Preparation of High-Purity Quartz Sand by Vein Quartz Purification and Characteristics: A Case Study of Pakistan Vein Quartz
Abstract
:1. Introduction
2. Hand Specimen of Sample
3. Purification Process
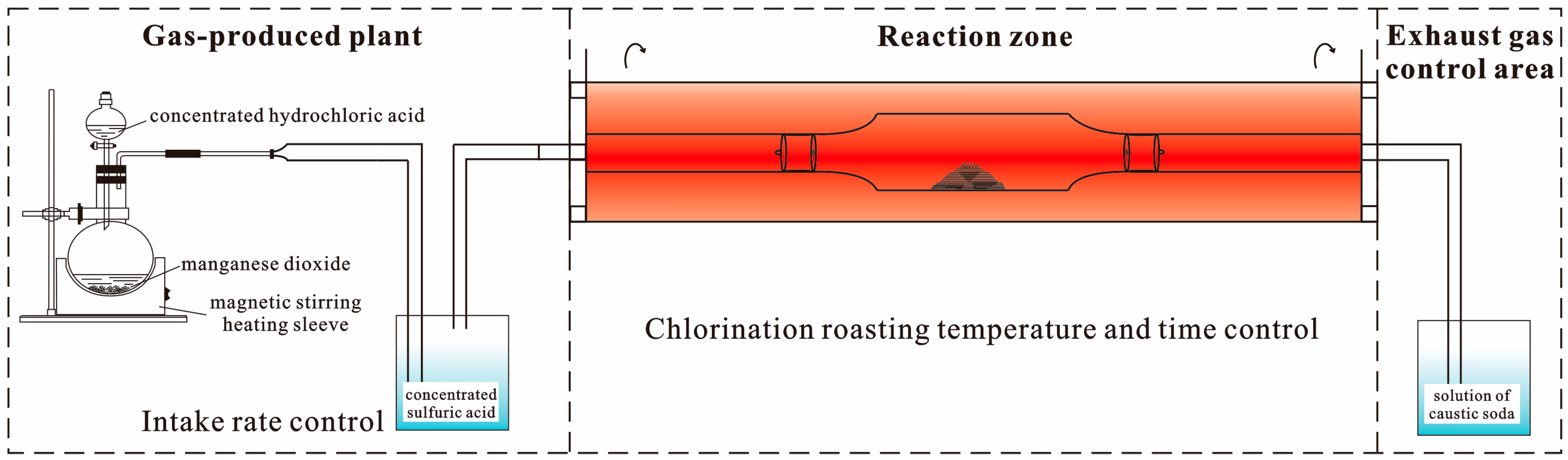
3.1. Experiment Reagents
3.2. Experimental Procedures
4. Analytical Method
4.1. Optical Microscopy Observation
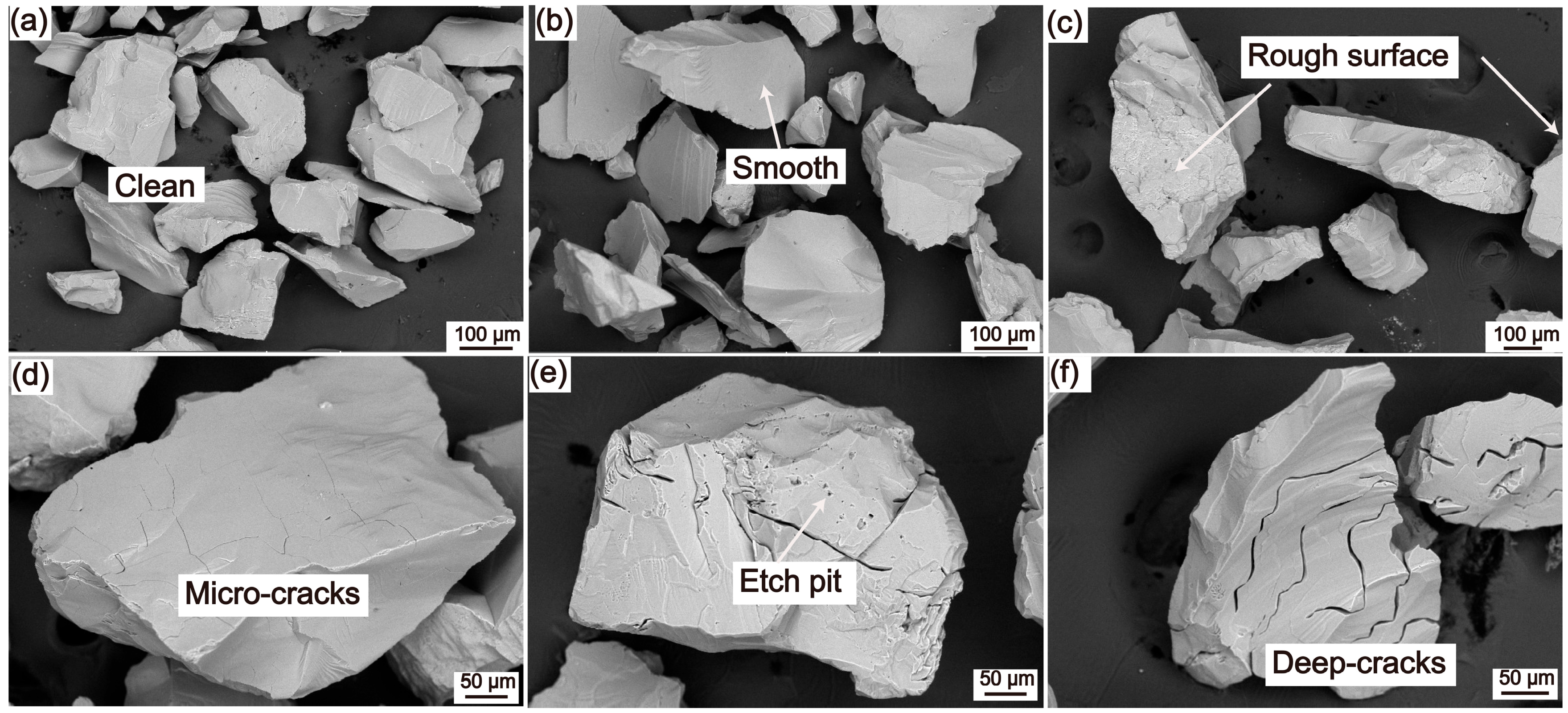
4.2. Scanning Electron Microscope
4.3. Raman Spectrometry Measurement
4.4. Bulk Chemical Composition Analysis by ICP-MS
5. Results and Discussion
5.1. Characteristics of Vein Quartz
5.1.1. Microstructure Characteristics
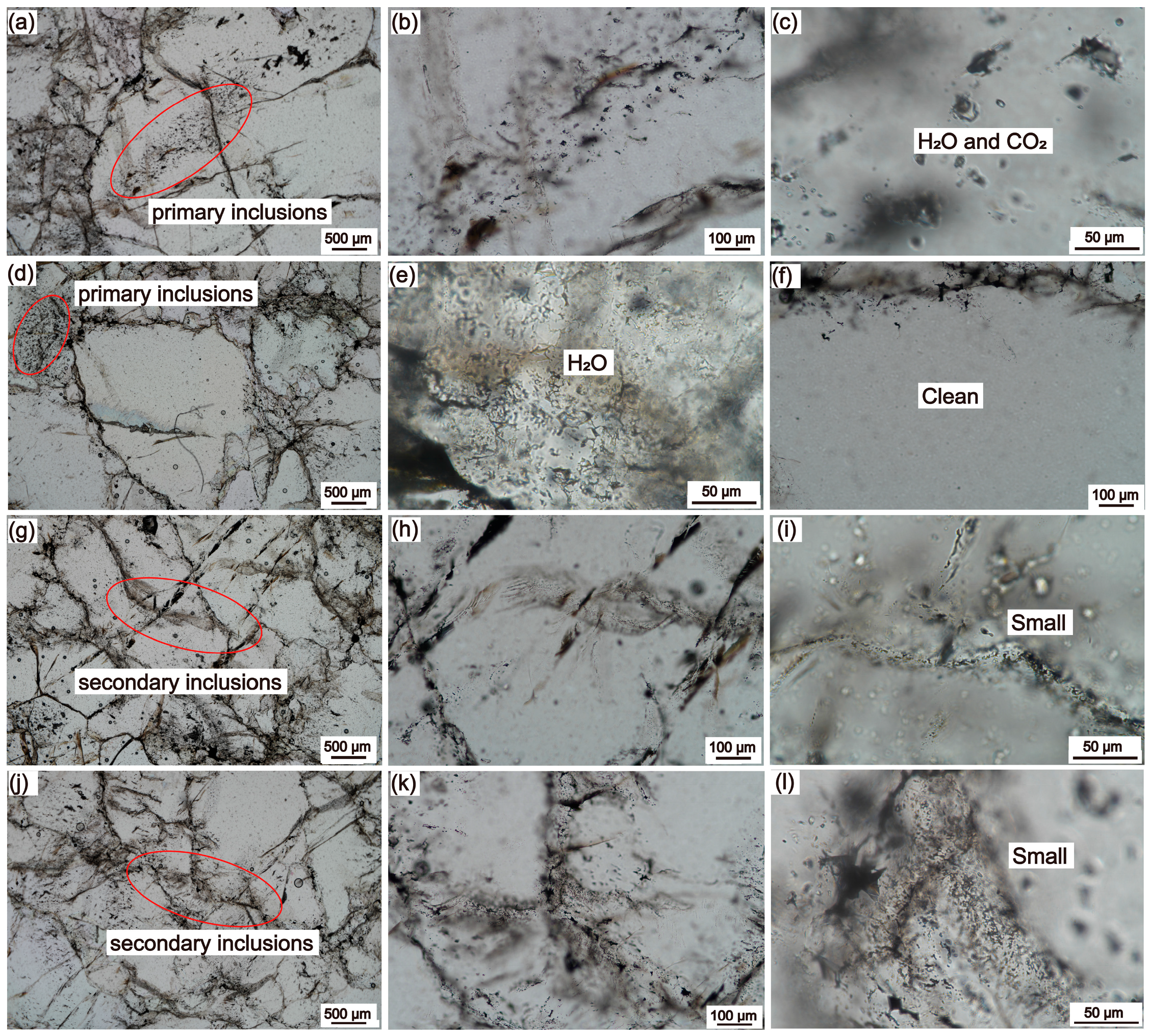
5.1.2. Inclusion Characteristics
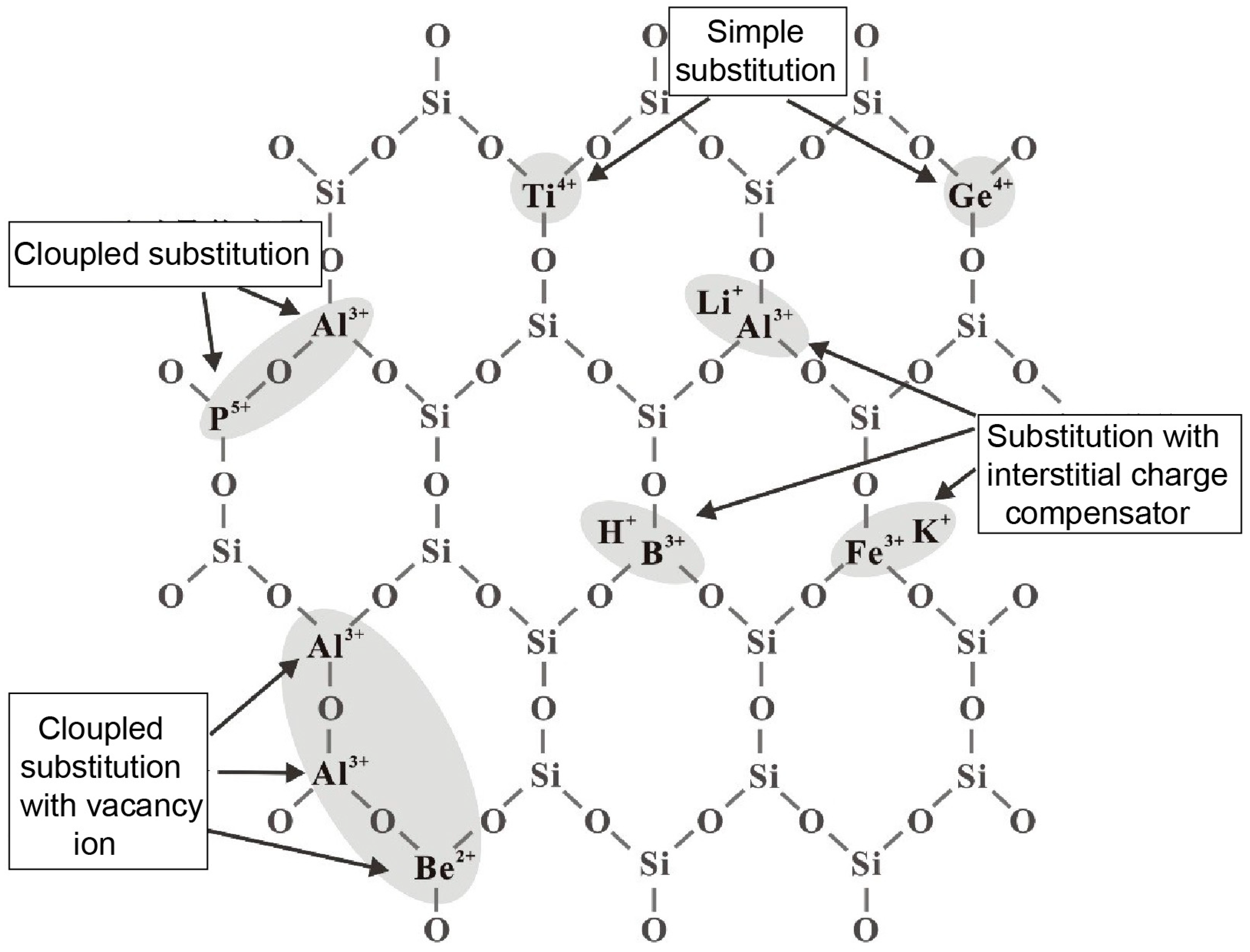
5.1.3. Chemical Composition
5.2. Processing of Quartz Sand
5.2.1. Crushing to Make Quartz Sand
5.2.2. Ultrasonic Scrubbing–Desliming
5.2.3. Flotation
5.2.4. High Temperature Calcination–Water Quenching
5.2.5. Hot Pressing and Acid Leaching
5.2.6. Chlorination Roasting
6. Summary and Conclusions
- (1)
- The raw ore of Pakistani vein quartz has a high content of quartz, with only a small amount of fine-grained K-feldspar, and the inclusions are mainly primary inclusions with large size, along with secondary fluid inclusions developed along micro-fractures. The majority of the grain areas have a very low inclusion percentage.
- (2)
- The processed quartz sand has a smooth surface, low gas–liquid inclusion content, with only a small quantity of impurity elements such as Ti and Li remaining; the total impurity elements are 24.23 µg·g−1, the impurity removal rate is 81.20%, and the purity of SiO2 reaches 99.998% (4N8).
- (3)
- The size of the primary inclusions in the raw quartz ore is relatively large, with low content, mainly containing secondary inclusions developed along the crystal micro-fractures, and the content of inclusions in most areas of the crystal is very low or even nonexistent. The quartz ore with such inclusion characteristics is considered a relatively good raw material for quartz.
- (4)
