Abstract
The continuous dumping of industrial solid wastes into the immediate environment is incommodious since these waste materials cause pollution and serious hazards to human health. In addition, these solid wastes are complex and consist of toxic chemical substances, heavy metals, and valuable metals, hence warranting treatment before disposal. Bioleaching is a green and sustainable technology for the solubilization and mobilization of metals from solid matrices. The leaching efficacy is contingent on the types and physiology of the organisms, the elemental content of the solid wastes, and the presence of appropriate bioprocess parameters at optimum conditions. Extremophilic microbes, including thermophiles, acidophiles, alkaliphiles, and halophiles, are recognized as excellent biological agents for the efficient bioextraction of metals from industrial solid wastes due to their aptitude for survival under harsh bioleaching conditions. Therefore, this review provides insights into the employability of extremophilic microorganisms as a biofactory for the recovery of valuable metals from various industrial solid wastes. More so, it discusses the sustainability of the bioleaching technique in terms of its life cycle assessment (LCA) and techno-economic analysis.
1. Introduction
The rise in urbanization and industrial growth coupled with an upsurge in the living standards of people have substantially accelerated the amount, rate, and quality of solid waste generation [1,2]. Solid wastes are discarded useless materials, or by-products, that are generated from various industrial and commercial operations [3]. These include fly ash (Cu, V, Ni), discarded batteries (Zn, Mn, Li, Ni, Cd), spent refinery catalysts (Al, Ni, Mo), electronic scraps (Au, Cu, La, Ag), metal-containing sludge (Pb, Cr, Ni), and metallurgical slags (Fe, Sb, As) with their respective representative metals [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] (Figure 1). These waste materials are heterogeneous and comprise a broad spectrum of toxic and non-fatal substances together with precious metals, base metals, and rare earth elements [14,22]. A wide variety of industries, such as electrical and electronics, electroplating, petrochemical, steel, and mining, discharge solid wastes, which, if poorly managed, threaten the environment and public health [22,23]. When solid wastes are indiscriminately dumped into landfills or incinerated, they have negative effects by contaminating surface and underground water and generating greenhouse gases [24]. The hazardous metal components of the solid wastes seep into the soil and travel up the food chain. Ingestion of such contaminated food by humans results in various health challenges, including cancer, cardiac failure, skin dermatitis, DNA damage, and birth defects [25] (Figure 2). As a result, the application of appropriate techniques for the recovery of the valuable metals present in solid wastes is an ideal approach to eliminate the toxic effects of waste materials and promote waste management and reuse [22]. Over the years, traditional physicochemical approaches, including pyrometallurgy, hydrometallurgy, precipitation, ion exchange, ion flotation, adsorption, and membrane separation, have been employed for the recovery of metals from solid wastes. Nevertheless, these techniques are unsatisfactory owing to operational difficulties, high downstream processing costs, release of toxic by-products, and risk of harmful gas emissions [3]. Therefore, the use of microorganisms is recognized as a preferred strategy that is gaining the attention of the scientific community for the extraction of metals from secondary solid wastes [14].

Figure 1.
Schematic diagrams elucidating different industrial solid wastes.
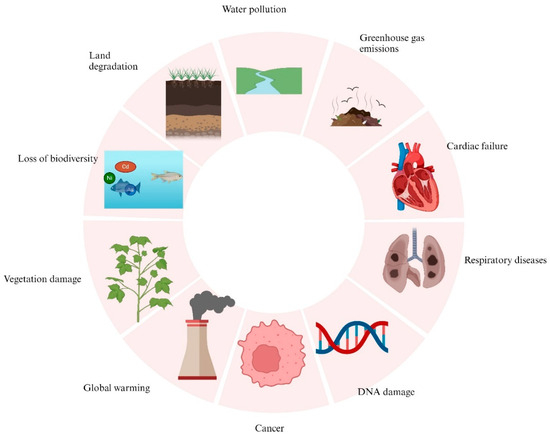
Figure 2.
Schematic diagrams illustrating some environmental and health effects of industrial solid wastes.
Bioleaching, also known as biohydrometallurgy, is the solubilization and mobilization of metals from insoluble secondary wastes using the metabolic potentials of microorganisms [26]. This technology is promising, simple, environmentally safe, effective, sustainable, and economical. In addition, it requires less energy and generates little secondary waste with ease of metal recovery from the solid matrices [27]. The leaching efficacy of the organisms is determined by the physiology of the microbes, the elemental composition of the solid wastes, and the availability of appropriate bioprocess parameters at optimum conditions [24]. Based on their metabolism and requirement for suitable physicochemical parameters, the microbes exist as acidophiles, alkaliphiles, psychrophiles, halophiles, thermophiles, and hyperthermophiles, which are collectively referred to as extremophiles [28] (Figure 3).
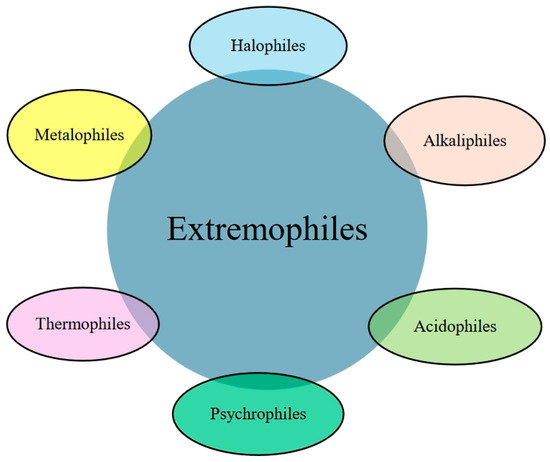
Figure 3.
Schematic diagrams showing various types of extremophiles.
Extremophiles are organisms that are adapted to survive in harsh environmental conditions that are beyond or very much below the limits required for human existence [29] (Table 1). These organisms can grow in natural conditions at high temperatures (60 to 80 °C) and low pH values (less than 3.0), and exhibit tolerance to high metal and salt concentrations (2–5 M NaCl) [30,31]. They are found in geothermal areas, hot acidic natural environments, deserts, deep seas, hypersaline lakes, hydrothermal vents, and polluted sites. For instance, Acidianus copahuensis, a novel thermoacidophilic crenarchaeota, found in Copahue volcano, oxidized sulfur compounds and ferrous ions at elevated temperatures, making it an ideal organism for metal bioleaching [32]. In addition, extremophilic microbes such as Acidiplasma aeolicum (hydrothermal pool), Sulfolobus acidocaldarius (acid hot spring), Picrophilus torridus (dry solfataric fields), Thermoplasma acidophilum (coal refuse piles), and Cuniculiplasma divulgatum (acid mine drainage), isolated from their respective habitats, are employed for metal bioleaching and biooxidation [33,34,35,36,37,38]. These extremophilic organisms are attractive due to their specific metabolic, biochemical, and genetic adaptations to thrive in the unfriendly environmental conditions of their ecological niche. These organisms possess distinct physiological and enzymatic properties that qualify them as suitable candidates for the mobilization of metals from solid waste materials [39]. The use of extremophiles for bioleaching processes increases the rate of mineral oxidation, minimizes operational costs, lessens the duration of metal solubilization, decreases microbial contamination, and reduces passivation of mineral surfaces [29,40,41]. Therefore, the present review focuses on assessing the potential of extremophiles in the recovery of valuable metals from different industrial solid wastes.

Table 1.
Properties of various types of extremophiles.
2. Extremophilic Microorganisms: Types and Survival Strategies
2.1. Halophiles
These are microorganisms that thrive in the presence of extreme saline environments. Salt is known to alter the solubility, stability, and conformation of cells’ proteins, thereby inhibiting the survival of the organisms owing to their osmolar imbalance and metabolism [28]. The halophilic microbes adapt to these conditions by accumulation of inorganic or organic osmo-protectants within the cell for maintaining osmotic equilibrium intracellularly and extracellularly [55]. In addition, under high-salt conditions, the halophiles utilize the adenosine triphosphate (ATP)-dependent K+ transport system, cationic amino acid transporter-3, and Na+ efflux antiporters for ensuring equilibrium in the osmotic gradient between the cell and environment [28]. This is typical of Halobacterium salinarum, found to survive in the presence of approximately 3.97 M K+ and 4.57 M Cl− [30,56]. In addition, halophiles possess anionic aspartate and glutamate residues on protein surfaces, which facilitate the retention of water molecules around the cells and prevent the organisms from dehydration and precipitation [30,57]. The halotolerant organisms utilize compatible solutes, including sucrose, polyols, trehalose, and glucosylglycerol, for maintaining osmotic equilibrium at high salt concentrations [58,59]. The secretion of appropriate organic solutes by Spiribacter salinus M19-40, under high salinity, reduced the thermodynamics of water to compensate for external osmotic pressure [60,61]. The presence of negative charges on the outer surface of halophiles’ proteins protects the organisms from water loss and agglomeration at high salinity [62]. More so, halotolerant microbes are structurally stable under hypersaline conditions due to the possession of P45 protein as well as the presence of H-bond networks and stable salt bridges in their enzymes [63,64]. Halophilic microorganisms such as Oceanobacillus sp. A22, Salinicoccus sp. A43, Halomonas smyrnensis KS802, Desulfovibrio halophilus, Halomonas elongata, and Bacillus pumilus have been reported for the recovery of metals from different salt-rich environments [65,66,67,68,69].
2.2. Acidophiles
These are microbes that survive in an extremely-low-pH (less than 3.0) environment through the homeostatic regulation of pH and control of proton permeation [30,46]. This is notable in microbial genera such as Sulfolobus, Thermoplasma, and Ferroplasma, which, due to possession of highly permeable cell membranes (consisting of tetraether lipids and a variety of polar head groups and an isoprenoid core), regulate proton permeation at very-low-pH conditions [70,71]. In addition, the acidophilic organisms thrive at low pH by modulating proton influx via a proton pump system and putative proton pump proteins (such as antiporters, symporters, and cell membrane H+-ATPase) [72,73]. In addition, F0F1-type ATP synthase from Leptospirillium ferriphilum and Thermoplasma acidophilum is well known for the regulation of proton permeation [74]. The acidophiles employed other strategies, including enhanced secretion of organic acids (uncouplers), cytoplasmic buffering capacity, and chaperone proteins for survival at extremely low pH levels by safeguarding certain molecules, including proteins, RNA, and DNA within the cell [40,74]. Acidophilic microorganisms such as Acidithiobacillus ferrooxidans, Acidomyces acidothermus, and Acidithiobacillus thiooxidans with metal removal potentials have been reported [42,43,44].
2.3. Thermophiles
These are organisms that grow optimally at temperatures between 60 and 80 °C. Among the defensive mechanisms developed by thermophiles for survival at high temperatures is the regulation of membrane lipid fluidity by enhancing the production of hopanoids, a characteristic demonstrated by thermophilic Bacillus acidocalidus [75]. In addition, the modulation of membrane lipid composition is a vital survival strategy for some thermophilic organisms. This is typical of a thermophilic archaeon, Metahnocaldococcus jannaschii, which, upon exposure to elevated temperatures, showed a decrease (80%–20%) in diether lipid content with an increase (10%–40%) in caldarchaeol- and cyclic archaeol-derived lipids [76,77]. Furthermore, the thermal stability of thermophiles is attained by the development of a robust heat-shock response for normal protein synthesis, generation of a positive supercoiled DNA structure, an increase in the concentration of the nitrogenous bases (guanine or cytosine) of DNA, oligomerization, DNA repair mechanisms, and a large hydrophobic core as well as an enhanced number of surface charges, disulfide bonds, and salt bridging [31,48]. Thermophilic microorganisms, including Anoxybacillus flavithermus and Brevibacillus borstelensis as well as species of the genera Sulfolobus, Leptospirillum, and Ferroplasma, are capable of extracting metals from solid matrices [29,45].
