Abstract
Conflict minerals are those mined in politically unstable regions of the world and are then sold to finance war or other illegal activities. Industrial manufacturers are required to show that minerals used in their applications are not derived from conflict areas. Several geochemical and geochronological methods have been suggested to fingerprint conflict minerals; however, all these methods require sophisticated and extensive laboratory procedures. Portable X-ray fluorescence data of 108 samples from various location in Democratic Republic of the Congo shows that cassiterite and wolframite ores from all studied regions can be fingerprinted using various discrimination diagrams. Coltan ore samples from several regions can also be discriminated using major and trace elements of these samples. In addition, patterns in chondrite-normalized spider diagrams for each region are unique and can be used as fingerprinting tools.
1. Introduction
Conflict minerals have always been an attractive topic, not only from a scientific point of view but also from political, international, and industrial views; these minerals can be used to fund war or illegal activities. Importantly, the end-user industries need to document the source of these minerals. In the 1990′s, the popular saleable item were so-called “blood diamonds,” with monies financing wars in some regions in Africa. Diamonds were easy to carry and smuggle, but this trade has been severely curtailed with the introduction of United Nations Security Council resolution 1173 that prohibited diamond sales from conflict regions (e.g., Angola) without a certificate of origin. Other illegally-traded materials have now become popular, such as cassiterite, wolframite, and coltan (tin, tungsten and niobium/tantalum ores) due to the widespread use of these metals in consumer electronics, medical devices, and military applications. Much of the illicit mining of these materials occurs in the eastern portion of the Democratic Republic of Congo (DCR).
With the advent of U.S. Securities and Exchange Commission (SEC) Conflict Minerals Law (sometimes called the 3TG rule), manufacturers have to submit a Conflict Minerals report every year for those conflict minerals used in their manufacturing process that document the supply chain trail. There has been a push back onto primary smelters to confirm that the ores they are using have come from a reputable source and are not part of the conflict mineral trade. This is done both through Certified Trading Chains (CTC) as well as determining an analytical fingerprint that provides a distinctive geochemical, geochronological [1], and mineralogical signature of individual production sites using multiple laboratory methods. All these methods require sophisticated and extensive sample preparation and laboratory analytical procedures. For example, the German Federal Agency for Geosciences and Natural Resources (BGR) developed a comprehensive method that requires a complete analysis of sample by several techniques including whole rock X-ray florescence (XRF), X-ray diffractometry (XRD), inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma optical emission spectrometry (ICP-OES), electron microprobe, U-Pb dating, and laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) [1].
Field-portable X-ray florescence (FPXRF), as handheld or benchtop portable analyzers, is a technique that has been used in various fields from geology/mineralogy to mining (green-field exploration to exploitation and ore grade control), environmental science, metallurgy, geo-archeology, and even oil/gas exploration and production [2,3,4,5,6,7,8,9,10,11]. Expansion of application of these instruments in many fields is mainly due to speed (fast, real time analysis), high sample density (high number of analyses), relatively inexpensive analyses, and ease of use in the field or remote labs. They offer a low-cost solution to rapidly determine the chemistry of materials, which could be used to determine the provenance of any given ore with region-specific element markers, or combinations of elements (“fingerprints”). This paper examines the use of HHXRF in delineating geochemical features of columbite, tantalite, cassiterite and wolframite ores from various regions of DRC.
Deposit Types
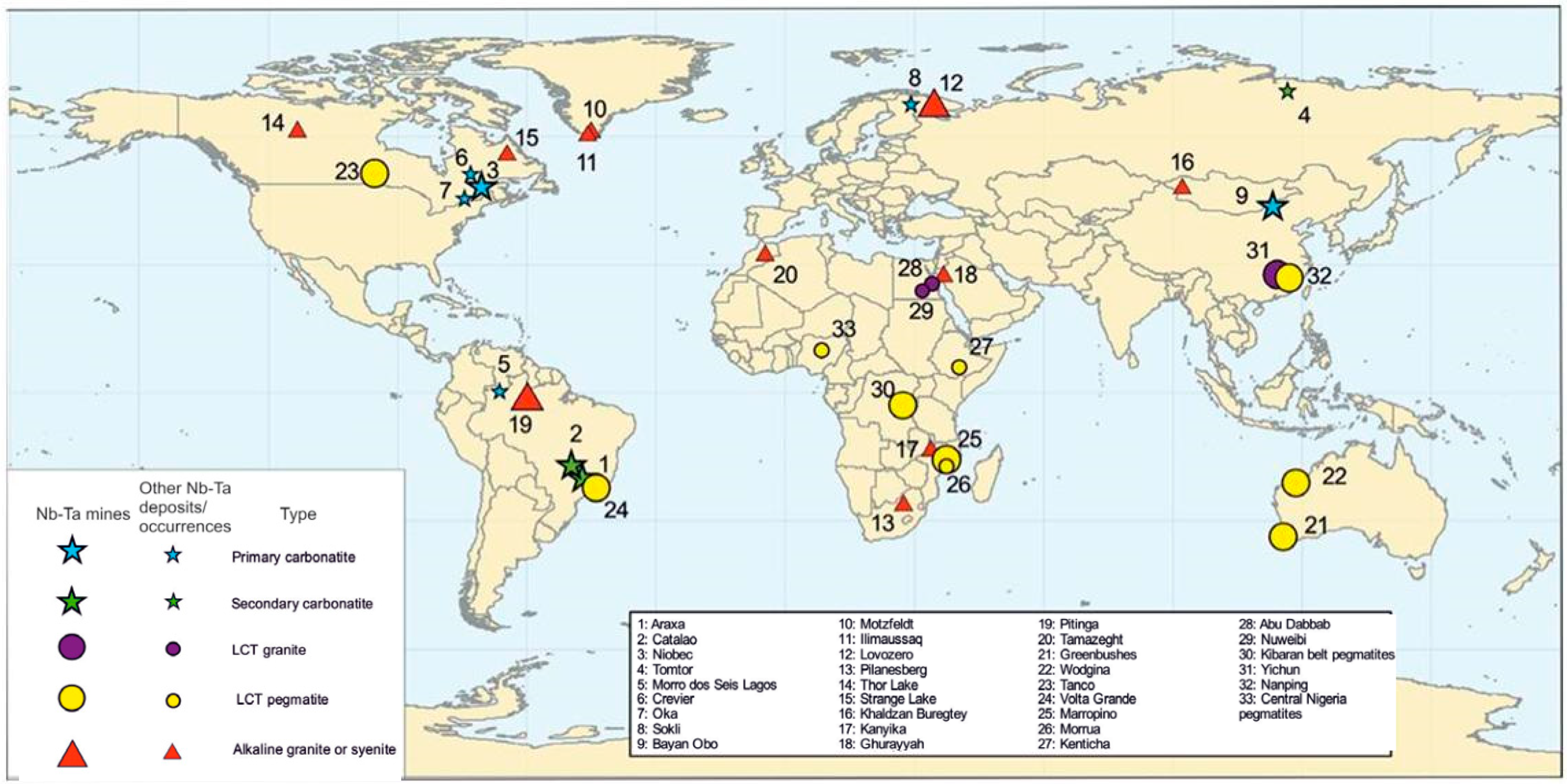
Niobium, Ta, Sn, and W are high field strength elements (HFSE) that are generally incompatible in silicate phases during magmatic differentiation, and as a result, are enriched in late-stage magmas where they have the potential to create economic ore deposits. Tantalum and Nb are commonly associated with three types of igneous rocks (Figure 1, Table 1) [12,13]: (1) carbonatites and associated rocks; (2) alkaline to peralkaline granites and syenites; and (3) granites and pegmatites enriched in Li, Cs and Ta (LCT granite family) [2]. In addition, placer deposits of these metals can form due to weathering, transportation, and deposition in a sedimentary environment. Generally, these secondary deposits occur relatively close to their primary source.
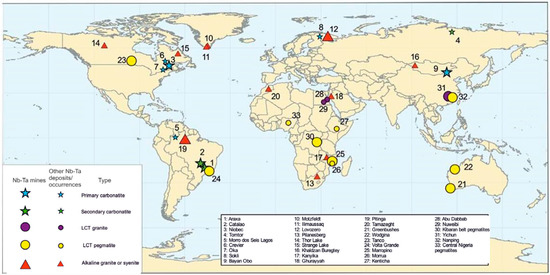
Figure 1.
Distribution of various types of Nb-Ta deposits worldwide [13].

Table 1.
Common types of Ta-Nb deposits with a brief description, grade, tonnage, and examples [13].
