Hydrothermal Retrogradation from Chlorite to Tosudite: Effect on the Optical Properties
Abstract
1. Introduction
2. Sampling and Geological Setting
3. Materials and Methods
3.1. Optics. Separation
3.2. X-Ray Diffraction (XRD)
3.3. Scanning Electron Microscopy (SEM)
3.4. Electron Microprobe (EPM)
3.5. Transmission Electron Microscopy (TEM)
4. Results
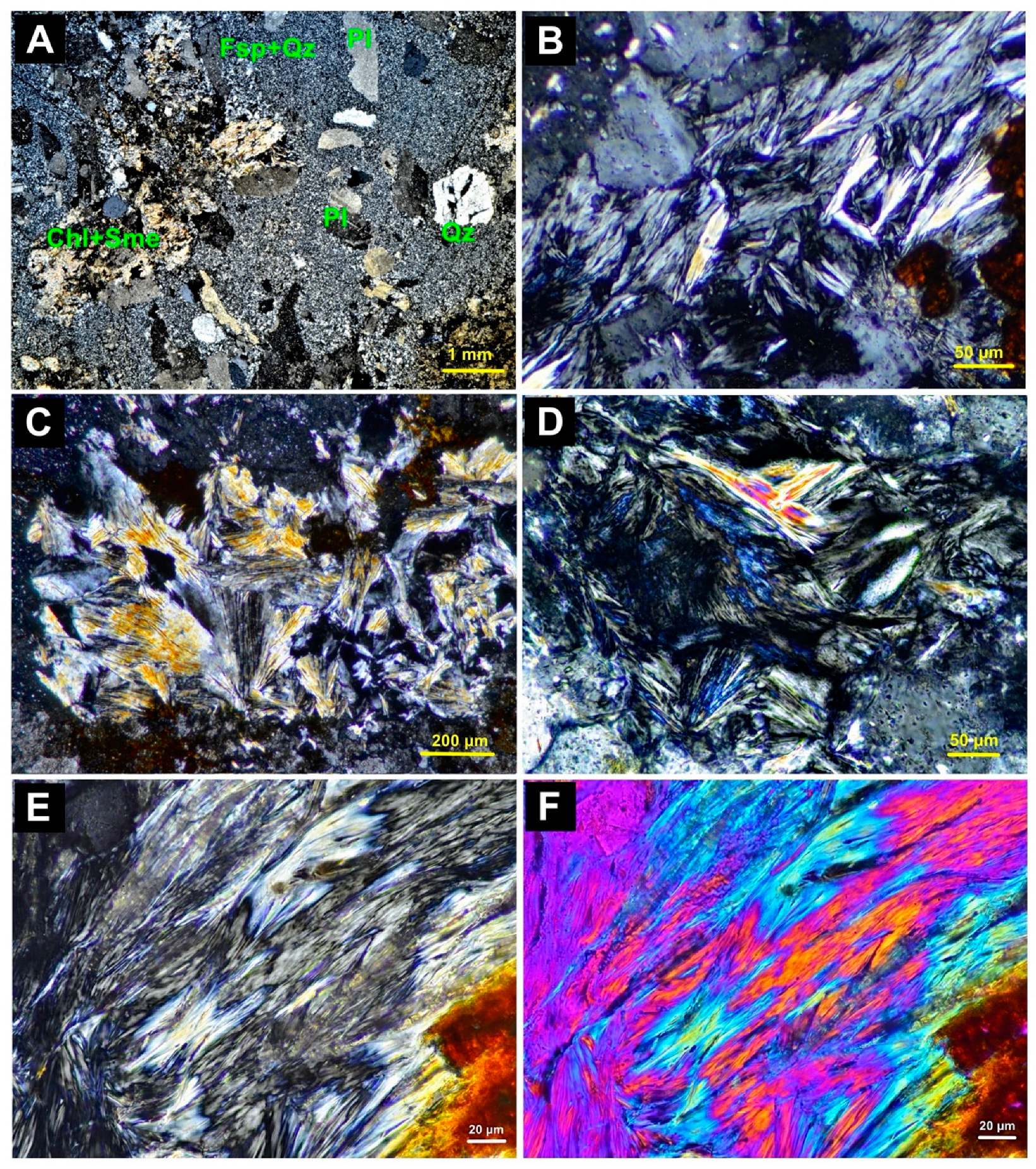
4.1. Optical Microscopy
4.2. X-Ray Diffraction (XRD)
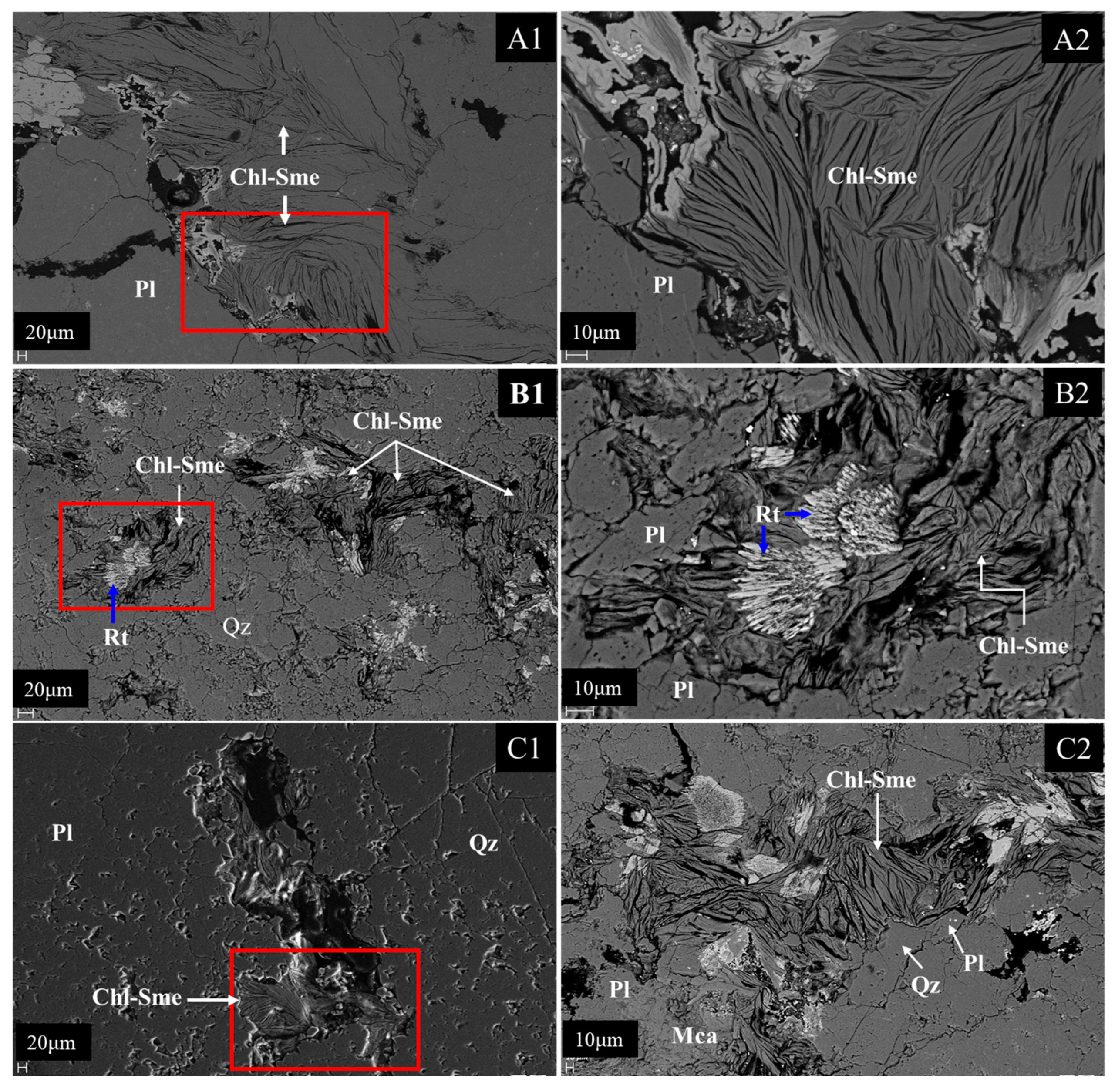
4.3. Scanning Electron Microscopy
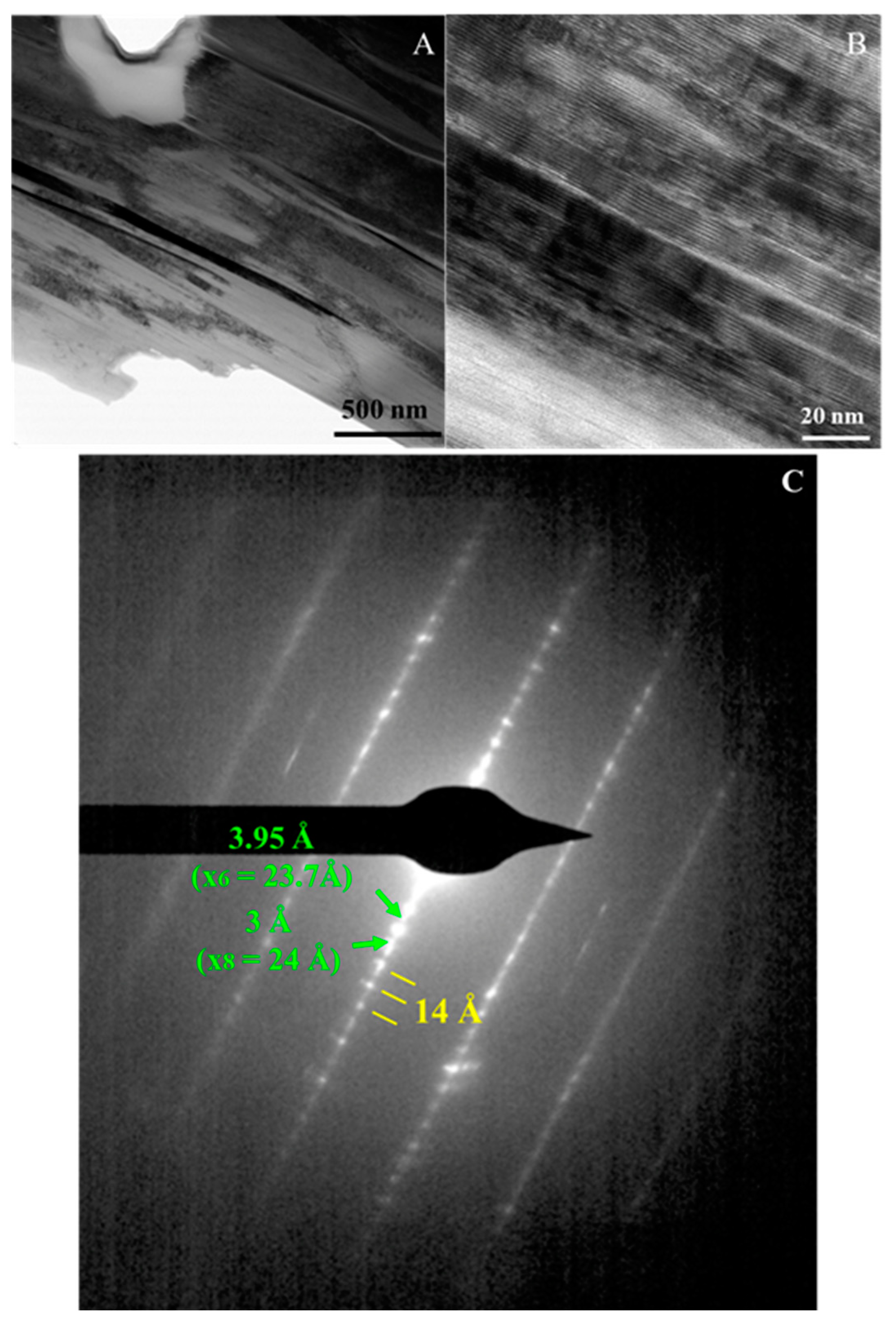
4.4. Transmission Electron Microscopy (TEM)

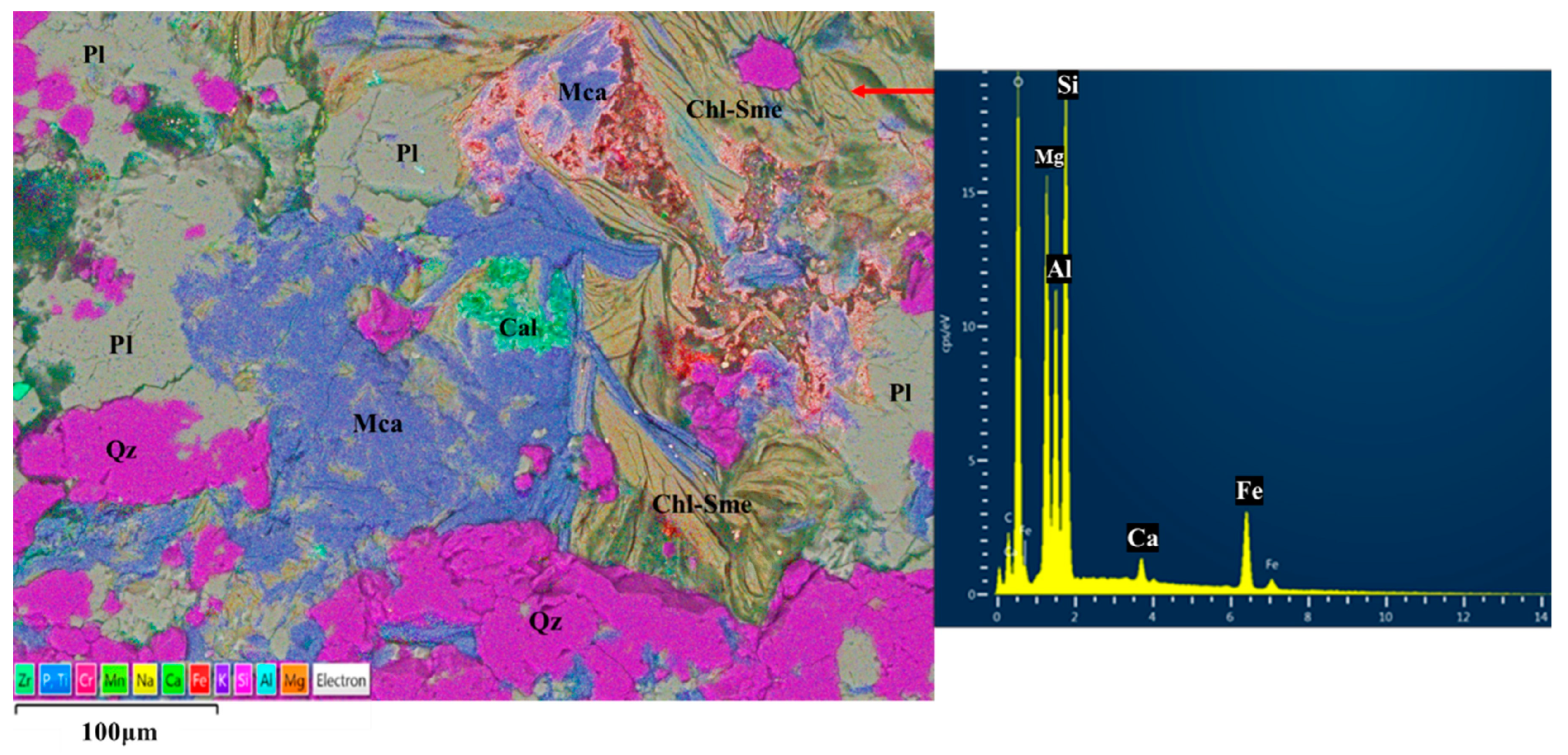
4.5. Chemical Composition of Chlorite and Chlorite/Smectite
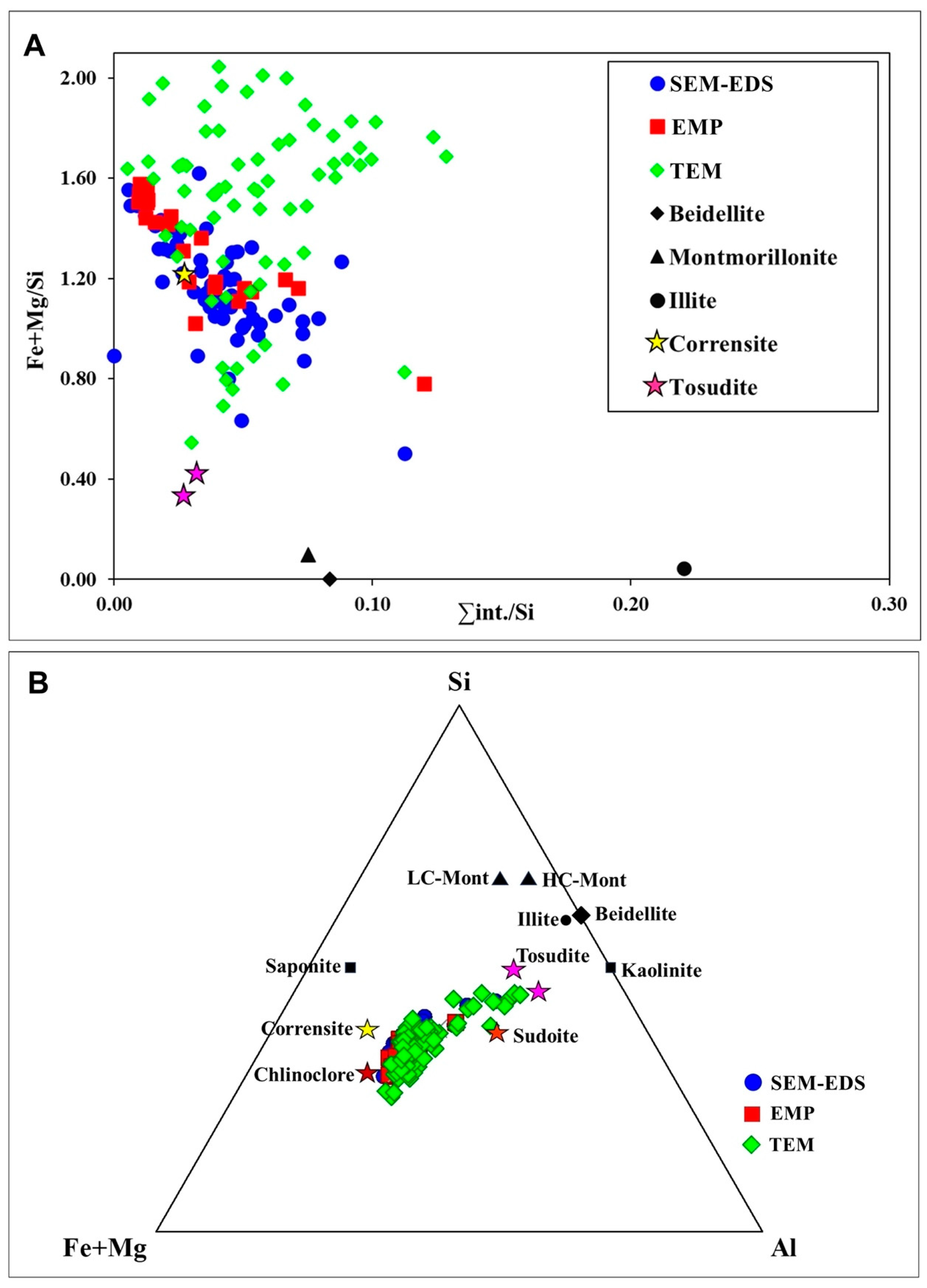
5. Discussion
5.1. Nature of the Mixed-Layer
5.2. Chemical Composition
5.3. Origin of the Mixed-Layer
5.4. Effect on the Optical Properties of Chlorite
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albee, A.L. Relationships between the mineral association, chemical composition, and physical properties of the chlorite series. Am. Mineral. 1962, 47, 851–870. [Google Scholar]
- Nesse, W.D. Introduction to Optical Mineralogy; Oxford University Press: Oxford, UK, 1991; 335p. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock Forming Minerals Vol. III: Sheet Silicates; Longman: Harlow, UK, 2013; 270p. [Google Scholar]
- Chatterjee, N.D. On the widespread occurrence of oxidized chlorites in the Pennine Zone of the Western Italian Alps. Contrib. Mineral. Petrol. 1966, 12, 325–339. [Google Scholar] [CrossRef]