2.4. Psychrophiles
Psychrophilic microbes such as Psychrobacter sp., Pseudoalteromonas sp., Deinococcus sp., and Oleispira sp. are adapted to survive at low temperatures in the range of 1 to 4 °C [30]. This group of microorganisms possesses different mechanisms for adaptation to extreme environmental conditions. These include the synthesis of antifreeze molecules, activation of molecular chaperones, alteration of protein structure, and control of membrane fluidity [48,78,79]. Psychrophilic organisms regulate membrane fluidity by modifying the composition of cellular lipids as well as enhancing the synthesis of polyunsaturated fatty acids and carotenoids [46,78]. In addition, the cold-tolerant microbes secrete a set of proteins known as growth acclimation proteins (CAPs) and cold-shock proteins (CSPs), which are overexpressed after extreme cold shocks (~4 °C) and under mild conditions, respectively, in response to continuous growth at low temperatures [80]. The cold-adapted enzymes are hyperactive, conformationally flexible with higher specific activity, and stable at low temperatures [28,31]. Abd-Elnaby et al. [47] and Ausuri et al. [49] reported on the metal removal efficiency of psychrophilic Dietzia psychralcaliphila JIID and Pseudomonas sp. H69A.
2.5. Alkaliphiles
These are a group of microorganisms that are tolerant of high pH levels. These organisms have developed some strategies for survival under extreme alkaline conditions. For instance, alkaliphilic Bacillus sp. enhances proton motive force generation by synthesis of acidic plasma membrane, consisting of teichurono-peptide, peptidoglycan, and teichuronic acid, which play a crucial role in pH balance and ATP production [30,81]. In addition, at extremely-high-pH conditions, sodium motive force in alkaliphiles promotes pH equilibrium [52,82]. At high sodium ion concentrations, the alkaliphiles modulate intracellular pH by employing Na+/H+ antiporters for the release of sodium ions while absorbing large quantities of hydrogen ions from outside the cell [83]. The secretion of organic acids by alkaliphilic microbes is a crucial metabolic activity that permits pH balance [84]. Furthermore, the presence of phosphoserine aminotransferase and anionic amino acid residues, as well as enhanced hydrogen linkages and hydrophobic interactions at the dimer interface, contributes to the survival of these organisms at extreme pH conditions [31,48]. Alkaliphiles survive at high alkalinity due to the presence of cytochrome c-552 (Gram-negative bacteria) and cytochrome c (Gram-positive bacteria), which regulate pH homeostasis by proton deposition, thereby promoting the growth of the organisms at high pH ranges [53]. The ATP synthase plays a crucial role in the survival mechanisms of alkaliphiles. The subunit c of the enzyme consists of amino acid motifs for the retention of hydrogen ions in the cytoplasm [85]. Several authors have reported on the metal removal potentials of alkaliphilic microbes such as Citricoccus alkalitolerans, Paenibacillus pabuli, and Micrococcus sp. [50,51,54].
3. Biorecovery of Metals from Industrial Solid Wastes Using Extremophiles
Extremophilic microorganisms have developed enormous strategies for adaptation to harsh environmental conditions that are noticeable during metal bioleaching processes. Iron- and sulfur-oxidizing bacteria, collectively known as acidophiles, produce Fe2(SO4)3 and H2SO4 (biolixiviants), respectively, as oxidizing agents for the solubilization and extraction of metals from industrial solid wastes using acidolysis and redoxolysis pathways (Equations (1)–(3)) [24,86,87].
H2SO4 + MeS → H2S + MeSO4
H2SO4 + MeO → H2O + MeSO4
Fe2(SO4)3 + MeS + H2O + 3/2O2 → Me2+ + SO42− + 2FeSO4 + H2SO4
Me denotes the metal ion.
The acidophilic organisms include Acidithiobacillus ferrooxidans, Acidithiobacillus caldus, Leptospirillum ferriphilum, Ferroplasma sp., and Acidithiobacillus thiooxidans, amongst others [88,89,90,91,92,93]. Thermophilic microorganisms such as Sulfobacillus thermosulfidooxidans, Thermoplasma acidophilum, and Ferroplasma thermophilum have potential for recovery of metals from solid wastes [6,94,95]. In general, extremophiles thrive optimally at a pH of less than 3.0 and temperatures up to 80 °C [31,96]. Conversely, the extracellular production of hydrogen cyanide by cyanogenic microbes (such as Chromobacterium violaceum) under alkaliphilic conditions triggers the recovery of precious metals from industrial solid wastes [5]. The hydrogen cyanide reacts with precious metals in the solid wastes, resulting in the formation of a soluble metal–cyanide complex, which prompts metal recovery from the solid matrix [97]. The precious metal solubilization from solid wastes is represented in Equations (4)–(6) [24].
4Au + 8CN− → 4Au (CN)2− + 4e−
O2 + 2H2O + 4e− → 4OH−
4Au + 8CN− + O2 + 2H2O → 4Au (CN)2− +4OH−
In addition, numerous microbes have been implicated in tolerating high environmental stress during metal bioleaching experiments. These organisms secrete organic acids that facilitate the mobilization of metals from industrial solid wastes [98]. The efficacy of the organisms in the recovery of metals from solid wastes is influenced by the presence of suitable physicochemical parameters, including temperature, pH, pulp density, and particle size, aeration, and culture medium [24].
- Mechanisms of metal bioleaching
Microorganisms employ different mechanisms of action for the recovery of metals from industrial solid wastes. These are discussed in detail below.
- Redoxolysis
This is an oxidation–reduction process involving oxidation of insoluble metal compounds into corresponding water-soluble form in the presence of an oxidizing agent [99]. This approach is mostly used by acidophilic bacteria (e.g., Acidithiobacillus sp.) for the oxidation of metal sulfides by Fe3+ (lixiviant) with subsequent solubilization of the metals [100]. At acidic pH and in the presence of specific redox enzymes, the Fe3+ is reduced to a ferrous ion (Fe2+), which is further oxidized back to the Fe3+ necessary for metal solubilization. The repeated biochemical oxidation and chemical reduction perpetually result in the release of lixiviant, required for metal solubilization [87]. The redox reaction is illustrated in Equation (7) [101].
Fe3+ + 0.5HO2C·CO2H → Fe2+ + H+ + CO2
- Acidolysis
This is an indirect bioleaching approach involving protonation of oxygen atoms on insoluble metal compounds, with subsequent oxidation and solubilization by an acidic compound [98]. Following protonation, the protons and oxygen atoms react to form a water molecule, causing recovery of metals from the solid matrices into solution [102]. Organic acids such as pyruvic acid, citric acid, formic acid, gluconic acid, and acetic acid facilitate protonation of oxygen atoms [99]. The metal solubilization by protonation in the aqueous phase is represented by Equation (8) [102].
Me0O + 2H+ → Men+ + H2O
Me denotes the metal ion.
- Complexolysis
This is a ligand-induced solubilization process involving the formation of metal–ligand complexes and chelates for the mobilization of metal ions from a particular solid matrix in solution [101]. Ligands such as organic acids, cyanides, and siderophores are utilized for the solubilization of desired metals. Notable metal–ligand complexes include oxalic acid with Mg, Al, and Fe; tartaric acid with Al, Mg, and Fe; siderophores with iron; and citric acid with Mg [99]. Cyanogenic bacteria employ this mechanism for the solubilization of precious metals [103]. The interaction of metal ions with organic acid (e.g., citric acid) is shown in Equation (9) [98].
Me2+ + C6H8O7 → Me(C6H8O7)− + 3H+
Me denotes the metal ion.
- Biosorption
This is an independent metabolic process in which microbes accumulate metals from a solid matrix by adsorption, ion exchange, precipitation, and complexation [98]. In the presence of certain functional groups (such as the carboxyl group, phosphate group, and amine group) on the cell surface, the microorganisms mobilize metals from the solid waste [104]. The microbial biomass is utilized as a biosorbent for the extraction of a variety of metals, including precious metals, base metals, and rare earth elements [24,105]. This process is simple, economical, and efficient, lessens waste generation, and permits biomass regeneration [106].
- Application of extremophiles in biorecovery of metals from industrial solid wastes
The application of extremophiles in the extraction of metals from industrial solid wastes is illustrated in Figure 4 and discussed in detail below.
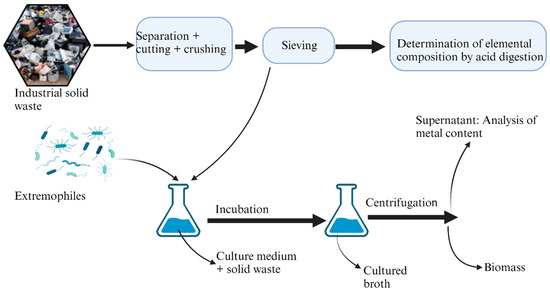
Figure 4.
Schematic diagrams depicting stepwise procedures for bioleaching of metals from industrial solid waste.
3.1. Spent Refinery Catalysts
Solid catalysts are utilized in large amounts for the conversion of crude oil into petroleum products, including petrol, kerosene, aviation fuel, gasoline, etc. The incessant reuse and reactivation of these catalysts cause them to lose their catalytic activity, thereby resulting in the dumping of the catalysts as solid wastes, eventually referred to as spent catalysts [107]. These waste materials are composed of different metals such as nickel, cobalt, copper, and molybdenum [107,108,109]. The bioextraction of these valuable metals from the solid matrices is vital before disposal. Numerous heterotrophic and chemolithotrophic extremophilic microorganisms have been reported for effective metal recovery from refinery catalyst waste [14,26,98] (Table 2). For instance, Bharadwaj and Ting [110] investigated the bioleaching of metals from spent hydrotreating catalyst using thermophilic Acidianus brierleyi at optimum bioprocess conditions of pulp density 1%, 70 °C, pH 1.5–2.0. The authors recorded enhanced recovery efficiencies of 100% Fe, 67% Al, 100% Mo, and 100% Ni. Acidithiobacillus ferrooxidans was reported to mobilize 99% Ni, 63% Al, 84% Mo, and 96% Co from spent refinery catalysts at pH 1.8–2.0 within 30 d [108]. At optimum conditions of 0.9% pulp density, 60.7 µm particle size, pH 2.0, and 209 mL/min aeration, Shahrabi-Farahani et al. [111] attained maximum recovery efficiencies of 15% Al, 37% Ni, and 87% Mo from hydrocracking spent catalyst using Acidithiobacillus thiooxidans after 7 d. Srichandan et al. [8] carried out an experiment on the bioleaching of metals from spent refinery catalysts using Acidithiobacillus thiooxidans under varying conditions of pulp density (1–10 g/L), initial pH (1.5–2.5), and sulfur concentration (0.5–3.0 g/L). A maximum extraction of 94% V, 44% Al, 34% Mo, and 93% Ni was recorded at optimum conditions of pH 1.5, sulfur concentration 1.5%, and pulp density 1%. However, the spent refinery catalyst contains oily substances on its surface, which, if not removed, can impede the penetration of oxidants into the solid matrix [110]. As a result, pretreatment of the solid wastes by techniques such as washing with acetone or decoking is imperative. Pradhan et al. [112] studied the extraction of metals from acetone-pretreated spent petroleum catalyst by acidophilic Acidithiobacillus thiooxidans at 35 °C (180 rpm), pH 2.5, pulp density 10%, and particle size 106 µm. The experimental results demonstrated maximum recovery efficiencies of 95% V, 46% Mo, and 88% Ni within 7 d.

