Abstract
The study object was slag from the Balkhash copper smelter, obtained by re-melting anode mud containing nonferrous metals. The process flow for processing these slags includes sintering with Na2SO4, Na2CO3, and coal, followed by soda-alkaline leaching of the sinter and extraction of metals from the solution into marketable products. Since sintering is the main operation providing high selectivity, the composition of the products of this process was studied. The main transformations during sintering were determined, and the optimal parameters were identified. The structures of slags and sintered materials obtained during the experiments were studied by electron-probe microanalysis. Sintering was performed at 600–800 °C. The best results for sulphidization of slag components were obtained at 800 °C; a further increase in temperature leads to the smelting of sinter particles and slows down sulphidization. The optimal quantities of additives, based on the weight of the slag, are Na2SO4—45%, Na2CO3—15%, and reducing agent—41%, with a sintering time of 2 h. These conditions enable the sulphidization of non-ferrous metals in the slag to the entire depth of the polymetallic globules. The distinct concentration of harmful impurities (Ni, As, and Sb) was observed in the fine structure of the polymetallic globules.
1. Introduction
A lot of attention is paid to the issue of processing oxidized slags after various types of smelting. For this purpose, methods of reduction smelting with additional extraction of metals are often used. One of the most common methods is flotation beneficiation of slags, including copper smelting. However, a significant part of the metal is in an oxidized form and does not lend itself to flotation. One way to address this problem is sulphidation of the slags for subsequent flotation enrichment. The processes of sulphidation of slags are often discussed in publications. For example, Lan et al. [1] report on the use of sulphidation for the extraction of heavy metals in the low-grade ores. The work [2] proposed the extraction of tellurium by directional sulphurization-vacuum distillation and discussed the principles and mechanisms of sulphidation, as well as practical examples of applying the process to treat various types of technogenic mineral waste. Compared with traditional methods, this proposed process is shorter and greatly reduces the loss of tellurium due to multiple dispersions. The process does not produce waste and is environmentally friendly, as demonstrated by the economic and sustainability evaluations. This research provides a clean and sustainable method for treating copper telluride slag for the metallurgical industry.
Sometimes, combined slag processing methods are employed, including pyrometallurgical processing, with the reduction of oxidized metals and sulphidation [3]. Numerous publications mention the use of sodium sulphide as a sulphidizing agent, but the mechanism of the process is not fully disclosed [4]. Antropova et al. [5] established the possibility of deep sulphidation of difficult-to-extract oxidized minerals of lead and zinc during their roasting with pyrite concentrate in an atmosphere of superheated water vapour. It has been shown that the reaction of oxidized compounds of lead and zinc with iron sulphide in an atmosphere of superheated water vapour with the formation of sulphides occurs at the solid-gas interface, namely: MeO–H2S—during the sulphidation of heterolite and bedantite; PbSO4 + H2S—during sulphidation of plumboyarosite. Sun et al. [6] explored the use of sulphidation to recover heavy metals like nickel and copper present in nickel-smelting slag. Kou et al. [7] investigated a process to separate silver and valuable metals by sulphidation and roasting and proposed the use of vacuum distillation to exploit the differences in saturated vapour pressure of the metal sulphides. This method utilized the physical properties of the various metal sulphides to selectively extract silver and other valuable metals from metallurgical waste. The authors in work [8] investigated the application of sulphidation to stabilize heavy metals in mineral processing wastes. The materials in articles [8,9] provide a fundamental understanding of the thermodynamic and kinetic aspects of sulphidation processes for the recovery of metals from various mineral sources. Ref. [10] studied the selective sulphidation-vacuum volatilization method for the recovery of tellurium and bismuth.
The complex composition of artificial raw materials, comprising various heavy, minor, rare, and noble metals, presents substantial obstacles to devising technologies to transform them into marketable products. There is considerable focus on further processing copper smelting residues, including those derived from anode mud in copper smelting [11,12,13,14,15,16]. Thiosalt metallurgy emerges as a promising solution to this challenge. Thiosalts, present in nearly all non-ferrous metals, display diverse physical and chemical attributes. Their solubility in sulphide-alkaline solutions varies greatly, offering avenues for segregating non-ferrous metals into separate products. Moreover, the functional qualities and novel attributes uncovered in thiosalt metallurgy hold considerable potential [17,18]. It is recognized that sulphides of heavy, noble, and rare metals exhibit semiconductor properties. When these sulphides interact with other sulphides, newly formed compounds acquire unique characteristics of superionic conductors. Research into equilibrium systems and phase equilibria enables the identification of methods for the targeted synthesis of novel phases. The examination of phase diagrams involving various combinations such as CuZnS2-FeZnS2, Cu2S-FeS-ZnS, Cu2S-La2S3-EuS, FeS-Sb2S3-Sm2S3, Cu2S-Cu3AsS4-S, Ag2S-SnS2-Sb2S3, Cu2S-PbS-Sb2S3, Nd2S3-Ga2S3-EuS, FeS-PbS, Cu2GeS3-Ag2GeS3, etc. It led to the discovery of new properties of thiosalts, which can be formed during sulphidizing sintering [19,20,21,22,23,24,25,26,27].