- Masci, L.; Dubacq, B.; Verlaguet, A.; Chopin, C.; De Andrade, V.; Herviou, C. A XANES and EPMA study of Fe3+ in chlorite: Importance of oxychlorite and implications for cation site distribution and thermobarometry. Am. Mineral. 2019, 104, 403–417. [Google Scholar] [CrossRef]
- Mellini, M.; Nieto, F.; Alvarez, F.; Gomez-Pugnaire, M.T. Mica-chlorite intermixing and altered chlorite from the Nevado-Filabride mica schists, southern Spain. Eur. J. Mineral. 1991, 3, 27–38. [Google Scholar] [CrossRef]
- Shau, J.H.; Peacor, D.R.; Essene, E.J. Corrensite and mixed-layer chlorite/corrensite in metabasalt from Northern Taiwan: TEM/AEM, EMPA, XRD and optical studies. Contrib. Mineral. Petrol. 1990, 105, 123–142. [Google Scholar] [CrossRef]
- Jiang, W.T.; Peacor, D.R. Formation of corrensite, chlorite and chlorite-mica stacks by replacement of detrital biotite in low-grade pelitic rocks. J. Metamorph. Geol. 1994, 12, 867–884. [Google Scholar] [CrossRef]
- Shimoda, S. New data for tosudite. Clays Clay Miner. 1969, 17, 179–184. [Google Scholar] [CrossRef]
- Nishiyama, T.; Shimoda, S.; Shimosaka, K.; Kanaoka, S. Lithium-bearing tosudite. Clays Clay Miner. 1975, 23, 337–342. [Google Scholar] [CrossRef]
- Ichikawa, A.; Shimoda, S. Tosudite from the Hokuno Mine, Hokuno, Gifu Prefecture, Japan. Clays Clay Miner. 1976, 24, 142–148. [Google Scholar] [CrossRef]
- Creach, M.; Meunier, A.; Beaufort, D. Tosudite crystallization in the kaolinized granitic cupola of Montebras, Creuse, France. Clay Miner. 1986, 21, 225–230. [Google Scholar] [CrossRef]
