Crystal Chemistry of a New Mineral-like Phosphate Na6.9Ni2+0.9V3+4.3Al0.8(PO4)8(H2O)2 in the Series of α-CrPO4 Derivatives
Abstract
:1. Introduction
2. Materials and Methods
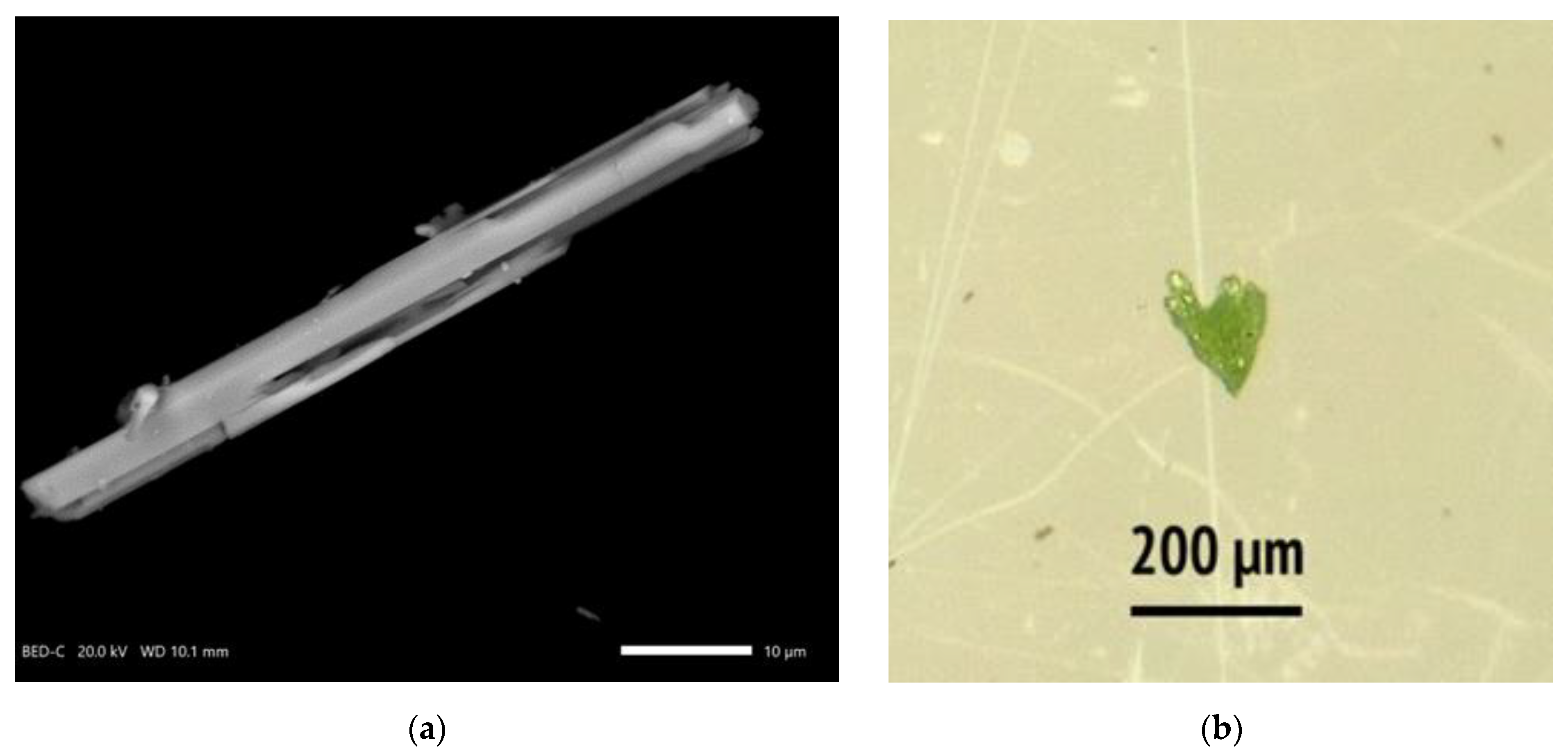
2.1. Hydrothermal Synthesis
2.2. X-Ray Energy-Dispersive Analysis
2.3. Single-Crystal X-Ray Diffraction
3. Results and Discussion
3.1. Chemical Composition
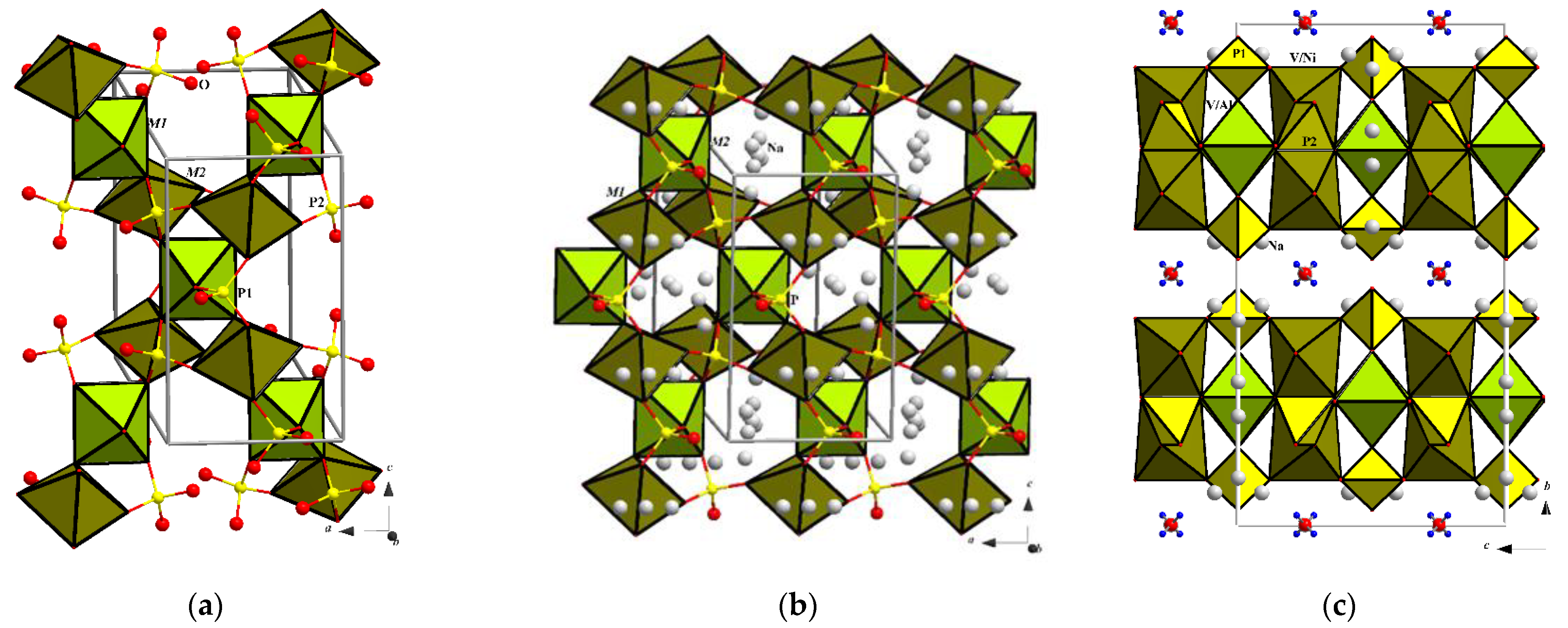
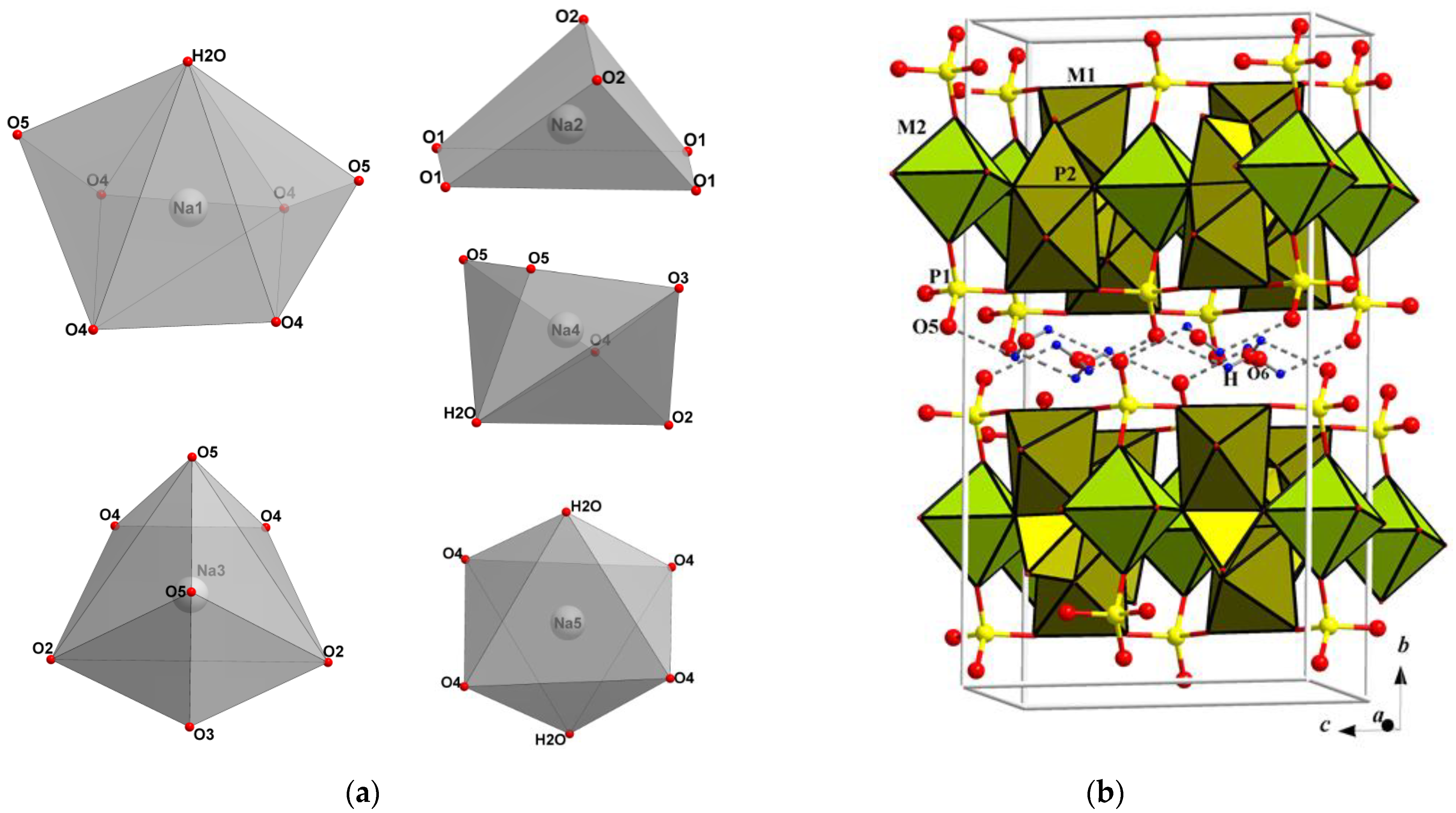
3.2. Crystal Structure Solution
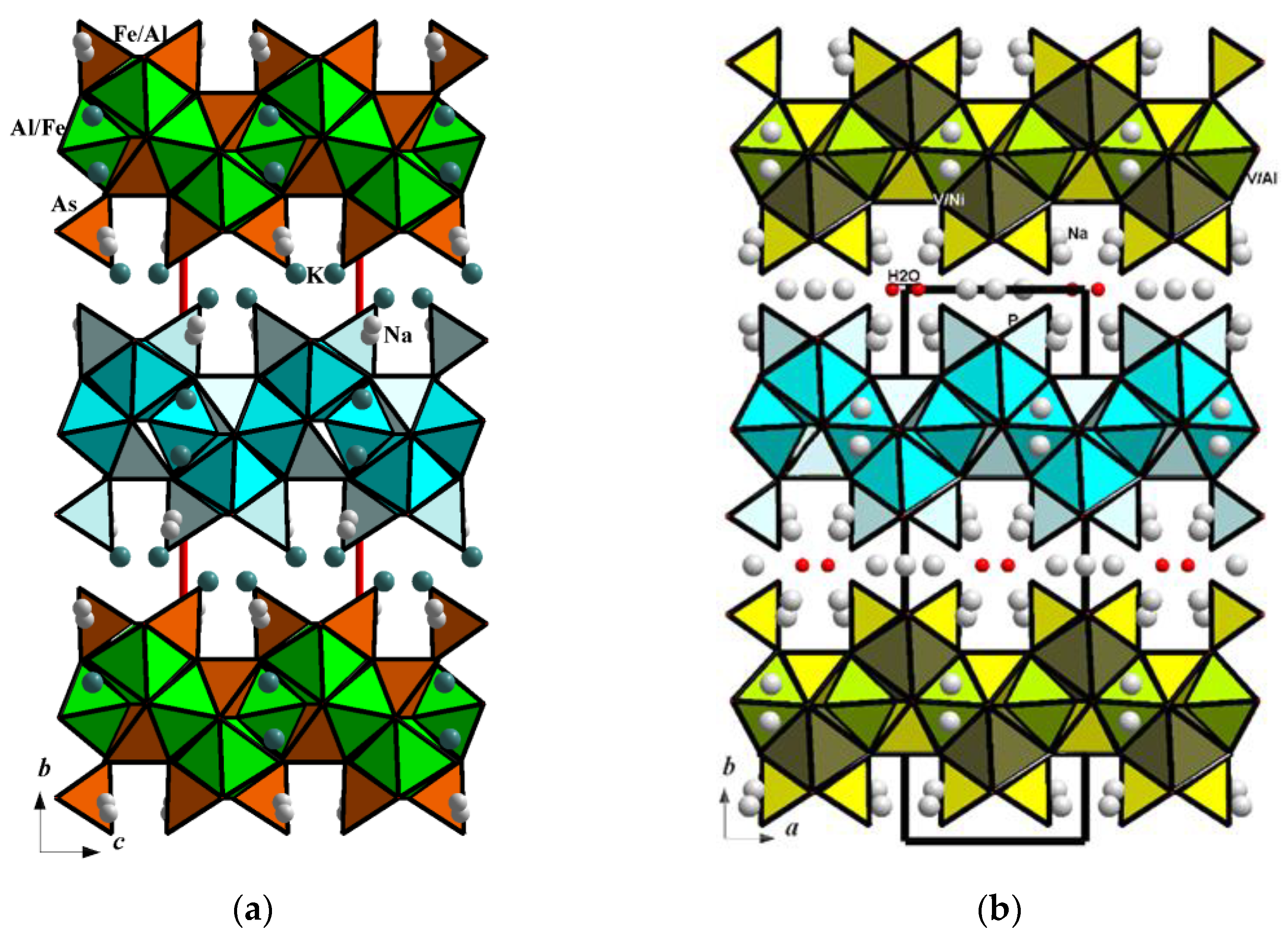
3.3. Crystal Chemistry in Comparison with Related Minerals and Synthetic Analogues
3.3.1. Analysis of Interatomic Distances and Crystal Structure Description
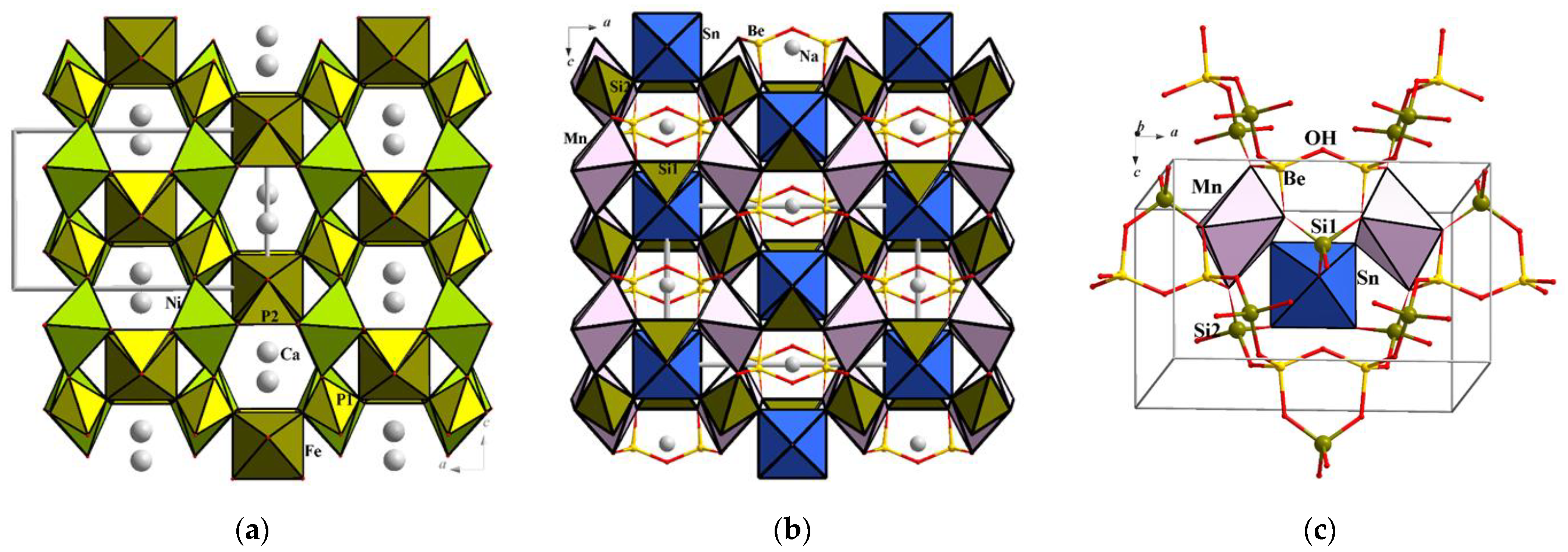
3.3.2. Structurally Related Synthetic and Mineral Phases
| Compound/Mineral | Unit Cell Parameters, Å | Space Group; Z; V, Å3 | Crystal Structure Description | Ref. |
|---|---|---|---|---|
| α-CrPO4 archetype | ||||
| α-CrPO4 | a = 10.403(2) b = 12.898(2) c = 6.299(1) | Imma 12 845.19 | CrPO4 tri-periodic framework | [14] |
| Synthetic V-bearing phosphates | ||||
| α-VPO4 | a = 10.5591(1) b = 13.1051(1) c = 6.33928(7) | Imma 12 877.22(2) | VPO4 tri-periodic framework | [22] |
| NaV2.67+3(PO4)3 | a = 10.488(2) b = 13.213(3) c = 6.455(1) | Imma 4 894.52 | [V2.67+3(PO4)3]− anionic tri-periodic framework with Na+ ions in the channels | [25] |
| NaFeV2(PO4)3 | a = 10.5067(4) b = 13.2437(6) c = 6.3946(2) | Imma 4 889.80(6) | [Fe2+V3+2(PO4)3]− anionic tri-periodic framework with Na+ ions in the channels | [33] |
| NaCoV2(PO4)3 | a = 10.495(1) b = 13.184(1) c = 6.3920(6) | Imma 4 884.4(1) | [Co2+V3+2(PO4)3]− anionic tri-periodic with Na+ ions in the channels | [33] |
| NaNiV2(PO4)3 | a = 10.5091(5) b = 13.1212(5) c = 6.3540(3) | Imma 4 876.16(6) | [Ni2+V3+2(PO4)3]− anionic tri-periodic with Na+ ions in the channels | [33] |
