A Critical Review on the Flotation Reagents for Phosphate Ore Beneficiation
Abstract
:1. Introduction
2. Flotation Reagents for Apatite
2.1. Regulators
2.1.1. Depressors
Depressors of Phosphate Minerals
Depressors of Carbonate Minerals
Depressors of Silicate Minerals
2.2. Collectors
2.2.1. Anionic Collectors
2.2.2. Cationic Collectors
2.2.3. Amphoteric Collector
2.3. Frothers
3. Summary
4. Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, 14–22. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.H.; Bao, S.X.; Ren, L.Y.; Zhu, Y.G.; Xu, W.; Yang, S.Y. Exploration of disodium methylenebis naphthalene sulfonatea as a novel depressant for the flotation separation of apatite from dolomite. Sep. Purif. Technol. 2024, 341, 126967. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2018; pp. 122–123.
- Li, H.Q.; Chen, H.F.; Huang, P.; Yang, P.J.; Chen, Q.; Weng, X.Q.; He, D.S.; Song, S.X. Effect of geological origin of apatite on reverse flotation separation of phosphate ores using phosphoric acid as depressant. Miner. Eng. 2021, 172, 107182. [Google Scholar] [CrossRef]
- Abouzeid, A.-Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Zafar, Z.I.; Anwar, M.M.; Pritchard, D.W. Innovations in beneficiation technology for low grade phosphate rocks. Nutr. Cycl. Agroecosys 1996, 46, 135–151. [Google Scholar] [CrossRef]
- Amirech, A.; Bouhenguel, M.; Kouachi, S. Two-stage reverse flotation process for removal of carbonates and silicates from phosphate ore using anionic and cationic collectors. Arab. J. Geosci. 2018, 11, 593. [Google Scholar] [CrossRef]
- Liu, R.Z.; Feng, Q.M.; Zhang, G.F.; He, X.W.; Pang, J.T.; Chen, Y.F. The studies of sodium oleate and sodium linoleate interaction characters on phosphate ores. Non-Met. Mines 2018, 41, 55–57. [Google Scholar]
- Abouzeid, A.-Z.M.; Negm, A.T.; Elgillani, D.A. Upgrading of calcareous phosphate ores by flotation: Effect of ore characteristics. Int. J. Miner. Process. 2009, 90, 81–89. [Google Scholar] [CrossRef]
- El-Shall, H.; Zhang, P.; Khalek, N.A.; El-Mofty, S. Beneficiation technology of phosphates: Challenges and solutions. Miner. Metall. Process. 2004, 21, 17–26. [Google Scholar] [CrossRef]
- Fan, T.Y.; Ma, T.L.; Wang, M.; Wang, S.; Wang, X.M.; Lu, A.K. Study on phosphorus release from medium- and low-grade phosphate ore powders by mechanical activation and low molecular weight organic acid activation. Physicochem. Probl. Miner. Process. 2024, 60, 183275. [Google Scholar] [CrossRef]
- Lan, S.Z.; Shen, P.L.; Zheng, Q.F.; Qiao, L.D.; Dong, L.Y.; Liu, D.W. Effective flotation separation of apatite from dolomite using a new eco-friendly depressant gallic acid. Green. Chem. 2024, 26, 1627–1636. [Google Scholar] [CrossRef]
- Zheng, H.F.; Chen, Y.X.; Weng, X.Q.; Jin, Y.F.; Kasomo, R.M.; Ao, S.F. Flotation Separation of Dolomite from Fluorapatite Using Sodium Dodecyl Benzene Sulfonate as the Efficient Collector under Low Temperature. Minerals 2022, 12, 228. [Google Scholar] [CrossRef]
- Hoang, D.H.; Kupka, N.; Peuker, U.A.; Rudolph, M. Flotation study of fine grained carbonaceous sedimentary apatite ore—Challenges in process mineralogy and impact of hydrodynamics. Miner. Eng. 2018, 121, 196–204. [Google Scholar] [CrossRef]
- Liu, S.B.; Ge, Y.Y.; Fang, J.; Yu, J.; Gao, Q. An investigation of froth stability in reverse flotation of collophane. Miner. Eng. 2020, 155, 106446. [Google Scholar] [CrossRef]
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid. Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.W.; Wang, Y.Y.; Wang, Y.M. A Review on Literatures of Phosphate Ores Analysis in China. Rock Miner. Anal. 2011, 30, 384–390. [Google Scholar]
- Derhy, M.; Taha, Y.; Ait-khouia, Y.; Elghali, A.; Benzaazoua, M.; Hakkou, R. Enhancing selective calcite and dolomite flotation in the phosphate ores: Investigation, modeling, and automated mineralogy assessment. Miner. Eng. 2024, 207, 108569. [Google Scholar] [CrossRef]
- Ruan, Y.R.; Zhang, Z.Q.; Luo, H.H.; Xiao, C.Q.; Zhou, F.; Chi, R. Effects of metal ions on the flotation of apatite, dolomite and quartz. Minerals 2018, 8, 141. [Google Scholar] [CrossRef]
- Yin, Y.L.; Ao, X.Q.; Xie, Y.; Chen, J.H. Effects of dissolved fluoride in phosphate ore flotation systems on the surfaces properties of dolomite. Colloids Surf. A 2022, 653, 129922. [Google Scholar] [CrossRef]
- Ruan, Y.Y.; He, D.S.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Colloids Surf. A 2019, 4, 253. [Google Scholar] [CrossRef]
