Utilisation of Biosilica as Active Silica Source for Metakaolin-Based Geopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Geopolymers
2.3. Characterisation
3. Results and Discussion
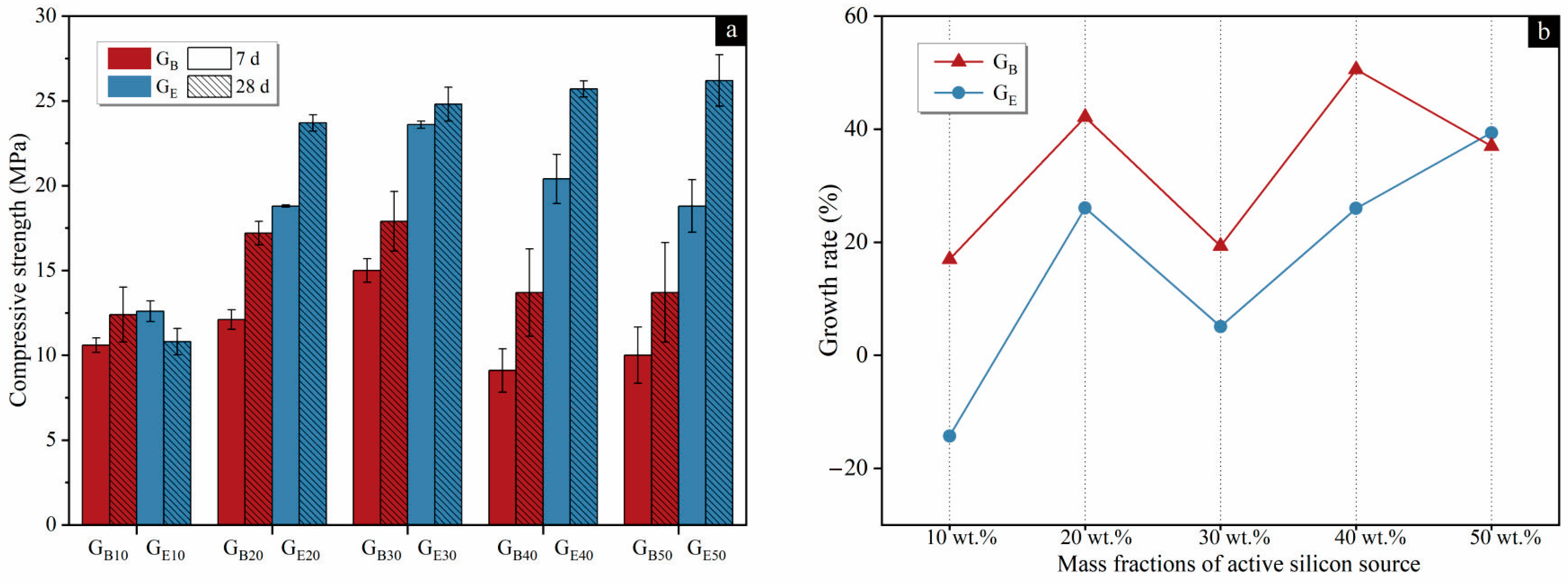
3.1. Compressive Strengths
3.1.1. Influence of Mass Fractions
3.1.2. Influence of Organic Matter
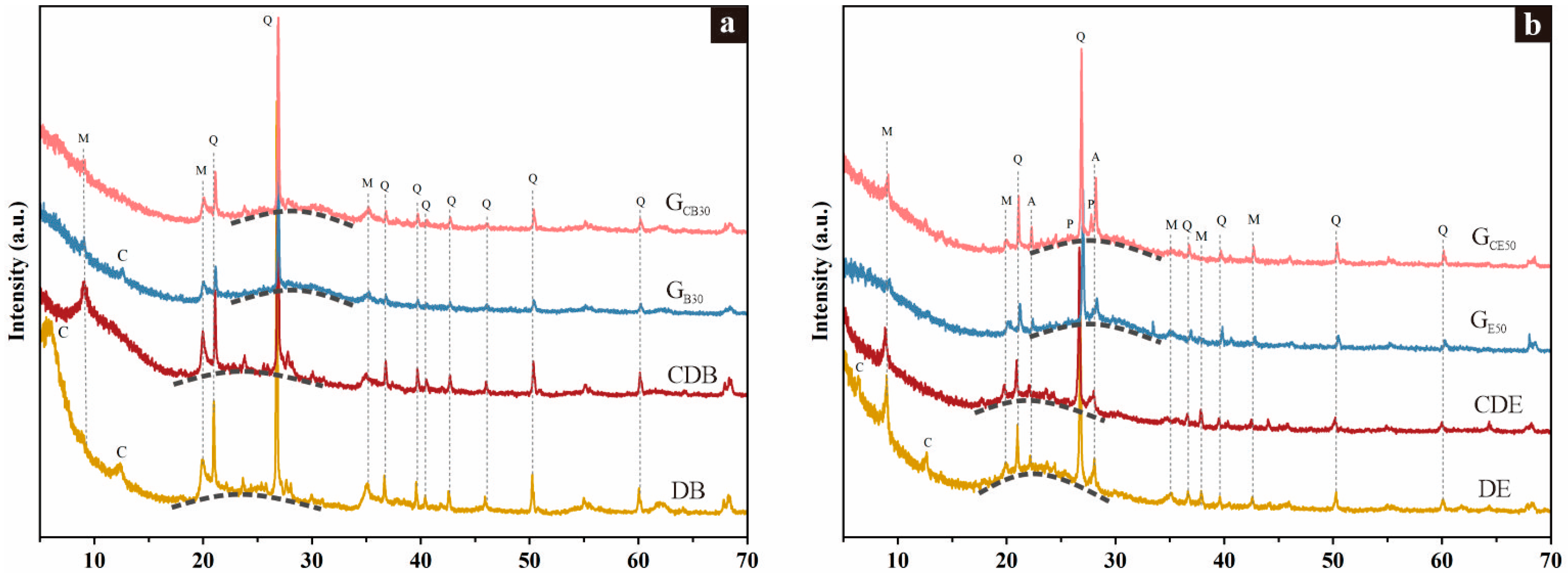
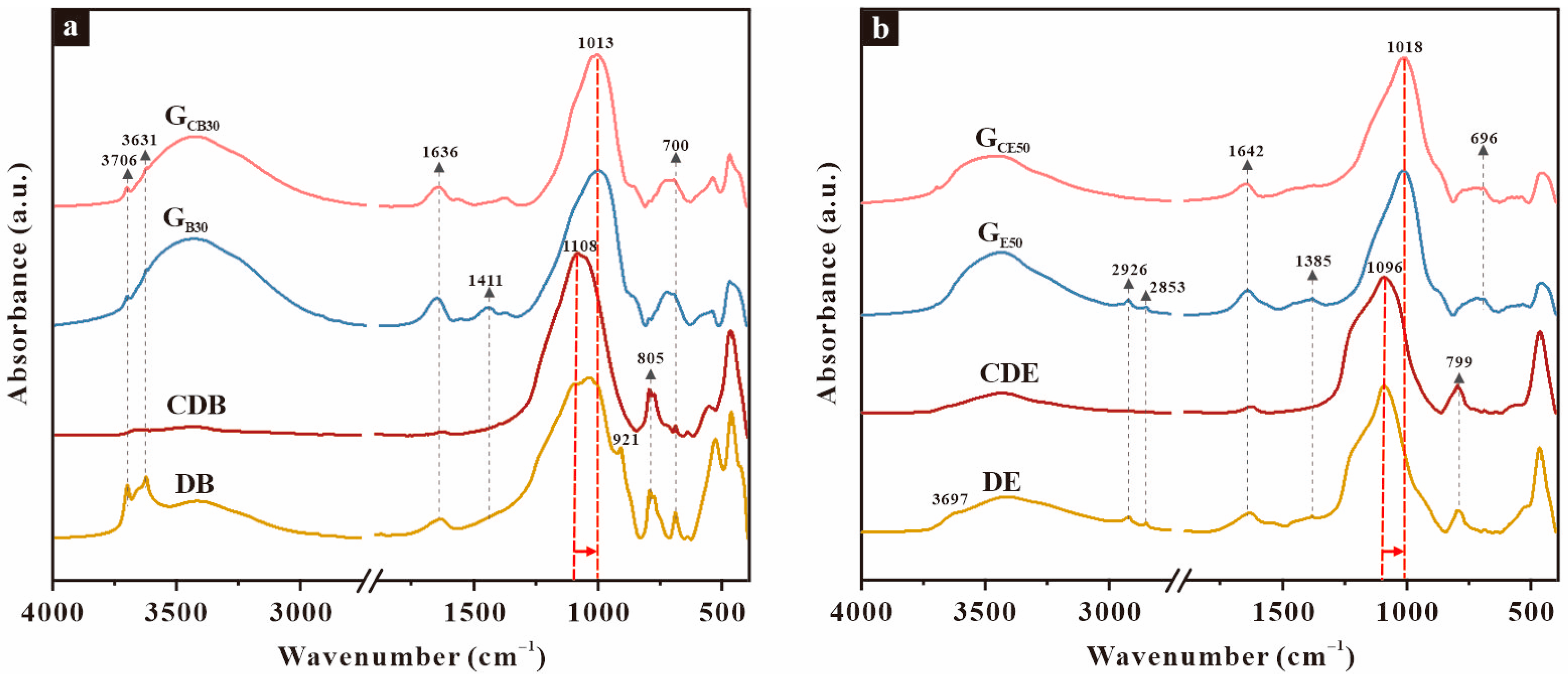
3.2. Compositions
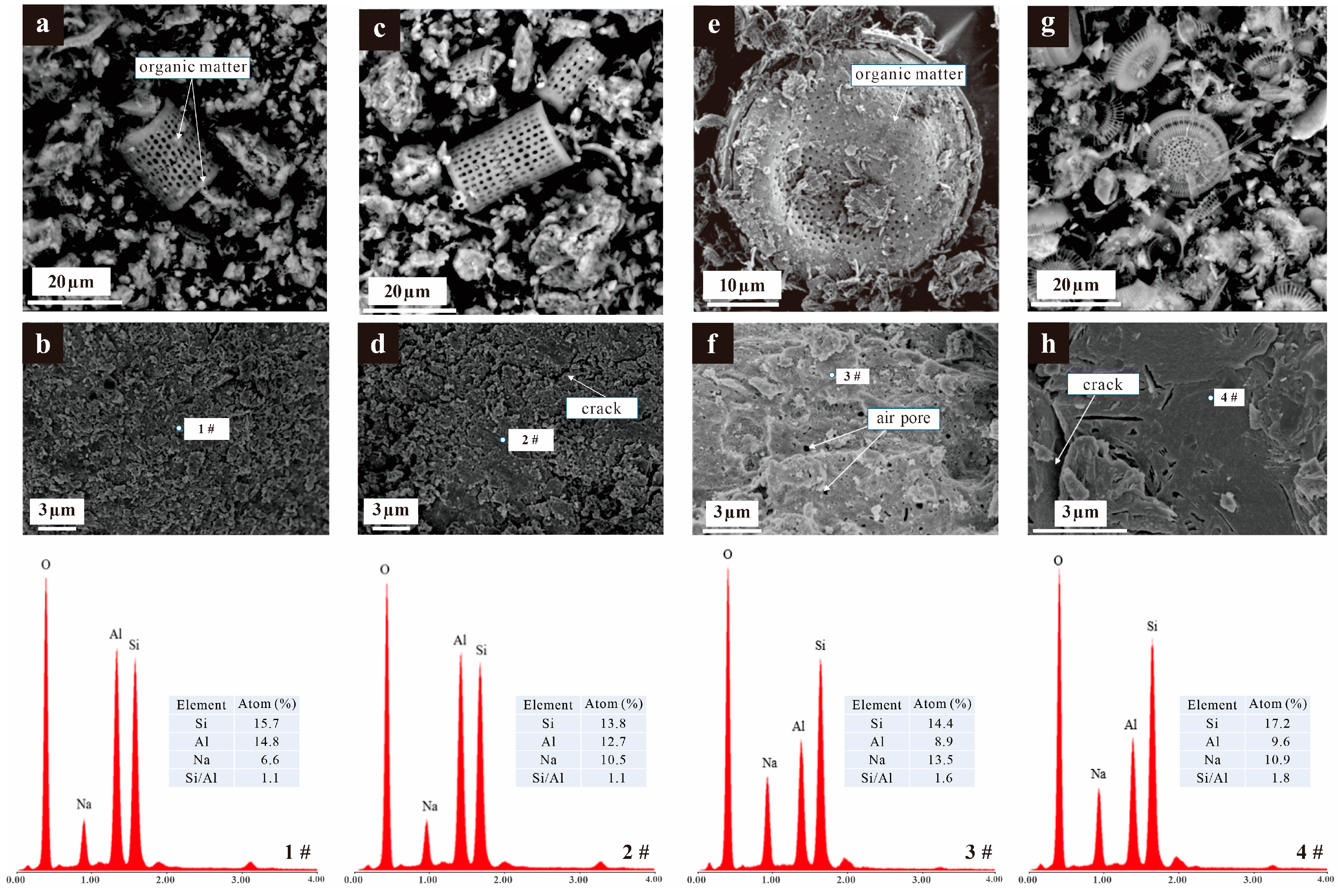
3.3. Microstructure
4. Conclusions
- The amorphous phase and organic matter present in biosilica are of critical importance in influencing the release of silicon monomers.
- Organic matter in biosilica exhibits chemical stability in strong alkaline environments, hindering the dissolution of frustules, which reduces the release amount of active silica while increasing the presence of impurity particles that need to be cemented.
- Calcination treatment for biosilica effectively removes organic matter, thereby enhancing the efficiency ratio of active silica content.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.G.; Lu, B.W.; Bai, T.; Wang, H.; Du, F.P.; Zhang, Y.F.; Cai, L.; Jiang, C.; Wang, W.J. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. 6—Nanostructure/microstructure of fly ash geopolymers. In Geopolymers; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 89–117. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Bai, C.; Shao, J.; Li, X.; Zhang, Z.; Qiao, Y.; Hao, J.; Li, H.; Zheng, T.; Colombo, P. Fabrication and properties of slag-based geopolymer syntactic foams containing hollow glass microspheres. Mater. Lett. 2022, 308, 131158. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, P.; Zhang, B.; Wang, Q.; Deng, L.; Liu, D. Realization of high-percentage addition of fly ash in the materials for the preparation of geopolymer derived from acid-activated metakaolin. J. Clean. Prod. 2021, 285, 125430. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.; Yong, H.C.; Ming, L.Y.; Hussin, K.; Surleva, A.; Azimi, E.A. Manufacturing parameters influencing fire resistance of geopolymers: A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 721–733. [Google Scholar] [CrossRef]
