An Overview of Authigenic Magnesian Clays
Abstract
:1. Introduction
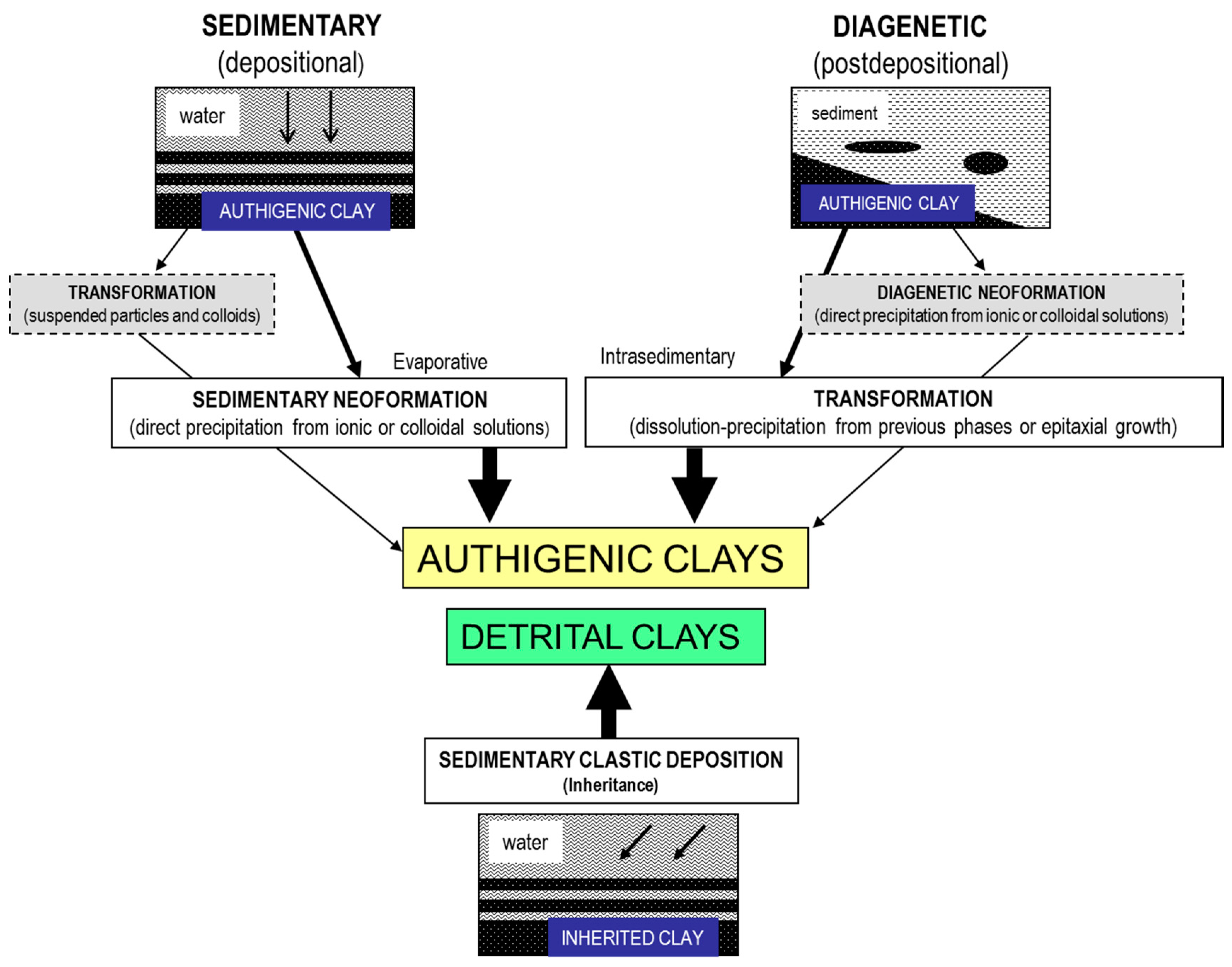
1.1. The “Authigenesis” Concept
1.2. Overview of Authigenic Clay Minerals
1.3. Magnesian Clays—General Concepts and Types
2. Authigenic Mg-Clay Minerals in Marine and Non-Marine Modern Environments
3. Authigenic Mg-Clay Minerals in Ancient Sedimentary and Non-Sedimentary Geological Formations
3.1. Mg-Clay Deposits in Sedimentary Environments
3.1.1. Peri-Marine Palygorskite Deposits
3.1.2. Continental Palygorskite Deposits
3.2. Continental Mg-Smectite and Sepiolite Deposits
3.3. Mg-Clay Occurrence in Ancient Non-Sedimentary Environments
4. Geochemical Pathways for Mg-Clay Minerals Formation
4.1. Pathway 1
4.2. Pathway 2
4.3. Pathway 3
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neuendorf, K.K.E.; Mehl, J.P.; Jackson, J.A. Glossary of Geology; American Geological Institute: Alexandria, VA, USA, 2005. [Google Scholar]
- Kalkowsky, E. Über die Erferschung der Archaeischen Formationen. Neues Jahrb. Mineral. 1880, 1, 1–28. [Google Scholar]
- Teodorovich, G.I. Authigenic Minerals in Sedimentary Rocks; Consultants Bureau: New York, NY, USA, 1961; p. 120. [Google Scholar]
- Fairbridge, R.W. Phases of diagenesis and authigenesis. In Diagenesis in Sediments, Developments in Sedimentology 8; Larsen, G., Chilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; pp. 2–89. [Google Scholar]
- Eslinger, E.; Pevear, D. Clay Minerals for Petroleum Geologists and Engineers; Short Course 22; Society for Economic Paleontologists and Mineralogists: Tulsa, OK, USA, 1988. [Google Scholar]
- Millot, G. Geology of Clays; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Jones, B.F. Clay mineral diagenesis in lacustrine sediments. U.S. Geol. Surv. Bull. 1986, 1578, 291–300. [Google Scholar]
- Galán, E.; Pozo, M. Palygorskite and sepiolite deposits in continental environments. Description, genetic patterns and sedimentary settings. In Developments in Palygorskite—Sepiolite Research, Developments in Clay Science 3; Elsevier: Amsterdam, The Netherlands, 2011; pp. 125–174. [Google Scholar]
- Tosca, N. Geochemical pathways to Mg-silicate formation. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; Digilabs: Bari, Italy, 2015; pp. 283–329. [Google Scholar]
- Nielsen, A.E. Kinetics of Precipitation; Pergamon Press: New York, NY, USA, 1964. [Google Scholar]
- Lasaga, A.C. Kinetic Theory in the Earth Sciences; Holland, H.D., Ed.; Princeton University Press: Princeton, NJ, USA, 1998. [Google Scholar]
- Stumm, W. Chemistry of the Solid-Water Interface; Processes at the Mineral-Water and Particle-Water Interface in Natural Systems; John Wiley and Son Inc.: Hoboken, NJ, USA, 1992. [Google Scholar]
- Zhang, J.; Huang, F.; Lin, Z. Progress of nanocrystalline growth kinetics based on oriented attachment. Nanoscale 2010, 2, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Eberl, D.; Hower, J. Kaolinite synthesis: The role of the Si/A1 and (alkali)/(H+) ratio in hydrothermal systems. Clays Clay Miner. 1975, 23, 301–309. [Google Scholar] [CrossRef]
- Siffert, B.; Wey, R. Synthèse d’une sepiolite à temperature ordinaire. C. R. Paris 1962, 254, 1460–1463. [Google Scholar]
- Wollast, R.; Mackenzie, F.T.; Bricker, O.P. Experimental precipitation and genesis of sepiolite at earth-surface conditions. Am. Mineral. 1968, 53, 1645–1662. [Google Scholar]
- Deocampo, D.M.; Cuadros, J.; Wing-Dudek, T.; Olives, J.; Amouric, M. Saline lake diagenesis as revealed by coupled mineralogy and geochemistry of multiple ultrafine clay phases: Pliocene Olduvai Gorge, Tanzania. Am. J. Sci. 2009, 309, 834–868. [Google Scholar] [CrossRef]