- Kou, Y.J.; Tao, D.; Xu, G. Fatty acid collectors for phosphate flotation and their adsorption behavior using QCM-D. Int. J. Miner. Process. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Lamghari, K.; Taha, Y.; Ait-Khouia, Y.; Elghali, A.; Hakkou, R.; Benzaazoua, M. Sustainable phosphate mining: Enhancing efficiency in mining and pre-beneficiation processes. J. Environ. Manag. 2024, 358, 120833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, Y.; Bogan, M. Challenging the “Crago” double float process II. Amine-fatty acid flotation of siliceous phosphates. Miner. Eng. 1997, 10, 983–994. [Google Scholar] [CrossRef]
- Quast, K. An investigation of the flotation minimum in the oleate flotation of hematite under alkaline conditions. Miner. Eng. 2017, 113, 71–82. [Google Scholar] [CrossRef]
- Bai, S.J.; Li, J.; Bi, Y.X.; Yuan, J.Q.; Wen, S.M.; Ding, Z. Adsorption of sodium oleate at the microfine hematite/aqueous solution. Int. J. Min. Sci. Technol. 2023, 33, 105–113. [Google Scholar] [CrossRef]
- Somasundaran, P. The role of ionomolecular surfactant complexes in flotation. Int. J. Miner. Process. 1976, 3, 35–40. [Google Scholar] [CrossRef]
- Joseph-Soly, S.; Quast, K.; Connor, J.N. Effects of Eh and pH on the oleate flotation of iron oxides. Miner. Eng. 2015, 83, 97–104. [Google Scholar] [CrossRef]
- Vučinić, D.R.; Radulović, D.S.; Deušić, S.Đ. Electrokinetic properties of hydroxyapatite under flotation conditions. Colloids Surf. A 2010, 343, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Henrique Santos, L.; Mércia Alves Santos, A.; Fernandes de Magalhães, L.; Fonseca de Souza, T.; Rodrigues da Silva, G.; Ed-uardo Clark Peres, A. Synergetic effects of fatty acids in amazon oil-based collectors for phosphate flotation. Miner. Eng. 2023, 191, 107932. [Google Scholar] [CrossRef]
- Owens, C.L.; Nash, G.R.; Hadler, K.; Fitzpatrick, R.S.; Anderson, C.G.; Wall, F. Apatite enrichment by rare earth elements: A review of the effects of surface properties. Adv. Colloid. Interface Sci. 2019, 265, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.A.; Ferreira, E.E.; Peres, A.E. Floatability of apatites and gangue minerals of an igneous phosphate ore. Miner. Eng. 2008, 21, 994. [Google Scholar] [CrossRef]
- Cheng, R.; Li, C.; Liu, X.; Deng, S. Synergism of octane phenol polyoxyethylene-10 and oleic acid in apatite flotation. Physicochem. Probl. Miner. Process. 2017, 53, 1214. [Google Scholar]
- Giuliani, G.; Fallick, A.; Rakotondrazafy, M.; Ohnenstetter, D.; Andriamamonjy, A.; Ralantoarison, T.; Rakotosamizanany, S.; Razanatseheno, M.; Offant, Y.; Garnier, V.; et al. Oxygen isotope systematics of gem corundum deposits in Madagascar: Relevance for their geological origin. Miner. Depos. 2007, 42, 251. [Google Scholar] [CrossRef]
- Mishra, S.K. The electrokinetics of apatite and calcite in inorganic electrolyte environment. Int. J. Miner. Process. 1978, 5, 69. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis of carbonate doped chlorapatite–ZnO nanocomposite with negative zeta potential. Ceram. Int. 2015, 41, 12323. [Google Scholar] [CrossRef]
- Somasundaran, P. Zeta potential of apatite in aqueous solutions and its change during equilibration. Adv. Colloid. Interface Sci. 1968, 27, 659. [Google Scholar] [CrossRef]
- Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Effect of Ca2+, Mg2+ and SO42− ions on the zeta potential of calcite and dolomite particles aged with stearic acid. Colloid. Surf. A 2015, 482, 290–299. [Google Scholar] [CrossRef]
- Aarab, I.; Derqaoui, M.; Amari, K.E.; Yaacoubi, A.; Abidi, A.; Etahiri, A.; Baçaoui, A. Influence of surface dissolution on reagents’ adsorption on low-grade phosphate ore and its flotation selectivity. Colloid. Surf. A-Physicochem. Eng. Asp. 2021, 631, 127700. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippova, I.V.; Filippov, L.O. Investigation of the depressants involved in the selective flotation of scheelite from apatite, fluorite, and calcium silicates: Focus on the sodium silicate/sodium carbonate system. Powder Technol. 2019, 352, 501–512. [Google Scholar] [CrossRef]
- Pawliszak, P.; Beheshti, A.; Møller, A.; Blencowe, A.; Beattie, D.A.; Krasowska, M. Increasing surface hydrophilicity with biopolymers: A combined single bubble collision, QCM-D and AFM study. Colloid. Surf. A 2024, 667, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Derhy, M.; Taha, Y.; Hakkou, R.; Benzaazoua, M. Review of the Main factors affecting the flotation of phosphate ores. Minerals 2020, 10, 1109. [Google Scholar] [CrossRef]
