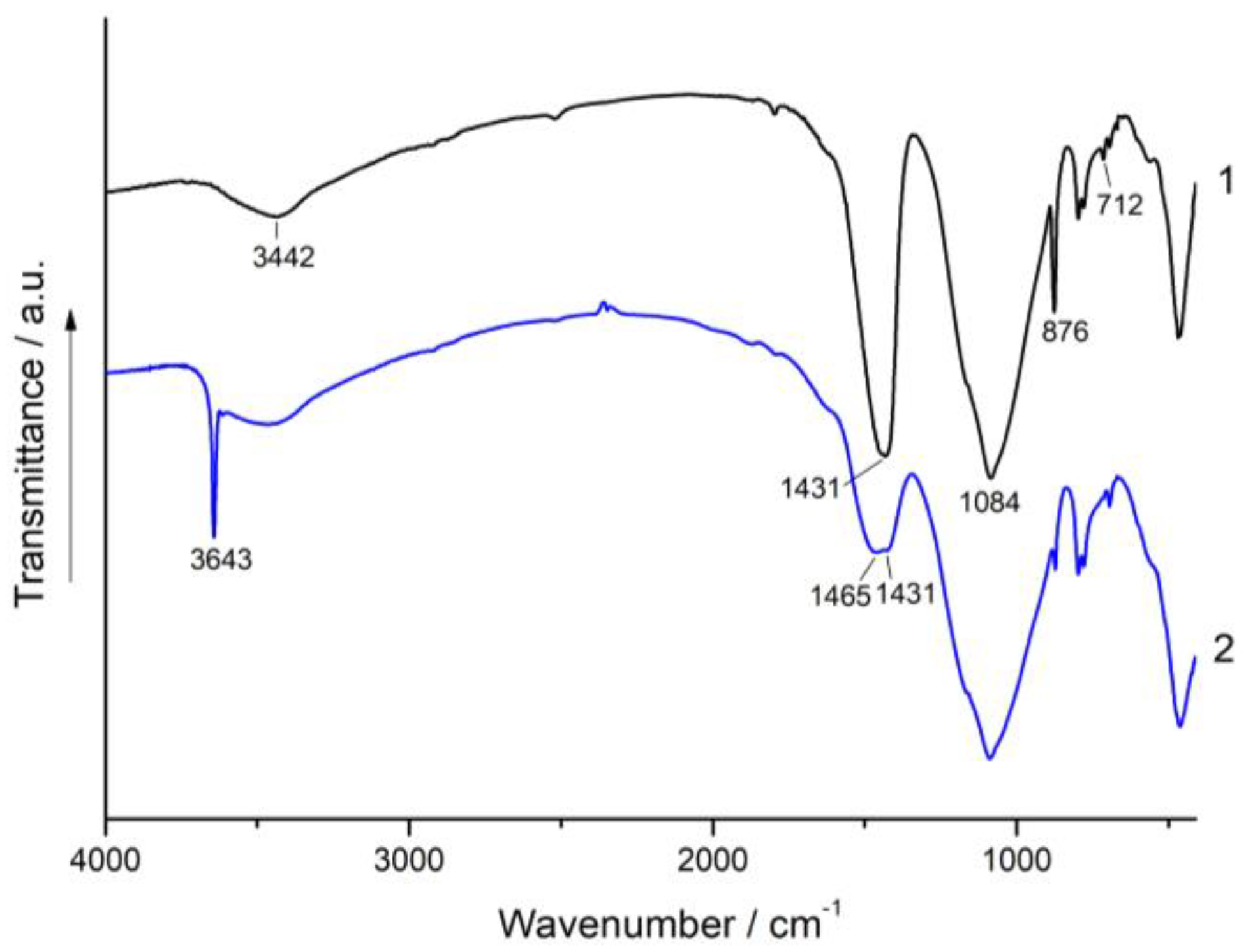
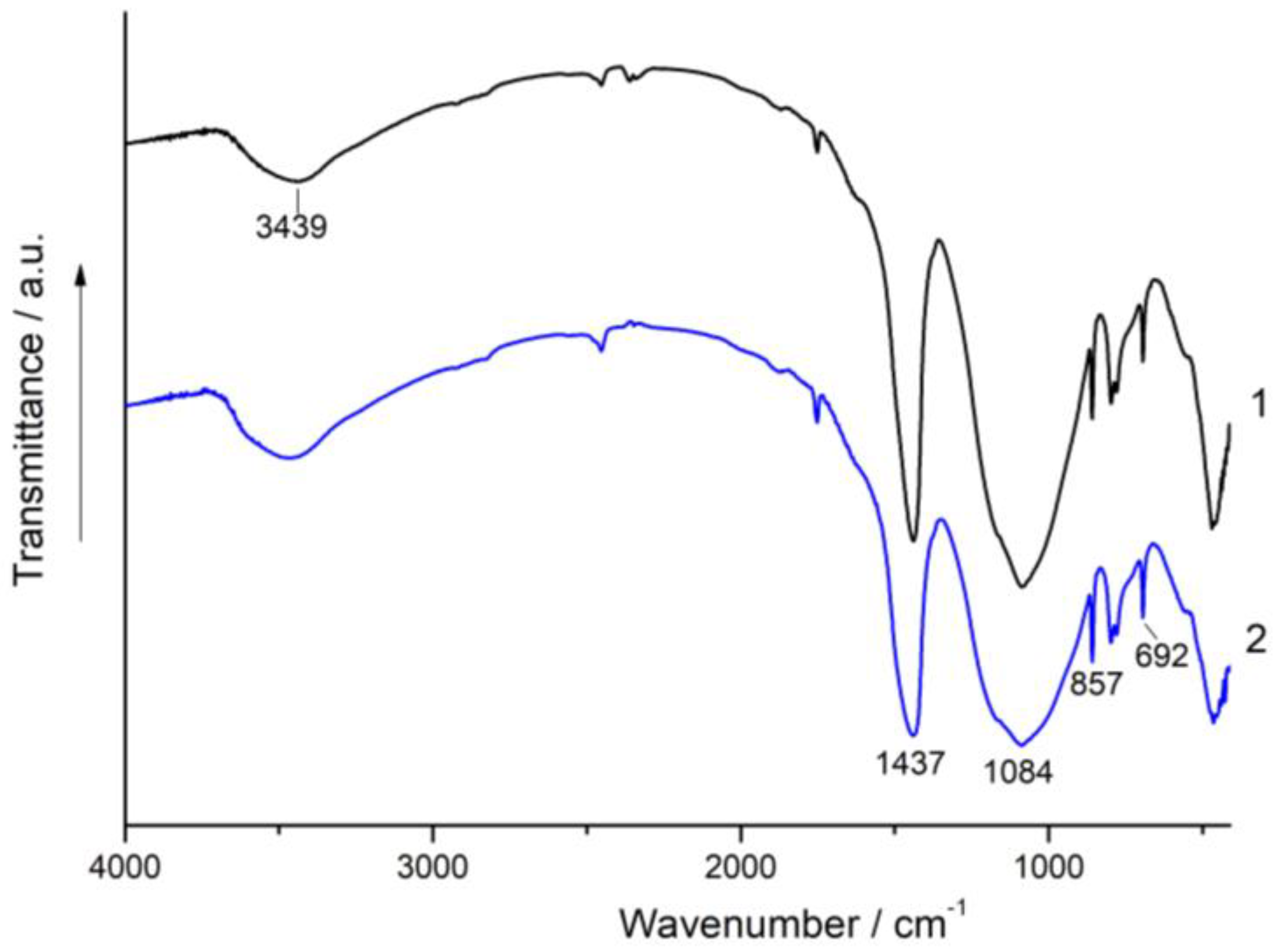
3.3. XRD and FT-IR Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FA | fly ash |
| MA | mechanical activation |
| XRD | X-ray diffraction |
| FTIR spectroscopy | Fourier transform infrared spectroscopy |
| SEM-EDS | scanning electron microscopy and energy dispersive X-ray spectroscopy |
| N-A-S-H gel | sodium aluminosilicate hydrogel |
References
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020; pp. 23–208. [Google Scholar]
- Kriven, W.M.; Leonelli, C.; Provis, J.L.; Boccaccini, A.R.; Attwell, C.; Ducman, V.S.; Ferone, C.; Rossignol, S.; Luukkonen, T.; van Deventer, J.S.J.; et al. Why geopolymers and alkali-activated materials are key components of a sustainable world: A perspective contribution. J. Am. Ceram. Soc. 2024, 107, 5159–5177. [Google Scholar] [CrossRef]
- Gaurav, G.; Kandpal, S.C.; Mishra, D.; Kotoky, N. A comprehensive review on fly ash-based geopolymer: A pathway for sustainable future. J. Sustain. Cement Based Mater. 2023, 13, 100–144. [Google Scholar] [CrossRef]
- Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. A comprehensive review on compressive strength and microstructure properties of GGBS-based geopolymer binder systems. Constr. Build. Mater. 2024, 417, 135242. [Google Scholar] [CrossRef]
- Shekhawat, P.; Sharma, G.; Singh, R.M. A Comprehensive Review of Development and Properties of Flyash-Based Geopolymer as a Sustainable Construction Material. Geotech. Geol. Eng. 2022, 40, 5607–5629. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.; Delaqua, G.C.G.; Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F. Performance of geopolymer tiles in high temperature and saturation conditions. Constr. Build. Mater. 2021, 286, 122994. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Yin, L.; Wang, Z. Review on mechanical properties of fiber-reinforced geopolymer concrete after high-temperature exposure. Iran. J. Sci. Technol. Trans. Civ. Eng. 2024, 1–23. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M.; Alomayri, T.; Pereira, S. Fire resistance characteristics of geopolymer concrete for environmental sustainability: A review of thermal, mechanical and microstructure properties. Environ. Dev. Sustain. 2023, 25, 8975–9010. [Google Scholar] [CrossRef]
