Geochemistry and Fluid Inclusion of Epithermal Gold-Silver Deposits in Kamchatka, Russia
Abstract
1. Introduction
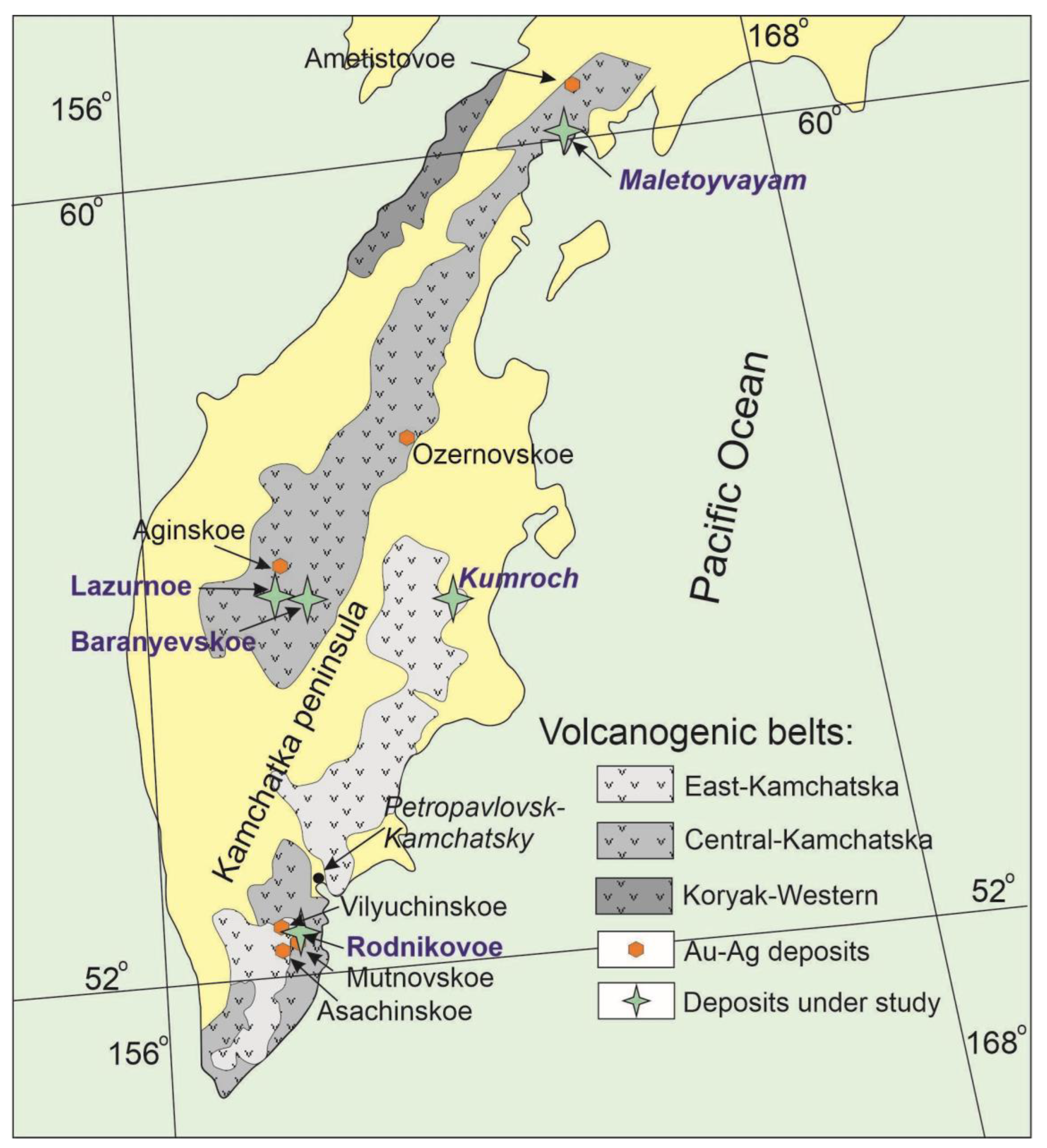
2. Geological and Mineralogical Background
3. Sampling and Analytical Methods
3.1. Geochemistry of Rare Elements
3.2. Fluid Inclusions Analysis
4. Results
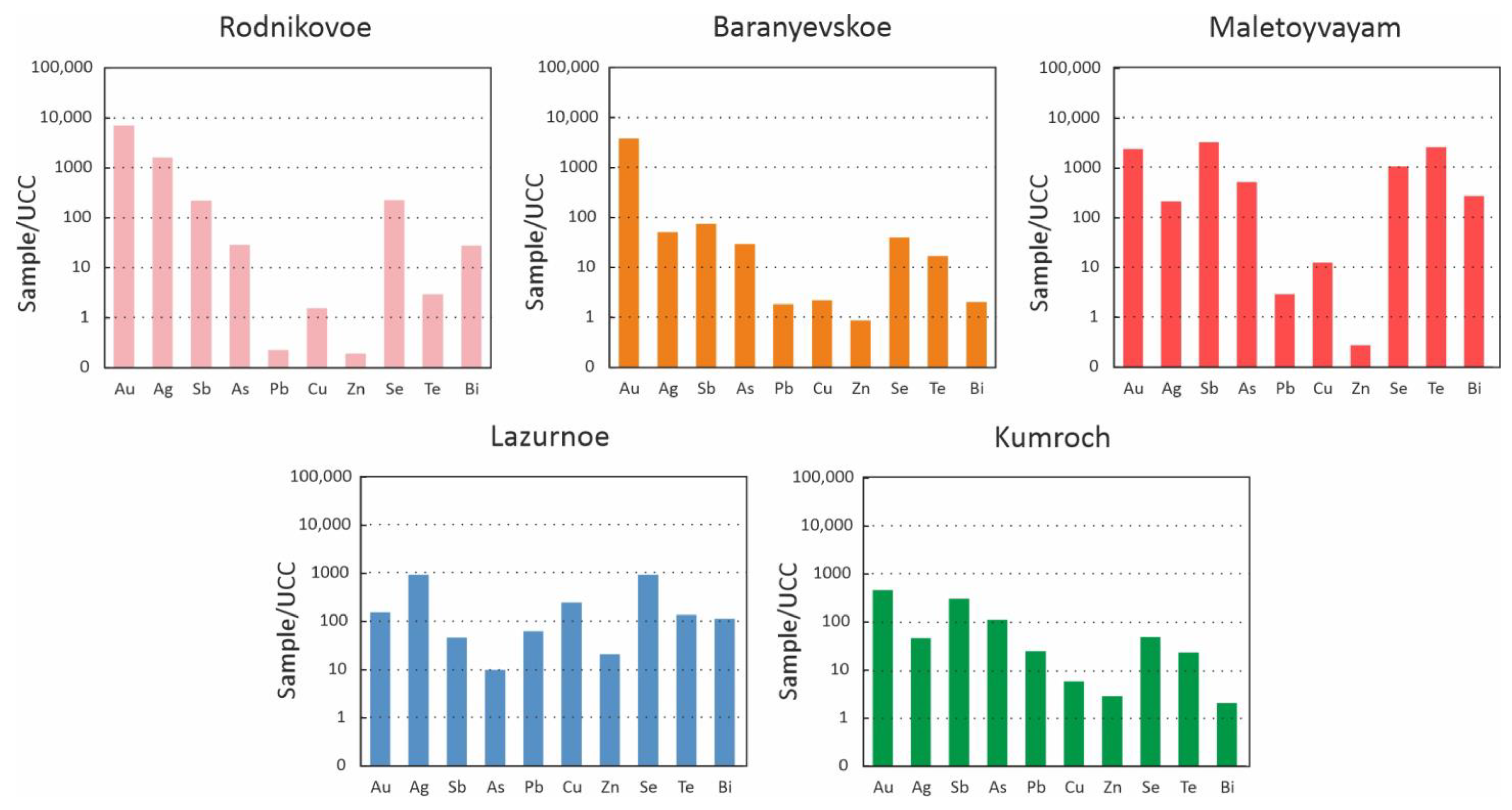
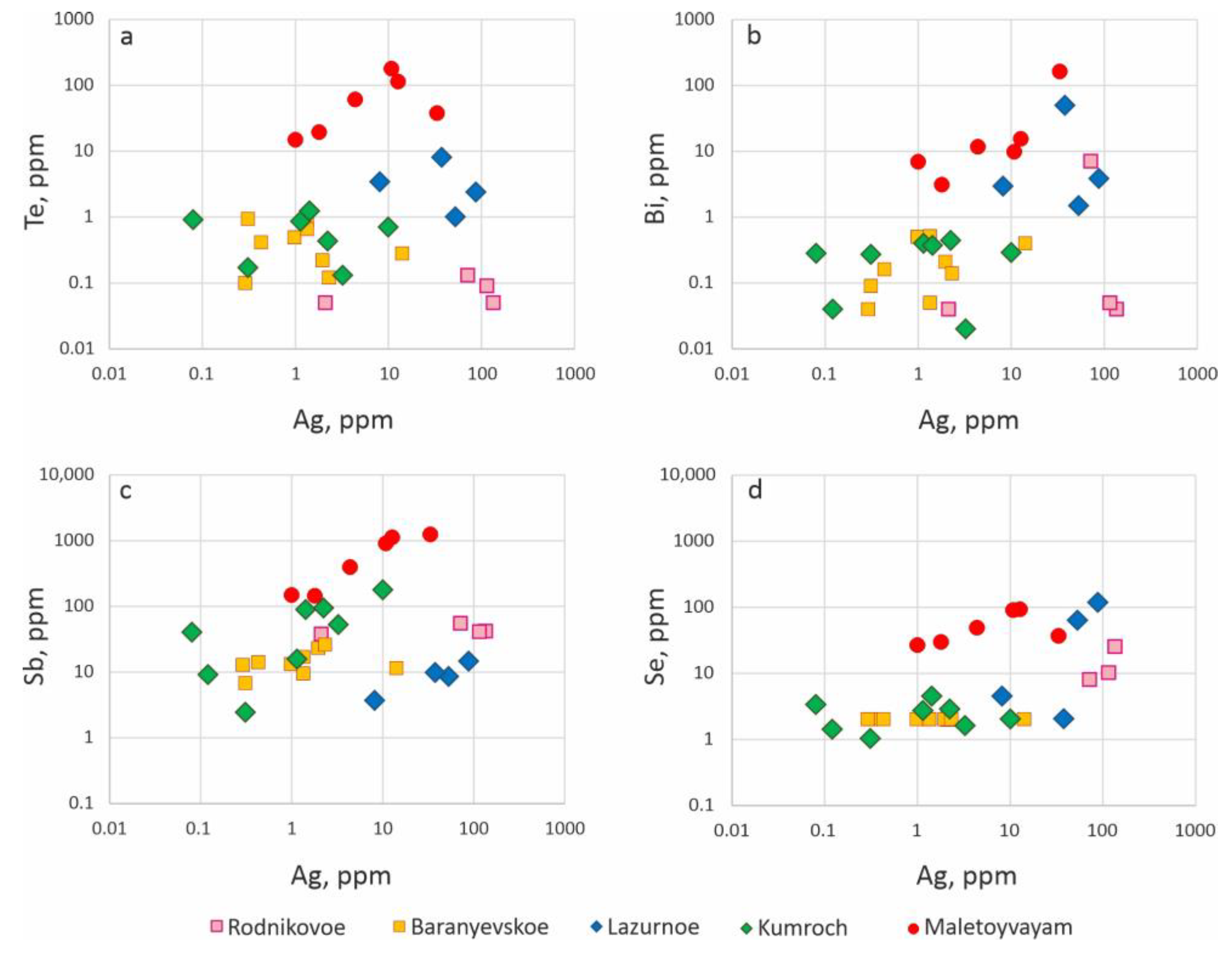
4.1. Geochemistry of Ore-Forming Elements
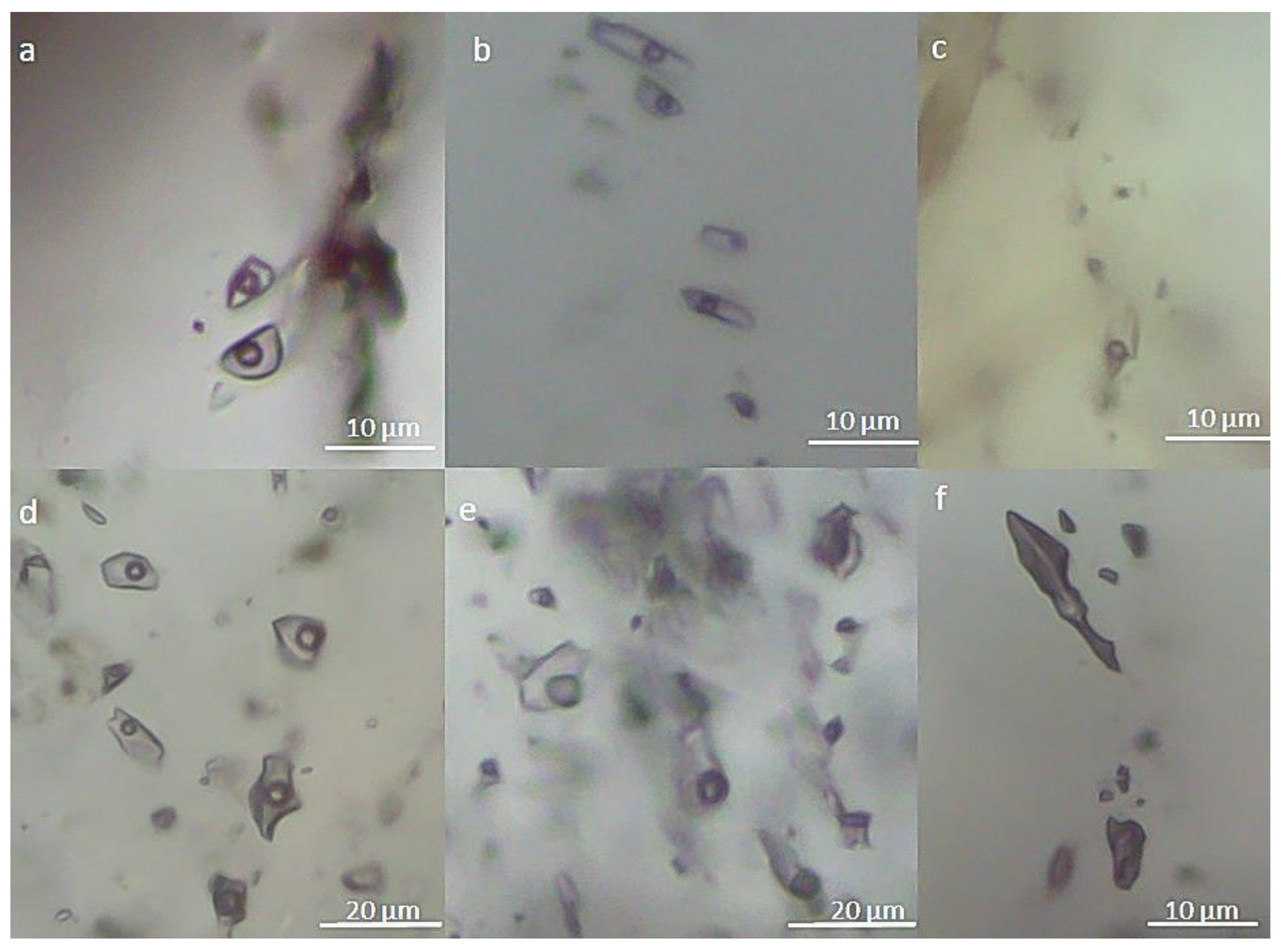
4.2. Fluid Inclusion Types
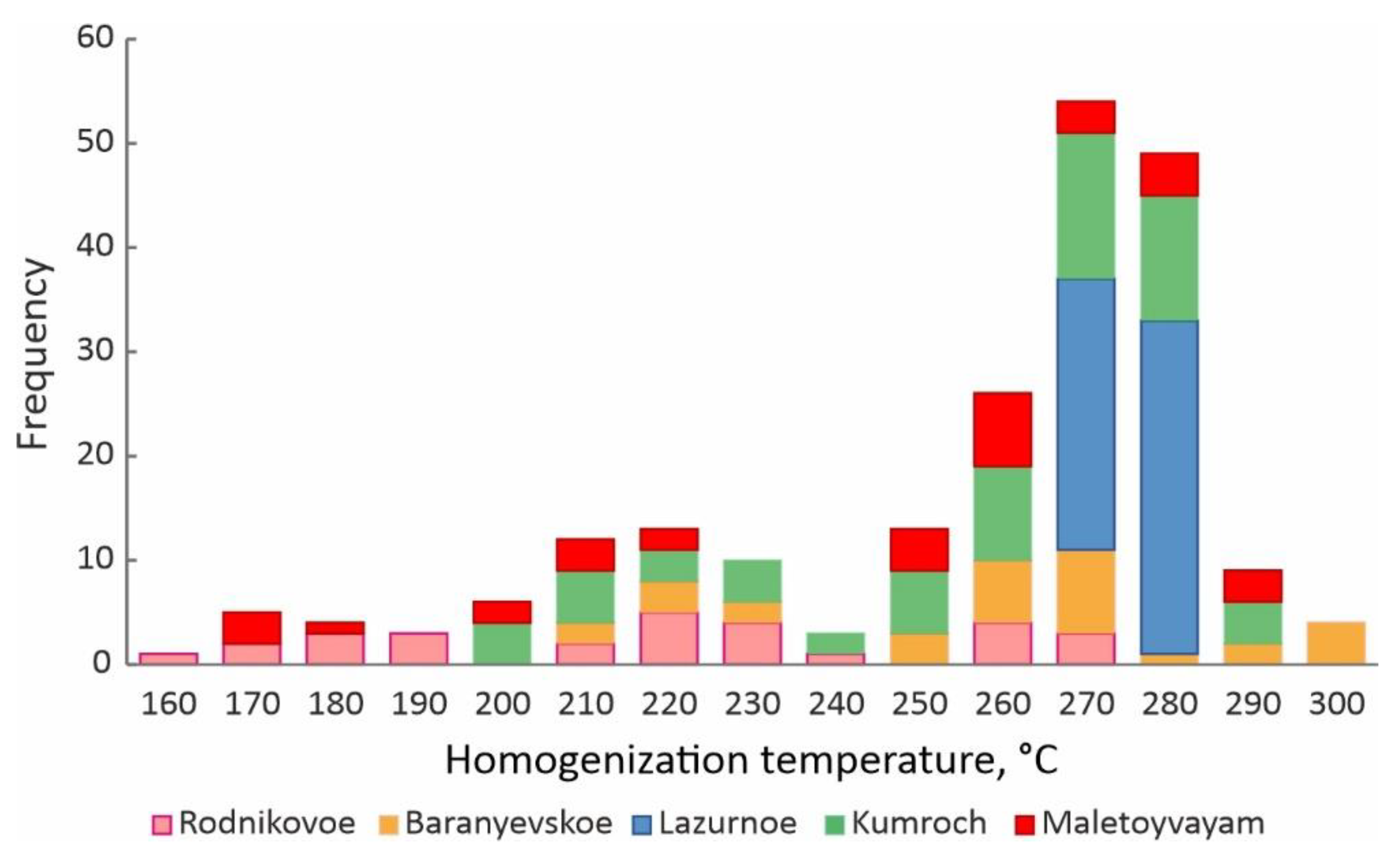
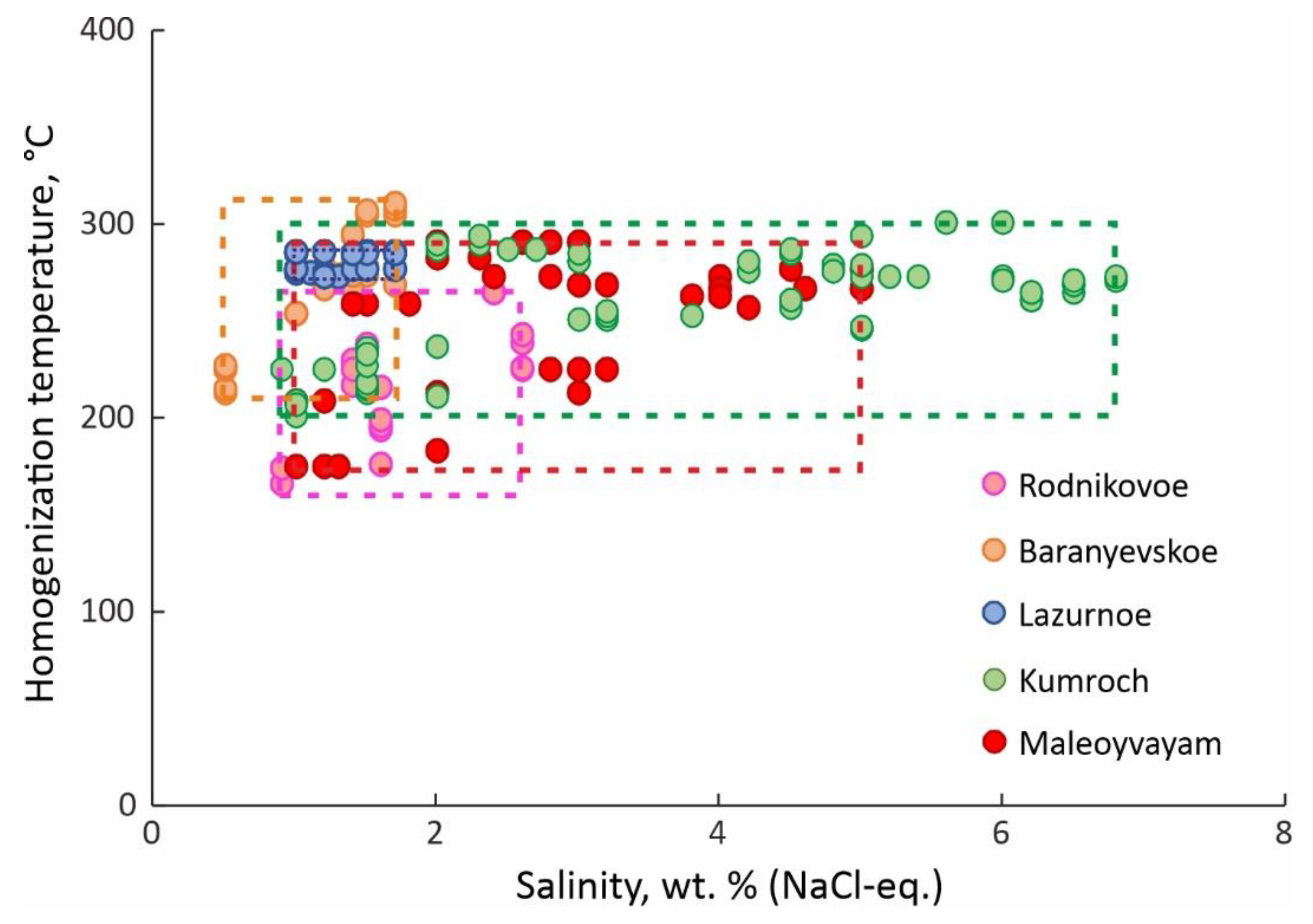
4.3. Homogenization Temperatures, Salinity and Pressure of Fluid
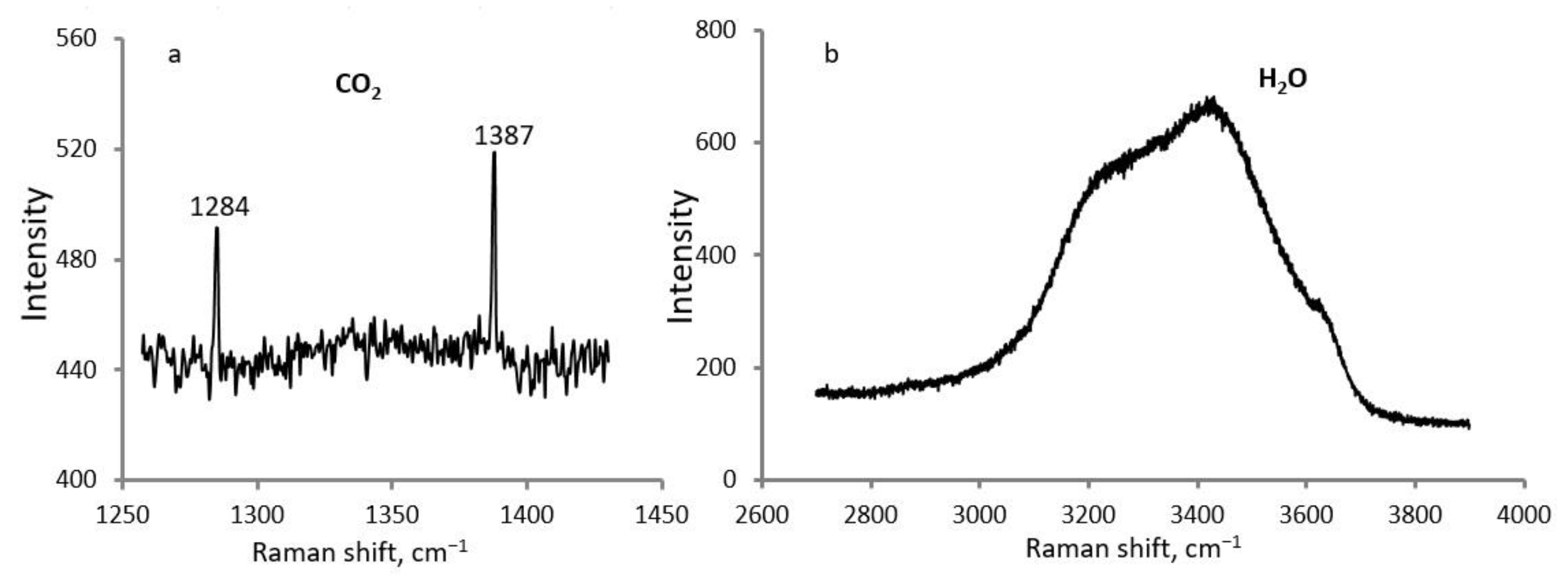
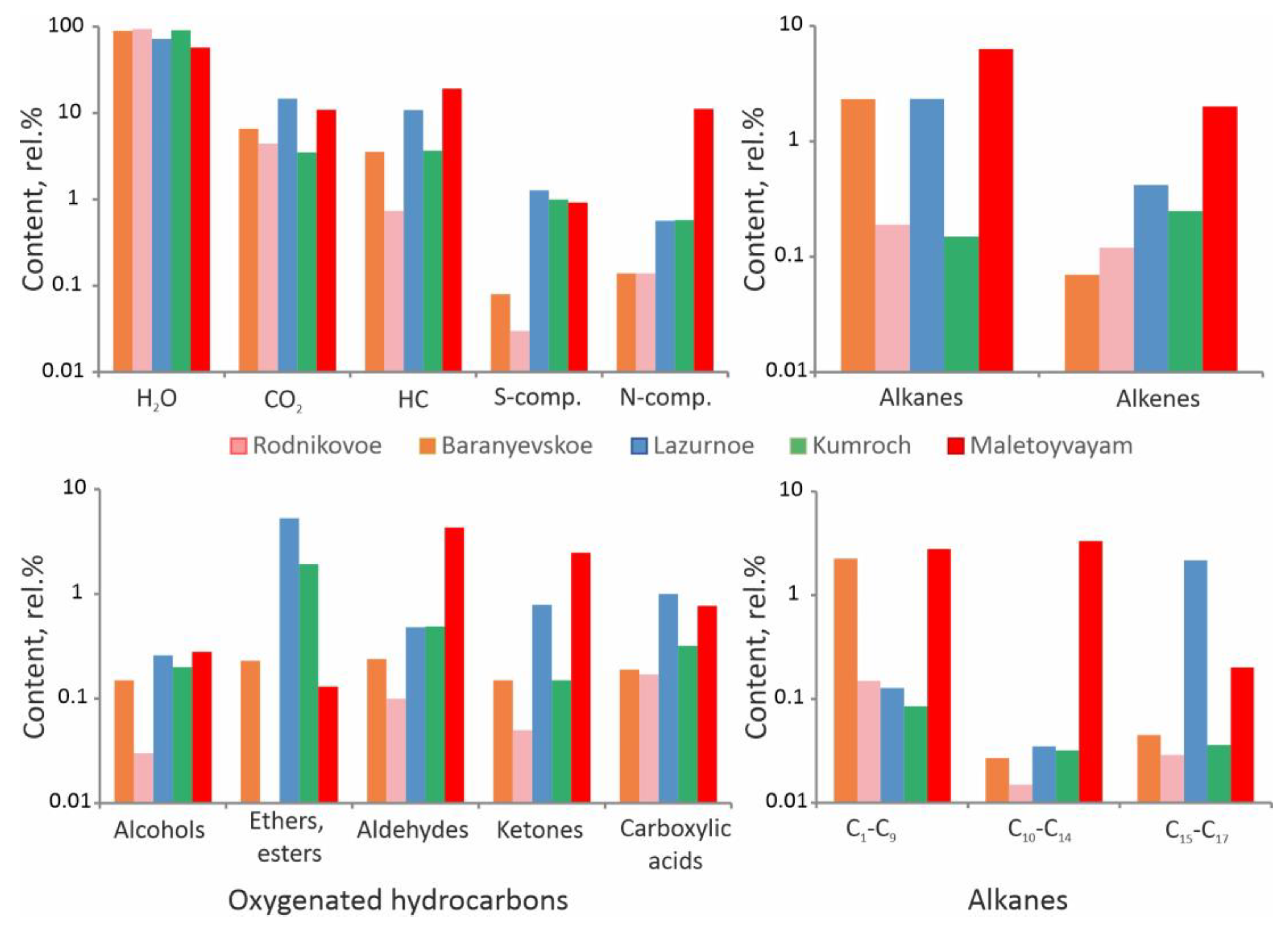
4.4. Composition of the Gaseous Phase of Fluid Inclusions
5. Discussion
5.1. Geochemistry of Ore-Forming Elements
5.2. Conditions of Au-Ag Mineralisation Formation
5.3. Origin of Hydrocarbons
5.4. The Role of Organic Compounds in the Accumulation of Ore-Forming Elements
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frimmel, H.E. Earth’s continental crustal gold endowment. Earth Planet. Sci. Lett. 2008, 267, 45–55. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Plotinskaya, O.Y. Te and Se mineralogy of Ozernovskoe and Prasolovskoe epithermal gold deposits (Kuril–Kamchatka volcanic belt). Geochem. Mineral. Petrol. 2005, 43, 118–124. [Google Scholar]
- Bortnikov, N.S.; Volkov, A.V.; Galyamov, A.L.; Vikent’ev, I.V.; Aristov, V.V.; Lalomov, A.V.; Murashov, K.Y. Mineral resources of high-tech metals in Russia: State of the art and outlook. Geol. Ore Depos. 2016, 58, 83–103. [Google Scholar] [CrossRef]
- Bortnikov, N.S. Geochemistry and origin of the ore-forming fluids in hydrothermal-magmatic systems in tectonically active zones. Geol. Ore Depos. 2006, 48, 1–22. [Google Scholar] [CrossRef]
- Jia, Y.; Li, X.; Kerrich, R. A fluid inclusion study of Au–bearing quartz vein systems in the Central and North Deborah deposits of the Bendigo gold field. Central Victoria, Autralia. Econ. Geol. 2000, 95, 467–494. [Google Scholar] [CrossRef]
- Kryazhev, S.G. Isotope–Geochemical Regime of the Rormation of the Gold Ore Deposit Muruntau. Ph.D. Thesis, TsNIGRI, Moscow, Russia, 2002. (In Russian). [Google Scholar]
- Safonov, Y.G.; Prokofiev, V.Y. Model of cosedimentation hydrothermal formation of gold–bearing reefs of the Witwatersrand basin. Geol. Ore Depos. 2006, 48, 475–511. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Gibsher, N.A.; Dublaynsky, Y.V.; Dallai, I. Geochemical and isotopic properties of fluid from gold–bearing and barren quartz veins of the Sovetskoye deposit (Siberia, Russia). Econ. Geol. 2010, 105, 375–394. [Google Scholar] [CrossRef]
- Dubessy, J.; Poty, B.; Ramboz, C. Advances in C–O–H–N–S fluid geochemistry based on micro–Raman spectrometric analysis of fluid inclusions. Eur. J. Miner. 1989, 1, 517–534. [Google Scholar] [CrossRef]
- Prokofiev, V.Y.; Naumov, V.B.; Mironova, O.F. Physicochemical parameters and geochemical features of fluids of Precamrbian gold deposits. Geochem. Int. 2017, 55, 1047–1065. [Google Scholar] [CrossRef]
- Kryazhev, S.G. Genetic Models and Criteria for Prediction of Gold Deposits in Carbon-Terrigenous Complexes. Ph.D. Thesis, TsNIGRI, Moscow, Russia, 2017. (In Russian). [Google Scholar]
