Reevaluating Hendy Test with Modern Cave Calcite from the Monsoon Region of China
Abstract
1. Introduction
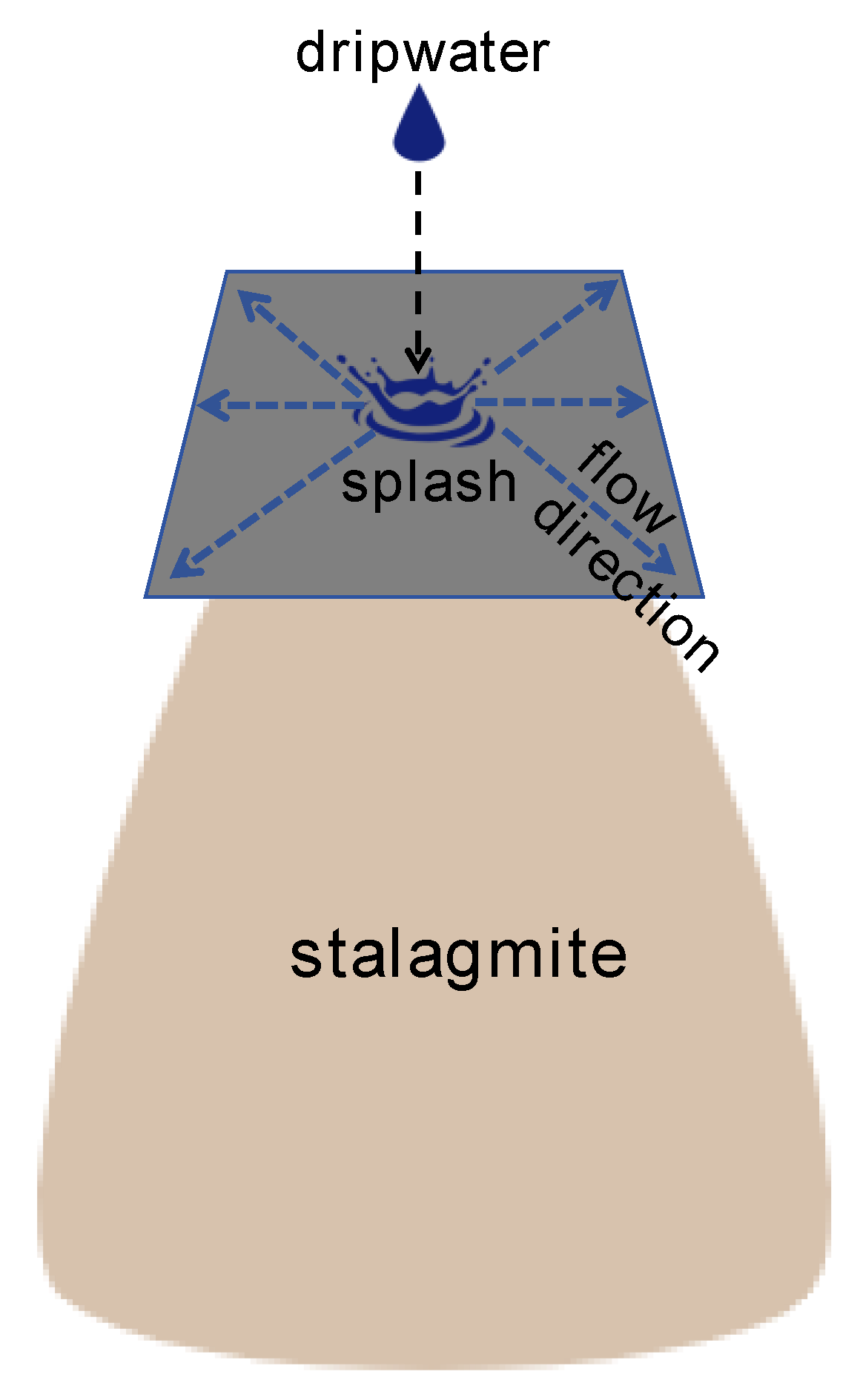
2. Materials and Methods
2.1. Cave Settings