| Na3.46(V3+1.54Ni2+0.46)M1(V3+0.61Al0.39)M2(PO4)4(H2O) | a = 10.504(1) b = 19.681(2) c = 6.4082(8) | Amaa * 4 1324.7(3) | [Ni2+0.46V3+2.15Al0.39(PO4)4]3.46− bi-periodic anionic slabs (double layers) joint together by Na+ ions and hydrogen bonds | This work |
| K3V3+3(PO4)4·H2O | a = 10.7161(4) b = 20.850(1) c = 6.5316(2) | Pnna 4 1459.36 | [V3+3(PO4)4]3− bi-periodic anionic slabs (double layers) joint together by K+ ions and hydrogen bonds | [31] |
| Na3V3+3(PO4)4 | a = 19.6724(4) b = 6.4041(1) c = 10.5890(2) β = 91.967(2) | C2/c 4 1323.24 | [V3+3(PO4)4]3− bi-periodic anionic slabs (double layers) joint together by Na+ ions | [32] |
| Minerals | ||||
| Yakubovichite CaNi2Fe3+(PO4)3 | a = 10.388(1) b = 13.088(1) c = 6.4794(6) | Imma 4 880.94(2) | [Ni2Fe3+(PO4)3]2− anionic tri-periodic framework with Ca2+ ions in the channels | [28] |
| Ozerovaite, Na2K(Al1.64Fe3+0.36)(AsO4)4 | a = 10.615(2) b = 20.937(3) c = 6.3932(9) | Cmca 4 1420.9(3) | [(Al, Fe3+)(AsO4)4]3− bi-periodic anionic slabs (double layers) joint together by Na+ and K+ ions | [34] |
| Pansnerite, Na1.6K1.4(Fe3+1.65Al1.35)(AsO4)4 | a = 10.7372(3) b = 20.8367(8) c = 6.4734(2) | Cmca 4 1448.27(7) | [(Fe3+, Al) (AsO4)4]3− bi-periodic anionic slabs (double layers) joint together by Na+ and K+ ions | [35] |
| Sverigeite, Na(Mn1.37Mg0.63)SnBe2 (SiO4)3(OH) | a = 10.815(8) b = 13.273(8) c = 6.818(6) | Imma 4 978.71(7) | [(Mn, Mg)SnBe2(SiO4)3OH]− tri-periodic framework with Na+ ions in the channels | [30] |
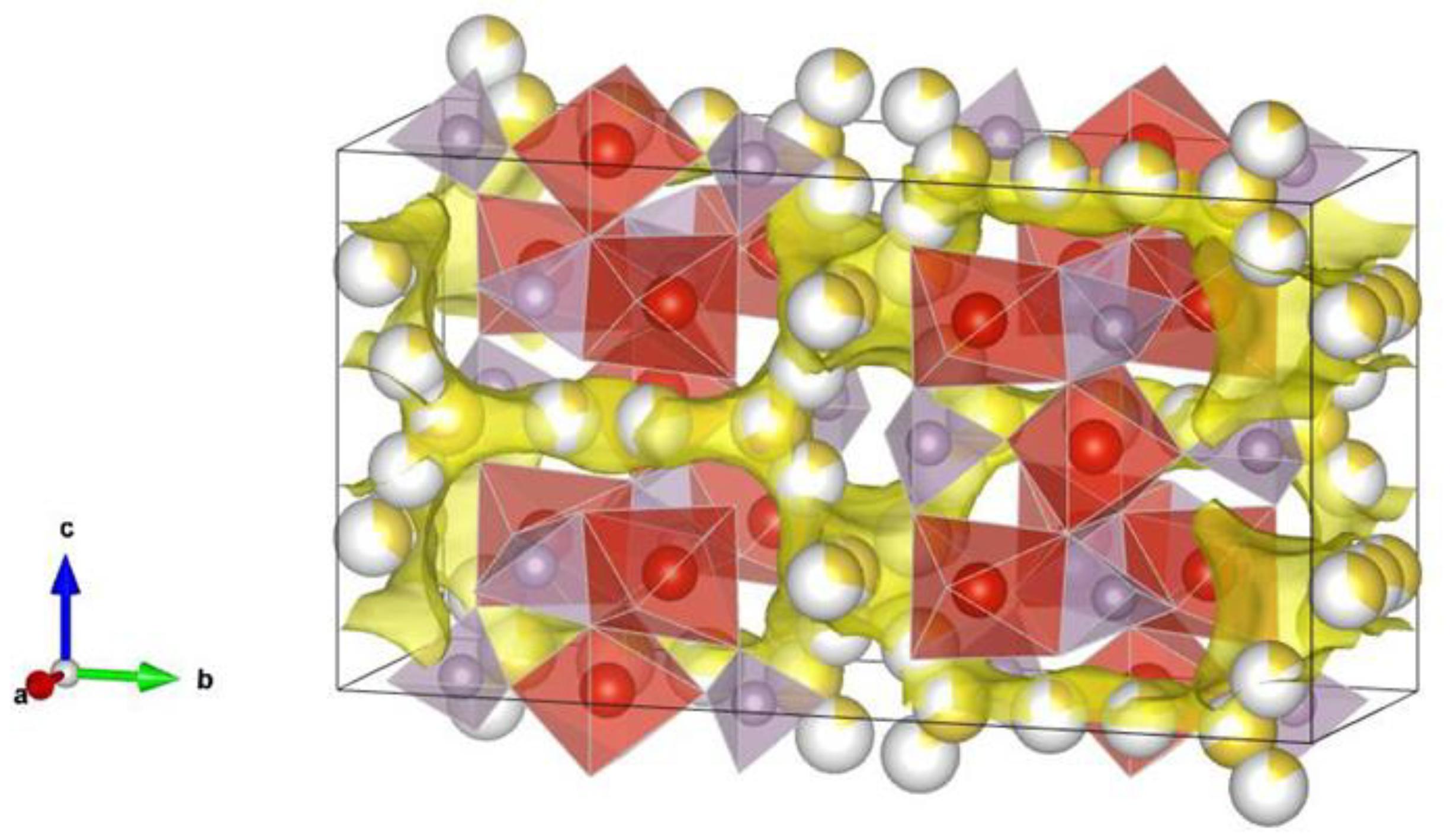
4. Ionic Conductivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krivovichev, S. (Ed.) Minerals as Advanced Materials I; Springer: Berlin/Heidelberg, Germany, 2008; 255p, ISBN 978-3-540-77122-7. [Google Scholar]
- Krivovichev, S. (Ed.) Minerals as Advanced Materials II; Springer: Berlin/Heidelberg, Germany, 2012; 427p. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef] [PubMed]