- Wilson, M.D.; Pittman, E.D. Authigenic clays in sandstones: Recognition and influence on reservoir properties and paleoenvironmental analysis. J. Sediment. Res. 1977, 47, 3–31. [Google Scholar]
- Guggenheim, S. Introduction to Mg-rich clay minerals: Structure and composition. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; Digilabs: Bari, Italy, 2015; pp. 1–62. [Google Scholar]
- Jones, B.F.; Galán, E. Sepiolite and palygorskite. Rev. Mineral. Geochem. 1988, 19, 631–674. [Google Scholar]
- Stoessell, R.K. 25 °C and 1 atm dissolution experiments of sepiolite and kerolite. Geochim. Cosmochim. Acta 1988, 52, 365–374. [Google Scholar] [CrossRef]
- Deocampo, D.M.; Ashley, G.M. Siliceous islands in a carbonate sea: Modern and Pleistocene records of spring-fed wetlands in Ngorongoro Crater and Olduvai Gorge, Tanzania. J. Sediment. Res. 1999, 69, 974–979. [Google Scholar] [CrossRef]
- Deocampo, D.M. Evaporative evolution of surface waters and the role of aqueous CO2 in magnesium silicate precipitation: Lake Eyasi and Ngorongoro Crater, northern Tanzania. S. Afr. J. Geol. 2005, 108, 493–504. [Google Scholar] [CrossRef]
- Tosca, N.J.; Macdonald, F.A.; Strauss, J.V.; Johnston, D.T.; Knoll, A.H. Sedimentary talc in Neoproterozoic carbonate successions. Earth Planet. Sci. Lett. 2011, 306, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Trauth, N. Argiles Évaporitiques dans les Sédimentation Carbonatée et Épicontinental Tertiaire. Bassin de Paris, Mormoiron et Salinelles (France), Jbel Ghassoul (Maroc); Sciences Geologiques Mémoire: Strasbourg, France, 1977; Volume 49, p. 195. [Google Scholar]
- Leguey, S.; Pozo, M.; Medina, J.A. Polygenesis of sepiolite and palygorskite in a fluvio-lacustrine environment in the Neogene basin of Madrid. Mineral. Petrogr. Acta 1985, 29A, 287–301. [Google Scholar]
- Williams, L.A.; Parks, G.A.; Crerar, D.A. Silica diagenesis. I. Solubility controls. J. Sediment. Petrol. 1985, 50, 301–311. [Google Scholar]
- Pozo, M.; Leguey, S.; Medina, J.A. Sepiolite and palygorskite genesis in carbonate lacustrine environments (Duero Basin, Spain). Chem. Geol. 1990, 84, 290–291. [Google Scholar] [CrossRef]
- Birsoy, R. Formation of sepiolite-palygorskite and related minerals from solution. Clays Clay Miner. 2002, 50, 736–745. [Google Scholar] [CrossRef]
- Khoury, H.M.; Eberl, D.D.; Jones, B.F. Origin of magnesium clays from the Amargosa Desert, Nevada. Clays Clay Miner. 1982, 30, 327–336. [Google Scholar] [CrossRef]
- Eberl, D.D.; Jones, B.F.; Khoury, H.N. Mixed layer Kerolite-stevensite from the Amargosa Desert, Nevada. Clays Clay Miner. 1982, 30, 321–326. [Google Scholar] [CrossRef]
- Post, J.L.; Janke, N.C. Barallat sepiolite in Inyo County California. In Palygorskite-Sepiolite. Occurrences, Genesis and Uses, Developments in Sedimentology; Singer, A., Galán, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 159–167. [Google Scholar]
- Chahi, A.; Duplay, J.; Lucas, J. Analysis of palygorskites and associated clays from the Jbel Rhassoul (Morocco): Chemical characteristics and origin of formation. Clays Clay Miner. 1993, 41, 401–411. [Google Scholar] [CrossRef]
- Pozo, M.; Casas, J.C. Origin of kerolite and associated Mg clays in palustrine-lacustrine environments. The Esquivias deposit (Neogene Madrid Basin, Spain). Clay Miner. 1999, 34, 395–418. [Google Scholar] [CrossRef]
- Pozo, M. Origin and evolution of magnesium clays in lacustrine environments: Sedimentology and geochemical pathways. In Proceedings of the 1st Latin American Clay Conference, Funchal, Portugal, September 2000; pp. 117–133. [Google Scholar]
- Kloprogge, J.T.; Komarmeni, S.; Amonette, J.E. Synthesis of smectite clay minerals: A critical review. Clays Clay Miner. 1999, 47, 529–554. [Google Scholar] [CrossRef]
