Regulatory Potential of Long Non-Coding RNAs (lncRNAs) in Boar Spermatozoa with Good and Poor Freezability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Media
2.2. Animal and Semen Collections
2.3. Semen Processing Procedure and Quality Assessment
2.3.1. Semen Cryopreservation
2.3.2. Motility Parameters Analyzed by the Computer-Assisted Sperm Analysis (CASA) System
2.3.3. Membrane Integrity Assessment
2.4. De Novo Transcriptome Assembly
2.5. Identification of DElncRNAs
2.6. Functional Enrichment of Potential Target Genes of DElncRNAs
2.7. Quantitative RT-qPCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Semen Quality Assessment
3.2. De Novo Transcriptome Assembly and Blast Statistics
3.3. Gene Ontology (GO) Mapping, Annotations and Visualization of lncRNAs
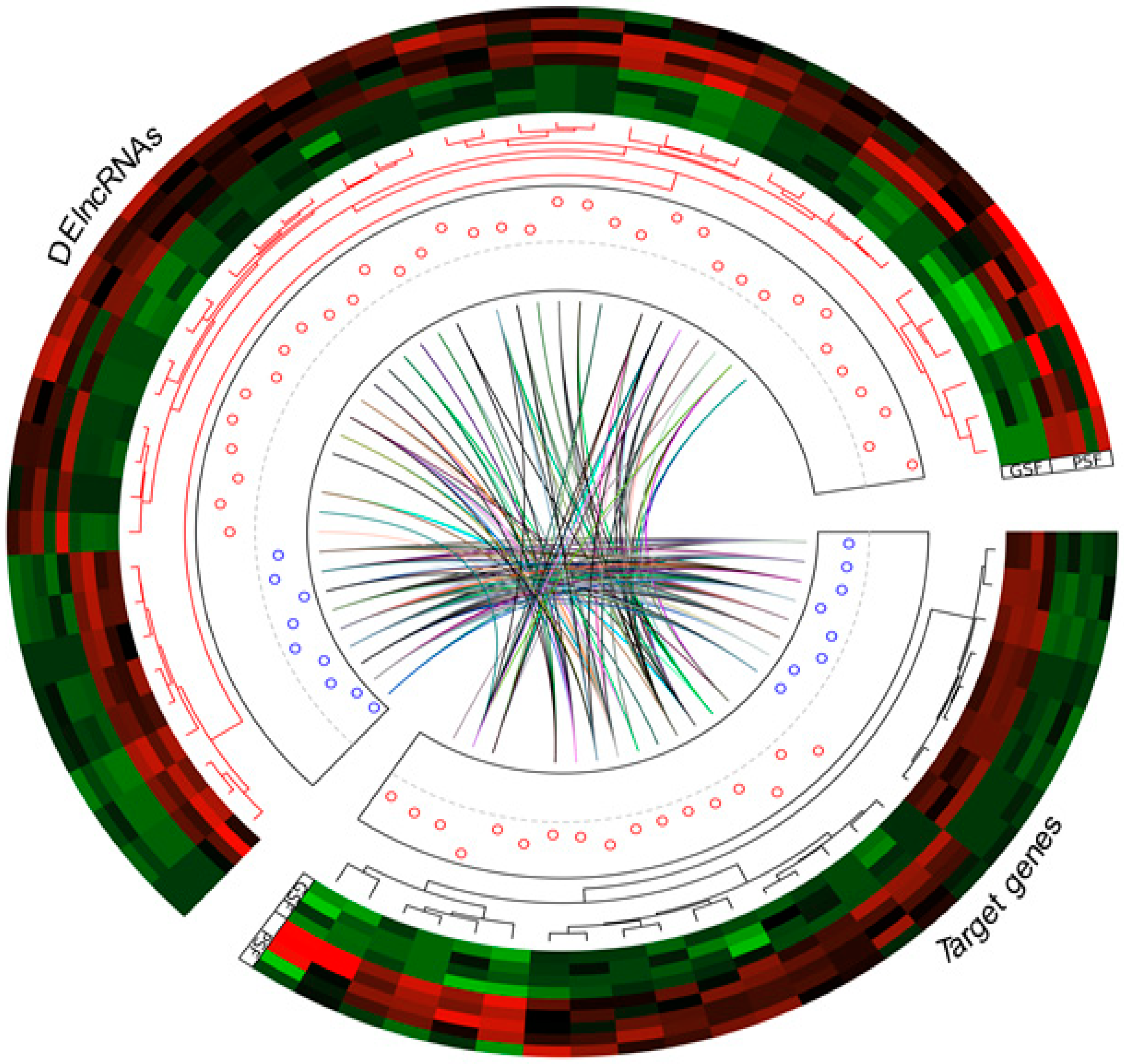
3.4. Functional Analysis of Regulatory Target Genes of DElncRNAs
3.5. Splicing Isoforms of lncRNAs
3.6. Functional Annotations of Potential Target Genes of DElncRNAs
3.7. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) Analysis
4. Discussion
4.1. Semen Freezability
4.2. Functional Characteristics of Potential Target Genes of DElncRNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Weng, B.; Ran, M.; Chen, B.; He, C.; Dong, L.; Peng, P. Genome-wide analysis of long non-coding RNAs and their role in postnatal porcine testis development. Genomics 2017, 109, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Wichman, L.; Somasundaram, S.; Breindel, C.; Valerio, D.M.; McCarrey, J.R.; Hodges, C.A.; Khalil, A.M. Dynamic expression of long noncoding RNAs reveals their potential roles in spermatogenesis and fertility. Biol. Reprod. 2017, 97, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Gòdia, M.; Swanson, G.; Krawetz, S.A. A history of why fathers’ RNA matters. Biol. Reprod. 2018, 99, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Sahlu, B.W.; Zhao, S.; Wang, X.; Umer, S.; Zou, H.; Huang, J.; Zhu, H. Long noncoding RNAs: New insights in modulating mammalian spermatogenesis. J. Anim. Sci. Biotechnol. 2020, 11, 16. [Google Scholar] [CrossRef]
- Sun, J.; Lin, Y.; Wu, J. Long non-coding RNA expression profiling of mouse testis during postnatal development. PLoS ONE 2013, 8, e75750. [Google Scholar] [CrossRef]
- Alessio, E.; Bonadio, R.S.; Buson, L.; Chemello, F.; Cagnin, S. A single cell but many different transcripts: A journey into the World of long non-coding RNAs. Int. J. Mol. Sci. 2020, 21, 302. [Google Scholar] [CrossRef]
- Kornienko, A.E.; Guenzi, P.M.; Barlov, D.P.; Pauler, F.M. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013, 11, 59. [Google Scholar] [CrossRef]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Fu, J.; Zhang, P.; Wang, Y.; Zeng, X. Systematic identification and characterization of long non-coding RNAs in mouse mature sperm. PLoS ONE 2017, 12, e0173402. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Lai, Z.; Zhou, Z.; Wu, F.; Huang, Y.; Lan, X.; Lei, C.; Chen, H.; Dang, R. Analysis of long non-coding RNA and mRNA expression profiling in immature and mature bovine (Bos Taurus) testes. Front. Genet. 2019, 10, 646. [Google Scholar] [CrossRef]
