Abstract
A general overview of the trends in structural and thermodynamic properties that have been identified within the hydrated normal rare earth carbonates and the rare earth hydroxycarbonates is presented. Based upon available literature, we demonstrate the trends in crystallographic unit cell parameters, thermal stability, aqueous solubility, and thermochemical properties. These trends can be attributed to both the unique chemistry and strong similarity of the rare earth elements. There are also inconsistent trends that signal research needs to better understand the structure–energy relationships of the rare earth carbonates.
1. Introduction
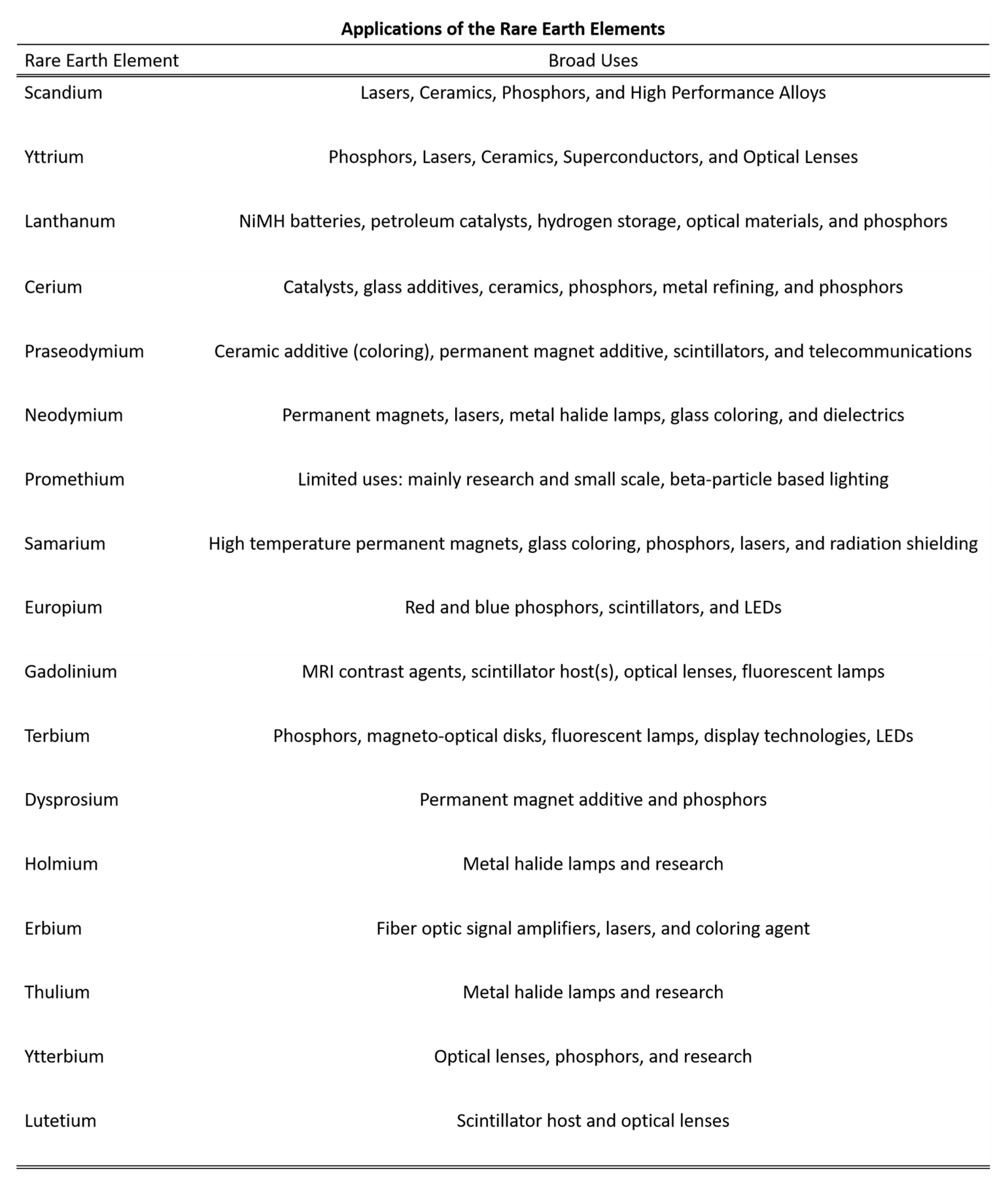
The rare earth elements have made their way into many aspects of modern life. From the gasoline in automobiles, the ubiquitous mobile phones, speakers, lights, to energy production, the rare earth elements are indispensable to current standards of living and technology. Common applications of the rare earth elements are summarized in Figure 1. The interesting properties that have allowed their application are largely due to the unique 4f electrons that have highly localized electronic states and very predictable electronic transitions that are weakly influenced by the coordination environment or crystal field. In general, this means that the unique physical properties of the rare earth ions are largely unaffected by their surroundings. However, it should be noted that slight variations and nuanced interactions of the rare earth ions with their surroundings are of great research interest [1].

Figure 1.
Applications of the rare earth elements broken down by element. Most applications are geared towards high-technology, such as lasers, magnets, phosphors, energy conversion, and catalysis. Adapted from Gschneidner, Jr. [2].
The International Union of Pure and Applied Chemistry (IUPAC) defines the rare earth elements as a series of 17 chemically similar elements in the periodic table [3] including scandium, yttrium, and the lanthanides. Scandium and yttrium are chemically similar to the lanthanides and often collocated with the lanthanides in mineral deposits. Scandium is not as widely utilized as the other rare earths as the process for obtaining metallic scandium is quite difficult. It is only relatively recently that scandium has found limited application in aluminum alloys. All but one of the lanthanides (lanthanum to lutetium) fill the 4f election shell. Depending upon classification and researchers’ preferences, either lanthanum, despite the namesake, or lutetium can be excluded from the lanthanide classification. Promethium was the last of rare earth elements to be formally discovered and is mainly utilized for its radioactivity in research and a small amount of applications [4].
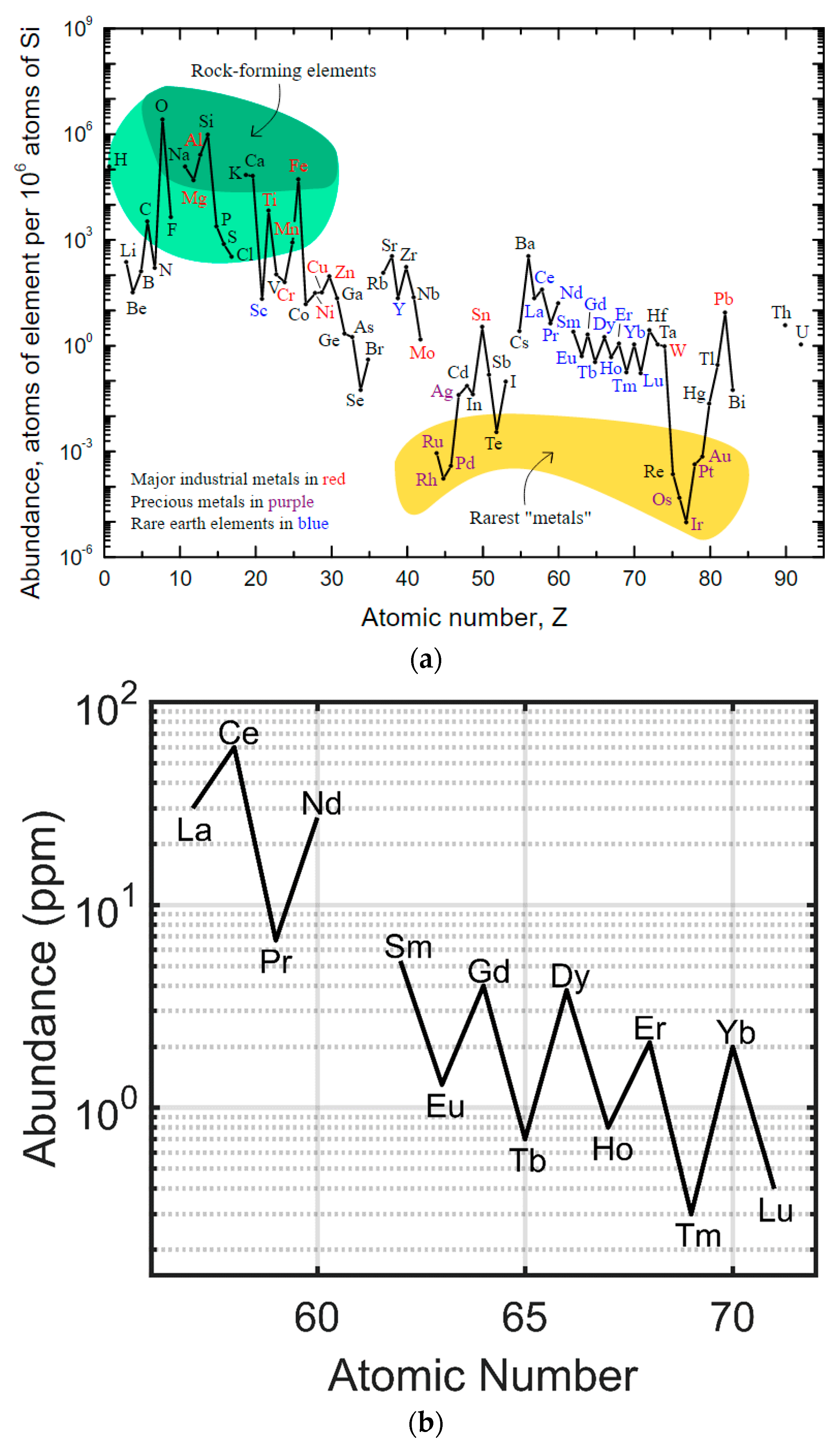
The rare earth elements are not actually rare in geologic abundance, despite their name [5] (Figure 2). In absolute terms, the rare earth elements are more abundant than many of the platinum group metals (e.g., platinum and palladium) and have similar abundances to tin, zinc, and tungsten. Lutetium and thulium are the least abundant and lanthanum, cerium, and yttrium are the most abundant. The rare earth element of even atomic number is more abundant than either of the corresponding rare earth elements of odd atomic number on either side (Figure 2) in the periodic table (e.g., cerium (58) is more abundant than both lanthanum (57) and praseodymium (59)). The rare earth elements are co-located with one another and usually found as part of a host mineral. Many of these rare earth enriched minerals are carbonate minerals [6], such as bastnaesite and lanthanite, and are found in large carbonatite deposits, such as those at Mountain Pass (California, USA) [7] and Bayan Obo (Inner Mongolia, China) [8,9]. Economically viable rare earth mineral deposits, large quantities of minerals with high rare earth concentrations and chemistries that allow for the relatively easy separation of the rare earth elements from the host, are mined and refined in only a few locations around the world. Large capital costs, high environmental impact, and specific mineral chemistries have resulted in China producing the majority of the world’s rare earths [5,10,11,12,13,14].
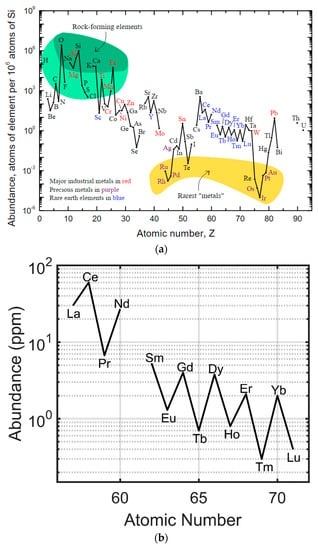
Figure 2.
(a) Crustal abundances of the rare earth elements (REEs) relative to silicon (adapted from USGS [5]) and each other (adapted from Gupta [15]). (b) REEs are relatively abundant compared to palladium group metals (e.g., palladium, platinum, and rhodium).
The ability to refine and produce rare earth products from the aforementioned carbonate mineral deposits begins with a fundamental understanding of the rare earth carbonates. The rare earth carbonates include both the rare earth bearing carbonate minerals and the synthetic rare earth carbonates that match the chemical composition of either the naturally occurring minerals or the pure single element carbonates. Understanding the behavior of the rare earth carbonates in geochemical systems begins with the behavior of the rare earths in the RE2O3-CO2-H2O ternary system. At standard temperature and pressure (25 °C, 1 atm), the rare earth carbonates are the hydrated normal rare earth carbonates (RE2(CO3)3·xH2O) and the rare earth hydroxycarbonates (RE(OH)CO3·xH2O, also known as basic carbonates, carbonate hydroxide, hydroxylcarbonates, and hydroxocarbonates). Anhydrous variants of the normal carbonates and hydroxycarbonates exist, but they readily absorb water to create their respective hydrated variants. The rare earth oxycarbonates are also an important class of rare earth carbonates. However, these oxycarbonates form at higher temperatures as a result of the thermal decomposition of either the normal carbonates or hydroxycarbonates. Within the framework of this study, the oxycarbonates and the anhydrous carbonates are treated as the thermal decomposition products of their respective rare earth carbonate and will not be thoroughly addressed.
The preponderance of CO2 and H2O in geological systems at ambient conditions has necessitated the analysis of the rare earth carbonates, especially with respect to phase stability, crystallography, thermodynamic stability, and behavior in water. Understanding which rare earth carbonate phase (normal vs. hexagonal hydroxycarbonate vs. orthorhombic hydroxycarbonate) will form in certain CO2, H2O, pressure, and temperature conditions is crucial to understanding the geochemistry and distribution of rare earths in natural systems. This is particularly important to nuclear fuel applications, as certain lanthanides are the fission products of nuclear fuels and other lanthanides, such as neodymium, serve as chemical homologues in studying the distribution of radioactive actinides in natural CO2-H2O hydrothermal systems [16,17,18,19]. In industrial rare earth production, understanding what phase the rare earth carbonates will assume in these mineral deposits (normal vs. hexagonal hydroxycarbonate vs. orthorhombic hydroxycarbonate) informs how the deposit can be processed. The refined rare earths are then precipitated as the rare earth carbonates to be used in the downstream production of other rare earth solids such as the rare earth chlorides, sulfates, and oxides. These industrially produced rare earth carbonate products utilize alkali or ammonium carbonates/bicarbonates to simultaneously adjust pH and precipitate [20,21,22,23,24,25,26,27,28,29,30,31,32,33] from process streams.
The purpose of this study is to present the most common synthesis methods of the rare earth carbonates, their crystallographic structure, thermochemical data, aqueous behavior, and thermal stability. Through this treatment, we will find trends that can be attributed to the unique chemistry of the rare earth elements and identify inconsistencies and research needs in the current body of literature.