- Antipov, E.V.; Khasanova, N.R.; Fedotov, S.S. Perspectives on Li and transition metal fluoride phosphates as cathode materials for a new generation of Li-ion batteries. IUCrJ 2015, 2, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yakubovich, O.V. Phosphates with amphoteric oxo-complexes: From structural features to genetic conclusions. Z. Kristallogr. Cryst. Mater. 2008, 223, 126–131. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Khasanova, N.R.; Antipov, E.V. Minerals as inspired materials: Synthetic phosphate analogues for battery application. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Peters, J.F.; Peña Cruz, A.; Weil, M. Exploring the economic potential of sodium-ion batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef]
- He, M.; Liu, S.; Wu, J.; Zhu, J. Review of cathode materials for sodium-ion batteries. Prog. Solid State Chem. 2024, 74, 100452. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The Scale-up and Commercialization of Nonaqueous Na-Ion Battery Technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 170043. [Google Scholar] [CrossRef]
- Serras, P.; Palomares, V.; Goni, A.; Gil de Muro, I.; Kubiak, P.; Lezama, L.; Rojo, T. High voltage cathode materials for Na-ion batteries of general formula Na3V2O2x(PO4)2F3−2x. J. Mater. Chem. 2012, 22, 22301–22308. [Google Scholar] [CrossRef]
- Glaum, R.; Gruehn, R.; Möller, M. Darstellung und Struktur von α-CrPO4. Beiträge zum thermischen Verhalten von wasserfreien Phosphaten. I. Z. Anorg. Allg. Chem. 1986, 543, 111–116. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- International Tables for Crystallography, 3rd ed.; Prince, E., Ed.; Kluwer: Dordrecht, The Netherlands, 2004; Table 4.2.6.8 and 6.1.14. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Fedotov, S.S.; Samarin, A.S.; Nikitina, V.A.; Stevenson, K.J.; Abakumov, A.M.; Antipov, E.V. α-VPO4: A Novel Many Monovalent Ion Intercalation Anode Material for Metal-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 12431–12440. [Google Scholar] [CrossRef]