There are two main types of primary deposits in DRC:
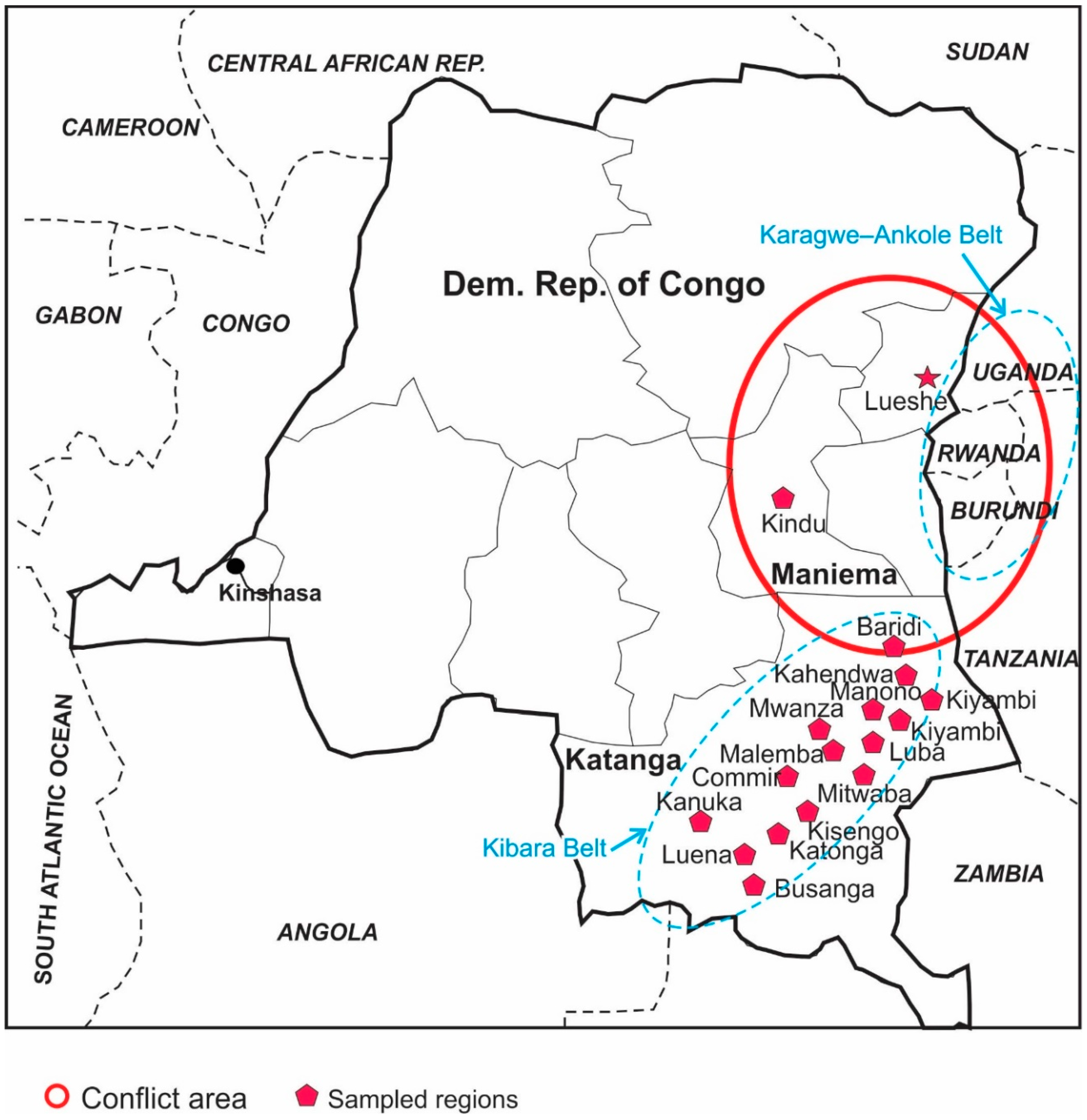
- Carbonatite-sourced secondary deposits: A good example of this type deposit is Lueshe Nb deposit, in the northeast DRC (Figure 2). This deposit is related to a Cambrian syenite-carbonatite intrusion with a core of syenite (~800 m in diameter), which is rimmed by a ring dyke of calcite carbonatite and dolomite carbonatite. This complex has experienced an intense weathering and supergene alteration, which resulted in the accumulation of weather-resistant pyrochlore (Na,Ca)2Nb2O6(OH,F) in the lateritic profile. This accumulation is accompanied by chemical transformations without any change in the mineralogical state [14].
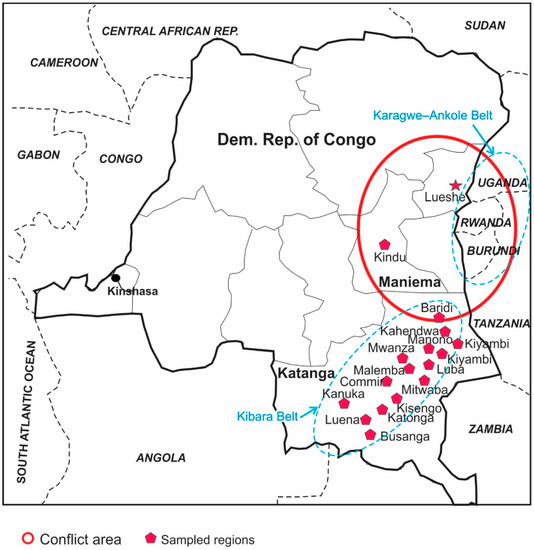 Figure 2. Location of regions in two provinces (Maniema, Katanga) that were sampled for this study. Lueshe Nb deposit in NE side, as well as Kibara and Karagwe-Ankole metalogenic belts (oval dash lines), are also shown.
Figure 2. Location of regions in two provinces (Maniema, Katanga) that were sampled for this study. Lueshe Nb deposit in NE side, as well as Kibara and Karagwe-Ankole metalogenic belts (oval dash lines), are also shown. - Pegmatites and quartz veins: Granite-related Ta-Nb-Sn-W mineralization is associated with pegmatites and quartz veins in the Kibara (the Katanga region of the DRC) and Karagwe–Ankole (part of eastern DRC, Burundi, Rwanda, Uganda) belts (Figure 2) [15,16,17,18]. Four generations of granites (G1 to G4) have been identified [17]: G1-3 intruded at 1380 ±10 Ma in the Palaeo- and Meso-proterozoic rocks of the Kibara belt followed by intrusion of the youngest granite, G4, at ~986 Ma, which was intruded by pegmatite at ~960 Ma. Some of these pegmatites have coltan mineralization. Quartz-tin mineralization occurred at ~940 Ma and its associated hydrothermal activity altered pegmatites intensely [17]. Columbite, tantalite and local cassiterite and wolframite are mined both from deeply weathered pegmatites and from secondary placer deposits derived from the pegmatites.
The secondary deposits formed from these two types of mineralization should have distinct geochemical fingerprints as ore mineralogy in these deposits varies significantly. Carbonatite-sourced secondary deposits can be recognized by high Nb and possibly high REE concentrations and low Ta, Sn and W concentrations, whereas pegmatite-related placer deposits would have lower REE and higher Ta, Sn and W.
2. Materials and Methods
A series of 99 fine-grained and 8 coarse-grained placer ore specimens were sampled from 15 sites in the DRC using panning and sieving methods, including 10 wolframite ore (from Katonga, Luena and Manono regions), 49 coltan ore (from Baridi, Kahendwa, Kanuka, Katonga, Kisengo, Kiyambi, Luba, Luena, Malemba, Manono, and Mwanza regions), and 48 tin samples (46 samples from Busanga, Commir, Kanuka, Luena, Malemba, Manono, Mitwaba, and Mwanza deposits and 2 samples from the Kindu region in the Maniema Province) (Table 2).

Table 2.
Samples used in the study. Locations are shown in Figure 2.
Each sample is not pure wolframite, columbite-tantalite or cassiterite minerals, rather, they may contain several minerals in each ore type (e.g., various Ta-Nb minerals in the coltan ore). The collected samples represent the true variations in mineralogy across different parts of the same deposit. These samples represent true ore that is traded in the mineral market of DRC.
Sub-samples were collected via handpicking using optical binoculars. All samples were pulverized to smaller than 200 mesh and packed into standard 32 mm cups fitted with 4 µm polypropylene film. At time of cupping, each sample was measured with the Thermo RadEye to determine radioactivity of material as some of the samples contain U and Th.
A series of certified reference materials, as well as the sampled specimens, were analyzed using a handheld ThermoFisher Niton XL3t-GOLDD (Figure 3) using Mining Mode so that spectra could be obtained in all 4 filters: Main, Low, High, Light (30 s analysis time in each filter). This mode uses fundamental parameters and relies on the detector’s response to pure element spectra. The factory calibration uses these spectra to identify the elements present in each sample and to calculate peak intensities from spectrum and showing results after applying corrections. The built-in TaHf calibration program was determined to be the most suitable method as it provides simultaneous analysis of suite of elements (Table 3), including the vital elements for discrimination purposes. This is a factory built-in special mode designed for coltan and associated minerals; in this mode, the performance of the analyzer is enhanced by eliminating elements with X-ray energies close to Nb and Ta. The energy values (in kilo electron volt, Kev) for Kα, Kβ, Lα, and Lβ lines are respectively 16.61, 18.62, 2.17, 2.26 for Nb and 57.52, 65.21, 8.15, 9.34 for Ta.

Figure 3.
Analysis of coltan ore samples by HHXRF showing a real time qualitative test by the user.

Table 3.
List of elements provided by the handheld analyzer. Limits of detection (LOD in ppm), as well as certified standards used for the calibration of the handheld analyzer, are included.
Factory calibration can produce reliable data for some elements (particularly base metals) in some rock/soil matrices; however, the analytical accuracy and precision can be low mainly due to peak overlap or matrix difference between the analyzed unknown sample and factory calibration samples. User calibration (i.e., calibration made by user) utilizing known samples (e.g., certified standards) was employed to verify FPXRF data quality. In order to increase the accuracy of the analytical data, the analyzer was calibrated for all the required elements and the analytical performance was checked against 35 certified reference materials (Table 3). All the specimens were analyzed twice, and a third analysis was carried out in cases where significant discrepancies existed between the previous analyses. Limits of detection for the elements of interest ranges from 3 ppm for trace elements to 3500 ppm for major elements (Table 3). Elements with potential spectral overlap (such as REE) or with values close to detection limit (1.5 times the detection limit) were not used during this study. Portable XRF is mostly a partial analysis technique that provides assay results for selected elements of interest. As a result, some elements may be missing and therefore the total of analyzed elements may not be 100%.