Leveraging the predictable formation patterns and distinctive physical and chemical characteristics of non-ferrous metal thiosalts holds the promise of devising innovative and efficient technologies for handling intricate synthetic materials. Exploiting the properties of non-ferrous metal thiosalts has laid the groundwork for developing technologies aimed at processing antimony oxide-sulphide concentrates, raw lump ores, and intermediates, along with secondary materials from lead production, anode mud, copper-molybdenum intermediates, and molybdenum-bismuth concentrates [28]. Proposed technologies aim to process arsenic copper speiss, coarse dehydration slag, and metallurgical waste, offering the potential to remove arsenic from metallurgical production in a less toxic form, leveraging the properties of thiosalts [29,30,31,32,33].
A comprehensive process flow, incorporating sintering, leaching of the sinter using a sulphide-alkali solution, fractional precipitation of non-ferrous metals from the solution, cake smelting, and subsequent processing for crude lead, represents a promising approach grounded in the properties of non-ferrous metal thiosalts. Research in this domain is pivotal. Precious metal slags from the Balkhash smelter emerge as particularly favourable material for such investigations. Figure 1 displays this process flow.
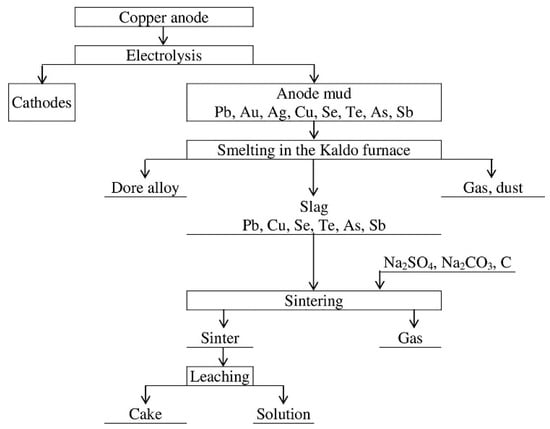
Figure 1.
Process flow of anode mud processing.
The composition of the initial slag fully corresponds to a typical slag obtained as a result of the smelting of anode mud in a Kaldo furnace. This technology consists of smelting anode mud to oxidize most of the lead. Noble metals concentrate in the remaining metallic lead, which is directed to further gold and silver extraction using standard technologies. The proposed technology intended for the processing of such slags includes sintering with sodium sulphate and carbonate, leaching of the sintered mass with a sulphate-carbonate solution, decomposition of the resulting solutions with the release of selenium, tellurium, antimony, arsenic, and melting of the sintered mass with the release of lead containing gold and silver, as well as matte containing copper.
In our perspective, the thiosalt formation reaction progresses through multiple elementary stages, primarily within the solid phase. These stages include the initial contact of reagents, activation of reagents facilitated by adsorption, formation of surface molecular films, deactivation of reagents, and the eventual crystalline structuring of the thiosalt molecule. The principal interaction during thiosalt formation is elucidated by the following scheme:
where Me′-Na, Ba; Me″-Pb, Sb, As, n, m, l, k, z, y, x—number of atoms.
Metal sulphides undergo a transformation into a novel structure endowed with specific physical and chemical properties.
2. Materials and Methods
The study object was smelting slag obtained by melting anode mud from the Balkhash copper smelter. Slag is an oxidized material with the following chemical composition, wt. %: 49.5 Pb, 17.3 SiO2, 1.5 Cu, 3.75 Sb, 13.3 Ba, 0.65 As, 4.5 Na, 0.2 Se, 0.24 Te, 0.0014 Au, 0.39 Ag, 7.3 O, 14.66, and others. Besides, the elemental composition of original slag was subjected to X-ray fluorescence analysis using a spectrometer with wave dispersion. Experimental studies aim to produce non-ferrous metal thiosalts during the sintering of a charge from slag, sodium sulphate, sodium carbonate, reducing agent—coal (with a carbon content of 74.3%).
The X-ray phase and electron probe microanalysis (EPMA) using the energy-dispersive spectroscopy (EDS) failed to detect sulphur in the original slag. Thus, the detection of sulphide and thio compounds in subsequent studies of the resulting sintered compounds will only be associated with the addition of sulphur-containing components to the sintering charge.
The sinter’s samples in the form of polished sections were studied by a JEOL JXA 8230 Electron Probe Microanalyzer (JEOL Ltd., Tokyo, Japan). Electron probe examination of the slag was carried out in the following modes: back-scattered electron image (COMPO), energy-dispersive spectroscopy (EDS), and qualitative wavelength-dispersive spectroscopy (WDS). To conduct research using EDS-WDS microanalysis methods, the thin carbon coating film was applied to the polished surface of the samples.