- Glasby, T.; Day, J.; Genrich, R.; Aldred, J. EFC Geopolymer Concrete Aircraft Pavements at Brisbane West Wellcamp Airport. In Proceedings of the Concrete 2015 Conference, Melbourne, Australia, 30 August–2 September 2015. [Google Scholar]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, K.; Mo, B.; Li, X.; Cui, X. Preparation and characterization of a reflective and heat insulative coating based on geopolymers. Energy Build. 2015, 87, 220–225. [Google Scholar] [CrossRef]
- Davidovits, J. 30 Years of successes and failures in geopolymer applications. Market trends and potential breakthroughs. In Proceedings of the Geopolymer 2002 Conference, Melbourne, Australia, 28–29 October 2002; Geopolymer Institute: Melbourne, Australia, 2002. [Google Scholar]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer—An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Mejía, J.M.; Mejía de Gutiérrez, R.; Montes, C. Rice husk ash and spent diatomaceous earth as a source of silica to fabricate a geopolymeric binary binder. J. Clean. Prod. 2016, 118, 133–139. [Google Scholar] [CrossRef]
- Bagci, C.; Kutyla, G.P.; Kriven, W.M. Fully Reacted High Strength Geopolymer made with Diatomite as a Fumed Silica Alternative. Ceram. Int. 2017, 43, 14784–14790. [Google Scholar] [CrossRef]
- Fulton, G.P.; Ebrary, I. Diatomaceous Earth Filtration for Safe Drinking Water; American Society of Civil Engineers: Reston, VA, USA, 2000. [Google Scholar]