3. Results
The tin ore samples from the eight different regions in the Katanga Province, DRC, range from 40 to 63% Sn, with 157 to 10,415 ppm Nb, <3 to 17,657 ppm Ta, and <3 to 19,320 ppm W (Table 4). Most of these samples are radioactive with up to 89 ppm U and 436 ppm Th. Visual examination of cassiterite ore specimens indicates that some regions have darker color samples. This may be partially related to their high Fe and Ti content [19]. The wolframite ore samples contain 36 to 51% W, 674 to 113,783 ppm Sn, 1249 to 2819 ppm Nb, and <3 ppm Ta (Table 5). Up to 1362 ppm Th was found in one sample from Katonga. In the coltan samples, Ta and Nb concentrations range from 9.5 to 30% and 2.6 to 36%, respectively (Table 6). Tungsten was detected only in 3 samples: from Katonga (47,250 ppm), Luena (83,688 ppm), and Malemba (3029 ppm). All samples contain Sn from 0.2 to 24%. Most samples are radioactive with Th and U ranging from 515 to 6427 ppm and 108 to 1368 ppm, respectively. Samples from Luba and Katonga do not contain Th.

Table 4.
Chemical analysis of cassiterite ore samples (in ppm). Nd, La, Ba were not detected. _Not detected.

Table 5.
Chemical analysis of wolframite ore samples (in ppm). Ta was not detected. —Not detected.

Table 6.
Chemical analysis of coltan ore samples (in ppm). _Not detected.
4. Discussion
Conflict minerals and their derivative metals (Ta, Na, Sn, W) are an integral part of many electronics and technology industries. According to many national and international laws, these industries are committed to responsible sourcing of these minerals to safeguard human rights and to use only conflict-free minerals, i.e., minerals that do not directly or indirectly benefit or finance armed groups in DRC or adjoining countries. As a result, discrimination of conflict minerals from conflict-free ones is an important task from industrial and social perspectives. Several analytical and laboratory methods have been proposed and tested to identify provenance of these minerals [1,20,21,22].
Geochemical composition of the analyzed samples (Table 4, Table 5 and Table 6) shows that some regions have specific elemental characteristics that reflect their unique mineralogy. Combination of these characteristics as single elements, ratios, or composite factors, can be used to discriminate various regions of ore types.
4.1. Fingerprinting Cassiterite Ore
The following discrimination diagrams are used to differentiate cassiterite ore from various regions.
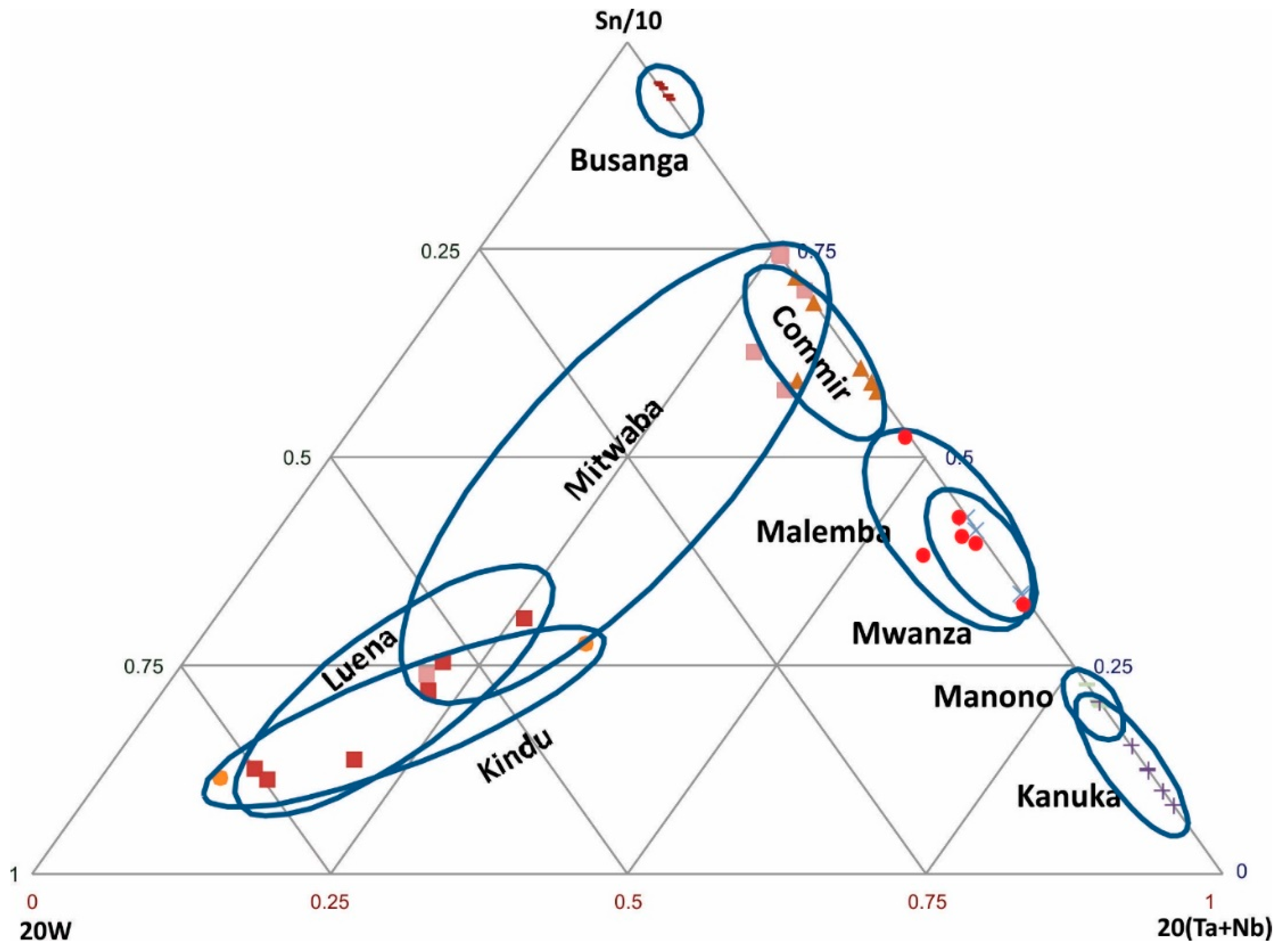
Sn-W-(Ta + Nb) ternary diagram: In many deposits, particularly pegmatite deposits, Sn may be associated with W, Nb, and Ta. On this basis, these elements are used to discriminate some of the deposits. In order to spread out data from various regions, the Sn-W-(Ta + Nb) ternary diagram is constructed using these factors: Sn/10, 20 W, and 20 (Nb + Ta) (Figure 4). Based on this diagram, the regions within the DRC are classified as follows:
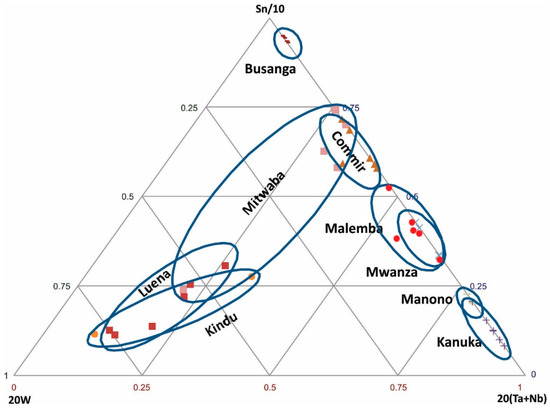
Figure 4.
Discrimination of cassiterite samples from various regions based on Sn-W-(Ta + Nb).
- (1)
- Sn-rich region: Busanga
- (2)
- Ta + Nb-rich regions: Manono, Kanuka
- (3)
- W-rich regions: Luena, Kindu
- (4)
- Sn and Ta + Nb-rich regions: Commir, Malemba, Mwanza
Cassiterite ore samples from the Mitwaba region show a wide range of W and Ta + Nb.
