Red Blood Cell Distribution Width Is Associated with Deterioration of Renal Function and Cardiovascular Morbidity and Mortality in Patients with Diabetic Kidney Disease
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Laboratory Analyses
4.3. CIMT Measurement
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Danese, E.; Lippi, G.; Montagnana, M. Red blood cell distribution width and cardiovascular diseases. J. Thorac. Dis. 2015, 7, E402–E411. [Google Scholar]
- Perkins, S.L. Chapter. Examination of the blood and bone marrow. In Wintrobe’s Clinical Hematoogy; Greer, J.P., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1999; pp. 9–35. [Google Scholar]
- Perlstein, T.S.; Weuve, J.; Pfeffer, M.A.; Beckman, J.A. Red Blood Cell Distribution Width and Mortality Risk in a Community-Based Prospective Cohort. Arch. Intern. Med. 2009, 169, 588–594. [Google Scholar] [CrossRef]
- Felker, G.M.; Allen, L.A.; Pocock, S.J.; Shaw, L.K.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; et al. Red Cell Distribution Width as a Novel Prognostic Marker in Heart Failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007, 50, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Sacks, F.; Arnold, M.; Moye, L.; Davis, B.; Pfeffer, M. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation 2008, 117, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ani, C.; Ovbiagele, B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J. Neurol. Sci. 2009, 277, 103–108. [Google Scholar] [CrossRef]
- Mucsi, I.; Ujszaszi, A.; Czira, M.E.; Novak, M.; Molnar, M.Z. Red cell distribution width is associated with mortality in kidney transplant recipients. Int. Urol. Nephrol. 2013, 46, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Park, J.T.; Kim, J.-K.; Yoo, D.E.; Kim, S.J.; Han, S.H.; Kang, S.-W.; Choi, K.H.; Yoo, T.-H. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol. Dial. Transplant. 2011, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Solak, Y.; Gaipov, A.; Turk, S.; Kayrak, M.; Yilmaz, M.I.; Caglar, K.; Verim, S.; Unal, H.U.; Gok, M.; Demirkaya, E.; et al. Red Cell Distribution Width Is Independently Related to Endothelial Dysfunction in Patients With Chronic Kidney Disease. Am. J. Med. Sci. 2014, 347, 118–124. [Google Scholar] [CrossRef]