2.2. Collection of Farmed Calcite
2.3. Stable Isotope Analysis
3. Results
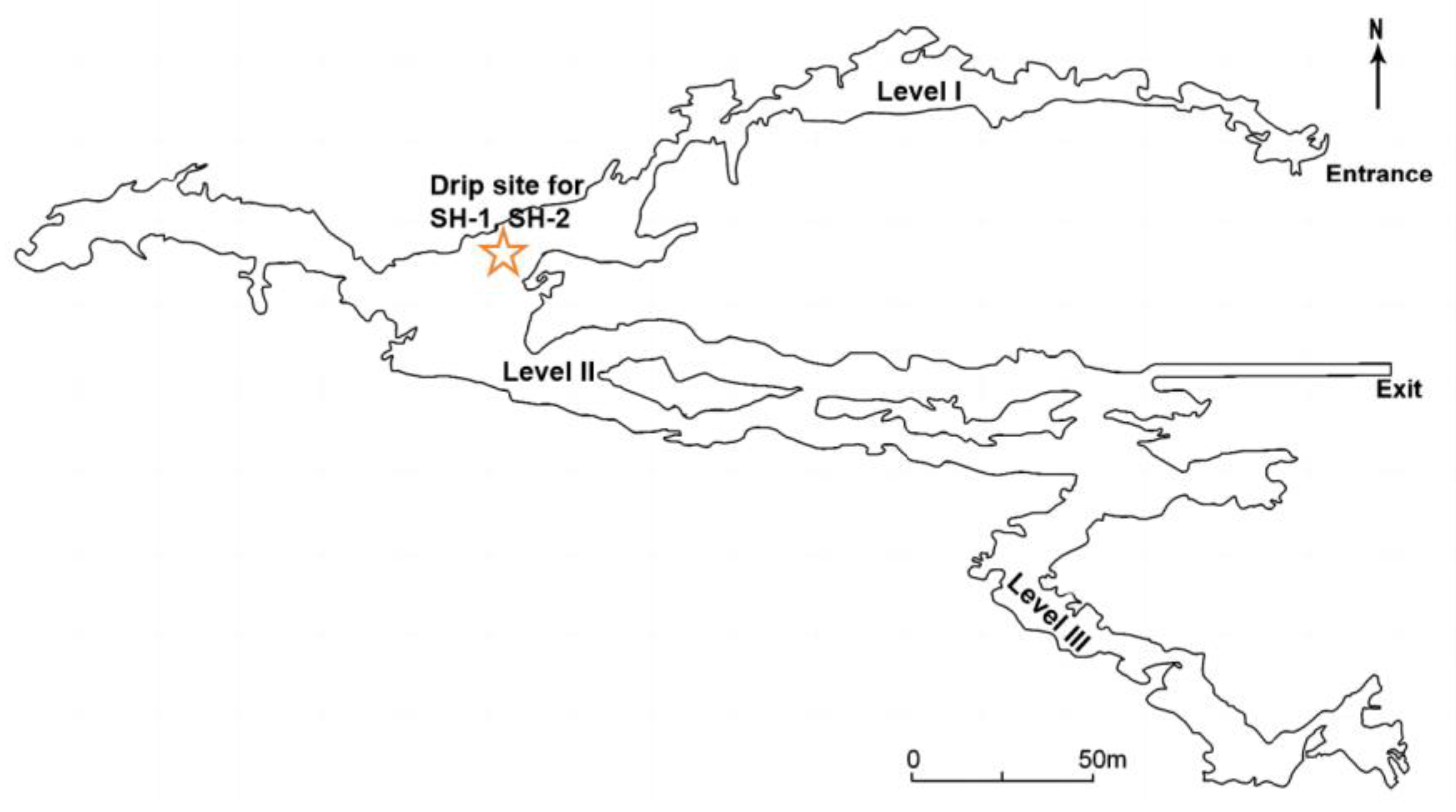
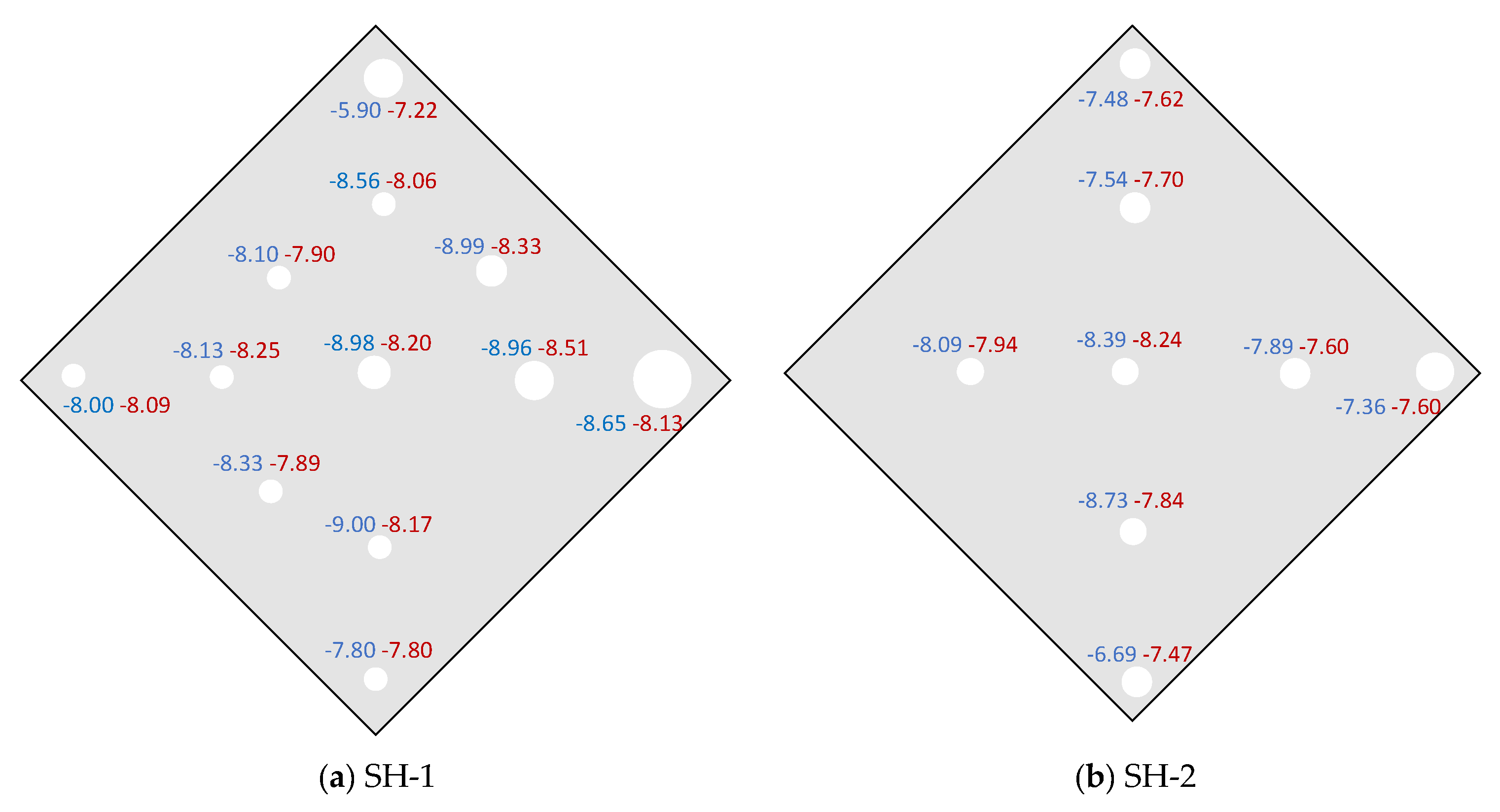
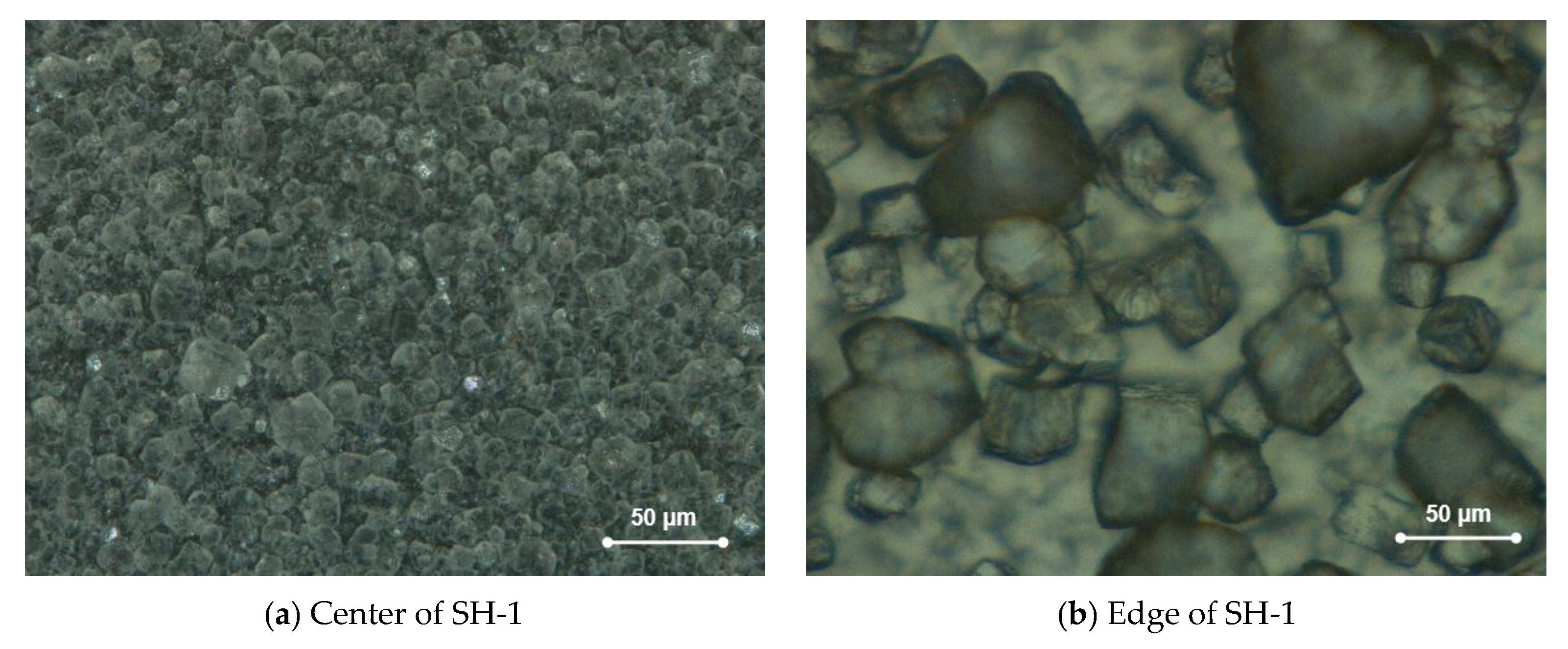
