Abstract
Nanofibrous clay minerals, specifically palygorskite (Pal) and sepiolite (Sep), have been becoming a new generation of rheological additives for drilling fluid systems due to their unique nanostructure, high performance, environmentally benign nature, and cost-effectiveness. These nanoclay minerals exhibit excellent colloidal and rheological properties in aqueous systems, even in saline and high-temperature environments. Although Pal and Sep have been employed as auxiliary rheological additives in a few cases to enhance the salt resistance of conventional water-based drilling fluids (WBDFs), these two clay minerals have not yet been used on a large scale due to a lack of understanding of their structures and properties, as well as the control of their performance. This paper presents a comprehensive review of the clay mineralogy, colloidal chemistry, rheological behaviors, and filtration properties of nanofibrous clay minerals in WBDFs, with critical comments. It also discusses the challenges and prospects for further research. This review provides new insights into fundamental and applied studies of nanofibrous clay minerals and helps promote the large-scale application of nanofibrous clay products in drilling fluids.
1. Introduction
Currently, oil and gas are the most and third-most prominent energy sources, respectively. The International Energy Agency (IEA) has forecast that oil and gas consumption demand will continue to rise until 2050. Consequently, the extraction of additional oil and gas represents one of the most urgent tasks which must be addressed before the advent of the new energy era. However, extracting oil and gas from the earth is becoming increasingly challenging. Drilling operations must overcome a number of challenges, including high temperatures, high pressure, unstable shales, and salty formations [1]. It is therefore evident that there is a need for high-performance drilling fluids in harsh environments.
Drilling fluids, also called drilling muds, are indispensable for the successful execution of drilling operations. The principal functions of drilling fluids are to suspend and transport drilling cuttings, clean the borehole, stabilize the wellbore by forming filter cakes, and balance the formation pressure [2,3]. In general, drilling fluids are classified into two categories: water-based drilling fluids (WBDFs) and oil-based drilling fluids (OBDFs). WBDFs are typically composed of fresh water, bentonite, and other additives [4]. OBDFs are generally formulated with base oils (principally diesel, low-toxicity mineral oils, and synthetic oils), organoclays, and other additives [5]. OBDFs are known for their excellent shale-inhibiting properties, lubricity, and salt resistance [5]. Nevertheless, they are frequently criticized for their high cost and toxicity derived from base oils. In contrast, WBDFs are inexpensive and environmentally friendly. Therefore, WBDFs are widely used, especially in challenging drilling operations and environmentally sensitive locations.
Despite the considerable advantages in terms of cost and environmental care offered by WBDFs, their application still encounters significant obstacles, particularly the difficulty of rheological control. The rheology of drilling fluids, including properties such as the viscosity, yield point, and gel strength, is a critical factor in the efficiency and safety of drilling operations in the oil and gas industry. Drilling fluids usually exhibit non-Newtonian behavior, with shear-thinning characteristics where the viscosity decreases with an increasing shear rate. This rheological behavior is essential for facilitating effective hole cleaning, as it allows the fluid to flow easily near the drill bit while maintaining sufficient viscosity to suspend and transport cuttings. The yield point and gel strength of the fluid further contribute to this by ensuring that cuttings are suspended even at low flow rates and that they do not settle when circulation stops. These properties also play a significant role in pressure control by influencing the equivalent circulating density (ECD), which helps balance hydrostatic pressure to prevent both formation fluid influx and formation fracturing. Consequently, the proper management of drilling fluid rheology is vital for maintaining wellbore stability, minimizing operational issues, and optimizing overall drilling efficiency.
The rheological behaviors of WBDFs are generally governed by bentonite, a natural clay with montmorillonite (Mt) as the primary component [6,7,8,9,10,11,12]. Mt can form a network of interconnected particles, namely a “house of cards” structure when it swells and even exfoliates in water. This property endows it with excellent rheological properties in aqueous systems, making it an invaluable component in WBDFs [6,13,14,15,16,17,18,19,20,21]. However, the properties of Mt-based drilling fluids are severely compromised in saline and high-temperature formations due to the large surface charge density and inadequate stability of Mt [6,18,19,20,22,23,24,25]. While water-soluble polymers, such as xanthan gum and carboxymethyl cellulose, can enhance specific aspects of fluid rheology, such as the low-shear viscosity, fluid loss control, and salt resistance, they are still limited due to several key factors related to performance, cost, and their functional properties. For example, the rheology of polymers is sensitive to temperature changes, especially at low and high temperatures. Therefore, it is urgent to develop new rheological additives for WBDFs with excellent rheological properties, thermal stability, and cost-effectiveness.
In recent years, plenty of new materials have been developed to improve the applied properties of WBDFs. These include improvements to the rheological properties under saline and high-temperature conditions, shale-inhibiting ability, and filtration properties. Nanomaterials are particularly interesting among these additives [26,27,28]. As a consequence, a novel concept, designated “nano-based drilling fluids” (also referred to as “nanomaterials-based drilling fluids” or “nano-enhanced drilling fluids”), is emerging [29,30,31]. Nanomaterials which have been reported for use in WBDFs include graphene nanoplatelets [32,33], nanocomposites [34,35], and nanoclay minerals [36,37,38,39,40,41,42,43,44,45,46]. Nanoclay minerals represent a promising class of additives for WBDFs, offering several advantages, such as their natural origin, environmental friendliness, low cost, and excellent rheological properties.
Nanofibrous clay minerals, specifically palygorskite (Pal) and sepiolite (Sep), are some of the most important rheological additives for high-performance WBDFs. Pal and Sep are unique fibrous clay minerals with distinct structures and properties which make them valuable in various industrial applications. Both minerals have the characteristic of a 2:1 layered structure. However, their fibrous morphology distinguishes them from other 2:1 clay minerals like Mt or illite. Pal has a needle-like structure consisting of ribbons of silica tetrahedra linked by magnesium, aluminum, or iron octahedra, creating channels along its fibers to accommodate water molecules and other ions. Similarly, Sep has a fibrous structure but with a more open, lath-like configuration, allowing for an even higher specific surface area and porosity. These structural characteristics impart several important properties; both minerals exhibit a high specific surface area, strong sorption capacities, and significant thermal stability.
Additionally, their fibrous nature provides excellent rheological properties, particularly in suspensions, which contribute to increased viscosity and gel formation. The ability of Pal and Sep to absorb water and other fluids and their chemical stability make them particularly useful in drilling fluids, where they enhance the fluid’s rheology. They exhibit excellent colloidal and rheological properties, even in saline and high-temperature environments [47]. These two clay minerals also offer notable advantages over other synthetic nanomaterials because they are naturally occurring, cost-effective, and environmentally friendly [48]. Indeed, Pal and Sep have been used in drilling fluids in some cases. They are included in the specifications of the American Petroleum Institute (API) standard. Neaman and Singer [49] conducted a study investigating the rheological properties of three Pal samples sourced from Sacalum (Mexico), Florida (USA), and Georgia (USA). Their findings indicated that the WBDFs with 5% (w/v) Pal exhibited an apparent viscosity (AV) of 16.63–23.97 mPa·s and a yield point (YP) of 13.06–16.39 Pa (at a shear rate of 1000 s−1), which satisfied the API requirements. A recent study [42] demonstrated that Turkish Sep outperformed Wyoming bentonite in WBDFs. The remarkable thickening ability of Pal and Sep in WBDFs contributes to their capacity to transport drill cuttings and suspend weight agents. Aside from this, WBDFs prepared with Pal or Sep also exhibit shear-thinning behavior [40,50,51,52,53], which is highly beneficial for improving the drilling rate and cutting carrying ability. Recent studies have demonstrated that Pal and Sep fluids presented thixotropy [52], with even more pronounced effects than those observed in Wyoming bentonite-based WBDFs [40]. It is postulated that Pal and Sep may be invaluable in overcoming drilling challenges, especially in high-temperature and high-pressure formations, shale formations, and saline formations [54].