- Afonso, L.; Zalawadiya, S.K.; Veeranna, V.; Panaich, S.S.; Niraj, A.; Jacob, S. Relationship between Red Cell Distribution Width and Microalbuminuria: A Population-Based Study of Multiethnic Representative US Adults. Nephron Clin. Pract. 2011, 119, c277–c282. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2018, 32, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Zalawadiya, S.K.; Veeranna, V.; Panaich, S.S.; Afonso, L.; Ghali, J.K. Gender and Ethnic Differences in Red Cell Distribution Width and Its Association With Mortality Among Low Risk Healthy United State Adults. Am. J. Cardiol. 2012, 109, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar]
- Douglas, S.W.; Adamson, J.W. The anemia of chronic disorders: Studies of marrow regulation and iron metabolism. Blood 1975, 45, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The Role of Inflammatory Cytokines in Diabetes and Its Complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Malandrino, N.; Wu, W.C.; Taveira, T.H.; Whitlatch, H.B.; Smith, R.J. Association between red blood cell distribution width and macrovascular and microvascular complications in diabetes. Diabetologia 2012, 55, 226–235. [Google Scholar] [CrossRef]
- Peng, F.; Li, Z.; Zhong, Z.; Luo, Q.; Guo, Q.; Huang, F.; Yu, X.; Yang, X. An increasing of red blood cell distribution width was associated with cardiovascular mortality in patients on peritoneal dialysis. Int. J. Cardiol. 2014, 176, 1379–1381. [Google Scholar] [CrossRef]
- Vashistha, T.; Streja, E.; Molnar, M.Z.; Rhee, C.M.; Moradi, H.; SooHoo, M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Red Cell Distribution Width and Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2016, 68, 110–121. [Google Scholar] [CrossRef]
- Chen, P.-C.; Sung, F.-C.; Chien, K.-L.; Hsu, H.-C.; Su, T.-C.; Lee, Y.-T. Red Blood Cell Distribution Width and Risk of Cardiovascular Events and Mortality in a Community Cohort in Taiwan. Am. J. Epidemiol. 2010, 171, 214–220. [Google Scholar] [CrossRef]
- Arbel, Y.; Raz, R.; Weitzman, D.; Steinvil, A.; Zeltser, D.; Berliner, S.; Chodick, G.; Shalev, V. Red blood cell distribution width and the risk of cardiovascular morbidity and all-cause mortality: A population-based study. Eur. Hear. J. 2013, 34, 300–307. [Google Scholar] [CrossRef]
- Patel, K.V.; Ferrucci, L.; Ershler, W.B.; Longo, D.L.; Guralnik, J.M. Red Blood Cell Distribution Width and the Risk of Death in Middle-aged and Older Adults. Arch. Intern. Med. 2009, 169, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V.; Semba, R.D.; Ferrucci, L.; Newman, A.B.; Fried, L.P.; Wallace, R.B.; Bandinelli, S.; Phillips, C.S.; Yu, B.; Connelly, S.; et al. Red Cell Distribution Width and Mortality in Older Adults: A Meta-analysis. