Thermodynamic and Experimental Substantiation of Comprehensive Processing of Zinc Sulfide Ore and Its Concentration Tailings to Extract Non-Ferrous Metals and Produce a Silicon Ferroalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- Under equilibrium conditions:
- -
- The silicon-containing products of SiO2 reduction are FeSi, Si, Fe3Si, Fe5Si3, FeSi2, FeSi2.33, and SiOg, which, in accordance with the reduction starting temperature, form an increasing series: Fe3Si (1200 °C); Fe5Si3, Si (1400 °C); and SiOg, FeSi2, FeSi2.33 (1500 °C);
- -
- The formation of low-silicon grades of ferrosilicon (FeSi15 and FeSi25) is possible at 1600–1979 °C in the presence of a large amount of magnetite (42.8%–55% of the mass of the ore and tailings mixture).
- -
- The ferrosilicon of FeSi45 grade is formed in the temperature interval of 1885–2000 °C in the presence of the smaller amount of magnetite (39.7%); in this case, the silicon extraction degree into the resulting alloy is 70.9–75.4%.
- Significant foaming, which becomes inactive when 40–60% of the iron in magnetite is replaced by the iron in steel shavings, is observed during electric smelting in the arc furnace of the mixture of the Shalkiya zinc–lead sulfide ore and its concentration tailings in the presence of magnetite concentrate and coke.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alshanov, R.A. Kazakhstan in the World Mineral Market: Problems and Their Solutions: Analytical Review; Print-S: Almaty, Kazakhstan, 2004; p. 220. (In Russian) [Google Scholar]
- Kaya, M.; Hussaini, S.; Kursunoglu, S. Critical review on secondary zinc resources and their recycling technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Utkin, N.I. Production of Non-Ferrous Metals; Intermet Engineering: Moscow, Russia, 2004; p. 443. (In Russian) [Google Scholar]
- Ignatkina, V.A.; Bocharov, V.A.; Milovich, P.F.O.; Ivanova, G.; Khachatryan, L.S. Base metals sulfides flotation response increase with application of sulfhydriccollectors combinations. Obogashchenie Rud 2015, 3, 18–24. [Google Scholar] [CrossRef]
- Algebraistova, N.K.; Prokopiev, I.V.; Markova, A.S.; Kolotushkin, D.M. Flow sheet and reactant treatment for lead-zinc ore bulk flotation. Gornyi Zhurnal 2017, 1, 50–54. [Google Scholar] [CrossRef][Green Version]
- Zimin, A.V.; Arustamyan, M.A. Method of Flotation Enrichment of Sulfide. Ores. Patent RU2588090C1, 27 June 2016. [Google Scholar]
- Zimin, A.V.; Pepersinkova, O.Y.; Arustamyan, M.A. Method of Flotation Enrichment of Polymetallic. Ores. Patent RU2588093C1, 27 June 2016. [Google Scholar]
- Smailov, B.B. Development of Methods for Assessing the Concentration and Modeling of Flotation Schemes for Processing Difficult-to-Process Lead-Zinc Ores. Ph.D. Dissertation, National University of Science and Technology, Moscow, Russia, 2019; p. 182. (In Russian). [Google Scholar]
- Prokopyev, I.V. Development of a Flotation Scheme for the Enrichment of Lead-Zinc Ore Using Microbiological Influence. Ph.D. Dissertation, Siberian Federal University, Krasnoyarsk, Russia, 2019; p. 121. (In Russian). [Google Scholar]
- Algebraistova, N.K.; Prokopiev, I.V.; Komarova, E.S. Preparation of collective lead-zinc concentrates for the selection cycle. Tsvetnye Met. 2021, 4, 12–17. [Google Scholar] [CrossRef]
- Chepushtanova, T.A.; Motovilov, I.Y.; Merkibayev, Y.S.; Polyakov, K.V.; Gostu, S. Flotation studies of the middling product of lead-zinc ores with preliminary sulfidizing roasting of oxidized lead and zinc compounds. Complex Use Miner. Resour. 2022, 323, 77–84. [Google Scholar] [CrossRef]
- Solozhelkin, P.M.; Trofimov, V.A. Ore Flotation. Method. Patent RU2564723C2, 10 October 2015. [Google Scholar]
- Telkov, S.A.; Motovilov, I.Y.; Barmenshinova, M.B.; Nurmanova, A.N. Determination of the conditions for using the enrichment process in heavy suspensions for the preliminary enrichment of crushed ores of the Shalkiya deposit. In Proceedings of the Effective Technologies of Non-Ferrous, Rare and Precious Metals Manufacturing, Almaty, Kazakhstan, 27–29 September 2018; pp. 104–108. [Google Scholar] [CrossRef]
- Telkov, S.A.; Motovilov, I.Y.; Barmenshinova, M.B.; Medyanik, N.L.; Daruesh, G.S. Substantiation of Gravity Concentration to the Shalkiya Deposit Lead-Zinc Ore. J. Min. Sci. 2019, 55, 430–436. [Google Scholar] [CrossRef]
- Komkov, N.M. Calculation of the gas distribution grid of a fluidized bed apparatus for evaporation of heat-sensitive materials. Complex Use Miner. Resour. 1998, 5, 40–44. (In Russian) [Google Scholar]
- Komkov, N.M. Features of calculating gas distribution of fluidization devices. Complex Use Miner. Resour. 1998, 3, 27–31. (In Russian) [Google Scholar]
- Komkov, N.M. The mechanism of dust formation during the operation of fluidized bed furnace. In XXXVII STC “Kazakhstan 2030: Deepening Reforms and Problems of Scientific and Technological Progress”; D. Serikbaev East Kazakhstan Technical University: Ust-Kamenogorsk, Kazakhstan, 1999; pp. 105–106. (In Russian) [Google Scholar]