- Decarreau, A. Cristallogenése expérimentale des smectites magnésiennes: Hectorite, stevensite. Bull. Minéral. 1980, 103, 579–590. [Google Scholar]
- Decarreau, A. Partitioning of divalent transition elements between octahedral sheets of trioctahedral smectites and water. Geochim. Cosmochim. Acta 1985, 49, 1537–1544. [Google Scholar] [CrossRef]
- Hay, R.L.; Hughes, R.E.; Kyser, T.K.; Glass, H.D.; Lin, J. Magnesium-rich clays of the Meerschaum mines in the Amboseli Basin, Tanzania and Kenya. Clays Clay Miner. 1995, 43, 455–466. [Google Scholar] [CrossRef]
- Brindley, G.W.; Bish, D.L.; Wan, H. The nature of kerolite: Its relation to talc and stevensite. Mineral. Mag. 1977, 41, 443–452. [Google Scholar] [CrossRef]
- Badaut, D.; Risacher, F. Authigenic smectite on diatom frustules in Bolivian saline lakes. Geochim. Cosmochim. Acta 1983, 47, 363–375. [Google Scholar] [CrossRef]
- Darragi, F.; Tardy, Y. Authigenic trioctahedral smectites controlling pH, alkalinity, silica an Mg-concentrations in alkaline lakes. Chem. Geol. 1987, 63, 59–72. [Google Scholar] [CrossRef]
- Tosca, N.J.; Masterson, A. Chemical controls on incipient Mg-silicate crystallization at 25 °C: Implications for early and late diagenesis. Clay Miner. 2014, 49, 165–194. [Google Scholar] [CrossRef] [Green Version]
- Isphording, W.C. Discussion of the occurrence and origin of sedimentary palygorskite-sepiolite deposits. Clays Clay Miner. 1973, 21, 391–401. [Google Scholar] [CrossRef]
- Singer, A. Palygorskite in sediments: Detrital, diagenetic or neoformed—A critical review. Geol. Rundsch. 1979, 68, 996–1008. [Google Scholar] [CrossRef]
- Paquet, H. Stability, instability and significance of attapulgite in the calcretes of Mediterranean and tropical areas with marked dry season. Sci. Géol. 1983, 72, 131–140. [Google Scholar]
- Weaver, C.E.; Beck, K.C. Miocene of the S.E. United States: A model for chemical sedimentation in a peri–marine environment. Sediment. Geol. 1977, 17, 1–234. [Google Scholar] [CrossRef]
- Singer, A. Pedogenic palygorskite in the arid environment. In Palygorskite-Sepiolite. Occurrences, Genesis and Uses, Developments in Sedimentology; Singer, A., Galán, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 169–177. [Google Scholar]
- Singer, A.; Norrish, K. Pedogenic palygorskite occurrences in Australia. Am. Mineral. 1974, 59, 508–517. [Google Scholar]
- Tazaki, K.; Fyfe, W.S.; Heath, G.R. Palygorskite formed on montmorillonite in North Pacific deep-sea sediments. Clay Sci. 1986, 6, 197–216. [Google Scholar]
- Tazaki, K.; Fyfe, W.S.; Tsuji, M.; Katayama, K. TEM observations of the smectite-to palygorskite transition in deep Pacific sediments. Appl. Clay Sci. 1987, 2, 233–240. [Google Scholar] [CrossRef]
- Suárez, M.; Robert, M.; Elsass, F.; Martín Pozas, J.M. Evidence of precursor in the neoformation of palygorskite-new data by analytical electron microscopy. Clay Miner. 1994, 29, 255–264. [Google Scholar] [CrossRef]
- Krekeler, M.P.S.; Guggenheim, S.; Rakovan, J. A microtexture study of palygorskite-rich sediments from the Hawthorne Formation; southern Georgia, by transmission electron microscopy (TEM) and atomic force microscopy (AFM). Clays Clay Miner. 2004, 52, 263–274. [Google Scholar] [CrossRef]
- Sautereau, M.; Decarreau, A. Genése des minéraux argileux. Géochemie des eléments majeurs, du chrome et du vanadium dans le Bartonien moyen du Bassin de Paris. These, 3éme cycle, Université Paris-Sud, Orsay, France, 1973; p. 79. [Google Scholar]
- Galán, E.; Ferrero, A. Palygorskite-sepiolite clays of Lebrija, Southern Spain. Clays Clay Miner. 1982, 30, 191–199. [Google Scholar] [CrossRef]