Although nanofibrous clay minerals exhibit promising rheological properties in WBDFs, their large-scale application is still constrained by both theoretical and engineering challenges. The use of these clay minerals in drilling fluids is a multidisciplinary issue which requires an interdisciplinary approach, incorporating insights from clay mineralogy, colloid chemistry, rheology, and filtrate control. Therefore, a comprehensive and critical understanding of the current research is essential to clarify fundamental scientific issues, performance regulation, challenges, and future directions for the application of nanofibrous clay minerals in WBDFs. The primary goal of this review is to comprehensively assess the current state of research on the application of nanofibrous clay minerals, specifically Pal and Sep, in WBDFs. With the increasing demand for more efficient and environmentally friendly drilling operations, it is crucial to understand how these unique clay minerals enhance the rheological, thermal, and filtration properties of drilling fluids. This paper aims to synthesize existing knowledge, identify the mechanisms by which Pal and Sep influence drilling fluid performance, and highlight their potential advantages over traditional additives. Moreover, this review will explore the challenges and limitations associated with their usage, providing insights into future research directions which could further optimize their application in the oil and gas industry.
2. Clay Mineralogy
2.1. Crystal Structure
Pal and Sep exhibit a layered structure comprising an octahedral sheet sandwiched by two tetrahedral sheets. The tetrahedral basal oxygen atoms are arranged in a continuous plane at a distance of approximately 6.6 Å. However, in contrast to the ideal 2:1 clay mineral, the apical oxygen atoms of the tetrahedra point away from the basal oxygen atom plane in opposing directions to form ribbons of joined pyroxene-like chains [55]. The apical oxygen atoms of the tetrahedra partially contribute to the formation of the coordination unit of the octahedral sheet. Consequently, there is a region of the structure where channels form adjacent to the basal oxygen planes between two 2:1 layers. The dimensions of these channels are 3.7 × 6.4 Å for Pal and 3.7 × 10.6 Å for Sep (Figure 1) [56]. The key features of Pal and Sep are as follows: (1) continuous tetrahedral basal oxygen planes, (2) an inverted tetrahedral arrangement which forms ribbons of joined pyroxene-like chains, and (3) a discontinuous octahedral sheet. Due to the distinctive structural attributes of Pal and Sep, they are generally regarded as a separate group (i.e., the palygorskite-sepiolite group).
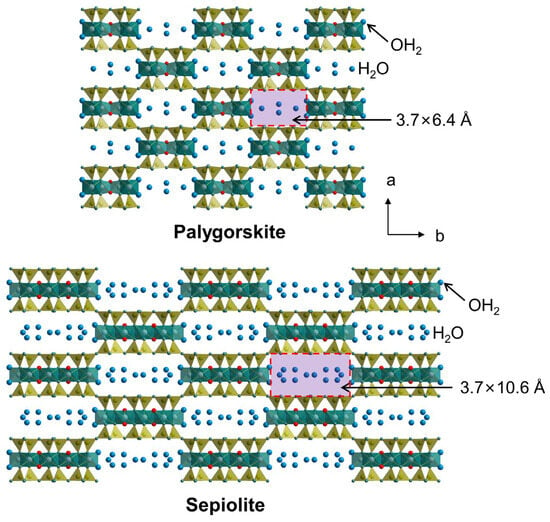
Figure 1.
Structures of Pal and Sep.
2.2. Chemical Composition
The chemical formula of Pal is , and that of Sep is . Si4+ ions predominantly occupy the centers of the tetrahedra, while the octahedral centers are primarily composed of Mg2+ and Al3+ ions. Therefore, Pal and Sep can be classified as Mg-rich aluminum silicate minerals. It should be noted, however, that other cations can replace the cations in the tetrahedra and octahedra. For example, Si4+ ions in the tetrahedra can be substituted with Al3+ ions, and the Al3+ ions in the octahedra can be replaced by Mg2+ and Fe3+ ions. In addition, there are also vacancies and different forms of water present in these two clay minerals’ structures, including hydroxyl (OH), coordinated water (OH2), and zeolitic water (H2O). For Pal, there is a general consensus that the OH groups are part of the octahedral anion coordination of the M1 and M2 sites, which occur well within the octahedral strips. The OH2 is part of the coordination unit around the M3 site along the edges of the octahedral strips, where the two hydrogen atoms are required for charge balance. In the case of Sep, the OH groups are part of the inner octahedral strip coordinating with M1, M2, and M3, whereas the OH2 groups are along the edges of the octahedral strip coordinating with M4, and this pattern is similar to that of Pal. H2O molecules occupy the channels of Pal and Sep, and they can be removed at low temperatures (e.g., below 200 °C).
2.3. Morphology
Pal and Sep are in a group of fibrous clay minerals which exhibit elongated and needle-like morphological features. Scanning and transmission electron microscopy (SEM and TEM) observations revealed that Pal and Sep are fibrous (Figure 2), with fibers developing along the c axis. These fibers, sometimes referred to as rods, are composed of several laths, which are the crystals of Pal and Sep. Aside from this, the laths and fibers often aggregate into bundles, forming a fibrous filamentous structure at the macroscopic level. Although Pal and Sep show fibrous morphological characteristics, samples from different deposits exhibit significant variations in their detailed features. These variations include the fiber length, width, thickness, and aggregation arrangements. Generally, Pal and Sep fibers have lengths of 0.2–2 μm, widths of 10–30 nm, and thicknesses of 5–10 nm [47,56,57].
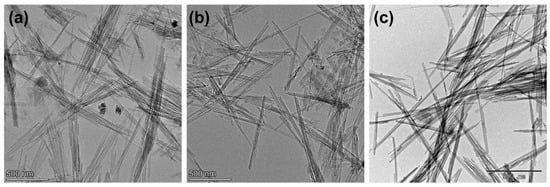
Figure 2.
TEM images of (a) Pal from Georgia, USA, (b) Pal from Mingguang, China, and (c) Sep from Spain.
2.4. Properties and Applications
Pal and Sep can be classified into two principal categories, namely colloidal and non-colloidal applications. Non-colloidal applications are primarily composed of fillers and adsorbents, which are mainly evaluated based on their color and adsorption capacity. Pal and Sep are mainly composed of O, Si, Al, and Mg, resulting in a predominantly white-gray color. However, they may exhibit a yellow-red hue when the Fe content is elevated. The color of Pal and Sep usually only affects the appearance of products, with no significant effect on their application properties. The adsorption capacity of Pal and Sep is primarily determined by their specific surface areas and pore volumes. Due to their nanofibrous morphology and abundance of micropores, Pal and Sep exhibit high specific surface areas, generally with values of 150 m2/g and 300 m2/g, respectively. The colloidal applications of Pal and Sep are based on their surface hydrophilicity and nanofibrous morphology. The numerous hydrophilic groups on the surface of Pal and Sep, including silanol and aluminol groups, facilitate the dispersion of clay mineral nanofibers in water, forming a haystack structure. This structure gives Pal and Sep remarkable rheological properties [47,58,59,60,61].
3. Rheological Properties of Pal and Sep in WBDFs
3.1. Network Formation Mechanism
The network formation mechanism of fibrous clay minerals in water is primarily driven by their distinctive crystal structure and surface chemistry. These minerals consist of elongated, needle-like fibers with high aspect ratios, which enable them to form intricate, three-dimensional networks when dispersed in an aqueous medium. Upon hydration, the surfaces of these fibers become charged due to the isomorphic substitution of cations and the dissociation of surface hydroxyl groups, leading to the development of an electrostatic double layer [53,62]. This charged surface facilitates repulsion among the fibers, preventing aggregation and promoting a stable suspension. As the concentration of these clay minerals increases in water, the fibers interact through van der Waals forces and hydrogen bonding, creating a physically entangled network. This network is further stabilized by the high specific surface area and the presence of exchangeable cations, which can bridge the fibers and enhance the network’s connectivity. The resulting structure significantly influences the rheological properties of the dispersion, imparting high viscosity and yield stress, which are advantageous for various applications, including WBDFs. Factors such as the pH level, ionic strength, and the presence of other additives can modify interparticle interactions and, consequently, the dispersion’s rheological behavior. Additionally, the fibrous network exhibits thixotropic behavior [40,42,52], where the structure aligns and disentangles under shear, reducing viscosity, and then reassembles when the shear is removed, ensuring stability and flowability. These properties make Pal and Sep highly effective in stabilizing suspensions and controlling the rheology of WBDFs, enhancing their utility in high-performance drilling operations.