2. Synthesis
The history of the rare earth carbonates begins in the latter half of the 19th century. Treatises on chemistry in the first half of the 19th century, such as those by Sylvester [34] and Reid [35], contain scant mention, if any, of the rare earth elements. Considering that Johan Gadolin’s discovery of yttrium dates to 1792, spread in knowledge concerning the chemistry of these new rare earths would have been limited. However, treatises on chemistry from the late 19th century onwards address the rare earth carbonates, along with other rare earth salts. Treatises such as those by Roscoe and Schorlermmer [36], Treadwell [37], Blitz and Blitz [38], and Fresenius [39] briefly detail the synthesis of the rare earth carbonates, though no specific mention of the stoichiometry is made. The most popular methods were precipitation from an aqueous rare earth salt solution using alkali/ammonia carbonates/bicarbonates or the conversion of the rare earth hydroxide to the carbonate using gaseous carbon dioxide. Efforts to synthesize the rare earth carbonates by alternative means yielded results in the 20th century. Starting in the 1950s, rare earth carbonates were synthesized by homogeneous precipitation from an aqueous solution of the rare earth salt plus a water soluble organic compound. Also known as decomposition synthesis, this method has been extensively used in laboratory settings to synthesize the carbonates. In laboratory settings with high purity requirements, homogeneous precipitation has been the synthesis method of choice as the conversion of hydroxide is a relatively slow process and precipitation using the alkali/ammonia carbonates/bicarbonate salts result in alkali or double carbonate contamination [36,37,38,39,40,41,42].
We have classified the various synthesis methods of the rare earth carbonates as conversion, precipitation, and decomposition. Based upon the findings by Kutty [43,44,45,46,47] and Caro [48,49,50], each of these synthesis types can be used to create the desired rare earth carbonate phase (normal vs. hydroxy). It should be noted that the most convenient means of creating the hydroxycarbonates are the decomposition methods. Both types of carbonates can be used in laboratory settings as host materials or as template materials in the formation of other nanocrystalline rare earth phases [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. In industrial settings, both normal and hydroxycarbonates have been equally useful as precursor materials.
2.1. Conversion
Conversion synthesis methods create rare earth carbonates by directly converting a colloidal solution of the insoluble precursor material into the rare earth carbonate of interest. Direct mention of this synthesis method can be found in chemistry treatises [36,37,38,39] dating to the late 19th century. In these cases, rare earth carbonates are created by flowing gaseous CO2 through a wet solution of the rare earth hydroxide. Later reports in the early 20th century will use this technique to begin the work of definitively characterizing the rare earth carbonates. Raikow et al. [76] demonstrated the formation of lanthanum, yttrium, and cerium carbonates in addition to the formation of other metal carbonates, by flowing relatively low pressure, gaseous CO2 over aqueous solutions of their respective metal hydroxides. By this method, normal lanthanum carbonate, yttrium hydroxycarbonate, and cerous/ceric carbonates were synthesized. Converting a RE hydroxide into its respective RE carbonate by this method is quite simple but is slow and has low conversion yields [77].
More recent advances in synthesizing rare earth carbonates from insoluble precursor materials (considering that solubility products of rare earth hydroxides range from 10−21 to 10−18) have come to include the conversion of the rare earth oxide [49,50,78]. Caro and coworkers [50] synthesized the entire array of normal rare earth carbonates at room temperature by equilibrating a colloidal solution of the oxides with a CO2 overpressure of 1 atm over a matter of days to weeks. Caro and coworkers later demonstrated [49] that the lighter normal rare earth carbonates can be hydrolyzed to create the hydroxycarbonates by allowing the lighter normal rare earth carbonates to sit in water without CO2 overpressure. Work by Fernando and coworkers [78] utilized supercritical CO2 at 2800 psi and at temperatures less than 100 °C to synthesize the normal carbonates of lanthanum, neodymium, samarium, europium, gadolinium, dysprosium, and holmium. It was demonstrated that working with very low solids loading of the oxides, at lower temperatures, increasing pCO2 increased conversion yields while keeping conversion times relatively short (> 95% conversion in 1 hour) with increasing reaction times not giving measurable increases in yield.
RE2O3-CO2-H2O systems have been studied using varied system pressure and temperature to determine hydrothermal phase equilibria of the system [43,44,45,46,47]. With sufficient pCO2 and mole fraction of CO2, the hydrated normal carbonates are preferred over the hydroxycarbonates at lower temperatures. Exact temperatures and mole fraction of CO2 at which each carbonate (e.g., normal carbonate vs. hydroxycarbonate vs. monoxycarbonate) becomes preferred changes with system pressure. In general, the normal carbonates form preferentially at temperatures less than 200 °C given sufficient pCO2/mole fraction of CO2. With insufficient amounts of CO2, the hydroxycarbonates are generally preferred regardless of temperature. As previously mentioned, the normal carbonates of the lighter rare earths can form their hydroxycarbonates at ambient conditions when exposed to water.
Gaseous CO2 is an integral part in the synthesis of the rare earth carbonates. Upon initial inspection, the conversion methods are relatively straightforward, facile means of creating the desired carbonates, especially the normal carbonates. Simple as they are, they are not necessarily the most popular or cost-effective solutions for creating the rare earth carbonates. Converting the hydroxides to the carbonates is a slow process (hours to days). Converting the oxides to the carbonates using supercritical CO2 is a faster process with high yields (hours), but requires high pressure vessels to contain the supercritical CO2 [78]. In either case, quick high-throughput synthesis of the carbonates is not possible. Synthesis of the carbonates from an aqueous solution of a rare earth salt is also not possible by these means. Yet, the importance of CO2 to the synthesis process, regardless of the type of synthesis, cannot be understated.
2.2. Decomposition
Decomposition synthesis, also known as homogeneous precipitation, create the rare earth carbonates by increasing the effective concentration of aqueous CO2/carbonate ions in solutions via the decomposition of rare earth organic salts or soluble organic compounds at elevated temperatures. Unlike the conversion of insoluble template materials in a colloidal solution, these precipitations occur from completely aqueous solutions of the rare earth salts plus an organic compound. An initial reason for finding decomposition-based methods for synthesizing the rare earth carbonates was to eliminate the contamination from carbonate/bicarbonate salt precipitations and the slow conversion process of the rare earth oxides/hydroxides. Hence, in principle, any organic compound that liberates CO2 upon decomposition/hydrolysis in water can serve as a CO2 source. The most popular organic compounds/salts that have been used are trichloroacetic acid and urea. Other organic sources such as gelatin, formic acid [79,80], acetic acid [81], and propionic acid [81] have been used but have not been as popular.
Rare earth trichloroacetate salts were one of the first rare earth organic salts used to synthesize the rare earth carbonates. Salutsky and Quill [82] first synthesized the normal carbonates of lanthanum, neodymium, and samarium using this method in 1950. The oxide is first dissolved in an excess of the trichloroacetic acid and then heated under CO2 bubbling until the excess trichloroacetic acid has been decomposed, after which precipitation can occur. Follow up studies on the normal rare earth carbonates by others such as Charles [83], Head [84,85], Sastry [86], Wakita [87], Shinn [88], and Eyring and coworkers [89,90] have utilized rare earth trichloroacetate decomposition to synthesize phase pure normal rare earth carbonates. For the purposes of characterizing the normal carbonates, such as diffraction analysis and thermal decomposition analysis, trichloroacetate decomposition has been the choice synthesis method. From these normal carbonates, the respective hexagonal hydroxycarbonates may be synthesized via hydrolysis; elevated temperatures and low pCO2 overpressure accelerate this hydrolysis. This hydrolysis occurs quickly and many steps during the normal carbonate synthesis are usually taken to ensure this does not occur, such as pCO2 overpressure during reaction and washing with CO2-laden water. To synthesize the orthorhombic hydroxycarbonates, alternative organic compounds other than trichloroacetic acid are used.
Urea decomposition was reported by Akinc and coworkers [91,92] and Matijevic and coworkers [61,71,72] in the late 1980s and early 1990s. Since then, it has become one of the most favored CO2-source organic compounds in the laboratory synthesis of rare earth carbonates [59,68,74,75,93,94,95,96,97,98], particularly the orthorhombic hydroxycarbonates. Urea hydrolysis is rather slow at even 90 °C, but is accelerated by the presence of lanthanide salts. Increasing the temperature beyond 100 °C results in uncontrolled, accelerated decomposition of urea. For nanoparticle synthesis, this has been shown to be an undesirable outcome as this affects particle size distribution, but this may not necessarily be a concern for purely synthesizing the hydroxycarbonate [99].
Other organic compounds such as gelatin, formic acid [79,80], acetic acid [81], and propionic acid [81] have been used to create the rare earth carbonates, but have only been utilized on a very limited scale, if at all. The most popular trichloroacetate and/or urea decompositions have been successfully used to synthesize the entire gamut of rare earth carbonates [59,61,68,71,72,74,75,82,83,84,85,86,87,88,89,90,94,95,96,97,98]. In laboratory settings where chemical purity is of utmost importance, they have been considered as choice precursor materials as the alkali precipitants will create double carbonate contaminants given sufficient contact time. Other modifications to reaction conditions such as stabilizing ligands, temperature, pressure, carbonate source content, and solvent have been used with great aplomb to achieve variations in particle morphology.
Decomposition syntheses are much faster than conversion syntheses and can be comparable in time to precipitations using carbonate/bicarbonate salts (minutes to hours). Some laboratory-based carbonate syntheses utilize these salts. Yet, compared to salt precipitations, decomposition syntheses are not as straightforward and difficult to scale to large quantities. This particular quality of the carbonate/bicarbonate salt precipitations has made these salts the choice methods for industrial scale precipitations from highly acidic rare earth salt solutions.
2.3. Precipitation
One of the most cost-effective ways of producing rare earth carbonates en masse from rare earth salt solutions (e.g., rare earth chlorides and nitrates) is by the precipitation of a rare earth carbonate using carbonate or bicarbonate salts. In an industrial setting, alkali or ammonia carbonate/bicarbonate salts are the most employed precipitation agents within the rare earth stripping/extraction/calcination process(es) [20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Given that many of these industrial rare earth salt solutions are highly acidic, the “dual” nature of these precipitation agents are very useful; they adjust pH to the carbonate/hydroxide precipitation pH regime (pH ≥ 6.0) and increase the aqueous carbonate/bicarbonate concentrations beyond saturation. Synthetic rare earth salt solutions, i.e., those derived directly from the rare earth salts, are also acidic and benefit from the ‘dual’ nature of the carbonate/bicarbonate salts. Achieving pH ≥ 6.0 is a necessary component to the precipitation process as the rare earth carbonates are soluble at even moderately acidic pH. In laboratory settings, the rare earth carbonates are also synthesized using these salts. Nagashima [97] reported that the use of bicarbonate salts improved the crystallinity of the final rare earth carbonate products. However, since research laboratories require high purity products and have other product requirements such as particle size, shape, and crystallinity, these parameters are more easily controlled using decomposition synthesis, and carbonate/bicarbonate salt precipitations are not as favored. Based upon our understanding of the RE2O3-CO2-H2O hydrothermal equilibria, the normal carbonates are the preferred carbonate phases in these precipitation processes. Most carbonate salt precipitation processes are conducted at ambient conditions in relatively short amounts of time. However, if the rare earth carbonate is allowed to remain in contact with aqueous alkali carbonate/bicarbonate salt solution, the carbonate will either hydrolyze to create hydroxycarbonate [100] or, more likely, create a double carbonate [36,37,38,39,40,41,42,101,102,103,104].
The double carbonates, single crystal phases characterized as a mixture of an alkali carbonate and rare earth carbonate, are a phase unique to the carbonate/bicarbonate salt precipitation methodologies. It has been understood since the beginnings of rare earth carbonate synthesis that the double carbonates form if a rare earth carbonate is allowed to sit in a solution of the alkali carbonate/bicarbonate salt [36,37,38,39]. Ammonium carbonate/bicarbonate solutions do not result in a double carbonate, but rather result in the formation of the perioxycarbonate [105]. It should be noted that the dissolution process occurs more rapidly if the excess salt solution is that of the bicarbonate salt. Yet, regardless of either carbonate or bicarbonate, the final carbonate product will be the double carbonate. This process occurs in two steps. The rare earth carbonates will dissolve in the salt solution and then precipitate as the double carbonate. It should be noted that this process is not quick and requires the rare earth carbonate to be in contact with carbonate salt solution for hours to days for appreciable amounts of precipitation. The best characterized rare earth/alkali double carbonates are those of the rare earth/sodium double carbonates [40,102,103,104]. Rare earth/potassium double carbonates also occur [41,42,102].
End product purity requirements, amongst other requirements, ultimately dictate what synthesis method is used to create the rare earth carbonate. Conversion methods are no longer as popular as the very large amounts of time require to achieve full conversions to the carbonates have seen them fall out of favor. The decomposition methods can be used to tailor product-specific properties such as particle size, morphology, and crystalline phase in laboratory settings. Decomposition methods are faster than conversion methods but still much slower than carbonate salt precipitations. Carbonate salt precipitations are generally not used to tailor product-specific properties such as particle size and shape, but are much more straightforward than decomposition methods, yet susceptible to double carbonate contamination.