- Komkov, N.M.; Komkov, S.Y. Development of methods for calculating the thermodynamic parameters of metallurgical reactions using the application program “EXCEL” [Razrabotka metodik rascheta termodinamicheskikh parametrov metallurgicheskikh reaktsiy s pomoshch’yu prikladnoy programmy «EXCEL»]. In Materials of the Republican Scientific and Practical Conference “Integration of the Science of Education and Production in Modern Conditions”; D. Serikbaev East Kazakhstan Technical University: Ust-Kamenogorsk, Kazakhstan, 2000; pp. 465–468. (In Russian) [Google Scholar]
- Komkov, N.M. Physico-Chemical Foundations of Solid-Phase Oxidation Processes of Metal Sulfides and Development of Technology for Roasting Low-Grade Zinc Concentrates. Ph.D. Dissertation, D. Serikbaev East Kazakhstan State Technical University, Almaty, Kazakhstan, 2007; p. 36. (In Russian). [Google Scholar]
- Kokaeva, A.G. Improving the Technology of Sulfide Zinc Concentrates with a High Content of Iron and Silicon. Ph.D. Dissertation, D. Serikbaev East Kazakhstan State Technical University, Almaty, Kazakhstan, 2010; p. 20. (In Russian). [Google Scholar]
- Turysbekov, D.; Tussupbayev, N.; Narbekova, S.; Kaldybayeva, Z. Combined microflotation effects in polymetallic ores beneficiation. SN Appl. Sci. 2023, 5, 124. [Google Scholar] [CrossRef]
- Lan, Z.-Y.; Lai, Z.-N.; Zheng, Y.-X.; Lv, J.-F.; Pang, J.; Ning, J.-L. Recovery of Zn, Pb, Fe and Si from a low-grade mining ore by sulfidation roasting-beneficiation-leaching processes. J. Cent. South Univ. 2020, 27, 37–51. [Google Scholar] [CrossRef]
- Bochkaryov, G.R.; Veigelt, Y.P.; Rostovtsev, R.I. Improvement in ore beneficiation technology of complex substance composition. J. Min. Sci. 1999, 35, 536–540. [Google Scholar] [CrossRef]
- Yang, K.; Shiwei; Peng, J.; Zhang, L.; Ma, A.; Chen, W.; Xie, F. Research on Leaching of Zinc Sulfide Ores through Synergistic Coordination. In 7th International Symposium on High-Temperature Metallurgical Processing; Hwang, J.-Y., Jiang, T., Pistorius, P.C., Alvear, F.G.R.F., Yücel, O., Cai, L., Zhao, B., Gregurek, D., Seshadri, V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 435–441. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Sun, W.; Hu, Y.; Ni, Z. New Innovative Method of Flotation Separation for High Sulfur Lead–Zinc Sulfide Ore. In Pb Zn 2020: 9th International Symposium on Lead and Zinc Processing; The Minerals, Metals & Materials Series; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Pan’shin, A.M.; Evdokimov, S.I.; Artemov, S.V. New technology of separation of the collective lead-zinc concentrate. Russ. J. Non-Ferr. Met. 2010, 51, 1–7. [Google Scholar] [CrossRef]
- Antropova, I.G.; Merinov, A.A.; Gulyashinov, P.A.; Damdinov, B.B. Sulfidation of Oxidized Lead and Zinc with Pyrite-Bearing Lead-and-Zinc Ore. J. Min. Sci. 2023, 59, 465–474. [Google Scholar] [CrossRef]
- Naboychenko, S.S.; Ni, L.P.; Shneerson, Y.M.; Chugaev, L.V. Autoclave Hydrometallurgy of Non-Ferrous Metals; USTU-UPI: Ekaterinburg, Russia, 2002; p. 940. (In Russian) [Google Scholar]
- Piskunov, V.M.; Sadykov, S.B.; Naboichenko, S.S. One-stage autoclave leaching of zinc concentrates in the presence of potassium-containing reagents. Theor. Found. Chem. Eng. 2007, 41, 734–736. [Google Scholar] [CrossRef]
- Shpaer, V.M.; Kalashnikova, M.I. Autoclave leaching of low-grade zinc concentrates. Non-Ferr. Met. 2010, 5, 23–27. (In Russian) [Google Scholar]
- Shneerson, J.M.; Vigdorchik, E.M.; Zhmarin, E.E.; Lapin, A.Y.; Shpaer, V.M. Mathematical modeling of pressure leaching of sulphide zinc concentrate. In Pressure Hydrometallurgy 2004: Proceedings of the International Conference on the Use of Pressure Vessels for Metal Extraction and Recovery; Metallurgical Society of the Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2004; pp. 983–997. [Google Scholar]
- Sadykov, S.B. Autoclave Processing of Low-Grade Zinc Concentrates; URO RAS: Ekaterinburg, Russia, 2006. (In Russian) [Google Scholar]
- Sadykov, S.B.; Bolton, G.L.; McConaughey, E.; Naboychenko, S.S. Enlarged tests of autoclave sulfuric acid leaching of low-grade zinc concentrates. Non-Ferr. Metall. 2006, 10, 9–14. (In Russian) [Google Scholar]
- Khaziyeva, E.B.; Naboychenko, S.S.; Sviridov, V.V. Study of the possibility of using surfactants in autoclave leaching of zinc sulfide concentrates. Bull. IRGTU 2016, 9, 147–155. (In Russian) [Google Scholar] [CrossRef]
- Garichev, S.N.; Novikov, D.N.; Bryksin, M.N.; Shekhirev, D.V.; Pankin, A.V. Procedure for Combined Processing of Rebellious Lead-Zinc. Ores. Patent RU2456357C1, 20 July 2012. [Google Scholar]
- Guanzhou, Q.; Jun, W.; Wenqing, Q.; Mingfei, H.; Yuping, C.; Zhineng, L.; Shuanghua, T. Flotation and Bacteria Leaching Combined Treatment Process for Low Grade Lead, Antimony and Zinc Sulfide. Ore. Patent CN101033507A, 12 September 2007. [Google Scholar]
- Borisov, V.A.; D’yachenko, A.N.; Kraydenko, R.I. Reaction of zinc oxide with ammonium chloride. Russ. J. Inorg. Chem. 2012, 57, 560–563. [Google Scholar] [CrossRef]
- Borisov, V.A.; Kraydenko, R.I. Processing of Zinc-Containing Ores by the Chlorammonium Method. Available online: http://portal.tpu.ru/SHARED/b/BORISOV/Tab2/Binder1.pdf (accessed on 7 January 2024). (In Russian).