- Tian, Q.; Bai, Y.; Pan, Y.; Chen, C.; Yao, S.; Sasaki, K.; Zhang, H. Application of geopolymer in stabilization/solidification of hazardous pollutants: A review. Molecules 2022, 27, 4570. [Google Scholar] [CrossRef]
- Rakhimova, N. Recent advances in alternative cementitious materials for nuclear waste immobilization: A review. Sustainability 2023, 15, 689. [Google Scholar] [CrossRef]
- Phillip, E.; Choo, T.F.; Khairuddin, N.W.A.; Abdel Rahman, R.O. On the sustainable utilization of geopolymers for safe management of radioactive waste: A review. Sustainability 2023, 15, 1117. [Google Scholar] [CrossRef]
- Komljenovic, M.; Tanasijevic, G.; Dzunuzovic, N.; Provis, J. Immobilization of cesium with alkali-activated blast furnace slag. J. Hazard Mater. 2020, 388, 121765. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-X.; He, Y.; Liu, Z.-H.; Ton, Z.-F.; Cui, X.-M. Preparation of geopolymer inorganic membrane and purification of pulppapermaking green liquor. App. Clay Sci. 2019, 168, 269–275. [Google Scholar] [CrossRef]
- Luukkonen, T.; Olsen, E.; Turkki, A.; Muurinen, E. Ceramiclike membranes without sintering via alkali activation of metakaolin, blast furnace slag, or their mixture: Characterization and cation-exchange properties. Ceram. Int. 2023, 49, 10645–10651. [Google Scholar] [CrossRef]
- Frahat, N.B.; Awed, A.S.; Kassem, S.M.; Abdel Maksoud, M.I.A.; Ibrahim, O.M.O. Innovative shielding solutions by geopolymer paste and fly ash as effective substitution of cement materials for sustainable protection. J. Build. Eng. 2024, 87, 108977. [Google Scholar] [CrossRef]
- Nurhasmi, H.; Heryanto, H.; Fahri, A.N.; Ilyas, S.; Ansar, A.; Abdullah, B.; Tahir, D. Study on optical phonon vibration and gamma ray shielding properties of composite geopolymer fly ash-metal. Radiat. Phys. Chem. 2021, 180, 109250. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Surface modification of fly ash by mechano-chemical treatment. Ceram. Int. 2019, 45, 849–853. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Garcia-Lodeiro, I.; Maltseva, O.; Palomo, A. Mechanical-chemical activation of coal fly ashes: An effective way for recycling and make cementitious materials. Front. Mater. 2019, 6, 51. [Google Scholar] [CrossRef]