- Merceron, T.; Inoue, A.; Bouchet, A.; Meunier, A. Lithium-bearing donbassite and tosudite from Echassières, Massif Central, France. Clays Clay Miner. 1988, 36, 39–46. [Google Scholar] [CrossRef]
- Bartier, D.; Meunier, A.; Liewig, N.; Morvan, G.; Addad, A. Hydrothermal alteration of the Soultz-sous-Forets granite (hot fractured rock geothermal exchanger) into a tosudite and illite assemblage. Eur. J. Mineral. 2008, 20, 131–142. [Google Scholar] [CrossRef]
- Hillier, S.; Son, B.K.; Velde, B. Effect of hydrothermal activity on clay mineral diagenesis in Miocene shales and sandstones from the Ulleung (Tsushima) back-arc basin, East Sea (Sea of Japan), Korea. Clay Miner. 1996, 31, 113–126. [Google Scholar] [CrossRef]
- Billon, S.; Patrier, P.; Beaufort, D.; Sardini, P.; Wattinne-Morice, A. Occurrence of tosudite in the Guezouman, Tarat, and Tchirezrine 2 formations, hosts of uranium deposits in Niger (Tim Mersoï basin). Clay Miner. 2016, 51, 635–651. [Google Scholar] [CrossRef]
- Abd Elmola, A.; Asaad, A.; Patrier, P.; Ballini, M.; Descostes, M. Clay mineral signatures of fault-related fluid flows in a sandstone reservoir: A case study from the Teloua Formation, Tim Mersoï Basin, Niger. J. Afr. Earth Sci. 2020, 168, 103840. [Google Scholar] [CrossRef]
- Maksimovic, Z.; Brindley, G.W. Hydrothermal alteration of a serpentinite near Takovo, Yugoslavia, to chromium-bearing illite/smectite, kaolinite, tosudite, and halloysite. Clays Clay Miner. 1980, 28, 295–302. [Google Scholar] [CrossRef]