3. Results and Discussion
It should be noted that the basis of this technology is the sintering operation, where the intensity of the low-temperature synthesis during the formation of non-ferrous metal thiosalts is of decisive importance. The efficiency of sintering is determined by the reactions of solid-phase synthesis that occurs in Na2S-MenSm systems, where Me is a non-ferrous metal, n is the number of non-ferrous metal atoms, and m is the number of sulphur atoms.
Reactions of the solid-phase synthesis of non-ferrous metal thiosalts proceed in several elementary stages, including contacting of reagents, activation of reagents due to adsorption, formation of surface molecular films, deactivation of the surface, activation of reagents, and formation of the crystal structure of the thiosalt molecule.
The thermodynamic characteristics of the main reactions of the sintering process are given in Table 1. The calculation of ΔG, ΔH, ΔS of possible reactions during sintering was carried out using the Outotec HSC Chemistry software (version 5). For all considered reactions, negative Gibbs energies were obtained. The sequential formation of Na2S and S can take place by reactions (2) and (3) (Table 1) during sintering. A significant excess of carbon seems to reduce the likelihood of oxidation of sulphur formed at a lower temperature during the heating of the charge. The excess carbon provides a sufficiently low partial pressure of oxygen in the reaction zone at 800 °C. This makes it possible to carry out reactions (4)–(6) and similar sulphidization reactions of other chalcophilic metals. In this case, the formation of metal thiosalts in sulphide form is possible. The form of thiosalts is unstable under normal conditions, including in the presence of oxygen. To prevent the decomposition of thiosalts and the oxidation of sulphides, the resulting sinters were stored before analysis and subsequent leaching in a vessel with an inert gas (Ar).

Table 1.
The thermodynamic characteristics of the main reactions of the sintering.
Figure 2 presents diagrams showing the areas of possible existence of sulphides, tellurides, and selenides, characteristic of the conditions of the slag sintering process. These diagrams were calculated using the Outotec HSC Chemistry software. As observed on the diagrams, the formation of these compounds occurs at low partial pressures of oxygen and high partial pressures of sulphur, selenium, and tellurium. Such conditions correspond to the sintering process, which is carried out in the presence of carbon, which reduces the atmosphere in the crucible. At the same time, during the sintering process, lead, arsenic, and antimony are sulphiding, and selenium and tellurium form selenides and tellurides, which are highly soluble in aqueous solutions, which is acceptable to subsequent leaching.
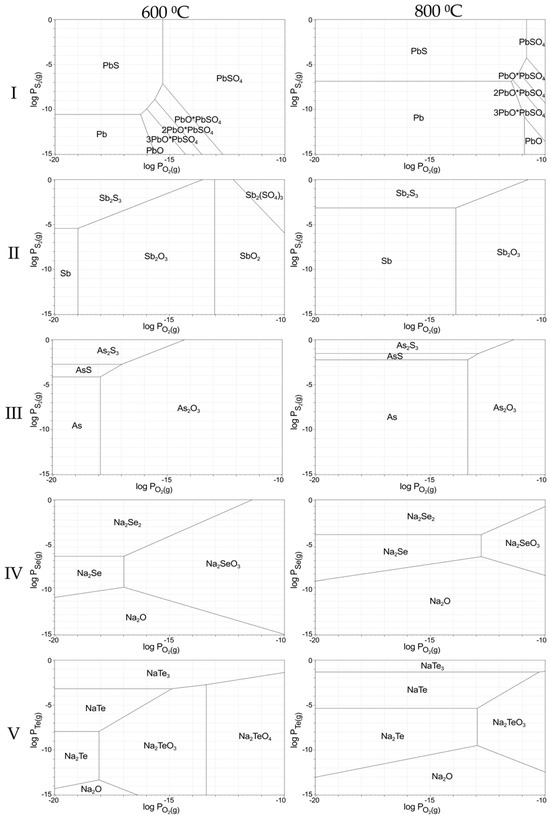
Figure 2.
Predominance area diagrams for systems: (I)—Pb-O-S; (II)—Sb-O-S; (III)—As-O-S; (IV)—Se-O-Na; (V)—Te-O-Na.
Experimental studies on the formation of thiosalts of non-ferrous metals during sintering of a slag charge were carried out in the presence of sodium sulphate, sodium carbonate, and a reducing agent with carbon. Sodium carbonate was added to facilitate the subsequent leaching of the sinter. Before mixing and sintering, all the components of the charge were milled. The size of more than 90% of the particles did not exceed 74 microns. Sintering was carried out in alumina crucibles in the NTS 08/16 Nabertherm GmbH (Nabertherm GmbH, Lilienthal, Germany) furnace by atmosphere pressure. The main technological parameters of the sintering process are specified in Table 2. The consumption coefficients of sodium sulfate and carbonate, as well as carbon, were determined as a result of a series of preliminary experiments.