Table 2.
Removal efficiencies of metals from bioleaching of spent refinery catalyst.
3.2. Wastewater Sludge
Large quantities of sewage sludge are generated during mechanical or biological treatment of municipal wastewater [14]. The sludge consists of different heavy metals, including Zn, Ni, Pb, Cr, Cu, Cd, etc., thereby making it unsafe for utilization as a soil conditioner. As a result, decontamination of the sewage sludge before land application is vital. The metal solubilization from the sewage sludge depends on the metal form, sludge type, and treatment techniques [22]. Bioleaching of sludge by extremophilic microbes has been reported for the recovery of valuable metals by several co-workers ([119,120] (Table 3). Kim et al. [121] assessed the recovery of heavy metals from dewatered sludge by Acidithiobacillus ferrooxidans at optimum conditions of 30 °C, pH 2.0, particle size < 2 mm, and Fe2+ concentration 9 g/L. The authors achieved the highest extraction of 40% Zn, 39% Cu, and 10% Cr in 40 d. Maximum recovery efficiencies of 78.2% Mn, 76% Cu, 84.2% Zn, and 79.5% Ni at 30 °C within 24 h from a secondary activated sludge using acidophilic sulfur-oxidizing bacteria have been reported [122]. Li et al. [123] studied the biorecovery of heavy metals from sewage sludge using a coculture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. The authors recorded an optimum extraction of 95.60% Zn, 98.54% Cu, 60.06% Ni, and 57.99% Cr at pH 2.0 after 14 d. Chen and Chen [6] investigated the thermophilic bioleaching of heavy metals from waste sludge by Sulfobacillus acidophilus, Sulfobacillus thermosulfidooxidans, and Acidithiobacillus caldus. Peak removal efficiencies of 78% Pb, 97% Cu, 99% Zn, and 99% Ni were obtained within 21 d when sludge content and sulfur concentration were optimum at 0.5% and 2.5%, respectively. The presence of toxic or precious metals in industrial wastewater sludge has been reported, thus necessitating an eco-friendly approach for treatment [26]. For instance, the bioleaching of Zn from paint industry sludge using Acidithiobacillus thiooxidans was reported at optimum conditions: particle size 1 mm, pH 4.2, and 32 °C (120 rpm) [92]. A highest recovery efficiency of above 22% was recorded. In another study, Wang et al. [4] reported a 99.7% Cr dissolution from tannery sludge in the presence of Acidithiobacillus thiooxidans in 5 d. Under acidophilic conditions (pH 0.8), Tian et al. [124] studied the bioleaching of valuable metals from electroplating sludge using a mixed culture of Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, and Leptospirillum ferriphilum at a pulp density of 2% and 35 °C. Complete (100%) recovery of Ni and Zn as well as 96.5% Cu and 76.1% Cr was reported. Rouchalova et al. [19] achieved the highest recovery efficiencies of 96.44% Cu, 98.73% Zn, and 85.42% Fe from mine sludge at pH 1.8 and 30 °C within 42 d in the presence of Acidithiobacillus ferrooxidans. Maes et al. [125] employed a halophilic microbial community for the recovery of 79%–99% Pt from refinery waste at 10–80 g/L K2Pt (II)Cl4 concentration at pH 2.3 and 28 °C within 7 d.

Table 3.
Removal efficiencies of metals from bioleaching of wastewater sludge.
3.3. Metallurgical Slags
Intensive industrial activities, especially pyrometallurgical extraction of metals from solid matrices, produce substantial amounts of waste (e.g., slags) which contains large quantities of metals that can be effectively recovered by bioleaching processes [14] (Table 4). For instance, Ahmadi et al. [12] reported complete (100%) and 95% extractions of Cu and Fe, respectively, from converter slags using Acidithiobacillus ferrooxidans at optimum conditions of pulp density 1.4%, initial pH 1.8, and Fe2+ concentration 7.3 g/L. Cheng et al. [128] studied the bioleaching of metals from Pb/Zn smelting slag under thermoacidophilic conditions at pH 1.5, 65 °C, pulp density 5% (w/v), and 120 rpm using a consortium of Pseudomonas sp., Sporosarcina sp., and Bacillus sp. The experimental results showed a maximum extraction of above 80% Zn, Cu, Al, Mn, As, and Fe as well as approximately 5% Pb after 6 d. Kinnunen et al. [16] reported a highest dissolution of 71% Cu, 41% Zn, 49% Co, 59% Fe, 2% Al, 11% Sb, 29% Ni, 37% Cr, and more than 100% Mn from the bioleaching of copper slag by a mixture of Marinobacter sp., Arcobacter sp., Acidithiobacillus sp., Leptospirillum sp., Sulfobacillus sp., Sulfurospirillum sp., and Cuniculiplasma sp. at a pulp density of 1%, 30 °C, and pH 1.5 within 21 d. Mikoda et al. [129] evaluated the extraction of metals from Polish copper metallurgical slags using acidophilic Acidithiobacillus thiooxidans at optimum conditions of pH 2.5, pulp density 1%, and particle size 0.25–0.5 mm. Maximum removal efficiencies of 88% Co, 40% Mo, 83% REE, and 55% V (lead slag); 100% Co, 44% Mo, 70% REE, and 70% V (shaft furnace slag); and 95% Co, 70% Mo, 99% REE, and 93% V (granulated slag) were achieved within 28 d. A culture supernatant of Acidithiobacillus thiooxidans was employed for the mobilization of metals from steel slag, yielding a maximum extraction of 28% Mg and 0.1% Mo after 6 d. Repeated bioleaching cycles enhanced metal recovery from the steel slag from 28% to 75% Mg, 14% to 60% Zn, and 11% to 27% Cu [130]. Hao et al. [131] investigated the biorecovery of metals from metallurgical industry waste using Acidithiobacillus caldus S1, Leptospirillum ferriphilum YSK, Acidithiobacillus thiooxidans AO1, and Ferroplasma thermophilum L1 at optimum conditions of pH 1.8, 175 rpm, 40 °C, and pulp density 5% (w/v) for 16 d. A maximum bioleaching efficiency of 58.7% Cu was reported.

Table 4.
Removal efficiencies of metals from bioleaching of metallurgical slags.
3.4. Waste Incineration Fly Ash
The incineration of solid waste in landfills reduces its volume, provides heat energy, and results in the production of finely divided residues known as fly ash and bottom ash [90]. The ash residues pose greater risks to the environment because of the presence of leachable heavy metals (such as Cr, Mn, Pb, and Cd), which contaminate surface and underground water when water flows through uncontrolled landfills. However, fly ash consists of huge amounts of heavy metals in comparison to bottom ash due to metal vaporization during combustion and adsorption of the vaporized metals on the surface of fly ash particles [11,134]. Therefore, fly ash is categorized as hazardous waste generated globally from solid waste [134]. The application of bioleaching is an effective, sustainable, and promising technology for the recovery of heavy metals from solid waste incineration fly ash [135]. Extremophilic microbes are ideal biological agents for the bioleaching of fly ash due to the toxic metal composition of the solid wastes [136] (Table 5). For instance, Ramanathan and Ting [11] investigated the bioleaching of metals from municipal solid waste incineration fly ash using Alkalibacterium sp. TRTYP6 under extreme alkaline conditions (pH 8.0 to 12.5). A maximum removal efficiency of 52% Cu was recorded at a fly ash tolerance of 20% (w/v) after 30 d. Rastegar et al. [10] assessed the recovery of metals from residual ash obtained from a thermal power plant by Acidithiobacillus ferrooxidans. The authors reported a higher extraction of 74% V, 95% Ni, and 88% Cu at optimum pH 1.3, initial Fe2+ concentration 2.6 g/L, 32 °C, 15 d, and pulp density 1% (w/v). Brombacher et al. [137] recorded recovery efficiencies of 81% Zn, 52% Al, 100% Cd, 89% Cu, 64% Ni, and 12% Cr during the bioleaching of metals from fly ash using a consortium of Acidithiobacillus (formerly Thiobacillus) thiooxidans and Acidithiobacillus (formerly Thiobacillus) ferrooxidans. Ishigaki et al. [138] assessed the bioextraction of metals from waste incineration fly ash in the presence of acidophilic Acidithiobacillus ferrooxidans and Acidithiobacillus (formerly Thiobacillus) thiooxidans at culture conditions of 28 °C (120 rpm), 1% pulp density, and incubation time 10 d. The authors reported maximum dissolutions of 78% Zn, 67% Cu, and 100% Cr and Cd. In addition, a consortium of acidophiles consisting of Sulfobacillus thermosulfidooxidans, Ferroplasma thermophilum, and Leptospirillum ferriphilum was employed for the recovery of metals from Shaoguan incineration ash in Guangdong province, China, at optimum conditions of pulp density 15% (w/v), 30 °C, pH 1.8, and Fe (II) concentration 200 g/L [95]. The experimental results showed maximum removal efficiencies of 13.8% Pb, 99.9% Mn, 93.2% Cd, and 88.7% Zn.

Table 5.
Removal efficiencies of metals from bioleaching of fly ash.
3.5. Discarded Batteries
The increase in global demand for electronic products (e.g., laptops, radios, and smartphones) has resulted in a huge need for electric storage systems (batteries). Depending on their metal contents, batteries are classified as Ni-Cd batteries, Li-ion batteries (LIBs), Pb-acid batteries, Ni-metal hydride batteries, and Zn-Mn batteries [140]. The LIBs are composed of an anode, cathode, electrolyte separator, organic compounds, and casing [141]. In addition, the LIBs consist of substantial amounts of metals, including Ni, Co, Li, Mn, Cu, and Al. These batteries are used as a power source due to their superior properties, including lightweight design, high discharge voltage, long-lasting stability, and high energy density [142,143]. The worldwide accumulation of LIBs is predicted to reach 9.16 × 105 tons by 2025, with a further increase to 1.6 million tons by 2030 [144]. On the other hand, Zn-Mn batteries are a non-rechargeable power source for small and low-energy-demand devices, including watches, remotes, radios, and clocks, used for a relatively short period. They contain metals such as Mn, Cu, Hg, and Zn [145,146]. The Ni-Cd batteries are rechargeable and employed as energy sources in wireless communication and computing devices. They consist of a cathode (NiOOH), anode, electrolyte (KOH), Ni, and Cd [140,147].
