Effects of Low-Temperature Heat Treatment on Mong Hsu Rubies
Abstract
1. Introduction
2. Materials, Experiments and Analytical Methods
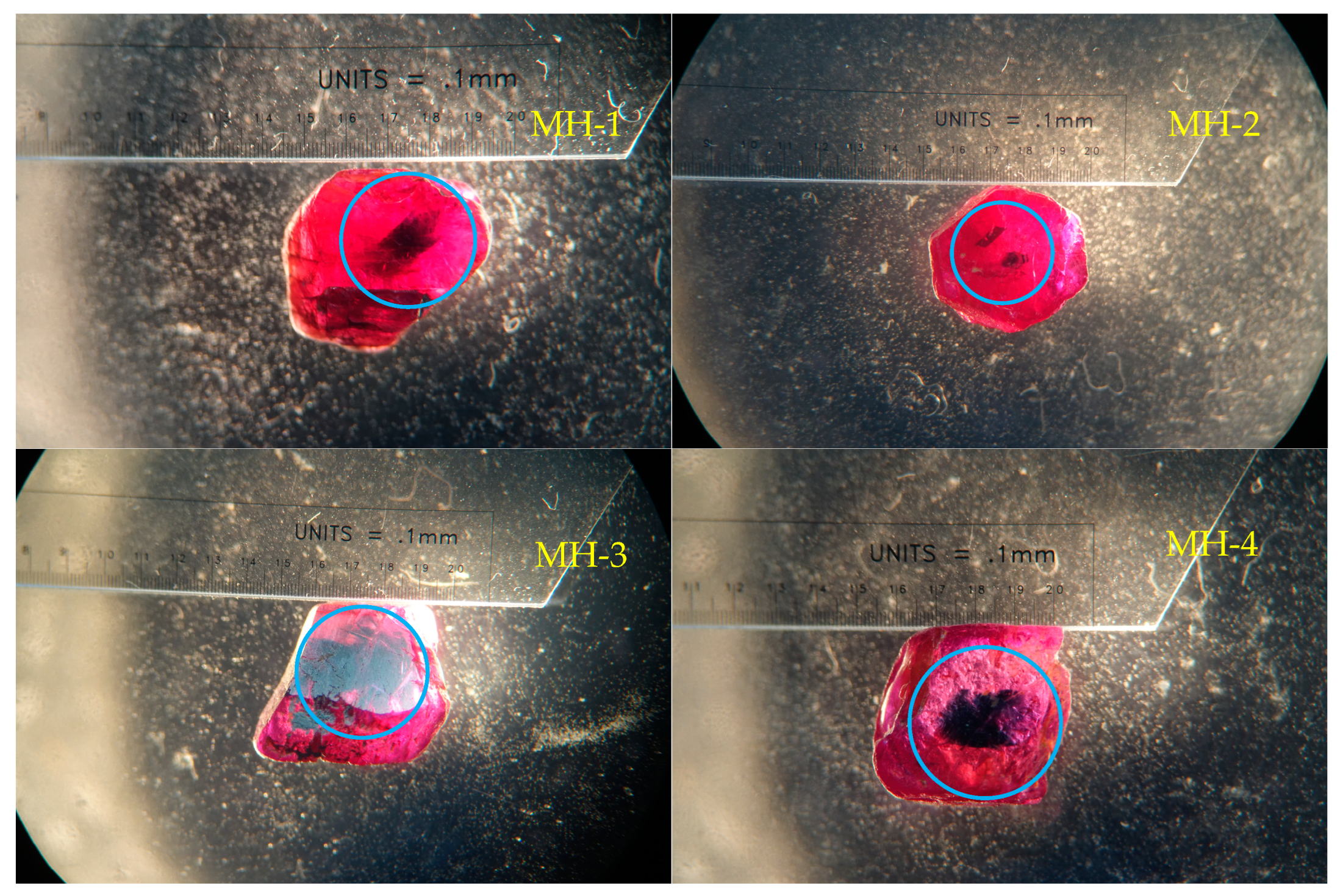
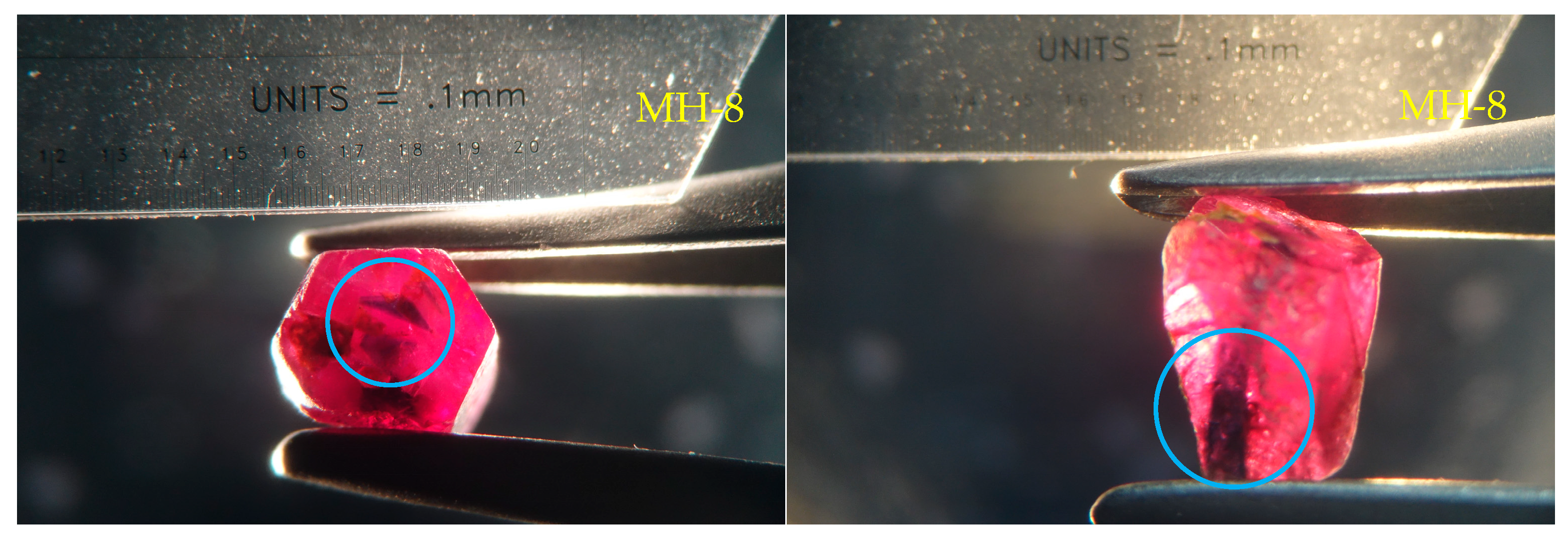
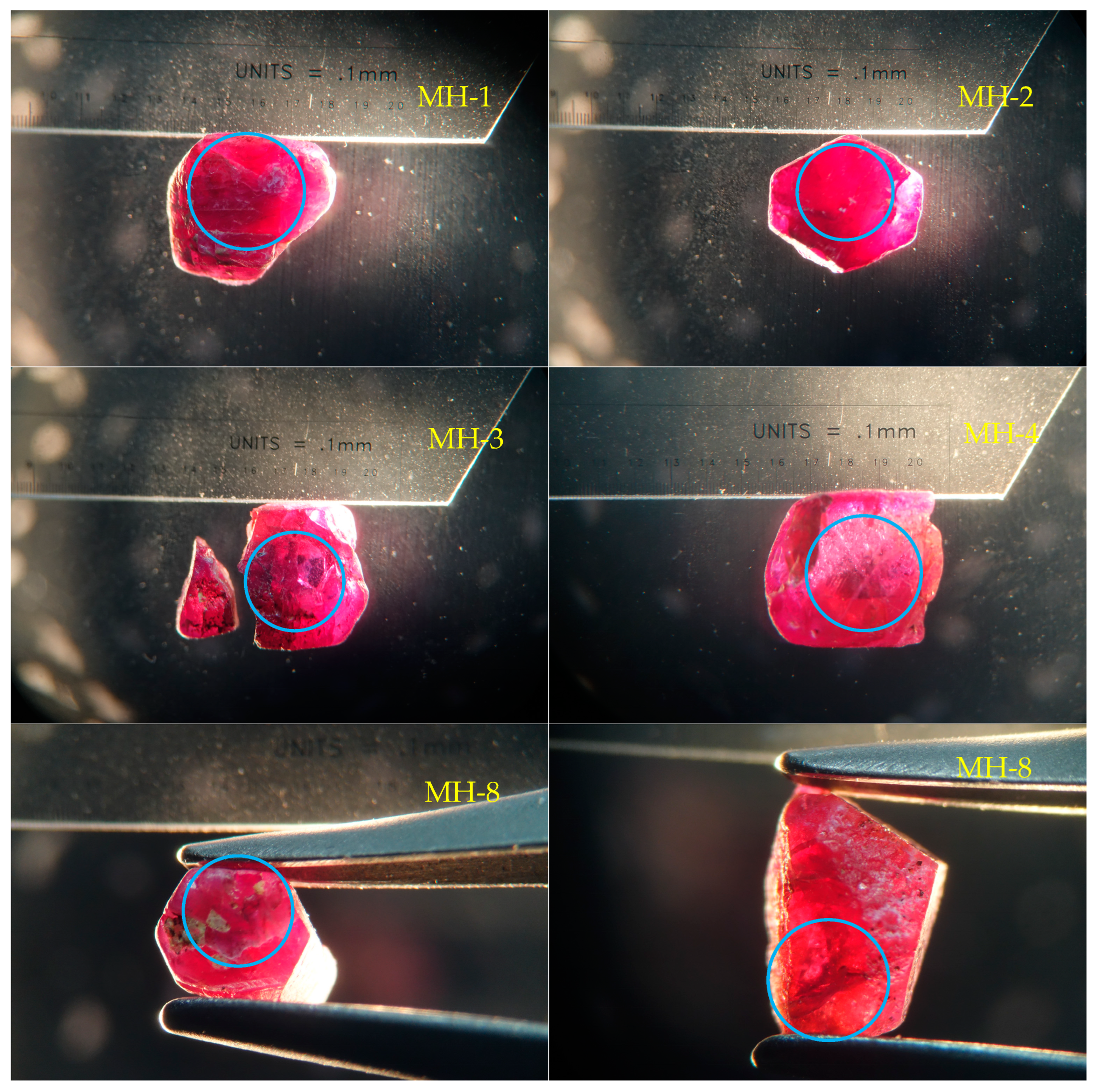
2.1. Materials
2.2. Experiments
2.3. Analytical Methods
2.3.1. EDXRF Spectra
2.3.2. UV–Vis Spectra
2.3.3. FTIR Spectra
2.3.4. Raman Spectra
2.3.5. PL Spectra
3. Results and Discussions
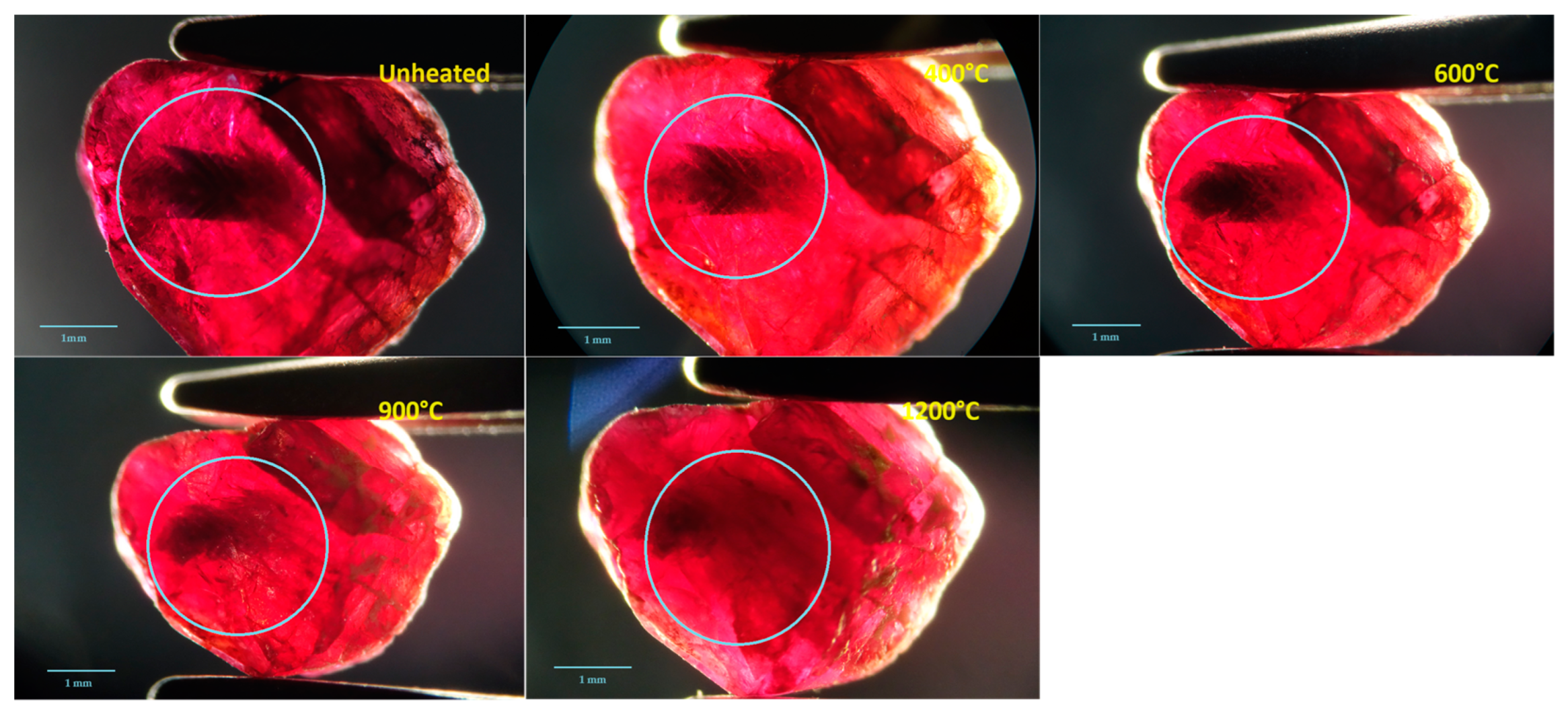
3.1. Appearance
3.2. EDXRF Spectra
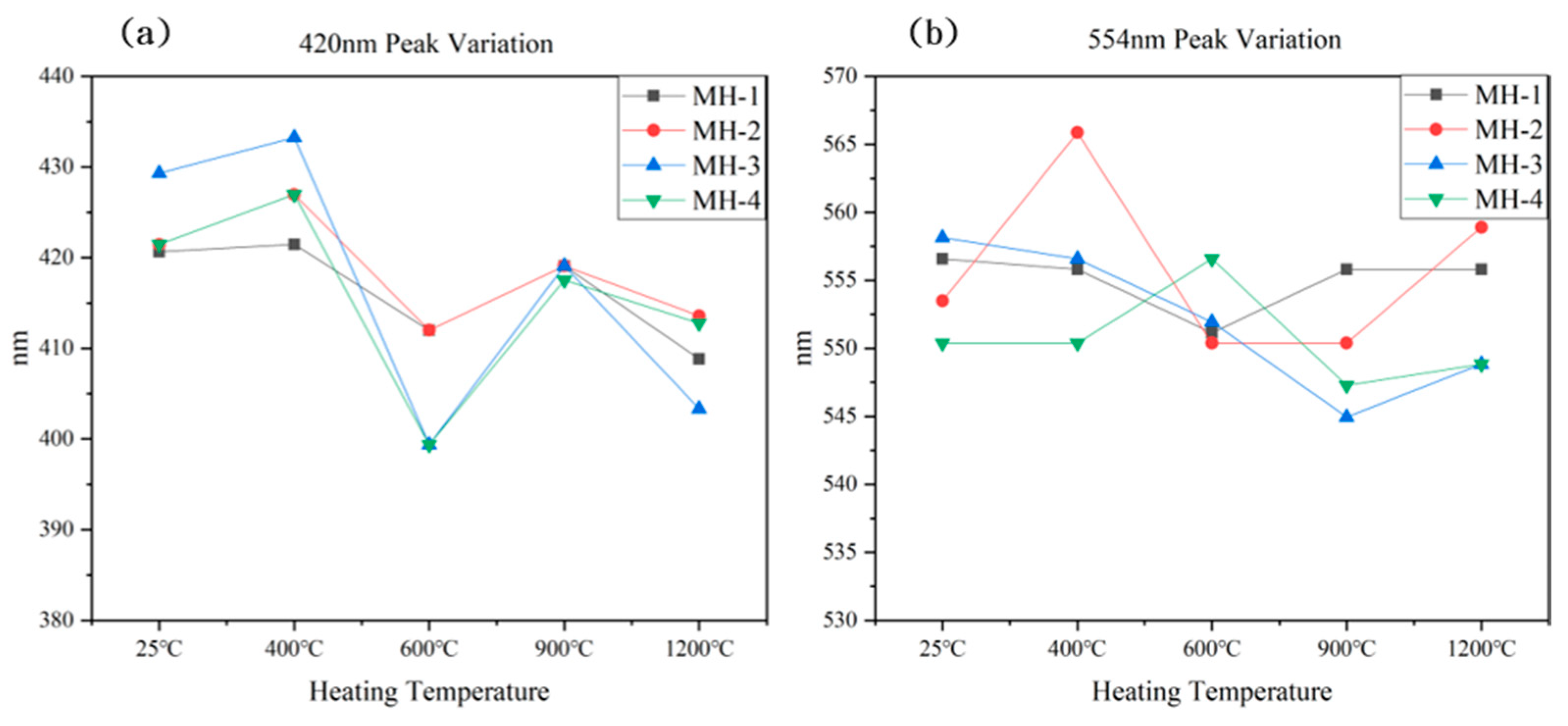
3.3. UV–Vis–NIR Spectra