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2010, 65, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Bujak, K.; Wasilewski, J.; Osadnik, T.; Jonczyk, S.; Kołodziejska, A.; Gierlotka, M.; Gąsior, M. The Prognostic Role of Red Blood Cell Distribution Width in Coronary Artery Disease: A Review of the Pathophysiology. Dis. Markers 2015, 2015, 824624. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Felker, G.M.; Mehra, M.R.; Chiong, J.R.; Dunlap, S.H.; Ghali, J.K.; Lenihan, D.J.; Oren, R.M.; Wagoner, L.E.; Schwartz, T.A.; et al. Validation and Potential Mechanisms of Red Cell Distribution Width as a Prognostic Marker in Heart Failure. J. Card. Fail. 2010, 16, 230–238. [Google Scholar] [CrossRef]
- Al-Najjar, Y.; Goode, K.M.; Zhang, J.; Cleland, J.G.F.; Clark, A.L. Red cell distribution width: An inexpensive and powerful prognostic marker in heart failure. Eur. J. Heart. Fail. 2009, 11, 1155–1162. [Google Scholar] [CrossRef]
- Aung, N.; Ling, H.Z.; Cheng, A.S.H.; Aggarwal, S.; Flint, J.; Mendonca, M.; Rashid, M.; Kang, S.; Weissert, S.; Coats, C.J.; et al. Expansion of the red cell distribution width and evolving iron deficiency as predictors of poor outcome in chronic heart failure. Int. J. Cardiol. 2013, 168, 1997–2002. [Google Scholar] [CrossRef]
- Van Kimmenade, R.R.J.; Mohammed, A.A.; Uthamalingam, S.; Van Der Meer, P.; Felker, G.M.; Januzzi, J.L. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur. J. Heart Fail. 2010, 12, 129–136. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Hu, Z.; Liu, S.-J.; Sun, Y.; Qin, Q.; Qin, B.-D.; Zhang, W.-W.; Zhang, J.-R.; Zhong, R.-Q.; Deng, A.-M. Prognostic Value of Red Blood Cell Distribution Width for Patients with Heart Failure: A Systematic Review and Meta-Analysis of Cohort Studies. PLoS ONE 2014, 9, e104861. [Google Scholar] [CrossRef]
- Ye, Z.; Smith, C.; Kullo, I.J. Usefulness of Red Cell Distribution Width to Predict Mortality in Patients With Peripheral Artery Disease. Am. J. Cardiol. 2011, 107, 1241–1245. [Google Scholar] [CrossRef]
- Azab, B.; Torbey, E.; Hatoum, H.; Singh, J.; Khoueiry, G.; Bachir, R.; McGinn, J.J.T.; Mccord, D.; Lafferty, J. Usefulness of Red Cell Distribution Width in Predicting All-Cause Long-Term Mortality after Non-ST-Elevation Myocardial Infarction. Cardiology 2011, 119, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dabbah, S.; Hammerman, H.; Markiewicz, W.; Aronson, D. Relation Between Red Cell Distribution Width and Clinical Outcomes After Acute Myocardial Infarction. Am. J. Cardiol. 