The use of extremophilic microorganisms for the recovery of valuable metals from spent batteries has been reported by many co-workers [148,149,150] (Table 6). For instance, Roy et al. [151] assessed the recovery of metals from spent LIBs using Acidithiobacillus ferrooxidans at a pulp density of 100 g/L, pH 1.93, 30 °C, and 160 rpm. The experimental results showed maximum recovery efficiencies of 89% Li, 82% Co, 92% Mn, and 90% Ni within 72 h. Mishra et al. [152] reported removal efficiencies of 10% Li and 65% Co from a spent LIB in the presence of Acidithiobacillus ferrooxidans at a pulp density of 0.5%, 32 °C, 180 rpm, and pH 2.5 when Fe2+ and So were employed as energy sources. Panda et al. [153] employed a consortium of acidophilic microbes consisting of Leptosprillum ferriphilum, Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, and Leptosprillum ferrooxidans for the bioleaching of metals from spent LIBs collected from a recycling plant in Turkey at bioprocess conditions of 500 µm particle size, initial pH 1.5–2.0, 30 °C (150 rpm), pulp density 0.5%, and incubation period 10 d. The authors recorded 98% Co and 80% Li. Heydarian et al. [154] studied the efficacy of a coculture of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans in the bioleaching of metals from spent Li-ion laptop batteries at pulp density 4%, particle size < 75 µm, and pH 1.5. The experimental results showed the highest recovery of 89% Ni, 99% Li, and 50% Co within 16 d. Naseri et al. [13] employed an actively growing acidophilic Acidithiobacillus thiooxidans PTCC for the bioleaching of metals from discarded coin cells obtained from a computer center in Iran. Recovery efficiencies of 60% Co, 99% Li, and 20% Mn were achieved at pH 2.0, inoculum volume 10% (v/v), pulp density 30 g/L, 30 °C, and 140 rpm. Under acidophilic conditions, Xin et al. [155] investigated the biorecovery of metals from Zn-Mn batteries using a mixed culture of Sulfobacillus spp. and Alicyclobacillus spp. The experimental results revealed maximum recovery efficiencies of 96% Zn (24 h) and 97% (Mn) (13 d) at pH 1.5 and 30 °C. Niu et al. [9] recovered 62.5% Zn and 62.4% Mn from spent Zn-Mn batteries by a mixed culture of Leptospirillum ferriphilum and Acidithiobacillus thiooxidans at a pulp density of 10% (w/v), pH 1.0, and 35 °C in the presence of a metallic catalytic ion (Cu2+). Cerruti et al. [156] investigated the bio-dissolution of discarded Ni-Cd batteries by acidophilic Acidithiobacillus (formerly Thiobacillus) ferrooxidans. The authors reported a maximum extraction of 100% Cd, 96.5% Ni, and 95% Fe after 93 d. Complete (100%) recovery of Cd from spent Ni-Cd batteries by acidophilic Acidithiobacillus ferrooxidans in the presence of ferric sulphate (substrate) has been reported [7].

Table 6.
Removal efficiencies of metals from bioleaching of discarded batteries.
3.6. Waste Electrical and Electronic Equipment
The upsurge in the development of electrical and electronic industries coupled with incessant demands for modern technologies gave rise to an increase in the disposal and accumulation of waste electrical and electronic equipment (WEEE), also known as electronic waste (e-waste) [24,86,91]. E-waste is recognized as the fastest-growing waste stream globally, with a projection of 82 million tons by 2030, accounting for a 32% increment from the 2022 statistics [159]. However, almost 9.3 million metric tons (17.4%) of the estimated amount of e-waste generated has so far been collected and recycled [87,160,161]. Based on elemental composition, age, source, types, and components, e-waste exists as cooling agents (e.g., air conditioners, refrigerators), smartphones, laptops, lamps, television sets, photocopiers, etc. These waste materials consist of poisonous gases (such as furan and dioxin), hazardous heavy metals, precious metals, and rare earth elements (REEs) [21,24,87]. The development of a green and sustainable technology for metal recovery from e-waste promotes waste management and reuse and further enhances the added value of the waste materials [26,103,162]. The use of extremophilic microbes in the bioleaching of metals from e-waste has been reported [102,163] (Table 7). For instance, Acidithiobacillus thiooxidans was employed for the recovery of 98% Cu and 82% Ni from a discarded mobile phone PCB at 30 °C (160 rpm) for 72 h [164]. Chandane et al. [165] investigated the bioextraction of metal from computer PCBs using Acidiphilium acidophilum. Complete (100%) recovery of copper was recorded at optimum conditions of 30% hydrogen peroxide concentration within 10 d. Benzal et al. [166] reported 95%–100% copper recovery efficiency from a mobile phone PCB when Acidithiobacillus ferrooxidans was inoculated into the culture medium at 30 °C (130 rpm) and 7.5 g/L pulp density for 48 h. Chi et al. [167] obtained highest recovery efficiencies of 24.6% Cu and 11.31% Au from waste mobile phone PCBs using alkaliphilic Chromobacterium violaceum at pH 8.0–11.0, 30 °C (150 rpm), H2O2 concentration 0.004% (v/v), and incubation time 8 d. Acidithiobacillus ferrooxidans CF3 achieved a maximum Cu recovery of 94.7% from electroformed copper tubes in 78 d at 22 °C, 20 mM Fe2+, and pH 1.9 [168]. A mixed culture of acidophilic Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans was employed at optimum experimental conditions of particle size 125 µm, inoculum volume 10% (v/v), leaching time 10 d, pulp density 10% (w/v), initial pH 1.8, and 30 °C for the mobilization of 94% Cu, 59% Al, 88% Zn, and 89% Ni from discarded PCBs obtained from printers and computers in Turkey [169]. Erust et al. [15] investigated the acidophilic bioleaching of metals from PCBs of spent mobile phones using a consortium of Acidithiobacillus thiooxidans DSMZ 9463, Leptosprillum ferrooxidans DSM 2705, and Acidithiobacillus ferrooxidans DSMZ 583 at optimum conditions of inoculum volume 10% (v/v), initial pH 1.8, pulp density 10%, and Fe2+ 9 g/L. The authors reported the highest dissolution of 66.9% Zn, 79.5% Ni, 55.9% Al, and 98.1% Cu. Tapia et al. [20] assessed the efficacy of a mixture of acidophilic Leptospirillum, Acidiphilium, and Tissierella bacteria for the mobilization of metals from waste PCBs for 18 d. The experimental results showed high bioleaching efficiencies of 91% Zn, 69% Cu, 28% Au, 16% Sn, and less than 0.25% Ag and Pb at bioprocess conditions of pulp density 10 g/L, 30 °C, 150 rpm, and airflow 500 mL/min.

Table 7.
Removal efficiencies of metals from bioleaching of waste electrical and electronic equipment (WEEE).
4. Sustainability of Metal Bioleaching from Industrial Solid Wastes
The sustainability of bioleaching processes can be evaluated by performing an explicit life cycle assessment (LCA) and techno-economic analysis of the technique. The LCA entails the impacts of bioleaching on the environment, especially when employed for industrial applications [172]. For instance, according to Alipanah et al. [173], LCA has been demonstrated to lessen the global warming potential during the bioleaching of discarded LIBs in comparison to hydrometallurgy. The economic feasibility of this bioprocess is determined by the overall cost of the industrial solid waste, cost, and consumption of oxidizing agents (FeSO4 and S) [148]. In a study to investigate the bioleaching of discarded Zn-Mn batteries by a mixed culture of Sulfobacillus sp. and Alicyclobacillus sp., marine ecotoxicity and human toxicity were identified as key environmental impact factors [174]. LCA showed that the reuse of coal fly ash caused a reduction in greenhouse gas emissions and heavy metal emissions by about 30% and 41%, respectively, with balanced energy consumption in comparison to landfilling [175]. In addition, the replacement of nitric acid with glycine significantly reduced the environmental footprints during the leaching of precious metals and base metals from PCBs [176]. Effective recycling of e-waste for the recovery of metals has been reported by several co-workers to lessen the environmental and health impacts of improper solid waste disposal [177,178]. In general, Priya and Hait [179] and Baniasadi et al. [180] reported that bioleaching for metal recovery has a lower environmental impact when compared to conventional approaches for metal recovery. LCA of the commercial bioheap leaching of 1 kg of nickel sulphate hexahydrate showed a carbon footprint of 1.75 kg CO2-eq when compared to the industry average of 5.4 kg CO2-eq, found to be 60% lower than that of the conventional technologies (smelting and high-pressure acid leaching). The bioleaching technique utilized about 90% less electricity and thermal energy in comparison to the traditional methods [181,182]. Villares et al. [183] investigated the environmental impact of recovery of Cu (1 kg) from PCBs (1% pulp density) using biohydrometallurgy and pyrometallurgy. The biohydrometallurgical process had higher environmental impacts when compared to pyrometallurgy, resulting in 1 × 10−1 and 3.5 × 102 kg CO2-eq emissions, respectively. In addition, Ganesan and Valderrama [184] reported a saving of 3539 kg CO2-eq from the recycling of silicon photovoltaic panel waste due to high-value recovery.
Kara et al. [185] studied the economic feasibility of two industrial-scale bioleaching technologies (an aerated bioreactor as well as an aerated and stirred bioreactor) for the recovery of metals from metallurgical by-products using Acidithiobacillus ferrooxidans. Results showed that bioleaching proved financially viable for Cu recovery at pulp densities of 5% and 10% (aerated bioreactor) and 10% (aerated and stirred bioreactor). However, when the aerated and stirred bioreactor was used, a net present value (NPV) of USD 127.4 million and an internal rate of return (IRR) of 65% were achieved over 20 years. Yken et al. [186] carried out a techno-economic analysis on metal recovery from 1000 tons/year of PCBs in Australia. The process flow sheet showed that base metal leaching at 5% pulp density with pH control gave the highest projected profit of USD 2749/tons of PCBs. Boxall et al. [157] recovered different metals (Mn, Li, Cu, Ni, and Co) worth USD 10,769 from the bioleaching of one ton of discarded LIBs using a mixed culture of iron-oxidizing bacteria and sulfur-oxidizing bacteria. Techno-economic and life cycle analysis of the bioleaching of the REEs from fluidized catalytic cracking catalysts in a continuous bioreactor system revealed glucose as the single largest expense for the bioleaching process, accounting for 44% of the estimated cost. Based on the LCA conducted, both the glucose and electricity required for the bioreactor had significant impacts on the environment [187].
5. Conclusions and Future Perspectives
Industrial solid wastes, including fly ash, spent refinery catalysts, discarded batteries, e-waste, wastewater sludge, and metallurgical slags, consist of a wide variety of toxic compounds as well as valuable metals. Bioleaching is an eco-friendly, cheap, sustainable, and efficient approach for the extraction of metals from waste materials, thus promoting waste management and reuse. The use of extremophilic microorganisms is a promising biofactory for the effective recovery of metals from industrial solid wastes. These microbes possess specific metabolic, biochemical, and genetic properties to survive in harsh environments of extreme temperatures, salt concentrations, and pH that are encountered during bioleaching processes. Further studies should be carried out on the use of halotolerant microbes that can recover metals from industrial solid wastes when cultivated in seawater media, especially in countries where access to fresh water is limited. Innovative strategies involving the application of synthetic biology, metabolic engineering, and nanotechnology are vital for the enhanced performance of extremophiles in the bioleaching of metals from industrial solid wastes. In addition, future research should be focused on the discovery of novel and hyperactive extremophiles capable of enhancing the recovery of different metals from industrial solid wastes on a pilot or industrial scale. Microorganisms possessing polyextremophilic properties in terms of tolerance to extreme salinity, acidity, alkalinity, heavy metal concentrations, and temperatures could be applied for efficient and high yields of metals from industrial solid wastes.