- Gibsher, N.A.; Tomilenko, A.A.; Sazonov, A.M.; Ryabukha, M.A.; Timkina, A.L. The Gerfed gold deposit: Fluids and PT–conditions for quartz vein formation (Yenisei Ridge, Russia). Russ. Geol. Geophys. 2011, 52, 1461–1473. [Google Scholar] [CrossRef]
- Gibsher, N.A.; Tomilenko, A.A.; Bul’bak, T.A.; Ryabukha, M.A.; Khomenko, M.O.; Shaparenko, E.O.; Sazonov, A.V.; Sil’yanov, S.A.; Nekrasova, N.A. The Olimpiadinskoe gold deposit (Yenisei ridge): Temperature, pressure, composition of ore–forming luids, 34S in sulfides, 3He/4He of fluids, Ar–Ar age, and duration of formation. Russ. Geol. Geophys. 2019, 60, 1043–1059. [Google Scholar] [CrossRef]
- Wen, H.-J.; Qiu, Y.-Z. Geology and Geochemistry of Se-Bearing Formations in Central China. Int. Geol. Rev. 2002, 44, 164–178. [Google Scholar] [CrossRef]
- Tsukanov, N.V. Tectonic-stratigraphic terranes, Kamchatka active margins: Structure, composition and geodynamics. In Proceedings of the Annual Conference “Volcanism and Related Processes”, IVS FEB RAS, Petropavlovsk-Kamchatsky, Russia, 30 March–1 April 2015; pp. 97–103. (In Russian). [Google Scholar]
- Volkov, A.V. Gold prospects of the Kamchatka region. Gold Tekhnol. 2019, 1, 66–73. (In Russian) [Google Scholar]
- Volkov, A.V.; Sidorov, A.A. Economic implications of epithermal gold-silver deposits. Her. Russ. Acad. Sci. 2013, 83, 365–374. [Google Scholar] [CrossRef]
- Okrugin, V.M.; Zelensky, M.E. Miocene-Quaternary center of volcanic, hydrothermal and ore-forming activity in Southern Kamchatka. In Metallogeny of the Pacific Northwest (Russian Far East). Tectonics, Magmatism and Metallogeny of the Active Continental Margin; Khanchuk, A.I., Gonevchuk, G.A., Selmann, G.R., Eds.; Dalnauka: Vladivostok, Russia, 2004; pp. 147–176. (In Russian) [Google Scholar]
- White, N.C.; Hedenquist, J.W. Epithermal environments and styles of mineralization: Variations and their causes, and guidelines for exploration. J. Geochem. Explor. 1990, 36, 445–474. [Google Scholar] [CrossRef]
- White, N.C.; Hedenquist, J.W. Epithermal gold deposits: Styles, characteristics and exploration. Publ. SEG Newsl. 1995, 23, 9–13. [Google Scholar] [CrossRef]
- Einaudi, M.T.; Hedenquist, J.W.; Inan, E.E. Sulfidation state of fluids in active and extinct hydrothermal systems: Transitions from porphyry to epithermal environments, Volcanic, Geothermal and Ore-Forming Fluids: Rulers and Witnesses of Processes within the Earth. Soc. Econ. Geol. Spec. Publ. 2003, 10, 285–313. [Google Scholar]
- Simmons, S.F.; White, N.C.; John, D.A. Geological characteristics of epithermal precious and base metal deposits. Econ. Geol. 2005, 100, 485–522. [Google Scholar]
- Hayba, D.O.; Bethke, P.M.; Heald, P.; Foley, N.F. Geologic, mineralogic, and geochemical characteristics of volcanic hosted epithermal precious-metal deposits. Rev. Econ. 1985, 2, 129–167. [Google Scholar]
- Heald, P.; Foley, N.K.; Hayba, D.O. Comparative anatomy of volcanic-hosted epithermal deposits; acid-sulfate and adularia-sericite types. Econ. Geol. 1987, 82, 1–26. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Tolstykh, N.D. Epithermal Deposits of Kamchatka, Russia. Geol. Ore Depos. 2023, 65, S124–S152. [Google Scholar] [CrossRef]
- Melkomukov, B.H.; Razumny, A.V.; Litvinov, A.F.; Lopatin, W.B. The new highly promising gold objects of Koryakiya. Min. Bull. Kamchatka 2010, 14, 70–74. (In Russian) [Google Scholar]