3. Trends in the Properties of the Rare Earth Carbonates
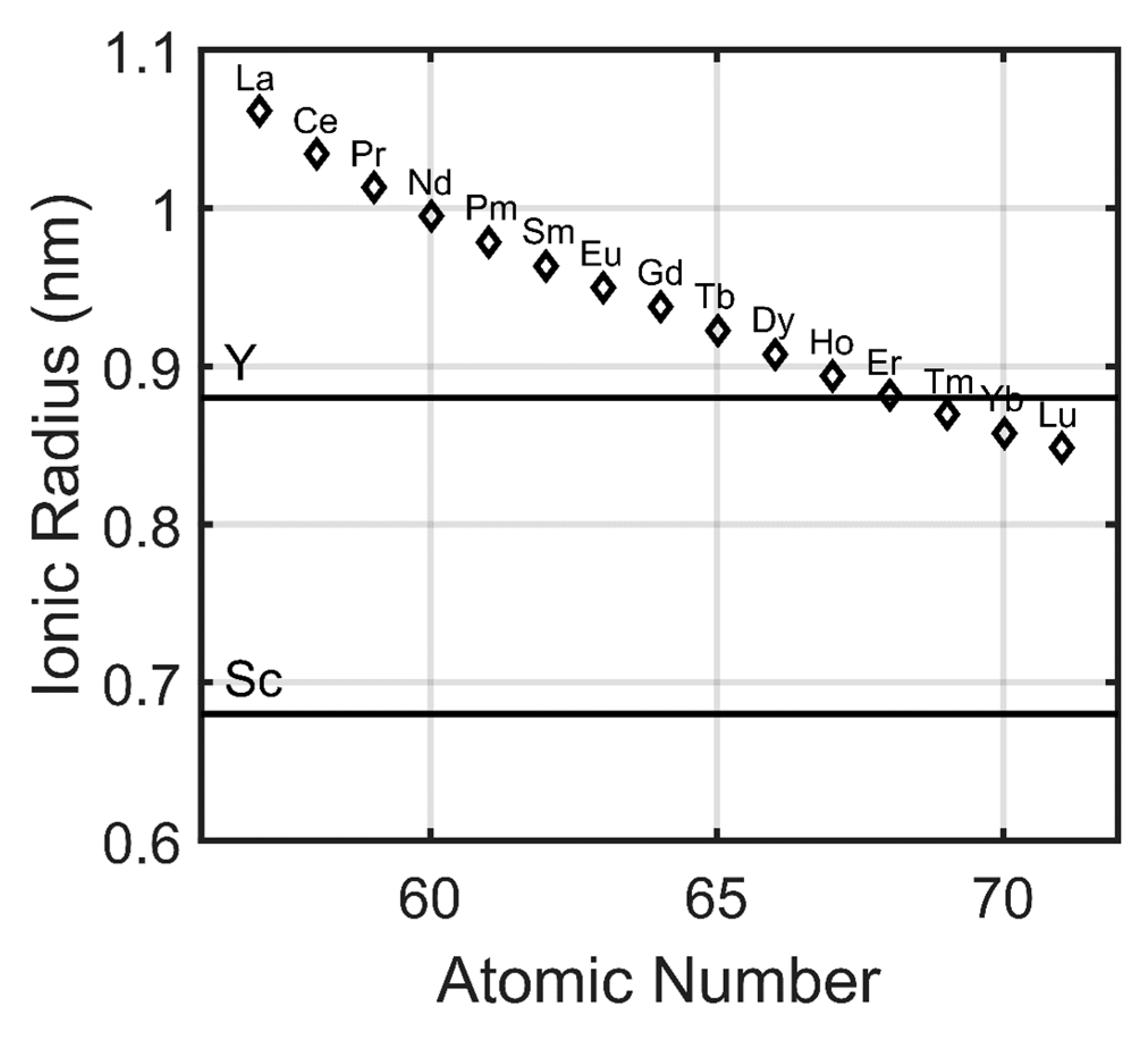
The rare earth elements, because of their chemically similar natures, exhibit a number of trends with respect to their atomic numbers. Many of these trends can be correlated with the lanthanide contraction, the decrease in ionic radii with increasing atomic number [15]. From lanthanum to lutetium, there is a demonstrable decrease in the ionic radius of the trivalent lanthanide cations due to the weak shielding of the valence electrons by the inner 4f electrons (Figure 3). This contraction manifests itself in the slight chemical differences between the rare earths, including yttrium and scandium, that allow for their chemical separation during industrial refining process. Based upon ionic radius, the properties of scandium are very different from those of all other rare earths and the properties of yttrium are somewhere between those of erbium and thulium. Though it may be difficult to separate immediately adjacent lanthanides (due to a small difference in ionic radius and chemistry), increasingly greater differences in ionic radius, and thus chemistry, allows for easier separation. It is relatively straightforward to separate lanthanum from erbium but extraordinarily difficult to separate neodymium from praseodymium.
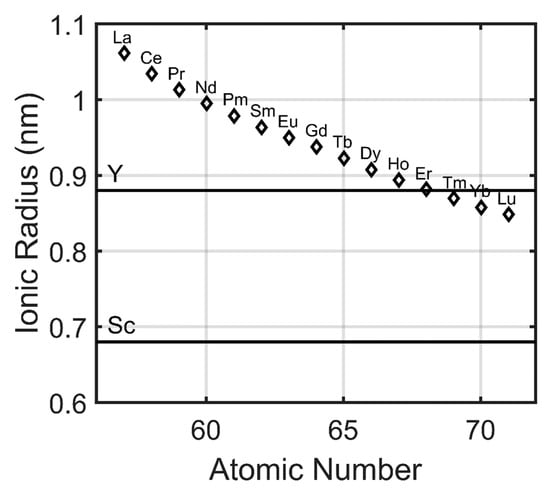
Figure 3.
Ionic radius of the trivalent rare earth (RE) cations (adapted from Gupta [15]). Lanthanide contraction is observed due to the weak shielding of the valence electrons by the 4f electrons. Certain properties of rare earth carbonate phases can be correlated with atomic number.
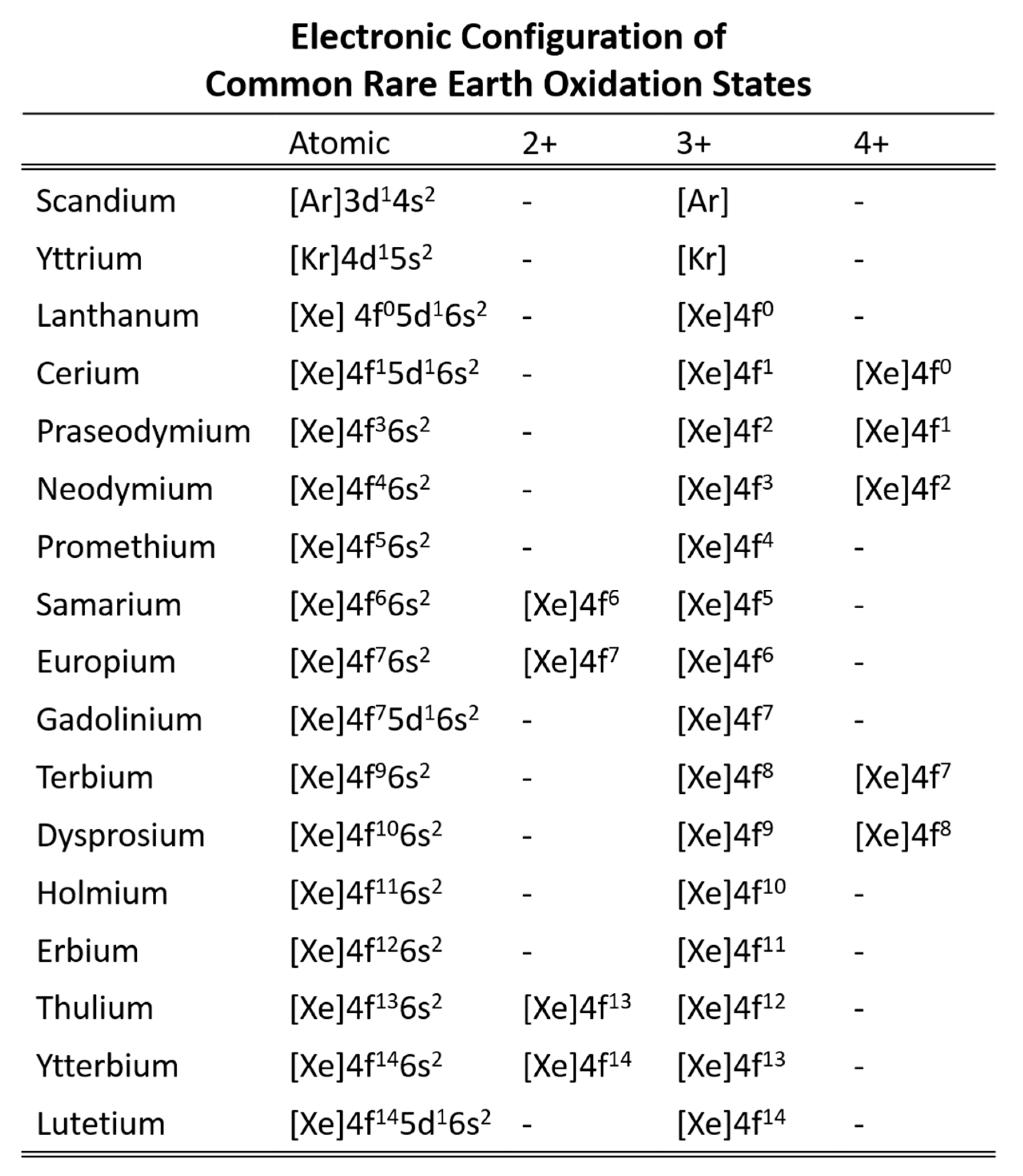
A number of lanthanide properties follows a general trend that is similar in principle to the lanthanide contraction. The metallic lanthanides have increasing Vickers hardness, density, and melting points with increasing atomic number. Europium and ytterbium are notable exceptions as they are divalent in the metallic state instead of the more common trivalent state (Figure 4). Cerium is also an exception as cerium can also be tetravalent, but the effect on Vickers hardness, density, and melting point is not as pronounced.
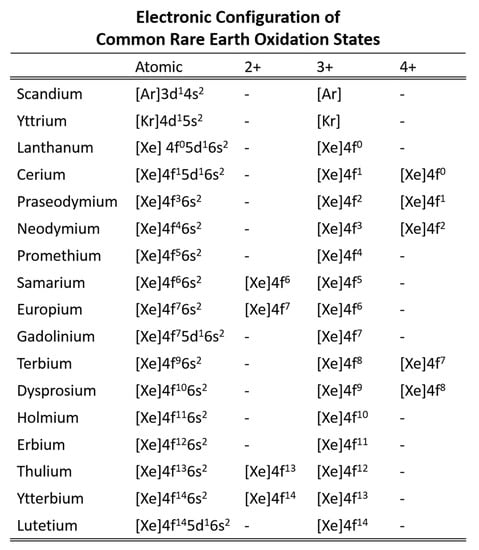
Figure 4.
Electron configuration of the REE cations (adapted from Gupta [15]).
The rare earth carbonates exhibit a number of trends that are similar in principle to that of the lanthanide contraction. Their crystallography, thermal stability, thermochemistry, and behavior in aqueous systems demonstrate some trends with increasing atomic number. Identifying these trends furthers our understanding of the influence of rare earth chemistry on materials and enables predictive capabilities in the general RE2O3-CO2-H2O system(s). The slight and major differences that arise in the rare earth carbonates, such as the unit cell parameters in the rare earth carbonates within the same isostructural group, the hydrolysis tendency of the normal rare earth carbonate to create the hydroxycarbonate, or solubility differences in aqueous solutions, enables our ability to separate the rare earths. In natural water systems that contain an abundance of water, carbon dioxide, and the rare earth oxides, a systematic understanding of rare earth carbonates can help us predict the distribution of the rare earths in these systems. As mentioned previously, rare earths are important in the study of nuclear fuel applications as rare earths are fission products of nuclear fuels and certain lanthanides are good chemical homologues for some actinides. As the carbonates are believed to be the solubility limiting factor in natural water systems, study of the synthetic carbonates is all the more relevant. Hydrolysis tendencies will affect which carbonate phase (normal carbonate vs. hydroxycarbonate) are found in natural water systems, solubility trends will demonstrate the distribution of the rare earths between solid and aqueous phases, and in mixed RE2O3-H2O-CO2 systems, quantified thermochemical differences enable predictions of which rare earth carbonate is more likely to form over the other(s), enabling separation techniques of mixed rare earths.
These property-specific trends are most evident within a systematic study and when quantification and analytical methods are self-consistent. Systematic studies generally encompass the whole rare earth spectrum or representative rare earths (yttrium + light rare earth(s) + heavy rare earth(s)) to demonstrate trends with respect to atomic number. Crystallographic trends are apparent when the same indexing methods and space groups are used. Thermal stability trends manifest with consistent atmospheres and heating conditions. Solubility and thermochemical trends require the use of the exact, desired rare earth carbonate phase to properly attribute these values.
3.1. Crystallography of the Rare Earth Carbonates
The normal rare earth carbonates, with the general chemical formula of RE2(CO3)3·xH2O, are hydrated carbonates in which the reported degree of hydration can vary from the theoretically determined values depending upon synthesis conditions. The rare earth hydroxycarbonates have the general chemical formula of RE(OH)CO3·xH2O that can assume the orthorhombic, hexagonal, or tetragonal structural variants depending upon reaction conditions. It should be noted that in the study of naturally occurring RE carbonates, particularly single RE carbonates, the hydroxycarbonates are of greater importance as they are the hydrolysis products of the normal carbonates at ambient conditions (pCO2 = 3 × 10−4 atm, 25 °C, total pressure = 1 atm) and are also the preferred carbonate phase at elevated temperatures [43,44,45,46,47,49].
Much prior literature regarding the crystallography of the rare earth carbonates has reported unit cell parameters, space groups, and crystal systems in isolation from one another, i.e., there is an abundance of literature that reports crystallographic parameters of one or two rare earth carbonates [88,106,107,108,109,110,111,112,113,114,115,116,117,118,119]. This fragmentation leads to confusion and inconsistencies in the available crystallographic data for the rare earth carbonates. Yet, literature that utilizes the same crystal systems and space groups often reports similar crystallographic parameters, such as those by Caro [120] and Shinn [88]. Authors that have systematically studied the rare earth carbonates (e.g., Caro, Wakita, Michiba, and Tahara) have utilized self-consistent indexing and synthesis methods, allowing trends in the crystallographic parameters to become apparent. For both hydroxycarbonates and normal carbonates, unit cell parameters shrink with increasing atomic number. Though such trends were not the main focus of these reports, the crystallographic data clearly indicate the trend is present.
Of the normal rare earth carbonates, the carbonates of lanthanum through neodymium are isostructural to lanthanite, an octahydrate carbonate, and the carbonates of samarium through thulium plus yttrium are isostructural to tengerite, a di-/trihydrate carbonate. Ytterbium carbonates and lutetium carbonates form hexahydrate carbonate phases that are unique from each other and the rest of the rare earths [50]. Scandium can form a carbonate, but is most likely to form a unique hydroxide phase. Attempts to reproducibly synthesize and characterize scandium carbonates have met with limited, if any, success [121,122].