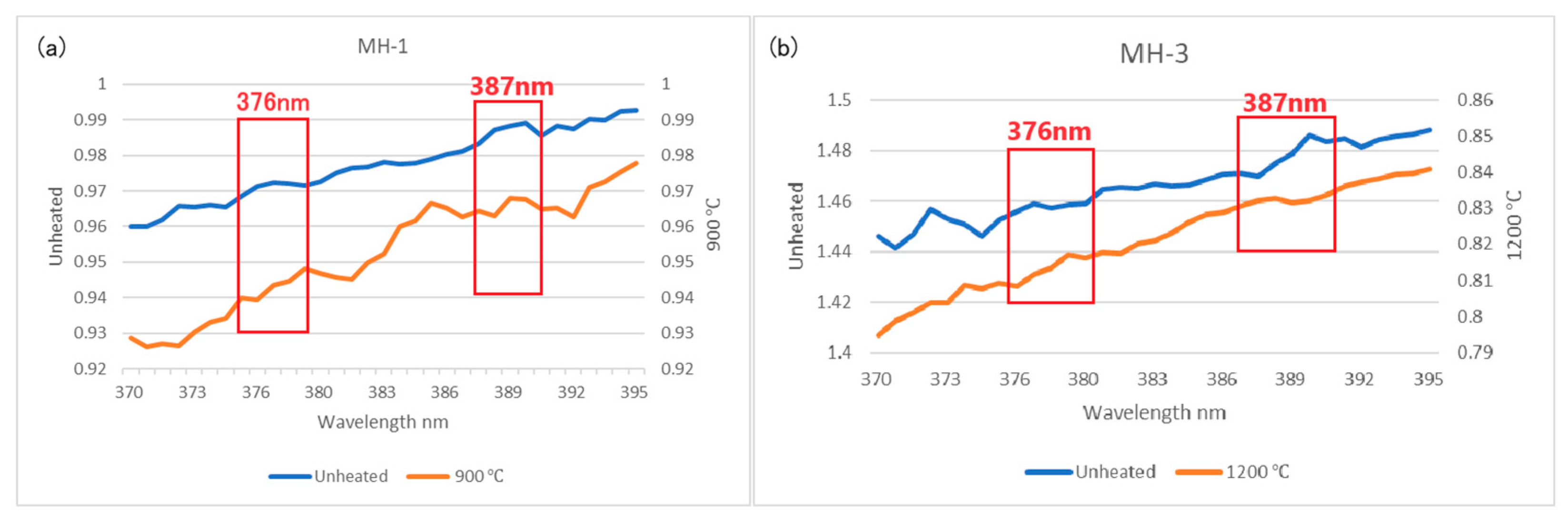
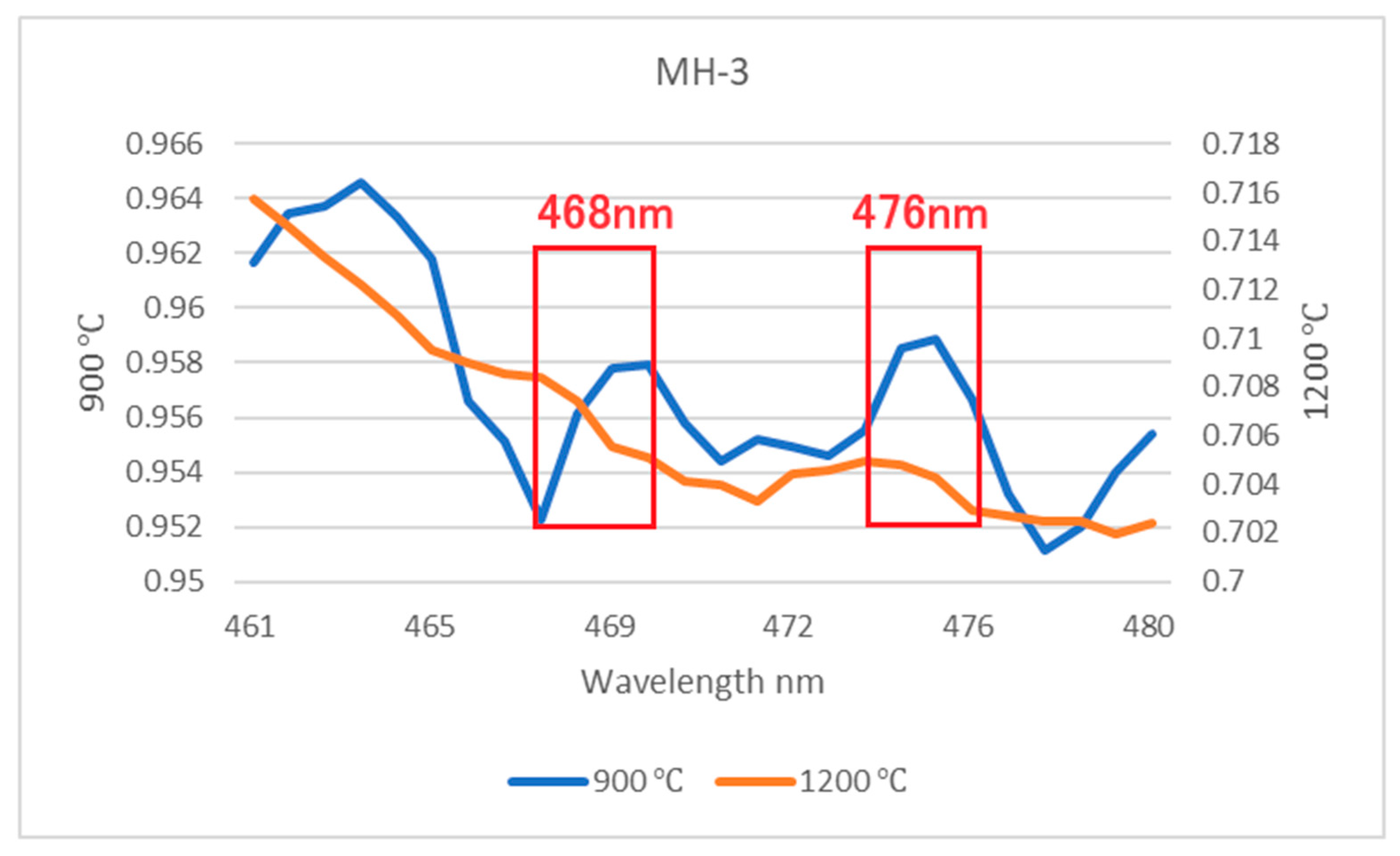
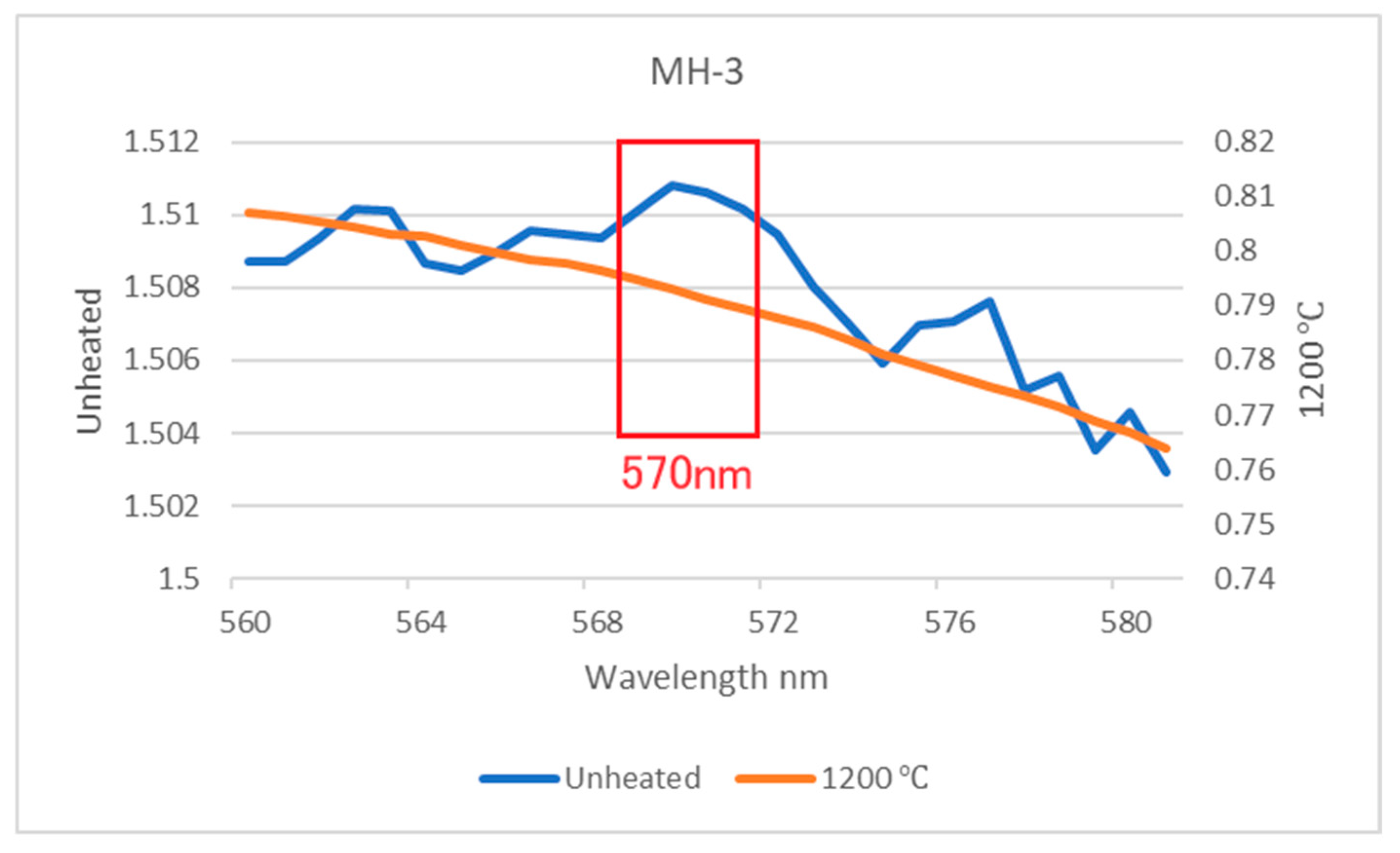
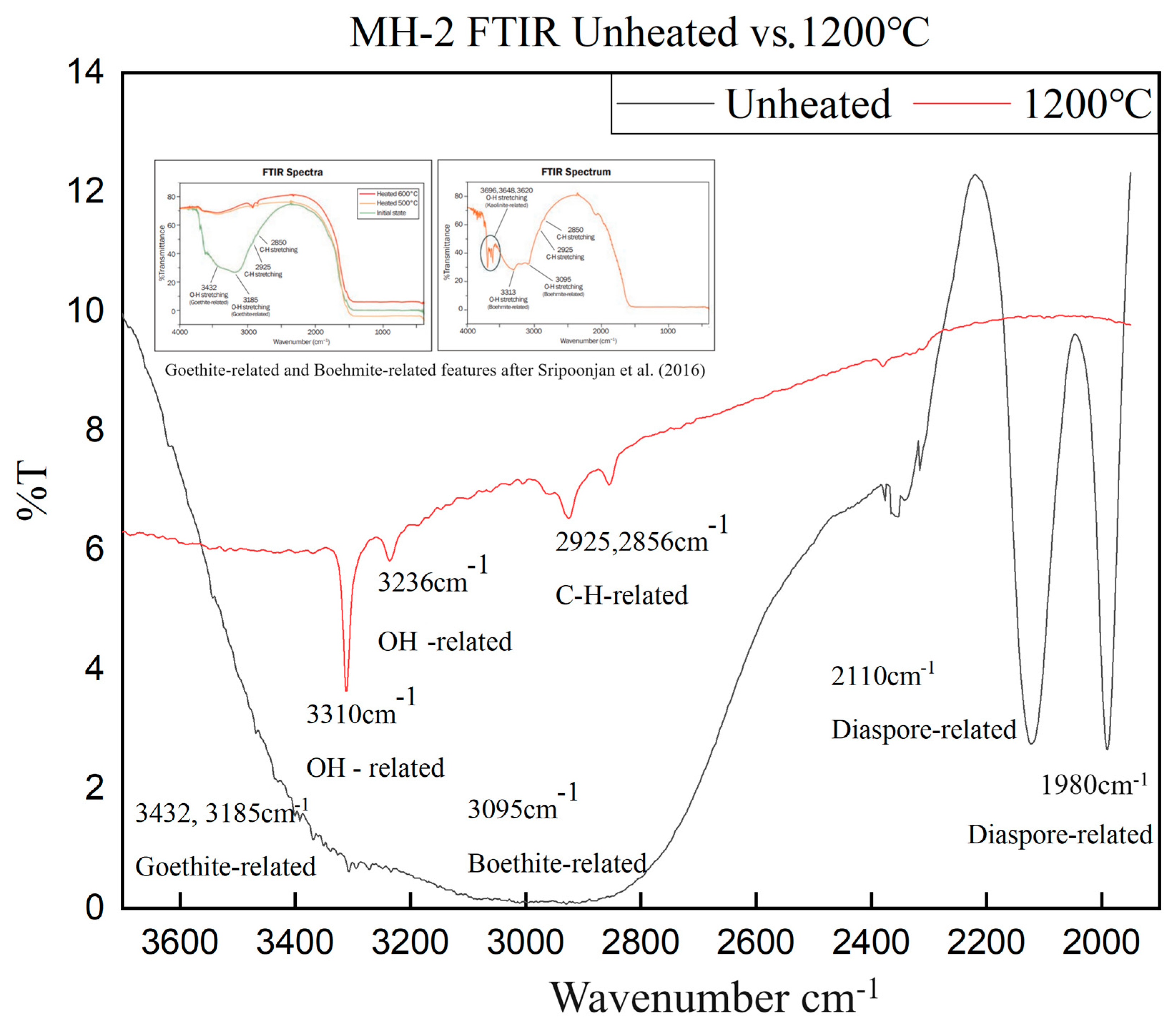
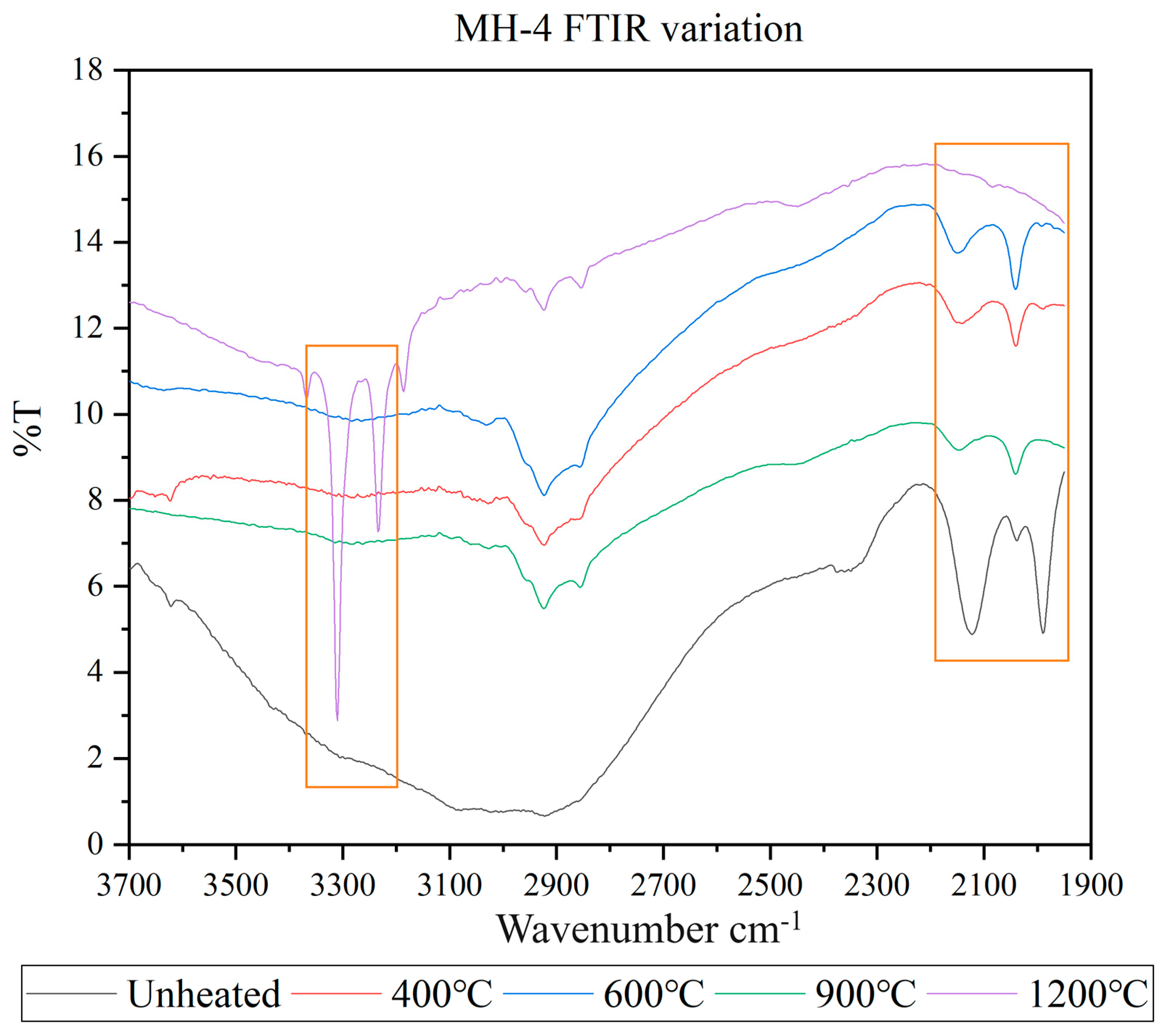
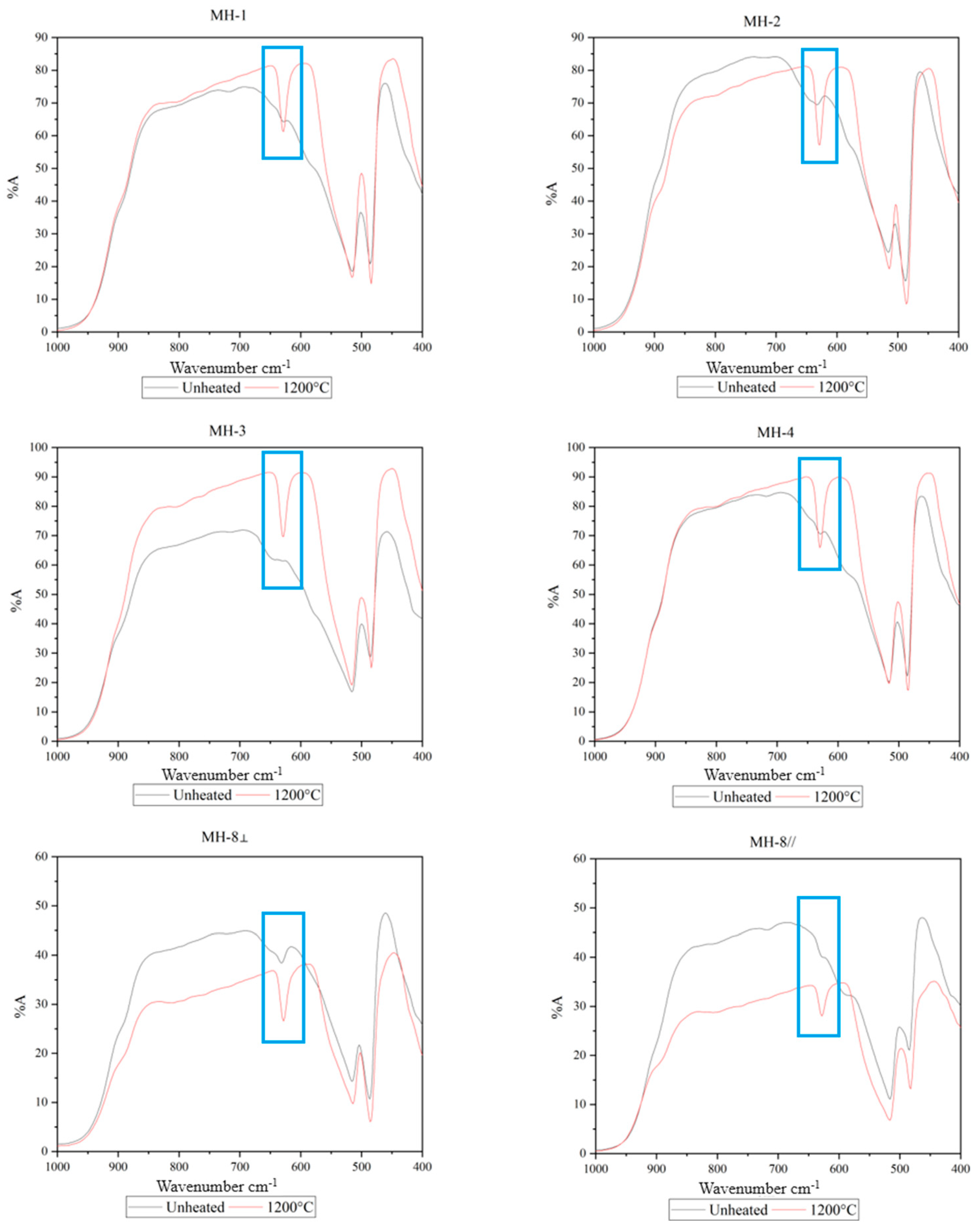
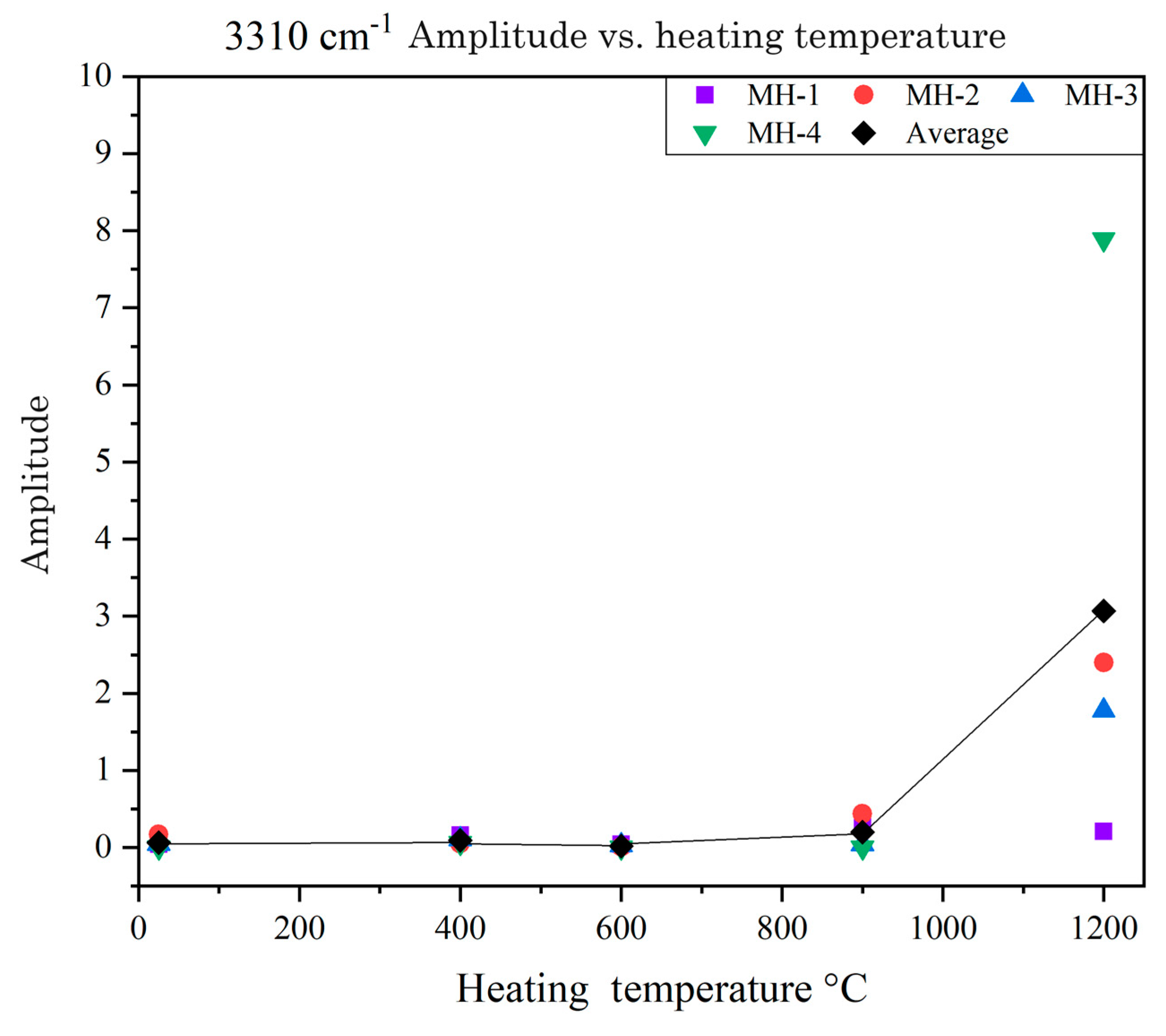
3.4. FTIR Spectra
3.5. Raman Spectra
3.6. The 532 nm PL Spectra
4. Conclusions
- Low-temperature heat treatment enhanced the ruby samples by causing the dark blue core to partially or completely fade away. It then increased the overall light transmittance and enhanced the fluorescence peak around 694 nm but did not improve the red hue of the samples.