- Ran, M.X.; Li, Y.; Zhang, Y.; Liang, K.; Ren, Y.N.; Zhang, M.; Zhou, G.B.; Zhou, Y.M.; Wu, K.; Wang, C.D.; et al. Transcriptome sequencing reveals the differentially expressed lncRNAs and mRNAs involved in cryoinjuries in frozen-thawed giant Panda (Ailuropoda melanoleuca) sperm. Int. J. Mol. Sci. 2018, 19, 3066. [Google Scholar] [CrossRef] [PubMed]
- Gòdia, M.; Estill, M.; Castelló, A.; Balasch, S.; Rodríguez-Gil, J.E.; Krawetz, S.A.; Sánchez, A.; Clop, A. A RNA-Seq Analysis to describe the boar sperm transcriptome and its seasonal changes. Front. Genet. 2019, 10, 1–10. [Google Scholar]
- Ran, M.X.; Zhou, Y.M.; Liang, K.; Wang, W.C.; Zhang, Y.; Zhang, M.; Yang, J.D.; Zhou, G.B.; Wu, K.; Wang, C.D.; et al. Comparative analysis of microRNA and mRNA profiles of sperm with different freeze tolerance capacities in boar (Sus scrofa) and giant panda (Ailuropoda melanoleuca). Biomolecules 2019, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.; Brym, P.; Pareek, C.S.; Mogielnicka-Brzozowska, M.; Paukszto, Ł.; Jastrzębski, J.P.; Wasilewska-Sakowska, K.; Mańkowska, A.; Sobiech, P.; Żukowski, K. Transcriptome analysis of boar spermatozoa with different freezability using RNA-Seq. Theriogenology 2020, 142, 400–413. [Google Scholar] [CrossRef]
- Fraser, L.; Strzeżek, J. Effect of different procedures of ejaculate collection, extenders and packages on DNA integrity of boar spermatozoa following freezing-thawing. Anim. Reprod. Sci. 2007, 99, 317–329. [Google Scholar] [CrossRef]
- Fraser, L.; Parda, A.; Filipowicz, K.; Strzeżek, J. Comparison of post-thaw DNA integrity of boar spermatozoa assessed with the neutral Comet assay and sperm-Sus Halomax test kit. Reprod. Domest. Anim. 2010, 45, e155–e160. [Google Scholar] [CrossRef]
- Wasilewska-Sakowska, K.; Zasiadczyk, Ł.; Fraser, L. Effect of fractionated seminal plasma on sperm characteristics following cryopreservation of boar semen. Ann. Anim. Sci. 2019, 19, 695–712. [Google Scholar] [CrossRef]
- Dziekońska, A.; Fraser, L.; Strzeżek, J. Effect of different storage temperatures on the metabolic activity of spermatozoa following liquid storage of boar semen. J. Anim. Feed. Sci. 2009, 18, 638649. [Google Scholar] [CrossRef]
- Fraser, L.; Strzeżek, J.; Kordan, W. Post-thaw sperm characteristics following long-term storage of boar semen in liquid nitrogen. Anim. Reprod. Sci. 2014, 147, 119–127. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Fraser, L.; Dziekońska, A.; Strzeżek, R.; Strzeżek, J. Dialysis of boar semen prior to freezing-thawing: Its effects on post-thaw sperm characteristics. Theriogenology 2007, 67, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, K.; Fraser, L. Boar variability in sperm cryo-tolerance after cooling of semen in different long term extenders at various temperatures. Anim. Reprod. Sci. 2017, 185, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Boeckmann, B.; Blatter, M.C.; Famiglietti, L.; Hinz, U.; Lane, L.; Roechert, B.; Bairoch, A. Protein variety and functional diversity: Swiss-Prot annotation in its biological context. C. R. Biol. 2005, 328, 882–899. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Parker, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Zhou, Z. PLEK: A tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinform. 2014, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talon, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, Z.; Knoll, R.; Hombach, P.; Becker, M.; Schultze, J.L.; Ulas, T. Shiny-seq: Advances guided transcriptome analysis. BMC Res. Notes 2019, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- BioBam Bioinformatics. OmicsBox-Bioinformatics Made Easy, (Version 1.3.11). 2019. Available online: https://www.biobam.com (accessed on 14 November 2020).