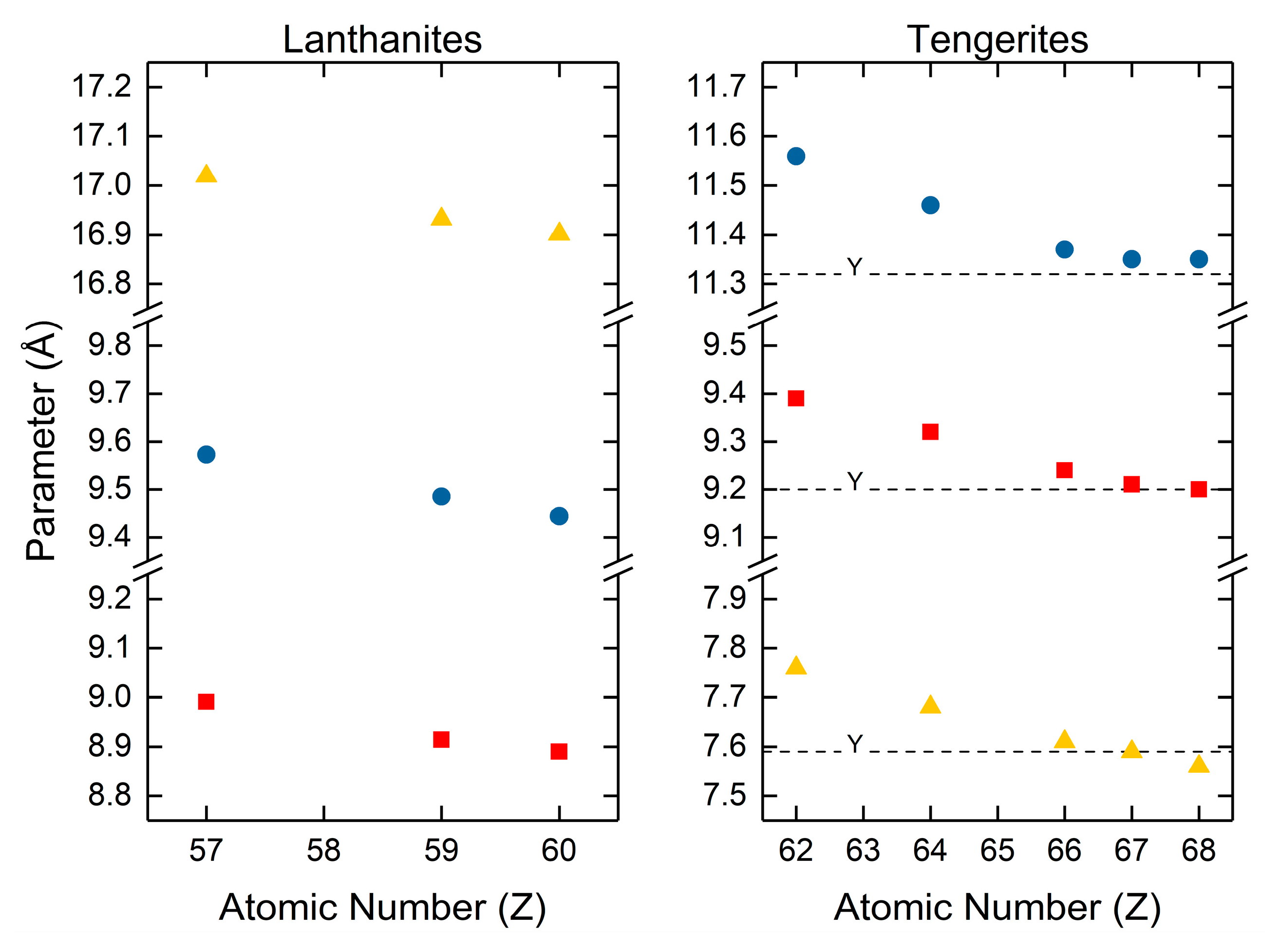
The most comprehensive studies on the normal carbonates are by Caro [120] and Wakita [123]. Caro [120] reported the unit cell parameters for lanthanum, praseodymium and neodymium carbonate by assigning them to the orthorhombic crystal system within the Pccn space group (Figure 5). Praseodymium and neodymium carbonate were found to be isostructural to lanthanite. Wakita [123] determined the unit cell parameters for the normal rare earth carbonates to be isostructural to tengerite. These were the normal carbonates of samarium, gadolinium, dysprosium, holmium, erbium, and yttrium. Wakita indexed all of the carbonates isostructural to tengerite using the “Battelle indexing charts for diffraction patterns of tetragonal, hexagonal and orthorhombic crystals” and assigned to the orthorhombic crystal system [123]. Both studies have shown that the crystallographic parameters shrink with increasing atomic number. Caro also reported increasing density with increasing atomic number, attributable to increasing atomic number and shrinking unit cell volumes. Wakita did not specifically calculate unit cell density but did mention that until cell densities closely follow the refractive indices, which increase with increasing atomic number. From Wakita’s explicitly reported unit cell volumes, it can also be assumed that density increases for the tengerites with increasing atomic number as unit cell volumes decreased with increasing atomic number.
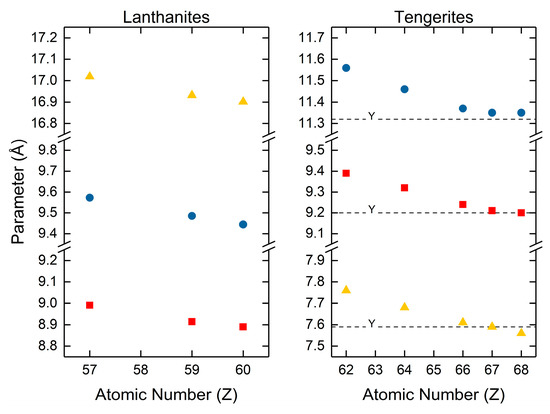
Figure 5.
Lattice parameter data for the lanthanites and tengerites, the normal rare earth carbonate hydrates. Values for the lanthanites are from Caro and coworkers [120]. Values for the tengerites are from Wakita and coworkers [123].
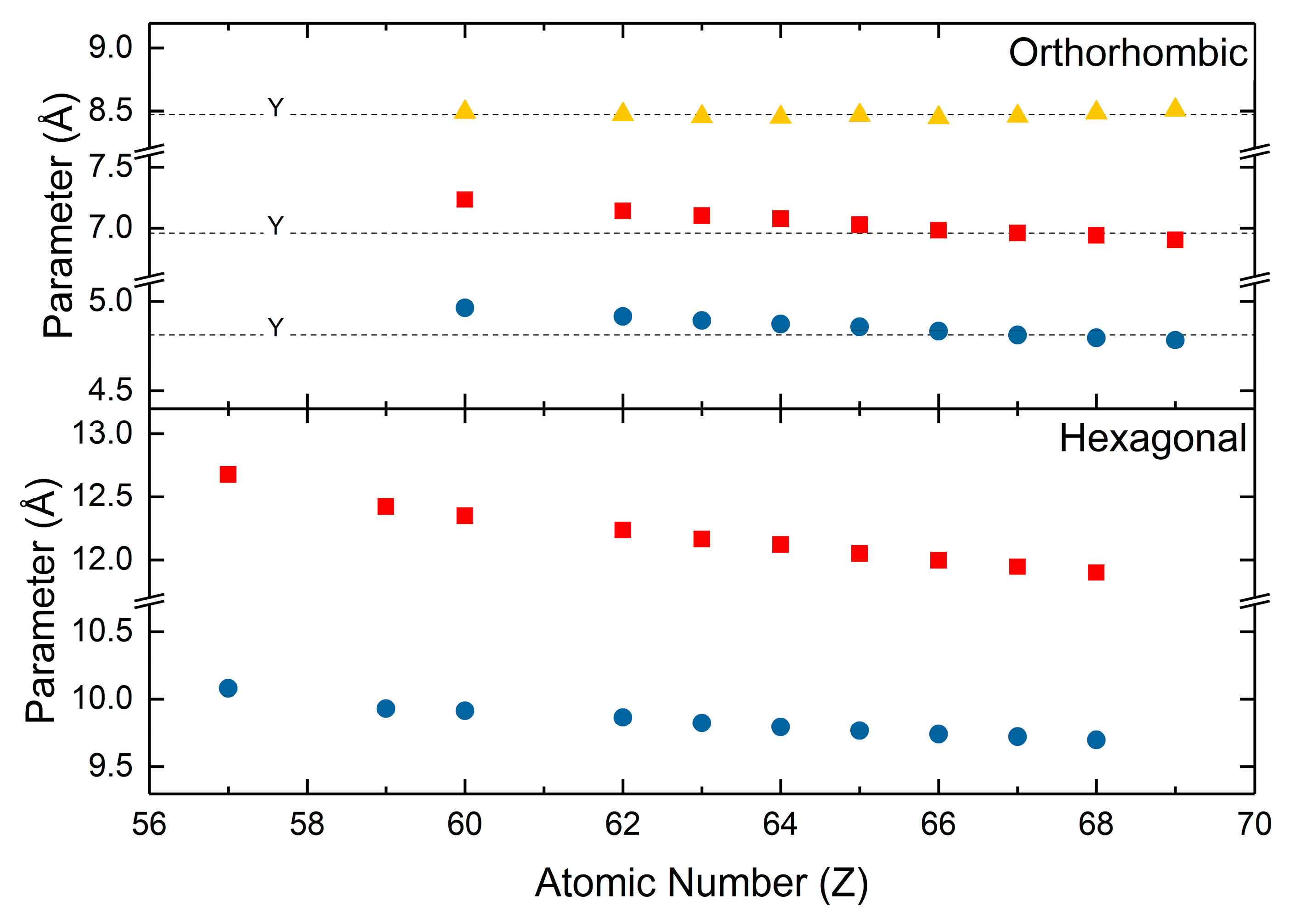
The hydroxycarbonates assume two polymorphs in nature. The hexagonal hydroxycarbonates are isostructural to the hydroxyl analogs of bastnäsite and the orthorhombic hydroxycarbonates are isostructural to the ancylite group of minerals plus kozoite. The most comprehensive examinations of the hydroxycarbonate crystal structures are by Tahara [124] and Michiba [79]. Tahara [124] synthesized the series of orthorhombic hydroxycarbonates by the decomposition of formic acid under hydrothermal conditions. Neodymium and samarium hydroxycarbonates were assigned the Pnma space group, europium through thulium hydroxycarbonates were assigned the P212121 space group, and thulium and ytterbium hydroxycarbonates were assigned the P42/nmc. Like those of the normal carbonates, the reported values for the orthorhombic unit cell parameters (Figure 6) shrink with increasing atomic number. Michiba [79] reported the unit cell parameters for the hexagonal hydroxycarbonates (Figure 6). The hexagonal hydroxycarbonates were synthesized via the hydrothermal decomposition of formic acid at temperatures greater than those for the orthorhombic hydroxycarbonates. All of the hydroxycarbonates were assigned the P6̅ space group. Like the normal carbonates, both polymorphs of the hydroxycarbonates have shrinking unit cell parameters with increasing atomic number. These shrinking parameters are also accompanied by hydroxycarbonate densities that increase with increasing atomic number, which is also attributable to shrinking unit cell volumes and increasing atomic mass.
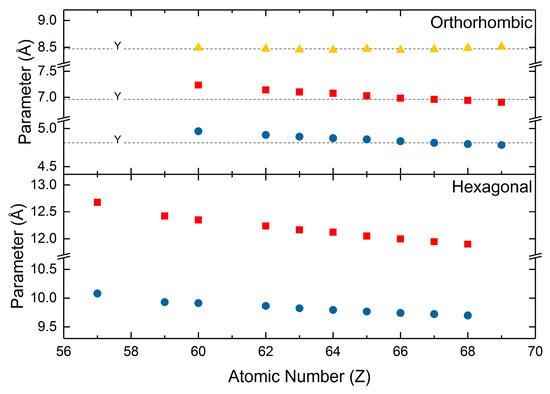
Figure 6.
Lattice parameters for the orthorhombic and hexagonal rare earth hydroxycarbonates. Values for the orthorhombic hydroxycarbonates are from Tahara [124]. Values for the hexagonal hydroxycarbonates are from Michiba [79].
Shrinking unit cell parameters and increasing carbonate density with increasing atomic number are found in all polymorphs of the rare earth carbonates. This is true for the normal carbonates, hexagonal hydroxycarbonates, and orthorhombic hydroxycarbonates. As previously discussed, these trends only exist when consistent methods of analysis and synthesis are applied to the systematic study of rare earth carbonate crystallography. For the hydroxycarbonates, lattice parameters reported by many different groups do not greatly vary from those of Tahara or Michiba. This can be attributed to the well understood crystallography and easily controllable chemical composition of the hydroxycarbonates. The values for the respective rare earth hydroxycarbonates from Beall [110], Dal Negro [125], Christensen [107], Dexpert [126], Doert [109], and Kutlu [108] (Figure 6) are very similar to those presented by Tahara [124] and Michiba [79].
Unlike the hydroxycarbonates, the reported values for the normal carbonates are rather inconsistent when considering a wider body of literature. These differing values can be attributed to different synthesis methods, degrees of hydration, particle size, and choice of crystal system. In many attempts to characterize the crystallography of the normal carbonates, the exact degree of hydration varies widely, and the stoichiometry of the carbonate will vary, though it may not be reported as such. Based upon the general chemical formula of RE2(CO3)3·xH2O, the RE2O3:CO2 ratio should be as close to 1:3 as possible, with the amount of water being released upon decomposition dependent upon the exact rare earth. The RE2O3:CO2 ratio is affected by the hydrolysis of the carbonates, of which those of cerium through europium tend to hydrolyze into the hydroxycarbonate, and degrees of hydration are sensitive to drying conditions [86]. Further deviations can be attributed to contamination by precipitation agents, such as sodium/potassium carbonates, where prolonged exposure of the rare earth carbonates to aqueous solutions of alkali carbonates will create the double carbonate [36,37,38,39,40,41,42].
The crystallographic parameters we have selected as representative of the carbonates are based upon the following. For the hydroxycarbonates, these are the values that have been accepted into the Inorganic Crystal Structure Database (ICSD) [79,124]. For the normal carbonates, the values for the lanthanites have been indexed using the same method for the diffraction pattern and crystallographic parameters accepted into the ICSD for lanthanum carbonate octahydrate by Shinn [88]. The values for the tengerites by Wakita should be approached with a little more caution than those of the lanthanites as the ICSD does not include these values. The diffraction pattern and crystallographic pattern for tengerite in the ICSD is that by Miyawaki [112], who assigned a different space group to the synthetic yttrium carbonate than that of Wakita.
3.2. Thermochemical Properties of the Rare Earth Carbonates
The thermochemical properties for the rare earth carbonates are differentiated into the distinct crystal systems for the normal carbonates and the hydroxycarbonates. Considering the distinct chemical nature(s) of the different rare earth carbonates, the thermochemical properties for the hydrated normal carbonates are not the same as those for the anhydrous normal carbonates. Likewise, the thermochemical properties for the hexagonal hydroxycarbonates are not the same as those for orthorhombic hydroxycarbonates. Thermochemical properties for the rare earth carbonates have been determined by both calorimetric and solubility means, with some studies utilizing both methods to demonstrate that both are valid methodologies [127] when carefully performed and can arrive at similar thermochemical values. When done correctly, calorimetric methods of deriving enthalpies of formation can be accomplished in relatively short time periods (minutes/hours vs. weeks/months) compared to solubility methods. Solubility methods [127,128,129,130,131] require extraordinarily long time points to ensure that thermodynamic equilibrium has been established. In addition, solubility experiments also dictate that the initial and final solid phase in contact with water are the same, i.e., the experimental conditions should not result in a change of the crystallographic phase or chemical composition during the course of equilibration. For phases with extremely low solubility, like the rare earth carbonates, appropriate adjustments in ionic strength and pH are utilized to increase the solubility of the solid phase(s) to concentration levels that can be accurately measured [130,131].