- Vasilevsky, M.M.; Stefanov, Y.M.; Shiroky, B.I.; Kutyev, F.S.; Okrugin, V.M. Metallogeny of Kamchatka upper structural story and the problem of ore specialization of folded regions tectonic-magmatic evolution. In Forecasting Estimation of Ore Content of Volcanogenic Formation; Nedra: Moscow, Russia, 1977; pp. 14–60. (In Russian) [Google Scholar]
- Petrenko, I.D. Gold-Silver Formation of Kamchatka; VSEGEI: St. Peterburg, Russia, 1999. (In Russian) [Google Scholar]
- Okrugin, V.M. Rodnikovskoe deposit. In Geodynamics, Magmatism and Metallogeny of the East of Russia; Dalnauka: Vladivostok, Russia, 2006; pp. 702–706. (In Russian) [Google Scholar]
- Okrugin, V.M. Mutnovsky Hydrothermal Field Uzon-Geyser Depression. In Proceedings of the 8th International Symposium Water-Rock Interact, Vladivostok, Russia, 15–19 August 1995. [Google Scholar]
- Tolstykh, N.; Shapovalova, M.; Shaparenko, E.; Bukhanova, D. The Role of Selenium and Hydrocarbons in Au-Ag Ore Formation in the Rodnikovoe Low-Sulfidation (LS) Epithermal Deposit, Kamchatka Peninsula, Russia. Minerals 2022, 12, 1418. [Google Scholar] [CrossRef]
- Andreeva, E.D.; Konovalova, N.S. Some features of micromorphology and composition of native gold from the Baranyevskoye deposit (Central Kamchatka). In Proceedings of the Russian Conference of Students, Graduate Students and Young Scientists Dedicated to the “Year of the Planet Earth”, Moscow, Russia, 6–7 April 2009; Volume 3, pp. 7–11. (In Russian). [Google Scholar]
- Bolshakov, N.M.; Frolov, A.I.; Mineev, S.D.; Gazizov, R.B.; Bezrukova, L.A.; Okrugin, V.M. Geological structure of the Baranyevskoe gold ore deposit (Central Kamchatka). Domest. Geol. 2010, 4, 15–22. (In Russian) [Google Scholar]
- Okrugin, V.M.; Okrugina, A.M.; Andreeva, E.D.; Takahashi, R.; Matsueda, H.; Ono, S. Epithermal Mineralization of the Zolotoye Ore Field in Central Kamchatka, Russia. In Proceedings of the Society of Resource Geology, Tokyo, Japan, 8–12 July 2007. [Google Scholar]
- Takahashi, R.; Matsueda, H.; Okrugin, V.M.; Shikazono, N.; Ono, S.; Imai, A.; Andreeva, E.D.; Watanabe, K. Ore-forming ages and sulfur isotope study of hydrothermal deposits in Kamchatka, Russia. Resour. Geol. 2012, 63, 210–223. [Google Scholar] [CrossRef]
- Sidorov, E.G.; Borovikov, A.A.; Tolstykh, N.D.; Bukhanova, D.S.; Chubarov, V.M. Gold mineralization at the Maletoyvayam Deposit (Koryak Highland, Russia) and Physicochemical Conditions of its Formation. Minerals 2020, 10, 1093. [Google Scholar] [CrossRef]
- Tolstykh, N.; Bukhanova, D.; Shapovalova, M.; Borovokov, A.; Podlipsky, M. The gold mineralization of the Baranyevskoe Au-Ag epithermal deposit in Central Kamchatka. Minerals 2021, 11, 1225. [Google Scholar] [CrossRef]
- Litvinov, A.F.; Markovsky, B.A. Geological Map of the Russian Federation; Scale 1:1,000,000 (Third Generation), Koryak-Kamchatskaya Series, Sheet N-57. Petropavlovsk-Kamchatsky. Explanatory Note; Publishing House of St. Petersburg Cartography: St. Petersburg, Russia, 2006; p. 376. (In Russian) [Google Scholar]
- Oleinik, V.I. Report on the Geological Survey and Prospecting of Mineral Resources on a Scale 1:50,000; Sheets N-57-IIV, G; N-57-12V; N-57-23 A, B; N-57-24 A, Performed by the Kumroch Team in 1981–1985; Kamchatskii TFGI: Petropavlovsk-Kamchatskii, Russia, 1985. (In Russian) [Google Scholar]