- Beisembaev, B.B.; Kapsalyamov, B.K.; Gorkun, V.I.; Govyadovskaya, O.Y.; Ignatiev, M.M. Deep Processing of Lead-Zinc Ores and Industrial Products to Obtain Products of Increased Marketability; Galym: Almaty, Kazakhstan, 2002; p. 220. (In Russian) [Google Scholar]
- Turkebaev, E.A.; Sadykov, G.K. Integrated Use of Raw Materials and Industrial Waste; Kazakhstan: Almaty, Kazakhstan, 1988. (In Russian) [Google Scholar]
- Shalkiya Zinc Ltd. Annual Report for 2016. Available online: https://kase.kz/files/emitters/SHZN/shznp_2016_rus.pdf (accessed on 7 January 2024).
- Evdokimov, V.I.; Toptygina, G.M. Application of Chloride Sublimation Processes for the Enrichment of Substandard Polymetallic Raw Materials. Complex Use of Ores and Concentrates; Nauka: Moscow, Russia, 1989; pp. 83–87. (In Russian) [Google Scholar]
- Algebraistova, N.K.; Kondratyeva, A.A.; Gubina, E.A.; Marchenko, A.A.; Vorontsova, E.A. Development of a technological scheme for the enrichment of sulfide polymetallic ore. Obogashchenie Rud 2012, 2, 3–9. (In Russian) [Google Scholar]
- Kupeeva, R.D. State and prospects for processing lead-zinc ores. Min. Inf. Anal. Bull. 2009, 12, 456–460. (In Russian) [Google Scholar]
- Seksenova, N.; Bykov, R.; Mamyachenkov, S.; Daumova, G.; Kozhakanova, M. Optimization of Conditions for Processing of Lead–Zinc Ores Enrichment Tailings of East Kazakhstan. Metals 2021, 11, 1802. [Google Scholar] [CrossRef]
- Pavlov, M.V.; Pavlov, I.V.; Lineitsev, A.V.; Pavlov, V.F. Glass Ceramic Materials Based on Beneficiation Tailings of Lead-Zinc Ore. Glass Ceram. 2017, 73, 459–464. [Google Scholar] [CrossRef]