However, the mechanism of network formation of Pal and Sep in an aqueous system is still unclear. On the one hand, conventional SEM or TEM cannot observe the real structure of aqueous samples in situ. Although cryo-SEM offers a novel approach to studying the microstructure of liquid samples, the results do not fully reflect the actual clay mineral dispersions. On the other hand, the formation mechanism of the fibrous clay mineral network structure is mainly based on an aqueous system without electrolytes. The network structure of these clay minerals under different electrolyte conditions is still unclear. Since a drilling fluid is a complex multi-phase dispersion system, understanding the network structure of the minerals themselves and the influence of other additives or contaminants (particularly electrolytes) is essential for an appreciation of the rheological properties of drilling fluids.
3.2. Influence of Clay Concentration
The concentration of Pal or Sep significantly influences the rheological behavior of water-based drilling fluids due to their unique fibrous structure and ability to form interconnected networks within the fluid. As the concentration of these nanofibrous clay minerals increases, the degree of network formation intensifies, leading to a more pronounced impact on the fluid’s viscosity and yield stress. At low concentrations, the number of nanofibers is insufficient to form a network structure [63] (Figure 3). Thus, the limited number of nanofibers does not apparently affect the flow behavior of drilling fluids. For example, the Pal fluid exhibits nearly Newtonian flow at a lower concentration of 1% (w/v), but the flow becomes pseudoplastic as the suspension concentration increases [50]. The shear rate threshold at which the flow transitions from non-Newtonian to linear depends on the clay concentration. High clay concentrations result in a preference for non-Newtonian fluids. The viscosity and gel strength of Pal and Sep in WBDFs typically increase with an increasing clay concentration [50,53,63,64] due to the increased internal friction of the fluid caused by the presence of a greater number of clay mineral particles. Thus, the concentration of Pal or Sep directly correlates with the thickening and gelation properties of the drilling fluid, influencing its performance in various drilling operations. However, the concentration of nanofibrous clay minerals added to WBDFs is not fixed; it varies with the origin, mineral content, physicochemical properties, and drilling fluid formulation.
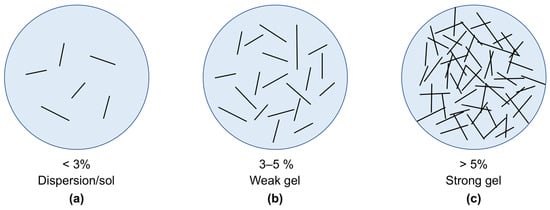
Figure 3.
Influence of the fibrous clay minerals’ concentration on the gel structure: (a) a dispersion or sol at a very low concentration, (b) a weak gel at a medium concentration, and (c) a strong gel at a high concentration.
3.3. Influence of Morphology and Fiber Arrangements
The morphology and fiber arrangements of Pal and Sep significantly influence the rheological behavior of their dispersions. Neaman and Singer [50] found that the viscosities and gel strengths of several Pal dispersions at a pH value of seven were linearly related to the ellipticity (L/W ratio) of the fibers. Later, Balter et al. demonstrated that Pal with longer fibers produced a greater viscosity in both fresh and saline water [65]. The length-to-width (L/W) ratio of the fibers is a crucial determinant of viscosity, with higher L/W ratios correlating with increased viscosity due to the greater surface area for interaction and entanglement, which facilitates a robust network structure within the fluid. This network formation, enhanced by longer fibers, increases internal friction, thereby elevating the viscosity and yield point. Effective dispersion of fiber bundles into individual fibers without breaking them is essential for maintaining high L/W ratios and achieving optimal rheological properties. Furthermore, the interactions between fibers, such as entanglement and electrostatic attractions, are more pronounced with higher L/W ratios, contributing to a stronger network. The application of higher shear rates during processing can also enhance fiber dispersion and alignment, promoting better network formation and improved rheological behavior. In summary, the rheological properties of Pal and Sep dispersions are intricately linked to their fiber morphology and arrangements, with longer, thinner fibers forming more effective networks which result in a higher viscosity and higher yield points. Proper processing techniques which preserve these morphological characteristics are essential for optimizing the performance of Pal and Sep in drilling fluids.
Since Pal and Sep nanofibers are typically found in bulk bundles, separating the fibers through special processing methods is essential. These include physical methods [66,67,68,69,70,71,72] and chemical methods [73,74,75]. For example, extrusion, slurring, surface modification, and high-pressure homogenization are effective methods for separating the fibers. Notably, excessive mechanical action may break the fibers into shorter ones, which is detrimental to the colloidal use of Pal and Sep [76]. It is therefore recommended that the disaggregation and dispersion of crystal bundles be carried out in a way that does not damage the L/W ratio. In the case of WBDFs, it was demonstrated that the dispersion of Pal or Sep at elevated shear rates resulted in enhanced rheological properties [77].
3.4. Influence of pH Conditions
The pH conditions significantly influence the rheological properties of Pal or Sep dispersions. On the one hand, the pH condition affects the flow behavior of the clay mineral dispersions. For example, Pal behaves as a nearly Newtonian fluid under basic conditions, whereas it behaves as a non-Newtonian fluid under acidic conditions [50]. On the other hand, the pH level regulates the viscosity of the fluid by affecting the association of nanofibers. A recent study [75] reported that the viscosity of Pal dispersions increased when the pH value increased from 1 to 11 but decreased when the pH value exceeded 12. Similarly, Liu et al. [53] observed that the yield stress of Sep dispersions slightly increased as the pH level increased from 2 to 8 and then decreased at pH values greater than 8. Sep dispersions typically exhibit zero yield stress at pH values above 11 [78,79]. The pH level influences the colloidal and rheological properties of Pal and Sep dispersions by altering the surface charge and fiber aggregation. The surface charge of Pal and Sep fibers is pH-dependent, affecting the electrostatic interactions between fibers. According to the literature [50,53,80,81], the point of zero charges (PZC) of Pal and Sep is usually between pH levels of 2 and 5. At a pH value equal to the PZC, the net electrostatic charge of the clay surface is zero. In this configuration, the van der Waals forces predominate the interaction between the nanofibers, resulting in the clay fibers being arranged in a parallel configuration [50]. At a pH level slightly above the PZC (e.g., approximately seven), Pal and Sep exhibit the optimal rheological properties. This is attributed to a dynamic equilibrium between the van der Waals forces and electrostatic repulsion between the nanofibers, resulting in a weak attraction between the nanofibers to form a network structure while maintaining reasonable repulsion to prevent a parallel arrangement (Figure 4). However, when the pH value is significantly greater than the PZC (e.g., above 11) or significantly smaller than the PZC, the nanofibers are endowed with many negative or positive charges on their surface. In this case, the electrostatic repulsive forces dominate, making the nanofibers entirely dispersed [79]. As the colloidal and rheological behaviors of Pal and Sep suspensions are less affected by common pH conditions (pH = 4–11), Pal and Sep are more popular in WBDFs for saline formations or offshore drilling. In practice, the PZC, as well as other properties of different Pal and Sep dispersions, may vary considerably. The response of these fibrous clay minerals to the pH conditions may also be different. Therefore, screening of different mineral resources is required.
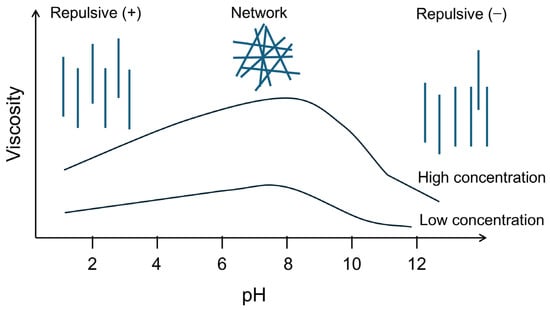
Figure 4.