- Zheng, X.; Arps, P.J.; Smith, R.W. Adhesion of two bacteria onto dolomite and apatite: Their effect on dolomite depression in anionic flotation. Int. J. Miner. Process. 2001, 62, 159–172. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, D.Z. Solution Chemistry: Minerals and Reagents, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 58–60. [Google Scholar]
- Prasad, M.; Majumder, A.K.; Rao, T.C. Reverse flotation of sedimentary calcareous/dolomitic rock phosphate ore—An overview. Miner. Metall. Process. 2000, 17, 49–55. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.Y.; Li, C.X.; Cheng, R.J. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Kaba, O.B.; Fornasiero, D. In-situ study of the kinetics of phosphoric acid interaction with calcite and fluorapatite by Raman spectroscopy and flotation. Miner. Eng. 2021, 162, 106729. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.X.; Luo, H.H.; Cheng, R.J.; Liu, F.Y. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Liu, R.; Liu, C.; Liu, L.; Li, R.; Zhang, H.; Pang, J.; Liu, D. Application of waste acid from phosphogysum dam as an eco-friendly depressant in collophane flotation. J. Clean. Prod. 2020, 267, 122184. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Abouzeid, A.-Z.M. Flotation of carbonates from phosphate ores in acidic media. Int. J. Miner. Process. 1993, 38, 235–256. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of sodium pyrophosphate on the reverse flotation of dolomite from apatite. Minerals 2018, 8, 278. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; Arafat, Y.; Abd El-Aleem, F.A.; El-Midany, A.A. Investigating sodium sulphate as a phosphate depressant in acidic media. Sep. Purif. Technol. 2014, 124, 163–169. [Google Scholar] [CrossRef]
- Soares, F.A.; Elves, M.; Teixeira, R.R. Flotation of calcite from apatite of a uranium-carbonate phosphate ore using carbon dioxide. Miner. Eng. 2021, 173, 107240. [Google Scholar]
- El-Mofty, S.E.; El-Midany, A.A. Role of calcium ions and their interaction with depressants in phosphate flotation. Chem. Pap. 2018, 72, 2641–2646. [Google Scholar] [CrossRef]
- Zhang, P.; Snow, R. Evaluation of phosphate depressants in the phosphate/silica system. Miner. Metall. Process. 2009, 26, 101–104. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Rothenberg, A.S.; Lipp, D.W.; Panzer, H.P. Low molecular weight polyacrylamide-based polymers as modifiers in phosphate beneficiation. Int. J. Miner. Process. 1987, 20, 291–308. [Google Scholar] [CrossRef]
- Zhang, J.Q. Research on the Adsorption Mechanism of Surface Collectors on Apatite and Dolomite. Master’s Thesis, Guizhou University, Guizhou, China, 2023. [Google Scholar]
- He, G.X.; Zhang, Q. Selective depression mechanism of amino trimethylene phosphonic acid on fluoroapatite and dolomite. Ind. Min. Process. 2023, 52, 12–17. [Google Scholar]
- Zhong, K.N.; Han, Y.; Xie, H.X. Flotation separation of apatite and dolomite. Nonferrous Met. 1994, 31–38. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CJFDTEMP&filename=YOUS402.006&dbcode=CJFQ (accessed on 12 August 2024).
- Asmae, E.-b.; Yassine, T.; Yassine, A.-K.; Rachid, H.; Mostafa, B. Advancing phosphate ore minerals separation with sustainable flotation reagents: An investigation into highly selective biobased depressants. Adv. Colloid. Interface Sci. 2023, 317, 102921. [Google Scholar]
- Dong, L.Y.; Wei, Q.; Jiao, F.; Qin, W.Q. Utilization of polyepoxysuccinic acid as the green selective depressant for the clean flotation of phosphate ores. J. Clean. Prod. 2020, 282, 124532. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Jiao, Q.; Liu, B.; Chen, H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J. Saudi Chem. Soc. 2019, 23, 61–74. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Flotation separation of apatite and calcite using gum arabic as a depressant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127723. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Yang, B.Q.; Guan, Z.W.; Zeng, L.Y.; Luo, H.H.; Deng, B. The selective adsorption of xanthan gum on dolomite and its implication in the flotation separation of dolomite from apatite. Appl. Surf. Sci. 2021, 551, 149301. [Google Scholar] [CrossRef]