- Danil de Namor, A.F.; El Gamouz, A.; Frangie, S.; Martinez, V.; Valiente, L.; Webb, O.A. Turning the volume down on heavy metals using tuned diatomite. A review of diatomite and modified diatomite for the extraction of heavy metals from water. J. Hazard. Mater. 2012, 241–242, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, N.; Yilmaz, A. Use of diatomite as partial replacement for Portland cement in cement mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Ergün, A. Effects of the usage of diatomite and waste marble powder as partial replacement of cement on the mechanical properties of concrete. Constr. Build. Mater. 2011, 25, 806–812. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Chindaprasirt, P.; Sata, V.; Sinsiri, T. High calcium fly ash geopolymer containing diatomite as additive. Indian J. Eng. Mat. Sci. 2013, 20, 310–318. [Google Scholar]
- Posi, P.; Lertnimoolchai, S.; Sata, V.; Phoo-ngernkham, T.; Chindaprasirt, P. Investigation of properties of lightweight concrete with calcined diatomite aggregate. KSCE J. Civ. Eng. 2014, 18, 1429–1435. [Google Scholar] [CrossRef]
- Li, J.; Jin, Q.; Zhang, W.; Li, C.; Monteiro, P.J.M. Microstructure and durability performance of sustainable cementitious composites containing high-volume regenerative biosilica. Resour. Conserv. Recycl. 2022, 178, 106038. [Google Scholar] [CrossRef]
- Sharma, N.; Simon, D.P.; Diaz-Garza, A.M.; Fantino, E.; Messaabi, A.; Meddeb-Mouelhi, F.; Germain, H.; Desgagné-Penix, I. Diatoms Biotechnology: Various Industrial Applications for a Greener Tomorrow. Front. Mar. Sci. 2021, 8, 636613. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, S.; Liu, P.; Deng, X.; Jing, Y.; Bai, C.; Li, J. Purification of biosilica from living diatoms by a two-step acid cleaning and baking method. J. Appl. Phycol. 2014, 26, 1511–1518. [Google Scholar] [CrossRef]
- Lutynski, M.; Sakiewicz, P.; Lutynska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Xun, B.; Jiang, Y.; Yang, D.; Shi, X. Carbonization Treatment of Diatomite with High Ignition Loss and Reinforcement of Natural Rubber. Chem. J. Chin. Univ. 2011, 32, 1617–1621. [Google Scholar]