General description of the influence of the pH level on the viscosity of nanofibrous clay minerals.
3.5. Influence of Electrolytes
Although Pal- and Sep-based WBDFs have better salt resistance than those fluids based on Mt, the effect of electrolytes on the rheological properties of Pal and Sep fluids cannot be ignored. Generally, the salt resistance of Pal and Sep is related to the surface charge and pH conditions. The rheological behavior of minerals with smaller charge densities is less affected by the electrolytes, whereas those with larger charge densities exhibit a more pronounced response. Typically, Pal and Sep exhibit the best salt resistance between pH levels of 4 and 8 [53]. In summary, electrolytes influence the rheological behaviors of Pal and Sep dispersions by compressing the electrical double layer, reducing electrostatic repulsion, and enhancing van der Waals attraction, which leads to increased fiber aggregation and network formation [81].
It is worth noting that although Pal and Sep have better salt resistance in WBDFs, most of the previous studies have been conducted using single-electrolyte systems (e.g., NaCl or KCl). There is a lack of comprehensive studies on systems with complex salts (e.g., brine-based drilling fluids and seawater-based drilling fluids). In recent years, brine-based and seawater-based drilling fluids have become increasingly crucial high-performance drilling fluids. Therefore, it is necessary to carry out an in-depth study on the rheological behavior and rheology control mechanism of fibrous clay minerals in complex electrolyte systems.
3.6. Influence of Temperatures
The extraction of oil and gas from deep and ultra-deep earth has increased steadily in recent years [82,83]. As the depth of a reservoir location increases, the corresponding pressure and temperature also increase. Reservoirs are classified as normal, high-temperature and high-pressure (HTHP) (150 °C, 68.95 MPa), ultra-HPHT (205 °C, 137.9 MPa), and extremely HPHT (260 °C, 241.32 MPa) reservoirs [84]. Consequently, drilling operations in HTHP fields are inherently risky and challenging. The management of rheological and filtration properties represents a significant factor contributing to the high cost of HTHP drilling operations [85].
Pal and Sep exhibit good thermal stability in WBDFs due to their unique fibrous structures. Studies have shown that Pal and Sep maintain their structural integrity and rheological properties at temperatures up to 200 °C [36,40,41,42,47,51,59,86,87,88,89]. This thermal stability ensures that WBDFs retain their viscosity and gel strength, which are essential for effective cutting transport and wellbore stability. However, the thermal stability of these clay minerals can be affected by the presence of electrolytes and other additives in a drilling fluid. Electrolytes can alter the surface charge of the clay particles, potentially leading to changes in colloidal interactions and aggregation behavior at elevated temperatures [90]. Although Pal and Sep show remarkable rheological properties at temperatures below 200 °C, the thermal stability of Pal and Sep in real WBDFs at higher temperatures (e.g., 250 °C) is unclear. In addition, high-temperature wells are generally accompanied by high pressure. Therefore, it is necessary to consider the effects of both temperature and pressure on the rheological behavior of these two clay minerals, especially at ultra-high temperatures.
4. Filtration Properties
The filtration properties of Pal and Sep in WBDFs are critical for maintaining wellbore stability and minimizing fluid loss into the formation. Studies have shown that Pal and Sep present more considerable fluid loss than Mt in WBDFs [9,38,41,63,86,87,91,92,93,94]. For instance, a real WBDF prepared with 4% Pal showed a fluid loss of 26.6 mL after aging at 180 °C for 16 h, whereas a control drilling fluid with 4% commercial bentonite exhibited a fluid loss of 16.4 mL [93]. This is due to the fibrous characteristic of Pal and Sep, which forms a filter cake with many pores that allow water to permeate easily (Figure 5).
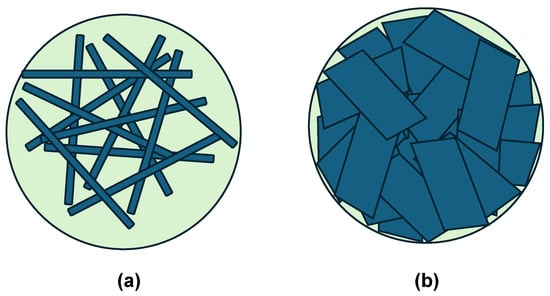
Figure 5.
Diagrams of the filter cake structures of (a) nanofibrous clay minerals and (b) layered Mt.
In order to improve the filtration properties of Pal and Sep in WBDFs, filtration control additives, such as cellulose, starches, and humic acids, are generally considered to be used [86,90,95]. It has been shown that adding polymeric filter loss reducers improves the filtration loss of Pal and Sep in WBDFs significantly, especially at high temperatures [89,96,97,98]. The use of polymers in drilling fluids is already widespread, and they are mainly used as rheology modifiers and fluid loss reducers. In controlling filtration loss, polymers generally interact with the surfaces of clay minerals to make the structure of the filter cake more compact, thereby preventing the loss of liquid. However, previous studies have all been about the interaction between Mt and polymers. Pal and Sep are quite different from Mt in terms of structure and properties. There is a need for detailed studies on the interaction of nanofibrous clay minerals with typical filter loss-reducing agents and their influence on the properties of a filter cake and the amount of fluid loss. Developing special filter loss-reducing agents for drilling fluids may be necessary based on Pal and Sep. Furthermore, it was demonstrated that the addition of nano Sep to a conventional WBDF (with Mt as the main rheological additive) could improve the overall stability of the drilling fluid by enhancing the dispersion and suspension of fine particles, thereby forming a low-permeability filter cake to decrease fluid loss.
5. Method of Applying Pal and Sep in WBDFs
Pal and Sep are used in WBDFs in a similar way to bentonite (i.e., clay minerals are directly mixed with other additives in water). Unlike bentonite, Pal and Sep do not need to be modified with soda or other sodium salts. Despite the remarkable rheological properties and thermal stability in WBDFs, their use as standalone agents is not recommended due to insufficient fluid loss control. Another reason is that Pal and Sep may display worse rheological properties than Mt in an aqueous system [99,100]. Pal and Sep are typically incorporated into WBDFs at a concentration based on Mt, as they have been demonstrated to enhance the salt resistance of drilling fluids. Therefore, in order to balance the rheological properties of Mt with the salt resistance of fibrous clay minerals, a mixture of layered Mt and fibrous clay minerals is generally used. It has been demonstrated that this method can effectively improve the rheological properties and stability of WBDFs under HTHP conditions [36,38,39,93,101,102]. The synergetic use of fibrous and layered clay minerals in WBDFs is typically achieved by combining the two types of clay minerals independently. However, natural Mt may be deposited with some Pal or Sep impurities in some situations. Such blended clays can be directly employed as the rheological additives for WBDFs, improving the rheological properties of drilling fluids [103]. This is important when using mixed clay resources because separation and purification may be unnecessary.
Although the use of layered and nanofibrous clay minerals in combination represents a promising avenue for enhancing the rheological and filtration characteristics of WBDFs, the optimal proportion and ultimate properties are contingent upon the provenance, physical attributes, and processing techniques employed for the clay minerals. For example, Alghareeb [9] found that adding Sep increased the viscosity and yield stress of bentonite dispersion, contrary to the findings of previous investigations [99,100]. Therefore, gaining a deeper understanding of the microstructure and colloidal forces which govern the clay mineral complexes in drilling fluids is essential. Unfortunately, a detailed study of the microstructure of mixed-layer fibrous clay mineral dispersions has yet to be conducted. Recently, the microstructure of mixed Laponite-Sep dispersions was revealed [79], and this may be helpful in understanding the microstructures of Mt-Pal and Mt-Sep mixtures. In that report, nanofibers were observed to be randomly orientated and interact attractively in a cross-fiber configuration, forming a three-dimensional network structure. The predominant interaction configuration appeared to be between the positive edges of Laponite layers (or flaky particles) and the negative silica face of Sep fibers. Mt probably interacts with Pal or Sep following a similar mechanism. However, it should be noticed that Mt particles are much larger than Laponite flakes (Figure 6). The Mt-Pal and Mt-Sep interactions may differ from the Laponite-Sep interaction. Consequently, further investigations are required to elucidate the microstructures and colloidal chemistry of Mt-Pal and Mt-Sep mixtures in WBDFs.