- The content of lattice impurity elements Al, Ti, and Li in quartz is the main factor restricting the purity and application direction of quartz. In industrial production, considering the economic cost, if quartz sand still contains high content of lattice impurity elements Al, Ti and Li after flotation, it cannot be used as a raw material for high-end HPQ.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, Y.Z.; Lu, J.W.; Yin, W.Z. Research on purifying low-grade quartz ore by flotation. Metal Mine 2009, 5, 84–87, (In Chinese with English Abstract). [Google Scholar]
- Haus, R. High demands on high purity. Ind. Miner. 2005, 10, 62–67. [Google Scholar]
- Haus, R.; Prinz, S.; Priess, C. Assessment of High Purity Quartz Resources. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–51. [Google Scholar]
- Müller, A.; Ihlen, P.M.; Wanvik, J.E.; Flem, B. High-purity quartz mineralisation in kyanite quartzites, Norway. Miner. Depos. 2007, 42, 523–535. [Google Scholar] [CrossRef]
- Shen, S.F. The actuality of study and manufacture in higher purity quartz. China Non-Met. Min. Ind. Her. 2006, 5, 13–16, (In Chinese with English Abstract). [Google Scholar]
- Moore, P. High-purity quartz. Ind. Miner. 2005, 455, 53–57. [Google Scholar]
- Götze, J.; Möckel, R. (Eds.) Quartz: Deposits, Mineralogy and Analytics.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2012; pp. 307–330. [Google Scholar]
- Yang, X.Y.; Sun, C.; Cao, J.Y.; Shi, J.B. Research progress and development trend of high purity quartz. Earth Sci. 2021, 1, 231–244. [Google Scholar] [CrossRef]
- Gao, B.; Nakano, S.; Kakimoto, K. Influence of reaction between silica crucible and graphite susceptor on impurities of multicrystalline silicon in a unidirectional solidification furnace. J. Cryst. Growth 2011, 314, 239–245. [Google Scholar] [CrossRef]
- Schreiber, A.; Kühn, B.; Arnold, E.; Schilling, F.J.; Witzke, H.D. Radiation resistance of quartz glass for VUV discharge lamps. J. Phys. D Appl. Phys. 2005, 38, 3242–3250. [Google Scholar] [CrossRef]
- Schlanz, J.W. High Pure and Ultra-High Pure Quartz. In Industrial Minerals & Rocks, 7th ed.; Kogel, J.E., Trivedi, N.C., Barker, J.M., Krukowski, S.T., Eds.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2009; pp. 833–837. [Google Scholar]
- Dal, M.E.; Tranell, G.; Gaal, S.; Raaness, O.S.; Tang, K.; Arnberg, L. Study of pellets and lumps as raw materials in silicon production from quartz and silicon carbide. Metall. Mater. Trans. B 2011, 42, 939–950. [Google Scholar]