- Behera, S.K.; Ghosh, C.N.; Mishra, K.; Mishra, D.P.; Singh, P.; Mandal, P.K.; Buragohain, J.; Sethi, M.K. Utilization of lead–zinc mill tailings and slag as paste backfill materials. Environ. Earth Sci. 2020, 79, 389. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Xiao, X.-M. Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation. J. Clean. Prod. 2017, 158, 73–80. [Google Scholar] [CrossRef]
- Li, R.; Yin, Z.; Lin, H. Research Status and Prospects for the Utilization of Lead–Zinc Tailings as Building Materials. Buildings 2023, 13, 150. [Google Scholar] [CrossRef]
- Huang, T.-M.; Li, S.-C.; Li, X.-H.; Zhang, P.-J.; Yang, H.-B.; Ruan, L.; Tang, F.-S. Study on resource utilization technology of nonferrous metals tungsten and lead-zinc tailings. Chin. J. Nonferrous Met. 2021, 31, 1057–1073. [Google Scholar] [CrossRef]
- Onukwuli, O.D.; Nnanwube, I.A. Optimization of Zinc Recovery from Sphalerite Using Response Surface Methodology and Particle Swarm Optimization. Period. Polytech. Chem. Eng. 2022, 66, 20–29. [Google Scholar] [CrossRef]
- Shevko, V.; Aitkulov, D.; Badikova, A. Comprehensive Processing of Vanadium-Containing Black Shale Tailings. Period. Polytech. Chem. Eng. 2022, 66, 617–628. [Google Scholar] [CrossRef]
- Roine, A. HSC Chemistry®, [Software] Metso: Outotec, Pori. 2021. Available online: www.mogroup.com/hsc (accessed on 1 February 2024).
- Akylbekov, Y.; Shevko, V.; Karatayeva, G. Thermodynamic prediction of the possibility of comprehensive processing of chrysotile-asbestos waste. Case Stud. Chem. Environ. Eng. 2023, 8, 100488. [Google Scholar] [CrossRef]
- Shevko, V.M.; Serzhanov, G.M.; Karataeva, G.E.; Amanov, D.D. Calculation equilibrium distribution elements in relation to To software complex HSC-5.1. In Certificate for the Object Protected by Copyright of the Republic of Kazakhstan; Mesto Finland Oy: Espoo, Finland, 2019; № 1501. (In Russian) [Google Scholar]