- Ruiz Cruz, M.D.; Andreo, B. Tosudite in very low-grade metamorphic graywackes from the Málaga area, Betic Cordilleras, Spain. Eur. J. Mineral. 1996, 8, 1391–1399. [Google Scholar] [CrossRef]
- Frank-Kamenetskii, V.A.; Logvinenko, N.V.; Drits, V.A. Tosudite—A new mineral, forming the mixed layer phase in alushtite. In Proceedings of the International Clay Conference, Stockholm, Sweden, 12–16 August 1965; Volume 2, pp. 181–186. [Google Scholar]
- De Windt, L.; Grizard, P.; Besançon, C.; Assalack, F.; Djibo Hama, I.; Reiller, P.E.; Seigneur, N.; Descostes, M. Modeling of hydrogeochemical processes influencing uranium migration in anthropized arid environments with application to the Teloua aquifer. J. Contam. Hydrol. 2025, 269, 104507. [Google Scholar] [CrossRef]
- Dimitrijevic, M.D. Geology of the Kerman Region; Report 52; Geology Survey of Iran: Tehran, Iran, 1973; pp. 245–334. [Google Scholar]
- Saric, V.; Mijalkovic, N. Metallogenic map of Kerman region, 1:500000 scale. In Exploration for Ore Deposits in Kerman Region; Report 53; Geology Survey of Iran: Tehran, Iran, 1973; pp. 244–247. [Google Scholar]
- Dercourt, J.; Zonenshain, L.; Ricou, L.E.; Kazmin, G.; Le Pichon, X.; Knipper, A.L.; Grandjacquet, C.; Sbortshikov, I.M.; Geyssant, J.; Lepvrier, C.; et al. Geological evolution of the Tethys belt from the Atlantic to Pamirs since the Lias. Tectonophysics 1986, 123, 241–315. [Google Scholar] [CrossRef]