- Götze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Bayaraa, B.; Greg, B.; Noriyoshi, T. Hydrothermal quartz vein formation, revealed by coupled SEM-CL imaging and fluid inclusion microthermometry: Shuteen complex, south Gobi, Mongolia. Resour. Geol. 2005, 55, 1–8. [Google Scholar]
- Yin, W.; Wang, D.; Drelich, J.W.; Yang, B.; Li, D.; Zhu, Z.; Yao, J. Reverse flotation separation of hematite from quartz assisted with magnetic seeding aggregation. Miner. Eng. 2019, 139, 105873. [Google Scholar] [CrossRef]
- Rohem Peçanha, E.; da Fonseca de Albuquerque, M.D.; Antoun Simao, R.; de Salles Leal Filho, L.; de Mello Monte, M.B. Interaction forces between colloidal starch and quartz and hematite particles in mineral flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 79–85. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Li, X.; Yan, X.; Zhang, H. Effect of the turbulent flow pattern on the interaction between dodecylamine and quartz. Appl. Surf. Sci. 2020, 507, 145012. [Google Scholar] [CrossRef]
- Khalifa, M.; Ouertani, R.; Hajji, M.; Ezzaouia, H. Innovative technology for the production of high-purity sand silica by thermal treatment and acid leaching process. Hydrometallurgy 2019, 185, 204–209. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R. Enhancing the Technical Qualifications of Egyptian White Sand Using Acid Leaching; Response Surface Analysis and Optimization. Int. J. Miner. Process. Extr. Metall. 2016, 1, 33–40. [Google Scholar]
- Lin, M.; Lei, S.; Pei, Z.; Liu, Y.; Xia, Z.; Xie, F. Application of hydrometallurgy techniques in quartz processing and purification: A review. Metall. Res. Technol. 2018, 115, 303. [Google Scholar] [CrossRef]
- Zhang, Q.D.; Li, X.L.; Song, Y.S.; Zhou, G.Y. Experimental Research on Preparation Technics of High-Purity Quartz Material. Key Eng. Mater. 2017, 748, 17–21. [Google Scholar] [CrossRef]
- Buttress, A.J.; Rodriguez, J.M.; Ure, A.; Ferrari, R.S.; Dodds, C.; Kingman, S.W. Production of high purity silica by microfluidic-inclusion fracture using microwave pretreatment. Miner. Eng. 2019, 131, 407–419. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zuo, Q.; Li, J.; Ban, B.; Chen, J. Purification mechanism of quartz sand by combination of microwave heating and ultrasound assisted acid leaching treatment. Silicon 2021, 13, 531–541. [Google Scholar] [CrossRef]