2010, 105, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Oh, J.; Kang, S.-M.; Kim, S.Y.; Won, H.; Youn, J.-C.; Park, S.; Jang, Y.; Chung, N. Red blood cell distribution width predicts early mortality in patients with acute dyspnea. Clin. Chim. Acta 2012, 413, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.T.; Kim, E.J.; Han, J.H.; Han, J.S.; Choi, J.; Han, S.H.; Yoo, T.-H.; Kim, Y.S.; Kang, S.-W.; et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care 2013, 17, R282. [Google Scholar] [CrossRef]
- Hou, H.; Sun, T.; Li, C.; Li, Y.; Guo, Z.; Wang, W.; Li, D. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci. Rep. 2017, 7, 43420. [Google Scholar] [CrossRef]
- Hsieh, Y.-P.; Chang, C.-C.; Kor, C.-T.; Yang, Y.; Wen, Y.-K.; Chiu, P.-F. The Predictive Role of Red Cell Distribution Width in Mortality among Chronic Kidney Disease Patients. PLoS ONE 2016, 11, e0162025. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Li, J.; Lin, Y.; Yang, H.; Cao, S. Association Between Red Blood Cell Distribution Width and All-cause Mortality in Chronic Kidney Disease Patients: A Systematic Review and Meta-analysis. Arch. Med. Res. 2017, 48, 378–385. [Google Scholar] [CrossRef]
- Fukasawa, H.; Ishibuchi, K.; Kaneko, M.; Niwa, H.; Yasuda, H.; Kumagai, H.; Furuya, R. Red Blood Cell Distribution Width Is Associated With All-Cause and Cardiovascular Mortality in Hemodialysis Patients. Ther. Apher. Dial. 2017, 21, 565–571. [Google Scholar] [CrossRef]
- Sun, I.O.; Chung, B.H.; Yoon, H.J.; Kim, J.H.; Choi, B.S.; Park, C.W.; Kim, Y.S.; Yang, C.W.; Lee, K.Y. Clinical significance of red blood cell distribution width in the prediction of mortality in patients on peritoneal dialysis: A Strobe-compliant article. Medicine. Kidney Res. Clin. Pract. 2016, 35, 114–118. [Google Scholar] [CrossRef][Green Version]
- SooHoo, M.; Molnar, M.Z.; Ujszaszi, A.; Obi, Y.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. Red blood cell distribution width and mortality and hospitalizations in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2018, 34, 2111–2118. [Google Scholar] [CrossRef]
- Cao, H.-X.; Zhao, X.-D.; Yan, L.; Fan, X.-G.; Shao, F.-M. Correlation between red blood cell distribution width and cardiovascular events in the patients receiving peritoneal dialysis. Medicine 2019, 98, e14376. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, T.S.; Vik, A.; Skjelbakken, T.; Brox, J.; Mathiesen, E.B.; Johnsen, S.H.; Brækkan, S.K.; Hansen, J.