- Guo, H.; Zhang, B.; Deng, L.; Yuan, P.; Li, M.; Wang, Q. Preparation of high-performance silico-aluminophosphate geopolymers using fly ash and metakaolin as raw materials. Appl. Clay Sci. 2021, 204, 106019. [Google Scholar] [CrossRef]
- Pereira, D.G.; Afonso, A.; Medeiros, F.M. Overview of Friedman’s Test and Post-hoc Analysis. Commun. Stat.—Simul. Comput. 2015, 44, 2636–2653. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yu, W.B.; Yuan, P.; Liu, D.; Deng, L.L.; Yuan, W.W.; Tao, B.; Cheng, H.F.; Chen, F.R. Facile preparation of hierarchically porous diatomite/MFI-type zeolite composites and their performance of benzene adsorption: The effects of NaOH etching pretreatment. J. Hazard. Mater. 2015, 285, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. A Review of the Classification of Opal with Reference to Recent New Localities. Minerals 2019, 9, 299. [Google Scholar] [CrossRef]
- Karuppaiyan, J.; Jeyalakshmi, R.; Kiruthika, S.; Wadaan, M.A.; Khan, M.F.; Kim, W. A study on the role of surface functional groups of metakaolin in the removal of methylene blue: Characterization, kinetics, modeling and RSM optimization. Environ. Res. 2023, 226, 115604. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 2005, 21, 3028–3036. [Google Scholar] [CrossRef]
- Obiora, S.C.; Umeji, A.C. Petrographic evidence for regional burial metamorphism of the sedimentary rocks in the Lower Benue rift. J. Afr. Earth Sci. 2004, 38, 269–277. [Google Scholar] [CrossRef]
- Gale, D.K.; Gutu, T.; Jiao, J.; Chang, C.-H.; Rorrer, G.L. Photoluminescence Detection of Biomolecules by Antibody-Functionalized Diatom Biosilica. Adv. Funct. Mater. 2009, 19, 926–933. [Google Scholar] [CrossRef]
- Rodriguez-Vidal, F.J.; Ortega-Azabache, B.; Gonzalez-Martinez, A.; Bellido-Fernandez, A. Comprehensive characterization of industrial wastewaters using EEM fluorescence, FT-IR and 1H NMR techniques. Sci. Total Environ. 2022, 805, 150417. [Google Scholar] [CrossRef]