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams (2007–2005). Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 14 November 2020).
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nuclei Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Zeng, C.; He, L.; Peng, W.; Ding, L.; Tang, K.; Fang, D.; Zhang, Y. Selection of optimal reference genes for quantitative RT-PCR studies of boar spermatozoa cryopreservation. Cryobiology 2014, 68, 113–121. [Google Scholar] [CrossRef]
- Koziorowska-Gilun, M.; Fraser, L.; Gilun, P.; Koziorowski, M.; Kordan, W. Activity of antioxidant enzymes and their mRNA expression in reproductive tract tissues of the male roe deer (Capreolus capreolus) during pre-rut and rut season. Small Rumin. Res. 2015, 129, 97–103. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J. Comput. Biol. 2005, 12, 1045–1062. [Google Scholar] [CrossRef]
- Mańkowska, A.; Brym, P.; Paukszto, Ł.; Jastrzębski, J.P.; Fraser, L. Gene polymorphisms in boar spermatozoa and their associations with post-thaw semen quality. Int. J. Mol. Sci. 2020, 21, 1902. [Google Scholar] [CrossRef] [PubMed]
- Ipsa, E.; Cruzat, V.F.; Kagize, J.N.; Yovich, J.L.; Keane, K.N. Growth hormone and insulin-like growth factors action in reproductive tissues. Front. Endocrinol. 2019, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Fumel, B.; Froment, P.; Holzenberger, M.; Livera, G.; Monget, P.; Fouchecourt, S. Expression of dominant-negative thyroid hormone receptor alpha1 in Leydig and Sertoli cells demonstrate no additional defect compared with expression in Sertoli cells only. PLoS ONE 2015, 10, e01119392. [Google Scholar]
- Gòdia, M.; Castelló, A.; Rocco, M.; Cabrera, B.; Rodríguez-Gil, J.E.; Sánchez, A.; Clop, A. Identification of circular RNAs in porcine sperm and their relation to sperm motility. Sci. Rep. 2020, 10, 7985. [Google Scholar] [CrossRef]
- Samant, G.V.; Schupp, M.O.; Francois, M.; Moleri, S.; Kothiniti, R.K.; Chun, C.Z.; Sinha, I.; Sellars, S.; Leigh, N.; Pramanik, K.; et al. Sox factors transcriptionally regulate ROBO4 gene expression in developing vasculature in Zebrafish. J. Biol. Chem. 2011, 286, 30740–30747. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell. Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Rezende, F.M.; Dietsch, G.O.; Penagaricano, F. Genetic dissection of bull fertility in US Jersey dairy cattle. Anim. Genet. 2018, 49, 393–402. [Google Scholar]
- Wu, Y.; Lee, S.H.; Williamson, E.A.; Reinert, B.L.; Cho, J.H.; Xia, F.; Jaiswal, A.S.; Srinivasan, G.; Patel, B.; Brantley, A.; et al. EEPD1 rescues stressed replication forks and maintains genome stability by promoting end resection and homologous recombination repair. PLoS Genet. 2015, 11, e1005675. [Google Scholar] [CrossRef]