-B.; Lappegård, J. Red cell distribution width and carotid atherosclerosis progression The Tromsø Study. Thromb. Haemost. 2015, 113, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, M.; Bo, H.; Hedblad, B.; Persson, M.; Engström, G. Red Cell Distribution Width in Relation to Incidence of Stroke and Carotid Atherosclerosis: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0124957. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, H.; Zhang, Y.; Li, C.; Chunfang, X.; Xia, C. Association between red blood cell distribution width (RDW) and carotid artery atherosclerosis (CAS) in patients with primary ischemic stroke. Arch. Gerontol. Geriatr. 2015, 61, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y. High red blood cell distribution width is closely associated with risk of carotid artery atherosclerosis in patients with hypertension. Exp. Clin. Cardiol. 2010, 15, 37–40. [Google Scholar]
- Sičaja, M.; Pehar, M.; Đerek, L.; Starčević, B.; Vuletić, V.; Romić, Ž.; Božikov, V. Red blood cell distribution width as a prognostic marker of mortality in patients on chronic dialysis: A single center, prospective longitudinal study. Croat. Med. J. 2013, 54, 25–32. [Google Scholar] [CrossRef]
- Khalil, A.; Shehata, M.; Abdeltawab, A.; Onsy, A. Red blood cell distribution width and coronary artery disease severity in diabetic patients. Future Cardiol. 2019, 15, 355–366. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3494867. [Google Scholar] [CrossRef]
- Arbos, K.A.; Claro, L.M.; Borges, L.; Santos, C.A.; Weffort-Santos, A.M. Human erythrocytes as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr. Res. 2008, 28, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Niki, E.; Komuro, E.; Takahashi, M.; Urano, S.; Ito, E.; Terao, K. Oxidative hemolysis of erythrocytes and its inhibition by free radical scavengers. J. Biol. Chem. 1988, 263, 19809–19814. [Google Scholar] [PubMed]
- Tozzi-Ciancarelli, M.G.; Di Giulio, A.; Troiani-Sevi, E.; D’Alfonso, A.; Amicosante, G.; Oratore, A. Human erythrocyte damage at the initial stages of oxidative stress. Cell Biophys. 1989, 15, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tziakas, D.N.; Chalikias, G.; Grapsa, A.; Gioka, T.; Tentes, I.; Konstantinides, S. Red blood cell distribution width–a strong prognostic marker in cardiovascular disease–Is associated with cholesterol content of erythrocyte membrane. Clin. Hemorheol. Microcirc. 2012, 51, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Yuan, H.; Zhou, T.; Kuang, Z.-M. Relationship between red blood cell distribution width and early-stage renal function damage in patients with essential hypertension. J. Hypertens. 2014, 32, 2450–2456. [Google Scholar] [CrossRef]