3.1. Shihua Cave
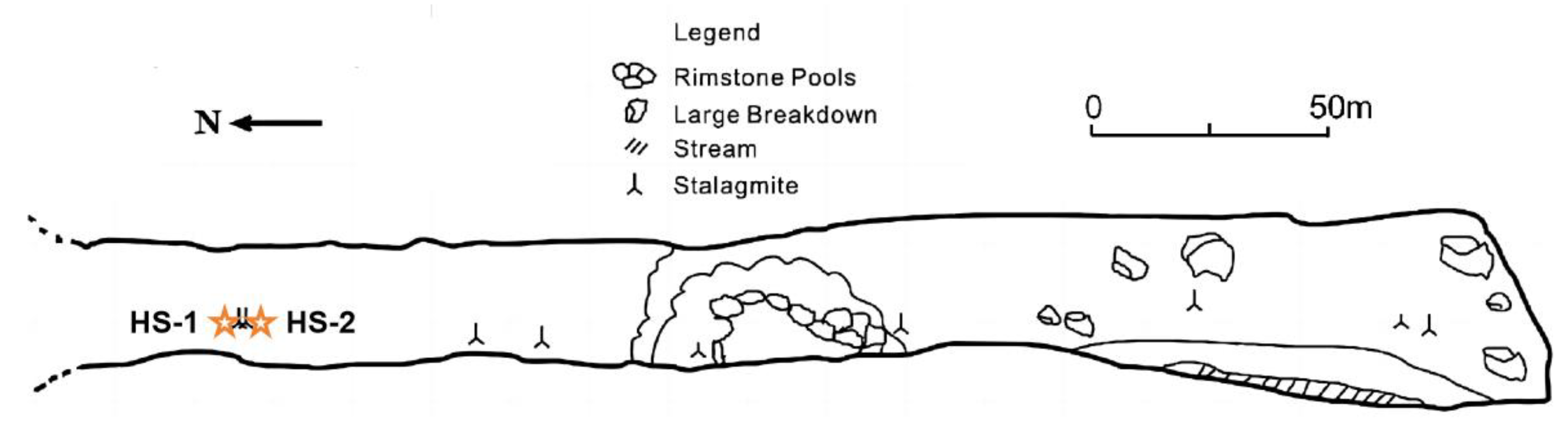
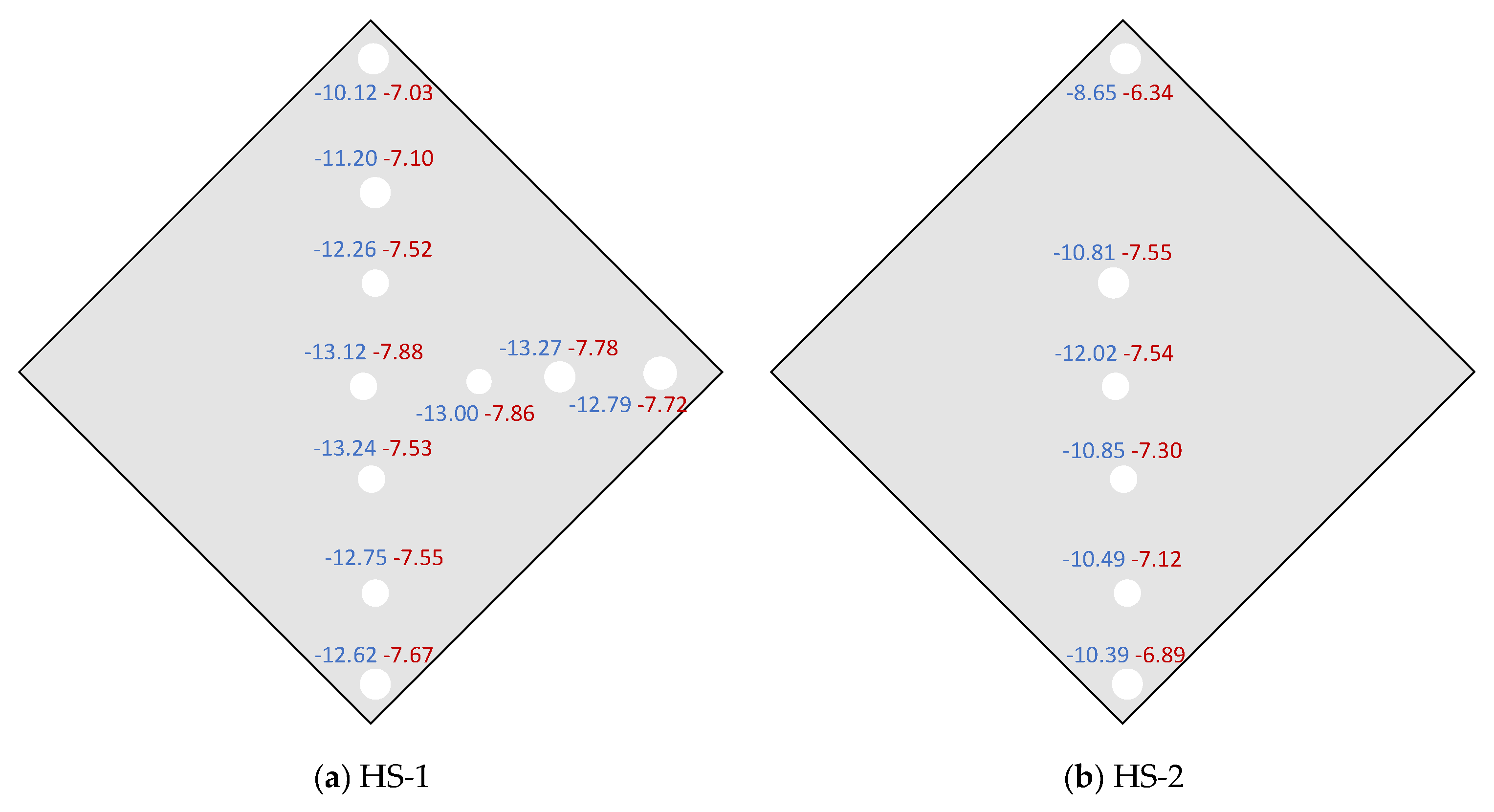
3.2. Heshang Cave
3.3. Baojinggong Cave
4. Discussion