- Vatalis, K.I.; Charalampides, G.; Platias, S.; Benetis, N.P. Market developments and industrial innovative applications of high purity quartz refines. Procedia Econ. Financ. 2014, 14, 624–633. [Google Scholar] [CrossRef]
- Zhou, Y.H. Study on refining quartz powder by leaching in HF acid solution. J. Mineral. Petrol. 2005, 25, 23–26. [Google Scholar]
- Wang, L. Industrial types and application characteristics of quartz deposits. Conserv. Util. Miner. Resour. 2019, 6, 39–47. [Google Scholar]
- Müller, A.; Wanvik, J.E.; Ihlen, P.M. Petrological and Chemical Characterisation of High-Purity Quartz Deposits with Examples from Norway. In Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 71–118. [Google Scholar]
- Zhong, T.S.; Yu, W.H.; Shen, C.; Wu, X.W. Research on Preparation and Characterisation of High-purity Silica Sands by Purification of Quartz Vein Ore from Dabie Mountain. Silicon 2022, 14, 4723–4729. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xie, Z.F.; Wang, C.L.; Hu, Y.F. Trace Element Concentrations and Mineralogy of Quartz Vein Deposits from Southeastern Hubei Province, China. Minerals 2022, 12, 814. [Google Scholar] [CrossRef]
- Ning, S.Y.; Pan, B.K.; Chen, Y.G.; Chen, H.R.; Zhang, Z.Y. Microstructure and inclusions characteristics of vein quartz in Yangjiang area, Guangdong. China Non-Met. Miner. Ind. 2020, 5, 65–68. [Google Scholar]
- Kohobhange, S.P.K.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. The effect of prolonged milling time on comminution of quartz. Powder Technol. 2018, 330, 266–274. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Li, W.; Yan, X.; Zhang, H. Intensification of interfacial adsorption of dodecylamine onto quartz by ultrasonic method. Sep. Purif. Technol. 2019, 227, 115701. [Google Scholar] [CrossRef]
- Al-maghrabi, N.H. Improvement of low-grade silica sand deposits in Jeddah Area. J. King Abdulaziz Univ.-Eng. Sci. 2004, 15, 113–128. [Google Scholar] [CrossRef]
- Götze, J. Mineralogy, geochemistry and cathodoluminescence of authigenic quartz from different sedimentary rocks. In Quartz: Deposits, Mineralogy and Analytics; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 287–306. [Google Scholar]
- Zhong, L.L. Study on Purifing Preparation and Mechanism. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2015. [Google Scholar]