- Banfi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand. J. Clin. Lab. Investig. 2008, 68, 745–748. [Google Scholar] [CrossRef]
- Ujszaszi, A.; Molnar, M.Z.; Czira, M.E.; Novak, M.; Mucsi, I. Renal function is independently associated with red cell distribution width in kidney transplant recipients: A potential new auxiliary parameter for the clinical evaluation of patients with chronic kidney disease. Br. J. Haematol. 2013, 161, 715–725. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, H.; Fu, S.; Wan, J.; Li, X. Red Blood Cell Distribution Width is an Independent Predictor of AKI and Mortality in Patients in the Coronary Care Unit. Kidney Blood Press. Res. 2017, 42, 1193–1204. [Google Scholar] [CrossRef]
- Gang, L.; Lifang, W. Association of the Elevated Red Blood Cell Distribution Width with the Risk of Developing Diabetes Mellitus. Intern. Med. 2016, 55, 1959–1965. [Google Scholar] [CrossRef]
- Magri, C.J.; Fava, S. Red blood cell distribution width and diabetes-associated complications. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 8, 13–17. [Google Scholar] [CrossRef]
- Chen, T.; Wang, X.; Bi, Q. Red Blood Cell Distribution Width is Associated with Glomerulonephritis in Diabetic Patients with Albuminuria. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924923-1. [Google Scholar]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Tavridou, A.; Georgoulidou, A.; Roumeliotis, A.; Roumeliotis, S.; Giannakopoulou, E.; Papanas, N.; Passadakis, P.; Manolopoulos, V.G.; Vargemezis, V. Association of Plasma Adiponectin and Oxidized Low-Density Lipoprotein with Carotid Intima-Media Thickness in Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 507265. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, A.; Roumeliotis, S.; Panagoutsos, S.; Theodoridis, M.; Argyriou, C.; Tavridou, A.; Georgiadis, G.S. Carotid intima-media thickness is an independent predictor of all-cause mortality and cardiovascular morbidity in patients with diabetes mellitus type 2 and chronic kidney disease. Ren. Fail. 2019, 41, 131–138. [Google Scholar] [CrossRef] [PubMed]

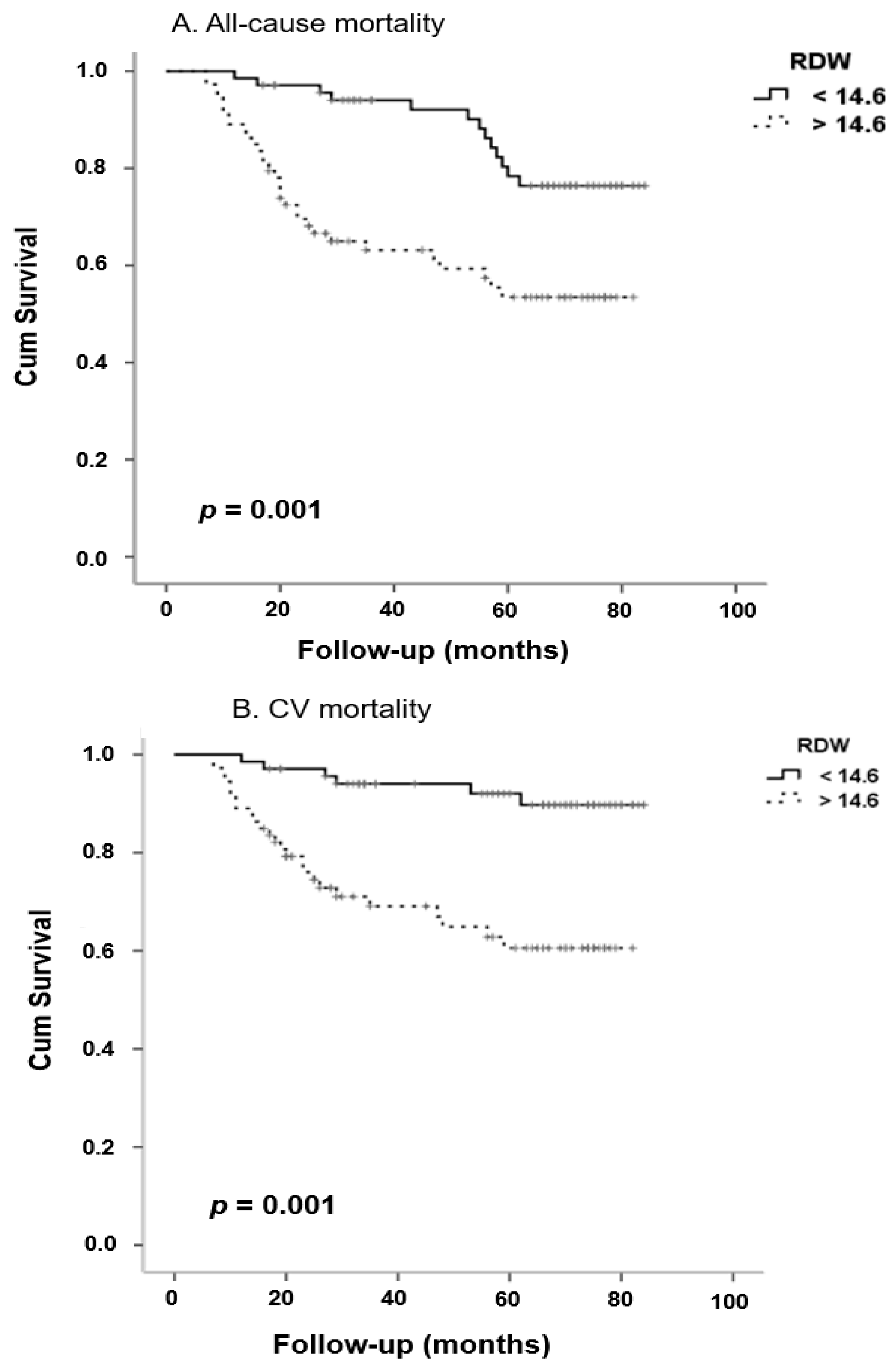
| All Patients (n = 142) | RDW < 14.6% (n = 69) | RDW ≥ 14.6% (n = 73) | p | |
|---|---|---|---|---|
| RDW (%) | 14.6 (12.0–21.5) | 13.4 (12.0–14.4) | 15.8 (14.6–21.5) | <0.0001 |