Author Contributions
Conceptualization and writing—original draft preparation, A.I.A.; funding acquisition, review, and editing, M.E. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support of the Directorate of Research and Development (DRD) of the University of the Free State (UFS) as well as the Centre for Mineral Biogeochemistry, UFS, and the Biogeochemistry Research Infrastructure Platform (BIOGRIP) of the Department of Science and Innovation of South Africa is acknowledged.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egyptian J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- He, Y.; Kiehbadroudinezhad, M.; Hosseinzadeh-Bandbafha, H.; Gupta, V.K.; Peng, W.; Lam, S.S.; Tabatabaei, M.; Aghbashlo, M. Driving sustainable circular economy in electronics: A comprehensive review on environmental life cycle assessment of e-waste recycling. Environ. Pollut. 2024, 342, 123081. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Md Din, M.F.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Pan, Z.-Y.; Lang, J.-M.; Xu, J.-M.; Zheng, Y.-G. Bioleaching of chromium from tannery sludge by indigenous Acidithiobacillus thiooxidans. J. Hazard. Mater. 2007, 147, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Lee, J.-C.; Pandey, B.D.; Jeong, J.; Yoo, K.; Huynh, T.H. Bacterial cyanide generation in presence of metal ions (Na+, Mg2+, Fe2+, Pb2+) and gold bioleaching from waste PCBs. J. Chem. Eng. 2011, 44, 692–700. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, W.-H. Thermophilic bioleaching of heavy metals from waste sludge using response surface methodology. J. Environ. Sci. Health Part A 2013, 48, 1094–1104. [Google Scholar]
- Velgosová, O.; Kaduková, J.; Marcinčáková, R.; Palfy, P.; Trpčevská, J. Influence of H2SO4 and ferric iron on bioleaching from spent Ni-Cd batteries. Waste Manag. 2013, 33, 456–461. [Google Scholar] [CrossRef]
- Srichandan, H.; Singh, S.; Pathak, A.; Kim, D.J.; Lee, S.W.; Heyes, G. Bioleaching of heavy metals from spent refinery petroleum catalyst using moderately thermophilic bacteria: Effect of particle size. J. Environ. Sci. Health A 2014, 49, 807–818. [Google Scholar] [CrossRef]
- Niu, Z.; Huang, Q.; Wang, J.; Yang, Y.; Xin, B.; Chen, S. Metallic ions catalysis for improving bioleaching yield of Zn and Mn from spent Zn-Mn batteries at high pulp density of 10%. J. Hazard. Mat. 2015, 298, 170–177. [Google Scholar] [CrossRef]
- Rastegar, S.O.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of an oil-fired residual: Process optimization and nanostructure NaV6O15 synthesis from the bioleachate. RSC Adv. 2015, 5, 41088–41097. [Google Scholar] [CrossRef]
- Ramanathan, T.; Ting, Y.-P. Alkaline bioleaching of municipal solid waste incineration fly ash by autochthonous extremophiles. Chemosphere 2016, 160, 54–61. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sefti, M.V.; Shadman, M.M.; Dijvejin, Z.A.; Hosseini, H. The optimization of Cu and Fe bioleaching from converter slag using Acidithiobacillus ferrooxidans. J. Adv. Environ. Health Res. 2017, 5, 154–162. [Google Scholar]
- Naseri, T.; Bahaloo-Horeh, N.; Mousavi, S.M. Environmentally friendly recovery of valuable metals from spent coin cells through two-step bioleaching using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 235, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. J. Chem. Technol. Biotechnol. 2020, 95, 2272–2285. [Google Scholar] [CrossRef]
- Kinnunen, P.; Makinen, J.; Salo, M.; Soth, R.; Komnitsas, K. Efficiency of chemical and biological leaching of copper slag for the recovery of metals and valorization of the leach residue as raw material in cement production. Minerals 2020, 10, 654. [Google Scholar] [CrossRef]
- Liao, X.J.; Ye, M.Y.; Liang, J.L.; Guan, Z.; Li, S.P.; Deng, Y.H.; Gan, Q.W.; Liu, Z.H.; Fang, X.D.; Sun, S.Y. Feasibility of reduced iron species for promoting Li and Co recovery from spent LiCoO2 batteries using a mixed-culture bioleaching process. Sci. Total Environ. 2022, 830, 154577. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Biometallurgical recovery of metals from waste printed circuit boards using pure and mixed strains of Acidithiobacillus ferrooxidans and Acidiphilium acidophilum. Proc. Saf. Environ. Prot. 2020, 143, 262–272. [Google Scholar] [CrossRef]
- Rouchalova, D.; Rouchalova, K.; Janakova, I.; Cablik, V.; Janstova, S. Bioleaching of iron, copper, lead, and zinc from the sludge mining sediment at different particle sizes, pH, and pulp density using Acidithiobacillus ferrooxidans. Minerals 2020, 10, 1013. [Google Scholar] [CrossRef]
- Tapia, J.; Dueñas, A.; Cheje, N.; Soclle, G.; Patiño, N.; Ancalla, W.; Tenorio, S.; Denos, J.; Taco, H.; Cao, W.; et al. Bioleaching of heavy metals from printed circuit boards with an acidophilic iron-oxidizing microbial consortium in stirred tank reactors. Bioengineering 2022, 9, 79. [Google Scholar] [CrossRef]
- Baez, A.G.; Muñoz, L.P.; Timmermans, M.J.T.N.; Garelick, H.; Purchase, D. Molding the future: Optimization of bioleaching of rare earth elements from electronic waste by Penicillium expansum and insights into its mechanism. Bioresour. Technol. 2024, 402, 130750. [Google Scholar]
- Lee, J.-C.; Pandey, B.D. Bio-processing of solid wastes and secondary resources for metal extraction—A review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef]
- Tawonezvi, T.; Nomnqa, M.; Petrik, L.; Bladergroen, B.J. Recovery and recycling of valuable metals from spent lithium-ion batteries: A comprehensive review and analysis. Energies 2023, 16, 1365. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of metals from E-waste using microorganisms. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. From garbage to treasure: A review on biorefinery of organic solid wastes into valuable biobased products. Bioresour. Technol. Rep. 2023, 24, 101610. [Google Scholar] [CrossRef]
- Naseri, T.; Beiki, V.; Mousavi, S.M.; Farnaud, S. A comprehensive review of bioleaching optimization by statistical approaches: Recycling mechanisms, factors affecting, challenges, and sustainability. RSC Adv. 2023, 13, 23570–23589. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, X.; Zhang, Y.; Li, S.; Ye, M.; Gan, Q.; Fang, X.; Mo, Z.; Huang, Y.; Liang, Z.; et al. A highly efficient process to enhance the bioleaching of spent lithium-ion batteries by bifunctional pyrite combined with elemental sulfur. J. Environ. Manag. 2024, 351, 119954. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.K.; Hadibarata, T.; Jusoh, M.N.H.; Sutiknowati, L.I.; Tan, I.S.; Foo, H.C.Y. Role of extremophiles in biodegradation of emerging pollutants. Topics Catal. 2024. [Google Scholar] [CrossRef]
- Donati, E.R.; Castro, C.; Urbieta, M.S. Thermophilic microorganisms in biomining. World J. Microbiol. Biotechnol. 2016, 32, 179. [Google Scholar] [CrossRef]
- Jeong, S.W.; Choi, Y.J. Extremophilic microorganisms for the treatment of toxic pollutants in the environment. Molecules 2020, 25, 4916. [Google Scholar] [CrossRef]
- Kochhar, N.; Kavya, I.K.; Shrivastava, S.; Ghosh, A.; Rawat, V.S.; Sodhi, K.K.; Kumar, M. Perspectives on the microorganism of extreme environments and their applications. Curr. Res. Microb. Sci. 2022, 3, 100134. [Google Scholar] [CrossRef]
- Giaveno, M.A.; Urbieta, M.S.; Ulloa, J.R.; Toril, E.G.; Donati, E.R. Physiologic versatility and growth flexibility as the main characteristics of a novel thermoacidophilic Acidianus strain isolated from Copahue geothermal area in Argentina. Microb. Ecol. 2013, 65, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Microbiol. 1972, 84, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Segerer, A.; Langworthy, T.A.; Stetter, K.O. Thermoplasma acidophilum and Thermoplasma volcanium sp. nov. from Solfatara Fields. Syst. Appl. Microbiol. 1988, 10, 161–171. [Google Scholar] [CrossRef]
- Fütterer, O.; Angelov, A.; Liesegang, H.; Gottschalk, G.; Schleper, C.; Schepers, B.; Dock, C.; Antranikian, G.; Liebl, W. Genome sequence of Picrophilus torridus and its implications for life around pH 0. Proc. Natl. Acad. Sci. USA 2004, 101, 9091–9096. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Yakimov, M.M.; Lünsdorf, H.; Ferrer, M.; Nimtz, M.; Timmis, K.N.; Wray, V.; Tindall, B.J.; Golyshin, P.N. Acidiplasma aeolicum gen. nov., sp. nov., a euryarchaeon of the family Ferroplasmaceae isolated from a hydrothermal pool, and transfer of Ferroplasma cupricumulans to Acidiplasma cupricumulans comb. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2815–2823. [Google Scholar] [CrossRef]
- Castro, C.; Urbieta, M.; Cazón, J.P.; Donati, E. Metal biorecovery and bioremediation: Whether or not thermophilic are better than mesophilic microorganisms. Bioresour. Technol. 2019, 279, 317–326. [Google Scholar] [CrossRef]
- Abdel Azim, A.; Bellini, R.; Vizzarro, A.; Bassani, I.; Pirri, C.F.; Menin, B. Highlighting the role of archaea in urban mine waste exploitation and valorisation. Recycling 2023, 8, 20. [Google Scholar] [CrossRef]
- Sun, J.; He, X.; Le, Y.; Al-Tohamy, R.; Ali, S.S. Potential applications of extremophilic bacteria in the bioremediation of extreme environments contaminated with heavy metals. J. Environ. Manag. 2024, 352, 120081. [Google Scholar] [CrossRef]
- Gumulya, Y.; Boxall, N.J.; Khaleque, H.N.; Santala, V.; Carlson, R.P.; Kaksonen, A.H. In a quest for engineering acidophiles for biomining applications: Challenges and opportunities. Genes 2018, 9, 116. [Google Scholar] [CrossRef]
- Varshney, S.; Bhattacharya, A.; Gupta, A. Halo-alkaliphilic microbes as an effective tool for heavy metal pollution abatement and resource recovery: Challenges and future prospects. 3 Biotech 2023, 13, 400. [Google Scholar] [CrossRef]
- Kremser, K.; Thallner, S.; Schoen, H.; Weiss, S.; Hemmelmair, C.; Schnitzhofer, W.; Aldrian, A.; Guebitz, G.M. Stirred-tank and heap-bioleaching of shredderlight-fractions (SLF) by acidophilic bacteria. Hydrometallurgy 2020, 193, 105315. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Vakilchap, F.; Baniasadi, M.; Mousavi, S.M.; Khodadadi Darban, A.; Farnaud, S. Green recovery of cerium and strontium from gold mine tailings using an adapted acidophilic bacterium in one-step bioleaching approach. J. Taiwan Inst. Chem. Eng. 2022, 138, 10448. [Google Scholar] [CrossRef]