- Zhang, L.Y.; Gao, H.M.; Liu, L.G. Experimental study on the preparation of high-purity quartz powder from high-grade vein quartzite. Glass 1996, 23, 6–9. [Google Scholar]
- Pang, Q.L.; Shen, J.X.; Cheng, C.B.; Guo, H.C.; Sun, X.; Li, W. Processing technology and application of high purity quartz. Jiangsu Ceram. Acad. Res. 2020, 53, 43–47. [Google Scholar]
- Zhu, Y.B.; Peng, Y.J.; Gao, H.M. Research on mineral processing of coastal quartz sand mine. J. Wuhan Polytech. Univ. 1999, 21, 37–39. [Google Scholar]
- Zhao, H.L. Experimental study on iron removal from quartz sand by ultrasonic wave. Glass Enamel 2004, 23, 44–49. [Google Scholar]
- Mckee, D.J. Automatic flotation control—A review of 20 years of effort. Miner. Eng. 1991, 4, 653–666. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; He, P.; Gu, G. Reverse flotation for removal of silicates from diasporic-bauxite. Miner. Eng. 2004, 17, 63–68. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.M.; Wang, H.; Feng, Q.C. Selective adsorption mechanism of salicylic acid on pyrite surfaces and its application in flotation separation of chalcopyrite from pyrite. Sep. Purif. Technol. 2020, 240, 116650. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the mechanism of the flotation of oxides and silicates. Miner. Eng. 2016, 95, 185–196. [Google Scholar] [CrossRef]
- Von Rybinski, W.; Schwuger, M. Adsorption of surfactant mixtures in froth flotation. Langmuir 1986, 2, 639–643. [Google Scholar] [CrossRef]
- Jiang, X.S.; Chen, J.; Ban, B.Y.; Song, W.F.; Chen, C.; Yang, X.Y. Application of competitive adsorption of ethylenediamine and polyetheramine in direct float of quartz from quartz feldspar mixed minerals under neutral pH conditions. Miner. Eng. 2022, 188, 107850. [Google Scholar] [CrossRef]
- Jiang, X.S.; Chen, J.; Wei, M.N.; Li, F.F.; Ban, B.Y.; Li, J.W. Effect of impurity content difference between quartz particles on flotation behavior and its mechanism. Powder Technol. 2020, 375, 504–512. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Xu, Y.; Shu, K.; Yang, J.; Luo, L.; Xu, L. Effect of dissolved fluorite and barite species on the flotation and adsorption behavior of bastnaesite. Sep. Purif. Technol. 2020, 237, 116387. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Sun, W.; Hu, Y. The flotation and adsorption of mixed collectors on oxide and silicate minerals. Adv. Colloid Interface Sci. 2017, 250, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shehu, N.; Spaziani, E. Separation of feldspar from quartz using EDTA as modifier. Miner. Eng. 1999, 12, 1393–1397. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 195–204. [Google Scholar] [CrossRef]