- Zheng, X.; Smith, R.W. Dolomite depressants in the flotation of apatite and collophane from dolomite. Miner. Eng. 1997, 10, 537–545. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, H.; Yang, B.Q.; Zeng, M.Y.; Zhang, H.S.; He, D.S.; Luo, H.H. Application and mechanism study of sodium lignosulfonate in direct flotation of phosphate ore for magnesium removal. Nonferrous Met. 2023, 152–157+180. [Google Scholar]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Yang, B.; Cao, S.H.; Zhu, Z.L.; Yin, W.Z.; Sheng, Q.Y.; Sun, H.R.; Yao, J.; Chen, K.Q. Selective flotation separation of apatite from dolomite utilizing a novel eco-friendly and efficient depressant for sustainable manufacturing of phosphate fertilizer. J. Clean. Prod. 2021, 286, 124949. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.L.; Sun, H.R.; Yin, W.Z.; Hong, J.S.; Cao, S.H.; Tang, Y.; Zhao, C.S. Improving flotation separation of apatite from dolomite using PAMS as a novel eco-friendly depressant. Miner. Eng. 2020, 156, 106492. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.D.; Lu, Q.F.; Yang, S.X.; Wang, X.; Zhang, X.M.; Yang, W. Reusability and selective adsorption of Pb2þ on chitosan/P(2-acryl amido-2-methyl-1-propanesulfonic acid-co-acrylic acid) hydrogel. Iran. Polym. J. 2016, 25, 1009–1019. [Google Scholar] [CrossRef]
- Kim, H.M.; Venkatesh, R.P.; Kwon, T.Y.; Park, J.G. Influence of anionic polyelectrolyte addition on ceria dispersion behavior for quartz chemical mechanical polishing. Colloid. Surf. A 2012, 411, 122–128. [Google Scholar] [CrossRef]
- Wang, C.T.; Liu, R.Q.; Xie, F.F.; Zhai, Q.L.; Sun, W.; Wen, X.F.; Li, J. Separation of sphalerite and dolomite using sodium alginate as an environmentally friendly depressant in a carbonate-hosted Pb-Zn ore system. J. Clean. Prod. 2022, 380, 135107. [Google Scholar] [CrossRef]
- Zhong, C.H.; Feng, B.; Zhang, W.P.; Zhang, L.Z.; Guo, Y.T.; Wang, T.; Wang, H.H. The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol. 2020, 373, 620–626. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, W.J.; Li, F.P.; Liu, J.X.; Zhang, H. Discrepant adsorption behavior of sodium alginate onto apatite and calcite surfaces: Implications for their selective flotation separation. Miner. Eng. 2022, 181, 107553. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.M.; Zhang, G.F.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jiao, F.; Qin, W.Q.; Zhu, H.L.; Jia, W.H. New insights into the carboxymethyl cellulose adsorption on scheelite and calcite: Adsorption mechanism, AFM imaging and adsorption model. Appl. Surf. Sci. 2018, 463, 105–114. [Google Scholar] [CrossRef]
- Jiao, F.; Dong, L.Y.; Qin, W.Q.; Liu, W.; Hu, C.Q. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Wang, T.; Feng, B.; Guo, Y.T.; Zhang, W.P.; Rao, Y.B.; Zhong, C.H.; Zhang, L.Z.; Cheng, C.; Wang, H.H.; Luo, X.P. The flotation separation behavior of apatite from calcite using carboxymethyl chitosan as depressant. Miner. Eng. 2019, 159, 106635. [Google Scholar] [CrossRef]
- Xie, R.Q.; Tong, X.; Xie, X.; Zhu, Y.; Liu, J. Flaxseed gum as new depressant in the separation of apatite and dolomite and its mechanism. Appl. Surf. Sci. 2022, 593, 153390. [Google Scholar] [CrossRef]
- Wang, X.; Xie, R.; Liu, J.; Zhu, Y. The utilization of tamarind seed gum as a novel dolomite depressant in the selective flotation of apatite from dolomite. Adv. Powder Technol. 2023, 34, 104022. [Google Scholar] [CrossRef]
- Giesekke, E.W.; Harris, P.J. The role of polyoxyethylene alkyl ethers in apatite flotation at Foskor, Phalaborwa (South Africa). Miner. Eng. 1994, 7, 1345–1361. [Google Scholar]
- Zhou, H.P.; Zhang, Y.B.; Tang, X.K.; Cao, Y.J.; Luo, X.P. Flotation separation of fluorite from calcite by using psyllium seed gum as depressant. Miner. Eng. 2020, 159, 106514. [Google Scholar] [CrossRef]
- Qun, W.; Heiskanen, K. Batch flotation tests by fatty acid on a phosphate-iron oxide-silicate regolith oresample from Sokli, Finland. Miner. Eng. 1990, 3, 473–481. [Google Scholar] [CrossRef]
- Silva, J.P.P.; Baltar, C.A.M.; Gonzaga, R.S.G.; Peres, A.E.C.; Leite, J.Y.P. Identification of sodium silicate species used as flotation depressants. Min. Met. Process 2012, 29, 207–210. [Google Scholar] [CrossRef]
- Rao, D.S.; Vijayakumar, T.V.; Angadi, S.; Prabhakar, S.; Raju, G.B. Effects of modulus and dosage of sodiumsilicate on limestone flotation. Maejo Int. J. Sci. Technol. 2010, 4, 397–404. [Google Scholar]
- Sayilgan, A.; Arol, A.I. Effect of carbonate alkalinity on flotation behavior of quartz. Int. J. Miner. Process. 2004, 74, 233–238. [Google Scholar] [CrossRef]