- Sutovsky, P.; Aarabi, M.; Miranda-Vizuete, A.; Oko, R. Negative biomarker-based male fertility evaluation: Sperm phenotypes associated with molecular-level anomalies. Asian J. Androl. 2015, 17, 554–560. [Google Scholar] [CrossRef]
- Walden, P.D.; Cowan, N.J. A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Mol. Cell Biol. 1993, 13, 7625–7635. [Google Scholar] [CrossRef]
- Amargant, F.; Barragan, M.; Vassena, R.; Vernos, I. Insights of the tubulin code in gametes and embryos: From basic research to potential clinical applications in humans. Biol. Reprod. 2019, 100, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, F.; Tian, G.; Piehl, M.; Cassimeris, L.; Lewis, S.A.; Cowan, N.J. Identification of a novel tubulin-destabilizing protein related to the chaperone cofactor E. J. Cell Sci. 2015, 118, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Shawki, H.H.; Ishikawa-Yamauchi, Y.; Kawashima, A.; Katoh, Y.; Matsuda, M.; Al-Soudy, A.S.; Minisy, F.M.; Kuno, A.; Gulibaikelamu, X.; Hirokawa, T.; et al. EFCAB2 is a novel calcium-binding protein in mouse testis and sperm. PLoS ONE 2019, 14, e0214687. [Google Scholar] [CrossRef] [PubMed]
- Codrington, A.M.; Hales, B.F.; Robaire, B. Chronic cyclophosphamide exposure alters the profile of rat sperm nuclear matrix proteins. Biol. Reprod. 2007, 77, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, N.; Li, X.; El-Ashram, S.; Wang, Z.; Zhu, L.; Jiang, W.; Peng, X.; Zhang, C.; Chen, Y.; et al. Identifying candidate genes associated with sperm morphology abnormalities using weighted single-step GWAS in a Duroc boar population. Theriogenology 2020, 141, 9–15. [Google Scholar] [CrossRef]
- Moeller, J.B.; Nielsen, M.J.; Reichardt, M.P.; Schlosser, A.; Sorensen, G.L.; Nielsen, O.; Tornøe, I.; Grønlund, J.; Nielsen, M.E.; Jørgensen, J.S.; et al. CD163-L1 is an endocytic macrophage protein strongly regulated by mediators in the inflammatory response. J. Immunol. 2012, 188, 2399–2409. [Google Scholar] [CrossRef]
- Johnson, K.J.; Zecevic, A.; Kwon, E.J. Protocadherin alpha3 acts at sites distinct from classic cadherins in rat testis and sperm. Biol. Reprod. 2004, 70, 303–312. [Google Scholar] [CrossRef]
- Buzanskas, M.E.; do Amral Grossi, D.; Ventura, R.V.; Schenkel, F.S.; Chud, T.C.S.; Stafuzza, N.B.; Rola, L.D.; Meirelles, S.L.C.; Mokry, F.B.; de Alvarenga Mudadu, M.; et al. Candidiate genes for male and female reproductive traits in Canchim beef cattle. J. Anim. Sci. Biotechnol. 2017, 8, 67. [Google Scholar] [CrossRef]
- Filippou, P.S.; Ren, A.H.; Soosaipillai, A.; Papaionnou, M.D.; Korbakis, D.; Safar, R.; Diamandis, E.P.; Conner, J.R. Expression profile of human tissue kallikrein 15 provides preliminary insights into its roles in the prostate and testis. Clin. Biochem. 2018, 59, 78–85. [Google Scholar] [CrossRef]
- Holt, W.V.; Medrano, A.; Thurston, L.M.; Watson, P.F. The significance of cooling rates andanimal variability for boar sperm cryopreservation: Insights from the cryomicroscope. Theriogenology 2005, 63, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Carle, B.; Mukai, T.; Simpson, A.; Vu, L.; Knight, J.W. Effect of exogenous glutathione supplementation on motility, viability, and DNA integrity of frozen-thawed boar semen. Anim. Reprod. 2008, 5, 127–131. [Google Scholar]