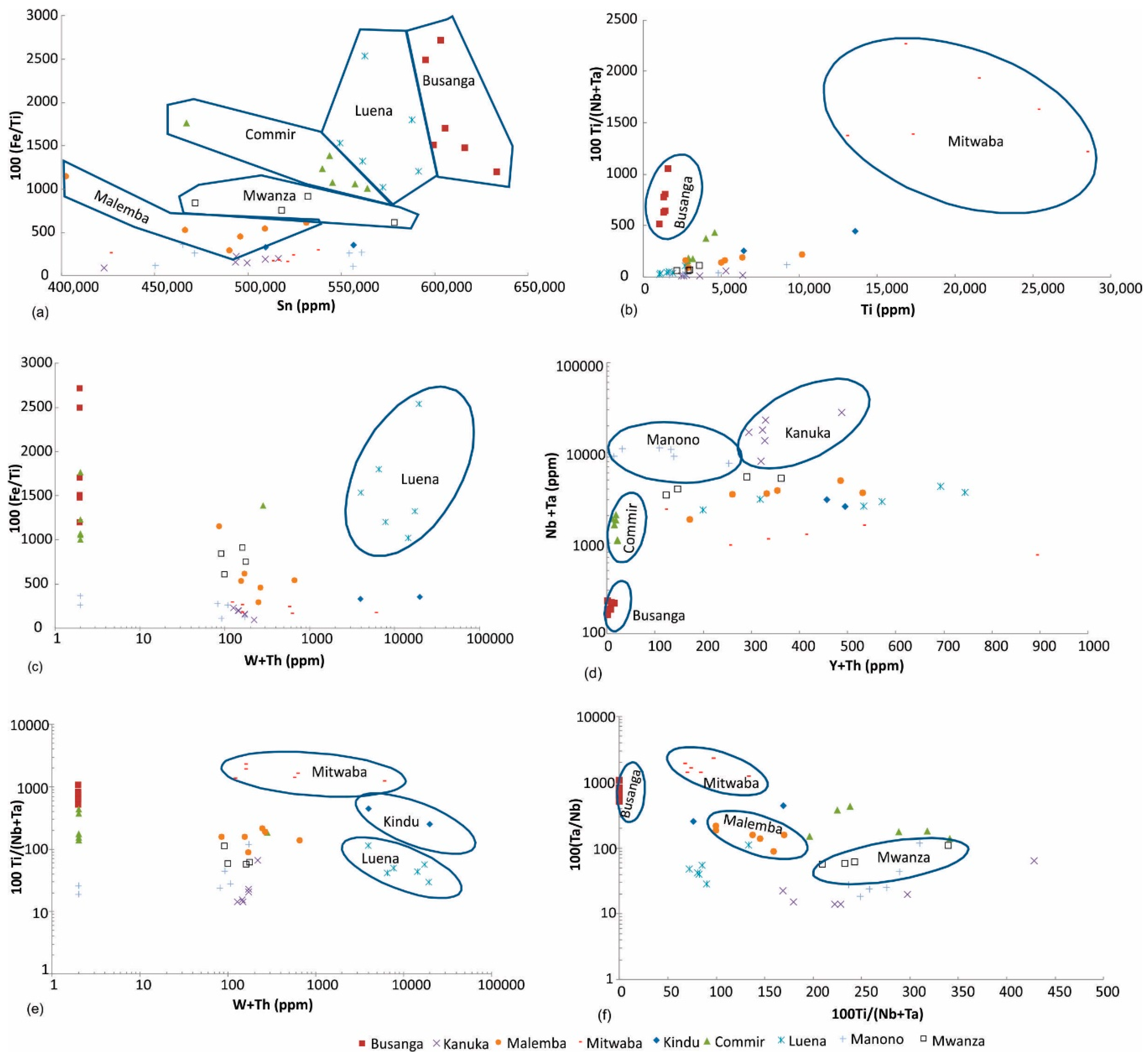
Sn vs. (Fe/Ti) diagram: Cassiterite ore from several regions can be discriminated using Sn vs. 100 (Fe/Ti) diagram (Figure 5a). Malemba, Mwanza, Commir, Luena, and Busanga cassiterite ore samples plot in discrete parts of this diagram, whereas rest of the regions (Kindu, Mitwaba, Kanuka, Manono) do not show considerable variation in Fe/Ti ratio by increasing Sn content.
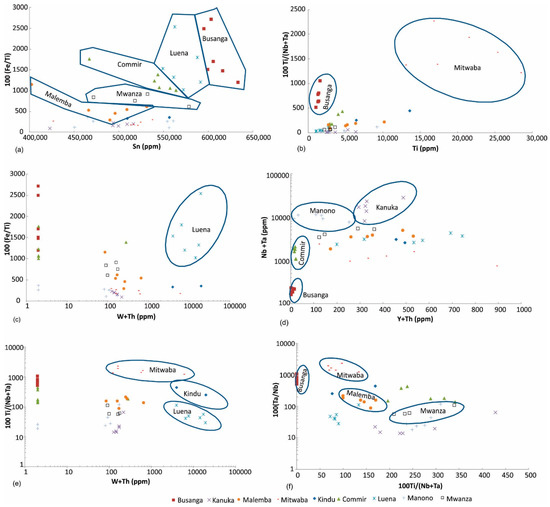
Figure 5.
Discrimination of (a) Malemba, Mwanza, Commir, Luena, and Busanga, (b) Mitwaba and Busanga, (c) Luena, (d) Busanga, Commir, Manono, and Kanuka, (e) Mitwaba, Kindu, and Luena, (f) Busanga, Mitwaba, Malemba, and Mwanza cassiterite ore samples.
Ti vs. (Ti/Nb + Ta) diagram: This diagram discriminates Mitwaba and Busanga tin ore from other regions (Figure 5b).
W + Th vs. Fe/Ti diagram: This diagram discriminates Luena cassiterite ore samples (Figure 5c).
Y + Th vs. Nb + Ta diagram: This diagram distinguishes Busanga, Commir, Manono and Kanuka cassiterite ore samples (Figure 5d).
W + Th vs. Ti/(Ta + Nb) diagram: This diagram differentiates Mitwaba, Kindu and Luena cassiterite ore samples (Figure 5e).
Ta/Nb vs. Ti/(Ta + Nb) diagram: This diagram discriminates Busanga, Mitwaba, Malemba and Mwanza tin ore samples (Figure 5f).
4.2. Fingerprinting Wolframite Ore
The following discrimination diagrams are constructed to fingerprint these wolframite ore samples.
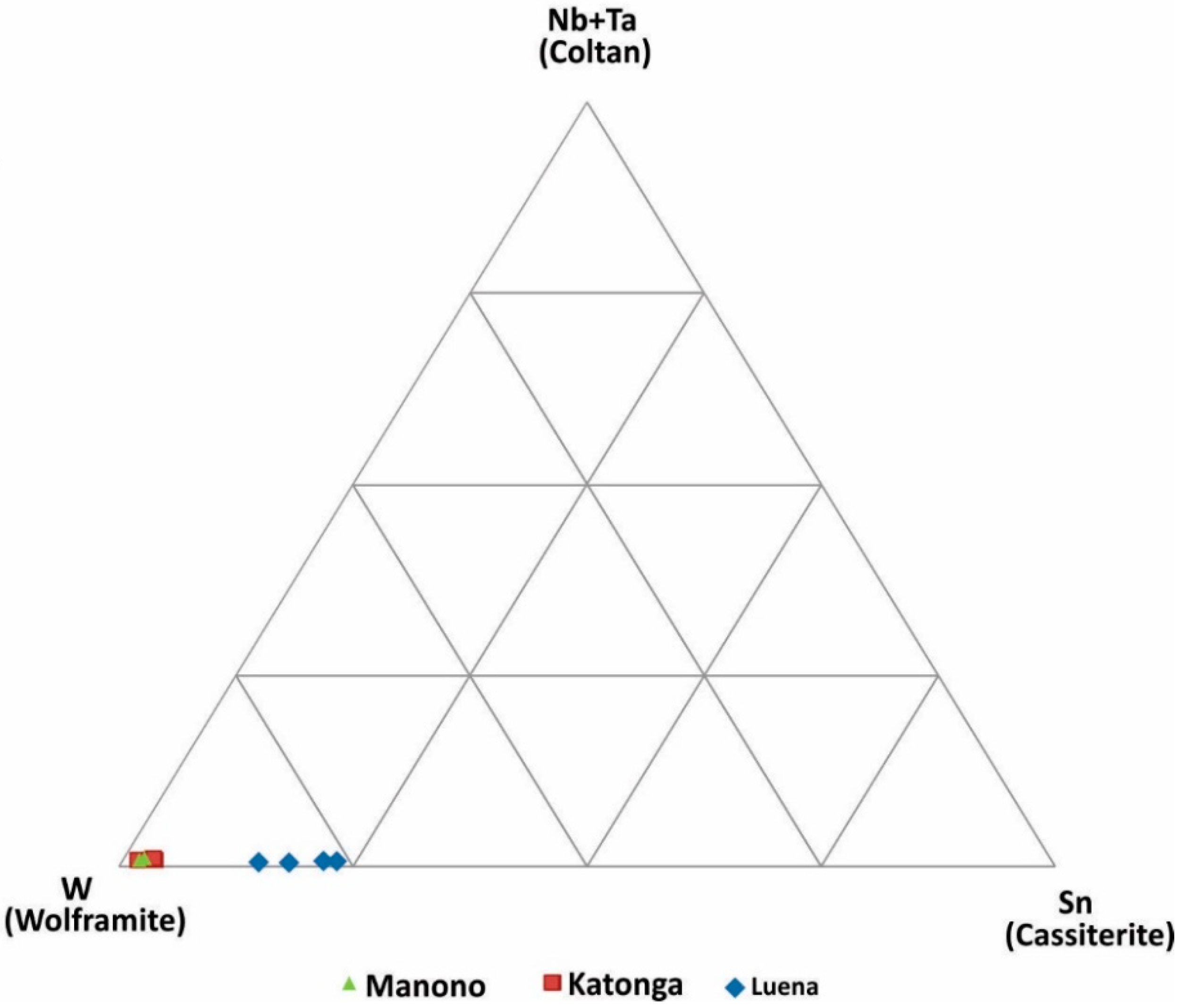
W-Sn-(Ta + Nb) ternary diagram: Tungsten may occur with Sn, Nb, and Ta in pegmatite deposits. In addition, W may occur in quartz veins and mix with Nb-Ta or Sn during weathering of primary deposits (i.e., placer formation processes). The W-Sn-(Ta + Nb) discrimination diagram is used to evaluate this possible association (Figure 6). Wolframite ore samples from all three regions have very low Ta + Nb. Samples from Katonga and Manono are similar and have low Sn concentrations, whereas Luena samples have the highest Sn concentration amongst the analyzed samples.