4.1. Equilibrium vs. Kinetic Isotope Fractionation
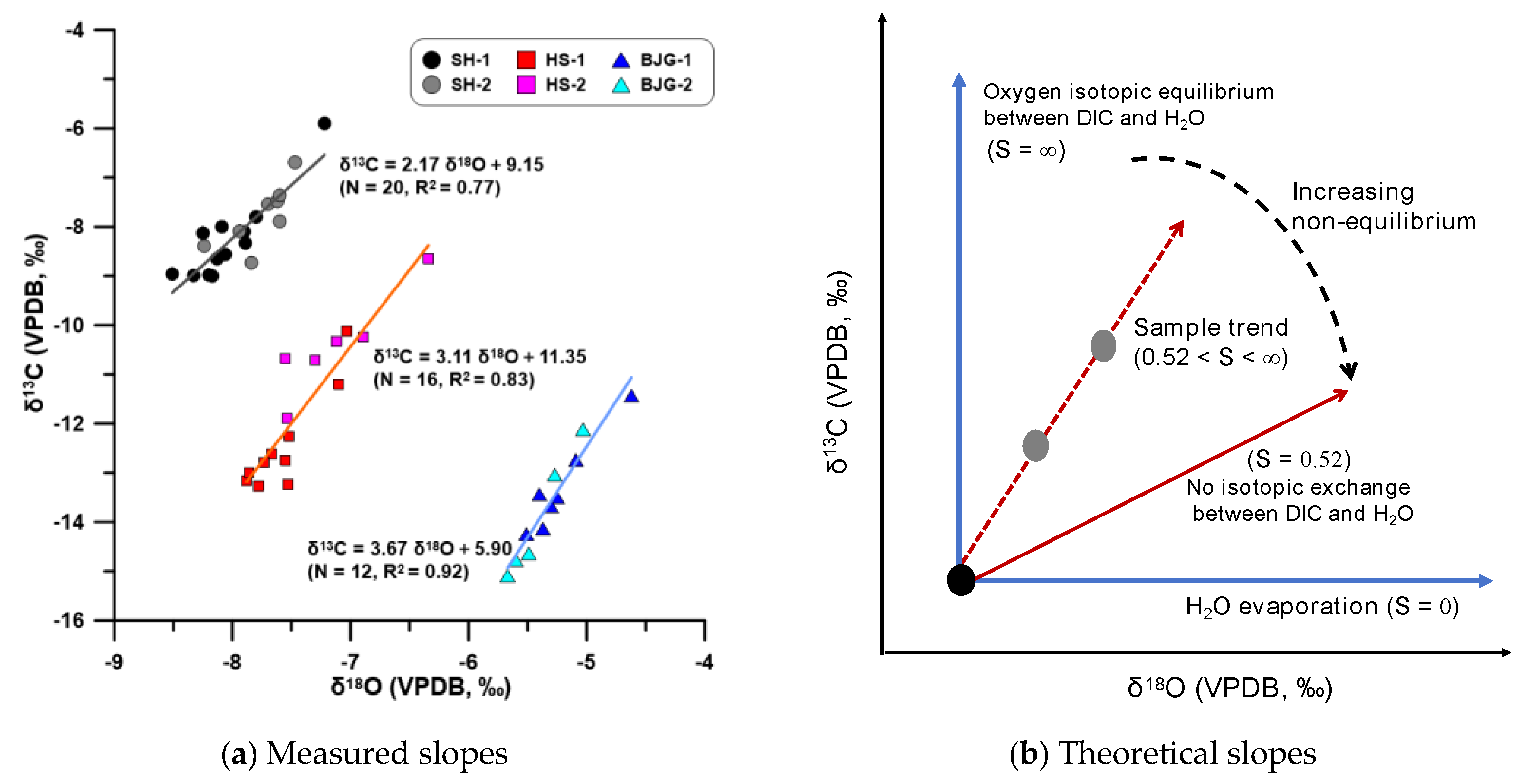
4.2. δ13C and δ18O Covariation
4.3. Implications for Speleothem-Based Paleoclimate Studies
5. Conclusions
- (1)
- The δ18O and δ13C correlations of farmed calcite from different cave sites are consistently strong, suggesting that kinetic fractionation effects are widely present in the MRC.