- El-Salmawy, M.S.; Nakahiro, Y.; Wakamatsu, T. The role of alkaline earth cations in flotation separation of quartz from feldspar. Miner. Eng. 1993, 6, 1231–1243. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, H.; Zhang, K. Mechanism of the function of sodium hexametaphophate. J. Northeast. Univ. Natural. Sci. 1993, 14, 231–235. [Google Scholar]
- Ata, S.; Jameson, G.J. The formation of bubble clusters in flotation cells. Int. J. Miner. Process. 2005, 76, 123–139. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Liu, J.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Sahoo, H.; Sinha, N.; Rath, S.S.; Das, B. Ionic liquids as novel quartz collectors: Insights from experiments and theory. Chem. Eng. 2015, 273, 46–54. [Google Scholar] [CrossRef]
- Shimoiizaka, J.; Nakatsuka, K.; Katayanagi, T. Separation of Feldspar from Quartz by a New Flotation Process; World Mining and Metals Technology Port City Press: Baltimore, MD, USA, 1976; pp. 428–438. [Google Scholar]
- Crozier, R.D. Non-metallic mineral flotation. Ind. Miner. 1990, 269, 55–65. [Google Scholar]
- Demir, C.; Gülgonul, I.; Bentli, I.; Çelik, M. Differential separation of albite from microcline by monovalent salts in HF medium. Min. Met. Process 2003, 20, 120–124. [Google Scholar] [CrossRef]
- Zuo, Q.X.; Liu, J.W.; Chen, J. Study on deep purification and kinetics of Fengyang quartz sand by calcination, quenching and acid leaching. Conserv. Util. Miner. Resour. 2022, 5, 76–81. [Google Scholar]
- Pan, X.D. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.Y.; Lei, S.M. Mineralogy and processing of hydrothermal vein quartz from hengche, Hubei Province (China). Minerals 2017, 7, 161. [Google Scholar] [CrossRef]
- Tuncuk, A.; Akcil, A. Iron removal in production of purified quartz by hydrometallurgical process. Int. J. Miner. Process 2016, 153, 44–50. [Google Scholar] [CrossRef]