- Okrugin, V.M.; Shshkanova, K.O.; Pholosophova, T.M. Mineralogical-geochemical ore features for Kumroch deposit, Eastern Kamchatka. Ores Met. 2019, 2, 84–96. (In Russian) [Google Scholar]
- Golyakov, V.I. Geological Map of the USSR Scale 1: 200 000; Pogozhev, A.G., Ed.; Series Koryak; Sheets P-5 8-XXXIII, O-58-III; VSEGEI Cartographic Factory: St. Peterburg, Russia, 1980. (In Russian) [Google Scholar]
- Kudaeva, S.S.; Kozlov, V.V.; Skilskaya, E.D.; Sergeeva, A.V.; Tolstykh, N.D.; Shkilev, A. New type of gold-bearing mineralization the Ozernovskoe Au-Te-Se epitermal deposit (Central Kamchatka, Russia). Geol. Ore Depos. 2024, 66, 547–569. [Google Scholar] [CrossRef]
- Tolstykh, N.; Shapovalova, M.; Podlipsky, M. Au-Ag-Se-Te-S mineralization in the Maletoyvayam High-Sulfidation epithermal deposit, Kamchatka peninsula. Minerals 2023, 13, 420. [Google Scholar] [CrossRef]
- Steele-MacInnis, M.; Lecumberri-Sanchez, P.; Bodnar, R.J. HokieFlincs_H2O-NaCl: A Microsoft Excel spreadsheet for interpreting microthermometric data from fluid inclusions based on the PVTX properties of H2O–NaCl. Comput. Geosci. 2012, 49, 334–337. [Google Scholar] [CrossRef]
- Borisenko, A.S. A Study of Salt Composition of Solutions Trapped in Vapor–Liquid Inclusions Using Cryometric Method. Russ. Geol. Geophys. 1977, 8, 16–27. (In Russian) [Google Scholar]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman spectroscopy for fluid inclusion analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Bul’bak, T.A.; Tomilenko, A.A.; Gibsher, N.A.; Sazonov, A.M.; Shaparenko, E.O.; Ryabukha, M.A.; Khomenko, M.O.; Sil’yanov, S.A.; Nekrasova, N.A. Hydrocarbons in Fluid Inclusions from Native Gold, Pyrite, and Quartz of the Sovetskoe Deposit (Yenisei Ridge, Russia) According to Pyrolysis-Free Gas Chromatography-Mass Spectrometry Data. Russ. Geol. Geophys. 2020, 61, 1260–1282. [Google Scholar] [CrossRef]
- Tomilenko, A.; Sonin, V.; Bul’bak, T.; Zhimulev, E.; Timina, T.; Chepurov, A.; Shaparenko, E.; Chepurov, A. Impact of Solid Hydrocarbon on the Composition of Fluid Phase at the Subduction (Experimental Simulation). Minerals 2023, 13, 618. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publ.: Oxford, UK, 1985; p. 330. [Google Scholar]
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Chi, G.; Diamond, L.W.; Lu, H.; Lai, J.; Chu, H. Common problems and pitfalls in fluid inclusion study: A review and discussion. Minerals 2020, 11, 7. [Google Scholar] [CrossRef]
- Roedder, E. Interpretation and Utilization of Inclusion Measurements-Compositional Data on Liquid and Gas Inclusions. In Fluid Inclusions; Ribbe, P.H., Ed.; Reviews in Mineralogy: Reston, VA, USA, 1984; Volume 12, pp. 221–250. [Google Scholar]
- Tolstykh, N.; Kasatkin, A.; Nestola, F.; Vymazalova, A.; Agakhanov, A.; Palyanova, G.; Korolyuk, V. Auroselenide, AuSe, a new mineral from Maletoyvayam deposit, Kamchatka peninsula, Russia. Mineral. Mag. 2022, 87, 284–291. [Google Scholar] [CrossRef]
- Nekrasov, I.Y. Volcano- kupol’naya structure of the Ametistovoye deposit and zoning of gold-silver mineralization. Dokl. Earth Sci. 1996, 347, 509–511. (In Russian) [Google Scholar]
- Okrugin, V.M.; Shishkanova, K.O.; Filosofova, T.M. New data on the ores of the Vilyuchinsk gld-silver-base metal ore occurrences, South Kamchatka. Rudy Met. 2017, 1, 40–54. (In Russian) [Google Scholar]