- (2)
- For the slower drip-rate site, the δ13C and δ18O covariations displayed a lower slope due to longer CO2 degassing. DIC depletion and H2O evaporation tend to cause shifts to higher δ18OC values.
- (3)
- Spatial distribution of farmed calcite isotopic values on glass plates indicates that isotopic equilibrium fractionation may take place in the stalagmite growth axis simultaneously with kinetic fractionation occurring at the flanks.
- (4)
- Covariations of speleothem δ13C and δ18O on short time scales may result from kinetic isotope effects, but this isotopic disequilibrium does not alter isotopic variations in response to climate change over longer time scales.
- (5)
- Hendy Test criteria have limitations as prerequisites to isotopic equilibrium. Instead, the Replication Test provides a more reliable indication of the integrity of isotopic proxies in paleoclimate research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henderson, G.M. Caving in to new chronologies. Science 2006, 313, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lawrence Edwards, R.; Shen, C.-C.; Polyak, V.J.; Asmerom, Y.; Woodhead, J.; Hellstrom, J.; Wang, Y.; Kong, X.; Spötl, C.; et al. Improvements in 230Th dating, 230Th and 234U half-life values, and U-Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth Planet. Sci. Lett. 2013, 371–372, 82–91. [Google Scholar] [CrossRef]
- Wang, Y.J.; Cheng, H.; Edwards, R.L.; An, Z.S.; Wu, J.Y.; Shen, C.-C.; Dorale, J.A. A high-resolution absolute-dated late Pleistocene monsoon record from Hulu Cave, China. Science 2001, 294, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, H.; Edwards, R.L.; He, Y.; Kong, X.; An, Z.; Wu, J.; Kelly, M.J.; Dykoski, C.A.; Li, X. The Holocene Asian monsoon: Links to solar changes and North Atlantic climate. Science 2005, 308, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, H.; Edwards, R.L.; Kong, X.; Shao, X.; Chen, S.; Wu, J.; Jiang, X.; Wang, X.; An, Z. Millennial- and orbital-scale changes in the East Asian monsoon over the past 224,000 years. Nature 2008, 451, 1090–1093. [Google Scholar] [CrossRef]
- Yuan, D.; Cheng, H.; Edwards, R.L.; Dykoski, C.A.; Kelly, M.J.; Zhang, M.; Qing, J.; Lin, Y.; Wang, Y.; Wu, J.; et al. Timing, duration, and transitions of the last interglacial Asian monsoon. Science 2004, 304, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cheng, H.; Edwards, R.L.; Chen, F.; Wang, Y.; Yang, X.; Liu, J.; Tan, M.; Wang, X.; Liu, J.; et al. A test of climate, sun, and culture relationships from an 1810-Year Chinese cave record. Science 2008, 322, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Griffiths, M.L.; Chiang, J.C.H.; Kong, W.; Wu, S.; Atwood, A.; Huang, J.; Cheng, H.; Ning, Y.; Xie, S. East Asian hydroclimate modulated by the position of the Westerlies during Termination I. Science 2018, 362, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Shen, C.C.; Li, H.C.; Li, J.Y.; Chiang, H.W.; Song, S.R.; Yuan, D.X.; Lin, C.D.J.; Gao, P.; Zhou, L.; et al. Oxygen and carbon isotopic systematics of aragonite speleothems and water in Furong Cave, Chongqing, China. Geochim. Cosmochim. Acta 2011, 75, 4140–4156. [Google Scholar] [CrossRef]
- Luo, W.; Wang, S. Transmission of oxygen isotope signals of precipitation-soil water-drip water and its implications in Liangfeng Cave of Guizhou, China. Sci. Bull. 2008, 53, 3364–3370. [Google Scholar] [CrossRef]
- Duan, W.; Ruan, J.; Luo, W.; Li, T.; Tian, L.; Zeng, G.; Zhang, D.; Bai, Y.; Li, J.; Tao, T.; et al. The transfer of seasonal isotopic variability between precipitation and drip water at eight caves in the monsoon regions of China. Geochim. Cosmochim. Acta 2016, 183, 250–266. [Google Scholar] [CrossRef]