- Shraer, S.D.; Luchinin, N.D.; Trussov, I.A.; Aksyonov, D.A.; Morozov, A.V.; Ryazantsev, S.V.; Iarchuk, A.R.; Morozova, P.A.; Nikitina, V.A.; Stevenson, K.J.; et al. Development of vanadium-based polyanion positive electrode active materials for high-voltage sodium-based batteries. Nat. Commun. 2022, 13, 4097. [Google Scholar] [CrossRef]
- Shraer, S.; Dembitskiy, A.; Trussov, I.; Komayko, A.; Aksyonov, D.; Luchinin, N.; Morozov, A.; Pollastri, S.; Aquilanti, G.; Ryazantsev, S.; et al. Designing a 3D framework NaVOPO4 as a high-power, low-strain and long-life positive electrode material for Na-ion batteries. Energy Storage Mater. 2024, 68, 103358. [Google Scholar] [CrossRef]
- Kinomura, N.; Matsui, N.; Kumada, N.; Muto, F. Synthesis and Crystal Ctructure of NaV3P3O12: A Stuffed Structure of α-CrPO4. J. Solid State Chem. 1989, 79, 232–237. [Google Scholar] [CrossRef]
- Wang, X.; Hu, P.; Chen, L.; Yao, Y.; Kong, Q.; Cui, G.; Shi, S.; Chen, L. An α-CrPO4-type NaV3(PO4)3 Anode for Sodium-Ion Batteries with Excellent Cycling Stability and the Exploration of Sodium Storage Behavior. J. Mater. Chem. A 2017, 5, 3839–3847. [Google Scholar] [CrossRef]
- Ishado, Y.; Inoishi, A.; Okada, S. Exploring Factors Limiting Three-Na+ Extraction from Na3V2(PO4)3. Electrochemistry 2020, 88, 457–462. [Google Scholar] [CrossRef]
- Britvin, S.N.; Murashko, M.N.; Krzhizhanovskaya, M.G.; Vapnik, Y.; Vlasenko, N.S.; Vereshchagin, O.S.; Pankin, D.V.; Zaitsev, A.N.; Zolotarev, A.A. Yakubovichite, CaNi2Fe3+(PO4)3, a New Nickel Phosphate Mineral of Non-Meteoritic Origin. Am. Mineral. 2023, 108, 2142–2150. [Google Scholar] [CrossRef]