- Shevko, V.; Kaskin, P.; Badikova, A.; Amanov, D. Obtaining of ferrosilicon from technogenic magnetite concentrate. Complex Use Miner. Resour. 2020, 313, 71–78. [Google Scholar] [CrossRef]
- Akhnazarova, S.A.; Kafarov, B.V. Methods for Optimizing Experiments in the Chemical Industry; Higher School Moscow: Moscow, Russia, 1978. (In Russian) [Google Scholar]
- Inkov, A.M.; Tapalov, T.; Umbetov, U.U.; Hu Wen Tsen, V.; Akhmetova, K.T.; Dyakova, E.T. Optimization Methods: E-Book; South Kazakhstan State University: Shymkent, Kazakhstan, 2003. (In Russian) [Google Scholar]
- Ochkov, V.F. Mathcad 14 for Students, Engineers and Designers; BHV-Petersburg: Saint Petersburg, Russia, 2007. (In Russian) [Google Scholar]
- Kaptay, G. On the equation of the maximum capillary pressure induced by solid particles to stabilize emulsions and foams and on the emulsion stability diagrams. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 387–401. [Google Scholar] [CrossRef]
- Nushtaeva, A.; Vilkova, N. Foams and emulsions stabilized by solid particles: Mechanisms of stability. In University Proceedings; Penza State University: Penza, Russia, 2017; Volga region. Natural sciences; pp. 74–85. [Google Scholar] [CrossRef]
- Akylbekov, Y.Y.; Shevko, V.M.; Aitkulov, D.K.; Karatayeva, G.E. Electrothermal processing of chrysotile-asbestos wastes with production of ferroalloy and extraction of magnesium into the gas phase. Complex Use Miner. Resour. 2023, 327, 74–81. [Google Scholar] [CrossRef]
- Uteyeva, R.; Shevko, V.; Aitkulov, D.; Badikova, A.; Tleuova, S. Electric smelting of phosphorites with production of a ferroalloy, calcium carbide and sublimation of phosphorus. Eng. J. Satbayev Univ. 2023, 145, 11–17. [Google Scholar] [CrossRef]