- Pu, J.; Wang, A.; Shen, L.; Yin, J.; Yuan, D.; Zhao, H. Factors Controlling the growth rate, carbon and oxygen isotope variation in modern calcite precipitation in a subtropical cave, Southwest China. J. Asian Earth Sci. 2016, 119, 167–178. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, Y.; Zhao, J.; Tian, N.; Feng, X. Potential ENSO effects on the oxygen isotope composition of modern speleothems: Observations from Jiguan Cave, Central China. J. Hydrol. 2018, 566, 164–174. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Zhao, K.; Chen, S.; Liu, D.; Zhang, Z.; Huang, W.; Yang, S.; Liang, Y. The transfer of oxygen isotopic signals from precipitation to drip water and modern calcite on the seasonal time scale in Yongxing Cave, Central China. Environ. Earth Sci. 2018, 77, 474. [Google Scholar] [CrossRef]
- Hendy, C.H. The Isotopic Geochemistry of speleothems—I. The calculation of the effects of different modes of formation on the isotopic composition of speleothems and their applicability as palaeoclimatic indicators. Geochim. Cosmochim. Acta 1971, 35, 801–824. [Google Scholar] [CrossRef]
- Dorale, J.A.; Liu, Z. Limitations of Hendy Test criteria in judging the paleoclimatic suitability of speleothems and the need for replication. J. Cave Karst Stud. 2009, 71, 73–80. [Google Scholar]
- Spötl, C.; Mangini, A. Stalagmite from the Austrian Alps Reveals Dansgaard-Oeschger events during Isotope Stage 3: Implications for the absolute chronology of Greenland ice cores. Earth Planet. Sci. Lett. 2002, 203, 507–518. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, J.; Qing, W.; Feng, Y.; Tang, J. Speleothem-derived Asian summer monsoon variations in Central China, 54–46 Ka. J. Quat. Sci. 2011, 26, 781–790. [Google Scholar] [CrossRef]
- Day, C.C.; Henderson, G.M. Oxygen isotopes in calcite grown under cave-analogue conditions. Geochim. Cosmochim. Acta 2011, 75, 3956–3972. [Google Scholar] [CrossRef]
- Li, X.; Cheng, H.; Tan, L.; Ban, F.; Sinha, A.; Duan, W.; Li, H.; Zhang, H.; Ning, Y.; Kathayat, G.; et al. The East Asian summer monsoon variability over the last 145 years inferred from the Shihua Cave record, North China. Sci. Rep. 2017, 7, 7078. [Google Scholar] [CrossRef]
- Cai, B.; Zhu, J.; Ban, F.; Tan, M. Intra-annual variation of the calcite deposition rate of drip water in Shihua Cave, Beijing, China and its implications for palaeoclimatic reconstructions. Boreas 2011, 40, 525–535. [Google Scholar] [CrossRef]
- Ruan, J.; Hu, C. Seasonal variations and environmental controls on stalagmite calcite crystal growth in Heshang Cave, Central China. Chin. Sci. Bull. 2010, 55, 3929–3935. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, H.; Duan, W. The evaporation effect on transmission of oxygen isotope signal of drip water in Baojinggong, Guangdong: Revealed by Cave Monitoring. Quat. Sci. 2013, 618, 618–620. (In Chinese) [Google Scholar]
- Chen, F.H.; Chen, J.H.; Holmes, J.; Boomer, I.; Austin, P.; Gates, J.B.; Wang, N.L.; Brooks, S.J.; Zhang, J.-W. Moisture changes over the last millennium in arid Central Asia: A review, synthesis and comparison with monsoon region. Quat. Sci. Rev. 2010, 29, 1055–1068. [Google Scholar] [CrossRef]
- Kim, S.T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Mickler, P.J.; Stern, L.A.; Banner, J.L. Large kinetic isotope effects in modern speleothems. Geol. Soc. Am. Bull. 2006, 118, 65–81. [Google Scholar] [CrossRef]
- Ban, F.; Pan, G.; Zhu, J.; Cai, B.; Tan, M. Temporal and spatial variations in the discharge and dissolved organic carbon of drip waters in Beijing Shihua Cave, China. Hydrol. Process. 2008, 22, 3749–3758. [Google Scholar] [CrossRef]
- Hu, C.; Henderson, G.; Huang, J.; Chen, Z.; Johnson, K. Report of a three-year monitoring programme at Heshang Cave, Central China. Int. J. Speleol. 2008, 37, 143–151. [Google Scholar] [CrossRef][Green Version]
- Tong, X.; Zhou, H.; Huang, Y.; He, H.; Zhu, L. Spatio-temporal variation of air CO2 concentration in Baojinggong Cave, Guangdong, China. Trop. Geogr. 2013, 33, 439–443. (In Chinese) [Google Scholar]
- Mickler, P.J.; Banner, J.L.; Stern, L.; Asmerom, Y.; Edwards, R.L.; Ito, E. Stable isotope variations in modern tropical speleothems: Evaluating equilibrium vs. kinetic isotope effects. Geochim. Cosmochim. Acta 2004, 68, 4381–4393. [Google Scholar] [CrossRef]