- Ouaatta, S.; Assani, A.; Saadi, M.; El Ammari, L. Crystal structure of calcium dinickel(II) iron(III) tris(orthophosphate): CaNi2Fe(PO4)3. Acta Crystallogr. 2017, E73, 893–895. [Google Scholar] [CrossRef]
- Rouse, R.C.; Peacor, D.R.; Metz, G.W. Sverigeite, a structure containing planar NaO4 groups and chains of 3- and 4-membered beryllosilicate rings. Am. Mineral. 1989, 74, 1343–1350. [Google Scholar]
- Jenkins, T.; Alarco, J.A.; Mackinnon, I.D.R. Synthesis and characterization of a Novel Hydrated Layered Vanadium(III) Phosphate Phase K3V3(PO4)4·H2O: A Functional Cathode Material for Potassium-Ion Batteries. ACS Omega 2021, 6, 1917–1929. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Sheng, T.; Zheng, S.; Zhong, G.; Zheng, G.; Liang, Z.; Ortiz, G.F.; Zhao, W.; Mi, J.; et al. Novel 3.9 V Layered Na3V3(PO4)4 Cathode Material for Sodium Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 3603–3606. [Google Scholar] [CrossRef]
- Alkhateeban, A.; BenYahia, H. Investigation of the inorganic compounds NaMV2(PO4)3 (M=Fe, Co, Ni) as anode materials for sodium-ion batteries. ACS Omega 2020, 5, 30799–30807. [Google Scholar] [CrossRef]
- Shablinskii, A.P.; Filatov, S.K.; Vergasova, L.P.; Avdontseva, E.Y.; Moskaleva, S.V.; Povolotskiy, A.P. Ozerovaite, Na2KAl3(AsO4)4, new mineral species from Tolbachik volcano, Kamchatka peninsula, Russia. Eur. J. Mineral. 2019, 31, 159–166. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Koshlyakova, N.N.; Agakhanov, A.A.; Belakovskiy, D.I.; Vigasina, M.F.; Yapaskurt, V.O.; Britvin, S.N.; Turchkova, A.G.; Sidorov, E.G.; et al. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. XIII. Pansnerite, K3Na3Fe3+6(AsO4)8. Mineral. Mag. 2020, 84, 143–151. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Dembitskiy, A.D.; Aksyonov, D.A.; Abakumov, A.M.; Fedotov, S.S. NH4+-based frameworks as a platform for designing electrodes and solid electrolytes for Na-ion batteries: A screening approach. Solid State Ion. 2022, 374, 115810. [Google Scholar] [CrossRef]