- Chu, Y.-S.; Davaabal, B.; Kim, D.-S.; Seo, S.-K.; Kim, Y.; Ruescher, C.; Temuujin, J. Reactivity of fly ashes milled in different milling devices. Rev. Adv. Mater. Sci. 2019, 58, 179–188. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yokoyama, K.; Okura, K.; Murayama, N.; Ueda, M.; Naito, M. Synthesis of geopolymers from mechanically activated coal fly ash and improvement of their mechanical properties. Minerals 2019, 9, 791. [Google Scholar] [CrossRef]
- Marjanovic, N.; Komljenovic, M.; Bascarevic, Z.; Nikolic, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Azimi, E.A.; Abdullah, M.M.A.B.; Vizureanu, P.; Salleh, M.A.A.M.; Sandu, A.V.; Chaiprapa, J.; Yoriya, S.; Hussin, K.; Aziz, I.H. Strength development and elemental distribution of dolomite/fly ash geopolymer composite under elevated temperature. Materials 2020, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Embong, R.; Kusbiantoro, A.; Shafiq, N.; Nuruddin, M.F. Strength and microstructural properties of fly ash based geopolymer concrete containing high-calcium and water-absorptive aggregate. J. Clean. Prod. 2016, 112, 816–822. [Google Scholar] [CrossRef]
- Rakhimova, N. Calcium and/or magnesium carbonate and carbonate-bearing rocks in the development of alkali-activated cements–a review. Constr. Build. Mater. 2022, 325, 126742. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Gurevich, B.I.; Myshenkov, M.S.; Chislov, M.V.; Kalinkina, E.V.; Zvereva, I.A.; Cherkezova-Zheleva, Z.; Paneva, D.; Petkova, V. Synthesis of fly ash-based geopolymers: Effect of calcite addition and mechanical activation. Minerals 2020, 10, 827. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Kalinkina, E.V.; Ivanova, A.G.; Kruglyak, E.A. Effect of magnesite addition and mechanical activation on the synthesis of fly ash-based geopolymers. Minerals 2022, 12, 1367. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Kalinkina, E.V.; Kruglyak, E.A.; Semushin, V.V.; Chislov, M.V.; Zvereva, I.A. Composite geopolymers based on mechanically activated fly ash blended with SrCO3 (strontianite) and BaCO3 (witherite). Minerals 2023, 13, 1493. [Google Scholar] [CrossRef]