- Wang, Y.F.; Khoso, S.A.; Luo, X.M.; Tian, M.J. Understanding the depression mechanism of citric acid in sodium oleate flotation of Ca2+-activated quartz: Experimental and DFT study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Pavlovic, S.; Brandão, P.R.G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz. Miner. Eng. 2003, 16, 1117–1122. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.C.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghan, R.; de Salles Leal Filho, L.; Chehreh Chelgani, S. Tannin: An eco-friendly depressant for the green flotation separation of hematite from quartz. Miner. Eng. 2021, 168, 106917+108022. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.Y.; Song, S.M.; Zhou, W.G.; Xue, N.; Nie, Y.M.; Feng, B. The depressing role of sodium alginate in the flotation of Ca2+− activated quartz using fatty acid collector. J. Mol. Liq. 2021, 343, 117618. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhang, H.F.; Xu, Y.L.; Bao, S.X.; Liu, C.; Yang, S.Y. The molecular structure effects of starches and starch phosphates in the reverse flotation of quartz from hematite. Carbohydr. Polym. 2023, 303, 120484. [Google Scholar] [CrossRef]
- Zhang, W.D.; Tu, R.Y.; Ren, Q.L.; Liu, S.; Guo, Z.H.; Liu, P.; Tian, M.J. The selective flotation separation of apatite from dolomite and calcite utilizing a combination of 2-chloro-9-octadecenoic acid collector and sodium pyrophosphate depressant. Sep. Purif. Technol. 2025, 353, 128446. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Yang, L.F.; Hu, X.X.; Zhang, X.R.; Zheng, G.B. Flotation separation of quartz from magnesite using carboxymethyl cellulose as depressant. Trans. Nonferrous Met. Soc. 2022, 32, 1623–1637. [Google Scholar] [CrossRef]
- Zhang, H.L.; Xu, Z.J.; Sun, W.; Chen, D.X.; Li, S.; Han, M.J.; Yu, H.; Zhang, C.Y. Selective adsorption mechanism of dodecylamine on the hydrated surface of hematite and quartz. Sep. Purif. Technol. 2021, 275, 119137. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, L.Z.Z.; Yang, S.Y.; Liu, C.; Xu, Y.L. Investigations on the reverse flotation of quartz from hematite using carboxymethyl chitosan as a depressant. Powder Technol. 2021, 393, 109–115. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.G.; Song, W.M.; Gao, H.Y.; Niu, F.S.; Zhang, J.X.; Ai, G.H. Selective separation of hematite from quartz with sodium oleate collector and calcium lignosulphonate depressant. J. Mol. Liq. 2021, 322, 114502. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.M.; Zhang, G.F.; Yang, Q.; Zhang, C.; Xu, F.P. The flotation separation of scheelite from calcite and fluorite using dextran sulfate sodium as depressant. Int. J. Miner. Process. 2017, 169, 53–59. [Google Scholar] [CrossRef]
- Zhang, C.H.; Sun, W.; Hu, Y.H.; Tang, H.H.; Gao, J.D.; Yin, Z.G.; Guan, Q.J. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid. Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.D.; Ge, P.; Sun, W.; Xu, L.H.; Tang, H.H.; Wang, L. Selective flotation of quartz from feldspar using hydroxypropyl starch as depressant. Miner. Eng. 2023, 195, 108022. [Google Scholar] [CrossRef]
- Silva, E.M.S.; Peres, A.E.C.; Silva, A.C.; Florencio, D.L.; Caixeta, V.H. Sorghum starch as depressant in mineral flotation: Part 2–flotation tests. J. Mater. Res. Technol. 2019, 8, 403–410. [Google Scholar] [CrossRef]
- Ai, G.; Liu, C.; Zhang, W. Utilization of sodium humate as selective depressants for calcite on the flotation of scheelite. Sep. Sci. Technol. 2018, 53, 2136–2143. [Google Scholar] [CrossRef]
- Dos Santos, I.D.; Oliveira, J.F. Utilization of humic acid as a depressant for hematite in the reverse flotation of iron ore. Miner. Eng. 2007, 20, 1003–1007. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhang, Y.B.; Li, G.H.; Wu, Y.D.; Jiang, T. A further study on adsorption interaction of humic acid on natural magnetite, hematite and quartz in iron ore pelletizing. Powder Technol. 2015, 271, 155–166. [Google Scholar] [CrossRef]
- Paiva, P.R.P.; Monte, M.B.M.; Simao, R.A.; Gaspar, J.C. In situ AFM study of potassium oleate adsorption andcalcium precipitate formation on an apatite surface. Miner. Eng. 2011, 24, 387–395. [Google Scholar] [CrossRef]