- Sitarz, M. The structure of liquation silico-phosphate glasses. J. Mol. Struct. 2008, 887, 229–236. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Deng, L.; Liu, D. Novel acid-based geopolymer synthesized from nanosized tubular halloysite: The role of precalcination temperature and phosphoric acid concentration. Cem. Concr. Compos. 2020, 110, 103601. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, P.; Guo, H.; Deng, L.; Li, Y.; Li, L.; Wang, Q.; Liu, D. Effect of curing conditions on the microstructure and mechanical performance of geopolymers derived from nanosized tubular halloysite. Constr. Build. Mater. 2020, 268, 121186. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated total reflectance Fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Jiang, Y.; Jiang, X.; Zhang, D. Preparation of biosilica structures from frustules of diatoms and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef]
- Huang, J.; Wu, B.; Lyu, S.; Li, T.; Han, H.; Li, D.; Wang, J.-K.; Zhang, J.; Lu, X.; Sun, D. Improving the thermal energy storage capability of diatom-based biomass/polyethylene glycol composites phase change materials by artificial culture methods. Sol. Energy Mater. Sol. Cells 2021, 219, 110797. [Google Scholar] [CrossRef]
- Umemura, K.; Noguchi, Y.; Ichinose, T.; Hirose, Y.; Kuroda, R.; Mayama, S. Diatom Cells Grown and Baked on a Functionalized Mica Surface. J. Biol. Phys. 2008, 34, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, C.; Shi, L.; Gao, T.; Song, X.; Bachmatiuk, A.; Zou, Z.; Deng, B.; Ji, Q.; Ma, D. Growing three-dimensional biomorphic graphene powders using naturally abundant diatomite templates towards high solution processability. Nat. Commun. 2016, 7, 13440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Zhang, W.; Pan, J.; Cai, J. Enlargement of diatom frustules pores by hydrofluoric acid etching at room temperature. J. Mater. Sci. 2011, 46, 5665–5671. [Google Scholar] [CrossRef]