- Hamer, W.J.; Wu, Y.C. Osmotic Coefficients and Mean Activity Coefficients of Uni-univalent Electrolytes in Water at 25 °C. J. Phys. Chem. Ref. Data 1972, 1, 1047–1100. [Google Scholar] [CrossRef]
- Goldberg, R.N. Evaluated activity and osmotic coefficients for aqueous solutions: Thirty-six uni-bivalent electrolytes. J. Phys. Chem. Ref. Data 1981, 10, 671–764. [Google Scholar] [CrossRef]
- Belov, G.V.; Iorish, V.S.; Yungman, V.S. Simulation of equilibrium states of thermodynamic systems using IVTANTERMO for windows. High Temp. 2000, 38, 191–196. [Google Scholar] [CrossRef]
- Tkáčová, K.; Heegn, H.; Števulová, N. Energy transfer and conversion during comminution and mechanical activation. Int. J. Miner. Process. 1993, 40, 17–31. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. Use of infrared spectroscopy to study geopolymerization of heterogeneous amorphous aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- White, W.B. The carbonate minerals. In The Infrared Spectra of Minerals; Farmer, W.C., Ed.; Mineralogical Society: London, UK, 1974; pp. 227–284. [Google Scholar]
- Hassan, R.S.; Eid, M.A.; El-Sadek, A.A.; Mansy, M.S. Preparation, characterization and application of Mg/Fe Hydrotalcite as gamma sealed source for spectroscopic measurements. Appl. Radiat. Isot. 2019, 151, 74–80. [Google Scholar] [CrossRef]
- Das, J.; Das, D.; Dash, G.P.; Parida, K.M. Studies on Mg/Fe hydrotalcite-like-compound (HTlc): I. Removal of inorganic selenite (SeO32−) from aqueous medium. J. Colloid Interface Sci. 2002, 251, 26–32. [Google Scholar] [CrossRef]
- Van der Marel, H.W.; Beutelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and Their Admixtures; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Kalinkin, A.M.; Kalinkina, E.V.; Zalkind, O.A.; Makarova, T.I. Chemical interaction of calcium oxide and calcium hydroxide with CO2 during mechanical activation. Inorg. Mater. 2005, 41, 1073–1079. [Google Scholar] [CrossRef]