- Yang, B.q.; Huang, P.l.; Song, S.x.; Luo, H.H.; Zhang, Y. Hydrophobic Agglomeration of Apatite Fines Induced by Sodium Oleate in Aqueous Solutions. Results Phys. 2018, 9, 970–977. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Yuan, J.q.; Yu, A.M.; Wen, S.M.; Bai, S.J. Insights into the influence of calcium ions on the adsorption behavior of sodium oleate and its response to flotation of quartz: FT-IR, XPS and AMF studies. Miner. Eng. 2023, 204, 108437. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Ambient temperature flotation of sedimentary phosphate ore using cottonseed oil as a collector. Minerals 2017, 7, 65. [Google Scholar] [CrossRef]
- Santos, E.P.; Dutra, A.J.B.; Oliveira, J.F. The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation. Int. J. Miner. Process. 2015, 143, 34–38. [Google Scholar] [CrossRef]
- Miller, J.D.; Wang, X.; Li, M. A new collector chemistry for phosphate flotation, Preprint 01-46. In Proceedings of the SME Annual Meeting, Denver, CO, USA, 26–28 February 2001. [Google Scholar]
- Huang, Q.; Huang, J.; Zhou, H.; Pan, Z.; Ping, X. Synthesis and application of a flotation collector for collophanite. In Beneficiation of Phosphates: New Thought, New Technology, New Development; Zhang, P., Miller, J., El-Shall, H., Eds.; SME: Denver, CO, USA, 2012; pp. 359–364. [Google Scholar]
- Oliveira, M.S.; Santana, R.C.; Ataíde, C.H.; Barrozo, M.A.S. Recovery of apatite from flotation tailings. Sep. Purif. Technol. 2011, 79, 79–84. [Google Scholar] [CrossRef]
- Leandro, H.S.; Adriele, M.A.S.; Luciano, F.d.M.; Tamíris, F.d.S.; Gilberto, R.d.S.; Antônio, E.C.P. Synergetic effects of fatty acids in amazon oil-based collectors for phosphate flotation. Miner. Eng. 2023, 191, 107932. [Google Scholar]
- Ding, Z.; Li, J.; Bi, Y.X.; Yu, P.; Dai, H.X.; Wen, S.M.; Bai, S.J. The adsorption mechanism of synergic reagents and its effect on apatite flotation in oleamide-sodium dodecyl benzene sulfonate (SDBS) system. Miner. Eng. 2021, 170, 107070. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yang, X.L.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid. Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, S.; Ren, Q.L.; Tu, R.Y.; Qiu, F.H.; Xu, S.H.; Sun, W.; Tian, M.J. Proposing a novel factor influencing flotation collector abilities to collect minerals by comparing two cationic collectors N,N-bis(2-hydroxy-3-chloropropyl) dodecylamine and dodecylamine. Appl. Surf. Sci. 2023, 640, 158284. [Google Scholar] [CrossRef]
- Zhao, P.X.; Liu, W.G.; Liu, W.B.; Shen, Y.B.; Cui, B.Y.; Zhao, Q. Novel low-foam viscous cationic collector 2-[2-(Tetradecylamino)ethoxy]ethanol: Design, synthesis, and flotation performance study to quartz. Sep. Purif. Technol. 2023, 307, 122633. [Google Scholar] [CrossRef]
- Zhang, W.D.; Ren, Q.L.; Tu, R.Y.; Liu, S.; Qiu, F.H.; Guo, Z.H.; Liu, P.; Xu, S.H.; Sun, W.; Tian, M.J. The application of a novel amine collector, 1-(dodecylamino)-2-propanol, in the reverse flotation separation of apatite and quartz. J. Mol. Liq. 2024, 133, 124377. [Google Scholar] [CrossRef]
- Liu, W.B.; Peng, X.Y.; Liu, W.G.; Shen, Y.B.; Zhao, Q.; Zhao, S.K.; Sun, W.H. Novel polyhydroxy cationic collector N-(2,3-propanediol)-Ndodecylamine: Synthesis and flotation performance to hematite and quartz. Int. J. Min. Sci. Technol. 2023, 33, 115–122. [Google Scholar] [CrossRef]
- Peng, X.; Liu, W.; Zhao, Q.; Liu, W.; Tong, K.; Zhao, P. Development and utilization of a novel hydrogen bonding enhanced collector in the separation of apatite from quartz. Miner. Eng. 2022, 180, 107477. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Sun, W.; Hu, Y. The Flotation and Adsorption of Mixed Collectors on Oxide and Silicate Minerals. Adv. Colloid. Interface Sci. 2017, 250, 1–14. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Kumari, N.; Bhagat, R.P. Adsorption mechanism of mixed collector systems on hematite flotation. Miner. Eng. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Severov, V.V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates. Miner. Eng. 2010, 23, 91–98. [Google Scholar] [CrossRef]
- Nogueira, S.C.S.; Matos, V.E.; Pereira, C.A.; Henriques, A.B.; Peres, A.E.C. Collector Mixtures and Their Synergistic Effect on Quartz Floatability. REM—Int. Eng. J. 2022, 75, 371–378. [Google Scholar] [CrossRef]
- Monte, M.B.M.; Pimentel, D.A.; De Albuquerque, M.D.D.F.; Neumann, R.S.; Lucas, A.; Correia, J.C.G.; Uliana, A. Synergism of mixed cationic collectors in the flotation of quartz unveiled by AFM, solution chemistry and quantum chemical calculations. J. Mol. Liq. 2023, 376, 121397. [Google Scholar] [CrossRef]