- Emami, M.H.; Mir Mohammad Sadeghi, M.; Omrani, S.J. Magmatic Map of Iran: 1: 2,500,000 Scale; Geological Survey of Iran: Tehran, Iran, 1993. [Google Scholar]
- Samani, B. Distribution, setting and metallogenesis of copper deposits in Iran. In Porphyry and Hydrothermal Copper and Gold Deposits: A Global Perspective; Porter, T.M., Ed.; Conference Proceedings; Perth PGC Publishing: Adelaide, Australia, 1998; pp. 135–158. [Google Scholar]
- Shafiei, B.; Haschke, M.; Shahabpour, J. Recycling of orogenic arc crust triggers porphyry Cu mineralization in Kerman Cenozoic arc rocks, southeastern Iran. Miner. Depos. 2009, 44, 265–283. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-Ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997; 378p. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. ‘PAP’ (φρZ) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Cliff, G.; Lorimer, G.W. The quantitative analysis of thin specimens. J. Microsc. 1975, 103, 203–207. [Google Scholar] [CrossRef]
- Laird, J. Chlorites: Metamorphic petrology. In Hydrous Phyllosilicates (Reviews in Mineralogy, vol 19); Bailey, S.W., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1988; pp. 405–453. [Google Scholar]
- Bourdelle, F.; Parra, T.; Chopin, C.; Beyssac, O. A new chlorite geothermometer for diagenetic to low-grade metamorphic conditions. Contrib. Mineral. Petrol. 2013, 165, 723–735. [Google Scholar]
- Inoue, A.; Inoué, S.; Utada, M. Application of chlorite thermometry to estimation of formation temperature and redox conditions. Clay Miner. 2018, 53, 143–158. [Google Scholar] [CrossRef]