- Volkov, A.V.; Prokof’ev, V.Y.; Vinokurov, S.F.; Andreeva, O.V.; Kiseleva, G.D.; Galyamov, A.L.; Murashov, K.Y.; Sidorova, N.V. Valunistoe Epithermal Au–Ag Deposit (East Chukotka, Russia): Geological Structure, Mineralogical–Geochemical Peculiarities and Mineralization Conditions. Geol. Ore Depos. 2020, 62, 97–121. [Google Scholar] [CrossRef]
- Liu, K.; Yang, R.; Chen, W.; Liu, R.; Tao, P. Trace Element and REE Geochemistry of the Zhewang Gold Deposit, Southeastern Guizhou Province, China. Chin. J. Geochem. 2014, 33, 109–118. [Google Scholar] [CrossRef]
- Volkov, A.V.; Prokof’ev, V.Y.; Sidorov, A.A.; Uyutnov, K.V.; Kolova, E.E.; Savva, N.E.; Bayankon, M.A. Ore formation at the Kupol epitermal gold-silver deposit in Northeastern Russia deduced from fluid inclusion study. Geol. Ore Depos. 2012, 54, 295–303. [Google Scholar] [CrossRef]
- Volkov, A.V.; Sidorov, A.A.; Savva, N.E.; Kolova, E.E.; Murashov, K.Y. Geochemical features of Paleozoic Au-Ag epitermal deposits (Northeastern Russia). Dokl. Earth Sci. 2017, 472, 178–183. [Google Scholar] [CrossRef]
- Volkov, A.V.; Savva, N.E.; Kolova, E.E.; Prokof’ev, V.Y.; Murashov, K.Y. Dvoinoe Au–Ag epithermal deposit, Chukchi peninsula, Russia. Geol. Ore Depos. 2018, 60, 527–545. [Google Scholar] [CrossRef]
- Takahashi, R.; Matsueda, H.; Okrugin, V.M.; Ono, S. Epithermal Gold-Silver Mineralization of the Asachinskoe Deposit in South Kamchatka, Russia. Resour. Geol. 2007, 57, 354–373. [Google Scholar] [CrossRef]
- Borovikov, A.; Lapukhov, A.; Borosenko, A.; Seryotkin, Y. The Asachinskoe epithermal Au-Ag deposit in Southern Kamchatka: Physicochemical conditions of formation. Russ. Geol. Geophys. 2009, 50, 693–702. [Google Scholar] [CrossRef]
- Takahashi, R.; Matsueda, H.; Okrugin, V.M. Hydrothermal gold mineralization at the Rodnikovoe deposit in South Kamchatka, Russia. Resour. Geol. 2002, 52, 359–369. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Volkov, A.V.; Savva, N.E.; Prokofiev, V.Y.; Kolova, E.E.; Dolomanova-Topol’, A.A.; Galyamov, A.L.; Murashov, K.Y. Epitermal Au-Ag-Se-Te deposits of the Chukchi peninsula (Atctic zone of Russia): Metallogeny, mineral assemblages, and fluid regime. Russ. Geol. Geophys. 2022, 63, 435–457. [Google Scholar] [CrossRef]
- Giordano, T.H. Organic Matter as a Transport Agent in Ore-Forming Systems. In Ore Genesis and Exploration: The Roles of Organic Matter; Giordano, T.H., Kettler, R.M., Scott, A., Wood, S.A., Eds.; Reviews in Economic Geology; Society of Economic Geologists: Littleton, CO, USA, 1997; Volume 9, pp. 319–354. [Google Scholar]
- Kizilshtein, L.Y. The role of organic matter in the forma tion of gold deposits (on the example of black shales). Russ. J. Gen. Chem. 2000, XLIV, 108–114. (In Russian) [Google Scholar]
- Huang, J.; Daniel, I.; Sverjensky, D.A.; Cardon, H.; Montagnac, G. Formation of hydrocarbons favored by high pressure at subduction zone conditions. Chem. Geol. 2023, 630, 121489. [Google Scholar] [CrossRef]
- Williams-Jones, A.; Bowell, R.; Migdisov, A. Gold in Solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Balikov, S.V.; Dementyev, V.E. Gold: Properties. Geochemical Aspects; Irgiredmet: Irkutsk, Russia, 2015; 328p. (In Russian) [Google Scholar]