| Hemoglobin (g/dL) | 12.38 ± 1.84 | 13.11 ± 1.55 | 11.69 ± 1.83 | <0.0001 |
| Age (years) | 68.04 ± 9.05 | 68.33 ± 9.39 | 67.77 ± 8.77 | 0.85 |
| Gender, Female (%) | 45.8 | 49.23 | 50.77 | 0.51 |
| Duration of HP (years) | 14.56 ± 7.51 | 14.57 ± 7.81 | 14.56 ± 7.26 | 0.78 |
| Duration of T2DM (years) | 25.26 ± 7.83 | 13.81 ± 7.70 | 16.65 ± 7.76 | 0.013 |
| Current smoking (%) | 21.13 | 21.74 | 20.55 | 0.51 |
| Presence of MI (%) | 32.39 | 30.43 | 34.24 | 0.38 |
| Presence of Stroke (%) | 12.70 | 8.70 | 16.44 | 0.13 |
| Presence of Angina (%) | 26.76 | 26.09 | 27.40 | 0.51 |
| Presence of PAD (%) | 44.36 | 39.13 | 49.32 | 0.15 |
| Presence of CVD (%) | 65.5 | 60.87 | 69.87 | 0.17 |
| SBP (mmHg) | 137.32 ± 17.50 | 135.93 ± 18.57 | 138.68 ± 16.40 | 0.33 |
| DBP (mmHg) | 77.29 ± 8.89 | 76.49 ± 8.88 | 78.06 ± 8.89 | 0.16 |
| BMI (kg/m2) | 30.71 ± 5.22 | 29.99 ± 4.84 | 31.39 ± 5.50 | 0.06 |
| Albumin (g/dL) | 4.15 ± 0.45 | 4.22 ± 0.39 | 4.09 ± 0.48 | 0.13 |
| Total cholesterol (mg/dL) | 179.41 ± 52.48 | 179.87 ± 50.85 | 178.99 ± 54.33 | 0.76 |
| LDL-cholesterol (mg/dL) | 101.83 ± 43.94 | 101.59 ± 39.93 | 102.06 ± 47.80 | 0.81 |
| HDL-cholesterol (mg/dL) | 45.65 ± 12.69 | 46.40 ± 12.11 | 44.94 ± 13.25 | 0.34 |
| Triglycerides (mg/dL) | 139.0 (27.0–966.0) | 149.72 (27.0–363.0) | 170.67 (59.0–966.0) | 0.61 |
| HbA1c (%) | 7.54 ± 1.14 | 7.61 ± 1.20 | 7.47 ± 1.10 | 0.94 |
| CRP (mg/dL) | 0.24 (0.0–14.0) | 0.20 (0.0–11.0) | 0.31 (0.0–14.0) | 0.016 |
| Urea (mg/dL) | 86.36 ± 57.13 | 66.87 ± 47.90 | 104.78 ± 59.29 | <0.0001 |
| Creatinine (mg/dL) | 2.48 ± 2.34 | 1.92 ± 1.99 | 3.03 ± 2.52 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 47.6 ± 31.81 | 57.84 ± 31.09 | 37.92 ± 29.54 | <0.0001 |
| UPCR | 0.20 (0.01–9.70) | 0.13 (0.01–5.50) | 0.46 (0.01–9.70) | 0.001 |
| UACR (mg/g) | 45.0 (1.0–9700.0) | 26.00 (1.0–1800.0) | 86.00 (2.4–9700.0) | 0.001 |
| cIMT (mm) | 0.86 (0.40–1.78) | 0.8 (0.40–1.61) | 0.90 (0.40–1.78) | 0.004 |
| Ox-LDL (U/L) | 61.07 (22.01–123.44) | 64.65 (21.04–123.44) | 59.91 (22.89–96.69) | 0.21 |
| Parameters | r | p |
|---|---|---|
| Anthropometric—Clinical Parameters | ||
| Age (years) | −0.05 | 0.58 |
| Duration of HP (years) | 0.02 | 0.82 |
| Duration of T2DM (years) | 0.19 | 0.023 |
| SBP (mmHg) | 0.15 | 0.09 |
| DBP (mmHg) | 0.18 | 0.03 |
| BMI (kg/m2) | 0.16 | 0.052 |
| Hematological Parameters | ||
| Hemoglobin (g/dL) | −0.41 | <0.0001 |
| Nutritional—Inflammatory Parameters | ||
| Albumin (g/dL) | −0.20 | 0.026 |
| Total cholesterol (mg/dL) | −0.03 | 0.76 |
| Triglycerides (mg/dL) | 0.09 | 0.27 |