- Torres, M.A.; Monterio, M.S.; Passarelli, M.S.; Papa, F.O.; Dell’Agua, J.A., Jr.; Alvarenga, M.A.; Martins, S.M.M.K.; de Andrade, A.F.C. The ideal holding time for boar semen is 24 h at 17 °C prior to short-cryopreservation protocols. Cryobiology 2019, 86, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Das, P.J.; McCarthy, F.; Vishnoi, M.; Paria, N.; Gresham, C.; Li, G.; Kachroo, P.; Sudderth, A.K.; Teague, S.; Love, C.C.; et al. Stallion sperm transcriptome comprises functionally coherent coding and regulatory RNAs as revealed by microarray analysis and RNA-seq. PLoS ONE 2013, 8, e56535. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Guo, F.; Zhang, Y.; Ju, Z.; Jiang, Q.; Zhao, X.; Liu, Y.; Zhao, H.; Wang, J.; et al. Integrated analysis of mRNAs and long noncoding RNAs in the semen from Holstein bulls with high and low sperm motility. Sci. Rep. 2019, 9, 2092. [Google Scholar] [CrossRef]
- Ma, H.; Hao, Y.; Dong, X.; Gong, Q.; Chen, J.; Zhang, J.; Tian, W. Molecular mechanisms and function prediction of long noncoding RNA. Sci. World J. 2012, 2012, 541786. [Google Scholar] [CrossRef]
- Hong, S.H.; Kwon, J.T.; Kim, J.; Jeong, J.; Kim, J.; Lee, S.; Cho, C. Profiling of testis specific long noncoding RNAs in mice. BMC Genom. 2018, 19, 539. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, F.; Chen, M.; Lan, X.; Yang, R.; Pan, C. Identification and characterization of male reproduction-related genes in pig (Sus scrofa) using transcriptome analysis. BMC Genom. 2020, 21, 381. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Baumgartner, A.; Anderson, D. Antioxidants modulate thyroid hormone- and noradrenaline-induced DNA damage in human sperm. Mutagenesis 2004, 19, 325–330. [Google Scholar]
- Naz, R.K.; Sellamuthu, R. Receptors in spermatozoa. J. Androl. 2006, 27, 627–636. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, J.; Chen, L.; Guo, X.; Du, Y.; Zhao, C.; Zhu, Y.; Lin, M.; Zhou, Z.; Sha, J. RGS22, a novel testes-specific regulator of GT-protein signaling involved in human and mouse spermiogenesis along with GNA12/13 subunits. Biol. Reprod. 2008, 79, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Baik, J.S.; Heo, K.; Kim, J.S.; Lee, K.; Rhee, M.H.; Kim, S.D. Changing of RGS transcripts levels by low-dose rate ionizing in mouse testis. J. Radiat. Prot. Res. 2015, 40, 187–193. [Google Scholar] [CrossRef]
- Liu, M.; Guan, Z.L.; Shen, Q.; Lalor, P.; Fitzgerald, U.; O’Brien, T.; Dockery, P.; Shen, S. Ulk4 is essential for ciliogenesis and CSF flow. J. Neurosci. 2016, 36, 7589–7600. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.O.; Yadav, R.K.; Kim, H.R.; Chae, H.J. ER stress: Autophagy induction, inhibition and selection. Autophagy 2015, 11, 1956–1977. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, M.; Espino, J.; Bejarano, I.; Gallardo-Soler, A.; Campo, M.L.; Salido, G.M.; Pariente, J.A.; Pena, F.J.; Tapia, J.A. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci. Rep. 2016, 6, 33647. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.; Hu, C.; Hao, H.; Zhang, J.; Li, K.; Zhao, X.; Qin, T.; Zhao, K.; Zhu, H.; et al. Identification of differentially expressed proteins in fresh and frozen–thawed boar spermatozoa by iTRAQ-coupled 2D LC–MS/MS. Reproduction 2014, 147, 321–330. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Zhu, J.; Shen, G.; Li, Z.; Dong, J. Long noncoding RNAs in the regulation of oxdiative stress. Oxid. Med. Cell. Longev. 2019, 2019, 1318795. [Google Scholar]