- Cao, Q.B.; Zou, H.; Chen, X.M.; Wen, S.M. Flotation selectivity of N-hexadecanoylglycine in the fluorapatite–dolomite system. Miner. Eng. 2019, 131, 353–362. [Google Scholar] [CrossRef]
- Li, J.; Nie, G.H.; Li, J.X.; Zhu, Z.X.; Wang, Z.G. Flotation separation of quartz and dolomite from collophane using sodium N-dodecyl-β-amino propionate and its adsorption mechanism. Colloids Surf. A 2022, 641, 128586. [Google Scholar] [CrossRef]
- Shao, X.; Jiang, C.L.; Parekh, B.K. Enhanced flotation separation of phosphate and dolomite using a new amphoteric collector. Miner. Metall. Process. 1998, 15, 11–14. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; El-Midany, A.A.; Abdel-Khalek, N.A. Application of amphoteric collector for dolomite separation by statistically designed experiments. Trans. Inst. Min. Met. C 2007, 116, 72–76. [Google Scholar] [CrossRef]
- Yang, J. Synthesis and Flotation Performance of Amphoteric Collectors. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2016. [Google Scholar]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Zohra, F.; Meryem, A.; Mohamed, A.; Noureddine, B.; M’hamed, S. Lecithin as an ecofriendly amphoteric collector foaming agent for the reverse flotation of phosphate ore. Chem. Eng. Res. Des. 2023, 200, 292–302. [Google Scholar]
- El-Shall, H.; Abdel-Khalek, N.A.; Svoronos, S. Collector–frother interaction in column flotation of Florida phosphate. Int. J. Miner. Process. 2000, 58, 187–199. [Google Scholar] [CrossRef]
- Ahmed, N.; Jameson, G. The effect of bubble size on the rate of flotation of fine particles. Int. J. Miner. Process. 1985, 14, 195–215. [Google Scholar] [CrossRef]
- Ahmed, N.; Jameson, G. Flotation kinetics. Miner. Procesing Extr. Metall. Rev. 1989, 5, 77–99. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P.B. Classification of flotation frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Improving froth characteristics and flotation recovery of phosphate ores with nonionic surfactants. Miner. Eng. 2003, 16, 587–595. [Google Scholar] [CrossRef]
- Lu, S.; Sun, K. Development of phosphate flotation reagents in China. In Beneficiation of Phosphates: Advances in Research and Practice; Zhang, P., El-Shall, H., Wiegel, R., Eds.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 1999; pp. 21–26. [Google Scholar]
- Lovell, V.M. Froth characteristics in phosphate flotation. In Flotation: Gaudin Memorial; Fuerstenau, M.C., Ed.; American Institute of Mining, Metallurgical, and Petroleum Engineers, Inc.: San Ramon, CA, USA, 1976; Volume 1, pp. 597–621. [Google Scholar]
- Somasundaran, P. Reagents in Mineral Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Khoshdast, H.; Sam, A.; Manafi, Z. The use of rhamnolipid biosurfactants as a frothing agent and a sample copper ore response. Miner. Eng. 2012, 26, 41–49. [Google Scholar] [CrossRef]
- Khoshdast, H.; Sam, A.; Vali, H.; Noghabi, K.A. Effect of rhamnolipid biosurfactants on performance of coal and mineral flotation. Int. Biodegrad. 2011, 65, 1238–1243. [Google Scholar] [CrossRef]
- Khoshdast, H.; Abbasi, H.; Sam, A.; Noghabi, K.A. Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem. Eng. J. 2012, 60, 127–134. [Google Scholar] [CrossRef]
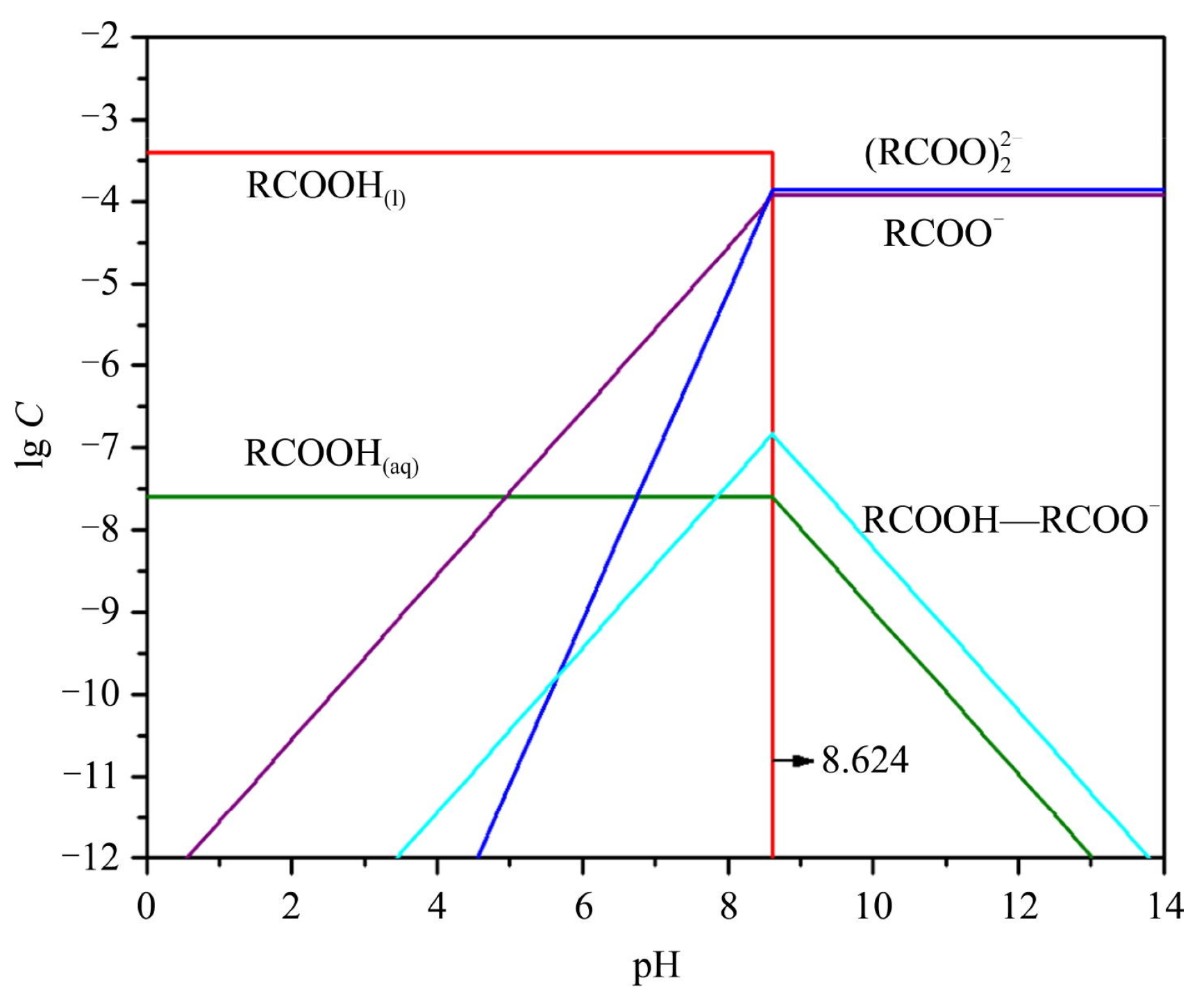
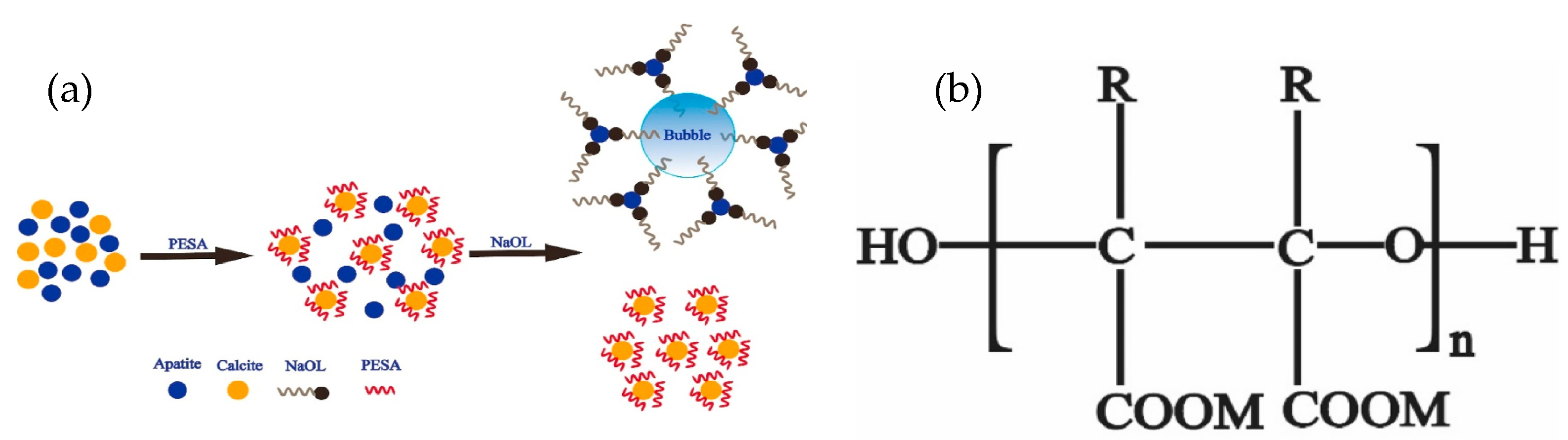
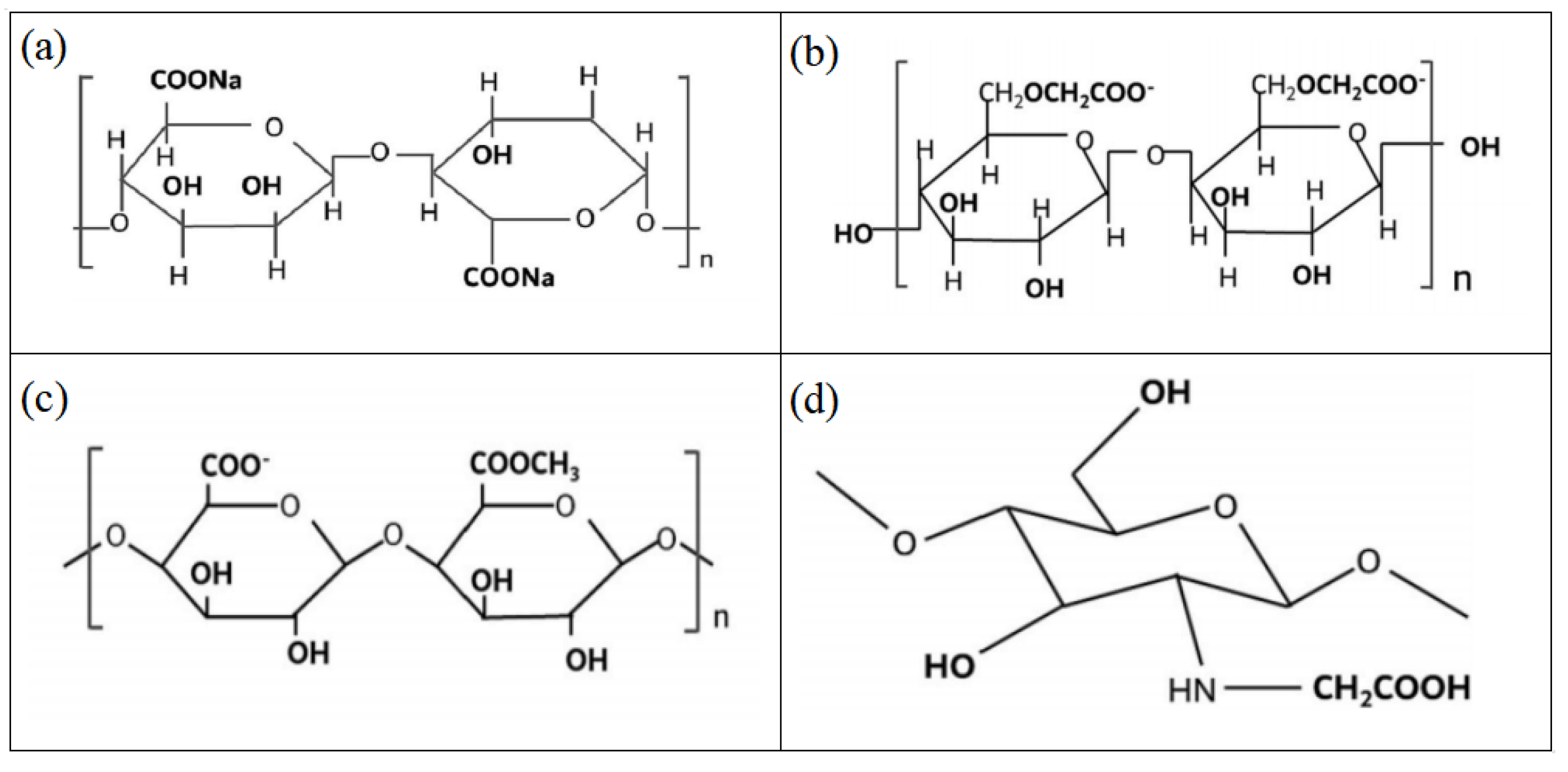
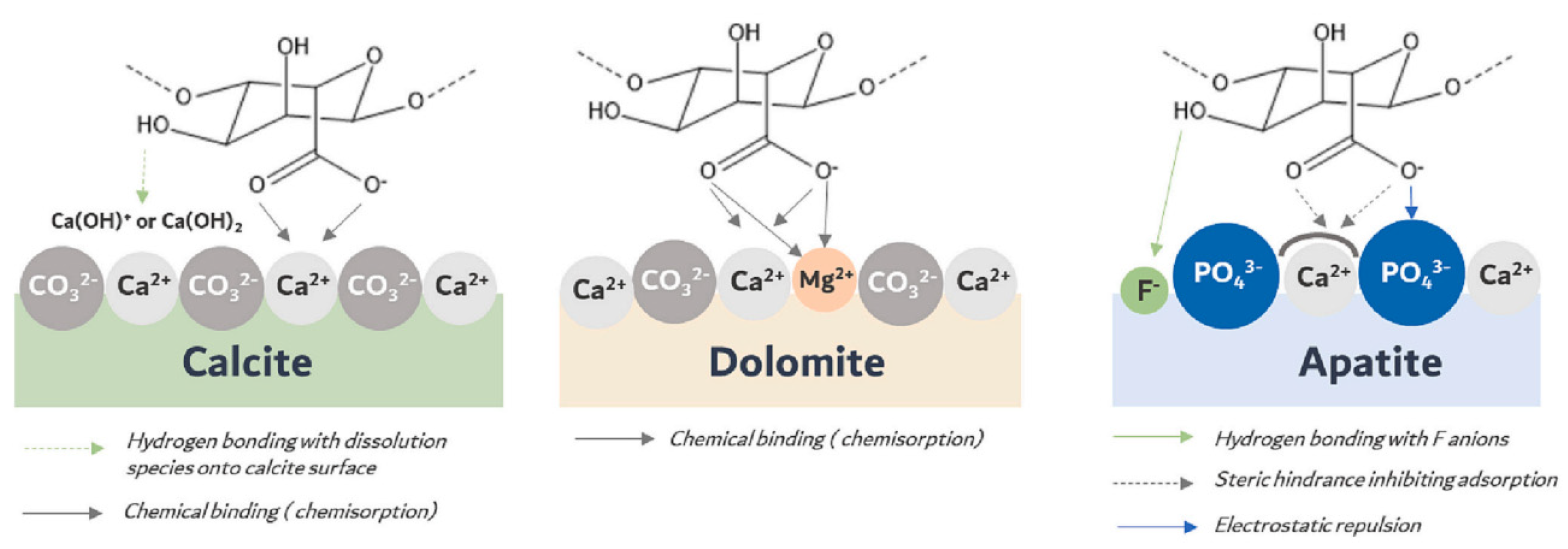
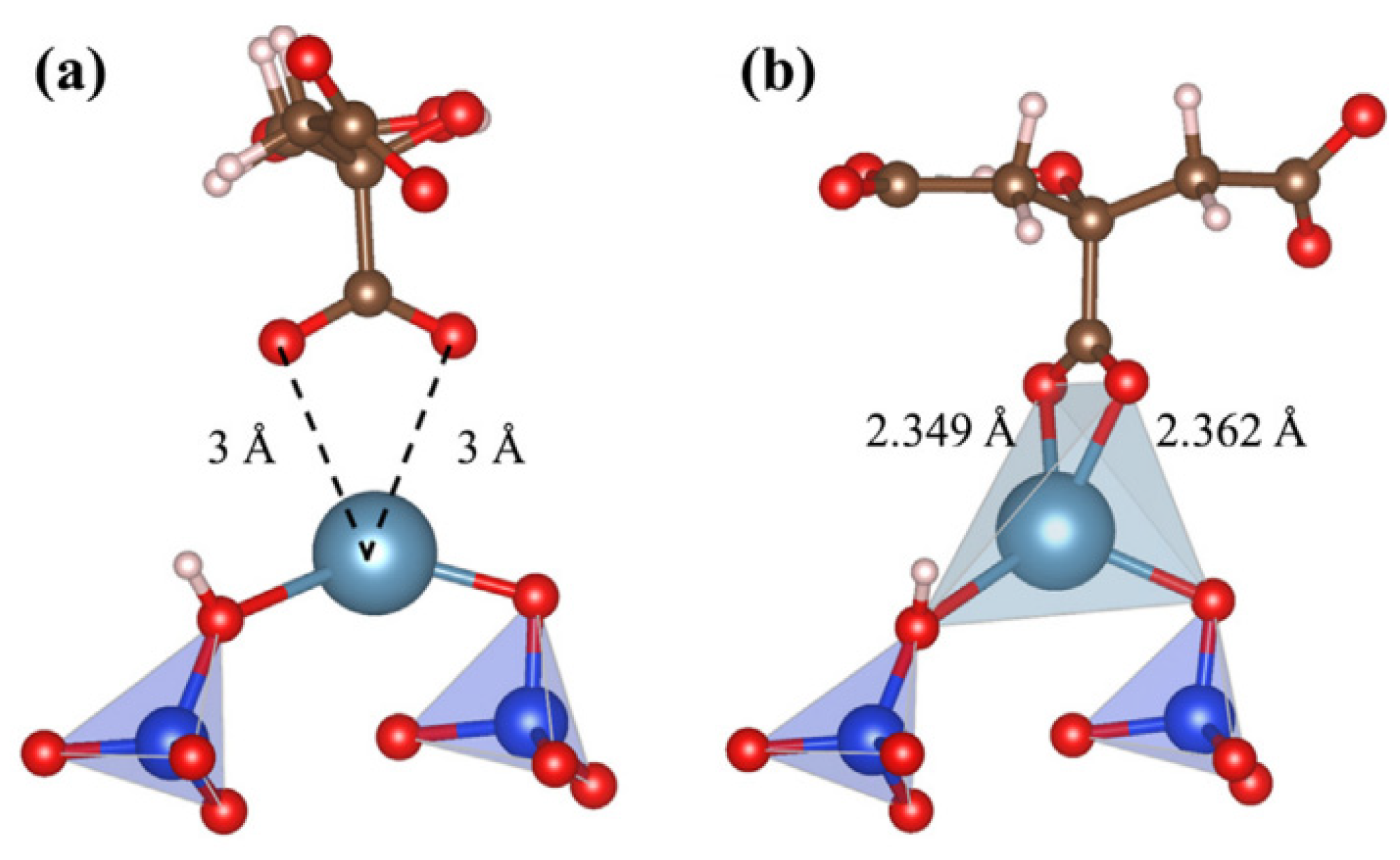


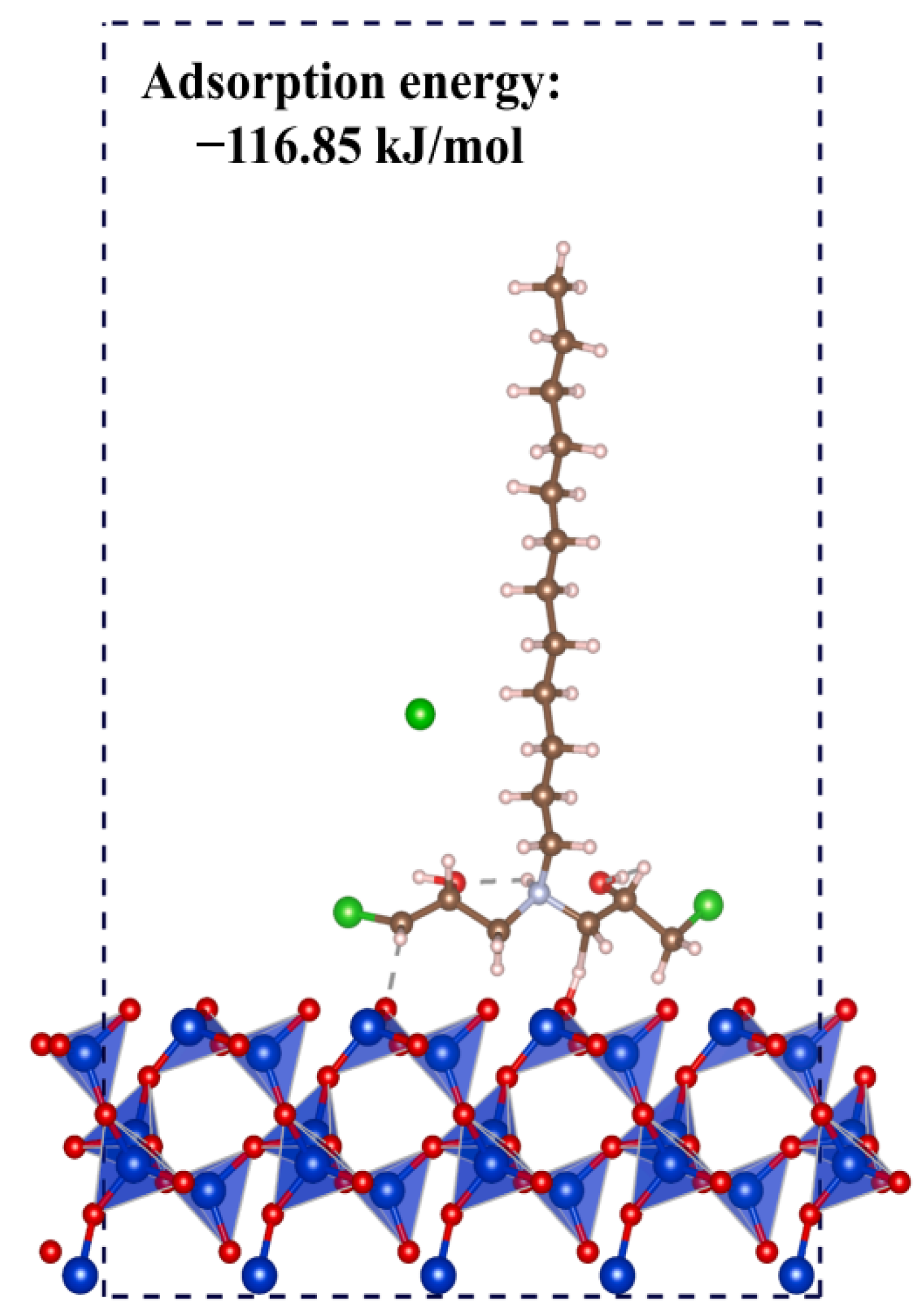
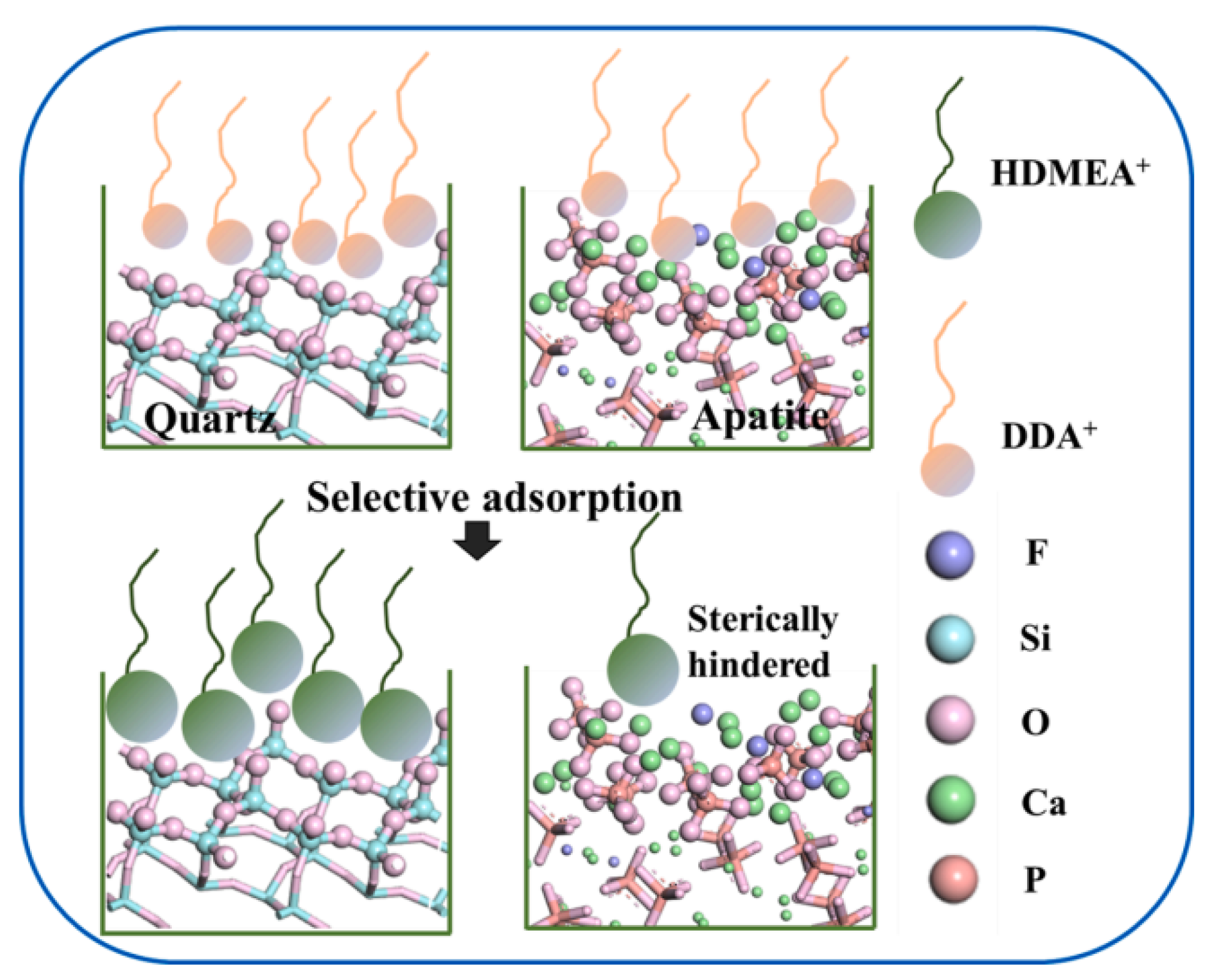
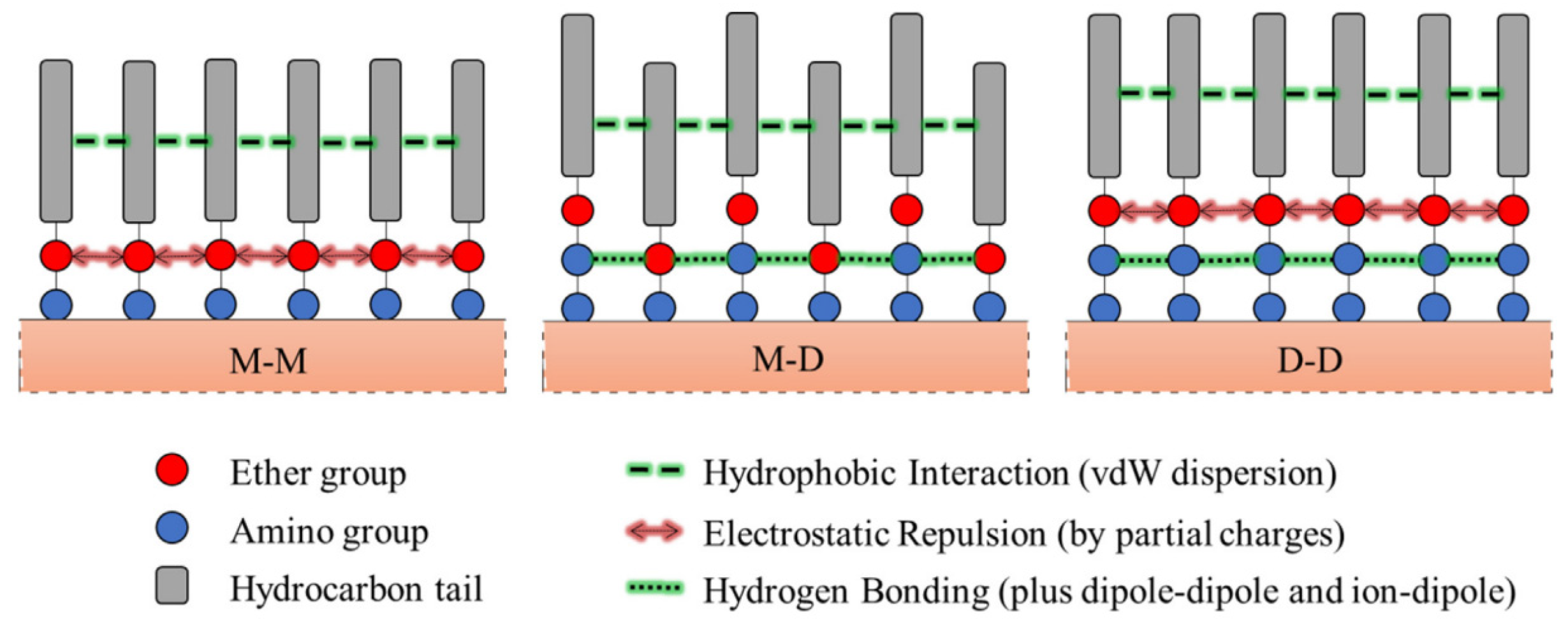

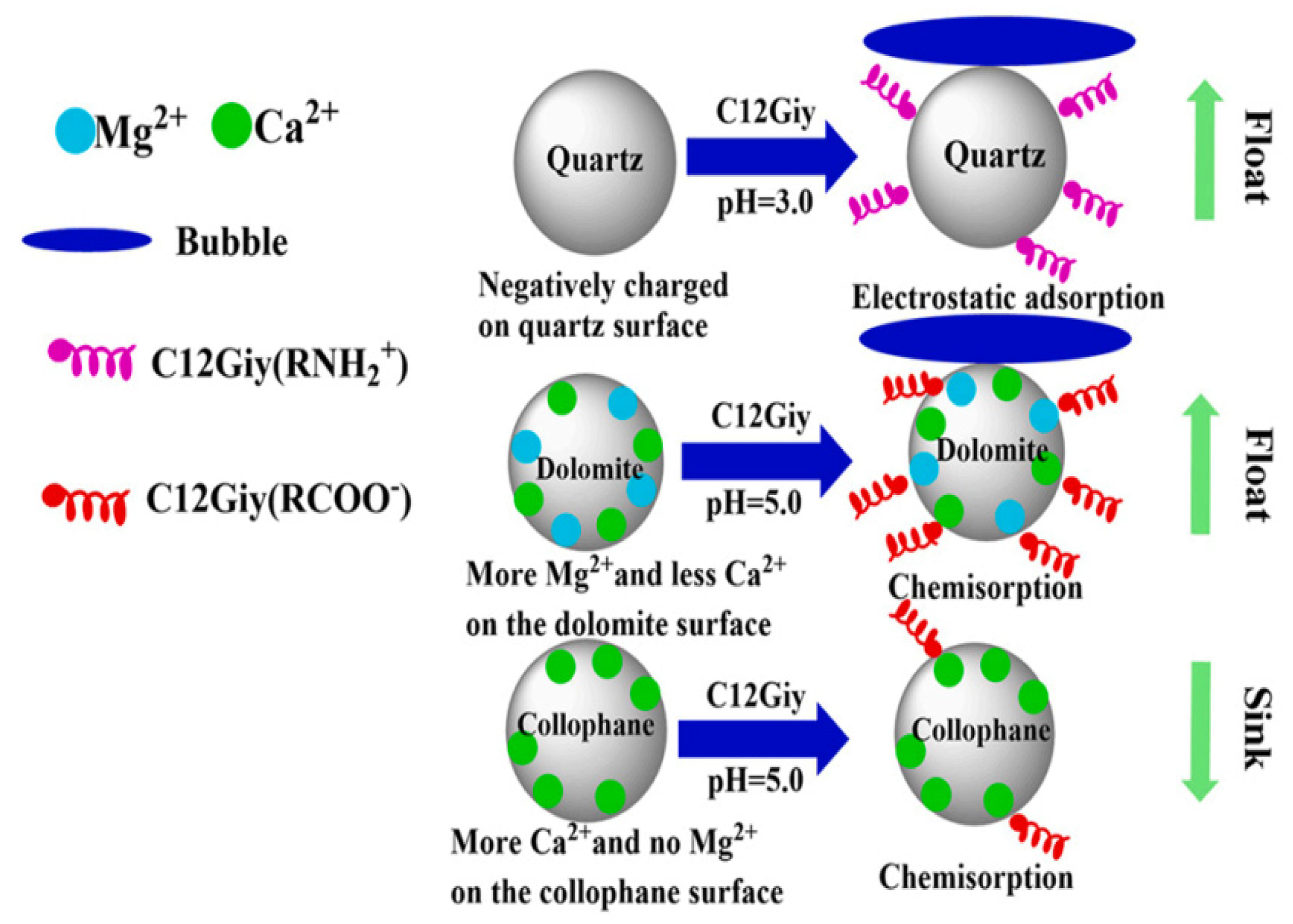
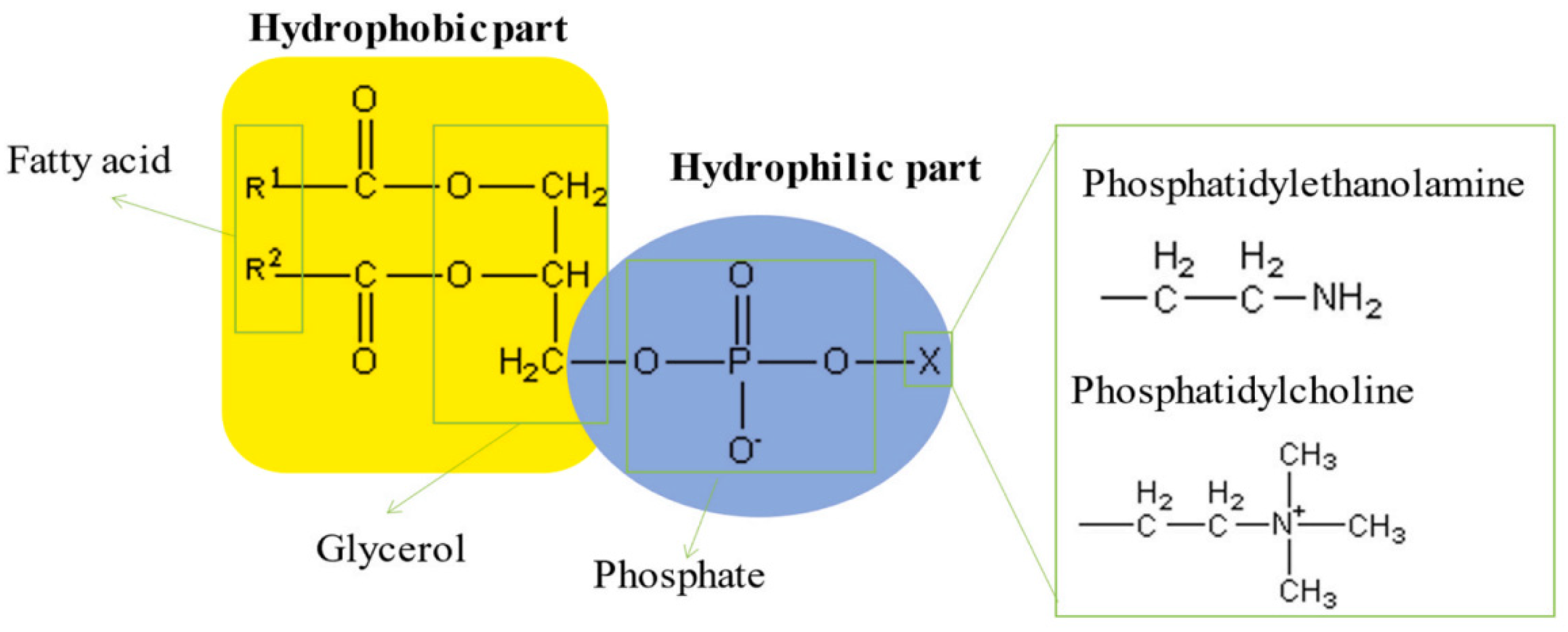
| Mineral System | Size/μm | Biobased Depressors | Dosage /mg/L | pH | Adsorption Dosage /mg/m2 | Selectivity | Refs |
|---|---|---|---|---|---|---|---|
| Apatite Calcite | −74 + 34 | CMC | 50 | 9 | 0.329 0.844 | Larger adsorption onto calcite surface | [79] |
| Apatite Dolomite | −74 + 37 | SA | 50 | 9 | 0.86 1.73 | Larger adsorption onto dolomite surface | [74] |
| Dolomite Apatite | −74 + 37 | XG | 30 | 9 | 0.75 1.8 | Larger adsorption onto dolomite surface | [59] |
| Calcite Fluorite | −74 + 37 | PSG | 200 | 7 | 14.9 12.14 | Similar adsorption onto both surfaces | [83] |
| Biobased Depressant | Quartz | Refs | Biobased Depressant | Quartz | Refs |
|---|---|---|---|---|---|
| Sodium alginate | * | [92] | Starch | *** | [89,93,94] |
| Carboxymethyl cellulose | * | [95] | Caustic Cassava starch | * | [96] |