- Yin, X.; Shan, X.; Liang, G.; Zhou, Q.; Lin, W. A newly acidophilic bacterium Acidomyces acidothermus was isolated to efficiently bioleach copper from waste printed circuit boards (WPCBs). Sustainability 2023, 15, 2709. [Google Scholar] [CrossRef]
- Khalil, A.; Shaikh, S.; Tawabini, B. Removal of heavy metals from contaminated water by thermophilic bacteria isolated from hot springs in Saudi Arabia. J. Bacteriol. Mycol. Open Access 2022, 10, 60–64. [Google Scholar] [CrossRef]
- Siliakus, M.F.; Van der Oost, J.; Kengen, S.W.M. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elnaby, M.; Abou-Elela, G.M.; Hussein, H.; Ghozlan, H.A.; Sabry, S.A. Characterization and Bioremediation potential of marine psychrotolerant Pseudomonas spp. isolated from the Mediterranean Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 669–683. [Google Scholar] [CrossRef]
- Basak, P.; Biswas, A.; Bhattacharyya, M. Exploration of extremophiles genomes through gene study for hidden biotechnological and future potential. In Physiological and Biotechnological Aspects of Extremophiles; Academic Press: Cambridge, MA, USA, 2020; pp. 315–325. [Google Scholar]
- Ausuri, J.; Dell’Anno, F.; Vitale, G.A.; Palma Esposito, F.; Funari, V.; Franci, G.; Galdiero, M.; Della Sala, G.; Tedesco, P.; Coppola, D.; et al. Bioremediation of multiple heavy metals mediated by antarctic marine isolated Dietzia psychralcaliphila JI1D. J. Mar. Sci. Eng. 2022, 10, 1669. [Google Scholar] [CrossRef]
- Benmalek, Y.; Fardeau, M.L. Isolation and characterization of metal-resistant bacterial strain from wastewater and evaluation of its capacity in metal-ions removal using living and dry bacterial cells. Int. J. Environ. Sci. Technol. 2016, 13, 2153–2162. [Google Scholar] [CrossRef]
- Chandra, P.; Bhimrao, B.; Prakash, A.; Bhimrao, R.B.; Singh, D.P. Isolation of alkaliphilic bacterium Citricoccus alkalitolerans CSB1: An efficient biosorbent for bioremediation of tannery wastewater arsenic adsorption and its mobilization in the soil of arsenic affected areas. Cell Mol. Biol. 2016, 62, 135. [Google Scholar]
- Matsuno, T.; Goto, T.; Ogami, S.; Morimoto, H.; Yamazaki, K.; Inoue, N.; Matsuyama, H.; Yoshimune, K.; Yumoto, I. Formation of proton motive force under low-aeration alkaline conditions in alkaliphilic bacteria. Front. Microbiol. 2018, 9, 2331. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Waghmode, M.S.; Patil, N.N. Role of extremozymes in bioremediation. Res. J. Biotechnol. 2021, 16, 240–252. [Google Scholar]
- Rangasamy, A.; Gandhi, S.; Tamilchelvan, V. Biosorption of hexavalent chromium by Paenibacillus pabuli and Bacillus cereus isolated from alkaline industrial contaminated soil in Puducherry, India. Nat. Environ. Pollut. Technol. 2021, 20, 729–735. [Google Scholar] [CrossRef]
- Czech, L.; Hermann, L.; Stöveken, N.; Richter, A.; Höppner, A.; Smits, S.H.J.; Heider, J.; Bremer, E. Role of the extremolytes ectoine and hydroxyectoine as stress protectants and nutrients: Genetics, phylogenomics, biochemistry, and structural analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.B.; Catchpole, H.R. A microprobe analysis of inorganic elements in Halobacterium salinarum. Cell. Biol. Int. 2005, 29, 616–622. [Google Scholar] [CrossRef]
- DasSarma, S.; DasSarma, P. Halophiles and their enzymes: Negativity put to good use. Curr. Opin. Microbiol. 2015, 25, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1, 5. [Google Scholar] [CrossRef]
- Barrau, C.; Di Lorenzo, F.; Menes, R.J.; Lanzetta, R.; Molinaro, A.; Silipo, A. The structure of the lipid A from the halophilic bacterium Spiribacter salinus M19–40T. Mar. Drugs 2018, 16, 124. [Google Scholar] [CrossRef]
- León, M.J.; Hoffmann, T.; Sánchez-Porro, C.; Heider, J.; Ventosa, A.; Bremer, E. Compatible solute synthesis and imported by the moderate halophile Spiribacter salinus: Physiology and genomics. Front. Microbiol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Vandrich, J.; Pfeiffer, F.; Alfaro-Espinoza, G.; Kunte, H.J. Contribution of mechanosensitive channels to osmoadaptation and ectoine excretion in Halomonas elongata. Extremophile 2020, 24, 421–432. [Google Scholar] [CrossRef]
- Ortega, G.; Diercks, T.; Millet, O. Halophilic protein adaptation results from synergistic residue-ion interactions in the folded and unfolded states. Chem. Biol. 2015, 22, 1597–1607. [Google Scholar] [CrossRef]
- Nayek, A.; Gupta, P.S.; Banerjee, S.S.; Mondal, B.; Bandyopadhyay, A.K. Salt-bridge energetics in halophilic proteins. PLoS ONE 2014, 9, e93862. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alam, A.; Tripathi, D.; Rani, M.; Khatoon, H.; Pandey, S.; Ehtesham, N.Z.; Hasnain, S.E. Protein adaptations in extremophiles: An insight into extremophilic connection of mycobacterial proteome. Semin. Cell Dev. Biol. 2018, 84, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Asksonthong, R.; Siripongvutikorn, S.; Usawakesmanee, W. Heavy metal removal ability of Halomonas elongata and Tetragenococcus halophilus in a media model system as affected by pH and incubation time. Int. Food Res. J. 2018, 25, 234–240. [Google Scholar]
- Biswas, J.; Bose, P.; Mandal, S.; Paul, A.K. Reduction of hexavalent chromium by a moderately halophilic bacterium, Halomonas smyrnensis KS802 under saline environment. Environ. Sustain. 2018, 1, 411–423. [Google Scholar] [CrossRef]
- Torbaghan, M.E.; Torghabeh, G.H.K. Biological removal of iron and sulfate from synthetic wastewater of cotton delinting factory by using halophilic sulfate-reducing bacteria. Heliyon 2019, 5, e02948. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Goli, D. Bioremediation of lead by a halophilic bacteria Bacillus pumilus isolated from the mangrove regions of Karnataka. Int. J. Sci. Res. 2020, 9, 1337–1343. [Google Scholar]
- Diba, H.; Cohan, R.A.; Salimian, M.; Mirjani, R.; Soleimani, M.; Khodabakhsh, F. Isolation and characterization of halophilic bacteria with the ability of heavy metal bioremediation and nanoparticle synthesis from Khara salt lake in Iran. Arch. Microbiol. 2021, 203, 3893–3903. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Rastädter, K.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. The cell membrane of Sulfolobus spp.–homeoviscous adaptation and biotechnological applications. Int. J. Mol. Sci. 2020, 21, 3935. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Tran, H.; Reva, O.N.; Lemak, S.; Yakunin, A.F.; Goesmann, A.; Nechitaylo, T.Y.; LaCono, V.; Smedile, F.; Slesarev, A.; et al. Metabolic and evolutionary patterns in the extreme acidophilic archaeon Ferroplasma acidiphilum Y T. Sci. Rep. 2017, 7, 3682. [Google Scholar]
- Vergara, E.; Neira, G.; González, C.; Cortez, D.; Dopson, M.; Holmes, D.S. Evolution of predicted acid resistance mechanisms in the extremely acidophilic Leptospirillum genus. Genes 2019, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef]
- Ranawat, P.; Rawat, S. Stress response physiology of thermophiles. Arch. Microbiol. 2017, 199, 391–414. [Google Scholar] [CrossRef]
- Sprott, G.D.; Meloche, M.; Richards, J.C. Proportions of diether, macrocyclic diether, and tetraether lipids in Methanococcus jannaschii grown at different temperatures. J. Bacteriol. 1991, 173, 3907–3910. [Google Scholar] [CrossRef]
- Mansilla, M.C.; Cybulski, L.E.; Albanesi, D.; de Mendoza, D. Control of membrane lipid fluidity by molecular thermosensors. J. Bacteriol. 2004, 186, 6681–6688. [Google Scholar] [CrossRef]
- De Maayer, P.; Anderson, D.; Cary, C.; Cowan, D.A. Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Yoshimune, K.; Galkin, A.; Kulakova, L.; Yoshimura, T.; Esaki, N. Cold-active DnaK of an Antarctic psychrotroph Shewanella sp. Ac10 supporting the growth of dnaK-null mutant of Escherichia coli at cold temperatures. Extremophiles 2005, 9, 145–150. [Google Scholar] [CrossRef]
- Aono, R. Assignment of facultatively alkaliphilic Bacillus sp. strain C-125 to Bacillus lentus group 3. Int. J. Syst. Bacteriol. 1995, 45, 582–585. [Google Scholar]
- Fang, H.; Qin, X.-Y.; Zhang, K.-D.; Nie, Y.; Wu, X.-L. Role of the Group 2 Mrp sodium/proton antiporter in rapid response to high alkaline shock in the alkaline- and salt-tolerant Dietzia sp. DQ12-45-1b. Appl. Microbiol. Biotechnol. 2018, 102, 3765–3777. [Google Scholar] [CrossRef]
- Stancik, L.M.; Stancik, D.M.; Schmidt, B.; Barnhart, D.M.; Yoncheva, Y.N.; Slonczewski, J.L. pH-Dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 2002, 184, 4246–4258. [Google Scholar] [CrossRef] [PubMed]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 317. [Google Scholar] [PubMed]
- Kulkarni, S.; Dhakar, K.; Joshi, A. Alkaliphiles: Diversity and bioprospection. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Elsevier: London, UK, 2019; pp. 239–263. [Google Scholar]
- Isildar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Rautela, R.; Gujjala, L.K.S.; Kundu, D.; Sharma, P.; Tembhare, M.; Kumar, S. A review on recovery processes of metals from E-waste: A green perspective. Sci. Total Environ. 2023, 859, 160391. [Google Scholar] [CrossRef]
- Paiment, A.; Leduc, L.G.; Ferroni, G.D. The effect of the facultative chemolithotrophic bacterium Thiobacillus acidophilus on the leaching of low-grade Cu-Ni sulfide ore by Thiobacillus ferrooxidans. Geomicrobiol. J. 2001, 18, 157–165. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidizing bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Wang, Q.; Wu, T. Effects of water-washing pretreatment on bioleaching of heavy metals from municipal solid waste incineration fly ash. J. Hazard. Mat. 2009, 162, 812–818. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of copper from waste printed circuit boards by bacteria-free cultural supernatant of iron-sulfur-oxidizing bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef]
- Barkusaraey, F.H.; Mafigholami, R.; Ghasemi, M.F.; Khayati, G. Optimization of zinc bioleaching from paint sludge using Acidithiobacillus thiooxidans based on response surface methodology. J. Environ. Sci. Health Part A 2021, 56, 1243–1252. [Google Scholar] [CrossRef]
- Magoda, K.; Mekuto, L. Biohydrometallurgical recovery of metals from waste electronic equipment: Current status and proposed process. Recycling 2022, 7, 67. [Google Scholar] [CrossRef]