- Tremaine, D.M.; Froelich, P.N.; Wang, Y. Speleothem calcite farmed in situ: Modern calibration of δ18O and δ13C paleoclimate proxies in a continuously-monitored natural cave system. Geochim. Cosmochim. Acta 2011, 75, 4929–4950. [Google Scholar] [CrossRef]
- Riechelmann, S.; Schröder-Ritzrau, A.; Wassenburg, J.A.; Schreuer, J.; Richter, D.K.; Riechelmann, D.F.C.; Terente, M.; Constantin, S.; Mangini, A.; Immenhauser, A. Physicochemical characteristics of drip waters: Influence on mineralogy and crystal morphology of recent cave carbonate precipitates. Geochim. Cosmochim. Acta 2014, 145, 13–29. [Google Scholar] [CrossRef]
- Genty, D.; Blamart, D.; Ghaleb, B.; Plagnes, V.; Causse, C.; Bakalowicz, M.; Zouari, K.; Chkir, N.; Hellstrom, J.; Wainer, K. Timing and dynamics of the last deglaciation from European and North African δ13C stalagmite profiles—Comparison with Chinese and South Hemisphere stalagmites. Quat. Sci. Rev. 2006, 25, 2118–2142. [Google Scholar] [CrossRef]
- Li, H.; Gu, D.; Ku, T.; Stott, L.D.; Chen, W. Applications of interannual-resolution stable isotope records of speleothem: Climatic changes in Beijing and Tianjin, China during the past 500 years—The δ18O record. Sci. China Ser. D Earth Sci. 1998, 41, 362–368. [Google Scholar] [CrossRef]
- Duan, W.; Cai, B.; Tao, T.; Yang, X.; Tan, M. Multi-timescale variations of δ18O–δ13C in stalagmites: Insights into isotopic disequilibrium and human activities. J. Geophys. Res. Atmos. 2023, 128, e2023JD039607. [Google Scholar] [CrossRef]




| Cave | Location | Altitude (m asl) | Annual Precipitation (mm) | Mean Surface Air T (°C) | Thickness of Bedrock (m) | Thickness of Soil (cm) | Climate | Vegetation |
|---|---|---|---|---|---|---|---|---|
| SH | 39.78° N, 115.93° E | 251 | 539 | 12.2 | 30–130 | 100 | Temperate monsoon climate | Shrub and grass |
| HS | 30.45° N, 110.42° E | 294 | 1343 | 16.5 | 300 | 40 | Subtropical monsoon climate | Woody perennial plant and shrub-grass |
| BJG | 24.12° N, 113.35° E | 610 | 1836 | 21.2 | >170 | 50 | Subtropical monsoon climate | Evergreen broad-leaf forest |
| Glass Plate | Date Placed | Date Collected | Drip Rate (drip/min) | RH (%) | CO2 Conc. (ppm) | Cave Air T (°C) | Measured δ18OC Range (VPDB, ‰) | Measured δ18OW (VSMOW, ‰) | Predicted δ18OC (VPDB, ‰) |
|---|---|---|---|---|---|---|---|---|---|
| SH-1 | 8 February 2013 | 8 March 2013 | 0.34 | 100 | 864 | 15.1 | (−8.51, −7.22) | −8.71 | −8.30 |
| SH-2 | 28 March 2012 | 14 June 2012 | 0.38 | 88.2 | 1142 | 16.4 | (−8.73, −6.69) | −8.62 | −8.49 |
| HS-1 | 30 May 2011 | 29 June 2011 | 4 | <80 | 495 | 19 | (−7.88, −7.03) | −7.65 | −8.10 |
| HS-2 | 30 November 2011 | 30 December 2011 | 3 | <80 | 505 | 17 | (−7.55, −6.34) | −7.76 | −7.78 |
| BJG-1 | 3 March 2012 | 2 June 2012 | 6.25 | 88.4 | 982 | 16.2 | (−5.51, −4.62) | −6.08 | −5.98 |
| BJG-2 | 3 March 2012 | 2 June 2012 | 52.20 | 88.4 | 982 | 16.2 | (−5.67, −5.03) | −6.12 | −6.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Tao, T.; Duan, W.; Ruan, J.; Hu, C.; Li, Y.; Li, X.; Cheng, H.; Tan, M. Reevaluating Hendy Test with Modern Cave Calcite from the Monsoon Region of China. Minerals 2024, 14, 747. https://doi.org/10.3390/min14080747
Tian L, Tao T, Duan W, Ruan J, Hu C, Li Y, Li X, Cheng H, Tan M. Reevaluating Hendy Test with Modern Cave Calcite from the Monsoon Region of China. Minerals. 2024; 14(8):747. https://doi.org/10.3390/min14080747
Chicago/Turabian StyleTian, Lijun, Tao Tao, Wuhui Duan, Jiaoyang Ruan, Chaoyong Hu, Yunxia Li, Xianglei Li, Hai Cheng, and Ming Tan. 2024. "Reevaluating Hendy Test with Modern Cave Calcite from the Monsoon Region of China" Minerals 14, no. 8: 747. https://doi.org/10.3390/min14080747
APA StyleTian, L., Tao, T., Duan, W., Ruan, J., Hu, C., Li, Y., Li, X., Cheng, H., & Tan, M. (2024). Reevaluating Hendy Test with Modern Cave Calcite from the Monsoon Region of China. Minerals, 14(8), 747. https://doi.org/10.3390/min14080747