- Stine, R.R.; Greenspan, L.J.; Ramachandran, K.V.; Matunis, E.L. Coordinate regulation of stem cell competition by Slit-Robo and JAK-STAT signaling in the Drosophila testis. PLoS Genet. 2014, 10, e1004713. [Google Scholar] [CrossRef]
- Hamidi, T.; Schmid, R.M.; Iovanna, J.L. Nucelar protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J. Clin. Investig. 2012, 122, 2092–2103. [Google Scholar] [CrossRef]
- Archana, S.S.; Selvaraju, S.; Binsila, B.K.; Arunachalam, A.; Krawetz, S.A. Immune regulatory molecules as modifiers of semen and fertility: A review. Mol. Reprod. Dev. 2018, 186, 1485–1504. [Google Scholar] [CrossRef]
- Noor, M.M.; Tawang, A.; Moore, H.D.M. Studies on tissue distribution of M1 antigen using sperm specific monoclonal antibody. Biomed. Res. 2006, 17, 133–138. [Google Scholar]
- Song, H.; Wang, L.; Chen, D.; Li, F. The function of pre-mRNA alternative splicing in mammal spermatogenesis. Int. J. Biol. Sci. 2020, 16, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Romero-Barrios, N.; Legascue, M.F.; Benhamedi, M.; Ariel, F.; Crespi, M. Splicing regulation by long coding RNAs. Nucleic Acids Res. 2018, 46, 2169–2184. [Google Scholar] [CrossRef] [PubMed]









| DElncRNA ID | Primer Sequence (5′-3′) | Temperature (°C) | Product Size (bp) |
|---|---|---|---|
| TRINITY_DN1365027_c0_g1_i2 | F: ccatatgctgtgggtgagg R: ttgtgatggaggataatttgagaa | 60 | 167 |
| TRINITY_DN1103286_c5_g1_i5 | F: aaaacagaaaggaaatgaaacca R: ggaaattaagccctcattgg | 60 | 100 |
| TRINITY_DN1278737_c0_g1_i1 | F: catatttctttacatcatggtcctg R: tttagagcttcagtgatgtgc | 60 | 150 |
| Sperm Parameters (df = 8) | F-Value | p-Value |
|---|---|---|
| Total motility (TMOT) | 34.641 | <0.001 |
| Progressive motility (PMOT) | 27.753 | <0.001 |
| Velocity straight line (VSL) | 16.916 | <0.001 |
| Velocity average path (VAP) | 6.944 | <0.001 |
| Mitochondrial membrane potential (MMP) | 8.785 | <0.001 |
| Plasma membrane integrity (PMI) | 13.316 | <0.001 |
| Normal apical ridge (NAR) acrosome integrity | 7.938 | <0.001 |
| DNA fragmentation | 3.712 | <0.006 |
| Gene and Transcript Counts, and guanine-cytosine (GC) content | Total trinity ‘genes’: | 1,879,557 |
| Total trinity transcripts: | 2,023,225 | |
| Percent GC | 36.49 | |
| Statistics Based on All Transcript Contigs: | Contig N10 (nt): | 660 |
| Contig N20 (nt): | 534 | |
| Contig N30 (nt): | 464 | |
| Contig N40 (nt): | 411 | |
| Contig N50 (nt): | 366 | |
| Median contig length (nt): | 310 | |
| Average contig (nt): | 353.78 | |
| Total assembled bases: | 715,783,046 | |
| Statistics Based on Only The Longest Isoform per ‘gene’: | Contig N40 (nt): | 662 |
| Contig N20 (nt): | 535 | |
| Contig N30 (nt): | 466 | |
| Contig N40 (nt): | 413 | |
| Contig N50 (nt): | 367 | |
| Median contig length (nt): | 312 | |
| Average contig (nt): | 355.03 | |
| Total assembled bases: | 667,299,497 |
| lncRNA ID | Locus | Cis-Regulation | Trans-Regulation |
|---|---|---|---|