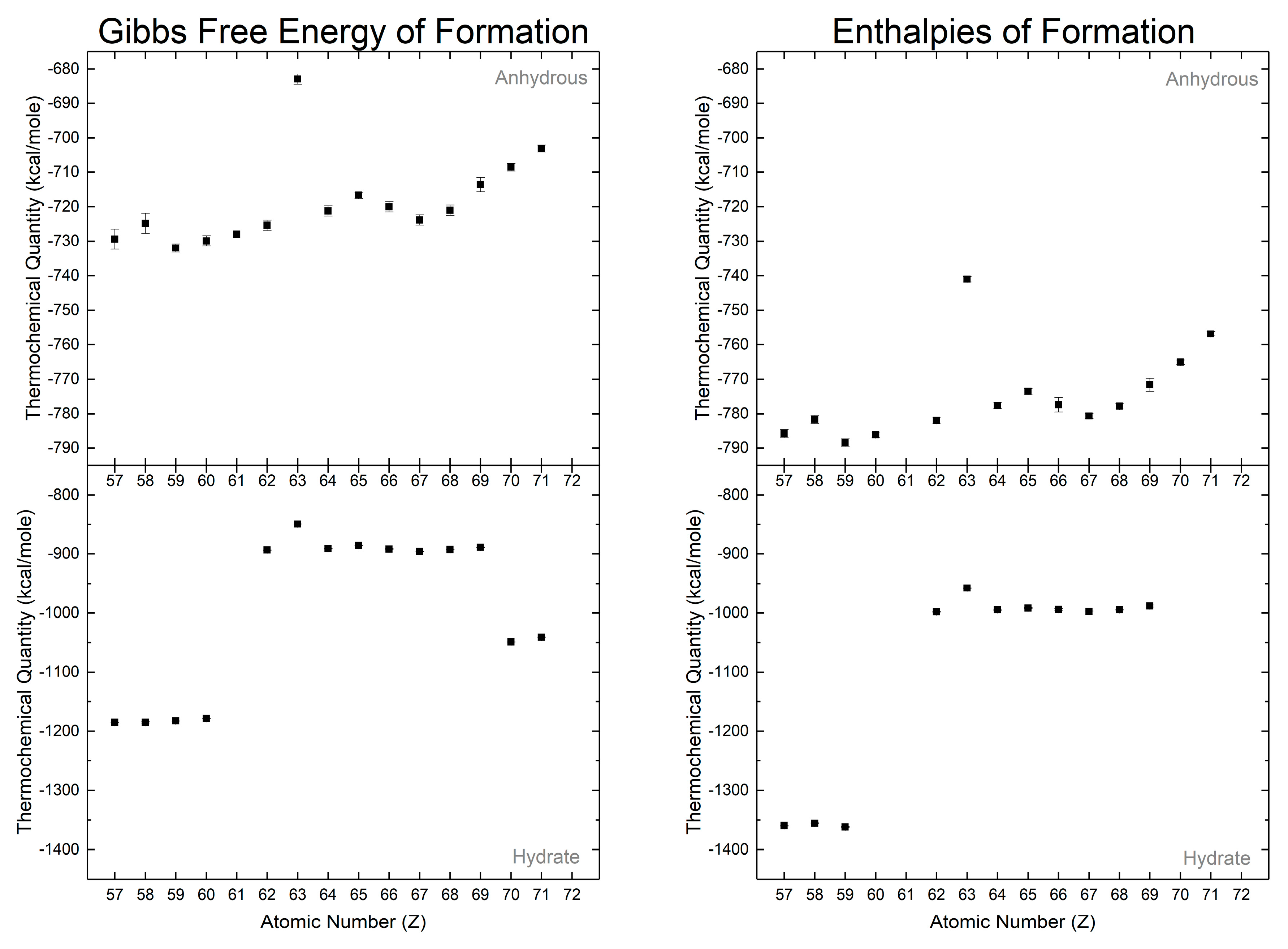
The hydrated normal carbonates can be classified along the same lines as per their crystallography: the octahydrate lanthanites, the di-/trihydrate tengerites, and the hexahydrates of ytterbium and lutetium. It should be noted that thermochemical properties for scandium carbonate are not presented as no definitive conclusion has been reached in literature regarding the exact composition of scandium carbonate, if it exists at all [121,122]. The thermochemical values of the hydrated normal carbonates by Karapet’yants [129,132] (Figure 7) make this apparent, with three distinct “levels” of thermochemical values (Gibbs free energies and enthalpies of formation). Though the report makes scant mention of the crystallographic identities, we assume that these hydrates are the isostructural groups associated with the degrees of hydration as the amount of water is strongly linked to the crystallographic phase [6].
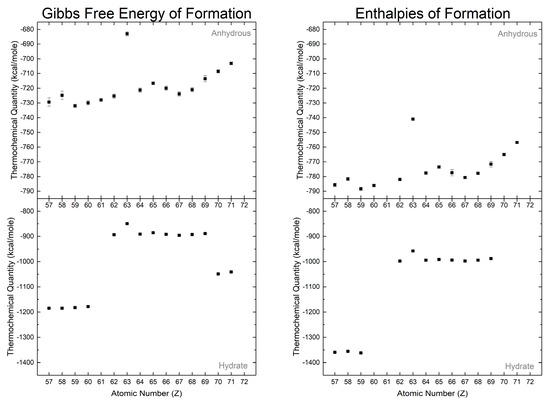
Figure 7.
Gibbs free energies of formations and enthalpies of formation from Karapet'yants [129], [132]. Hydration of the rare earth carbonates masks decreasing thermochemical values with respect to atomic number as observed in the anhydrous phases.
The Gibbs free energies and enthalpies for the anhydrous normal carbonates are smaller in magnitude than those for the hydrated normal carbonates (Figure 7). The difference in the thermochemical quantities between the respective anhydrous and hydrated carbonates can be correlated with the enthalpy of hydration. Both Gibbs free energies and enthalpies of formation for the anhydrous normal carbonates decrease in magnitude with increasing atomic number, with a europium exception attributed to a strong tendency to form the divalent cation. This trend directly carries over to the hydrated carbonates and is found within the isostructural carbonate groups (i.e., lanthanites, tengerites, and hexahydrates).
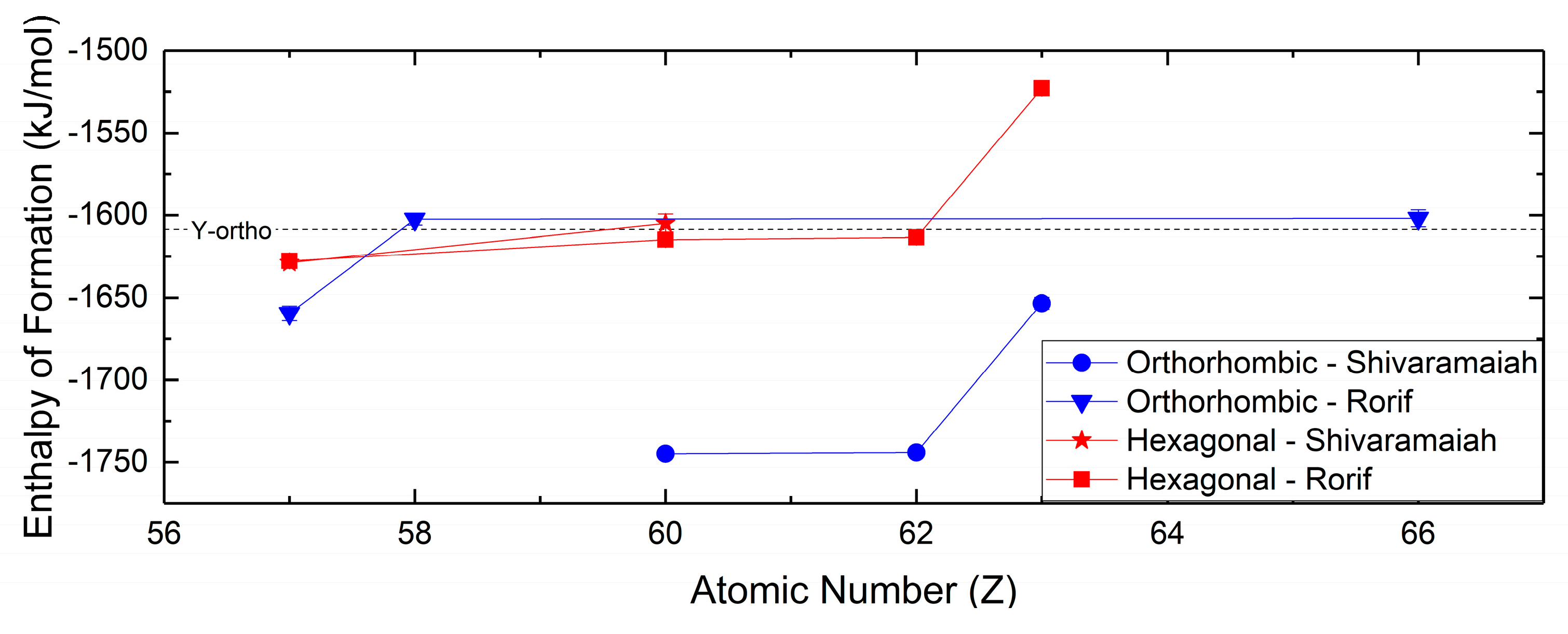
The thermochemistry of the hydroxycarbonates is separated between the orthorhombic and hexagonal polymorphs, which are isostructural to ancylite and hydroxyl-bastnaesite, respectively. This requires that the presented thermochemical values account for the distinction between these two phases. Compared to studies of the thermochemical properties for the normal carbonates, studies of the thermochemistry of the hydroxycarbonates are not as extensive, but have been conducted more recently. Studies by Rorif [127] and Shivaramaiah [99] (Figure 8) have determined the enthalpies of formation for a number of hydroxycarbonates spanning the rare earths. In general, the enthalpies of formation for both hexagonal and orthorhombic hydroxycarbonates decrease in magnitude with increasing atomic number, and the hexagonal hydroxycarbonates are lower in magnitude than the orthorhombic hydroxycarbonates. The comparison of the orthorhombic and hexagonal hydroxycarbonates by Rorif [127] has led to the determination that though the orthorhombic hydroxycarbonates may be isolated at standard conditions, the orthorhombic carbonates are metastable compared to their hexagonal polymorphs.
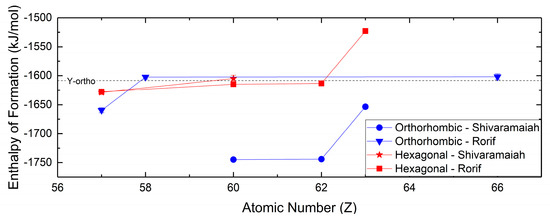
Figure 8.
Enthalpies of formation for hexagonal and orthorhombic hydroxycarbonates from Rorif [127] and Shivaramaiah [99]. Value for orthorhombic yttrium hydroxycarbonate is from Shivaramaiah [99].
Overall, the present body of thermochemical values has allowed us to draw the following conclusions: the hydrated normal carbonates form preferentially over the hydroxycarbonates at standard conditions, the orthorhombic hydroxycarbonates are preferred over the hexagonal hydroxycarbonates at standard conditions, and thermochemical properties for normal carbonates and hydroxycarbonates decrease in magnitude with increasing atomic number. However, additional studies into the thermochemistry of other hydroxycarbonates such as those of holmium, erbium, ytterbium, and gadolinium will flesh out the body of literature available. Literature on the thermochemical parameters for the normal carbonates may require revisiting as the work by Karapet’yants [129,132] was conducted over 40 years ago. More recent analytical methodologies may prove beneficial to refining and improving the thermochemical quantities for the hydrated normal carbonates [130,131].
3.3. Thermal Behavior of the Rare Earth Carbonates
Literature regarding the thermal behavior of the rare earth carbonates has attempted to identify certain trends, intermediate phases, or simply show the decomposition profile to demonstrate that they have synthesized the desired rare earth carbonate. In general, all rare earth carbonates will follow the sequence of dehydration, partial decarbonation, and full decarbonation. The dehydration process can account for 5% to 20% of the mass loss; the lanthanites can lose up to 23% of their initial mass due to dehydration. Dehydration mainly occurs below 100 °C with any additional water, or crystalline water in the hydroxycarbonate, being lost between 200 and 500 °C. Following dehydration, carbonates will partially decompose into the oxycarbonates. The oxycarbonates will then decompose into the respective oxide.
The normal carbonates in general follow the decomposition pathway of:
The hydroxycarbonates in general follow the decomposition pathway of:
Thermal decomposition profiles are affected by the decomposition atmosphere and heating rate. Relative to air, humidified air will not stabilize the hydrates or water bearing phases, CO2 atmospheres will stabilize all carbonate phases, and vacuum atmospheres will expedite the decomposition of all phases [84,85]. High heating rates can mask the existence of intermediate phases [89,90,133,134] and make the accurate identification of phase transitions difficult. With all that said, most systematic studies on the thermal decomposition of both hydrated normal carbonates and hydroxycarbonates do not necessarily present specific values according to which the onset of thermal decomposition, and therefore trends across the rare earths, can be identified. This makes the specific identification of trends difficult, but correlations with increasing atomic number have been reported. In general, the decomposition temperature for all rare earth carbonates and all intermediate phases (anhydrous and oxycarbonate) trends downwards with increasing atomic number. It should be noted that the thermal decomposition of the rare earth oxycarbonates have been previously characterized [86,135,136,137].
Comprehensive studies of the normal carbonates by Head [84,85], Domingues and coworkers [133,134], Wendlandt [138], and Foger [139] demonstrate correlations in the stability of the representative intermediate carbonate with respect to atomic number. In general, the intermediate carbonate phases, the anhydrous normal carbonate and dioxymonocarbonate, will trend toward lower decomposition temperatures in air with increasing atomic number. It should be noted that this correlation is not perfect [84,85] and these exceptions, such as the greater decomposition temperature of gadolinium carbonate over the europium carbonate, multiple dehydrations steps for neodymium carbonate [89], and the complex decomposition of praseodymium carbonate [90], can be attributed to the unique electron configurations of the respective rare earths.
In contrast to the normal carbonates, the thermal decomposition behavior of the hydroxycarbonates has not been as comprehensively studied. A limited number of studies on the hydroxycarbonates by Eyring [89,90], Charles [83], and D’Assuncao [140] have demonstrated the general decomposition pathway as outlined by equations 3–5. Charles [83] gives a relatively qualitative view on the decomposition pathways of the hydroxycarbonates and the studies by Eyring [89,90] are in general agreement with this decomposition pathway.
D’Assuncao [140] has given one of the most comprehensive reports on the decomposition profiles of the rare earth hydroxycarbonates, but it should be noted that no mention of crystal phase for the rare earth hydroxycarbonates was given and thus it is difficult to assess to which hydroxycarbonate phase the results can be ascribed. Specific transition temperature(s) were also not reported as the high heating rates, which have also been known to shift the transition/decomposition temperature higher [89,90,133,134], most likely made this very difficult. Based on the provided decomposition profiles and associated decomposition temperature ranges, we make the following conclusions:
- (1)
- Decreasing carbonate product crystallinity (lack of distinct plateaus in decomposition profiles) with increasing atomic number, with the relatively high heating rate (~ 20 °C/min), makes decomposition transition temperature difficult to identify.
- (2)
- Dehydration of the hydroxycarbonates, due to their greater amorphous nature, occurs at much lower temperatures than those of the lighter hydroxycarbonates.
- (3)
- Within the lighter hydroxycarbonates (La-Eu), the temperature at which the partial decarbonation to form an oxycarbonate occurs trends downwards with increasing atomic number.