| Carboxymethyl chitosan | * | [97] | Calcium lignosulphonate | * | [98] |
| Dextran | * | [99] | Tannic acid | * | [100] |
| Hydroxypropyl starch | * | [101] | Tannin | * | [91] |
| Sorghum starch | * | [102] | Humic acid/salt | *** | [103,104,105] |
| Separation System | Depressant Dosage | Collector Dosage | pH | Flotation Index | Refs |
|---|---|---|---|---|---|
| Depressors of phosphate minerals | |||||
| Apatite/Dolomite | H3PO4: 80 mg/L | NaOL: 25 mg/L | 5.5 | GP2O5: 27.32% RP2O5: 66.60% | [47] |
| Apatite/Dolomite in collophanite | H2SO4: 17 kg/t H3Cit: 500 g/t | Gutter oil fatty acid: 1.5 kg/t | 6.0 | GP2O5: 28.46% RP2O5: 87.20% | [49] |
| Apatite/Dolomite | NaPP: 100 m/L | NaOL: 60 mg/L | 7.0 | GP2O5: 34.1% RP2O5: 96.5% | [52] |
| A low-grade phosphate ore | Na2SO4: 7.5 kg/t | Oleic acid: 1.5~2.0 kg/t | 4.5 | GP2O5: >32% RP2O5: 84%~87% | [53] |
| Fluorapatite/Dolomite | ATMP: 1.5 mmol/L | NaOL: 80 mg/L | 9~10 | Rdolomite: 66.9% Rapatite: 15.3% | [59] |
| Depressors of carbonate minerals | |||||
| Apatite/Calcite | PESA: 7 mg/L | NaOL: 0.1 mmol/L | 8 | GP2O5: 29.26% RP2O5: 72.03% | [62] |
| Apatite/Calcite | GA: 50 mg/L | NaOL: 0.05 mmol/L | 9 | GP2O5: 26.86% RP2O5: 88.84% | [64] |
| Apatite/Dolomite | XG: 10 mg/L | NaOL: 0.3 mmol/L | 10 | GP2O5: 36.77% RP2O5: 83.6% | [65] |
| collophane/dolomite | NSFC: 30 mg/L | NaOL: 15 mg/L | 9 | GP2O5: 32.58% RP2O5: 86.01% | [68] |
| Dolomite-bearing phosphate ore | P(AA-AMPS): 0.24 kg/t | NaOL: 1 kg/t | 8.5 | GP2O5: 38.53% RP2O5: 82.56% | [69] |
| Apatite/Dolomite | PAMS: 14 mg/L | NaOL: 100 mg/L | 9 | GP2O5: 35.43% RP2O5: 88.40% | [70] |
| Apatite/Dolomite | SA: 20 mg/L | NaOL: 0.1 mmol/L | 9 | Rdolomite: 0.05% Rapatite: 88% | [74] |
| Apatite/Calcite | Carboxymethyl chitosan: 10 mg/L | NaOL: 0.1 mmol/L | 9 | Rapatite: 87.8% Rcalcite: 10.1% | [79] |
| Apatite/Dolomite | Flaxseed gum: 40 mg/L | NaOL: 40 mg/L | 8 | GP2O5: 39.39% RP2O5: 76.43% | [80] |
| Apatite/Dolomite | TSG: 10 mg/L | NaOL: 40 mg/L | 9 | GP2O5: 35.86% RP2O5: 78.76% | [81] |
| Depressors of silicate minerals | |||||
| Ca2+ activated quartz | Citric acid: 1 mmol/L | NaOL: 0.5 mmol/L | 12 | Rquartz decreased by 92.15% | [88] |
| Ca2+ activated quartz | NaAl: 20 mg/L | NaOL: 60 mg/L | 11.5 | Rquartz: <10% | [92] |
| Quartz/Hematite | CMCS: 12 mg/L | DDA: 60 mg/L | 7.5 | Rquartz: >90% Rhematite: <5% | [97] |
| Anionic Collectors | |||||
| Quartz | - | NaOL: 0.5 mmol/L | 12.5 | Rquartz: 84.50% | [108] |
| Phosphate flotation tailings | Corn starch: 500 g/t | ROS/KE: 80 g/t | 11.5 | GP2O5: 29.4% RP2O5: 46.2% | [113] |
| Apatite | - | Oleamide/sodium dodecyl benzene sulfonate | 9 | Rapatite improved by 4.87% | [115] |
| Cationic collectors | |||||
| Apatite/Quartz | - | BHCPDA: 0.1 mmol/L | 6 | Rquartz: ~100% Rapatite: ~0% | [117] |
| Apatite/Quartz | - | DDAIP: 0.01 mmol/L | 7 | Rquartz: 90% Rapatite: 10% | [119] |
| Quartz/Hematite | Starch | PDDA: 20 mg/L | 7.38 | Rquartz: 94.9% RHematite: 62.4% | [120] |
| Apatite/Quartz | - | HDMEA: 20 mg/L | 6.43 | GP2O5: 38.57% RP2O5: 95.8% | [121] |
| Amphoteric collector | |||||
| Apatite/Dolomite | - | C16Gly: 0.1 mmol/L | 9 | Rgap was 80% | [127] |
| Collophane/Quartz /Dolomite | - | C12Giy: 30 mg/L | 3.5 | Rgap of collophane and dolomite was 82.26% at pH 3; Rgap of dolomite and collophane was 74.57% at pH 5 | [128] |
| Francolite/Dolomite | - | Dodecyl-N-carboxyethyl-N-hydroxyethyl-imidazoline: 290 g/t | 10.5 | GP2O5: 35.2% RP2O5: 90% | [129] |
| Apatite/Calcite | - | BABP: 0.36 mM | 9 | Rapatite: >90% Rcalcite: <20% | [132] |
| Phosphate ore | H2SO4 | Lecithin: 40 mg/L | 5 | GP2O5: 31.05% RP2O5: 98.21% | [133] |
| Phosphate ore | - | Lecithin: 40 mg/L | 8.5 | GP2O5: 32.39% RP2O5: 96% | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Yu, P.; Bai, S. A Critical Review on the Flotation Reagents for Phosphate Ore Beneficiation. Minerals 2024, 14, 828. https://doi.org/10.3390/min14080828
Yu L, Yu P, Bai S. A Critical Review on the Flotation Reagents for Phosphate Ore Beneficiation. Minerals. 2024; 14(8):828. https://doi.org/10.3390/min14080828
Chicago/Turabian StyleYu, Liangmou, Pan Yu, and Shaojun Bai. 2024. "A Critical Review on the Flotation Reagents for Phosphate Ore Beneficiation" Minerals 14, no. 8: 828. https://doi.org/10.3390/min14080828
APA StyleYu, L., Yu, P., & Bai, S. (2024). A Critical Review on the Flotation Reagents for Phosphate Ore Beneficiation. Minerals, 14(8), 828. https://doi.org/10.3390/min14080828