- Sánchez, C.; Galán, E. An approach to the genesis of palygorskite ia a Neogene-Quaternary continental basin using principal factor analysis. Clay Miner. 1995, 30, 225–238. [Google Scholar] [CrossRef]
- Galán, E.; Brell, J.M.; la Iglesia, A.; Robertson, R.H.S. The Cáceres palygorskite deposits, Spain. In Proceedings of the International Clay Conference, Mexico City, Mexico, 16–23 July 1975; pp. 91–94. [Google Scholar]
- Meunier, A. Clays; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Calvo, J.P.; Pozo, M. Geology of magnesian clays in sedimentary and non-sedimentary environments. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; Digilabs: Bari, Italy, 2015; ISSN 2283-687X. ISBN 978-88-7522-093-8. [Google Scholar]
- Chamley, H. Clay Sedimentology; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Warren, J.K. Evaporites. A Geological Compendium, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Bohacs, K.M.; Carroll, A.R.; Neal, J.E.; Mankiewicz, P.J. Lake-basin type, source potential, and hydrocarbon character: An integrated sequence-stratigraphic-geochemical framework. In Lake Basins through Space and Time; Gierlowski-Kordesch, E., Kelts, K., Eds.; Studies in Geology; American Association of Petroleum Geologists: Tulsa, OK, USA, 2000; Volume 46, pp. 3–34. [Google Scholar]
- Calvo, J.P.; Blanc-Valleron, M.M.; Rodríguez-Aranda, J.P.; Rouchy, J.M.; Sanz, M.E. Authigenic clay minerals in continental evaporitic environments. In Palaeoweathering, Palaeosurfaces and Related Continental Deposits; Thiry, M., Simon-Coinçon, R., Eds.; Special Publications of the International Association of Sedimentologists: Oxford, UK, 1999; pp. 129–151. [Google Scholar]
- Jones, B.F.; Deocampo, D.M. Geochemistry of Saline Lakes. In Surface and Groundwater, Weathering and Soils, Treatise on Geochemistry; Drever, J.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 5, Chapter 5.13; pp. 393–424. [Google Scholar]
- Jones, B.F.; Weir, A.H. Clay minerals at Lake Abert, an alkaline, saline lake. Clays Clay Miner. 1983, 31, 161–172. [Google Scholar] [CrossRef]
- Deocampo, D.M. Authigenic clay minerals in lacustrine mudstones. Geol. Soc. Am. Spec. Pap. 2015, 515, 49–64. [Google Scholar]
- Jones, B.F.; Spencer, R.J. Clay mineral diagenesis at Great Salt Lake, Utah, USA. In Geochemistry of the Earth’s Surface; Armannsson, O., Ed.; Balkema: Amsterdam, The Netherlands, 1999; pp. 293–297. [Google Scholar]
- Banfield, J.F.; Jones, B.F.; Veblen, D.R. An AEM-TEM study of weathering and diagenesis, Abert Lake, Oregon: II. Diagenetic modification of the sedimentary assemblage. Geochim. Cosmochim. Acta 1991, 55, 2795–2810. [Google Scholar] [CrossRef]
- Gac, J.Y.; Droubi, A.; Fritz, B.; Tardy, Y. Geochemical behavior of silica and magnesium during the evaporation of waters in Chad. Chem. Geol. 1977, 19, 215–228. [Google Scholar] [CrossRef]
- Jones, B.F.; Eugster, H.P.; Rettig, S.L. Hydrochemistry of the Lake Magadi basin, Kenya. Geochim. Cosmochim. Acta 1977, 41, 53–72. [Google Scholar] [CrossRef]
- Stoessell, R.K.; Hay, R.L. The geochemical origin of sepiolite and kerolite at Amboseli, Kenya. Contrib. Mineral. Petrol. 1978, 65, 255–267. [Google Scholar] [CrossRef]
- Deocampo, D.M.; Jones, B.F. Geochemistry of Saline Lakes. In Surface and Groundwater, Weathering and Soils, Treatise on Geochemistry; Drever, J.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, Chapter 7.13; pp. 437–469. [Google Scholar]
- Higgins, J.A.; Schrag, D.P. Constraining magnesium cycling in marine sediments using magnesium isotopes. Geochim. Cosmochim. Acta 2010, 74, 5039–5053. [Google Scholar] [CrossRef]