| TRINITY_DN1035446_c0_g1 | chr13:15027825-15028258 | ENSSSCG0000033794 | CAAP1, GAS2, COX7A2L |
| TRINITY_DN1044754_c0_g1 | chr6:165435006-165435571 | MAST2 | n/a |
| TRINITY_DN1045510_c0_g1 | chr16:27228522-27229268 | GHR | COX7A2L |
| TRINITY_DN1361685_c0_g1 | chr1:258475511-258475820 | U6 | n/a |
| TRINITY_DN1059433_c0_g1 | chr11:14840949-14841766 | n/a | CAAP1 |
| TRINITY_DN157051_c1_g1 | chr13:11234377-11234878 | THRB | COX7A2L, EFCAB11 (EFCAB2) |
| TRINITY_DN1778675_c1_g1 | chr6:99002552-99002962 | n/a | COX7A2L, KLK15, EFCAB11 (EFCAB2) |
| TRINITY_DN1778721_c0_g1 | chr1:146979892-146981163 | n/a | CAAP1 |
| TRINITY_DN241239_c0_g1 | chr10:597871-598247 | CDC73 | EFCAB11 (EFCAB2) |
| TRINITY_DN263649_c0_g1 | chr10:1545624-1546297 | RGS18 | GAS2, COX7A2L |
| TRINITY_DN4560_c0_g1 | chr14:106830465-106891960 | ENSSSCG0000033600 | n/a |
| TRINITY_DN416076_c0_g1 | chr7:94728590-94729707 | n/a | CAAP1, COX7A2L |
| TRINITY_DN538320_c0_g1 | chr5:63499679-63500175 | CD163L1 | n/a |
| TRINITY_DN559365_c0_g1 | chr1:251338735-251339236 | TXNDC8 | GAS2 |
| TRINITY_DN600110_c0_g2 | chr18:37853087-37853635 | EEPD1 | n/a |
| TRINITY_DN625807_c0_g1 | chr7:10417725-10418388 | n/a | CAAP1, EFCAB11 (EFCAB2) |
| TRINITY_DN640486_c0_g1 | chr18:41489497-41491323 | ITPRID1 | CAAP1, GAS2 |
| TRINITY_DN740880_c0_g1 | chr8:16645743-16646197 | n/a | CAAP1, COX7A2L |
| TRINITY_DN848488_c0_g1 | chr17:4530969-4531377 | n/a | COX7A2L |
| TRINITY_DN869342_c0_g1 | chr1:30107201-30107838 | n/a | EFCAB11 (EFCAB2) |
| TRINITY_DN934504_c0_g1 | chr6:98298308-98298817 | RAB31 | n/a |
| TRINITY_DN942459_c0_g1 | chr13:109345030-109346972 | n/a | CAAP1, GAS2 |
| TRINITY_DN962891_c0_g1 | chr6:129774162-129774633 | TTLL7 | COX7A2L |
| TRINITY_DN971351_c0_g1 | chrX:6727378-6736952 | n/a | CAAP1 |
| TRINITY_DN976443_c0_g1 | chr13:43295569-43296123 | n/a | COX7A2L, KLK15, EFCAB11 (EFCAB2) |
| TRINITY_DN987550_c1_g1 | chr13:25727243-25727879 | ULK4 | COX7A2L |
| TRINITY_DN1058184_c4_g1 | AEMK02000598.1:14135-15082 | ENSSSCG00000038136 | CAAP1, COX7A2L, KLK15 |
| TRINITY_DN792292_c0_g1 | AEMK02000514.1: 18098-64313 | ENSSSCG00000034138 | n/a |
| lncRNA ID | Locus | Cis-Regulation | Trans-Regulation |
|---|---|---|---|
| TRINITY_DN1022003_c0_g2 | chr2:142618997-142620529 | LOC100621701 | TECTA, SOX-7, NUPR2 |
| TRINITY_DN1094887_c21_g2 | chr13:177656907-177657455 | ROBO2 | TECTA, SOX-7, NUPR2 |
| TRINITY_DN1225954_c0_g1 | chr11:31386574-31386891 | n/a | TECTA, SOX-7, NUPR2 |
| TRINITY_DN1278737_c0_g1 | chr6:2487124-24872979 | n/a | SOX-7, NUPR2 |
| TRINITY_DN652283_c0_g1 | chr17:9809518-9810042 | ZMAT4 | TECTA, NUPR2 |
| TRINITY_DN698757_c0_g1 | chr6:13294125-132941663 | n/a | TECTA, NUPR2 |
| TRINITY_DN742894_c0_g1 | chr3:60110813-60111197 | ENSSSCG00000023812 | TECTA, SOX-7, NUPR2 |
| TRINITY_DN980890_c0_g1 | chr15:27098747-27099086 | CNTNAP5 | TECTA, NUPR2 |
| Ensembl | Gene Name | Gene Description | Sperm/Reproductive Traits | References |
|---|---|---|---|---|
| ENSSSCG00000016866 | GHR | growth hormone receptor | steroidogenesis and spermatogenesis | [44] |
| ENSSSCG00000036033 | THRB | thyroid hormone receptor beta | steroidogenesis | [45] |
| ENSSSCG00000033945 | RGS18 | regulator of G protein signaling 18 | uncharacterized | n/a |