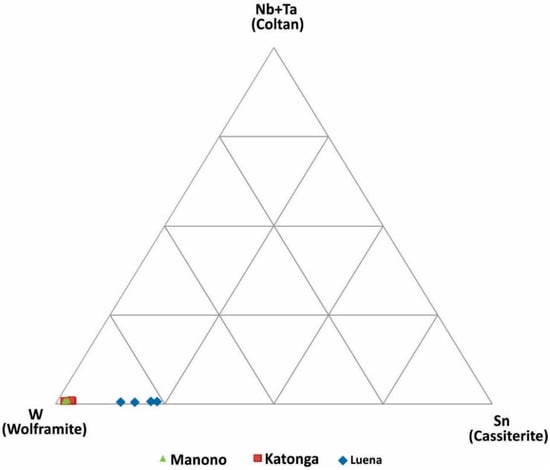
Figure 6.
Discrimination of wolframite ore samples based on W, Sn and Nb+Ta concentrations.
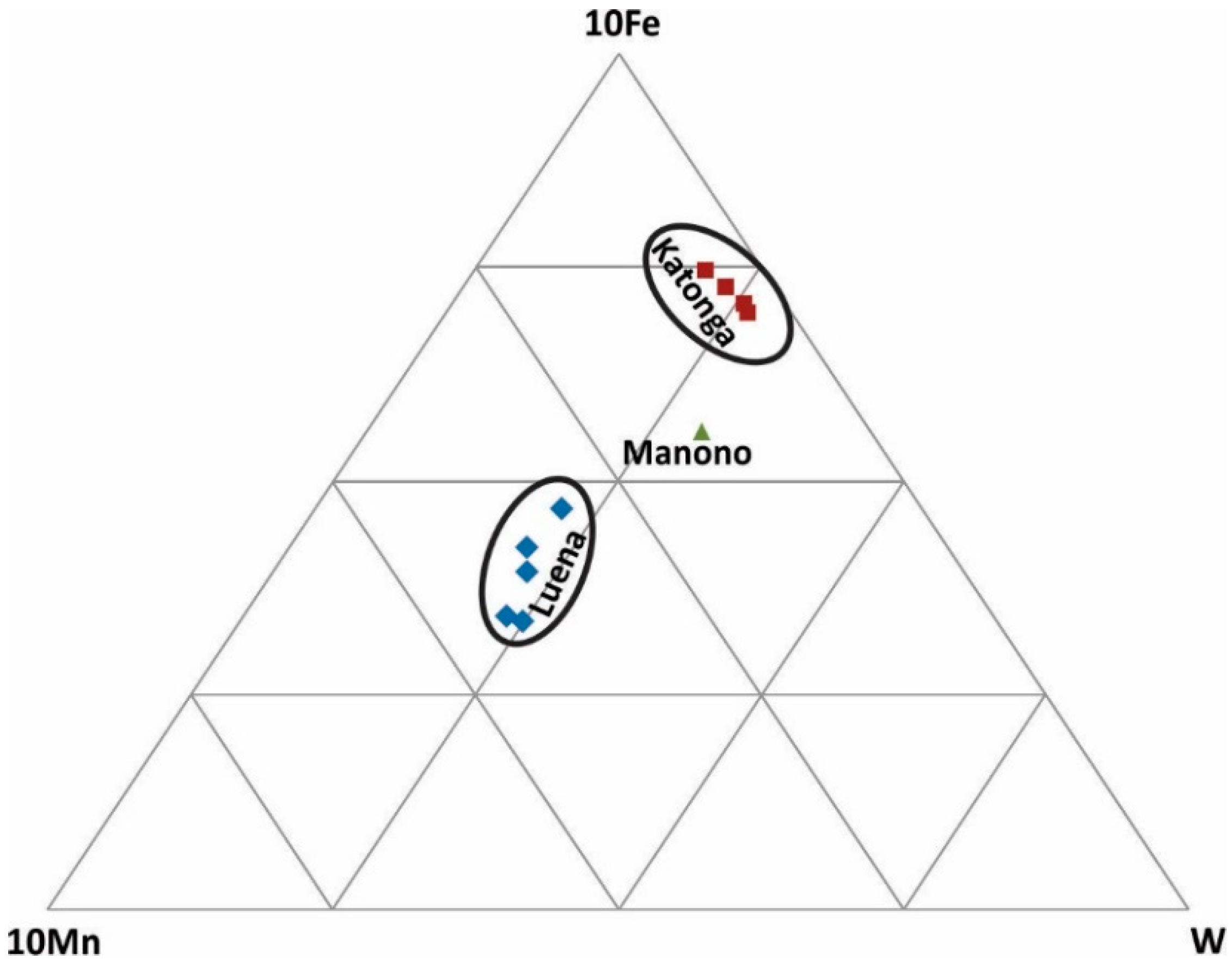
W-Fe-Mn ternary diagram: This diagram (Figure 7) is used to discriminate various wolframite ore samples because natural wolframite grains are composed of three main metals: Fe, Mn, and W. Katonga and Luena wolframite ore samples are characterized by high Fe and high Mn, respectively. Only one sample from Manono was analyzed and it may not be a representative of this region.
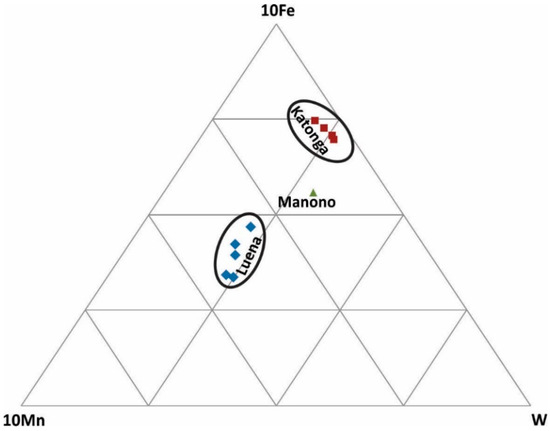
Figure 7.
Discrimination of wolframite ore samples based on Fe, Mn, and W concentrations.
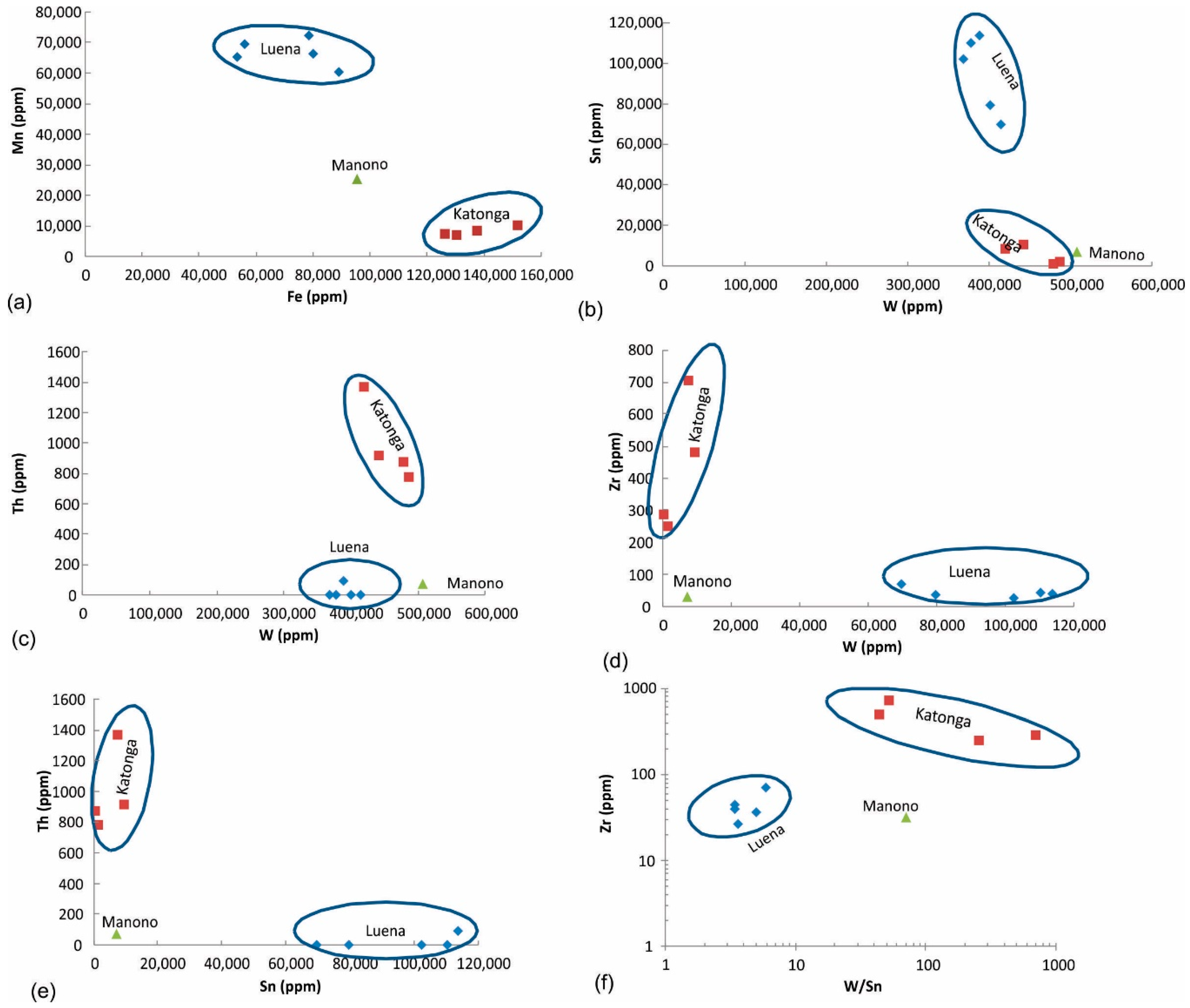
Fe vs. Mn diagram: Based on the above-mentioned criteria, Fe-Mn diagram can clearly discriminate wolframite ore from these three regions (Figure 8a).