- Dunlea, A.G.; Murray, R.W.; Santiago Ramos, D.P.; Higgins, J.A. Cenozoic global cooling and increased seawater Mg/Ca via reduced reverse weathering. Nat. Commun. 2017, 8, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, H.H.; Pozo, M.; Galán, E. An introduction to palygorskite and sepiolite deposits-location, geology and uses. In Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 85–99. [Google Scholar]
- Singer, A.; Galán, E. (Eds.) Palygorskite-Sepiolite. Occurrences, Genesis and Uses. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37. [Google Scholar]
- Galán, E.; Singer, A. (Eds.) Developments in Palygorskite—Sepiolite Research. In Developments in Clay Science 3; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Pozo, M.; Galán, E. Magnesian clay deposits: Mineralogy and origin. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; Digilabs: Bari, Italy, 2015; pp. 175–227. [Google Scholar]
- Weaver, C.E. Origin and geologic implications of the palygorskite of the SE United States. In Palygorskite—Sepiolite. Occurrences, Genesis and Uses: Developments in Sedimentology 37; Singer, A., Galán, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 39–58. [Google Scholar]
- Krekeler, M.P.S.; Hammerley, E.; Rakovan, J.; Guggenheim, S. Microscopy studies of the palygorskite-to-smectite transformation. Clays Clay Miner. 2005, 53, 92–99. [Google Scholar] [CrossRef]
- García-Romero, E.; Suárez, M.; Santarén, J.; Álvarez, A. Crystallo-chemical characterization of the palygorskite and sepiolite from the Allou Kagne deposit (Senegal). Clays Clay Miner. 2007, 55, 606–617. [Google Scholar] [CrossRef]
- Zhou, H.; Murray, H.H. Overview of Chinese Palygorskite Clay Resources-Their Geology, Mineralogy, Depositional Environment, Applications and Processing. In Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 239–263. [Google Scholar]
- Chen, T.; Xu, H.; Lu, A.; Xu, X.; Peng, S.; Yue, S. Direct evidence of transformation from smectite to palygorskite: TEM investigation. Sci. China Ser. D Earth Sci. 2004, 47, 985–994. [Google Scholar] [CrossRef]
- Kastritis, D.; Mposkos, E.; Gionis, V.; Kacandes, G. The palygorskite and Mg-Fe-smectite clay deposits of the Ventzia basin, western Macedonia, Greece. In Mineral Exploration and Sustainable Development, Proceedings of the Seventh Biennial SGA Meeting, Athens, Greece, 24–28 August 2003; Mill Press: Rotterdam, The Netherlands, 2003. [Google Scholar]
- Kastritis, D.; Chryssikos, G.D.; Mposkos, E.; Gionis, V.; Kacandes, G. The genesis and geochemistry of palygorskite clays from western Macedonia, Greece. In Proceedings of the 42nd Annual Meeting of the Clay Mineral Society, Burlington, VT, USA, June 2005; p. 62. [Google Scholar]
- Suárez, M.; Navarrete, J.; Martín-Pozas, J.M. Estudio mineralógico del yacimiento de palygorskita de Bercimuel (Segovia) y de su entorno. Bol. Geol. Minero 1993, 104, 407–415. [Google Scholar]
- Galán, E.; Castillo, A. Sepiolite-Palygorskite in Spanish Tertiary Basins: Genetical Patterns in Continental Environments. In Palygorskite-Sepiolite. Occurrences, Genesis and Uses. Developments in Sedimentology; Singer, A., Galán, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 87–124. [Google Scholar]
- Ordoñez, S.; Calvo, J.P.; García del Cura, M.A.; Alonso Zarza, A.M.; Hoyos, M. Sedimentology of sodium sulphate deposits and special clays from the Tertiary Madrid Basin (Spain). In Lacustrine Facies Analysis; Anadón, P., Cabrera, L., Kelts, K., Eds.; Blackwell Scientific Publications: Oxford, UK, 1991; Volume 13, pp. 39–55. [Google Scholar]
- Doval, M.; Dominguez, M.C.; Brell, J.M.; García, E. Mineralogía y sedimentología de las Facies distales del borde norte de la Cuenca del Tajo. Bol. Soc. Esp. Mineral. 1985, 8, 257–269. [Google Scholar]