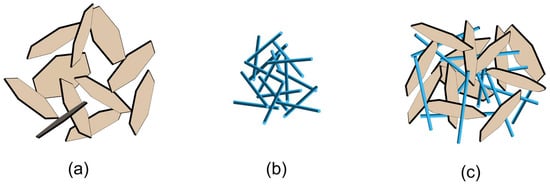
Figure 6.
Structures of (a) Mt dispersion, (b) fibrous clay mineral dispersion, and (c) mixed-layer fibrous clay mineral dispersion.
6. Challenges
6.1. Cost
One of the significant challenges in the application of nanofibrous clay minerals in WBDFs is the cost. Although these minerals offer unique properties which can enhance drilling fluid performance, their production and processing costs can be relatively high compared with conventional additives. For example, the prices of Pal and Sep usually range from USD 200 to 600 per ton, while bentonite ranges from USD 50 to 150 per ton. In addition, the refinement and activation processes for optimizing the rheological and filtration properties of nanofibrous clay minerals also increase the total cost. Moreover, scaling up from laboratory to industrial production can introduce further financial barriers. In order to make these nanofibrous minerals more economically viable, it is necessary to develop cost-effective processing techniques and gain a deeper understanding of how to maximize their performance with minimal quantities.
6.2. Demand and Supply
The demand for Pal and Sep in the drilling industry is growing as operators seek more efficient and environmentally friendly drilling fluid additives. However, the supply of these minerals can be inconsistent, depending on geographical availability and mining conditions. Pal and Sep deposits are not uniformly distributed worldwide, leading to potential supply chain issues, especially in regions where these minerals are not readily available. This uneven distribution can cause price volatility and supply bottlenecks, hindering widespread adoption. More importantly, the properties of natural minerals give rise to enormous variations in their genesis, deposits, and chemical compositions. Such variations can further reinforce the imbalance between supply and demand for Pal and Sep.
6.3. Environmental Concerns
The environmental impact of using nanofibrous clay minerals in drilling fluids is a critical consideration. While these minerals are naturally occurring and generally considered environmentally benign, the processes involved in their extraction, refinement, and disposal can raise environmental concerns. For instance, acids or surfactants may be employed to remove impurities (e.g., carbonates and quartz). The mining of Pal and Sep can lead to land degradation and habitat destruction if not managed sustainably. Additionally, the disposal of drilling fluids containing these minerals poses challenges, particularly in terms of potential contamination of soil and water bodies. Research into more sustainable mining practices, along with the development of biodegradable or easily recyclable drilling fluid formulations, is necessary to address these environmental concerns.
6.4. Technical Requirements
From a technical perspective, the integration of nanofibrous clay minerals into water-based drilling fluids requires careful consideration of several factors, including compatibility with other fluid components, optimal concentration levels, and the impact on fluid properties under various drilling conditions (particularly under high temperatures and high pressures). The unique fibrous structure of Pal and Sep can significantly influence the rheological behavior of drilling fluids, but achieving the desired balance of viscosity, yield stress, and filtration control is complex. Moreover, the effectiveness of these minerals can vary depending on the specific conditions of the drilling operation, such as temperature, pressure, and salinity. Addressing these technical challenges demands extensive research and field trials to develop standardized formulations which can consistently deliver the desired performance across different drilling scenarios.
6.5. Field Application
Field application of nanofibrous clay minerals in drilling fluids presents both opportunities and challenges. While laboratory studies have demonstrated their potential benefits, translating these findings into real-world drilling operations requires overcoming practical obstacles. Deployment of these minerals in the field must account for the variability in drilling environments, including different geological formations and operational parameters. Ensuring consistent dispersion and performance in large-scale operations requires the development of specialized equipment and procedures. Furthermore, real-time monitoring and adjustment of drilling fluid properties are essential to optimize the use of Pal and Sep in diverse field conditions. Despite these challenges, successful field applications can lead to significant improvements in drilling efficiency and environmental compliance.
7. Conclusions and Perspectives
Overall, the application of nanofibrous clay minerals, specifically Pal and Sep, in water-based drilling fluids presents significant potential for enhancing the performance and sustainability of drilling operations. These minerals offer unique rheological properties, thermal stability, and filtration control which can improve the efficiency of drilling fluids under a variety of challenging conditions. However, their widespread adoption is currently limited by several factors, including high costs, supply chain variability, environmental concerns, and technical challenges related to their integration into drilling fluid formulations. Despite these obstacles, ongoing research and technological advancements suggest that these challenges can be mitigated with appropriate strategies, paving the way for broader application in the industry.
Regarding weaknesses and challenges, the following suggestions are proposed:
- (1)
- It is recommended that future research focuses on developing more cost-effective processing methods for Pal and Sep. This could include optimizing the refinement processes to reduce energy consumption and exploring the potential of using smaller quantities of these minerals in drilling fluid formulations without compromising performance. Furthermore, developing new processing technologies to enhance low-grade or underperforming minerals is essential to reducing costs and ensuring a sufficient supply.
- (2)
- To mitigate environmental concerns, it is crucial to promote sustainable mining practices which minimize the ecological footprint of extracting Pal and Sep. Additionally, research should explore the development of environmentally friendly drilling fluid formulations which incorporate these minerals. This could include designing biodegradable or easily recyclable fluids, thereby reducing the environmental impact of their disposal.
- (3)
- In terms of technical requirements, further research is needed to fully understand the interactions between nanofibrous clay minerals and other drilling fluid components. Developing standardized formulations which are optimized for different drilling conditions will be essential for ensuring consistent performance in the field. Collaboration between academia, industry, and field operators could accelerate the development of these standardized formulations and their testing in real-world conditions.