Table 2.
Main technological parameters of sintering.
The resulting sintered mass is very heterogeneous in composition, so microprobe analysis was used for further studies of the sintered mass samples. Figure 3 shows a microphotograph of sintered mass sample No. 3, with element EDS mapping, where Na, Ba, Pb, Cu, Se, and Te are in an oxidized form in the polymetallic globule (sinter No. 3, t—600 °C). Silver is present in a metallic form. There are no traces of sulphur in this globule. Consequently, the formation of sulphides at temperature 600 °C has not yet begun. Noticeable changes in the sintered mass structure occurred at temperatures above 700 and 800 °C. The most characteristic changes occurred in sintered mass samples 6 and 7, so further analysis with the use of the microprobe method was performed specifically for these samples. As follows from the above analysis of the original slag, no sulphur was detected in it. Figure 4 shows a microphotograph of sintered mass sample No. 6, with element EDS mapping, where the distribution of Na, Cu, Pb, and S shows the areas of initial formation of thiosalts at a sintering temperature of 700 °C for 2 h. Individual slag globules containing copper and lead are associated with sulphur. Thus, the sintering temperature and the corresponding holding of the charge at this temperature do not ensure a sufficiently complete formation of thiosalts. Figure 5 shows the results of the EDS analysis of sintered mass sample No. 7 at some points. The ratio of non-ferrous metals and sulphur at these points indicates the presence of thiosalts. However, the ratio between sulphur and metals indicates that the process of the sulphidization of lead and copper was not complete.
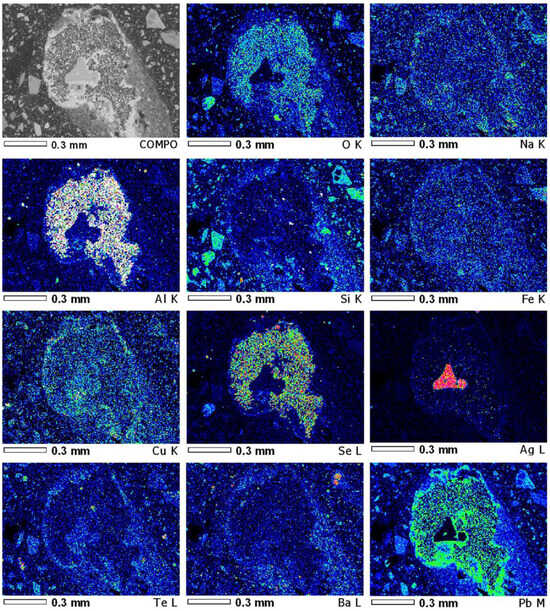
Figure 3.
EDS element mapping of polymetallic globule (sinter No. 3, t—600 °C).
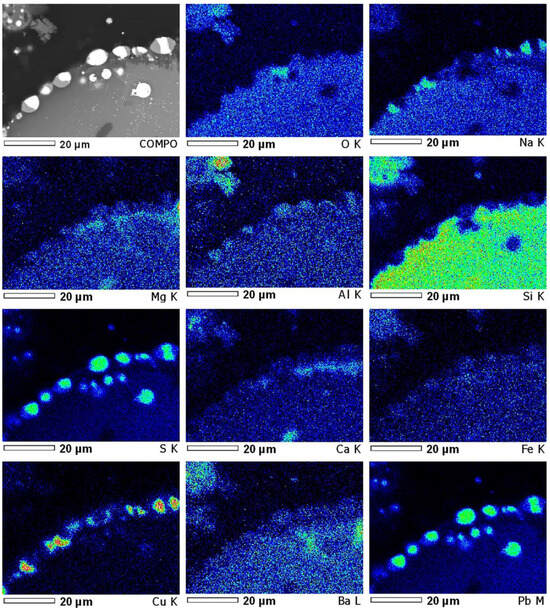
Figure 4.
EDS element mapping of fragment of polymetallic globule (sinter No. 6, t—700 °C).
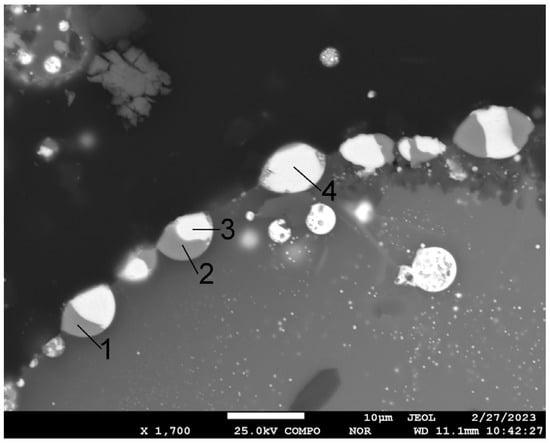
Figure 5.