- Nakajima, T.; Volcani, B.E. 3,4-Dihydroxyproline: A New Amino Acid in Diatom Cell Walls. Science 1969, 164, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Volcani, B.E. ϵ-N-trimethyl-L-δ-hydroxylysine phosphate and its nonphosphorylated compound in diatom cell walls. Biochem. Biophys. Res. Commun. 1970, 39, 28–33. [Google Scholar] [CrossRef]
- Loucaides, S.; Behrends, T.; Van Cappellen, P. Reactivity of biogenic silica: Surface versus bulk charge density. Geochim. Cosmochim. Acta 2010, 74, 517–530. [Google Scholar] [CrossRef]
- Rozek, P.; Krol, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Silva, P.D.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.; Weng, L. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J. Mater. Sci. 2007, 42, 3007–3014. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I—Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
| Materials | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | Na2O | MgO | TiO2 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| DB | 64.00 | 15.95 | 0.39 | 5.14 | 2.15 | 0.63 | 0.71 | 0.82 | 0.26 | 9.95 |
| DE | 67.90 | 4.92 | 0.41 | 2.67 | 0.88 | 0.56 | 0.60 | 0.21 | 1.11 | 20.74 |
| Metakaolin | 52.54 | 43.50 | 0.05 | 0.76 | 0.74 | 0.14 | 0.12 | 0.56 | 0.33 | 1.26 |
| Sodium silicate | 26.50 | – | – | – | – | 8.50 | – | – | 65.00 | – |
| Solid Raw Materials | Activator | Molar Ratio | L/S | Sample | ||||
|---|---|---|---|---|---|---|---|---|
| Main Source | wt% | Silica Source | wt% | SiO2/Al2O3 | Na2O/Al2O3 | |||
| Metakaolin | 90.0 | DB | 10.0 | 10 M NaOH | 2.2 | 0.6 | 0.7 | GB10 |
| 80.0 | 20.0 | 2.5 | 0.7 | 0.7 | GB20 | |||
| 70.0 | 30.0 | 2.7 | 0.7 | 0.7 | GB30 | |||
| 60.0 | 40.0 | 3.0 | 0.8 | 0.7 | GB40 | |||
| 50.0 | 50.0 | 3.3 | 0.9 | 0.7 | GB50 | |||
| 70.0 | CDB | 30.0 | 2.8 | 0.8 | 0.7 | GCB30 | ||
| 90.0 | DE | 10.0 | 2.3 | 0.6 | 0.6 | GE10 | ||