- Pozo, M.; Moreno, A.; Casas, J.; Martín Rubí, J.A. Mineralogy and geochemistry of sedimentary bentonites related to alluvial fan arkosic facies (Neogene Madrid Basin, Spain). Chem. Geol. 1993, 107, 457–461. [Google Scholar] [CrossRef]
- Galán, E.; Álvarez, A.; Esteban, M.A. Occurrence of stevensite at the Vallecas sepiolite deposit (Madrid). In Proceedings of the 7th Internation Clay Conference, Bologna-Pavía, Italy, 6–12 September 1981; pp. 98–99. [Google Scholar]
- Martín de Vidales, J.L.; Pozo, M.; Alia, J.M.; García Navarro, F.; Rull, F. Kerolite-stevensite mixed-layers from the Madrid Basin, Central Spain. Clay Miner. 1991, 26, 329–342. [Google Scholar] [CrossRef]
- Pozo, M.; Medina, A.; Leguey, S. Mineralogénesis de palygorskita en la zona central de la Cuenca de Madrid. Bol. Soc. Esp. Mineral. 1985, 8, 271–283. [Google Scholar]
- Pozo, M.; Calvo, J.P.; Pozo, E.; Moreno, A. Genetic constraints on crystallinity, thermal behaviour and surface area of sepiolite from Cerro de los Batallones deposit (Madrid Basin, Spain). Appl. Clay Sci. 2014, 91–92, 30–45. [Google Scholar] [CrossRef]
- Calvo, J.P.; Alonso Zarza, A.M.; García del Cura, M.A. Depositional sedimentary controls on sepiolite ocurrence in Paracuellos del Jarama, Madrid Basin. Geogaceta 1986, 1, 25–28. [Google Scholar]
- Cuevas, J.; Vigil de la Villa, R.; Ramírez, S.; Petit, S.; Meunier, A.; Leguey, S. Chemistry of Mg smectites in lacustrine sediments from the Vicálvaro sepiolite deposit, Madrid Neogene Basin (Spain). Clays Clay Miner. 2003, 51, 457–472. [Google Scholar] [CrossRef]
- Yalçin, H.; Bozkaya, Ö. Sepiolite—Palygorskite occurrences in Turkey. In Developments in Palygorskite—Sepiolite Research. A New Outlook on These Nanomaterials. Developments in Clay Research 3; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 175–200. [Google Scholar]
- Yeniyol, M. Geology, mineralogy and genesis of Yenidoğan (Sivrihisar) sepiolite deposit. Min. Res. Expl. Bull. Turk. 1992, 114, 51–64. [Google Scholar]
- Chahi, A.; Fritz, B.; Duplay, J.; Weber, F.; Lucas, J. Textural transition and genetic relationship between precursor stevensite and sepiolite in lacustrine sediments (Jbel Rhassoul, Morocco). Clays Clay Miner. 1997, 45, 378–389. [Google Scholar] [CrossRef]
- Benhammou, A.; Tanouti, B.; Nibou, L.; Yaacoubi, A.; Bonnet, J.P. Mineralogical and physicochemical investigation of Mg-smectite from Jbel Ghassoul, Morocco. Clays Clay Miner. 2009, 57, 269–270. [Google Scholar] [CrossRef]
- Chahi, A.; Duringer, P.; Ais, M.; Bouabdelli, M.; Gauthier-Lafaye, F.; Fritz, B. Diagenetic transformation of dolomite into stevensite in lacustrine sediments from Jbel Rhassoul, Morocco. J. Sediment. Res. 1999, 69, 1123–1135. [Google Scholar] [CrossRef]
- Miles, W.J. Amargosa sepiolite and saponite: Geology, mineralogy and markets. In Developments in Palygorskite—Sepiolite Research. Developments in Clay Science 3; Galán, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 265–277. [Google Scholar]
- Singer, A.; Stahr, K.; Zarei, M. Characteristics and origin of sepiolite (Meerschaum) from Central Somalia. Clays Clay Miner. 1998, 33, 349–362. [Google Scholar] [CrossRef]
- Inoue, A.; Utada, M. Smectite to chlorite transformation in thermally metamorphosed volcanoclastic rocks in the Kamikita area, northern Honshu, Japan. Am. Mineral. 1991, 76, 628–640. [Google Scholar]
- Inoue, A.; Utada, M.; Nagata, H.; Watanabe, T. Conversion of trioctahedral smectite to interstratified chlorite/smectite in Pliocene acidic pyroclastic sediments of the Ohyu district, Akita Prefecture, Japan. Clay Sci. 1984, 6, 103–106. [Google Scholar]
- McMurtry, G.M.; Wang, C.-H.; Yeh, H.-W. Chemical and isotopic investigations into the origin of clay minerals from the Galapagos hydrothermal mounds field. Geochim. Cosmochim. Acta 1983, 47, 475–489. [Google Scholar] [CrossRef]