- (4)
- Oxycarbonate decomposition temperature trends downwards with increasing atomic number, which was also found for the oxycarbonates of the hydrated normal carbonates.
It should be noted that few if any of these observed decompositions represent equilibrium reactions, with these observations being kinetic rather than thermodynamic. These reactions have not been shown in these studies to be reversible and particle sizes were not necessarily controlled. Since particle size and therefore particle packing was not necessarily controlled, deviations in decomposition temperature due to these factors were also not controlled. Yet, these studies provide valuable insight into the thermal decomposition of the carbonates.
In comparing the available literature on the thermal decomposition of the rare earth carbonates, the normal carbonates have been better studied with many reporting the same downward trend in decomposition temperature of all intermediate phases with increasing atomic number. No distinct trend across the entire rare earth series could be established for the hydroxycarbonates due to the isolated nature of the literature, studying only one hydroxycarbonate at a time, and the high heating rate used in the most encompassing study. To firmly establish decomposition temperature trends, for either normal carbonates or hydroxycarbonates, with respect to atomic number, future studies should utilize extremely low heating rates (e.g., 0.25 °C/min) [84,85,89,90], same crystallography (i.e., normal carbonates vs. hexagonal hydroxycarbonates vs. orthorhombic hydroxycarbonates), and same atmosphere (e.g., air or CO2) [84,85].
3.4. Behavior of Rare Earth Carbonates in Aqueous Environments
The rare earth carbonates are known for two particular behavior patterns in water: they are very insoluble but undergo hydrolysis. Both normal carbonates and hydroxycarbonates are very insoluble water, with systematic studies such as those by Jordanov [141], Caro [48], Spahiu [142], and Rorif [127] showing Ksp values on the order of 10−35–10−20. Yet, insolubility does not translate to resistance to hydrolysis. At ambient conditions, Caro demonstrated the tendency of the lighter normal rare earth carbonates (La-Eu) to readily hydrolyze while the heavier normal carbonates are resistant to hydrolysis [49]. All normal rare earth carbonates will hydrolyze into their respective hydroxycarbonates in water when temperature is increased close to 100 °C. Studies on the hydrothermal behavior of the RE2O3-CO2-H2O ternary system by Kutty and coworkers [43,44,45,46,47] show that at isobaric conditions (fixed mole fraction CO2) the hydroxycarbonates are the preferred phase at elevated temperatures.
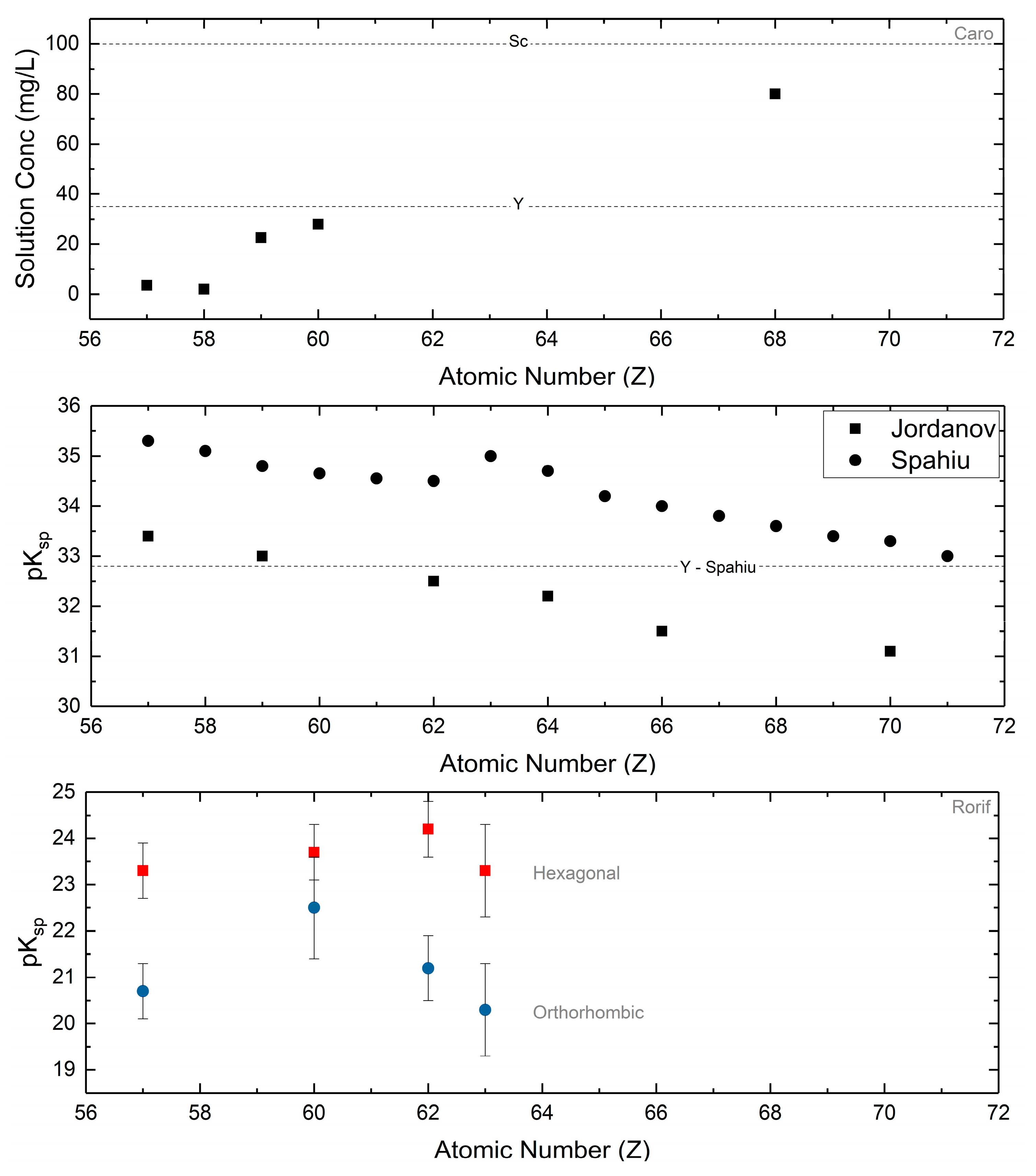
The normal carbonates have Ksp on the order of 10−35–10−30 (Figure 9), much lower than the those of the hydroxycarbonates (10−20–10−15), as determined at neutral pH and with sufficient pCO2. Since solubility measurement requires that the solid in contact with water does not undergo a chemical change, this extremely low normal carbonate solubility is relevant with the caveat that the applied pCO2 prevents hydrolysis into the hydroxycarbonate. Caro demonstrated that the normal carbonates are stable at a pCO2 of 1 atm [50]. Both Jordanov [141] and Caro [48] utilized this knowledge to obtain solubility data on the entire series of normal carbonates, demonstrating increasing solubility with increasing atomic number. Normal yttrium carbonate falls within the expected range based upon ionic radius of the trivalent cation (Figure 9). Analysis of multiple solubility reports by Spahiu [142] have also concluded this general trend. Other collections of systematically acquired solubility data all show varying degrees of solubility for each of the carbonates, but in general demonstrate increasing solubility with increasing atomic number.
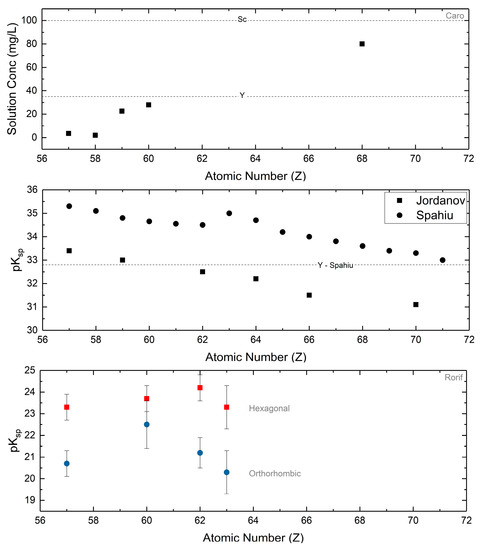
Figure 9.
Solubility products and raw data for the normal carbonates are presented from Caro and coworkers [48], Jordanov [141], and Spahiu [142]. In general, the solubility of the normal carbonates increases with increasing atomic number. The dotted lines are the values for scandium carbonate and yttrium carbonate as reported by the respective authors and are presented for reference. Solubility products for the hexagonal and orthorhombic hydroxycarbonates are from Rorif [127]. No trend with respect to atomic number may be established, but the orthorhombic carbonates are in general more soluble than their hexagonal counterparts.
Hydroxycarbonate solubility, for both orthorhombic and hexagonal phases, has been explored on a limited basis. Many of these studies have focused on the lighter lanthanide carbonates, particularly neodymium carbonate as it is a chemical homologue for radioactive actinide carbonates [16,19]. As such, understanding their aqueous solubility has been crucial as the hydroxycarbonate phases are the solubility limiting phases in natural water systems in the distribution of actinides/lanthanides. Yet, comprehensive studies on a scale similar to those of the normal carbonates are scarce. Rorif [127] presented a comparison of the aqueous solubilities of the hexagonal and orthorhombic lanthanum, neodymium, samarium, and europium hydroxycarbonates. Though no trends with respect to atomic number can be established, there is a marked difference in solubility between the hexagonal and orthorhombic hydroxycarbonates (Ksp = 10−25–10−23 vs. 10−22–10−20, respectively). Across the board, the orthorhombic hydroxycarbonates are more soluble than their respective hexagonal hydroxycarbonates (Figure 9).
4. Final Remarks
The rare earth carbonates, both normal and hydroxycarbonates, are important in understanding the distribution of rare earths in geological settings. Since the rare earths are chemically related to the actinides, understanding the behavior of the rare earths also furthers our knowledge of the distribution and chemistry of nuclear fuels and radioactive actinides in natural water systems, which contain CO2. Industrial production of rare earth solids fundamentally requires a consistent and sound understanding of the crystallographic, thermochemical, thermal decomposition, and aqueous properties of the rare earth carbonates. Since many geological sources of rare earths are enriched carbonate minerals and the downstream production of other rare earth solids begins with the rare earth carbonates, the fully comprehensive understanding of these properties is of utmost importance. With that said, the current understanding of physical and chemical properties of the rare earth carbonates has been limited to extrapolations based upon what has been explored, i.e., representative rare earths for the light, middle, and heavy rare earths. As in the case of nuclear fuel applications, neodymium and europium carbonates/hydroxycarbonates have been studied as chemical homologues for americium.
Trends in crystallography, thermochemistry, aqueous behavior, and thermal decomposition of the rare earth carbonates have informed us that physical and chemical parameter trends with respect to atomic number found for the rare earth elements also carry over to the carbonates. The shrinking lattice parameters, decreasing magnitude in thermochemical parameter(s), downward trending decomposition temperatures, and increasing solubility with increasing atomic number have basis within the concept of the lanthanide contraction and the chemical implications thereof. However, these parameters and trends can yet benefit from further refinement and studies. The lattice parameters for the normal carbonates, besides those for lanthanite-(La) and tengerite-(Y), are not found in ICSD, whilst those for the hydroxycarbonates for both hexagonal and orthorhombic polymorphs can be accessed through ICSD. The most comprehensive studies of normal carbonate thermochemistry can only be found in obscure compilations such as that by Karapet’yants. More easily accessible compilations contain the thermochemical values for singular carbonates or the anhydrous variants. Hydroxycarbonate thermochemistry is limited to the lighter rare earths with a small sampling of the heavier rare earths. The aqueous solubility of the carbonates is subject to the same limitations as the available thermochemical literature, i.e., normal carbonate solubility is found in obscure literature or data for anhydrous variants are reported as valid for hydrated variants, and hydroxycarbonate solubility is limited to the lighter rare earths plus a sampling of the heavier rare earths. The thermal decomposition profiles and our understanding thereof for all carbonates would benefit from reevaluation. By utilizing extraordinarily slow heating rates, standardized atmospheres, and standardized transition analyses, firmer correlations may be established in the already established general correlation in the downward trending decomposition temperature with increasing atomic number. Updating these values and making them readily accessible would benefit our understanding of the rare earth elements.
Acknowledgments
Funding from the Critical Materials Institute, an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office (AMES LABORATORY–SC-13-394) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Critical Materials Institute (CMI). Critical Materials Institute—Grand Challenge Problems; CMI: Ames, IA, USA, 2014. [Google Scholar]
- Gschneidner, K.A., Jr. The Rare Earth Crisis—The Supply/Demand Situation for 2010–2015. Mater. Matters 2011, 6, 32–37. [Google Scholar]
- Connelly, N.G.; Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Nomenclature of Inorganic Chemistry—IUPAC Recommendations 2005; Division of Chemical Nomenclature and Structure Respresentation; RSC Publishing: London, UK, 2005. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Haxel, G.B.; Hedrick, J.B.; Orris, G. Rare Earth Elements—Critical Resources for High Technology; United States Geological Survey: Reston, VA, USA, 2002.
- Railsback, L.B. Patterns in the Compositions, Properties, and Geochemistry of Carbonate Minerals. Carbonates Evaporites 1999, 14, 1–20. [Google Scholar] [CrossRef]
- Castor, S.B. The Mountain Pass rare-earth carbonatite and associated ultrapotassic rocks, California. Can. Mineral. 2008, 46, 779–806. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Kellere, J.; Kejie, T.; Wall, F.; William, C.T.; Peishan, Z. Carbonatite dykes at bayan Obo, inner Mongolia, China. Mineral. Petrol. 1992, 46, 195–228. [Google Scholar] [CrossRef]
- Zhou, Z.; Gongyuan, L.; Tongyun, S.; Yuguan, L. On the geological characteristics and the genesis of the dolomitic carbonatites at Bayan Obo, Inner Mongolia. Geol. Rev. 1980, 26, 35–42. [Google Scholar]
- U.S. Geological Survey. Mineral Commodities Summary 2011; United States Geological Survey: Reston, VA, USA, 2011.
- U.S. Geological Survey. Mineral Commodity Summaries 2012; United States Geological Survey: Reston, VA, USA, 2012.
- U.S. Geological Survey. Mineral Commodities Summaries 2015—Rare Earths. Earth; United States Geological Survey: Reston, VA, USA, 2015.
- U.S. Geological Survey. Mineral Commodity Summaries 2016. Mineral Commodity Summaries; United States Geological Survey: Reston, VA, USA, 2016.