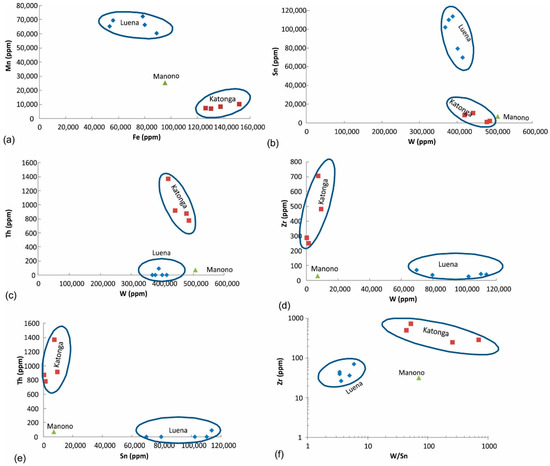
Figure 8.
Discrimination of wolframite ore samples based on (a) Fe and Mn, (b–f) combination of W, Sn, Th, and Zr concentrations.
Other diagrams: Other trace elements that can occur in the late-stage pegmatite deposits and may show variation in the analyzed wolframite ore samples include Sn, Th, and Zr. Combinations of different discrimination diagrams (Figure 8b–f) can be used to successfully differentiate wolframite ore from these three regions.
4.3. Fingerprinting Coltan Ore
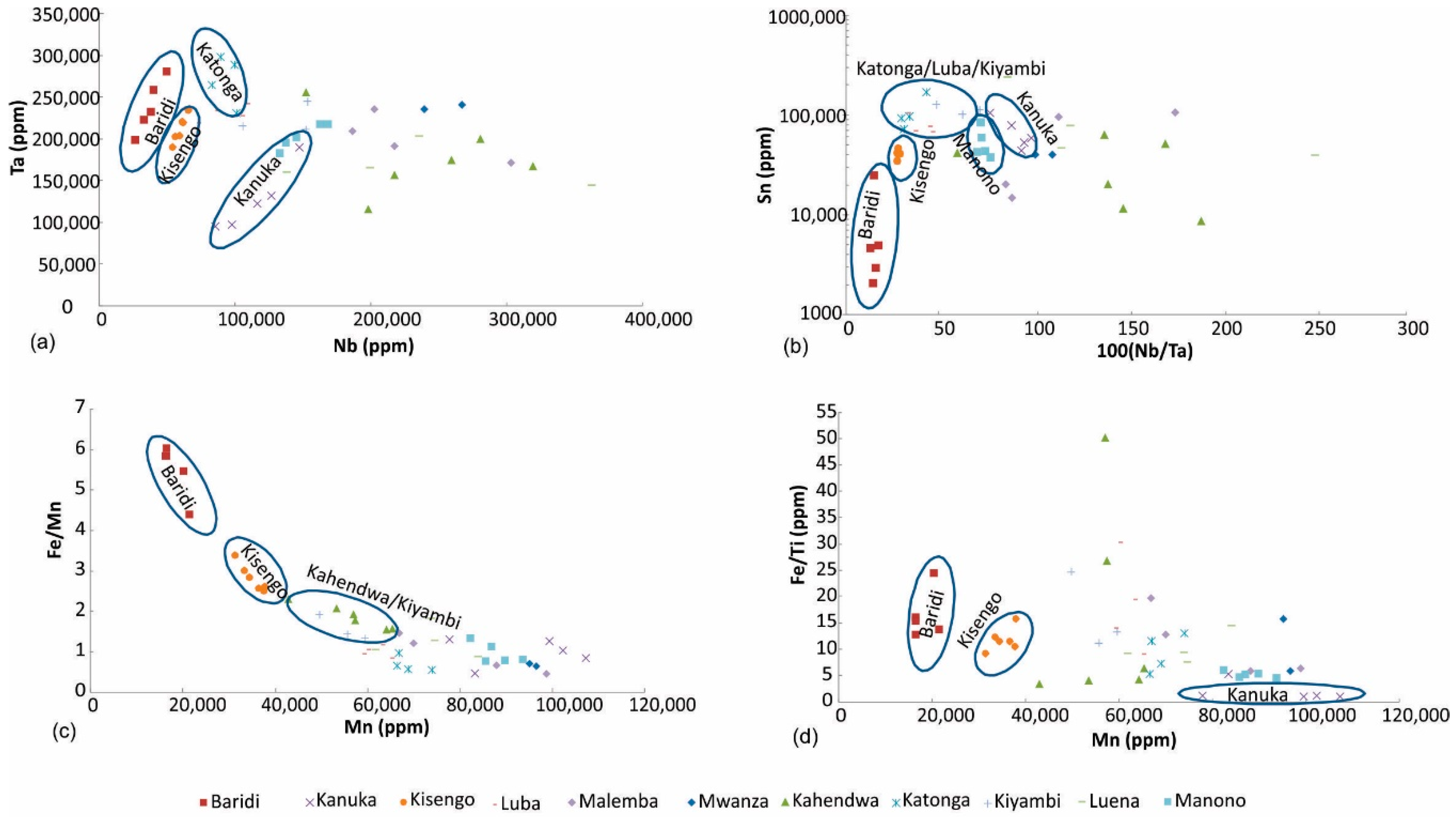
Nb vs. Ta diagram: Nb and Ta are the main economic metals of coltan ore, and the concentrations of these elements can be used to discriminate the sampled regions (Figure 9a). This diagram can differentiate Baridi and Kisengo coltan ore samples, which have the lowest Nb concentrations. Most of Kanuka samples plot on the low Nb-low Ta part of the diagram. Katonga samples are characterized by ~10% Nb and >23% Ta.
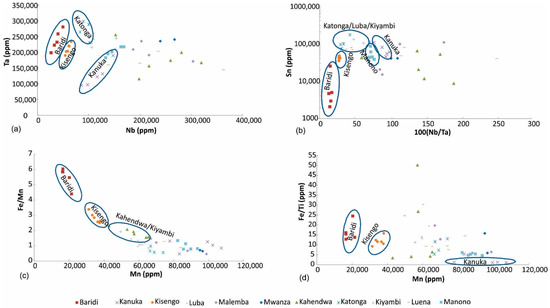
Figure 9.
Discrimination of coltan ore samples based on (a) Nb and Ta, (b) 100 (Nb/Ta) vs. Sn, (c) Mn vs. Fe/Mn, (d) Mn vs. Fe/Ti.
100 (Nb/Ta) vs. Sn diagram: Tin is commonly associated with coltan and can be used as a discriminator (Figure 9b). Coltan ore samples from Baridi, Kisengo, Manono, and Kanuka plot in discrete areas in this diagram. Most samples from Kahendwa have high Nb/Ta ratio. Katonga, Luba and Kiyambi samples are characterized by high Sn and 100 Nb/Ta of ~50. Samples from other regions show overlap.
Mn vs. Fe/Mn diagram: Iron and Mn are two major elements of coltan minerals; however, their concentrations and relative abundances may vary significantly depending on the geology of the area. The Mn vs. Fe/Mn diagram (Figure 9c) can be used to fingerprint coltan ore samples from Baridi, Kisengo and Kahendwa/Kiyambi regions. Samples from Kahendwa and Kiyambi plot in the same area in this diagram. Coltan from the remaining regions have low Fe/Mn ratios, which cannot be used to discriminate them.
Mn vs. Fe/Ti diagram: Titanium may substitute for Nb and Ta in the structure of coltan minerals. In addition, Ti can accompany these minerals as its own discrete minerals such as rutile (TiO2). The Mn vs. Fe/Ti diagram (Figure 9d) can be used to fingerprint coltan ore samples from Baridi, Kisengo and Kanuka regions. One Kanuka sample plots with Manono samples, and it is likely that it has been mis-labeled.