- Abashina, T.; Vainshtein, M. Current trends in metal biomining with a focus on genomics aspect and attention to arsenopyrite leaching- A review. Microorganisms 2023, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Z.; Liu, R.; Zhou, W.; Cheng, H.; Sun, J.; Zhao, K.; Wang, Y.; Zhou, H. Effective multi-metal removal from plant incineration ash via the combination of bioleaching and brine leaching. RSC Adv. 2020, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of microbial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.-P. Gold biorecovery from e-waste: An improved strategy through spent medium leaching with pH modification. Chemosphere 2015, 136, 232–238. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of fungal bioleaching of metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Desmarais, M.; Pirade, F.; Zhang, J.; Rene, E.R. Biohydrometallurgical processes for the recovery of precious and base metals from waste electrical and electronic equipments: Current trends and perspectives. Bioresour. Technol. Rep. 2020, 11, 100526. [Google Scholar] [CrossRef]
- Ilyas, S.; Chi, R.; Lee, J.-C. Fungal bioleaching of metals from mine tailing. Miner. Process. Extra Metall. Rev. 2013, 34, 185–194. [Google Scholar] [CrossRef]
- Dong, Y.; Zan, J.; Lin, H. Bioleaching of heavy metals from mine tailings utilizing bacteria and fungi: Mechanisms, strengthen measures, and development prospect. J. Environ. Manag. 2023, 344, 118511. [Google Scholar] [CrossRef]
- Trivedi, A.; Vishwakarma, A.; Saawarn, B.; Mahanty, B.; Hait, S. Fungal biotechnology for urban mining of metals from waste printed circuit boards: A review. J. Environ. Manag. 2022, 323, 116133. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ramsay, J.; Ghahreman, A. A review of biocyanidation as a sustainable route for cold recovery from primary and secondary low-grade resources. J. Clean. Prod. 2021, 296, 126457. [Google Scholar] [CrossRef]
- Vo, P.H.N.; Danaee, S.; Hai, H.T.N.; Huy, L.N.; Nguyen, T.A.H.; Nguyen, H.T.M.; Kuzhiumparambil, U.; Kim, M.; Nghiem, L.D.; Ralph, P.J. Biomining for sustainable recovery of rare earth elements from mining waste: A comprehensive review. Sci. Total Environ. 2024, 908, 168210. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Lee, J.C. Biometallurgical recovery of metals from waste electrical and electronic equipment: A review. ChemBioEng. Rev. 2014, 1, 148–169. [Google Scholar] [CrossRef]
- Ji, X.; Yang, M.; Wan, A.; Yu, S.; Yao, Z. Bioleaching of typical electronic waste-printed circuit boards (WPCBs): A short review. Int. J. Environ. Res. Public Health 2022, 19, 7508. [Google Scholar] [CrossRef] [PubMed]
- Nagar, N.; Garg, H.; Sharma, N.; Awe, S.A.; Gahan, C.S. Effect of pulp density on the bioleaching of metals from petroleum refinery spent catalyst. 3 Biotech 2021, 11, 143. [Google Scholar] [CrossRef]
- Gholami, R.M.; Borghei, S.M.; Mousavi, S.M. Bacterial leaching of a spent Mo–Co–Ni refinery catalyst using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 2011, 106, 26–31. [Google Scholar] [CrossRef]
- Mouna, H.M.; Baral, S.S.; Mohapatra, P. Leaching of metals from spent fluid catalytic cracking catalyst using Acidothiobacillus ferrooxidans and comparing its leaching efficiency with organic and inorganic acids. J. Environ. Chem. Eng. 2021, 9, 105522. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Ting, Y.P. Bioleaching of spent hydrotreating catalyst by acidophilic thermophile Acidianus brierleyi: Leaching mechanism and effect of decoking. Bioresour. Technol. 2013, 130, 673–680. [Google Scholar] [CrossRef]
- Shahrabi-Farahani, M.; Yaghmaei, S.; Mousavi, S.M.; Amiri, F. Bioleaching of heavy metals from a petroleum spent catalyst using Acidithiobacillus thiooxidans in a slurry bubble column bioreactor. Sep. Purif. Technol. 2014, 132, 41–49. [Google Scholar] [CrossRef]
- Pradhan, D.; Mishra, D.; Kim, D.J.; Roychaudhury, G.; Lee, S.W. Dissolution kinetics of spent petroleum catalyst using two different acidophiles. Hydrometallurgy 2009, 99, 157–162. [Google Scholar] [CrossRef]
- Srichandan, H.; Mishra, S.; Singh, P.K.; Blight, K.; Singh, S. Sequential-anaerobic and sequential-aerobic bioleaching of metals (Ni, Mo, Al and V) from spent petroleum catalyst in stirred tank reactor: A comparative study. Indian J. Microbiol. 2022, 62, 70–78. [Google Scholar] [CrossRef]
- Mikoda, B.; Potysz, A.; Kucha, H.; Kmiecik, E. Vanadium removal from spent sulfuric acid plant catalyst using citric acid and Acidithiobacillus thiooxidans. Archiv. Civ. Mech. Eng. 2020, 20, 132. [Google Scholar] [CrossRef]
- Ferreira, P.F.; Sérvulo, E.F.C.; da Costa, A.C.A.; Ferreira, D.M.; Godoy, M.L.D.P.; Oliveira, F.J.S. Bioleaching of metals from a spent diesel hydrodesulfurization catalyst employing Acidithiobacillus thiooxidans FG-01. Braz. J. Chem. Eng. 2017, 34, 119–129. [Google Scholar] [CrossRef]
- Kim, D.J.; Srichandan, H.; Gahan, C.S.; Lee, S.-W. Thermophilic bioleaching of spent petroleum refinery catalyst using Sulfolobus metallicus. Can. Metall. Q. 2012, 51, 403–412. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ralph, D.E.; Ahn, J.G.; Rhee, Y.H. Bioleaching of spent hydro-processing catalyst using Acidophilic bacteria and its kinetics aspect. J. Hazard. Mater. 2008, 152, 1082–1091. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Teng, S.; Shi, G.; Cheng, J.; Zhang, N.; Shao, Q.; Cui, Y.; Wang, J.; Xin, B. Intensified bioleaching of a spent Co-Mo catalyst through the addition of extracellular polymeric substances (EPSs) and its mechanism exploration. Dalton Trans. 2024, 53, 11787–11799. [Google Scholar] [CrossRef]
- Viller, L.D.; Garcia, O.J. Effect of anaerobic digestion and initial pH on metal bioleaching from sewage sludge. J. Env. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2006, 41, 211–212. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, J.U.; Jang, A. Bioleaching of heavy metals from dewatered sludge by Acidithiobacillus ferrooxidans. J. Chem. Technol. Biotechnol. 2005, 80, 1339–1348. [Google Scholar] [CrossRef]
- Jain, R.; Pathak, A.; Sreekrishnan, T.R.; Dastidar, M.G. Autoheated thermophilic aerobic sludge digestion and metal bioleaching in a two-stage reactor system. J. Environ. Sci. 2010, 22, 230–236. [Google Scholar] [CrossRef]
- Li, H.; Ye, M.; Zheng, L.; Xu, Y.; Sun, S.; Du, Q.; Zhong, Y.; Ye, S.; Zhang, D. Optimization of kinetics and operating parameters for the bioleaching of heavy metals from sewage sludge, using co-inoculation of two Acidithiobacillus species. Water Sci. Technol. 2018, 2017, 390–403. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Qin, Z.; Wen, L.; Li, Z.; Chu, H.; Xin, B. Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J. Environ. Manag. 2022, 312, 114927. [Google Scholar] [CrossRef]
- Maes, S.; Claus, M.; Verbeken, K.; Wallaert, E.; De Smet, R.; Vanhaecke, F.; Boon, N.; Hennebel, T. Platinum recovery from industrial process streams by halophilic bacteria: Influence of salt species and platinum speciation. Water Res. 2016, 105, 436–443. [Google Scholar] [CrossRef]
- Bayat, B.; Sari, B. Comparative evaluation of microbial and chemical leaching processes for heavy metal removal from dewatered metal plating sludge. J. Hazard. Mater. 2010, 174, 763–769. [Google Scholar] [CrossRef]
- Zhou, L.X.; Fang, D.; Wang, S.M.; Wong, J.W.C.; Wang, D.Z. Bioleaching of chromium from tannery sludge: The effect of initial acid addition and recycling of acidified bioleached sludge. Environ. Technol. 2005, 26, 227–284. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, Z.; Liu, X.; Yin, H.; Qiu, G.; Pan, F.; Liu, H. The bioleaching feasibility of Pb/Zn smelting slag and community characteristics of indigenous moderate-thermophilic bacteria. Bioresour. Technol. 2009, 100, 2737–2740. [Google Scholar] [CrossRef]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal values from polish copper metallurgical slags using Acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef]
- Hocheng, H.; Su, C.; Jadhav, U.U. Bioleaching of metals from steel slag by Acidithiobacillus thiooxidans culture supernatant. Chemosphere 2014, 117, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, X.; Yang, Q.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Comparative Study on Bioleaching of Two Different Types of Low-Grade Copper Tailings by Mixed Moderate Thermophiles. Trans. Nonferrous Met. Soc. China 2018, 28, 1847–1853. [Google Scholar] [CrossRef]
- Carranza, F.; Romero, R.; Mazuelos, A.; Iglesias, N.; Forcat, O. Biorecovery of copper from converter slag: Slags characterization and exploratory ferric leaching tests. Hydrometallurgy 2009, 97, 39–45. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Lavonen, L.; Kuusenaho, M.; Kolli, A.; Närhi, H.; Vestola, E.; Puhakka, J.A.; Tuovinen, O.H. Bioleaching and recovery of metals from final slag waste of the copper smelting industry. Miner. Eng. 2011, 24, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, X.; Hao, Q.; Si, R. Bioleaching of heavy metals from municipal solid waste incineration fly ash: Availability of recoverable sulfur prills and form transformation of heavy metals. Metals 2020, 10, 815. [Google Scholar] [CrossRef]
- Kanesalingam, B.; Fernando, W.A.M.; Panda, S.; Jayawardena, C.; Attygalle, D.; Amarasinghe, D.A.S. Harnessing the capabilities of microorganisms for the valorisation of coal fly ash waste through biometallurgy. Minerals 2023, 13, 724. [Google Scholar] [CrossRef]
- Ramanathan, T.; Ting, Y.P. Fly Ash and the Use of Bioleaching for Fly Ash Detoxification, Fly Ash: Chemical Composition, Sources and Potential Environmental Impacts; Nova Publishers: New York, NY, USA, 2014. [Google Scholar]
- Brombacher, C.; Bachofen, R.; Brandl, H. Development of a laboratory-scale leaching plant for metal extraction from fly ash by Thiobacillus strains. Appl. Environ. Microbiol. 1998, 64, 1237–1241. [Google Scholar] [CrossRef]
- Ishigaki, T.; Nakanishi, A.; Tateda, M.; Ike, M.; Fujita, M. Bioleaching of metal from municipal waste incineration fly ash using a mixed culture of sulfur-oxidizing and iron-oxidizing bacteria. Chemosphere 2005, 60, 1087–1094. [Google Scholar] [CrossRef]