- Hurst, C. China’s Rare Earth Elements Industry: What Can the West Learn? Institute for the Analysis of Global Security: Washington, DC, USA, 2010. [Google Scholar]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Carroll, S.A. Precipitation of Nd-Ca carbonate solid solution at 25 °C. Geochim. Cosmochim. Acta 1993, 57, 3383–3393. [Google Scholar] [CrossRef]
- Meinrath, G.; Takeishi, H. Solid-liquid equilibria of Nd3+ in carbonate solutions. J. Alloys Compd. 1993, 194, 93–99. [Google Scholar] [CrossRef]
- Meinrath, G.; Kim, J.I. Solubility products of different Am (III) and Nd (III) carbonates. Eur. J. Solid State Inorg. Chem. 1991, 28, 383–388. [Google Scholar]
- Runde, W.; Meinrath, G.; Kim, J.I. A Study of Solid-Liquid Phase-Equilibria of Trivalent Lanthanide and Actinide Ions in Carbonate Systems. Radiochim. Acta 1992, 58–59, 93–100. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, X. Precipitation of Rare Earth with Carbonic Acid. CN Patent 1055395a, 16 October 1991. [Google Scholar]
- Yu, Q.; Li, X. Formation of crystalline rare earth carbonates from ore leaching liquors. Zhongguo Xitu Xuebao 1993, 11, 171–173. [Google Scholar]
- Vladescu, C.M.; Iusein, G. Separation of cerium from trivalent lanthanides. Italian patent RO 86901, 29 June 1985. [Google Scholar]
- Tselik, I.N.; Shvartsman, V.Y.; Fedorenko, V.D. Composition and thermal stability of carbonates of yttrium group rare earth elements. Zhurnal Neorganicheskoi Khimii 1968, 13, 106–112. [Google Scholar]
- Tong, Z.; Chen, W.; Guan, X. Manufacture of Rare Earth Carbonates from Rare Earth Sulfates. CN Patent CN1094380A, 2 November 1994. [Google Scholar]
- Schmitt, A.; Lorenz, H.; Richter, H.; Kunze, G. Discontinuous process for the precipitation of rare earth carbonates from aqueous solutions containing other anions and cations. DE patent, DD292216, 25 July 1991. [Google Scholar]
- Osipova, T.P.; Kharakoz, A.E.; Bleshinskii, S.V. Composition of precipitates obtained during the reaction of sodium carbonate with the salts of rare earth elements. Fiz.-Khim. Issled. Redkozemel. Elem. 1972, 52–60. [Google Scholar]
- Osipova, T.P.; Bleshinskii, S.V.; Kharakoz, A.E. Sodium carbonate as a reagent for the precipitation of rare earth elements. In Khim. Svoistva Soedin. Redkozemel. Elem.; Nauka: Moscow, Russia, 1973. [Google Scholar]
- Liu, S.; Ma, R. Preparation by crystalline precipitation of mixed rare earth carbonates. Zhongguo Youse Jinshu Xuebao 1998, 8, 331–334. [Google Scholar]
- Li, Y.; Li, M.; He, X.; Hu, P.; Gu, Z. Precipitation and crystallization of rare earth carbonate. Zhongguo Youse Jinshu Xuebao 1999, 9, 165–170. [Google Scholar]
- He, L.; Gu, Z. Carbonate in Separation of Mixed Rare Earth Metals. CN Patent 1054269A, 4 September 1991. [Google Scholar]
- He, D.; Liao, L.; Xu, R. Precipitation of Rare Earths with a Mixed Precipitant. CN Patent CN1033976A, 19 July 1989. [Google Scholar]
- Fischer, W.; Muller, J.; Niemann, K.E. Separation of the rare earths by fractional precipitation of their carbonates. Z. Anorgan. Allg. Chem. 1955, 282, 63–79. [Google Scholar] [CrossRef]
- Da Silva Queiroz, C.A.; Abrao, A. Behavior of rare earth (La, Ce, Pr, Nd, Sm) carbonates in ammonium carbonate and ammonium carbonate/ammonium hydroxide solution. Publ. ACIESP 1993, 89, 187–200. [Google Scholar]
- Sylvester, C. An Elementary Treatise on Chemistry Comprising the Most Important Facts of the Science, with Tables of Decomposition; To Which Is Added, An Appendix, Giving an Account of the Latest Discoveries, 1st ed.; Liverpool: London, UK, 1809. [Google Scholar]
- Reid, H. Popular Treatise on Chemistry. I. Chemistry of Nature; Simpkin, Marshall & Co.: London, UK, 1834. [Google Scholar]
- Roscoe, H.E.; Schorlemmer, C. A Treatise on Chemistry. Volume II.—Metals; Macmillan and Co.: London, UK, 1879. [Google Scholar]
- Treadwell, F.P. Analytical Chemistry Quantitative Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1910. [Google Scholar]
- Blitz, H.; Blitz, W. Laboratory Methods of Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1909. [Google Scholar]
- Fresenius, C.R. Manual of Qualitative Chemical Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1913. [Google Scholar]
- Mochizuki, A.; Nagashima, K.; Wakita, H. The synthesis of crystalline hydrated double carbonates of rare earth elements and sodium. Bull. Chem. Soc. Jpn. 1974, 47, 755–756. [Google Scholar] [CrossRef]
- Fridman, Y.D.; Sorochan, R.I.; Sarbaev, D.S. Solubility of rare earth carbonates in potassium carbonate solutions. Issled. Khim. Redk. Soputstv. Elem. 1966, 175–184. [Google Scholar]
- Tselik, I.N.; Deineka, G.F.; Fedorenko, V.D.; Shvartsman, V.Y. Reaction of rare-earth chlorides with potassium carbonate in solution. Ukrainskiĭ Khimicheskii Zhurnal 1969, 35, 1042–1045. [Google Scholar]
- Kutty, T.R.N.; Viswanathiah, M.N.; Tareen, J.A.K. Hydrothermal equilibria in Nd2O3-H2O-CO2 System. Proc. Indian Acad. Sci. Chem. Sci. 1978, 87, 69–74. [Google Scholar]
- Mohamed, I.; Tareen, J.A.K.; Kutty, T.R.N. Hydrothermal phase equilibria in Er2O3-H2O-CO2 and Tm2O3-H2O-CO2 systems. Proc. Indian Acad. Sci. Chem. Sci. 1984, 93, 785–793. [Google Scholar]
- Kutty, T.R.N.; Mohamed, I.; Tareen, J.A.K. Hydrothermal phase equilibria in Ln2O3-H2O-CO2 systems for Tm, Yb and Lu. Mater. Chem. Phys. 1984, 10, 425–441. [Google Scholar] [CrossRef]
- Tareen, J.A.K.; Kutty, T.R.N. Hydrothermal phase equilibria in Ln2O3-H2-CO2 Systems. J. Cryst. Growth 1980, 50, 527–532. [Google Scholar] [CrossRef]
- Tareen, J.A.K.; Kutty, T.R.N.; Mohamed, I. The Stable Lanthanide Carbonates in the Ln2O3-H2O-CO2 Systems. Indian Mineral. 1980, 21, 43–48. [Google Scholar]
- Trombe, F.; Blaise, M.; Caro, P.E. Sur la solubilite des carbonate d’yttrium, de scandium et de quelques elements du groupe des terres rares dans l’eau charge de gaz carbonique. C. R. Hebdomadaires Seances L’acad. Sci. Sér. C 1966, 263, 521–524. [Google Scholar]
- Caro, P.E.; Lemaitre-Blaise, M. Hydroxycarbonates de terres rares Ln2(CO3)x(OH)2(3-x)·nH2O (Ln = terres rares). C. R. Hebdomadaires Seances L’acad. Sci. Sér. C 1969, 269, 687–690. [Google Scholar]
- Caro, P.; Lemaitre-Blaise, M.; Trombe, F. Identification et solubilites des phase soslides a l’equilibre sous une atmosphere de gaz carbonique dans les systemes temaires oxydes de terres rares-gaz carbon. C. R. Hebdomadaires Seances L’acad. Sci. Sér. C 1968, 267, 1594–1597. [Google Scholar]
- Sprycha, R.; Jablonski, J.; Matijević, E. Zeta potential and surface charge of monodispersed colloidal yttrium(III) oxide and basic carbonate. J. Colloid Interface Sci. 1992, 149, 561–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, K.; Cheng, T.; Fang, Z. Synthesis, characterization, and photoluminescence property of LaCO3OH microspheres. Inorg. Chem. 2007, 46, 4713–4717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, M.; Han, K.; Fang, Z.; Yin, X.; Xu, Z. Synthesis, characterization and formation mechanism of dumbbell-like YOHCO3 and rod-like Y2(CO3)3·2.5H2O. J. Alloys Compd. 2009, 474, 598–604. [Google Scholar] [CrossRef]
- Li, K.; Zhao, P. Synthesis of single-crystalline Ce(CO3)(OH) with novel dendrite morphology and their thermal conversion to CeO2. Mater. Res. Bull. 2010, 45, 243–246. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Hosseinzadeh, G.; Davar, F. Synthesis of lanthanum hydroxide and lanthanum oxide nanoparticles by sonochemical method. J. Alloys Compd. 2011, 509, 4098–4103. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Du, J.; Gao, H.; Gao, Y.; Mu, T.; Han, B. Synthesis of LaCO3OH nanowires via a solvothermal process in the mixture of water and room-temperature ionic liquid. Mater. Lett. 2005, 59, 963–965. [Google Scholar] [CrossRef]
- Qi, R.J.; Zhu, Y.J.; Cheng, G.F.; Huang, Y.H. Sonochemical synthesis of single-crystalline CeOHCO3 rods and their thermal conversion to CeO2 rods. Nanotechnology 2005, 16, 2502–2506. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Javidi, J.; Davar, F. Sonochemical synthesis of Dy2(CO3)3 nanoparticles, Dy(OH)3 nanotubes and their conversion to Dy2O3 nanoparticles. Ultrason. Sonochem. 2010, 17, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Z.; Shao, M.; Liu, X.; Qian, Y. Preparation of cerium hydroxycarbonate by a surfactant-assisted route. J. Phys. Chem. Solids 2003, 64, 295–297. [Google Scholar] [CrossRef]
- Kawahashi, N.; Matijević, E. Preparation and properties of uniform coated colloidal particles. V. Yttrium basic carbonate on polystyrene latex. J. Colloid Interface Sci. 1990, 138, 534–542. [Google Scholar] [CrossRef]
- Aiken, B.; Matijević, E. Preparation and properties of uniform coated inorganic colloidal particles. IV. Yttrium basic carbonate and yttrium oxide on hematite. J. Colloid Interface Sci. 1988, 126, 645–649. [Google Scholar] [CrossRef]
- Matijevic, E.; Hsu, W.P. Preparation and Properties of Monodispersed of Lanthanide Compounds Colloidal Particles of Lanthanide Compounds. J. Colloid Interface Sci. 1987, 118, 506–523. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, J.; Xing, X.; Xu, L.; Chen, Y. Microemulsion-assisted solvothermal synthesis of Nd2(CO3)3·8H2O microstructures. Mater. Res. Bull. 2011, 46, 830–834. [Google Scholar] [CrossRef]
- Yang, X.; Zhai, Z.; Xu, L.; Li, M.; Zhang, Y.; Hou, W. LaCO3OH microstructures with tunable morphologies: EDTA-assisted hydrothermal synthesis, formation mechanism and adsorption properties. RSC Adv. 2013, 3, 3907–3926. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, Y.Y.; Tong, D.Q.; Tian, L.; Wang, Z.H. Hydrothermal synthesis of single-crystal CeCO3OH and their thermal conversion to CeO2. Chin. Chem. Lett. 2014, 25, 383–386. [Google Scholar] [CrossRef]
- Han, Z.H.; Guo, N.; Tang, K.B.; Yu, S.H.; Zhao, H.Q.; Qian, Y.T. Hydrothermal crystal growth and characterization of cerium hydroxycarbonates. J. Cryst. Growth 2000, 219, 315–318. [Google Scholar] [CrossRef]
- Zhong, S.-L.; Zhang, L.F.; Jiang, J.W.; Lv, Y.H.; Xu, R.; Xu, A.W.; Wang, S.-P. Gelatin-mediated hydrothermal synthesis of apple-like LaCO3OH hierarchical nanostructures and tunable white-light emission. CrystEngComm 2011, 13, 4151–4160. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.F.; Jiang, J.W.; Lv, Y.H.; Xu, R.; Xu, A.W.; Wang, S.-P. Eu3+/Tb3+-Doped La2O2CO2/La2O3 Nano/Microcrystals with Multiform Morphologies: Facile Synthesis, Growth Mechanism, and Luminescence Properties. Inorg. Chem. 2010, 49, 10522–10535. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Wang, Z.; Han, D.; Guo, G.; Guo, H. Crystallization of rare earth carbonate nanostructures in the reverse micelle system. Cryst. Growth Des. 2007, 7, 1452–1458. [Google Scholar] [CrossRef]
- Qian, L.W.; Wang, X.; Zheng, H.G. Controlled synthesis of three-fold dendrites of Ce(OH)CO3 with multilayer caltrop and their thermal conversion to CeO2. Cryst. Growth Des. 2012, 12, 271–280. [Google Scholar] [CrossRef]
- Her, Y.-S.; Matijević, E.; Wilcox, W.R. Continuous precipitation of monodispersed yttrium basic carbonate powders: Part III. Kinetics. J. Mater. Res. 1992, 7, 2269–2272. [Google Scholar] [CrossRef]
- Her, Y.S.; Matijević, E.; Wilcox, W.R. Continuous precipitation of monodispersed yttrium basic carbonate powders. Powder Technol. 1990, 61, 173–177. [Google Scholar] [CrossRef]
- Matijevic, E. Colloid science of ceramic powders. Pure Appl. Chem. 1988, 60, 1479–1491. [Google Scholar] [CrossRef]
- Cress, C.D.; Redino, C.S.; Landi, B.J.; Raffaelle, R.P. Alpha-particle-induced luminescence of rare-earth-doped Y2O3 nanophosphors. J. Solid State Chem. 2008, 181, 2041–2045. [Google Scholar] [CrossRef]
- Di, W.; Ren, X.; Zhang, L.; Liu, C.; Lu, S. A facile template-free route to fabricate highly luminescent mesoporous gadolinium oxides. CrystEngComm 2011, 13, 4831–4833. [Google Scholar] [CrossRef]
- Raikow, P.N. The Action of Carbon Dioxide upon the Hydroxides of the Metals. Chem. Zeitung 1907, 31, 55–57. [Google Scholar]
- Trombe, F.; Caro, P. Process for Separating Rare Earths. U.S. Patent 3,492,084A, 27 January 1970. [Google Scholar]
- Fernando, Q.; Yanagihara, N.; Dyke, J.T.; Vemulapalli, K. Formation of Rare Earth Carbonates Using Supercritical Carbon Dioxide. U.S. Patent 5,045,289A, 3 September 1991. [Google Scholar]
- Michiba, K.; Tahara, T.; Nakai, I.; Miyawaki, R.; Matsubara, S. Crystal structure of hexagonal RE(CO3)OH. Z. Kristallogr. 2011, 226, 518–530. [Google Scholar] [CrossRef]
- Tahara, T.; Hokura, A.; Nakai, I.; Miyawaki, R.; Matsubara, S. Hydrothermal synthesis and crystal structure analysis of RE(CO3)OH (RE = La, Ce, Nd, Sm, Gd, Dy, Y, Er, Yb). Kidorui 2003, 42, 216–217. [Google Scholar]
- Head, E.L. Preparation of the carbonates of the rare earths from some of their organic acid salts. Inorg. Nucl. Chem. Lett. 1966, 2, 33–37. [Google Scholar] [CrossRef]
- Salutsky, M.L.; Quill, L.L. The Rare Earth Metals and their Compounds. XII. Carbonates of Lanthanum, Neodymium and Samarium. J. Am. Chem. Soc. 1950, 72, 3306–3307. [Google Scholar] [CrossRef]
- Charles, R.G. Rare-earth carbonates prepared by homogeneous precipitation. J. Inorg. Nucl. Chem. 1965, 27, 1489–1493. [Google Scholar] [CrossRef]
- Head, E.L.; Holley, C.E., Jr. The Preparation and Thermal Decomposition of Some Rare Earth Carbonates; Los Alamos National Laboratory: Los Alamos, NM, USA, 1963.