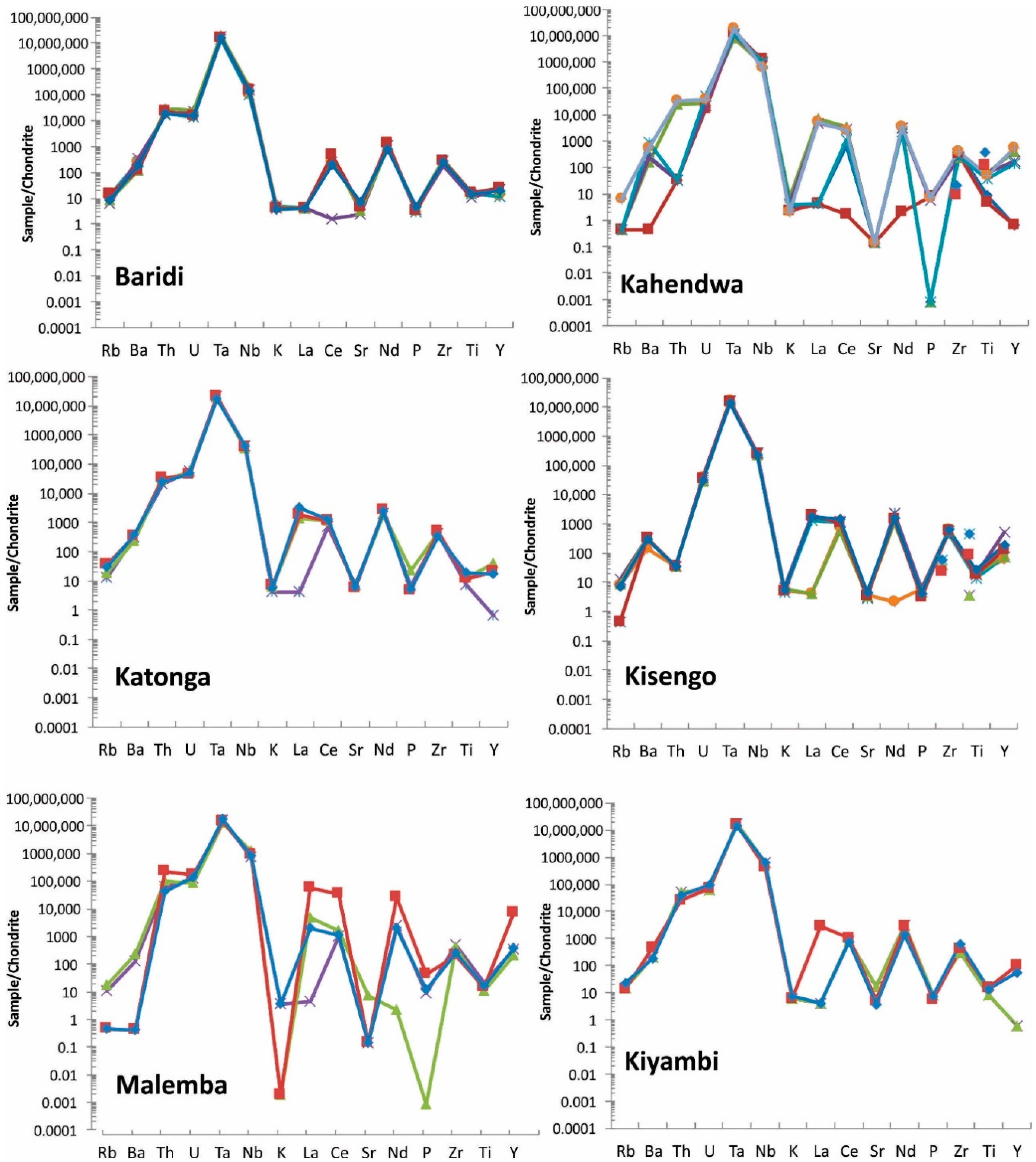
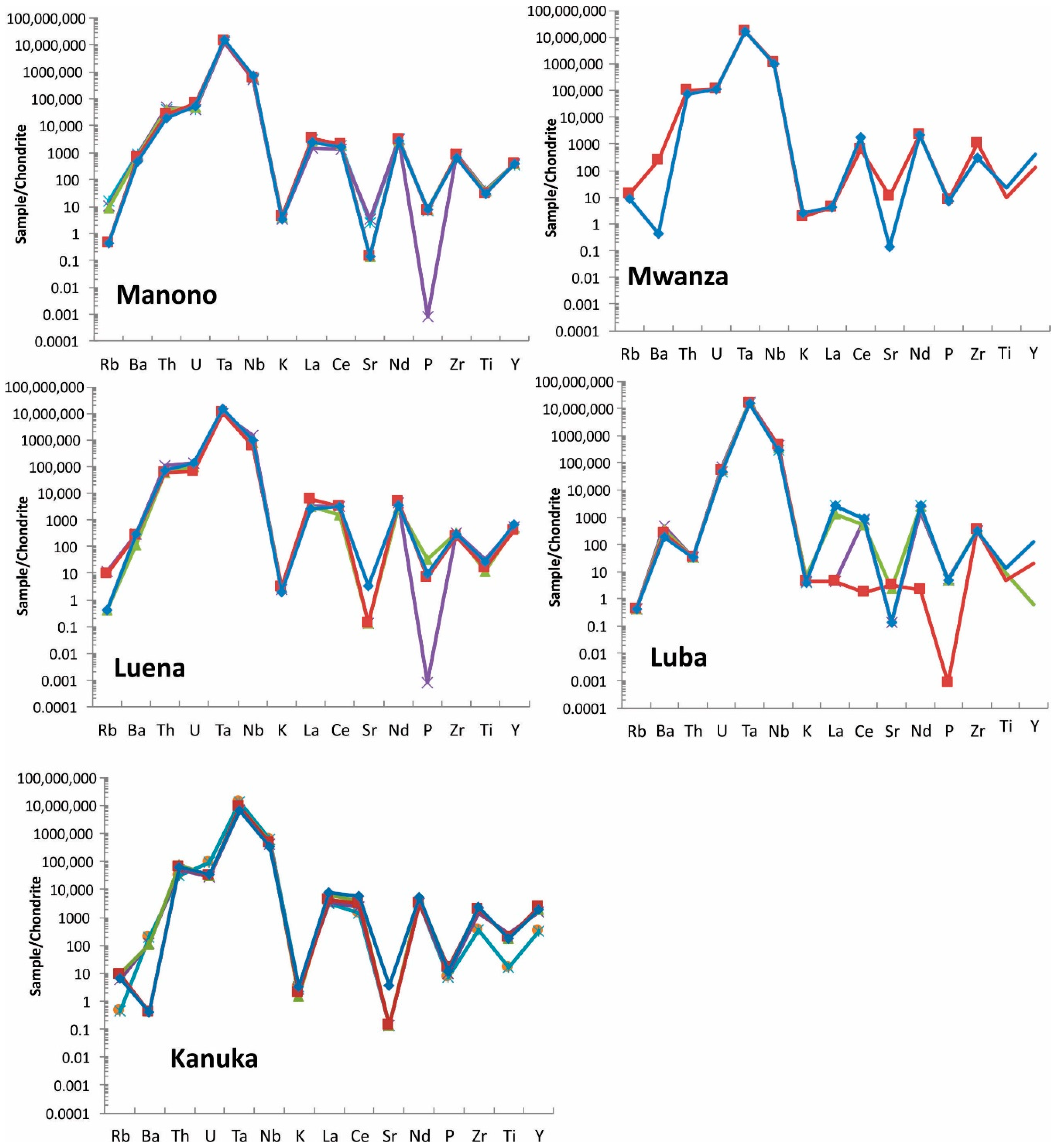
Spider diagrams: These diagrams are used in geology to investigate elemental composition compared to a source such as Bulk Earth or Chondrite Meteorite. Concentrations of trace elements (Rb, Ba, Th, U, Ta, Nb, K, La, Ce, Sr, Nd, P, Zr, Ti, and Y) determined by FPXRF are plotted relative to Chondrite Meteorite [23] in the spider diagrams (Figure 10).
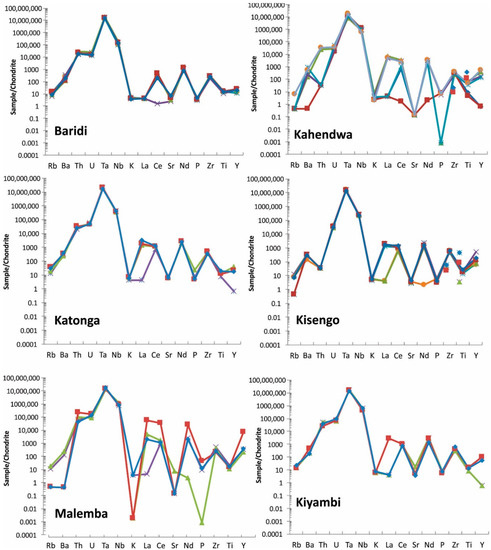
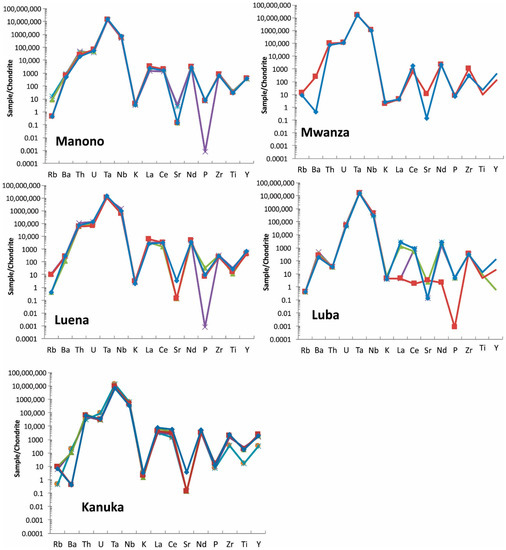
Figure 10.
Spider diagram of coltan ore samples from all studied regions. Note that Manono and Luena samples show very similar pattern. For elements <LOD, half of detection limit is used for graphing purpose. Each sample is shown with a different color.
Coltan from most regions show a unique pattern in spider diagrams and this can be used as a fingerprinting tool. Manono and Luena samples show a very similar spider pattern and, therefore, the spider diagram of these regions should be used with other discrimination diagrams for effective discrimination.