Microstructure of fragment of polymetallic globule and EDS point analysis (sinter No. 6, t—700 °C). Content in points, mass %: 1—25.6 S, 74.4 Cu; 2—26.9 S, 71.5 Cu, 1.6 Pb; 3—14.6 C, 13.1 S, 1.2 Cu, 2.3 Se, 68.8 Pb; 4—9.3 C, 14.0 S, 1.2 Cu, 2.6 Se, 72.9 Pb.
As it follows from the above study results, the thiosalt formation process during sintering occurs only on the surface of slag particles, even at 700 °C; therefore, further sintering was performed only at 800 °C.
Microphotographs of sintered mass sample no. 7 with element EDS mapping are shown in Figure 6 and Figure 7. In Figure 6, the zone consisting mainly of metals—lead, iron, antimony, copper, and chromium—coincides with the sulphur distribution zone, indicating a fairly complete sulphidization of these metals to the entire depth of the polymetallic globule, surrounded by slag. Of interest in this globule is the annular distribution of antimony together with sulphur around the central part of the globule, which consists mainly of lead sulphide. Copper and iron, also apparently in the form of sulphides, are evenly distributed throughout the entire volume of this lead globule, where the distribution of As, Ba, Pb, and S shows thiosalt formation areas at a sintering temperature of 800 °C for 2 h. Figure 7 shows the results of EDS analysis at four characteristic points. If the first two points correspond mainly with lead sulphide with an admixture of barium and carbon, then at the third and fourth points, some of the lead, arsenic, and antimony is possibly present in metallic form.
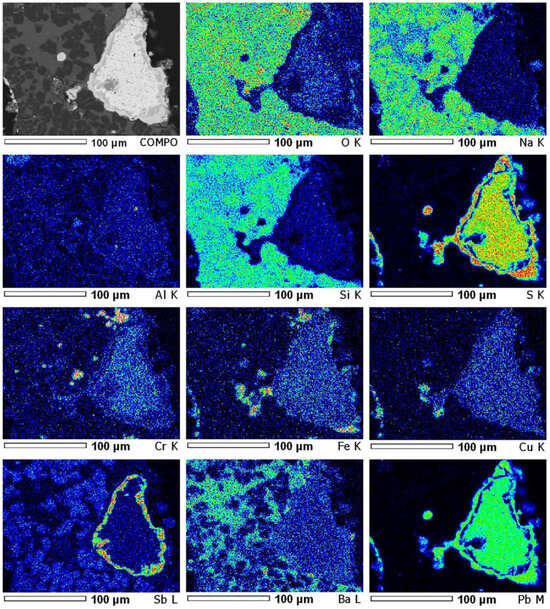
Figure 6.
EDS element mapping of polymetallic globule (sinter No. 7, t—800 °C).
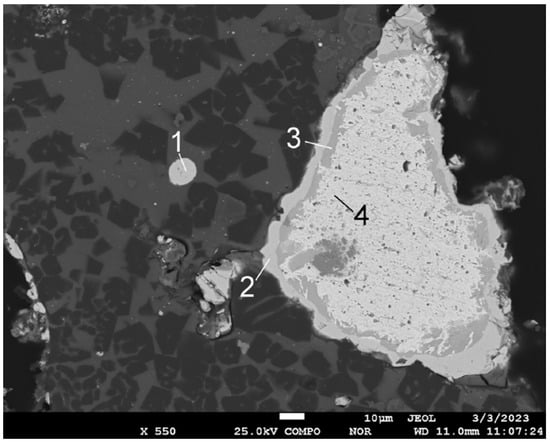
Figure 7.
Microstructure of polymetallic globule and EDS point analysis (sinter No. 7, t—800 °C). Content in points, mass %: 1—9.5 C, 16.5 S, 3.7 Ba, 70.3 Pb; 2—9.5 C, 16.0 S, 3.4 Ba, 71.1 Pb; 3—5.0 C, 2.5 S, 27.4 As, 49.8 Sb, 15.3 Pb; 4—5.8 C, 5.0 O;, 2.4 S, 1.0 Cr, 2.3 Sb, 83.5 Pb.
The most remarkable feature of this area was the multi-shell structure of the polymetallic globule. The presence of Cr and O likely occurred due to a particle of polishing substance being imprinted onto the soft fragment of globule. Moreover, in the fine structure of polymetallic globules, areas with concentrated contents of harmful impurities of heavy elements such as Ni, As, Sb, and Bi or Te were also observed (Figure 8). Their presence was confirmed by means of wavelength-dispersive spectroscopy (WDS), as shown in Figure 9.