Author Contributions
Conceptualization, G.Z.; writing—original draft preparation, G.Z., J.Z., and J.C.; writing—review and editing, Q.L. (Qian Liu), W.F., and Q.L. (Qiang Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangdong Basic and Applied Basic Research Foundation (grant number 2019A1515110263), and the APC was funded by Guangdong University of Technology.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Q.; Zhuang, G.; Yuan, P.; Bergaya, F. Chapter 12—Future challenges related to clay minerals in drilling and drilling fluids. In Clay Science in Drilling and Drilling Fluids; Zhuang, G., Yuan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 313–338. [Google Scholar]
- Zhuang, G.; Li, Q.; Bergaya, F.; Yuan, P. Chapter 1—The significance of clay minerals in drilling and drilling fluids. In Clay Science in Drilling and Drilling Fluids; Zhuang, G., Yuan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–19. [Google Scholar]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Chapter 1—Introduction to Drilling Fluids. In Composition and Properties of Drilling and Completion Fluids, 7th ed.; Caenn, R., Darley, H.C.H., Gray, G.R., Eds.; Gulf Professional Publishing: Boston, MA, USA, 2017; pp. 1–34. [Google Scholar]
- Tabatabaee Moradi, S.S. Development of a water-based drilling fluid for chemical enhancement of drilling rate in a dolomite rock sample. J. Pet. Sci. Eng. 2022, 216, 110768. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Jaber, M. Organoclays used as colloidal and rheological additives in oil-based drilling fluids: An overview. Appl. Clay Sci. 2019, 177, 63–81. [Google Scholar] [CrossRef]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Kok, M.V. Rheological and thermal analysis of bentonites for water base drilling fluids. Energy Sources 2004, 26, 145–151. [Google Scholar]
- Xu, T.; Bezuijen, A. Bentonite slurry infiltration into sand: Filter cake formation under various conditions. Géotechnique 2019, 69, 1095–1106. [Google Scholar] [CrossRef]
- Alghareeb, A. A Review on Controlling Bentonite-Based Drilling Mud Properties. Int. J. Archit. Energy Urban. 2020, 1, 44–55. [Google Scholar]
- Vipulanandan, C.; Mohammed, A. Effect of drilling mud bentonite contents on the fluid loss and filter cake formation on a field clay soil formation compared to the API fluid loss method and characterized using Vipulanandan models. J. Pet. Sci. Eng. 2020, 189, 107029. [Google Scholar] [CrossRef]
- Temraz, M.G.; Hassanien, I. Mineralogy and rheological properties of some Egyptian bentonite for drilling fluids. J. Nat. Gas Sci. Eng. 2016, 31, 791–799. [Google Scholar] [CrossRef]
- Larsen, D.H. Use of Clay in Drilling Fluids. Clays Clay Miner. 1952, 1, 269–281. [Google Scholar] [CrossRef]
- Leong, Y.K.; Du, M.; Au, P.I.; Clode, P.; Liu, J. Microstructure of Sodium Montmorillonite Gels with Long Aging Time Scale. Langmuir 2018, 34, 9673–9682. [Google Scholar] [CrossRef]
- Au, P.-I.; Du, M.; Liu, J.; Haq, M.B.; Leong, Y.-K. Surface chemistry, rheology and microstructure of as-received SHCa-1 hectorite gels. Clay Miner. 2019, 54, 269–275. [Google Scholar] [CrossRef]
- Du, M.; Liu, P.; Clode, P.L.; Liu, J.; Haq, B.; Leong, Y.-K. Impact of additives with opposing effects on the rheological properties of bentonite drilling mud: Flow, ageing, microstructure and preparation method. J. Pet. Sci. Eng. 2020, 192, 107282. [Google Scholar] [CrossRef]
- Du, M.; Liu, P.; Wong, J.-E.; Clode, P.L.; Liu, J.; Leong, Y.-K. Colloidal forces, microstructure and thixotropy of sodium montmorillonite (SWy-2) gels: Roles of electrostatic and van der Waals forces. Appl. Clay Sci. 2020, 195, 105710. [Google Scholar] [CrossRef]
- Caenn, R.; Darley, H.C.H.; Gray, G.R. Chapter 4—Clay Mineralogy and the Colloid Chemistry of Drilling Fluids. In Composition and Properties of Drilling and Completion Fluids, 7th ed.; Caenn, R., Darley, H.C.H., Gray, G.R., Eds.; Gulf Professional Publishing: Boston, MA, USA, 2017; pp. 93–134. [Google Scholar]
- Kelessidis, V.C.; Christidis, G.; Makri, P.; Hadjistamou, V.; Tsamantaki, C.; Mihalakis, A.; Papanicolaou, C.; Foscolos, A. Gelation of water–bentonite suspensions at high temperatures and rheological control with lignite addition. Appl. Clay Sci. 2007, 36, 221–231. [Google Scholar] [CrossRef]
- Kelessidis, V.C.; Tsamantaki, C.; Dalamarinis, P. Effect of pH and electrolyte on the rheology of aqueous Wyoming bentonite dispersions. Appl. Clay Sci. 2007, 38, 86–96. [Google Scholar] [CrossRef]
- Kelessidis, V.C.; Tsamantaki, C.; Michalakis, A.; Christidis, G.E.; Makri, P.; Papanicolaou, K.; Foscolos, A. Greek lignites as additives for controlling filtration properties of water–bentonite suspensions at high temperatures. Fuel 2007, 86, 1112–1121. [Google Scholar] [CrossRef]
- Kelessidis, V.C.; Maglione, R. Yield stress of water–bentonite dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 217–226. [Google Scholar] [CrossRef]
- Chen, J.S.; Cushman, J.H.; Low, P.F. Rheological Behavior of Na-Montmorillonite Suspensions at Low Electrolyte Concentration. Clays Clay Miner. 1990, 38, 57–62. [Google Scholar] [CrossRef]
- Santoyo, E.; Santoyo-Gutiérrez, S.; García, A.; Espinosa, G.; Moya, S.L. Rheological property measurement of drilling fluids used in geothermal wells. Appl. Therm. Eng. 2001, 21, 283–302. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Zhou, F.; Amutenya Evelina, L.M.; Long, W.; Chen, S.; He, L.; Yi, X.; Yang, X. Evaluation method of thermal stability of bentonite for water-based drilling fluids. J. Pet. Sci. Eng. 2022, 208, 109239. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Sun, Y.; Lin, D.; Feng, X.; Wang, F. Insights into the high temperature-induced failure mechanism of bentonite in drilling fluid. Chem. Eng. J. 2022, 445, 136680. [Google Scholar] [CrossRef]
- Afolabi, R.O.; Orodu, O.D.; Seteyeobot, I. Predictive modelling of the impact of silica nanoparticles on fluid loss of water based drilling mud. Appl. Clay Sci. 2018, 151, 37–45. [Google Scholar] [CrossRef]
- Cheraghian, G.; Wu, Q.; Mostofi, M.; Li, M.-C.; Afrand, M.; Sangwai, J.S. Effect of a novel clay/silica nanocomposite on water-based drilling fluids: Improvements in rheological and filtration properties. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 339–350. [Google Scholar] [CrossRef]
- Elkatatny, S.; Kamal, M.S.; Alakbari, F.; Mahmoud, M. Optimizing the rheological properties of water-based drilling fluid using clays and nanoparticles for drilling horizontal and multi-lateral wells. Appl. Rheol. 2018, 28, 43606. [Google Scholar]
- Aftab, A.; Ismail, A.R.; Ibupoto, Z.; Akeiber, H.; Malghani, M. Nanoparticles based drilling muds a solution to drill elevated temperature wells: A review. Renew. Sustain. Energy Rev. 2017, 76, 1301–1313. [Google Scholar] [CrossRef]
- Vryzas, Z.; Kelessidis, V.C. Nano-Based Drilling Fluids: A Review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- Ali, M.; Jarni, H.H.; Aftab, A.; Ismail, A.R.; Saady, N.M.C.; Sahito, M.F.; Keshavarz, A.; Iglauer, S.; Sarmadivaleh, M. Nanomaterial-Based Drilling Fluids for Exploitation of Unconventional Reservoirs: A Review. Energies 2020, 13, 3417. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Ceriotti, G.; Wilson, K.C.; Lomeda, J.R.; Scorsone, J.T.; Patel, A.D.; Friedheim, J.E.; Tour, J.M. Graphene oxide as a high-performance fluid-loss-control additive in water-based drilling fluids. ACS Appl. Mater. Interfaces 2012, 4, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ridha, S.; Ibrahim, A.; Shahari, R.; Fonna, S. Graphene nanoplatelets as high-performance filtration control material in water-based drilling fluids. IOP Conf. Ser.-Mater. Sci. Eng. 2018, 352, 012025. [Google Scholar] [CrossRef]