- State Standard 1415-93; Ferrosilicon. Technical Requirements and Delivery Conditions. Standartinform: Moscow, Russia, 2011. (In Russian)
- Kozlov, P.A. The Welz Process; Ore and Metals: Moscow, Russia, 2002; p. 176. (In Russian) [Google Scholar]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
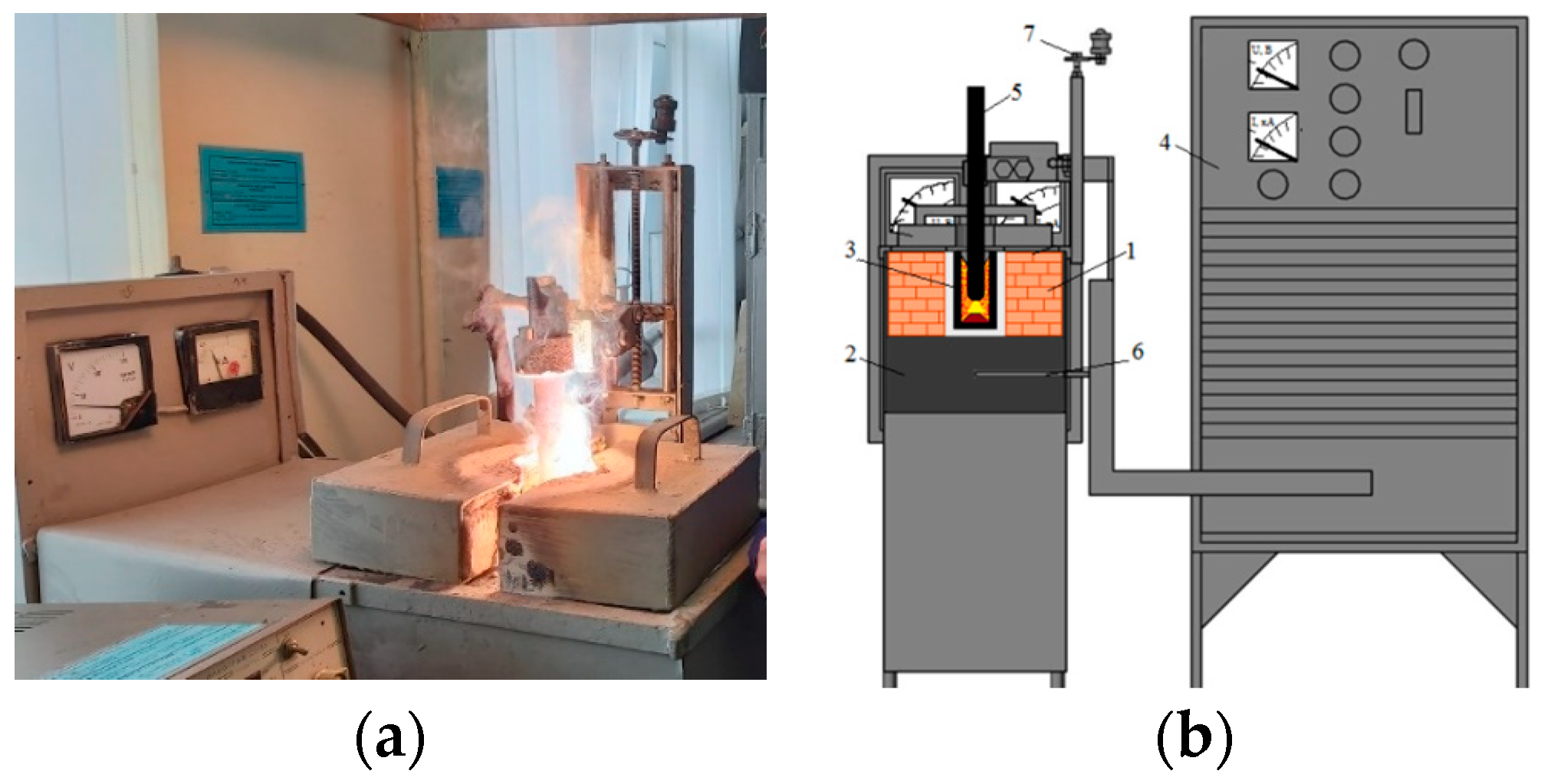
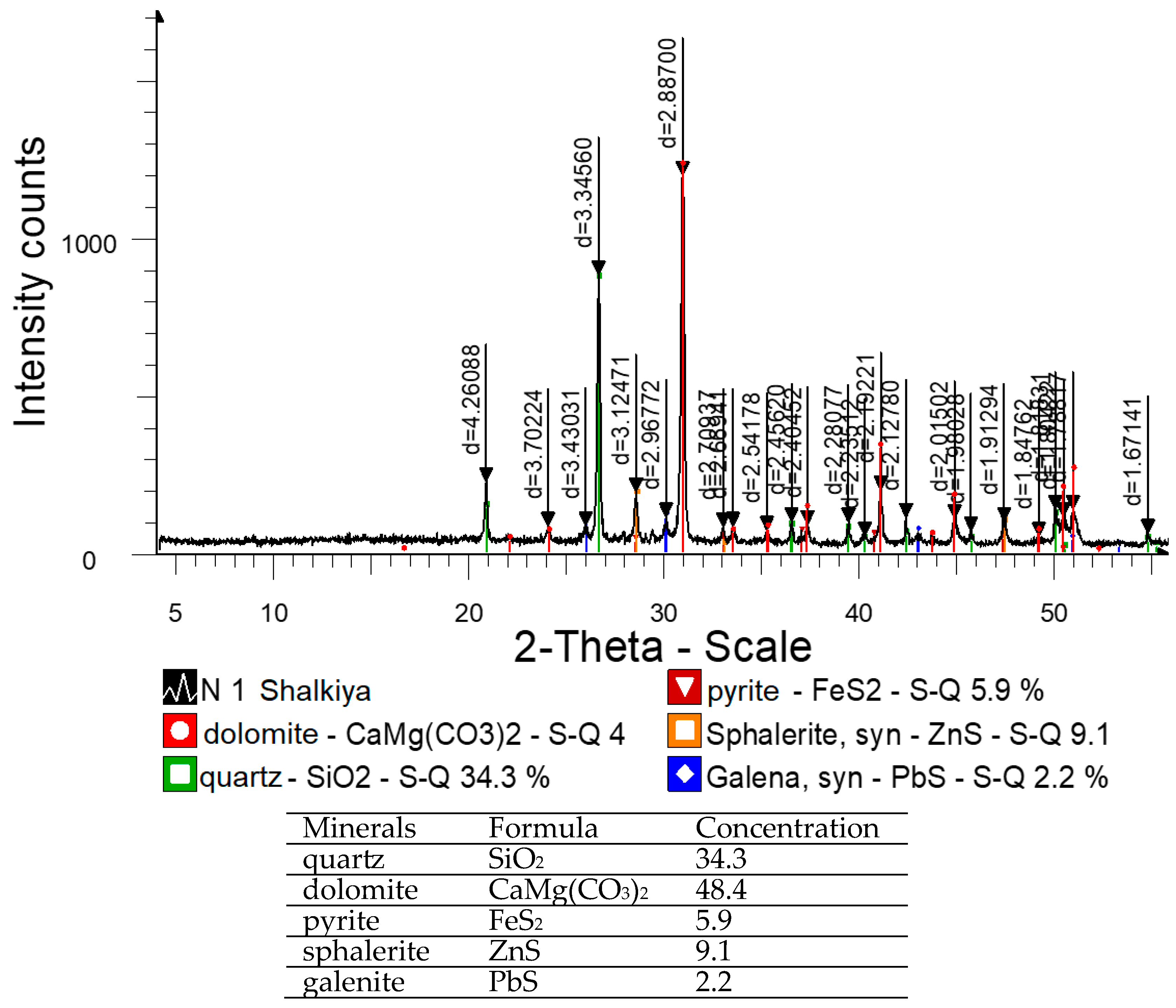
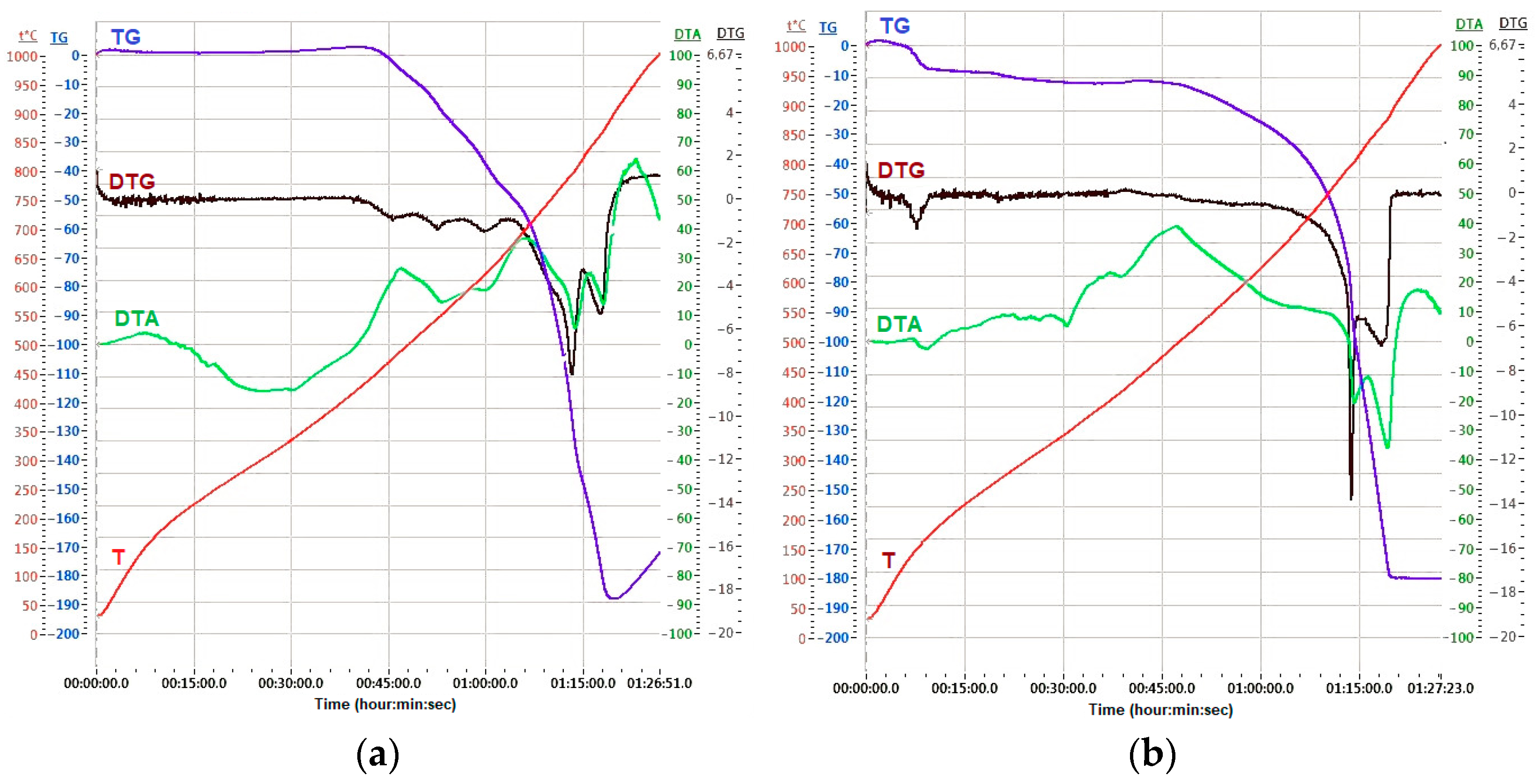

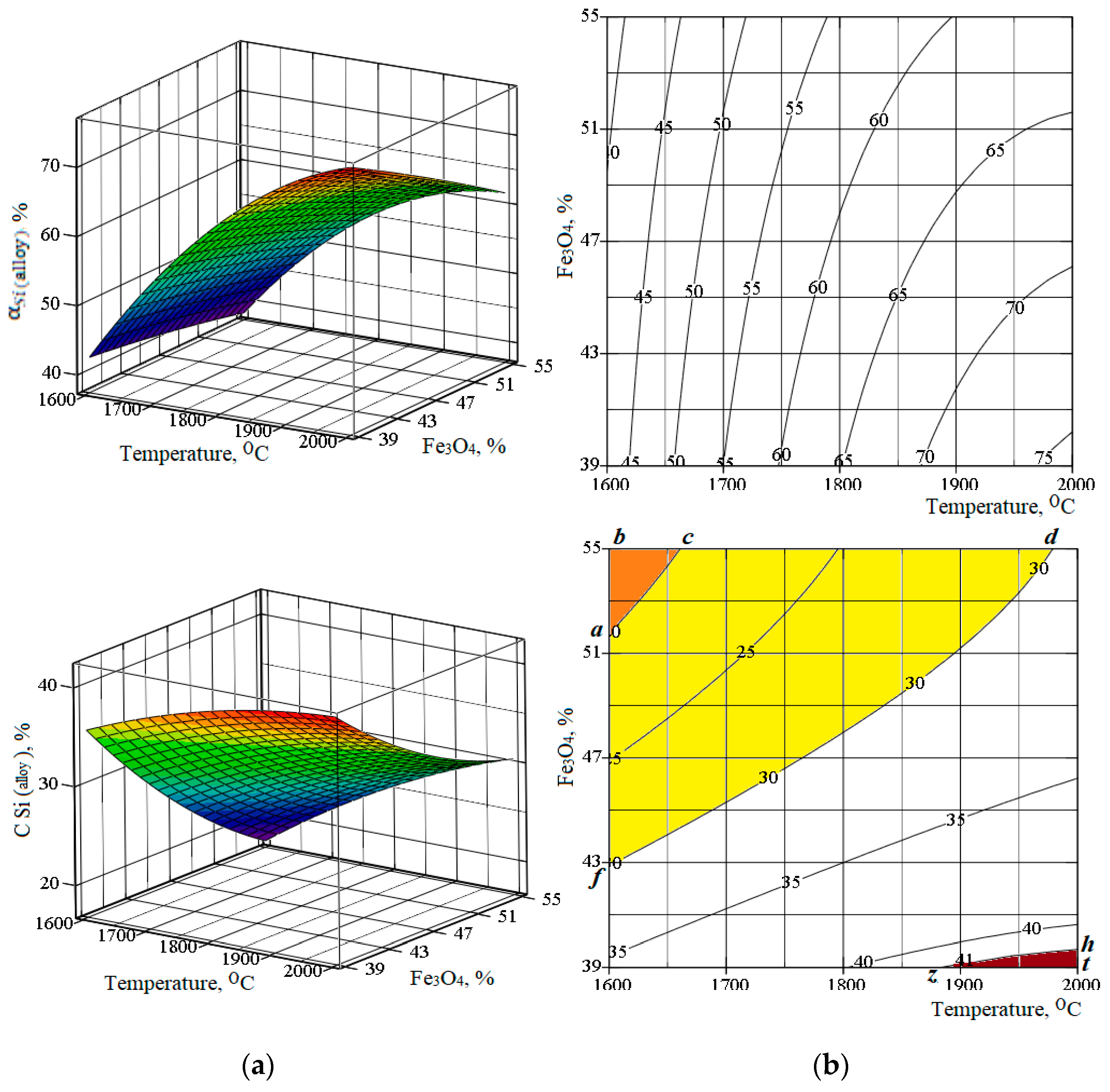

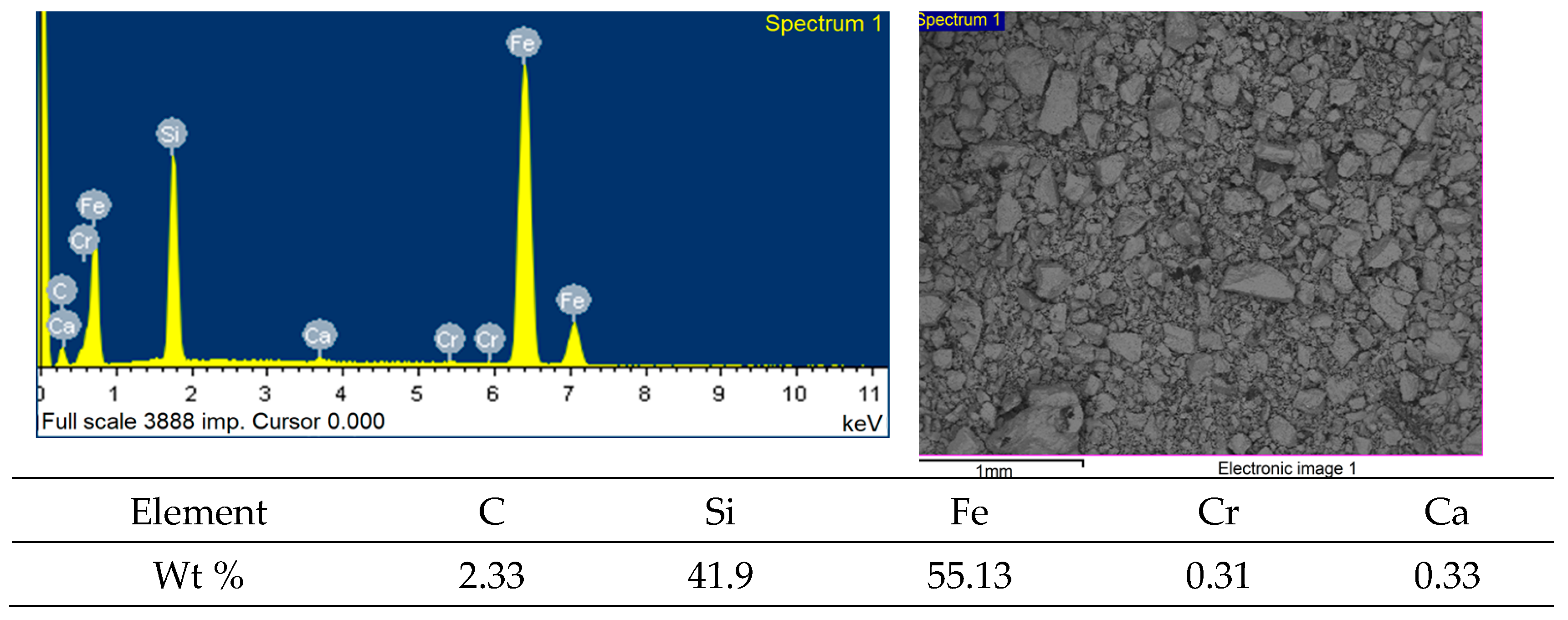
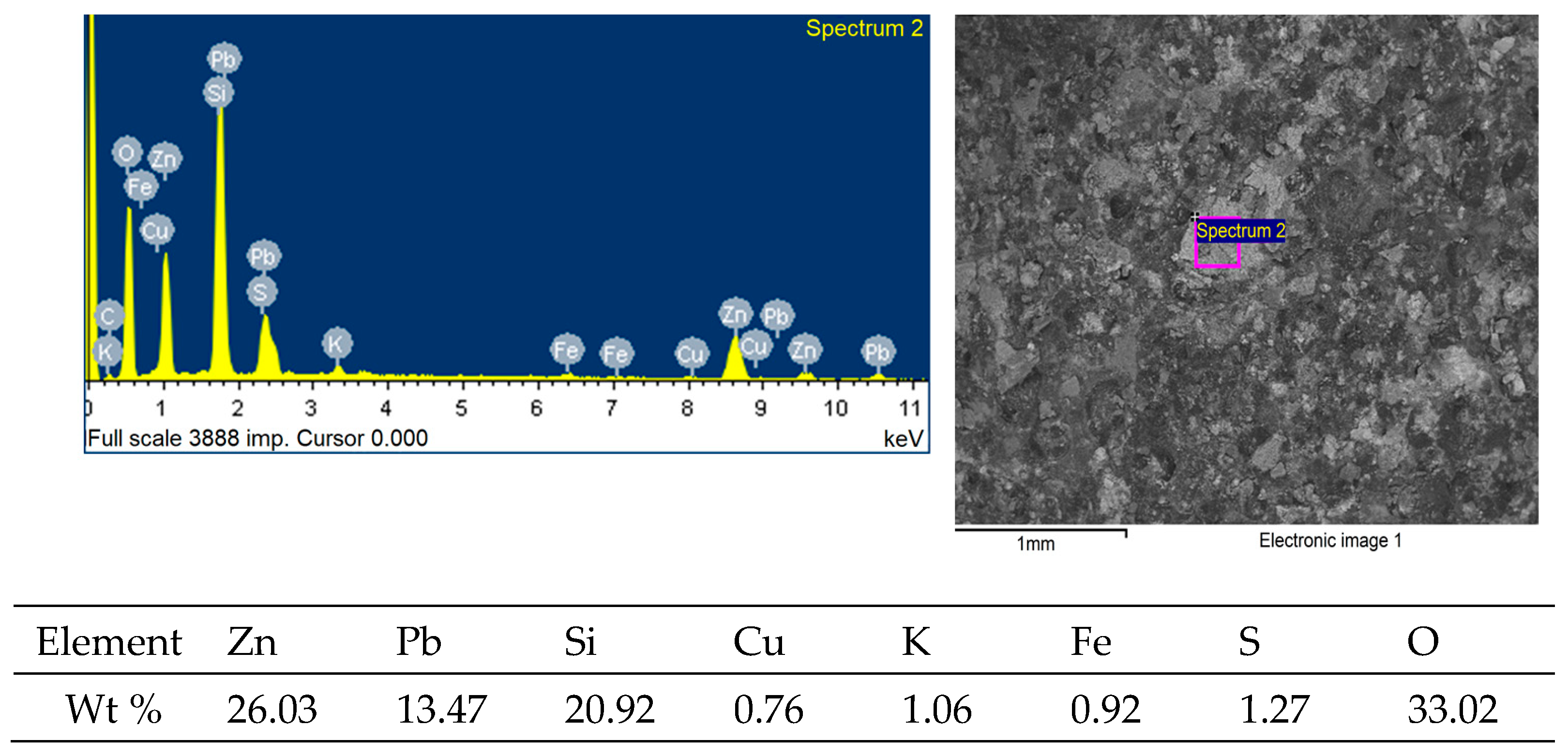
| Content, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ZnS | PbS | FeS2 | SiO2 | Al2O3 | CaCO3 | MgCO3 | Fe2O3 | TiO2 | Na2O |
| 3.3 | 0.8 | 2.0 | 55.6 | 4.6 | 18.8 | 9.5 | 4.5 | 0.3 | 0.6 |
| Temperature, °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 500 | 700 | 900 | 1100 | 1400 | 1600 | 1800 | 1877 | 1900 | 2000 | 2100 |
| 3933.2 | 3332.6 | 2742.6 | 2160.9 | 1308.6 | 746.2 | 204.9 | 0 | −60.71 | −324.9 | −588.3 |
| Substance | Temperature, °C | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 800 | 900 | 1000 | 1100 | 1200 | 1300 | 1400 | 1500 | 1600 | 1700 | 1800 | 1900 | |
| ZnS | 99.89 * | 99.84 | 98.73 | 86.96 | 44.78 | 9.84 | 1.4 | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| 99.82 ** | 99.8 | 98.8 | 87.8 | 45.8 | 10.1 | 1.37 | 0.14 | <0.1 | <0.1 | <0.1 | <0.1 | |
| ZnO | 0.12 | 0.10 | 0.15 | 0.21 | 0.13 | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 0.18 | 0.13 | 0.17 | 0.22 | 0.14 | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Zn | 0.01 | 0.06 | 0.64 | 4.73 | 12.33 | 12.09 | 7.64 | 3.52 | 1.55 | 0.83 | 0.49 | 0.3 |