- BVlain Python Package. Available online: https://github.com/dembart/BVlain (accessed on 19 December 2024).
- Adams, S.; Rao, R.P. High power lithium-ion battery materials by computational design. Phys. Status Solidi 2011, A208, 1746–1753. [Google Scholar] [CrossRef]
- He, B.; Ye, A.; Chi, S.; Mi, P.; Ran, Y.; Zhang, L.; Zou, X.; Pu, B.; Zhao, Q.; Zou, Z.; et al. CAVD, towards better characterization of void space for ionic transport analysis. Sci. Data 2020, 7, 153. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]


| Crystal Data | |
|---|---|
| Formula weight | 2500.51 |
| Crystal system, space group | Orthorhombic, Cccm |
| Unit cell parameters, Å | a = 6.4082(8) |
| b = 19.6813(19) | |
| c = 10.5035(11) | |
| Volume, Å3 | 1324.7(3) |
| Z, Calculated density, g/cm3 | 2, 3.134 |
| Crystal size, mm | 0.042 × 0.155 × 0.437 |
| Data Collection | |
| Radiation | Mo-Kα, graphite monochromator |
| Temperature, K | 110(2) |
| Scanning mode | ω |
| Measuring range | max θ = 29.99° |
| Reflections (total) | 6683 |
| Rint, Rσ | 0.1084, 0.0620 |
| Refinement | |
| Refinement method | Full-matrix least-squares on F2 |
| Reflections unique/observed | 1025/804 |
| Parameters | 89 |
| Absorption correction, Tmax/Tmin | Numerical, 0.648/0.536 |
| Goodness-of-fit, S | 1.109 |
| Residuals [I > 2sigma(I)] | R1 = 0.0516, wR2 = 0.1073 |
| Δρ (max/min), e/Å3 | 0.862 and −0.794 |
| Atom | x | y | z | Uiso */Ueq | Occ. (<1) |
|---|---|---|---|---|---|
| V1 | 0.750000 | 0.750000 | 0.500000 | 0.0102(5) | 0.609(15) |
| Al1 | 0.750000 | 0.750000 | 0.500000 | 0.0102(5) | 0.391(15) |
| V2 | 0.000000 | 0.65880(5) | 0.750000 | 0.0105(3) | 0.787(17) |