- Head, E.L.; Holley, C.E., Jr. The Preparation and Thermal Decomposition of the Carbonates of Tb, Dy, Ho, Er, Tm, Yb, Lu, Y, AND Sc; Los Alamos National Laboratory: Los Alamos, NM, USA, 1963.
- Sastry, R.L.N.; Yoganarasimhan, S.R.; Mehrotra, P.N.; Rao, C.N.R. Preparation, characterization and thermal decomposition of praseodymium, terbium and neodymium carbonates. J. Inorg. Nucl. Chem. 1966, 28, 1165–1177. [Google Scholar] [CrossRef]
- Wakita, H.; Kinoshita, S. A synthetic study of the solid solution in the systems La2(CO3)3·8H2O-Ce2(CO3)3·8H2O and La(OH)CO3-Ce(OH)CO3. Bull. Chem. Soc. Jpn. 1979, 52, 428–432. [Google Scholar] [CrossRef]
- Shinn, D.B.; Eick, H.A. The Crystal Structure of Lanthanum Carbonate Octahydrate. Inorg. Chem. 1968, 7, 1340–1345. [Google Scholar] [CrossRef]
- Hinode, H.; Sharma, R.; Eyring, L. A study of the decomposition of neodymium hydroxy carbonate and neodymium carbonate hydrate. J. Solid State Chem. 1990, 84, 102–117. [Google Scholar] [CrossRef]
- Sharma, R.; Hinode, H.; Eyring, L. A study of the decomposition of praseodymium hydroxy carbonate and praseodymium carbonate hydrate. J. Solid State Chem. 1991, 92, 401–419. [Google Scholar] [CrossRef]
- Sordelet, D.; Akinc, M. Preparation of spherical, monosized Y2O3 precursor particles. J. Colloid Interface Sci. 1988, 122, 47–59. [Google Scholar] [CrossRef]
- Akinc, M.; Sordelet, D. Preparation of Yttrium, Lanthanum, Cerium, and Neodymium Basic Carbonate Particles by Homogeneous Precipitation. Adv. Ceram. Mater. 1987, 2, 232–238. [Google Scholar] [CrossRef]
- Refat, M.S. A novel method for the synthesis of rare earth carbonates. Synth. React. Inorg. Met. Chem. 2004, 34, 1605–1613. [Google Scholar] [CrossRef]
- Deng, H.-M.; Xu, Y.; Gu, Y.-D. Growth kinetics of ultrafine monodispersed colloidal particles of rare earth compounds. Huaxue Xuebao 1995, 53, 867–870. [Google Scholar]
- Kang, Z.C.; Eyring, L. Sintering in colloidal particles of rare earth hydroxycarbonate and its decomposition products. J. Alloys Compd. 1995, 225, 190–192. [Google Scholar] [CrossRef]
- Mochizuki, A. Synthetic study of crystalline rare earth carbonates. Numazu Kogyo Koto Senmon Gakko Kenkyu Hokoku 1974, 9, 57–62. [Google Scholar]
- Nagashima, K.; Wakita, H.; Mochizuki, A. The synthesis of crytalline rare earth carbonates. Bull. Chem. Soc. Jpn. 1973, 46, 152–156. [Google Scholar] [CrossRef]
- Shibata, J.; Noda, S.; Mashimo, M. Crystallization of Rare Earths from Organic Phase by Urea Decomposition. Shigen-to-Sozai 1994, 110, 185–189. [Google Scholar] [CrossRef][Green Version]
- Shivaramaiah, R.; Anderko, A.; Riman, R.E.; Navrotsky, A. Thermodynamics of bastnaesite: A major rare earth ore mineral. Am. Mineral. 2016, 101, 1129–1134. [Google Scholar] [CrossRef]
- Sklyarenko, Y.S.; Ruzaikina, L.V. Formation of neodymium, europium, and ytterbium carbonates and their behavior in aqueous potassium carbonate solutions. Zhurnal Neorganicheskoi Khimii 1970, 15, 778–784. [Google Scholar]
- Goff, G.S.; Cisneros, M.R.; Kluk, C.; Williamson, K.; Scott, B.; Reilly, S.; Runde, W. Synthesis and structural characterization of molecular Dy(III) and Er(III) Tetra-carbonates. Inorg. Chem. 2010, 49, 6558–6564. [Google Scholar] [CrossRef] [PubMed]
- Philippini, V.; Vercouter, T.; Chaussé, A.; Vitorge, P. Precipitation of ALn(CO3)2, xH2O and Dy2(CO3)3, xH2O compounds from aqueous solutions for A+ = Li+, Na+, K+, Cs+, NH4+. J. Solid State Chem. 2008, 181, 2143–2154. [Google Scholar] [CrossRef]
- Vercouter, T.; Vitorge, P.; Trigoulet, N.; Giffcaut, E.; Moulin, C. Eu(CO3)33+ and the limiting carbonate complexes of other M3+ f-elements in aqueous solutions: A solubility and TRLFS study. New J. Chem. 2005, 29, 544–553. [Google Scholar]
- Ben Ali, A.; Maisonneuve, V.; Houlbert, S.; Silly, G.; Buzaré, J.Y.; Leblanc, M. Cation and anion disorder in new cubic rare earth carbonates Na2LiLn(CO3)3 (Ln = Eu-Er, Yb, Lu, Y); Synthesis, crystal structures, IR, Raman and NMR characterizations. Solid State Sci. 2004, 6, 1237–1243. [Google Scholar] [CrossRef]
- De Vasconcellos, M.E.; da Rocha, S.M.R.; Pedreira, W.R.; Queiroz, C.A.D.S.; Abrão, A. Solubility behavior of rare earths with ammonium carbonate and ammonium carbonate plus ammonium hydroxide: Precipitation of their peroxicarbonates. J. Alloys Compd. 2008, 451, 426–428. [Google Scholar] [CrossRef]
- Wickleder, M.S. Inorganic lanthanide compounds with complex anions. Chem. Rev. 2002, 102, 2011–2087. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.N. Hydrothermal preperation of rare earth hydroxy-carbonates. The crystal structure of NdOHCO3. Acta Chem. Scand. 1973, 27, 2973–2982. [Google Scholar] [CrossRef][Green Version]
- Kutlu, I.; Meyer, G. Basic Carbonates of Dysprosium: Dy2O2(CO3) and Dy(OH)(CO3). ZAAC 1999, 2, 402–406. [Google Scholar]
- Doert, T.; Rademacher, O.; Getzschmann, J. Crystal structure of dysprosium hydroxide carbonate, DyOHCO3. Z. Kristallogr. 1999, 214, 11–12. [Google Scholar] [CrossRef]
- Beall, G.; Milligan, W.; Mroczkowski, S. Yttrium carbonate hydroxide. Acta Crystallogr. Sect. B 1976, 8, 3143–3144. [Google Scholar] [CrossRef]
- Sungur, A.; Kizilyalli, M. Synthesis and Structure of Gd2(CO3)3·nH2O (n = 2, 3). J. Less Common Met. 1983, 93, 419–423. [Google Scholar] [CrossRef]
- Miyawaki, R.; Kuriyama, J.; Nakai, I. The redefinition of tengerite-(Y), Y2(CO3)3·2-3H2O, and its crystal structure. Am. Mineral. 1993, 78, 425–432. [Google Scholar]
- Liu, S.; Ma, R. Synthesis of lutetium carbonate. Acta Chem. Scand. 1997, 51, 893–895. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R. Synthesis of a Crystalline Hydrated Basic Ytterbium Carbonate, Yb2O3·2.17CO2·6.17H2O. Synth. React. Inorg. Met. Chem. 1997, 27, 1183–1190. [Google Scholar]
- Liu, S.; Ma, R.; Jiang, R.; Luo, F. Synthesis and Structure of Hydrated Yttrium Carbonate, Y2(CO3)3·2.79H2O. Synth. React. Inorg. Met. Chem. 2000, 30, 271–279. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R. Synthesis and characterization of hydrated holmium and erbium carbonates. Asian J. Chem. 2007, 19, 1883–1887. [Google Scholar]
- Liu, S.; Ma, R.; Jiang, R.; Luo, F. Synthesis and structure of hydrated neodymium carbonate. J. Cryst. Growth 1999, 203, 454–457. [Google Scholar] [CrossRef]
- Song, L.; Rongjun, M. Synthesis and structure of hydrated terbium carbonate. Indian J. Chem. Sect. A 1996, 35, 992–994. [Google Scholar]
- Song, L.; Rongjun, M. Synthesis and structure of hydrated europium carbonate. J. Cryst. Growth 1996, 169, 190–192. [Google Scholar]
- Caro, P.E.; Sawyer, J.O.; Eyring, L. The infrared spectra of rare earth carbonates. Spectrochim. Acta Part A Mol. Spectrosc. 1972, 28, 1167–1173. [Google Scholar] [CrossRef]
- Head, E.L. The preparation of scandium carbonate. J. Inorg. Nucl. Chem. 1971, 33, 1201–1203. [Google Scholar] [CrossRef]
- Crookes, W. II. On Scandium. Philos. Trans. R. Soc. Lond. Ser. A 1908, 209, 15–48. [Google Scholar] [CrossRef][Green Version]
- Wakita, H.; Nagashima, K. Synthesis of Tengerite Type Rare Earth Carbonates. Bull. Chem. Soc. Jpn. 1972, 45, 2476–2479. [Google Scholar] [CrossRef]
- Tahara, T.; Nakai, I.; Miyawaki, R.; Matsubara, S. Crystal chemistry of RE(CO3)OH. Z. Kristallogr. 2007, 222, 326–334. [Google Scholar] [CrossRef]
- Dal Negro, A.; Rossi, G.; Tazzoli, V. The Crystal Structure of Ancylite, (RE)x(Ca,Sr)2-x(CO3)2(OH)x(2-x)H2O. Am. Mineral. 2015, 60, 280–284. [Google Scholar]
- Dexpert, H.; Caro, P. Determination de la structure cristalline de la variete a des hydroxycarbonates de terres rares LnOHCO3 (Ln = Na). Mater. Res. Bull. 1974, 9, 1577–1585. [Google Scholar] [CrossRef]
- Rorif, F.; Fuger, J.; Desreux, J.F. Thermochemistry of selected trivalent lanthanide and americium compounds: Orthorhombic and hexagonal hydroxycarbonates. Radiochim. Acta 2005, 93, 103–110. [Google Scholar] [CrossRef]
- Batkibekova, M.; Usubaliev, D. Thermal chemistry of rare earth carbonates. Zhurnal Fizicheskoi Khimii 1974, 48, 1615. [Google Scholar]
- Karapet’yants, M.K.; Maier, A.I.; Bas’kova, N. Gibbs Standard Energies of Formation of Rare Earth Element Yttrium Carbonates and their Entropies. Neorg. Mater. 1977, 13, 1055–1058. [Google Scholar]
- Gamsjäger, H.; Marhold, H.; Königsberger, E.; Tsai, Y.J.; Kolmer, H. Solid-Solute Phase Equilibria in Aqueous Solutions IX. Thermodynamic Analysis of Solubility Measurements: La(OH)m(CO3)q·rH2O. Z. Naturforsch. A 1995, 50, 59–64. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Königsberger, E.; Marhold, H.; Gamsjäger, H. Solid-Solute Phase Equilibria in Aqueous Solutions, VIII: The Standard Gibbs Energy of La2(CO3)3·8H2O. Monatshefte Chem. 1993, 124, 1011–1018. [Google Scholar] [CrossRef]
- Kaгapet’yants, М.K.; Maier, А.I.; Bas’kova, N. Standard heats of formation of rare earth element and yttrium carbonates. Neorg. Mater. 1977, 13, 1279–1281. [Google Scholar]
- Wilfong, R.L.; Domingues, L.P.; Furlong, L.R. Thermogravimetric Analysis of Five Salts of Praseodymium, Neodymium, and Samarium. J. Am. Ceram. Soc. 1964, 47, 240–241. [Google Scholar] [CrossRef]
- Domingues, L.P.; Wilfong, R.L.; Furlong, L.R. Pyrolysis of Five Salts of Yttrium, Lanthanum, and Cerium; Bureau of Mines College Park Metallurgy Research Center: College Park, MD, USA, 1962. [Google Scholar]
- Patil, K.C.; Chandrashekhar, G.V.; George, M.V.; Rao, C.N.R. Infrared spectra and thermal decompositions of metal acetates and dicarboxylates. Can. J. Chem. 1968, 46, 257–265. [Google Scholar] [CrossRef]
- Watanabe, Y.; Miyazaki, S.; Maruyama, T.; Saito, Y. Dissociation pressure of lanthanum dioxide carbonate. J. Mater. Sci. Lett. 1986, 5, 135–136. [Google Scholar] [CrossRef]
- Olafsen, A.; Fjellvag, H. Synthesis of rare earth oxide carbonates and thermal stability of Nd2O2CO3 II. J. Mater. Chem. 1999, 9, 2697–2702. [Google Scholar] [CrossRef]
- Wendlandt, W.; George, T.D. The thermal decomposition of inorganic compounds. IV. Rare earth carbonates. Tex. J. Sci. 1961, 13, 316–328. [Google Scholar]
- Foger, K.; Hoang, M.; Turney, T.W. Formation and thermal decomposition of rare-earth carbonates. J. Mater. Sci. 1992, 27, 77–82. [Google Scholar] [CrossRef]
- D’Assunção, L.M.; Ionashiro, M.; Rasera, D.E.; Giolito, I. Thermal decomposition of the hydrated basic carbonates of lanthanides and yttrium in CO2 atmosphere. Thermochim. Acta 1993, 219, 225–233. [Google Scholar] [CrossRef]
- Jordanov, N.; Havezov, I. Löslichkeitsprodukte der normalen Carbonate einiger dreiwertiger Seltener Erden (Er2(CO3)3·nH2O). Z. Anorgan. Allg. Chem. 1966, 347, 101–106. [Google Scholar] [CrossRef]
- Spahiu, K.; Bruno, J. A Selected Thermodynamic Database for REE to Be Used in HLNW Performance Assessment Exercises; SKB: Orange City, CA, USA, 1995. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).