- Do Campo, M.; Nieto, F.; Albanesi, G.L.; Ortega, G.; Monaldi, C.R. Outlining the thermal postdepositional evolution of the Ordovician successions of northwestern Argentina by clay mineral analysis, chlorite geothermometry, and Kübler index. Andean Geol. 2017, 44, 179–212. [Google Scholar] [CrossRef]
- Arostegui, J.; Arroyo, X.; Nieto, F.; Bauluz, B. Evolution of clays in the Cretaceous marly series (Alava Block, Basque Cantabrian Basin, Spain): Diagenesis and detrital input control. Minerals 2020, 9, 40. [Google Scholar] [CrossRef]
- Petruk, W. Determination of the heavy atom content in chlorite by means of X-ray diffractometer. Am. Mineral. 1964, 49, 61–67. [Google Scholar]
- Nieto, F. Chemical composition of metapelitic chlorites; X-ray diffraction and optical property approach. Eur. J. Mineral. 1997, 9, 829–841. [Google Scholar] [CrossRef]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and chloritization processes through mixed-layer mineral series in low-temperature geological systems—A review. Clay Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Hullaster, D.P.; Soreghan, G.S.; Kukkadapu, R.K.; Dumont, B.S.; Dee, K.T.; Elwood Madden, A.S. Red-green-bleached redox interfaces in the proximal Permian Cutler red beds: Implications for regional fluid alteration. Front. Earth Sci. 2023, 11, 1219966. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. (Eds.) Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1980; Available online: https://handbookofmineralogy.org/ (accessed on 1 March 2025).