- Sadeghalvaad, M.; Sabbaghi, S. The effect of the TiO2/polyacrylamide nanocomposite on water-based drilling fluid properties. Powder Technol. 2015, 272, 113–119. [Google Scholar] [CrossRef]
- Aftab, A.; Ali, M.; Arif, M.; Panhwar, S.; Saady, N.M.C.; Al-Khdheeawi, E.A.; Mahmoud, O.; Ismail, A.R.; Keshavarz, A.; Iglauer, S. Influence of tailor-made TiO2/API bentonite nanocomposite on drilling mud performance: Towards enhanced drilling operations. Appl. Clay Sci. 2020, 199, 105862. [Google Scholar] [CrossRef]
- Abdo, J.; Haneef, M.D. Nanomaterials Modified Drilling Fluid for Improving Deep Drilling Conditions. J. Energy Resour. Technol. 2022, 144, 073202. [Google Scholar] [CrossRef]
- Abdo, J. Nano-attapulgite for improved tribological properties of drilling fluids. Surf. Interface Anal. 2014, 46, 882–887. [Google Scholar] [CrossRef]
- Abdo, J.; AL-Sharji, H.; Hassan, E. Effects of nano-sepiolite on rheological properties and filtration loss of water-based drilling fluids. Surf. Interface Anal. 2016, 48, 522–526. [Google Scholar] [CrossRef]
- Al-Malki, N.; Pourafshary, P.; Al-Hadrami, H.; Abdo, J. Controlling bentonite-based drilling mud properties using sepiolite nanoparticles. Pet. Explor. Dev. 2016, 43, 717–723. [Google Scholar] [CrossRef]
- Ettehadi, A. A Comparative Study on Thixotropic Behavior of Clay Based Drilling Fluids. Annu. Trans. Nord. Rheol. Soc. 2020, 28, 99–107. [Google Scholar]
- Ettehadi, A. An Experimental Study on Structural and Thermal Stability of Water-Based Drilling Fluids. Eur. J. Sci. Technol. 2021, 23, 70–80. [Google Scholar]
- Ettehadi, A.; Ülker, C.; Altun, G. Nonlinear viscoelastic rheological behavior of bentonite and sepiolite drilling fluids under large amplitude oscillatory shear. J. Pet. Sci. Eng. 2022, 208, 109210. [Google Scholar] [CrossRef]
- Weng, J.; Gong, Z.; Liao, L.; Lv, G.; Tan, J. Comparison of organo-sepiolite modified by different surfactants and their rheological behavior in oil-based drilling fluids. Appl. Clay Sci. 2018, 159, 94–101. [Google Scholar] [CrossRef]
- Buriti, B.; Barsosa, M.E.; Buriti, J.D.; Cartaxo, J.D.; Ferreira, H.S.; Neves, G.D. Modification of palygorskite with cationic and nonionic surfactants for use in oil-based drilling fluids. J. Therm. Anal. Calorim. 2022, 147, 2935–2945. [Google Scholar] [CrossRef]
- Huang, X.; Shen, H.; Sun, J.; Lv, K.; Liu, J.; Dong, X.; Luo, S. Nanoscale Laponite as a Potential Shale Inhibitor in Water-Based Drilling Fluid for Stabilization of Wellbore Stability and Mechanism Study. ACS Appl. Mater. Interfaces 2018, 10, 33252–33259. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-B.; Sun, J.-S.; Huang, Y.; Yan, B.-C.; Dong, X.-D.; Liu, F.; Wang, R. Laponite: A promising nanomaterial to formulate high-performance water-based drilling fluids. Pet. Sci. 2021, 18, 579–590. [Google Scholar] [CrossRef]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Bergaya, F.; Yuan, P. Chapter 3—Application of fibrous clay minerals in water-based drilling fluids. In Clay Science in Drilling and Drilling Fluids; Zhuang, G., Yuan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 55–82. [Google Scholar]
- Neaman, A.; Singer, A. Possible use of the Sacalum (Yucatan) palygorskite as drilling muds. Appl. Clay Sci. 2004, 25, 121–124. [Google Scholar] [CrossRef]
- Neaman, A.; Singer, A. Rheological properties of aqueous suspensions of palygorskite. Soil Sci. Soc. Am. J. 2000, 64, 427–436. [Google Scholar] [CrossRef]
- Echt, T.; Plank, J. An improved test protocol for high temperature carrying capacity of drilling fluids exemplified on a sepiolite mud. J. Nat. Gas Sci. Eng. 2019, 70, 102964. [Google Scholar] [CrossRef]
- García-Villén, F.; Sánchez-Espejo, R.; López-Galindo, A.; Cerezo, P.; Viseras, C. Design and characterization of spring water hydrogels with natural inorganic excipients. Appl. Clay Sci. 2020, 197, 105772. [Google Scholar] [CrossRef]
- Liu, P.; Du, M.; Clode, P.; Li, H.; Liu, J.; Leong, Y.K. Surface Chemisty, Microstructure, and Rheology of Thixotropic 1-D Sepiolite Gels. Clays Clay Miner. 2020, 68, 9–22. [Google Scholar] [CrossRef]
- Choupani, M.A.; Tabatabaee Moradi, S.S.; Tabatabaei Nejad, S.A. Study on Attapulgite as Drilling Fluid Clay Additive in Persian Gulf Seawater. Int. J. Eng. 2022, 35, 587–595. [Google Scholar]
- Guggenheim, S.; Krekeler, M.P.S. Chapter 1—The Structures and Microtextures of the Palygorskite–Sepiolite Group Minerals. In Developments in Clay Science; Galàn, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–32. [Google Scholar]
- Álvarez, A.; Santarén, J.; Esteban-Cubillo, A.; Aparicio, P. Chapter 12—Current Industrial Applications of Palygorskite and Sepiolite. In Developments in Clay Science; Galàn, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 281–298. [Google Scholar]
- García-Romero, E.; Suárez, M. Sepiolite–palygorskite: Textural study and genetic considerations. Appl. Clay Sci. 2013, 86, 129–144. [Google Scholar] [CrossRef]
- Murray, H.H. Applied clay mineralogy today and tomorrow. Clay Miner. 1999, 34, 39–49. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Haden, W. Attapulgite: Properties and Uses. Clays Clay Miner. 1961, 10, 284–290. [Google Scholar] [CrossRef]
- Haden, W.L.; Schwint, I.A. Attapulglte: Its Properties and Applications. Ind. Eng. Chem. 1967, 59, 58–69. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Z.; Han, F. Effects of pH on the gel properties of montmorillonite, palygorskite and montmorillonite-palygorskite composite clay. Appl. Clay Sci. 2020, 190, 105543. [Google Scholar] [CrossRef]
- Santanna, V.C.; Silva, S.L.; Silva, R.P.; Castro Dantas, T.N. Use of palygorskite as a viscosity enhancer in salted water-based muds: Effect of concentration of palygorskite and salt. Clay Miner. 2020, 55, 48–52. [Google Scholar] [CrossRef]
- Dahab, A.S. Thermal-Stability of Drilling-Fluids Prepared from Saudi Palygorskite. J. Can. Pet. Technol. 1991, 30, 49–52. [Google Scholar] [CrossRef]
- Baltar, C.A.M.; da Luz, A.B.; Baltar, L.M.; de Oliveira, C.H.; Bezerra, F.J. Influence of morphology and surface charge on the suitability of palygorskite as drilling fluid. Appl. Clay Sci. 2009, 42, 597–600. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Wang, Q.; Wang, A. Disaggregation of palygorskite crystal bundles via high-pressure homogenization. Appl. Clay Sci. 2011, 54, 118–123. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Wang, A. Superior dispersion properties of palygorskite in dimethyl sulfoxide via high-pressure homogenization process. Appl. Clay Sci. 2013, 86, 174–178. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Wang, A. Effects of solvent treatment and high-pressure homogenization process on dispersion properties of palygorskite. Powder Technol. 2013, 235, 652–660. [Google Scholar] [CrossRef]
- Xu, J.X.; Wang, W.B.; Wang, A.Q. Dispersion of palygorskite in ethanol-water mixtures via high-pressure homogenization: Microstructure and colloidal properties. Powder Technol. 2014, 261, 98–104. [Google Scholar] [CrossRef]
- Boudriche, L.; Chamayou, A.; Calvet, R.; Hamdi, B.; Balard, H. Influence of different dry milling processes on the properties of an attapulgite clay, contribution of inverse gas chromatography. Powder Technol. 2014, 254, 352–363. [Google Scholar] [CrossRef]