| 0.01 | 0.05 | 0.45 | 3.09 | 7.93 | 7.60 | 4.57 | 2.04 | 0.9 | 0.48 | 0.29 | 0.2 | |
| Zn(g) | <0.01 | 0.02 | 0.49 | 8.08 | 42.77 | 78.08 | 91.04 | 96.40 | 98.50 | 99.23 | 99.57 | 99.76 |
| <0.01 | 0.03 | 0.57 | 8.86 | 46.06 | 82.30 | 94.11 | 97.87 | 99.15 | 99.58 | 99.76 | 99.86 | |
| PbS | 24.25 | 11.12 | 2.87 | 0.74 | 0.36 | 0.19 | 0.08 | 0.03 | 0.01 | <0.01 | <0.01 | <0.01 |
| 24.27 | 12.71 | 4.02 | 1.13 | 0.57 | 0.29 | 0.13 | 0.05 | 0.02 | <0.01 | <0.01 | <0.01 | |
| Pb | 75.99 | 88.65 | 96.63 | 98.51 | 98.13 | 97.03 | 94.72 | 87.49 | 71.42 | 51.65 | 33.82 | 20.68 |
| 75.92 | 86.69 | 94.64 | 97.00 | 96.13 | 94.19 | 90.35 | 79.25 | 58.47 | 37.82 | 23.27 | 14.32 | |
| Pb(g) | 0.01 | 0.01 | 0.06 | 0.21 | 0.63 | 1.66 | 4.10 | 11.53 | 28.27 | 48.55 | 66.52 | 79.70 |
| 0.01 | 0.02 | 0.09 | 0.34 | 1.03 | 2.70 | 6.77 | 18.30 | 40.14 | 61.76 | 76.72 | 85.81 | |
| PbS(g) | 0.17 | 0.62 | 0.85 | 0.94 | 1.28 | 1.53 | 1.50 | 1.35 | 0.7 | 0.21 | 0.07 | 0.03 |
| 0.22 | 0.99 | 1.65 | 1.94 | 2.68 | 3.22 | 3.50 | 2.81 | 1.78 | 0.82 | 0.41 | 0.28 | |
| # | Independent Factors | , % | ||||||
|---|---|---|---|---|---|---|---|---|
| Coded Form | Natural Form | According to Research | According to Equation (2) | According to Research | According to Equation (3) | |||
| X1 | X2 | T, °C | Fe3O4,% | |||||
| 1 | −1 | −1 | 1658 | 41.3 | 50.3 | 49.48 | 37 | 33.85 |
| 2 | 1 | −1 | 1941 | 41.3 | 71.7 | 72.21 | 39.5 | 38.89 |
| 3 | −1 | 1 | 1658 | 52.7 | 46.3 | 45.51 | 23 | 21.55 |
| 4 | 1 | 1 | 1941 | 52.7 | 62.6 | 63.13 | 29 | 30.09 |
| 5 | 1.414 | 0 | 2000 | 47 | 70 | 69.20 | 35.2 | 34.42 |
| 6 | −1.414 | 0 | 1600 | 47 | 39.6 | 40.60 | 22 | 24.82 |
| 7 | 0 | 1.414 | 1800 | 55 | 55.5 | 55.62 | 25.3 | 25.12 |