- The color of the dark blue cores of the Mong Hsu rubies gradually faded during the heat treatment because of iron oxidation, which weakened the intervalence charge transfer between the Fe2+ and Ti4+. This charge transfer weakened considerably or disappeared at 1200 °C. For the crystals with polysynthetic twins, the dark blue cores were diffused in the parting planes, whereas the single crystals faded at their original locations.
- The main inclusions of the Mong Hsu rubies examined in this study were diaspore, boehmite and secondary goethite. The aforementioned inclusions slowly decomposed and released OH during the heating process, thus causing gradual changes in the FTIR spectra of the rubies. Therefore, observation of the release of OH is an effective method of detecting the effect of low-temperature heat treatment on rubies.
- The 630 cm−1 peaks in the fingerprint region of the FTIR spectra of the ruby samples were highly sensitive to low-temperature heat treatment. The 3310 cm−1 peaks in the characteristic region of these spectra became sensitive to temperature when the temperature reached 1200 °C. Therefore, when determining whether the Mong Hsu rubies are being heated, one should observe the changes in both of the aforementioned types of peaks.
5. Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardieu, V.; Saeseaw, S.; Detroyat, S.; Raynaud, V.; Sangsawong, S.; Bhusrisom, T.; Engniwat, S.; Muyal, J. GIA Lab Reports on Low-Temperature Heat Treatment of Mozambique Ruby. Gia News Res. 2015. [Google Scholar]
- Saeseaw, S.; Kongsomart, B.; Atikarnsakul, U.; Khowpong, C.; Vertriest, W.; Soonthorntantikul, W. Update on “Low-Temperature” Heat Treatment of