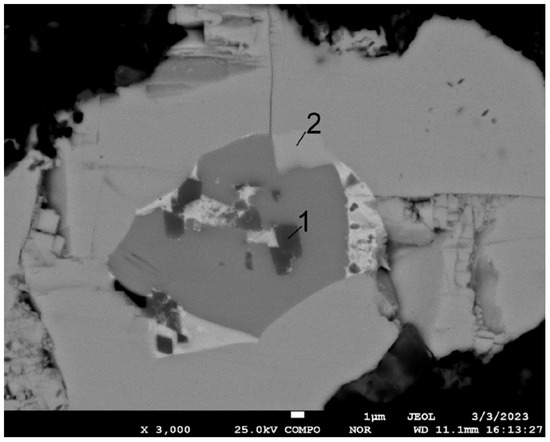
Figure 8.
Microstructure of polymetallic globule and EDS point analysis (sinter No. 7, t—800 °C). Content in points, mass %: 1—7.6 C, 7.7 Ni, 76.0 As, 6.8 Sb, 1.9 Bi; 2—5.4 C, 3.8 S, 2.0 Sb, 28.4 Te, 60.4 Pb.
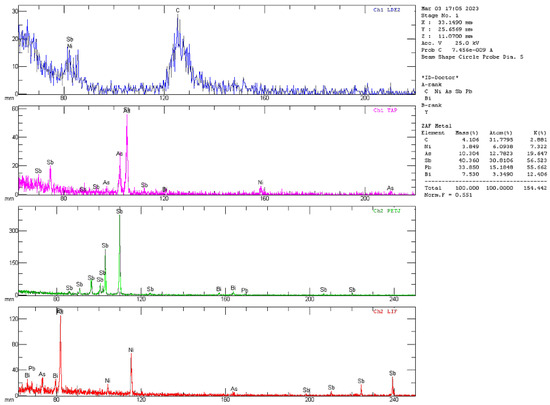
Figure 9.
WDS analysis of selected area of polymetallic globule (sinter No. 7, t—800 °C).
It should be noted that barium is present in both the oxidized slag zone and the sulphidized zone. Copper and lead are present mainly in the sulphidized zone. Sodium and silicon are present in the oxidized slag zone. Iron is primarily found in the sulphidized zone, but it is also present in the oxidized zone. The basis of the sulphidized polymetallic globules is lead sulphide. Inside it in the near-surface layer, a metallized alloy of antimony and arsenic is present in the form of a thin layer (Figure 7, point 3). Carbon is present in almost all points of sulphidized phases, which indicates its penetration into the depth of polymetallic globules (Figure 5, Figure 7 and Figure 8).
Thus, a sintering temperature of 800 °C and the corresponding holding of the charge at this temperature ensure a fairly complete formation of thiosalts and metallic form of some metals.
4. Conclusions
Thermodynamic analysis has shown the possibility of sulphidization of slag metal oxides under sintering conditions. The conducted studies have shown that sintering of slags of this type with Na2SO4, Na2CO3 and carbon in the temperature range 600–800 °C is technically feasible. However, no traces of sulphur were found in globules containing chalcophilic metals obtained at 600 °C. At 700 °C, the appearance of sulphur on the outer surface of the globules was noted. At a temperature of 800 °C, the globules were sulphidated to the entire depth.
Thus, the results obtained from the study of the slag sintering process with sodium carbonate and sulphate in the presence of carbon indicate the possibility of obtaining a sinter containing metal thiosalts. The preferred process temperature is 800 °C, since a decrease in the activity of the charge components is observed at lower temperatures. A higher temperature results in partial melting of the slag components and a sharp slowdown in the sulphidization process. The optimal amount of additives based on the weight of the slag is Na2SO4—45%, Na2CO3—15%, reducing agent—41%, sintering time—2 h. These conditions make it possible to perform sulphidization of non-ferrous metals in the slag to the entire depth of the polymetallic globules. The distinctive concentration of harmful impurities (Ni, As, Sb, and Bi or Te) was observed in the fine structure of polymetallic globules. Copper is present in the sinter, mainly in the sulphidization zone along with lead. The oxidized slag zone contains sodium, silicon, aluminum, and partially barium. Iron is primarily found in the sulphidized zone, but it is also present in the oxidized zone. The basis of the sulphidized polymetallic globules is lead sulphide. Inside it in the near-surface layer a metallized alloy of antimony and arsenic is present in the form of a thin layer. Carbon is present at almost all points of the sulphided phases, which indicates its penetration to the entire depth of the polymetallic globules. As a result of sintering the slag of the Kaldo furnace with reagents, a redistribution of the forms of metal content took place in accordance with the Goldschmidt classification—in the slag form—Si, Al, and Na remained, in the sulphide form—Pb, Cu, Sb, As, Se, and Te.
Thus, the resulting cake is completely ready for subsequent hydrometallurgical processing. This technology of sulphidization of metals in slag can be used for other types of slag before its flotation beneficiation.