- Phillips, G.N.; Evans, K.A. Role of CO2 in the formation of gold deposits. Nature 2004, 429, 860–863. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Kokh, M.A.; Guillaume, D.; Borisova, A.Y.; Gisquet, P.; Hazemann, J.-L.; Lahera, E.; Del Net, W.; Proux, O.; Testemale, D.; et al. Sulfur radical species form gold deposits on Earth. Proc. Natl. Acad. Sci. USA 2015, 112, 13484–13489. [Google Scholar] [CrossRef]
- Korshunova, V.A.; Charykova, M.V. Organometallic forms of gold and satellite elements in podzolic soils on the territory of the New Sands gold deposit (South Karelia). Bull. St. Petersburg Univ. Geosci. 2018, 63, 22–35. (In Russian) [Google Scholar]
- Galimov, E.M.; Sevast’yanov, V.S.; Kamaleeva, A.I.; Kuznetsova, O.V.; Konopleva, I.V.; Vlasova, L.N.; Karpov, G.A. Hydrocarbons from a volcanic area. Oil seeps in the Uzon caldera, Kamchatka. Geochem. Int. 2015, 53, 1019–1027. [Google Scholar] [CrossRef]
- Poturay, V.A. Organic Material in Peninsular and Continental Hydrothermal Systems of the Far East. Ph.D. Thesis, Institute for Complex Analysis of Regional Problems FEB RAS, Birobidzhan, Russia, 2019; 160p. (In Russian). [Google Scholar]
- Poturay, V.A.; Kompanichenko, V.N. Composition and distribution of saturated hydrocarbons in the thermal waters and vapor-water mixture of the Mutnovsii geothermal field and Uzon caldera, Kamchatka. Geochem. Int. 2019, 57, 74–82. [Google Scholar] [CrossRef]
- Shulga, N.A.; Peresypkin, V.I.; Revelskii, I.A. Composition research of n-alkanes in the samples of hydrothermal deposits of the Mid-Atlantic ridge by means of gas chromatography-mass spectrometry. Oceanology 2010, 50, 479–487. [Google Scholar] [CrossRef]
- Kompanichenko, V.N.; Poturay, V.A.; Karpov, G.A. Organic Compounds in Thermal Water: The Mutnovskii Area and the Uzon Caldera. J. Volcanol. Seismol. 2016, 10, 305–319. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Guo, X.; Xu, H.; Williams-Jones, A.E.; Sun, C.J.; Vasyukova, O.; Sugiyama, I.; Fuchs, S.; Pearce, K.; Roback, R. Hydrocarbons as ore fluids. Eur. Assoc. Geochem. Geochem. Perspect. Lett. 2017, 5, 47–52. [Google Scholar] [CrossRef]
- Lyakhov, Y.V.; Pavlun’, N.N. Some geological and geochemical features of gold concentration processes in metamorphic-hydrothermal and magmatic-hydrothermal mineral-forming systems. In Ore-Forming Processes: From Genetic Concepts to Forecasting and Discovery of New Ore Provinces and Deposits; IGEM RAS: Moscow, Russia, 2013; p. 144. (In Russian) [Google Scholar]
- Karpov, G.A.; Pavlov, A.L. Uzon-Geysernaya Hydrothermal Ore-Forming System of Kamchatka; Nauka: Moscow, Russia, 1976; p. 89. (In Russian) [Google Scholar]
- Tolstykh, N.D.; Bortnikov, N.S.; Shapovalova, M.O.; Shaparenko, E.O. The role of hydrocarbons in the formation of epitermal gold-silver deposits in Kamchatka, Russia. Dokl. Earth Sci. 2022, 507, 994–1000. [Google Scholar] [CrossRef]
- Seward, T.M. Thio complex of gold and the transfer of gold in hydrothermal ore solutions. Geochim. Cosmochim. Acta 1973, 53, 379–399. [Google Scholar] [CrossRef]