- Percival, J.B.; Ames, D.E. Clay mineralogy of active hydrothermal chimneys and an associated mound, Middle Valley, northern Juan de Fuca Ridge. Can. Mineral. 1993, 31, 957–971. [Google Scholar]
- Lackschewitz, K.S.; Singer, A.; Botz, R.; Garbe-Schönberg, D.; Stoffers, P.; Horz, K. Formation and transformation of clay minerals in the hydrothermal deposits of Middle Valley, Juan de Fuca Ridge, ODP Leg 169. Econ. Geol. 2000, 95, 361–389. [Google Scholar] [CrossRef]
- D’Orazio, M.; Boschi, C.; Brunelli, D. Talc-rich hydrothermal rocks from the St. Paul and Conrad fracture zones in the Atlantic Ocean. Eur. J. Mineral. 2004, 16, 73–83. [Google Scholar] [CrossRef]
- Dekov, V.M.; Cuadros, J.; Shanks, W.C.; Koski, R.A. Deposition of talc, kerolite–smectite, smectite at seafloor hydrothermal vent fields: Evidence from mineralogical, geochemical and oxygen isotope studies. Chem. Geol. 2008, 247, 171–194. [Google Scholar] [CrossRef]
- Cuadros, J.; Dekov, V.M.; Fiore, S. Crystal chemistry of the mixed-layer sequence talc–talc–smectite–smectite from submarine hydrothermal vents. Am. Mineral. 2008, 93, 1228–1348. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Ishibashi, J.; Faure, K.; Maeto, K.; Matsukura, S.; Omura, A.; Shimada, K.; Sato, H.; Sakamoto, T.; Uehara, S.; et al. Mg-rich clay mineral formation associated with marine shallow-water hydrothermal activity in an arc volcanic caldera setting. Chem. Geol. 2013, 355, 28–44. [Google Scholar] [CrossRef]
- Cuadros, J.; Michalski, J.R.; Dekov, V.; Bishop, J.; Fiore, S.; Dyar, M.D. Crystal-chemistry of interstratified Mg/Fe-clay minerals from seafloor hydrothermal sites. Chem. Geol. 2013, 360–361, 142–158. [Google Scholar] [CrossRef]
- Marumo, K.; Hattori, K. Seafloor hydrothermal clay alteration at Jade in the backarc Okinawa Trough: Mineralogy, geochemistry and isotope characteristics. Geochim. Cosmochim. Acta 1999, 63, 2785–2804. [Google Scholar] [CrossRef]
- Dekov, V.; Scholten, J.; Garbe-Schonberg, C.-D.; Botz, R.; Cuadros, J.; Schmidt, M.; Stoffers, P. Hydrothermal sediment alteration at a seafloor vent field: Grimsey Graben, Tjornes Fracture Zone, north of Iceland. J. Geophys. Res. 2008, 113, B11101. [Google Scholar] [CrossRef]
- Imai, N.; Otsuka, R.; Nakamura, T. An occurrence of well-crystallized sepiolite from Akatani mine, Niigata Prefecture, Northeastern Japan. J. Jpn. Assoc. Miner. Petrol. Econ. Geol. 1967, 57, 39–56. [Google Scholar] [CrossRef]
- Imai, N.; Otsuka, R. Sepiolite and palygorskite in Japan. In Palygorskite-Sepiolite. Occurrences, Genesis and Uses. Developments in Sedimentology; Singer, A., Galán, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 211–232. [Google Scholar]
- Yeniyol, M. Vein-like sepiolite occurrence as a replacement of magnesite in Konya, Turkey. Clay. Clay Miner. 1986, 34, 353–356. [Google Scholar] [CrossRef]
- Yalçın, H.; Bozkaya, Ö. Ultramafic-rock-hosted vein sepiolite occurrences in the Ankara ophiolitic mélange, Central Anatolia, Turkey. Clays Clay Miner. 2004, 52, 227–239. [Google Scholar] [CrossRef]
- Pozo, M.; Calvo, J.P. Madrid Basin (Spain): A natural lab for the formation and evolution of magnesian clay minerals. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; Digilabs: Bari, Italy, 2015. [Google Scholar]
- Jones, B.F.; Bowser, C.J. The mineralogy and related chemistry of lake sediments. In Lakes; Lerman, E., Ed.; Springer: New York, NY, USA, 1978; pp. 179–235. [Google Scholar]
- Clauer, N.; Fallick, A.; Galán, E.; Pozo, M.; Taylor, C. Crystallization of sepiolite and associated Mg-clays from Madrid Basin (Spain) traced by oxygen and hydrogen isotope geochemistry. Geochim. Cosmochim. Acta 2012, 94, 181–198. [Google Scholar] [CrossRef]