- Bloss, F.D. Crystallography and Crystal Chemistry; Mineralogical Society of America: Chantilly, VA, USA, 1994; 545p. [Google Scholar]



| Chlorite (Clinochlore) | Mixed-Layer Chlorite/Smectite | |
|---|---|---|
| Refractive index | γ ≈ 1.588, β ≈ α ≈ 1.58 | γ ≈ β = 1.578, αmin ≈ 1.557 |
| Birefringence (max.) | 0.007 | 0.021 |
| Colour | pale green | colourless or very pale green |
| Pleochroism | weak, X = Y < Z | very weak, X< Y = Z |
| Optic orientation | X~a, Y = b, Z~c | X~c, Y = b, Z~a |
| Sign of the elongation | length-fast (−) | length-slow (+) |
| Optic sign | (+) | (−) |
| Optic angle 2V | <5° | <5° |
| Data Set/Point | 16 | 21 | 29 | 33 | 14 | 22 | 31 | 26 | 23 | 11 | 15 |
| SiO2 | 30.76 | 31.13 | 29.45 | 27.56 | 29.33 | 33.82 | 34.47 | 32.73 | 32.71 | 28.24 | 22.22 |
| Al2O3 | 21.40 | 20.41 | 18.75 | 18.73 | 16.57 | 19.76 | 18.45 | 17.64 | 16.83 | 15.50 | 12.95 |
| MgO | 25.07 | 24.99 | 24.58 | 24.46 | 23.18 | 22.81 | 22.68 | 21.32 | 19.65 | 17.96 | 14.56 |
| FeOt | 8.35 | 8.21 | 7.23 | 7.53 | 6.49 | 7.36 | 8.53 | 7.56 | 9.88 | 5.48 | 5.82 |
| TiO2 | 0.03 | 0.02 | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 |
| MnO | 0.12 | 0.11 | 0.16 | 0.12 | 0.12 | 0.14 | 0.17 | 0.13 | 0.17 | 0.08 | 0.13 |
| CaO | 0.16 | 0.42 | 0.47 | 0.22 | 0.63 | 0.54 | 0.98 | 0.76 | 1.12 | 0.86 | 1.04 |
| Na2O | 0.03 | 0.05 | 0.03 | 0.03 | 0.09 | 0.06 | 0.05 | 0.07 | 0.12 | 0.10 | 0.12 |
| K2O | 0.11 | 0.08 | 0.07 | 0.05 | 0.11 | 0.22 | 0.16 | 0.25 | 0.23 | 0.19 | 0.11 |
| Sum | 86.02 | 85.41 | 80.75 | 78.72 | 76.52 | 84.73 | 85.52 | 80.48 | 80.73 | 68.42 | 56.96 |
| Data Set/Point | 16 | 21 | 29 | 33 | 14 | 22 | 31 | 26 | 23 | 11 | 15 |
| Numbers of cations based on O10(OH)8 | |||||||||||
| Si | 2.99 | 3.06 | 3.06 | 2.94 | 3.21 | 3.31 | 3.38 | 3.40 | 3.45 | 3.43 | 3.31 |
| Aliv | 1.01 | 0.94 | 0.94 | 1.06 | 0.79 | 0.69 | 0.62 | 0.60 | 0.55 | 0.57 | 0.69 |
| Alvi | 1.45 | 1.42 | 1.35 | 1.30 | 1.34 | 1.60 | 1.52 | 1.55 | 1.54 | 1.65 | 1.58 |
| Mg | 3.64 | 3.66 | 3.80 | 3.89 | 3.78 | 3.33 | 3.32 | 3.30 | 3.09 | 3.25 | 3.23 |
| Fe * | 0.68 | 0.67 | 0.63 | 0.67 | 0.59 | 0.60 | 0.70 | 0.66 | 0.87 | 0.56 | 0.72 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 |
| ∑ Oct | 5.78 | 5.76 | 5.80 | 5.88 | 5.72 | 5.54 | 5.55 | 5.52 | 5.51 | 5.46 | 5.55 |
| Ca | 0.02 | 0.04 | 0.05 | 0.03 | 0.07 | 0.06 | 0.10 | 0.08 | 0.13 | 0.11 | 0.17 |
| Na | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 |
| K | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 |
| Int ch. | 0.05 | 0.11 | 0.12 | 0.06 | 0.18 | 0.15 | 0.24 | 0.22 | 0.31 | 0.28 | 0.38 |
| Point | SEM-EDS Formulae |
| Site4Sp13 | [Si3.45Al0.54]4O10[Al1.63Mg2.63Fe1.10Mn0.02Ti0.02]5.42[K0.02Ca0.15]0.17(OH)8 |
| Site3Sp3 | [Si3.30Al0.69]4O10[Al1.61Mg3.08Fe0.81Mn0.03]5.54[K0.04Ca0.08]0.12(OH)8 |
| Site1Sp2 | [Si3.17Al0.82]4O10[Al1.34Mg3.73Fe0.63Mn0.02]5.74[K0.01Ca0.06]0.07(OH)8 |
| Site6Sp22 | [Si3.28Al0.71]4O10[Al1.44Mg3.57Fe0.61]5.63[K0.03Ca0.07]0.10(OH)8 |
| Site2Sp3 | [Si2.92Al1.07]4O10[Al1.19Mg3.11Fe1.62]5.93[K0.02Ca0.06]0.08(OH)8 |
| Site6Sp15 | [Si3.15Al0.84]4O10[Al1.50Mg3.65Fe0.51]5.67[K0.02Ca0.03]0.05(OH)8 |
| Site4Sp7 | [Si3.15Al0.84]4O10[Al1.34Mg3.83Fe0.56]5.74[K0.02Ca0.04]0.06(OH)8 |