| 80.0 | 20.0 | 2.6 | 0.7 | 0.6 | GE20 | |||
| 70.0 | 30.0 | 3.0 | 0.8 | 0.6 | GE30 | |||
| 60.0 | 40.0 | 3.5 | 0.9 | 0.6 | GE40 | |||
| 50.0 | 50.0 | 4.2 | 1.1 | 0.6 | GE50 | |||
| 50.0 | CDE | 50.0 | 4.7 | 0.9 | 0.6 | GCE50 | ||
| 100.0 | – | – | NaOH and sodium silicate solution | 2.7 | 0.8 | 0.9 | GS2.7 | |
| 100.0 | – | – | 10 M NaOH | 2.0 | 0.5 | 1.0 | GMK | |
| Sample | Compressive Strength (MPa) | ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 wt% | 20 wt% | 30 wt% | 40 wt% | 50 wt% | F | p | ||
| GB | 7 days | 10.6 bc 1 | 12.1 b | 15.0 a | 9.1 c | 10.0 bc | 15.043 | 0.000 |
| 28 days | 12.4 b | 17.2 ab | 17.9 a | 13.7 ab | 13.7 ab | 4.871 | 0.002 | |
| GE | 7 days | 12.6 c | 18.8 b | 23.8 a | 20.4 b | 18.8 b | 44.632 | 0.000 |
| 28 days | 10.8 b | 23.7 a | 24.6 a | 25.7 a | 26.2 a | 85.327 | 0.000 | |
| Active Silica Source | DB | CDB | DE | CDE |
|---|---|---|---|---|
| Specific surface area (m2/g) | 27.79 | 44.84 | 18.85 | 25.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Huang, Z.; Pantongsuk, T.; Yu, T.; Zhang, B.; Luo, J.; Yuan, P. Utilisation of Biosilica as Active Silica Source for Metakaolin-Based Geopolymers. Minerals 2024, 14, 816. https://doi.org/10.3390/min14080816
Guo H, Huang Z, Pantongsuk T, Yu T, Zhang B, Luo J, Yuan P. Utilisation of Biosilica as Active Silica Source for Metakaolin-Based Geopolymers. Minerals. 2024; 14(8):816. https://doi.org/10.3390/min14080816
Chicago/Turabian StyleGuo, Haozhe, Zhihao Huang, Thammaros Pantongsuk, Ting Yu, Baifa Zhang, Jinghan Luo, and Peng Yuan. 2024. "Utilisation of Biosilica as Active Silica Source for Metakaolin-Based Geopolymers" Minerals 14, no. 8: 816. https://doi.org/10.3390/min14080816
APA StyleGuo, H., Huang, Z., Pantongsuk, T., Yu, T., Zhang, B., Luo, J., & Yuan, P. (2024). Utilisation of Biosilica as Active Silica Source for Metakaolin-Based Geopolymers. Minerals, 14(8), 816. https://doi.org/10.3390/min14080816