| Ni1 | 0.000000 | 0.65880(5) | 0.750000 | 0.0105(3) | 0.213(17) |
| P1 | 0.8422(3) | 0.58964(8) | 0.500000 | 0.0122(4) | |
| P2 | 0.500000 | 0.70237(8) | 0.250000 | 0.0128(4) | |
| O1 | 0.5403(5) | 0.75461(15) | 0.3611(3) | 0.0133(7) | |
| O2 | 0.3126(5) | 0.65892(15) | 0.2849(3) | 0.0166(7) | |
| O3 | 0.7066(7) | 0.6539(2) | 0.500000 | 0.0188(10) | |
| O4 | 0.9847(6) | 0.58914(17) | 0.6170(3) | 0.0231(8) | |
| O5 | 0.7004(8) | 0.5290(3) | 0.500000 | 0.0391(16) | |
| O6 | 0.572(3) | 0.500000 | 0.750000 | 0.104(10) | 0.5 |
| Na1 | 0.8413(18) | 0.500000 | 0.750000 | 0.032(2) | 0.351(5) |
| Na2 | 0.245(2) | 0.7844(9) | 0.500000 | 0.057(7) | 0.252(12) |
| Na3 | 0.1606(7) | 0.9072(3) | 0.500000 | 0.0362(13) | 0.660(4) |
| Na4 | 0.133(2) | 0.9366(8) | 0.5880(15) | 0.0362(13) | 0.170(4) |
| Na5 | 0.000000 | 0.500000 | 0.750000 | 0.032(2) | 0.298(10) |
| H1 | 0.679(6) | 0.519(4) | 0.783(7) | 0.150 * | 0.5 |
| P1—tetrahedron | P2—tetrahedron | M1 *—octahedron | M2 **—octahedron | Na1—seven-vertex polyhedron | |||||
| P1—O5 | 1.499(5) | P2—O2 | 1.519(3) × 2 | M1—O4 | 1.960(3) × 2 | M2—O3 | 1.911(5) × 2 | Na1—O4 | 2.423(6) × 2 |
| —O4 | 1.532(3) × 2 | —O1 | 1.577(3) × 2 | —O2 | 2.037(3) × 2 | —O1 | 1.985(3) × 4 | —O4′ | 2.503(6) × 2 |
| —O3 | 1.535(5) | —O1 | 2.082(3) × 2 | —O6 | 2.65(2) | ||||
| —O5 | 2.835(4) × 2 | ||||||||
| Na2—octahedron | Na3—seven-vertex polyhedron | Na4—octahedron | Na5—octahedron | ||||||
| Na2—O1 | 2.46(1) × 4 | Na3—O5 | 2.410(8) | Na4—O5 | 2.09(2) | Na5—O4 | 2.245(3) × 4 | ||
| —O2 | 2.548(9) × 2 | —O4 | 2.584(6) × 2 | —O2 | 2.33(2) | —O6 | 2.75(2) × 2 | ||
| —O2 | 2.614(4) × 2 | —O5 | 2.42(1) | ||||||
| —O5 | 2.632(8) | —O6 | 2.48(2) | ||||||
| —O3 | 2.644(7) | —O4 | 2.52(1) | ||||||
| —O3 | 2.96(1) | ||||||||