| Site6Sp2 | [Si3.05Al0.94]4O10[Al1.44Mg3.77Fe0.53]5.75[K0.01Ca0.03]0.04(OH)8 |
| Site1 Sp14 | [Si2.93Al1.06]4O10[Al1.30Mg4.03Fe0.53]5.87[Ca0.01]0.01(OH)8 |
| Sites.sp710 | [Si3.29Al0.70]4O10[Al1.36Mg2.67Fe1.63]5.67[Ca0.15]0.15(OH)8 |
| Site1Sp9 | [Si2.99Al1]4O10[Al1.35Mg3.82Fe0.64]5.82[Ca0.02]0.02(OH)8 |
| Site5Sp4 | [Si3.68Al0.31]4O10[Al1.91Mg2.71Fe0.57]5.20(OH)8 |
| Point | TEM Formulae |
| map1.35sp2 | [Si3.01Al0.98]4O10[Al1.44Mg3.40Fe0.91Mn0.01]5.77[Na0.01K0.03Ca0.07]0.11(OH)8 |
| 1002bsp1b | [Si3.18Al0.81]4O10[Al1.58Mg3.43Fe0.57Mn0.01]5.61[K0.04Ca0.03]0.07(OH)8 |
| 1002bsp2b | [Si3.88Al0.11]4O10[Al2.98Mg1.04Fe0.52Mn0.01]4.56[K0.06Ca0.10]0.16(OH)8 |
| 1011bsp1b | [Si3.73Al0.26]4O10[Al2.85Mg1.57Fe0.27]4.70[Na0.04K0.04Ca0.06]0.14(OH)8 |
| 1352sp2 | [Si3.20Al0.79]4O10[Al1.54Mg3.17Fe0.90]5.62[Na0.03K0.01Ca0.19]0.23(OH)8 |
| aro3sp4 | [Si3.15Al0.84]4O10[Al1.48Mg3.21Fe0.97]5.67[Na0.05K0.02Ca0.13]0.20(OH)8 |
| 1110sp1 | [Si3.09Al0.90]4O10[Al1.43Mg4.11Fe0.16Ti0.01]5.72[K0.05Ca0.09]0.14(OH)8 |
| 1039sp3 | [Si2.66Al1.33]4O10[Al1.25Mg4.46Fe0.30Mn0.01]6.04[Ca0.04]0.04(OH)8 |
| 1234225f | [Si2.87Al1.12]4O10[Al1.38Mg3.91Fe0.58]5.87[K0.02Ca0.12]0.14(OH)8 |
| 1039sp1 | [Si2.94Al1.05]4O10[Al1.32Mg4.32Fe0.19]5.86[K0.01Ca0.02]0.03(OH)8 |
| 1039sp2 | [Si3.12Al0.87]4O10[Al1.44Mg3.98Fe0.27]5.71[K0.01Ca0.07]0.08(OH)8 |
| 1359sp3 | [Si2.87Al1.12]4O10[Al1.50Mg4.07Fe0.22]5.80(OH)8 |
| Point | Si | Aliv | Alvi | Al | Mg | Fe * | Mn | Ti | ∑oct | Na | K | Ca | ∑c.int. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP2b | 6.74 | 1.26 | 4.14 | 5.4 | 1.81 | 0.91 | 0.02 | 0.00 | 6.89 | 0.00 | 0.12 | 0.18 | 0.47 |
| SP7 | 6.68 | 1.32 | 4.12 | 5.44 | 1.97 | 0.84 | 0.00 | 0.00 | 6.93 | 0.03 | 0.33 | 0.07 | 0.51 |
| SP18 | 6.68 | 1.32 | 4.12 | 5.44 | 1.97 | 0.84 | 0.00 | 0.00 | 6.93 | 0.03 | 0.33 | 0.07 | 0.51 |
| SP28 | 6.64 | 1.36 | 4.02 | 5.39 | 2.00 | 0.94 | 0.00 | 0.00 | 6.97 | 0.00 | 0.10 | 0.18 | 0.46 |
| SP1 | 6.78 | 1.22 | 4.43 | 5.65 | 2.25 | 0.38 | 0.00 | 0.00 | 7.05 | 0.00 | 0.09 | 0.11 | 0.31 |
| SP1b | 6.56 | 1.44 | 4.04 | 5.47 | 2.76 | 0.48 | 0.00 | 0.00 | 7.27 | 0.08 | 0.08 | 0.11 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, Z.; Nieto, F.; Khormali, F.; Velilla, N.; Einali, M.; Maghsoudi, A.; Amini, A. Hydrothermal Retrogradation from Chlorite to Tosudite: Effect on the Optical Properties. Minerals 2025, 15, 326. https://doi.org/10.3390/min15030326
Ahmadi Z, Nieto F, Khormali F, Velilla N, Einali M, Maghsoudi A, Amini A. Hydrothermal Retrogradation from Chlorite to Tosudite: Effect on the Optical Properties. Minerals. 2025; 15(3):326. https://doi.org/10.3390/min15030326
Chicago/Turabian StyleAhmadi, Zahra, Fernando Nieto, Farhad Khormali, Nicolás Velilla, Morteza Einali, Abbas Maghsoudi, and Arash Amini. 2025. "Hydrothermal Retrogradation from Chlorite to Tosudite: Effect on the Optical Properties" Minerals 15, no. 3: 326. https://doi.org/10.3390/min15030326
APA StyleAhmadi, Z., Nieto, F., Khormali, F., Velilla, N., Einali, M., Maghsoudi, A., & Amini, A. (2025). Hydrothermal Retrogradation from Chlorite to Tosudite: Effect on the Optical Properties. Minerals, 15(3), 326. https://doi.org/10.3390/min15030326