Author Contributions
Conceptualization, L.S.; methodology, L.S. and S.K. (Sergey Kvyatkovskiy); validation, M.D. and A.S. (Alexander Shakhalov); formal analysis, B.S.; investigation, A.S. (Anastassiya Semenova); resources, M.D.; data curation, S.K. (Sergey Kvyatkovskiy); writing—original draft preparation, S.K. (Sergey Kvyatkovskiy); writing—review and editing, S.K. (Sultanbek Kozhakhmetov); visualization, B.S.; supervision, S.K. (Sergey Kvyatkovskiy); project administration, S.K. (Sergey Kvyatkovskiy); funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been/was/is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP14869407).
Data Availability Statement
The data is available upon the request to the corresponding author due to our internal policy.
Conflicts of Interest
The coauthor Alexander Shakhalov is working at the Kazakhmys Corporation LLP company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lan, Z.Y.; Lai, Z.N.; Zheng, Y.X.; Lv, J.F.; Pang, J.; Ning, J.L. Recovery of Zn, Pb, Fe and Si from a low-grade mining ore by sulphidation roasting-beneficiation-leaching processes. J. Cent. S. Univ. 2020, 27, 37–51. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Liu, D.; Jiang, W.; Zha, G.; Huang, D.; Deng, P.; Li, B. Waste-free separation and recovery of copper telluride slag by directional sulphidation-vacuum distillation. J. Clean. Prod. 2022, 335, 130356. [Google Scholar] [CrossRef]
- Matkarimov, S.T.; Safarov, A.K.H. Processing of copper slag by sulphidation of its oxidized compounds. Molod. Uchenii. 2019, 17, 32–34. [Google Scholar]
- Nadirov, R.K. Recovery of Valuable Metals from Copper Smelter Slag by Sulfation Roasting. Trans. Indian Inst. Met. 2019, 72, 603–607. [Google Scholar] [CrossRef]
- Antropova, I.G.; Dambayeva, A.Y.U. Method of sulphidation of difficult-to-enrich oxidized minerals of lead and zinc in an atmosphere of water vapor. Fiziko_tehn. Probl. Razr. Polez. Iskop. 2015, 1, 163–167. [Google Scholar]
- Sun, W.; Li, X.; Liu, R.; Zhai, Q.; Li, J. Recovery of valuable metals from nickel smelting slag based on reduction and sulfurization modification. Minerals 2021, 11, 1022. [Google Scholar] [CrossRef]
- Kou, H.; Yang, H.; Xu, W.; Li, Y. Recovery of Crude Silver and Valuable Metals from Tin Anode Slime. JOM 2024, 23, 1–12. [Google Scholar] [CrossRef]
- Liang, Y.; Chai, L.Y.; Min, X.; Tang, C.J. Hydrothermal sulphidation and floatation treatment of heavy-metal-containing sludge for recovery and stabilization. J. Hazard. Mater. 2012, 217–218, 307–314. [Google Scholar] [CrossRef]
- Ma, J.; Shi, T.; Zhang, Y.; Huang, D.; Li, Y.; Yang, B.; Chen, C. Thermodynamic and kinetic analyses and experimental verification of tellurium and bismuth recovery from bismuth telluride waste by selective sulphidation-vacuum volatilization. J. Mater. Res. Technol. 2023, 26, 3003–3014. [Google Scholar] [CrossRef]
- Ma, J.; Shi, T.; Li, Y.; Yang, B. Selective sulphidation-vacuum volatilization processes for tellurium and bismuth recovery from bismuth telluride waste thermoelectric material. J. Environ. Manag. 2023, 327, 116845. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Kvyatkovskiy, S.A.; Dyussebekova, M.A.; Semenova, A.S.; Nurhadiyanto, D. Analysis of Existing Technologies for Depletion of Dump Slags of Autogenous Melting. Comp. Use Min. Res. 2022, 323, 23–29. [Google Scholar] [CrossRef]
- Koizhanova, A.; Kenzhaliyev, B.; Magomedov, D.; Erdenova, M.; Bakrayeva, A.; Abdyldaev, N. Hydrometallurgical studies on the leaching of copper from man-made mineral formations. Comp. Use Min. Res. 2023, 330, 32–42. [Google Scholar] [CrossRef]
- Gorai, B.; Jana, R.K.; Premchand. Characteristics and utilisation of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Wu, T.; Zhang, F. Improving Beneficiation of Copper and Iron from Copper Slag by Modifying the Molten Copper Slag. Metals 2016, 6, 86. [Google Scholar] [CrossRef]