- Cwirzen, A.; Provis, J.L.; Penttala, V.; Habermehl-Cwirzen, K. The effect of limestone on sodium hydroxide-activated metakaolin-based geopolymers. Constr. Build. Mater. 2014, 66, 53–62. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Properties of alkali activated slag–fly ash blends with limestone addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Canfield, G.M.; Eichler, J.; Griffith, K.; Hearn, J.D. The role of calcium in blended fly ash geopolymers. J. Mater. Sci. 2014, 17, 5922–5933. [Google Scholar] [CrossRef]





| SiO2 | Al2O3 | Fe2O3 | FeO | CaO | MgO | SO3 | Na2O | K2O | C | P2O5 | TiO2 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 56.26 | 18.39 | 8.58 | 0.69 | 2.14 | 2.60 | 0.18 | 4.04 | 1.32 | 0.88 | 0.32 | 1.13 | 2.28 |
| Calcite | 0.24 | 0.47 | 0.67 | - | 52.1 | 1.44 | 0.15 | 1.76 | 0.56 | - | 0.05 | 0.05 | 43.0 |
| Magnesite | 1.50 | - | 0.36 | - | 0.32 | 46.20 | 0.16 | 0.07 | 0.03 | - | - | 0.02 | 50.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinkin, A.M.; Kalinkina, E.V.; Kruglyak, E.A.; Ivanova, A.G. Synthesis of Geopolymers Incorporating Mechanically Activated Fly Ash Blended with Alkaline Earth Carbonates: A Comparative Analysis. Minerals 2024, 14, 726. https://doi.org/10.3390/min14070726
Kalinkin AM, Kalinkina EV, Kruglyak EA, Ivanova AG. Synthesis of Geopolymers Incorporating Mechanically Activated Fly Ash Blended with Alkaline Earth Carbonates: A Comparative Analysis. Minerals. 2024; 14(7):726. https://doi.org/10.3390/min14070726
Chicago/Turabian StyleKalinkin, Alexander M., Elena V. Kalinkina, Ekaterina A. Kruglyak, and Alla G. Ivanova. 2024. "Synthesis of Geopolymers Incorporating Mechanically Activated Fly Ash Blended with Alkaline Earth Carbonates: A Comparative Analysis" Minerals 14, no. 7: 726. https://doi.org/10.3390/min14070726
APA StyleKalinkin, A. M., Kalinkina, E. V., Kruglyak, E. A., & Ivanova, A. G. (2024). Synthesis of Geopolymers Incorporating Mechanically Activated Fly Ash Blended with Alkaline Earth Carbonates: A Comparative Analysis. Minerals, 14(7), 726. https://doi.org/10.3390/min14070726