- Sobolev, O.I.; Gutyj, B.V.; Sobolieva, S.V.; Borshch, O.O.; Nedashkivsky, V.M.; Kachan, L.M.; Karkach, P.M.; Nedashkivska, N.V.; Poroshinska, O.A.; Stovbetska, L.S.; et al. Selenium in natural environment and food chains. A Review. Ukr. J. Ecol. 2020, 10, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Akinfiev, N.N.; Tagirov, B.R. Effect of selenium on silver transport and precipitation by hydrothermal solutions: Thermodynamic description of the Ag-Se-S-Cl-O-H system. Geol. Ore Depos. 2006, 48, 460–472. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Li, L.; D’Ulivo, A.; Belzile, N. Extraction and determination of elemental selenium in sediments—A comparative study. Anal. Chim. Acta 2006, 577, 126–133. [Google Scholar] [CrossRef]
- Shaparenko, E.; Gibsher, N.; Tomilenko, A.; Sazonov, A.; Bul’bak, T.; Ryabukha, M.; Khomenko, M.; Silyanov, S.; Nekrasova, N.; Petrova, M. Ore–Bearing Fluids of the Blagodatnoye Gold Deposit (Yenisei Ridge, Russia): Results of Fluid Inclusion and Isotopic Analyses. Minerals 2021, 11, 1090. [Google Scholar] [CrossRef]
- Tomilenko, A.A.; Bul’bak, T.A.; Timina, T.Y.; Shaparenko, E.O.; Simonov, V.A.; Laptev, Y.V. Composition of volatiles of sulfide deposits and carbonate structures in submarine hydrothermal fields of the Mid-Atlantic Ridge. Mar. Geol. 2022, 444, 106713. [Google Scholar] [CrossRef]
- Yuningsih, E.T.; Matsueda, H.; Rosana, M.F. Diagnostic genesis features of Au-Ag selenide-telluride mineralization of Western Java Deposits. Indones. J. Geosci. 2016, 3, 67–76. [Google Scholar] [CrossRef]
- Slobodskoy, R.M. Organo-Element Compounds in Magmatic and Ore-Forming Processes; Nauka: Novosibirsk, Russia, 1981. (In Russian) [Google Scholar]
- Hustor, D.L.; Sieb, S.H.; Suterb, G.F. Selenium theoretical and its importance to the study of ore genesis: The basis and its application to volcanic-hosted massive sulfide deposits using pixeprobe analysis. Nucl. Instrum. Methods Phys. Res. 1995, 104, 476–480. [Google Scholar] [CrossRef]
- Simon, G.; Kesler, S.E.; Essene, E.J. Phase Relations among Selenides, Sulfides, Tellurides, and Oxides: II. Applications to Selenide-Bearing Ore Deposits. Econ. Geol. 1997, 92, 468–484. [Google Scholar] [CrossRef]


| Deposit | Rodnikovoe | Baranyevskoe | Kumroch | Lazurnoe | Maletoyvayam | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Avg. Value N = 4 | Sam./ UCC | Avg. Value N = 9 | Sam./ UCC | Avg. Value N = 8 | Sam./ UCC | Avg. Value N = 4 | Sam./ UCC | Avg. Value N = 6 | Sam./ UCC | |
| Co | 2.00 | 0.20 | 6.17 | 0.62 | 5.13 | 0.51 | 6.55 | 0.66 | 2.70 | 0.27 |
| Ni | 17.60 | 0.88 | 16.74 | 0.84 | 4.48 | 0.22 | 10.93 | 0.55 | 9.25 | 0.46 |
| Zn | 13.75 | 0.19 | 62.33 | 0.88 | 206 | 2.90 | 1501 | 21.13 | 19.58 | 0.28 |
| As | 43.50 | 29.00 | 44.44 | 29.63 | 166 | 111 | 15.00 | 10.00 | 792 | 528 |
| Se | 11.35 | 227 | 2.00 | 40.00 | 2.43 | 48.58 | 46.85 | 937 | 54.08 | 1082 |
| Ag | 80.90 | 1618 | 2.56 | 51.24 | 2.32 | 46.35 | 46.53 | 931 | 10.67 | 213 |
| Sb | 44.23 | 221 | 15.00 | 74.99 | 60.67 | 303 | 9.23 | 46.13 | 662 | 3312 |
| Te | 0.08 | 2.96 | 0.45 | 16.83 | 0.63 | 23.44 | 3.70 | 137 | 70.90 | 2626 |
| Ba | 66.25 | 0.12 | 121 | 0.22 | 654 | 1.19 | 312 | 0.57 | 1056 | 1.92 |
| Pb | 4.53 | 0.23 | 36.84 | 1.84 | 502 | 25.10 | 1250 | 62.48 | 58.42 | 2.92 |
| Bi | 3.54 | 27.87 | 0.26 | 2.03 | 0.26 | 2.08 | 14.50 | 114 | 34.98 | 275 |
| Cu | 39.23 | 1.57 | 54.81 | 2.19 | 148 | 5.90 | 6231 | 249 | 314 | 12.56 |
| Au | 12.65 | 7029 | 6.92 | 3846 | 0.83 | 461 | 0.28 | 154 | 4.36 | 2423 |
| Au/Ag | 0.16 | 2.70 | 0.36 | 0.01 | 0.41 | |||||
| Te/Bi | 0.02 | 1.76 | 2.40 | 0.26 | 2.03 | |||||