- Viseras, C.; Meeten, G.H.; Lopez-Galindo, A. Pharmaceutical grade phyllosilicate dispersions: The influence of shear history on floc structure. Int. J. Pharm. 1999, 182, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, Z.; Morsali, A. Sonochemical preparation of palygorskite nanoparticles. Appl. Clay Sci. 2011, 51, 51–53. [Google Scholar] [CrossRef]
- Pardo-Canales, L.; Essih, S.; Cecilia, J.A.; Domínguez-Maqueda, M.; Olmo-Sánchez, M.I.; Pozo-Rodríguez, M.; Franco, F. Modification of the textural properties of palygorskite through microwave assisted acid treatment. Influence of the octahedral sheet composition. Appl. Clay Sci. 2020, 196, 105745. [Google Scholar] [CrossRef]
- Myriam, M.; Suárez, M.; Martín-Pozas, J.M. Structural and Textural Modifications of Palygorskite and Sepiolite Under Acid Treatment. Clays Clay Miner. 1998, 46, 225–231. [Google Scholar] [CrossRef]
- Oliveira, R.; Acchar, W.; Soares, G.; Barreto, L. The increase of surface area of a Brazilian palygorskite clay activated with sulfuric acid solutions using a factorial design. Mater. Res. 2013, 16, 924–928. [Google Scholar] [CrossRef]
- Zhou, H.; Murray, H.H. Chapter 10—Overview of Chinese Palygorskite Clay Resources—Their Geology, Mineralogy, Depositional Environment, Applications and Processing. In Developments in Clay Science; Galàn, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 239–263. [Google Scholar]
- Altun, G.; Serpen, U. Investigating improved rheological and fluid loss performance of sepiolite muds under elevated temperatures. In Proceedings of the World Geothermal Congress, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Cinar, M.; Can, M.F.; Sabah, E.; Karaguzel, C.; Celik, M.S. Rheological properties of sepiolite ground in acid and alkaline media. Appl. Clay Sci. 2009, 42, 422–426. [Google Scholar] [CrossRef]
- Liu, P.; Du, M.; Clode, P.; Liu, J.; Leong, Y.-K. Rod–plate interactions in sepiolite–LAPONITE® gels: Microstructure, surface chemistry and rheology. Soft Matter 2021, 17, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Ferraz, E.; Santarén, J.; Rasteiro, M.G.; Gamelas, J.A.F. Improving Colloidal Stability of Sepiolite Suspensions: Effect of the Mechanical Disperser and Chemical Dispersant. Minerals 2020, 10, 779. [Google Scholar] [CrossRef]
- Sabah, E.; Mart, U.; Çınar, M.; Çelik, M.S. Zeta Potentials of Sepiolite Suspensions in Concentrated Monovalent Electrolytes. Sep. Sci. Technol. 2007, 42, 2275–2288. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, V.K. A state of the art review on the performance of high-pressure and high-temperature drilling fluids: Towards understanding the structure-property relationship of drilling fluid additives. J. Pet. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Y.; Zhang, H.; Zhang, J.; Huang, Y. A critical review of the possible effects of physical and chemical properties of subcritical water on the performance of water-based drilling fluids designed for ultra-high temperature and ultra-high pressure drilling applications. J. Pet. Sci. Eng. 2020, 187, 106795. [Google Scholar] [CrossRef]
- Belani, A.; Orr, S. A Systematic Approach to Hostile Environments. J. Pet. Technol. 2008, 60, 34–39. [Google Scholar] [CrossRef]
- Mohamed, A.; Salehi, S.; Ahmed, R. Significance and complications of drilling fluid rheology in geothermal drilling: A review. Geothermics 2021, 93, 102066. [Google Scholar] [CrossRef]
- Carney, L.L.; Meyer, R.L. A New Approach to High Temperature Drilling Fields. In Proceedings of the SPE Annual Fall Technical Conference and Exhibition, New Orleans, LA, USA, 3–6 October 1976. SPE-6025-MS. [Google Scholar]
- Ettehadi, A.; Altun, G. Extending thermal stability of calcium carbonate pills using sepiolite drilling fluid. Pet. Explor. Dev. 2017, 44, 477–486. [Google Scholar] [CrossRef]
- Ettehadi, A.; Altun, G. In-Situ Thermal Rheological Properties of Drilling Muds. In Proceedings of the SPE/IADC Middle East Drilling Technology Conference and Exhibition, Abu Dhabi, United Arab Emirates, 29–31 January 2018. SPE/IADC-189349-MS. [Google Scholar]
- Altun, G.; Osgouei, A.E.; Ozyurtkan, M.H. Customization of sepiolite based drilling fluids at high temperatures. In Proceedings of the 48th US Rock Mechanics/Geomechanics Symposium, Minneapolis, MN, USA, 1–4 June 2014. ARMA 14-7031: American Rock Mechanics Association. [Google Scholar]
- Carney, L.L.; Guven, N. Investigation of changes in the structure of clays during hydrothermal study of drilling fluids. Soc. Pet. Eng. J. 1980, 20, 385–390. [Google Scholar] [CrossRef]
- Zhao, X.; Li, W.; Li, S.; Ji, Y. Application of Several Kinds of Clays and a New Type of Nano-Modified Bontonite in Drilling Fluids. Adv. Mater. Res. 2013, 746, 489–495. [Google Scholar] [CrossRef]
- Tao, Z.; Tianqi, L.; Yuechao, L.; Yang, L.; Gaowei, G.; Jingcheng, C.; Fengshan, Z. Preparation and Rheological Properties of Attapulgite Gel for Aqueous Suspensions. In Proceedings of the 2016 7th International Conference on Education, Management, Computer and Medicine (EMCM 2016), Shenyang, China, 29–31 December 2016. [Google Scholar]
- Wenlong, Z.; Xiaoming, W.; Yuming, H.; Jie, X.; Wenshi, W. Research and Application of High-Temperature Drilling Fluid for Scientific Core Drilling Project. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13 November 2017. SPE-188906-MS. [Google Scholar]
- Christidis, G.E.; Athanasakis, N.; Marinakis, D. Rheological properties of magnesium bentonite and sepiolite suspensions after dynamic ageing at high temperatures. Clay Miner. 2024. [Google Scholar] [CrossRef]
- Guven, N.; Panfil, D.J.; Carney, L.L. Comparative Rheology of Water-Based Drilling Fluids with Various Clays. In Proceedings of the International Meeting on Petroleum Engineering, Tianjin, China, 31 October 1988. SPE-17571-MS. [Google Scholar]
- Osgouei, A.E.; Ozyurtkan, M.H.; Altun, G.; Dilsiz, E.A. Dynamic Filtration Properties of Clay Based Drilling Muds under Elevated Temperatures. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2012. SPE-163325-MS. [Google Scholar]
- Osgouei, A.E.; Ozyurtkan, M.H.; Altun, G. Dynamic Filtration Properties of Fresh Water Sepiolite-based Muds. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 2079–2086. [Google Scholar] [CrossRef]
- Altun, G.; Osgouei, A.E. Investigation and remediation of active-clay contaminated sepiolite drilling muds. Appl. Clay Sci. 2014, 102, 238–245. [Google Scholar] [CrossRef]
- Neaman, A.; Singer, A. Rheology of mixed palygorskite-montmorillonite suspensions. Clays Clay Miner. 2000, 48, 713–715. [Google Scholar] [CrossRef]
- İşçi, E.; Turutoğlu, S.İ. Stabilization of the mixture of bentonite and sepiolite as a water based drilling fluid. J. Pet. Sci. Eng. 2011, 76, 1–5. [Google Scholar] [CrossRef]
- Abdo, J.; Haneef, M.D. Nano-Enhanced Drilling Fluids: Pioneering Approach to Overcome Uncompromising Drilling Problems. J. Energy Resour. Technol. 2012, 134, 014501. [Google Scholar] [CrossRef]
- Abdo, J.; Haneef, M.D. Clay nanoparticles modified drilling fluids for drilling of deep hydrocarbon wells. Appl. Clay Sci. 2013, 86, 76–82. [Google Scholar] [CrossRef]
- Huang, W.; Leong, Y.-K.; Chen, T.; Au, P.-I.; Liu, X.; Qiu, Z. Surface chemistry and rheological properties of API bentonite drilling fluid: pH effect, yield stress, zeta potential and ageing behaviour. J. Pet. Sci. Eng. 2016, 146, 561–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