| 8 | 0 | −1.414 | 1800 | 39 | 64.7 | 64.85 | 37.8 | 40.03 |
| 9 | 0 | 0 | 1800 | 47 | 61 | 60.58 | 30.6 | 30.88 |
| 10 | 0 | 0 | 1800 | 47 | 59.6 | 60.58 | 29 | 30.88 |
| 11 | 0 | 0 | 1800 | 47 | 59.8 | 60.58 | 32 | 30.88 |
| 12 | 0 | 0 | 1800 | 47 | 61.1 | 60.58 | 31.8 | 30.88 |
| 13 | 0 | 0 | 1800 | 47 | 61.4 | 60.58 | 31 | 30.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevko, V.; Makhanbetova, B.; Aitkulov, D.; Badikova, A.; Amanov, D. Thermodynamic and Experimental Substantiation of Comprehensive Processing of Zinc Sulfide Ore and Its Concentration Tailings to Extract Non-Ferrous Metals and Produce a Silicon Ferroalloy. Minerals 2024, 14, 819. https://doi.org/10.3390/min14080819
Shevko V, Makhanbetova B, Aitkulov D, Badikova A, Amanov D. Thermodynamic and Experimental Substantiation of Comprehensive Processing of Zinc Sulfide Ore and Its Concentration Tailings to Extract Non-Ferrous Metals and Produce a Silicon Ferroalloy. Minerals. 2024; 14(8):819. https://doi.org/10.3390/min14080819
Chicago/Turabian StyleShevko, Viktor, Baktygul Makhanbetova, Dosmurat Aitkulov, Alexandra Badikova, and Daniel Amanov. 2024. "Thermodynamic and Experimental Substantiation of Comprehensive Processing of Zinc Sulfide Ore and Its Concentration Tailings to Extract Non-Ferrous Metals and Produce a Silicon Ferroalloy" Minerals 14, no. 8: 819. https://doi.org/10.3390/min14080819
APA StyleShevko, V., Makhanbetova, B., Aitkulov, D., Badikova, A., & Amanov, D. (2024). Thermodynamic and Experimental Substantiation of Comprehensive Processing of Zinc Sulfide Ore and Its Concentration Tailings to Extract Non-Ferrous Metals and Produce a Silicon Ferroalloy. Minerals, 14(8), 819. https://doi.org/10.3390/min14080819