| Co/Ni | 0.11 | 0.37 | 1.15 | 0.60 | 0.29 | |||||
| Deposit | FIA Type | N | Thom, °C | Tmelt, °C | Teut, °C | Salinity, wt. % (NaCl-eq.) | Pressure, Bar |
|---|---|---|---|---|---|---|---|
| Rodnikovoe | P,PS | 28 | −1.5…−0.5 | - | 0.9–2.6 | 6–50 | |
| Baranyevskoe | P,PS | 31 | −1…−0.6 | - | 0.5–1.7 | 19–98 | |
| Lazurnoe | P | 58 | −1…−0.6 | −22.5…−21.7 | 1–1.7 | 54–85 | |
| Kumroch | P,PS | 63 | −4.2…−0.5 | −32…−29.5 | 0.9–6.8 | 17–85 | |
| Maletoyvayam | P,PS | 30 | −3…−0.6 | - | 1–5 | 8–72 |
| Components | MW * | Baranyevskoe | Rodnikovoe | Lazurnoe | Kumroch | Maletoyvayam |
|---|---|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||||
| Paraffins (alkanes) (CH4–C17H36) | 16–240 | 2.33 (18) | 0.19 (17) | 2.34 (20) | 0.15 (19) | 6.33 (40) |
| C1-C9 | 2.26 | 0.15 | 0.13 | 0.09 | 2.79 | |
| C10-C14 | 0.03 | 0.02 | 0.05 | 0.03 | 3.34 | |
| C15-C17 | 0.05 | 0.03 | 2.18 | 0.04 | 0.20 | |
| Olefins (alkenes) (C2H2–C17H34) | 26–238 | 0.07 (20) | 0.12 (24) | 0.42 (29) | 0.25 (28) | 2.01 (31) |
| Cyclic hydrocarbons | ||||||
| Cycloalkanes, cycloalkenes, arenes, PAH (C5H10–C15H24) | 70–204 | 0.19 (18) | 0.90 (23) | 0.23 (17) | 0.18 (17) | 2.97 (24) |
| Oxygenated hydrocarbons | ||||||
| Alcohols (CH4O–C8H10O3) | 32–154 | 0.15 (9) | 0.03 (4) | 0.26 (15) | 0.20 (13) | 0.28 (7) |
| Ethers and esters (C5H8O–C14H26O2) | 84–226 | 0.23 (12) | <0.01 (6) | 5.32 (13) | 1.93 (14) | 0.13 (3) |
| Aldehydes (CH2O–C18H36O) | 30–268 | 0.24 (22) | 0.10 (18) | 0.48 (28) | 0.49 (27) | 4.31 (23) |
| Ketones (C3H6O–C15H30O) | 58–226 | 0.15 (17) | 0.05 (12) | 0.79 (22) | 0.15 (21) | 2.48 (13) |
| Carboxylic acids (CH2O2–C14H28O2) | 46–228 | 0.19 (14) | 0.17 (15) | 1.00 (16) | 0.32 (13) | 0.77 (13) |
| Heterocyclic compounds | ||||||
| Dioxanes, furans (C4H4O–C11H18O) | 68–166 | 0.01 (7) | <0.01 (8) | 0.02 (9) | 0.01 (10) | 0.05 (9) |
| Nitrogenated compounds | ||||||
| N2, ammonia, nitriles (N2–C10H21NO) | 17–171 | 0.14 (12) | 0.14 (12) | 0.57 (23) | 0.58 (21) | 11.17 (14) |
| Sulfonated compounds | ||||||
| H2S, SO2, CS2, COS, thiophenes (H2S–C12H20S) | 34–196 | 0.08 (15) | 0.03 (13) | 1.28 (15) | 1.00 (15) | 0.92 (12) |
| Inorganic compounds | ||||||
| CO2 | 44 | 6.60 | 4.44 | 14.75 | 3.50 | 10.93 |
| H2O | 18 | 89.63 | 94.65 | 72.54 | 91.25 | 57.67 |
| Ar | 40 | 0.02 | - | 0.001 | 0.001 | - |
| Number of components | 167 | 155 | 210 | 201 | 192 | |
| CO2/(CO2+H2O) | 0.04 | 0.04 | 0.17 | 0.04 | 0.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapovalova, M.; Shaparenko, E.; Tolstykh, N. Geochemistry and Fluid Inclusion of Epithermal Gold-Silver Deposits in Kamchatka, Russia. Minerals 2025, 15, 2. https://doi.org/10.3390/min15010002
Shapovalova M, Shaparenko E, Tolstykh N. Geochemistry and Fluid Inclusion of Epithermal Gold-Silver Deposits in Kamchatka, Russia. Minerals. 2025; 15(1):2. https://doi.org/10.3390/min15010002
Chicago/Turabian StyleShapovalova, Maria, Elena Shaparenko, and Nadezhda Tolstykh. 2025. "Geochemistry and Fluid Inclusion of Epithermal Gold-Silver Deposits in Kamchatka, Russia" Minerals 15, no. 1: 2. https://doi.org/10.3390/min15010002
APA StyleShapovalova, M., Shaparenko, E., & Tolstykh, N. (2025). Geochemistry and Fluid Inclusion of Epithermal Gold-Silver Deposits in Kamchatka, Russia. Minerals, 15(1), 2. https://doi.org/10.3390/min15010002