- Yang, C.; Li, S.; Yang, R.; Bai, J.; Guo, Z. Recovery of silicon powder from kerf loss slurry waste using superconducting high gradient magnetic separation technology. J. Mater. Cycles Waste Manag. 2018, 20, 937–945. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Li, X.; Zhang, Z. Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid. Ultrason. Sonochem. 2011, 18, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Tran, T.; Jung, B.H.; Kim, S.J.; Kim, M.J. Dissolution of iron oxide using oxalic acid. Hydrometallurgy 2007, 87, 91–99. [Google Scholar] [CrossRef]
- Zeng, H.; Lei, S.; Liu, Y.; Zhang, F. Effect and Complexation Mechanism of Complex Ion in Quartz Purification by Oxidation Leaching. Min. Res. Dev. 2012, 32, 67–70. [Google Scholar]
- Su, Y.; Zhou, Y.; Huang, W.; Gu, Z. Study on reaction kinetics between silica glasses and hydrofluoric acid. J. Chin. Ceram. Soc. 2004, 32, 287–293. [Google Scholar]
- Zhong, L.; Lei, S.; Wang, E.; Pei, Z.; Li, L.; Yang, Y.Y. Research on removal impurities from vein quartz sand with complexing agents. Appl. Mech. Mater. 2013, 454, 194–199. [Google Scholar] [CrossRef]
- Luo, X.; Yang, W.; Li, R.; Gao, L. Effects of pH on the solubility of the feldspar and the development of secondary porosity. Bull. Mineral. Petrol. Geochem. 2001, 20, 103–107. [Google Scholar]
- Xiong, K.; Pei, Z.; Zang, F.; Lin, M. Process and Mechanism of High-purity Quartz Prepared by Mixed Acid Leaching. Non-Met. Mines 2016, 39, 60–62. [Google Scholar]
- Lin, M.; Pei, Z.; Li, Y.; Liu, Y.; Wei, Z.; Lei, S. Separation mechanism of lattice-bound trace elements from quartz by KCl-doping calcination and pressure leaching. Miner. Eng. 2018, 125, 42–45. [Google Scholar] [CrossRef]
- Wu, X. Study on Raw Material Selection Evaluation and Purification Technology of High Purity Quartz. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2013. [Google Scholar]
- Pan, J.L. Experimental Study on Preparation of 4N8 Standard High Purity Quartz by Chlorination Roasting. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2015. [Google Scholar]
- Zhang, D.H. Experimental Study on Processing 5N High Purity Quartz with Vein Quartz as Raw Material. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2016. [Google Scholar]
- Mao, L.W.; Gu, C.H.; Wu, J.X. Experimental study on production of high purity quartz sand by replacing crystal with vein quartz. World Build. Mater. 2010, 31, 1–4. [Google Scholar]