Mozambican Ruby: A Focus on Inclusions and FTIR Spectroscopy, GIA NEWS FROM RESEARCH, April 25, 2018. Available online: https://www.gia.edu/doc/low_HT_Moz_report.pdf (accessed on 13 August 2024).
- Lu, Q.; Li, X.; Sun, L.; Qin, B. Chemical and Spectral Variations between Untreated and Heat-Treated Rubies from Mozambique and Madagascar. Minerals 2022, 12, 894. [Google Scholar] [CrossRef]
- Hughes, E.B.; Vertriest, W. A Canary in the Ruby Mine: Low-Temperature Heat Treatment Experiments on Burmese Ruby. Gems Gemol. 2022. Available online: https://www.gia.edu/gems-gemology/winter-2022-burmese-ruby (accessed on 13 August 2024). [CrossRef]
- Peretti, A.; Schmetzes, K.; Bernhadt, H.-J.; Mouawad, F.; Gem-Institute of America. RUBIES FROM MONG HSU. Gems Gemol. 1995, 31, 2–26. [Google Scholar] [CrossRef]
- Emmett, J.L. Fluxes and the Heat Treatment of Ruby and Sapphire. Gems Gemol. 1999, 35, 90–92. [Google Scholar]
- Song, Q.; Shen, A.H. Comparative Study of Unheated and Heated Rubies from Myanmar. 2021. Available online: http://jogg.cug.edu.cn/en/article/doi/10.15964/j.cnki.027jgg.2021.03.002 (accessed on 13 August 2024). (In Chinese with English abstract).