In addition, elements used in these diagrams can be sorted out based on their chondrite-normalized concentrations to provide a powerful tool for fingerprinting coltan. The sorted arrays of elements are unique for coltan ore samples from each region (Table 7). The array of elements based on the normalized values are not the same. For example, the third highest element (based on the normalized value) in most deposits is U, whereas it is Th in Baridi and Kanuka.

Table 7.
Elements in chondrite-normalized coltan ore samples in order of increasing from left to right. Average values were used in the calculations. Based on chondrite-normalized values, Ta and Nb are the first and second highest elements, respectively, followed by other elements in each deposit.
5. Conclusions
- HHXRF assay data shows that cassiterite and wolframite ores from all regions can be fingerprinted using various discrimination diagrams (Table 8). Although ore from some regions may be fingerprinted using a single discriminator (e.g., W + Th vs. Fe/Ti diagram for the Luena cassiterite ore), a combination of a few diagrams are often required to discriminate ore from most of the regions.
 Table 8. Discriminators that are used for fingerprinting conflict minerals.
Table 8. Discriminators that are used for fingerprinting conflict minerals. - Coltan ore samples from several regions can also be discriminated using major and trace elements of these samples. Baridi and Kisengo stand out chemically among the coltan ore samples. For other coltan regions, combinations of discrimination diagrams can be used to distinguish them. Coltan ore from some regions such as Luena, Malemba, and Mwanza have similar major element composition (Ta, Nb, Fe, Mn); however, distinctive spider diagram patterns can be used as a fingerprinting tool. In addition, Chondrite-normalized elemental arrays reflect characteristic trace element distributions in coltan ore from various regions, which in turn is related to the specific ore and mineral chemistry in each region.
This study demonstrates that a handheld XRF has the potential to provide immediate screening and indication of the origin of the mineral, and may provide an additional tool for rapidly determining provenance of some conflict minerals. However, this test is based on limited samples; with the speed of analysis that handheld XRF offers, more samples from other parts of the world can be analyzed to create a global library/data set for further detailed fingerprinting. Such a library can be used to fingerprint conflict mineral in-situ and in real time. Such a discriminating handheld analyzer would be invaluable for various industries and custom offices around the world.
Funding
This research was funded by ThermoFisher Scientific.
Acknowledgments
Samples for this case study were provided by MMR (Mining Mineral Resources), Democratic Republic of Congo and I am very grateful for logistics and having access to MMR lab. Also, Ingo Steinhage (United Spectrometer Technologies) and John Kande (Ets United Technologies & Ets United Scientific) are thanked for their field and logistic help. ThermoFisher Scientific is greatly appreciated for providing the handheld analyzer. The manuscript benefited from constructive comments made by three anonymous reviewers. Special thanks to Anderson for her edits and comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melcher, F.; Sitnikova, M.A.; Graupner, T.; Martin, N.; Oberthür, T.; Henjes-Kunst, F.; Gäbler, E.; Gerdes, A.; Brätz, H.; Davis, D.W.; et al. Fingerprinting of conflict minerals: Columbite-tantalite (“coltan”) ores. SGA News 2008, 23, 7–14. [Google Scholar]
- Conrey, R.M.; Goodman-Elgar, M.; Bettencourt, N.; Seyfarth, A.; Van Hoose, A.; Wolff, J.A. Calibration of a portable X-ray fluorescence spectrometer in the analysis of archaeological samples using influence coefficients. Geochem. Explor. Environ. Anal. 2012, 14, 291–301. [Google Scholar] [CrossRef]
- Arne, D.C.; Mackie, R.A.; Jones, S.A. The use of property-scale portable X-ray fluorescence data in gold exploration: Advantages and limitations. Geochem. Explor. Environ. Anal. 2014, 14, 233–244. [Google Scholar] [CrossRef]
- Cheng, Q. Vertical distribution of elements in regolith over mineral deposits and implications for mapping geochemical weak anomalies in covered areas. Geochem. Explor. Environ. Anal. 2014, 14, 277–289. [Google Scholar] [CrossRef]
- Gazley, M.F.; Tutt, C.M.; Brisbout, L.I.; Fisher, L.A.; Duclaux, G. Application of portable X-ray fluorescence analysis to characterize dolerite dykes at the Plutonic Gold Mine, Western Australia. Geochem. Explor. Environ. Anal. 2014, 14, 223–231. [Google Scholar] [CrossRef]
- Lemiere, B.; Laperche, V.; Haouche, L.; Auger, P. Portable XRF and wet materials: Application to dredged contaminated sediments from waterways. Geochem. Explor. Environ. Anal. 2014, 14, 257–264. [Google Scholar] [CrossRef]
- Quiniou, T.; Laperche, V. An assessment of field-portable X-ray fluorescence analysis for nickel and iron in laterite ore (New Caledonia). Geochem. Explor. Environ. Anal. 2014, 14, 245–255. [Google Scholar] [CrossRef]
- Simandl, G.J.; Paradis, S.; Stone, R.S.; Fajber, R.; Kressall, R.D.; Grattan, K.; Crozier, J.; Simandl, L.J. Applicability of handheld X-Ray fluorescence spectrometry in the exploration and development of carbonatite-related niobium deposits: A case study of the Aley Carbonatite, British Columbia, Canada. Geochem. Explor. Environ. Anal. 2014, 14, 211–221. [Google Scholar] [CrossRef]
- Vaillant, M.L.; Barnes, S.J.; Fisher, L.; Fiorentini, M.L.; Caruso, S. Use and calibration of portable X-Ray fluorescence analysers: Application to lithogeochemical exploration for komatiite-hosted nickel sulphide deposits. Geochem. Explor. Environ. Anal. 2014, 14, 199–209. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, Q.; Xia, Q.; Yao, L.; Chen, Z.; Zuo, R.; Xu, D. Spatial patterns of geochemical elements measured on rock surfaces by portable X-ray fluorescence: Application to hand specimens and rock outcrops. Geochem. Explor. Environ. Anal. 2014, 14, 265–276. [Google Scholar] [CrossRef]
- Hunt, A.M.W.; Speakman, R.J. Portable XRF analysis of archaeological sediments and ceramics. J. Archaeol. Sci. 2015, 53, 626–638. [Google Scholar] [CrossRef]
- Kuster, D. Granitoid-hosted Ta mineralization in the Arabian-Nubian Shield: Ore deposit types, tectonometallogenetic setting and petrogenetic framework. Ore Geol. Rev. 2009, 35, 68–86. [Google Scholar] [CrossRef]
- Shaw, R.; Goodenough, K.; Gunn, G.; Brown, T.; Rayner, D. Niobium and tantalum. Br. Geol. Surv. Publ. 2011, 2011, 27. [Google Scholar]
- Nasraoui, M.; Bilal, E. Pyrochlore from the Lueshe carbonatite complex (Democratic Republic of Congo): A geochemical record of different alteration stages. J. Asian Earth Sci. 2000, 18, 237–251. [Google Scholar] [CrossRef]
- Tack, L.; Wingate, M.T.D.; De Waele, B.; Meert, J.; Belousova, E.; Griffin, B.; Tahon, A.; Fernandez-Alonso, M. The 1375 Ma Kibaran event’ in Central Africa: Prominent emplacement of bimodal magmatism under extensional regime. Precambrian Res. 2010, 180, 63–84. [Google Scholar] [CrossRef]
- Hulsbosch, N.; Hertogen, J.; Dewaele, S.; Andre, L.; Muche, P. Petrographic and mineralogical characterization of fractionated pegmatites culminating in the Nb-Ta-Sn pegmatites of the Gatumba area (western Rwanda). Geol. Belg. 2013, 16, 105–117. [Google Scholar]
- Dewaele, S.; Fernandez-Alonso, M.; Tack, L. Cassiterite and columbo-tantalite (Coltan) mineralisation in the Proterozoic rocks of the northern part of the Kibara orogen (Central Africa): Preliminary results. Bull. Scéanc. Acad. R. Sci. Outre-Mer 2008, 54, 341–357. [Google Scholar]
- Romer, R.L.; Lehmann, B. U-Pb Columbite Age of Neoproterozoic Ta-Nb Mineralisation in Burundi. Econ. Geol. 1995, 90, 2303–2309. [Google Scholar] [CrossRef]
- Somarin, A.K.; Ashley, P. Hydrothermal alteration and mineralization of the Glen Eden Mo-W-Sn deposit: A leucogranite-related hydrothermal system, Southern New England Orogen, NSW, Australia. Miner. Deposita 2004, 39, 282–300. [Google Scholar]
- Gäbler, H.; Melcher, F.; Graupner, T.; Bahr, A.; Sitnikova, M.A.; Henjes-Kunst, F.; Oberthür, T.; Brätz, H.; Gerdes, A. Speeding up the analytical workflow for coltan fingerprinting by an integrated mineral liberation analysis/LA-ICP-MS approach. Geostand. Geoanalytical Res. 2011, 35, 431–448. [Google Scholar] [CrossRef]
- Harka, R.; Remus, J.J.; East, L.J.; Harmon, R.S.; Wise, M.; Tansi, B.M.; Shughrue, K.M.; Dunsin, K.; Liu, C. Geographical analysis of “conflict minerals” utilizing laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2012, 74, 131–136. [Google Scholar] [CrossRef]
- Arnaud, C.H. Fingerprinting conflict minerals: Spectroscopic method could help identify mineral origins. Chem. Engin. News 2012, 90, 36–37. [Google Scholar]
- Su, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. In Magmatism in Ocean Basins; Saunders, A.D., Norry, M.J., Eds.; Geological Society, London, Special Publications: London, UK, 1989; pp. 313–345. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).