- Zhang, S.X. Study on purification of quartz minerals. J. Jinzhou Norm. Univ. Nat. Sci. Ed. 2001, 22, 28–30. [Google Scholar]
- Zhang, H.Q.; Tan, X.M.; Ma, Y.M.; Chen, C.L.; Zhang, S.H.; Wang, L.; Liu, L.; Zhu, L.K.; Guo, L.X.; Zhang, H.L.; et al. Geological characteristics and 4N8 grade product preparation technology of Altai pegmatite type high purity quartz deposit in Xinjiang. Conserv. Util. Miner. Resour. 2022, 5, 1–7. [Google Scholar]
- Wang, L. The concept of high purity quartz and the classification of raw materials. Conserv. Util. Miner. Resour. 2022, 5, 55–63. [Google Scholar]





| Element | Raw Ore | Flotation Sand | Acid Leaching Sand | Chlorinated Sand | IOTA-STD |
|---|---|---|---|---|---|
| Li | 2.38 | 2.44 | 2.26 | 2.37 | 0.90 |
| B | bdl | 0.19 | 0.48 | 0.55 | 0.08 |
| Na | 6.65 | 6.47 | 5.09 | 0.72 | 0.90 |
| Mg | 0.50 | 1.11 | 0.20 | 0.30 | <0.05 |
| Al | 54.05 | 34.85 | 13.08 | 13.50 | 16.20 |
| K | 34.66 | 16.32 | 1.22 | 0.35 | 0.60 |
| Ca | 22.55 | 6.09 | 0.95 | 0.78 | 0.50 |
| Ti | 5.06 | 5.13 | 5.22 | 5.23 | 1.10 |
| Cr | bdl | 0.52 | 0.02 | 0.01 | <0.05 |
| Mn | 0.02 | 0.10 | 0.06 | 0.06 | <0.05 |
| Fe | 2.82 | 4.06 | 0.30 | 0.24 | 0.23 |
| Ni | 0.02 | 0.17 | 0.15 | 0.10 | <0.05 |
| Cu | 0.16 | bdl | 0.03 | 0.02 | <0.05 |
| Σ | 128.86 (Σ0) | 77.42 (Σ1) | 29.06 (Σ2) | 24.23(Σ3) | <19.66 |
| Removal rate | - | 39.92% | 77.45% | 81.20% | - |
| SiO2 (wt%) | 99.987 | 99.992 | 99.997 | 99.998 | >99.998 |
| Size Range (mm) | Yield (g) | Productive Rate/% |
|---|---|---|
| +0.425 | 400 | 40.0 |
| −0.425 +0.074 | 520 | 52.0 |
| −0.074 | 80 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, M.; Yang, X.; Hou, Z. Preparation of High-Purity Quartz Sand by Vein Quartz Purification and Characteristics: A Case Study of Pakistan Vein Quartz. Minerals 2024, 14, 727. https://doi.org/10.3390/min14070727
Xia M, Yang X, Hou Z. Preparation of High-Purity Quartz Sand by Vein Quartz Purification and Characteristics: A Case Study of Pakistan Vein Quartz. Minerals. 2024; 14(7):727. https://doi.org/10.3390/min14070727
Chicago/Turabian StyleXia, Mei, Xiaoyong Yang, and Zhenhui Hou. 2024. "Preparation of High-Purity Quartz Sand by Vein Quartz Purification and Characteristics: A Case Study of Pakistan Vein Quartz" Minerals 14, no. 7: 727. https://doi.org/10.3390/min14070727