- Subrata, R.; Sandeep, R. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Klaffenbach, E.; Montenegro, V.; Guo, M.; Blanpain, B. Sustainable and Comprehensive Utilization of Copper Slag: A Review and Critical Analysis. J. Sustain. Met. 2023, 9, 468–496. [Google Scholar] [CrossRef]
- Volodin, V.N.; Burabaeva, N.M.; Trebukhov, S.A.; Ersaiynova, A.A. Phase diagram of the selenium-sulphur system in the pressure range 1×10−5–1×10−1 MPa. Russ. J. Phys. Chem. A 2016, 90, 1663–1668. [Google Scholar] [CrossRef]
- Naimanbayev, M.A.; Lokhova, N.G.; Abisheva, A.E.; Baltabekova, Z.A.; Maldybaev, G.K. Study of low-titanium slag and soda baking process. Non-Ferr. Metall. 2017, 4, 12–20. [Google Scholar] [CrossRef][Green Version]
- Lampeka, Y.D.; Tsymbal, L.V. Preparation, structure and functional properties of MoS2 and WS2 nanocomposites with inorganic chalcogenide semiconductors: A review. Theor. Exp. Chem. 2017, 53, 211–234. [Google Scholar] [CrossRef]
- Lampeka, Y.D.; Tsymbal, L.V. Nanocomposites of two-dimensional molybdenum and tungsten dichalcogenides with metal particles: Preparation and prospects for application Theor. Exp. Chem. 2015, 51, 141–162. [Google Scholar] [CrossRef]
- Štirbanovic, Z.; Uroševic, D.; Ðordevic, M.; Sokolovic, J.; Aksic, N.; Živadinovic, N.; Milutinovic, S. Application of Thionocarbamates in Copper Slag Flotation. Metals 2022, 12, 832. [Google Scholar] [CrossRef]
- Abdullaeva, S.S.; Mammadov, F.M.; Bakhtiyarly, I.B. Quasi-binary section CuInS2-FeIn2S4. Russ. J. Inorg. Chem. 2020, 65, 100–105. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Andreev, O.V. Phase equilibria in the Cu2S-La2S3-EuS. Russ. J. Inorg. Chem. 2017, 62, 610–618. [Google Scholar] [CrossRef]
- Aliev, O.M.; Asadov, M.M.; Azhdarova, D.S.; Mamedov, S.G.; Ragimova, V.M. Polythermal section FeSb2S4-FeSm2S4 the FeS-Sb2S3-Sm2S3 system. Russ. J. Inorg. Chem. 2018, 63, 833–836. [Google Scholar] [CrossRef]
- Gasanova, Z.T.; Mashadieva, L.F.; Babanly, M.B.; Yusibov, Y.A. Phase equilibria in the Cu2S-Cu3AsS4-S system. Russ. J. Inorg. Chem. 2017, 62, 591–597. [Google Scholar] [CrossRef]
- Mashadieva, L.F.; Babanly, M.B.; Gasanova, Z.T.; Yusibov, Y.A. Phase equilibria in the Cu-Cu2Se-As system. Russ. J. Inorg. Chem. 2017, 62, 598–603. [Google Scholar] [CrossRef]
- Mammadov, S.H.; Mammadov, A.N.; Kurbanova, R.C. Quasi-binary section Ag2SnS-AgSbS2. Russ. J. Inorg. Chem. 2020, 65, 217–221. [Google Scholar] [CrossRef]
- Aminov, T.G.; Busheva, E.V.; Shabunina, G.G.; Novotortsev, V.M. Magnitic phase diagram of solid solution in the CoCr2S4-Cu0.5Ga0.5Cr2S4 system. Russ. J. Inorg. Chem. 2018, 63, 519–529. [Google Scholar] [CrossRef]
- Gapanovich, M.V.; Agapkin, M.D.; Rakitin, V.V.; Kolesnikova, A.M.; Novikov, G.F.; Odin, I.N.; Sedlovets, D.M. Effect of precursor mixture composition on the phase composition and electrical transport properties of Cu1.85ZnSnS4 and Cu1.5Zn1.15Sn0.85S4 kesterite solid solutions prepared in molten KI. Inorg. Mat. 2018, 54, 760–766. [Google Scholar] [CrossRef]
- Serikbaeva, A.K.; Aimova, M.Z.; Mamyrbaeva, K.K.; Busurmanova, A.C.; Suleimenova, B.S.; Seidalieva, L.K. Development of a method for reprocessing technogenic lead production raw materials to extract rhenium and arsenic. Metallurgist 2021, 65, 340–348. [Google Scholar] [CrossRef]
- Raevskaya, A.E.; Stroyuk, O.L.; Kuchmy, S.Y. Nanoparticles of Ag-In-S and Cu-In-S in aqueous media: Preparation, spectral and luminescent properties. Theor. Exp. Chem. 2017, 53, 338–348. [Google Scholar] [CrossRef]
- Gasanova, U.A.; Aliev, O.M.; Bakhtiyarly, I.B.; Mamedov, S.G. The FeS-PbS system. Russ. J. Inorg. Chem. 2019, 64, 242–246. [Google Scholar] [CrossRef]
- Kopylov, N.I. Processing of commercial arsenic-containing products of metallurgy of heavy nonferrous metals with withdrawal of arsenic into iron-arsenic alloy. Rev. Khimich. Tekhn. 2021, 3, 114–122. (In Russian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).