| ENSSSCG00000010801 | CDC73 | cell division cycle 73 | embryo development | [46] |
| ENSSSCG00000025408 | ULK4 | unc-51 like kinase 4 | motility | [46] |
| ENSSSCG00000031889 | SOX-7 | SRY-box transcription factor 7 | embryo development and implantation | [47] |
| ENSSSCG00000037264 | RAB31 | RAB31, member RAS oncogene family | autophagy | [48] |
| ENSSSCG00000008466 | COX72AL | cytochrome c oxidase subunit 7A-related protein, mitochondrial | motility | [49] |
| ENSSSCG00000039703 | EEPD1 | endonuclease/exonuclease/phosphatase family domain containing 1 | maintenance of genome stability | [50] |
| ENSSSCG00000005454 | TXNDC8 | thioredoxin domain containing 8 (spermatozoa) | protection against oxidative stress | [51] |
| ENSSSCG00000003913 | MAST2 | microtubule associated serine/threonine kinase 2 | cytoskeletal regulation | [52] |
| ENSSSCG00000003760 | TTLL7 | tubulin tyrosine ligase like 7 | cytoskeletal regulation | [53] |
| ENSSSCG00000023728 | TECTA | tubulin-specific chaperone cofactor E-like protein | cytoskeletal regulation | [54] |
| ENSSSCG00000002430 | EFCAB11 (EFCAB2) | EF-hand calcium binding domain 11 | motility | [55] |
| ENSSSCG00000031940 | GAS2 | growth arrest-specific protein 2 | cytoskeletal regulation and apoptosis | [56] |
| ENSSSCG00000012002 | ROBO2 | roundabout guidance receptor 2 | predicted: fertility | [57] |
| ENSSSCG00000007741 | NUPR2 | nuclear protein 2, transcriptional regulator | uncharacterized | n/a |
| ENSSSCG00000034914 | CD163L1 | CD163 molecule-like 1 | immune response | [58] |
| ENSSSCG00000016671 | ITPRID1 | inositol 1,4,5-trisphosphate receptor (ITPR) interacting domain containing 1 | predicted: receptor binding | n/a |
| ENSSSCG00000029281 | LOC100621701 | protocadherin alpha-C2 | predicted: spermatogenesis | [59] |
| ENSSSCG00000007010 | ZMAT4 | zinc finger matrin-type 4 | predicted: fertilization | [60] |
| ENSSSCG00000030539 | KLK15 | kallikrein related peptidase 15 | spermatogenesis | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraser, L.; Paukszto, Ł.; Mańkowska, A.; Brym, P.; Gilun, P.; Jastrzębski, J.P.; Pareek, C.S.; Kumar, D.; Pierzchała, M. Regulatory Potential of Long Non-Coding RNAs (lncRNAs) in Boar Spermatozoa with Good and Poor Freezability. Life 2020, 10, 300. https://doi.org/10.3390/life10110300
Fraser L, Paukszto Ł, Mańkowska A, Brym P, Gilun P, Jastrzębski JP, Pareek CS, Kumar D, Pierzchała M. Regulatory Potential of Long Non-Coding RNAs (lncRNAs) in Boar Spermatozoa with Good and Poor Freezability. Life. 2020; 10(11):300. https://doi.org/10.3390/life10110300
Chicago/Turabian StyleFraser, Leyland, Łukasz Paukszto, Anna Mańkowska, Paweł Brym, Przemysław Gilun, Jan P. Jastrzębski, Chandra S. Pareek, Dibyendu Kumar, and Mariusz Pierzchała. 2020. "Regulatory Potential of Long Non-Coding RNAs (lncRNAs) in Boar Spermatozoa with Good and Poor Freezability" Life 10, no. 11: 300. https://doi.org/10.3390/life10110300
APA StyleFraser, L., Paukszto, Ł., Mańkowska, A., Brym, P., Gilun, P., Jastrzębski, J. P., Pareek, C. S., Kumar, D., & Pierzchała, M. (2020). Regulatory Potential of Long Non-Coding RNAs (lncRNAs) in Boar Spermatozoa with Good and Poor Freezability. Life, 10(11), 300. https://doi.org/10.3390/life10110300