- Pozo, M.; Carretero, M.I.; Galán, E. Approach to the trace element geochemistry of non-marine sepiolite deposits: Influence of the sedimentary environment (Madrid Basin, Spain). Appl. Clay Sci. 2016, 131, 27–43. [Google Scholar] [CrossRef]
- Wright, V.P.; Barnett, A. An abiotic model for the development of textures in some South Atlantic early Cretaceous lacustrine carbonates. Geol. Soc. Lond. Spec. Publ. 2014, 418, 209–219. [Google Scholar] [CrossRef]
- Tosca, N.; Wright, V.P. Diagenetic pathways linked to labile Mg-clays in lacustrine carbonate reservoirs: A model for the origin of secondary porosity in the Cretaceous Pre-salt Barra Velha Formation, offshore Brazil. Geol. Soc. Lond. Spec. Publ. 2015, 435, 33–46. [Google Scholar] [CrossRef]
- Herlinger, R.; Zambonato, E.E.; De Ros, L.F. Influence of diagenesis on the quality of Lower Cretaceous Pre-salt lacustrine carbonate reservoirs from northern Campos Basin, offshore Brazil. J. Sediment. Res. 2017, 87, 1285–1313. [Google Scholar] [CrossRef]








| Clay Minerals | Composition |
|---|---|
| Trioctahedral Smectites | Saponite: Mg3(Si3, 67Al0,33)O10(OH)2M+0,33 Hectorite: (Mg2.67Li0,33)Si4O10(OH)2M+0,33 Stevensite: (Mg2.67□0,33)Si4O10(OH)2M+0,33 |
| Palygorskite | (Mg, Al, Fe3+)5(Si, Al)8O20(OH)2(OH2)4·4H2O |
| Sepiolite (Loughlinite) | Mg8Si12O30(OH)4(OH2)4·8H2O (Na4Mg6Si12O30(OH)4(OH2)4·8H2O) |
| Kerolite | Mg3Si4O10(OH)2 |
| Deposit/AGE | Mineralogical Assemblage | Environment | Origin |
|---|---|---|---|
| Eskisehir (Turkey) (Miocene) | Sep-(Dol-Qz-Ilt-Fsp) | Lacustrine | Neoformation Diagenetic |
| Amargosa (USA) (Pliocene-Pleistocene) | Sep-Sap-(Ilt-Stv/Tlc-Dol-Cal) | Lacustrine (playa) | Neoformation Diagenetic |
| Vicálvaro-Yunclillos (Spain) (Miocene) | Sep-(Sap-Stv-Ilt-Cal-Dol-Qz-Fsp) | Lacustrine/alluvial | Neoformation Diagenetic |
| Batallones (Spain) (Miocene) | Sep-(Plg-Sap-Ilt-Qz-Fsp-Opl-Cal) | Palustrine | Neoformation Diagenetic |
| Cabañas-Yuncos (Spain) (Miocene) | Sap-(Ilt-Sep-Kln-Qz-Fsp-Dol-Cal-Stv/Tlc) | Lacustrine/alluvial | Diagenetic Neoformation |
| Magan (Spain) (Miocene) | Sap-(Ilt-Sep-Stv-Kln-Qz-Fsp-Cal-Brt) | Lacustrine (mudflat) | Diagenetic Neoformation |
| Esquivias (Spain) (Miocene) | Tlc/Stv-(Sep-Sap-Ilt-Qz-Cal-Dol-Zeo) | Lacustrine (mudflat) | Neoformation Diagenetic |
| Jbel Rhassoul (Morocco) (Miocene) | Stv-(Sep-Dol-Qz-Gp-Clt) | Lacustrine | Diagenetic |
| Guanshan (China) (Miocene) | Plg-(Sme(Al)-Qz-Sep-Ms-Dol) | Lacustrine (alteration profile) | Diagenetic (basaltic ash) |
| Bercimuel (Spain) (Miocene) | Plg-(Ilt-Qz-Kln-Sme(Al)-Ilt/Sme) | Alluvial | Diagenetic (Al-smectite) |
| Torrejón el Rubio (Spain) (Paleogene) | Plg-(Ilt-Sep-Chl-Dol-Sap-Qz-Fsp) | Lacustrine-palustrine (alteration profile) | Diagenetic (chlorite) |
| High Mg/Si (~6) | High Salinity (0.46 mol·kg−1) | Low Salinity (0.00 mol·kg−1) |
| pH ≥ 9 | Stevensite | Kerolite |
| pH < 9 | Kerolite | Sepiolite |
| Low Mg/Si (≤1) | High Salinity (0.46 mol·kg−1) | Low Salinity (0.00 mol·kg−1) |
| pH ≥ 9 | Stevensite | Kerolite |
| pH < 9 | Sepiolite | Sepiolite |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozo, M.; Calvo, J.P. An Overview of Authigenic Magnesian Clays. Minerals 2018, 8, 520. https://doi.org/10.3390/min8110520
Pozo M, Calvo JP. An Overview of Authigenic Magnesian Clays. Minerals. 2018; 8(11):520. https://doi.org/10.3390/min8110520
Chicago/Turabian StylePozo, Manuel, and José Pedro Calvo. 2018. "An Overview of Authigenic Magnesian Clays" Minerals 8, no. 11: 520. https://doi.org/10.3390/min8110520
APA StylePozo, M., & Calvo, J. P. (2018). An Overview of Authigenic Magnesian Clays. Minerals, 8(11), 520. https://doi.org/10.3390/min8110520