| Atom | M1 | M2 | P1 | P2 | Na1 | Na2 | Na3 | Na4 | Na5 | H1 | Σ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O1 | 0.385↓2 | 0.474↓4 | 1.114↓2 | 0.021↓4→2 | 1.99 | ||||||
| O2 | 0.435↓2 | 1.303↓2 | 0.017↓2 | 0.074↓2 | 0.082 | 1.91 | |||||
| O3 | 0.580↓2 | 1.248 | 0.068 | 0.015 | 1.91 | ||||||
| O4 | 0.536↓2 | 1.258↓2 | 0.107↓2 0.132↓2 | 0.080↓2 | 0.050 | 0.051↓4 | 2.22 | ||||
| O5 | 1.376 | 0.043↓2→2 | 0.070 0.128 | 0.166→2 0.064→2 | 0.085 | 2.20 | |||||
| O6 (H2O) | 0.072 | 0.054 | 0.022↓2 | 0.415 | 0.56 | ||||||
| Σ | 2.71 | 3.06 | 5.14 | 4.83 | 0.64 | 0.12 | 0.64 | 0.41 | 0.25 | 0.5 |
| D—H ……‥A | D—H, Å | H ……‥ A, Å | D ……‥A, Å | angle D—H ……‥ A, ° |
|---|---|---|---|---|
| O6—H1 ……. O5 | 0.850(1) | 2.47(9) | 2.811(6) | 105(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubovich, O.; Kiriukhina, G.; Nesterova, V.; Volkov, A.; Fedotov, S.; Dimitrova, O. Crystal Chemistry of a New Mineral-like Phosphate Na6.9Ni2+0.9V3+4.3Al0.8(PO4)8(H2O)2 in the Series of α-CrPO4 Derivatives. Minerals 2025, 15, 3. https://doi.org/10.3390/min15010003
Yakubovich O, Kiriukhina G, Nesterova V, Volkov A, Fedotov S, Dimitrova O. Crystal Chemistry of a New Mineral-like Phosphate Na6.9Ni2+0.9V3+4.3Al0.8(PO4)8(H2O)2 in the Series of α-CrPO4 Derivatives. Minerals. 2025; 15(1):3. https://doi.org/10.3390/min15010003
Chicago/Turabian StyleYakubovich, Olga, Galina Kiriukhina, Valentina Nesterova, Anatoly Volkov, Stanislav Fedotov, and Olga Dimitrova. 2025. "Crystal Chemistry of a New Mineral-like Phosphate Na6.9Ni2+0.9V3+4.3Al0.8(PO4)8(H2O)2 in the Series of α-CrPO4 Derivatives" Minerals 15, no. 1: 3. https://doi.org/10.3390/min15010003
APA StyleYakubovich, O., Kiriukhina, G., Nesterova, V., Volkov, A., Fedotov, S., & Dimitrova, O. (2025). Crystal Chemistry of a New Mineral-like Phosphate Na6.9Ni2+0.9V3+4.3Al0.8(PO4)8(H2O)2 in the Series of α-CrPO4 Derivatives. Minerals, 15(1), 3. https://doi.org/10.3390/min15010003