- Achiwawanich, S.; Brack, N.; James, B.D.; Liesegang, J. Surface analysis of heat-treated Mong Hsu rubies. Appl. Surf. Sci. 2006, 252, 8646–8650. [Google Scholar] [CrossRef]
- Smith, C.P. A contribution to understanding the infrared spectra of Mong Hsu rubies. J. Gemmol. 1995, 24, 321–335. [Google Scholar] [CrossRef]
- Themelis, T. The Heat Treatment of Ruby & Sapphire Experiments & Observations, 3rd ed.; Published by the author: Thailand, 2018; Volume 1: Attributes of Ruby-Sapphire and Related Heating Technology, ISBN 0-940965-57-7. [Google Scholar]
- Hughes, R.W. Ruby: An Expensive Mistake. Gems Gemol. 2022, 58, 258. [Google Scholar]
- Karampelas, S.; Hennebois, U.; Mevellec, J.-Y.; Pardieu, V.; Delaunay, A.; Fritsch, E. Pink to Purple Sapphires from Ilakaka, Madagascar: Insights to Separate Unheated from Heated Samples. Minerals 2023, 13, 704. [Google Scholar] [CrossRef]
- Hughes, R.W.; Manorotkul, W.; Hughes, E.B. Ruby & Sapphire: A Gemologist’s Guide; RWH Publishing/Lotus Publishing: Bangkok, Thailand, 2017. [Google Scholar]
- Long, C.; He, L.-Y.; Hou, S.-Y.; Zhao, B.-W.; Tu, C.; LÜ, X.-Y. The Characteristics of Hydrous Mineral Inclusions in Low-Temperature Heated Corundum. Spectrosc. Spectr. Anal. 2021, 41, 1530–1536. [Google Scholar]
- Nassau, K. Heat Treating Ruby and Sapphire: Technical Aspects. Gems Gemol. Fall 1981, 17, 121–131. [Google Scholar] [CrossRef]
- The Gemmological Association of Great Britain (FGA or Gem-A); The Diploma in Gemmology Course, 2012.
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T.; Duong, L. Far-infrared spectroscopy of alumina phases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Koivula, J. Useful Visual Clue Indicating Corundum Heat Treatment. Gems Gemol. Fall 2013, 49, 160. [Google Scholar] [CrossRef]
- Sripoonjan, T.; Wanthanachaisaeng, B.; Leelawatanasuk, T. Phase Transformation of Epigenetic Iron Staining: Indication of Low-Temperature Heat Treatment in Mozambique Ruby. J. Gemmol. 2016, 35, 156–161. [Google Scholar] [CrossRef]
- Kammerling, R.C.; Scarratt, K.; Bosshart, G.; Jobbins, E.A.; Kane, R.E.; Gubelin, E.J.; Levinson, A.A. Myanmar and its gems-An update. J. Gemmol. 1994, 24, 3–40. [Google Scholar] [CrossRef]
- Yanagida, H.; Yamaguchi, G. A Discussion on the Phase Diagram of the System Al2O3-H2O Considering the Transformation Mechanism of the Polymorphs Appearing in It. J. Ceram. Assoc. Japan. 1966, 74, 1966. [Google Scholar] [CrossRef]
- Shaw, C.L. An evaluation of the infrared 630 cm−1 OH libration band in bone mineral as evidence of fire in the archaeological record. J. Archaeol. Sci. Rep. 2022, 46, 103655. [Google Scholar] [CrossRef]
- Lowry, S. Analysis of Rubies and Sapphires by FT-IR Spectroscopy, Thermo Fisher Scientific Application Note: 51124. Available online: https://assets.thermofisher.com/TFS-Assets/CAD/Application-Notes/D10280~.pdf (accessed on 13 August 2024).
- Lowry, S. Using FTIR Spectroscopy to Analyze Gemstones, American Laboratory, 2008. Available online: https://www.americanlaboratory.com/913-Technical-Articles/730-Using-FTIR-Spectroscopy-to-Analyze-Gemstones/ (accessed on 13 August 2024).











| Temperature Sample | Stage 1 400 °C | Stage 2 600 °C | Stage 3 900 °C | Stage 4 1200 °C |
|---|---|---|---|---|
| MH-1 | 12 h | 12 h | 12 h | 12 h |
| MH-2 | 24 h | 24 h | 24 h | 12 h |
| MH-3 | 4 h | 4 h | 4 h | 4 h |
| MH-4 | 1 h | 1 h | 1 h | 1 h |
| MH-8 | 12 h | 12 h | 12 h | 12 h |
| Element Sample | Fe (ppm) | Ti (ppm) | Cr (ppm) | Al (ppm) | ||||
|---|---|---|---|---|---|---|---|---|
| Unheated | 1200 °C | Unheated | 1200 °C | Unheated | 1200 °C | Unheated | 1200 °C | |
| MH-1 | 1331 | 710 | 451 | 688 | 3420 | 7639 | 388,897 | 564,185 |
| MH-2 | 497 | 471 | 1231 | 1468 | 11,601 | 11,515 | 546,237 | 496,440 |
| MH-3 | 1485 | 2110 | 466 | 1034 | 4407 | 6071 | 427,334 | 472,278 |
| MH-4 | 595 | 500 | 1506 | 1249 | 2069 | 2368 | 507,254 | 496,193 |
| MH-8 | 798 | 1084 | 1171 | 1285 | 2434 | 3688 | 378,017 | 382,673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Ding, Y.-C. Effects of Low-Temperature Heat Treatment on Mong Hsu Rubies. Minerals 2024, 14, 829. https://doi.org/10.3390/min14080829
Fan C, Ding Y-C. Effects of Low-Temperature Heat Treatment on Mong Hsu Rubies. Minerals. 2024; 14(8):829. https://doi.org/10.3390/min14080829
Chicago/Turabian StyleFan, Chen, and Yung-Chin Ding. 2024. "Effects of Low-Temperature Heat Treatment on Mong Hsu Rubies" Minerals 14, no. 8: 829. https://doi.org/10.3390/min14080829
APA StyleFan, C., & Ding, Y.-C. (2024). Effects of Low-Temperature Heat Treatment on Mong Hsu Rubies. Minerals, 14(8), 829. https://doi.org/10.3390/min14080829