- Pangayao, D.C. Bioleaching of Trace Metals from Coal Ash Using Acithiobacillus albertensis, Acithiobacillus thiooxidans, and Local Isolates from Coal Ash Pond. Ph.D. Thesis, Gokongwei College of Engineering, Manila, Philippines, 2016. [Google Scholar]
- Moosakazemi, F.; Ghassa, S.; Jafari, M.; Chelgani, S.C. Bioleaching for recovery of metals from spent batteries—A review. Miner. Proc. Extr. Metall. Rev. 2023, 44, 511–521. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Wang, H.; Zhang, B. Research progress on bioleaching recovery technology of spent lithium-ion batteries. Environ. Res. 2023, 238, 117145. [Google Scholar] [CrossRef]
- Ghassa, S.; Farzanegan, A.; Gharabaghi, M.; Abdollahi, H. Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent. Hydrometallurgy 2020, 197, 105465. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, N.-W.; Wang, X.-H. Comparison of bio-dissolution of spent Ni-Cd batteries by sewage sludge ferrous ions and elemental sulfur as substrate. Chemosphere 2008, 70, 974–981. [Google Scholar] [CrossRef]
- Verma, V.; Joseph, J.R.; Chaudhary, R.; Srinivasan, M. Upcycling spent cathode materials from Li-ion batteries to precursors: Challenges and opportunities. J. Environ. Chem. Eng. 2023, 11, 110216. [Google Scholar] [CrossRef]
- Sayilgan, E.; Kukrer, T.; Civelekoglu, G.; Ferella, F.; Akcil, A.; Veglio, F.; Kitis, M. A review of technologies for the recovery of metals from spent alkaline and zinc–carbon batteries. Hydrometallurgy 2009, 97, 158–166. [Google Scholar] [CrossRef]
- Calin, L.; Catinean, A.; Bilici, M.; Samuila, A. A corona-electrostatic technology for zinc and brass recovery from the coarse fraction of the recycling process of spent alkaline and zinc–carbonbatteries. J. Clean. Prod. 2021, 278, 123477. [Google Scholar] [CrossRef]
- Chakankar, M.; Jadhav, U.; Hocheng, H. Assessment of bio-hydrometallurgical metal recovery from Ni-Cd batteries. Adv. Eng. Res. 2017, 115, 539–545. [Google Scholar]
- Biswal, B.K.; Balasubramanian, R. Recovery of valuable metals from spent lithium-ion batteries using microbial agents for bioleaching: A review. Front. Microbiol. 2023, 14, 1197081. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Tan, N.; Zhu, M.; Tan, W.; Daramola, D.; Gu, T. Advances in bioleaching of waste lithium batteries under metal ion stress. Bioresour. Bioprocess. 2023, 10, 19. [Google Scholar] [CrossRef]
- Lalropuia, L.; Kucera, J.; Rassy, W.Y.; Pakostova, E.; Schild, D.; Mandl, M.; Kremser, K.; Guebitz, G.M. Metal recovery from spent lithium-ion batteries via two-step bioleaching using adapted chemolithotrophs from an acidic mine pit lake. Front. Microbiol. 2024, 15, 1347072. [Google Scholar] [CrossRef]
- Roy, J.J.; Srinivasan, M.; Cao, B. Bioleaching as an eco-friendly approach for metal recovery from spent NMC-based lithium-ion batteries at a high pulp density. ACS Sustain. Chem. Eng. 2021, 9, 3060–3069. [Google Scholar]
- Mishra, D.; Kim, D.-J.; Ralph, D.E.; Ahn, J.-G.; Rhee, Y.-H. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Gaydardzhiev, S.; van Hullebusch, E.D.; Gönen, M.; Dembele, S. Biotechnological recycling and recovery of metals from waste printed circuit boards and spent Li-ion batteries—Selected results from the ERAMIN EU BaCLEM project. Mat. Proc. 2024, 15, 76. [Google Scholar]
- Heydarian, A.; Mousavi, S.M.; Vakilchap, F.; Baniasadi, M. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J. Power Sources 2018, 378, 19–30. [Google Scholar] [CrossRef]
- Xin, B.; Jiang, W.; Aslam, H.; Zhang, K.; Liu, C.; Wang, R.; Wang, Y. Bioleaching of zinc and manganese from spent Zn-Mn batteries and mechanism exploration. Bioresour. Technol. 2012, 106, 147–153. [Google Scholar] [CrossRef]
- Cerruti, C.; Curutchet, G.; Donati, E. Bio-dissolution of spent nickel-cadmium batteries using Thiobacillus ferrooxidans. J. Biotechnol. 1998, 62, 209–219. [Google Scholar] [CrossRef]
- Boxall, N.J.; Cheng, K.Y.; Bruckard, W.; Kaksonen, A.H. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries. J. Hazard. Mater. 2018, 360, 504–511. [Google Scholar] [CrossRef]
- Putra, R.A.; Al Fajri, I.; Hariyadi, A. Metal bioleaching of used lithium ion battery using acidophilic ferrooxidans isolated from acid mine drainage. Key Eng. Mater. 2022, 937, 193–200. [Google Scholar] [CrossRef]
- The Global E-Waste Monitor 2024—Electronic Waste Rising Five Times Faster than Documented E-Waste Recycling: UN. 2024. Available online: https://ewastemonitor.info/the-global-e-waste-monitor-2024/ (accessed on 12 August 2024).
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Vienna, Austria, 2020. [Google Scholar]
- Singh, N.; Ogunseitan, O.A. Disentangling the worldwide web of e-waste and climate change co-benefits. Circ. Econ. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Ilyas, S.; Ruan, C.; Bhatti, H.N.; Ghauri, M.A.; Anwar, M.A. Column bioleaching of metals from electronic scrap. Hydrometallurgy 2010, 101, 135–140. [Google Scholar] [CrossRef]
- Anaya-Garzon, J.; Hubau, A.; Joulian, C.; Guezennec, A.-G. Bioleaching of e-waste: Influence of printed circuit boards on the activity of acidophilic iron-oxidizing bacteria. Front. Microbiol. 2021, 12, 669738. [Google Scholar] [CrossRef]
- Kadivar, S.; Pourhossein, F.; Mousavi, S.M. Recovery of valuable metals from spent mobile phone printed circuit boards using biochar in indirect bioleaching. J. Environ. Manag. 2021, 280, 111642. [Google Scholar] [CrossRef]
- Chandane, P.; Jori, C.; Chaudhari, H.; Bhapkar, S.; Deshmukh, S.; Jadhav, U. Bioleaching of copper from large printed circuit boards for synthesis of organic-inorganic hybrid. Environ. Sci. Pollut. Res. 2020, 27, 5797–5808. [Google Scholar] [CrossRef]
- Benzal, E.; Solé, M.; Lao, C.; Gamisans, X.; Dorado, A.D. Elemental copper recovery from e-waste mediated with a two-step bioleaching process. Waste Biomass Valoriz. 2020, 11, 5457–5465. [Google Scholar] [CrossRef]
- Chi, T.D.; Lee, J.C.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Pakostova, E.; Herath, A. A bioleaching process for sustainable recycling of complex structures with multi-metal layers. Sustainability 2023, 15, 14068. [Google Scholar] [CrossRef]
- Arslan, V. Bacterial leaching of copper, zinc, nickel and aluminum from discarded printed circuit boards using acidophilic bacteria. J. Mat. Cycl. Waste Manag. 2021, 23, 2005–2015. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Q.; Kamali, A.R.; Sand, W.; Yang, H. Effect of graphite on copper bioleaching from waste printed circuit boards. Minerals 2020, 10, 79. [Google Scholar] [CrossRef]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.-I.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.; Srinivasan, M. Green recycling methods to treat lithium-ion batteries E-waste: A circular approach to sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, M.; Reed, D.; Thompson, V.; Fujita, Y.; Jin, H. Sustainable bioleaching of lithium-ion batteries for critical materials recovery. J. Clean. Prod. 2023, 382, 135274. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Hong, J.; Dai, J.; Wang, R.; Niu, Z.; Xin, B. Life cycle assessment of a bio-hydrometallurgical treatment of spent Zn–Mn batteries. J. Clean. Prod. 2016, 129, 350–358. [Google Scholar] [CrossRef]
- Deng, B.; Meng, W.; Advincula, P.A.; Eddy, L.; Ucak-Astarlioglu, M.G.; Wyss, K.M.; Chen, W.; Carter, R.A.; Li, G.; Cheng, Y.; et al. Heavy metal removal from coal fly ash for low carbon footprint cement. Comm. Eng. 2023, 2, 13. [Google Scholar] [CrossRef]
- Rezaee, M.; Saneie, R.; Mohammadzadeh, A.; Abdollahi, H.; Kordloo, M.; Rezaee, A.; Vahidi, E. Eco-friendly recovery of base and precious metals from waste printed circuit boards by step-wise glycine leaching: Process optimization, kinetics modeling, and comparative life cycle assessment. J. Clean. Prod. 2023, 389, 136016. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Z.; Li, J.; Zeng, X. The life cycle assessment of an e-waste treatment enterprise in China. J. Mater. Cycles Waste Manag. 2013, 15, 469–475. [Google Scholar] [CrossRef]
- Fiore, S.; Ibanescu, D.; Teodosiu, C.; Ronco, A. Improving waste electric and electronic equipment management at full-scale by using material flow analysis and life cycle assessment. Sci. Total Environ. 2019, 659, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Hait, S. Comparative assessment of metallurgical recovery of metals from electronic waste with special emphasis on bioleaching. Environ. Sci. Pollut. Res. 2017, 24, 6989–7008. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Terrafame, Terrafame’s Nickel Sulphate Production Offers the Lowest Carbon Footprint in the Industry-Altogether 60% Lower than in Existing Conventional Processes. 2020. Available online: https://www.terrafame.com/newsroom/media-releases/terrafames-nickel-sulphate-production-offers-the-lowest-carbon-footprint-in-the-industry-altogether-60-lower-than-in-existing-conventional-processes.html?p461=12 (accessed on 22 August 2024).
- Kinnunen, P.; Hedrich, S. Biotechnological strategies to recover value from waste. Hydrometallurgy 2023, 222, 106182. [Google Scholar] [CrossRef]
- Villares, M.; Işildar, A.; Mendoza Beltran, A.; Guinee, J. Applying an ex-ante life cycle perspective to metal recovery from e-waste using bioleaching. J. Clean. Prod. 2016, 129, 315–328. [Google Scholar] [CrossRef]
- Ganesan, K.; Valderrama, C. Anticipatory life cycle analysis framework for sustainable management of end-of-life crystalline photovoltaic panels. Energy 2022, 245, 123207. [Google Scholar] [CrossRef]
- Kara, I.T.; Wagland, S.T.; Coulon, F. Techno-economic assessment of bioleaching for metallurgical by-products. J. Environ. Manag. 2024, 358, 120904. [Google Scholar] [CrossRef]
- Yken, J.V.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.; Kaksonen, A.H. Techno-economic analysis of an integrated bio- and hydrometallurgical process for base and precious metal recovery from waste printed circuit boards. Hydrometallurgy 2023, 222, 106193. [Google Scholar] [CrossRef]
- Thompson, V.S.; Gupta, M.; Vahidi, E.; Yim, M.; Jindra, M.A.; Nguyen, V.; Fujita, Y.; Sutherland, J.W.; Jiao, Y.; Reed, D.W. Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain. Chem. Eng. 2018, 6, 1602–1609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).