| Ox-LDL (U/L) | −0.90 | 0.35 |
| HbA1c (%) | 0.08 | 0.37 |
| CRP (mg/dL) | 0.24 | 0.004 |
| DKD Markers | ||
| Urea (mg/dL) | 0.38 | <0.0001 |
| Creatinine (mg/dL) | 0.32 | <0.0001 |
| eGFR (mL/min/1.73 m2) | −0.33 | <0.0001 |
| UPCR | 0.30 | 0.001 |
| UACR (mg/g) | 0.29 | 0.002 |
| Vascular Calcification Markers | ||
| cIMT (mm) | 0.22 | 0.008 |
| β | SE | p | CI | |
|---|---|---|---|---|
| Model 1 (Unadjusted) | ||||
| Age | 0.008 | 0.03 | 0.004 | 0.003–0.013 |
| Model 2 (Adjusted) a | ||||
| Age | 0.008 | 0.03 | 0.001 | 0.003–0.013 |
| RDW | 0.031 | 0.012 | 0.012 | 0.007–0.056 |
| BMI | 0.009 | 0.004 | 0.042 | 0.0–0.018 |
| β | SE | p | CI | |
|---|---|---|---|---|
| Model 1 (Unadjusted) | ||||
| Hemoglobin | −0.44 | 0.10 | <0.001 | −0.65 to −0.24 |
| Model 2 (Adjusted) a | ||||
| Hemoglobin | −0.38 | 0.10 | <0.001 | −0.58 to −0.18 |
| UPCR | 0.30 | 0.10 | 0.003 | 0.10–0.49 |
| cIMT | 1.59 | 0.64 | 0.014 | 0.33–2.86 |
| B | HR | 95% CI | p | |
|---|---|---|---|---|
| All-Cause Mortality | ||||
| RDW | 0.24 | 1.27 | 1.1–1.61 | 0.04 |
| eGFR | −0.03 | 0.96 | 0.94–0.99 | 0.02 |
| History of CV event | 2.33 | 10.27 | 1.32–80.1 | 0.03 |
| Cardiovascular Mortality | ||||
| RDW | 0.26 | 1.30 | 1.1–1.56 | 0.01 |
| eGFR | −0.03 | 0.97 | 0.94–0.99 | 0.009 |
| Age | −0.08 | 1.1 | 1.01–1.16 | 0.02 |
| History of CV event | 1.40 | 4.0 | 1.1–14.77 | 0.04 |
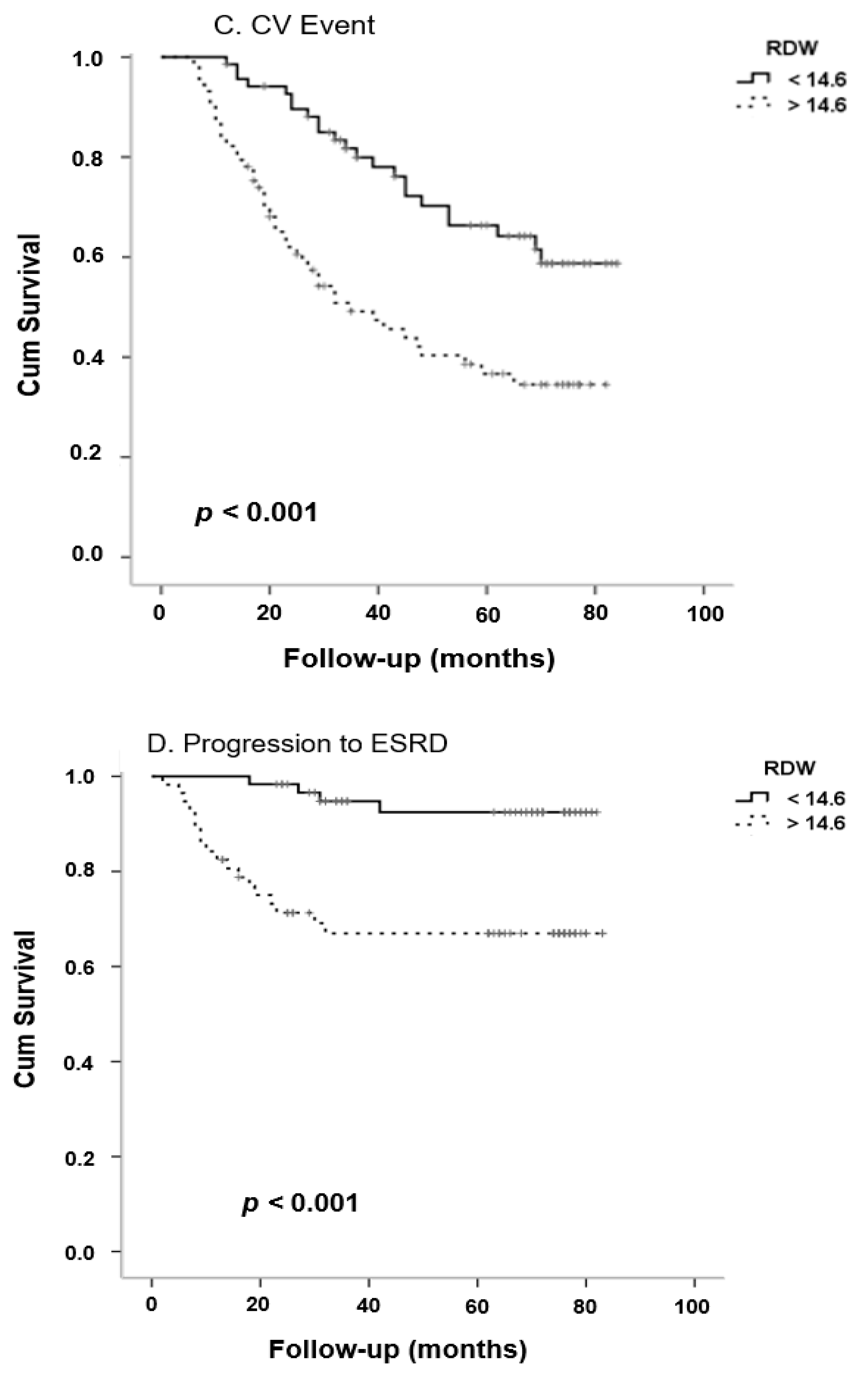
| Cardiovascular Events | ||||
| RDW | 0.33 | 1.40 | 1.17–1.66 | <0.0001 |
| History of CV event | 1.95 | 7.00 | 2.69–18.21 | <0.0001 |
| Progression to ESRD | ||||
| RDW | 0.31 | 1.36 | 1.11–1.66 | 0.003 |
| eGFR | −0.05 | 0.95 | 0.92–0.98 | 0.001 |
| BMI | −0.13 | 0.88 | 0.80–0.98 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumeliotis, S.; Stamou, A.; Roumeliotis, A.; Theodoridis, M.; Leivaditis, K.; Panagoutsos, S.; Liakopoulos, V. Red Blood Cell Distribution Width Is Associated with Deterioration of Renal Function and Cardiovascular Morbidity and Mortality in Patients with Diabetic Kidney Disease. Life 2020, 10, 301. https://doi.org/10.3390/life10110301
Roumeliotis S, Stamou A, Roumeliotis A, Theodoridis M, Leivaditis K, Panagoutsos S, Liakopoulos V. Red Blood Cell Distribution Width Is Associated with Deterioration of Renal Function and Cardiovascular Morbidity and Mortality in Patients with Diabetic Kidney Disease. Life. 2020; 10(11):301. https://doi.org/10.3390/life10110301
Chicago/Turabian StyleRoumeliotis, Stefanos, Aikaterini Stamou, Athanasios Roumeliotis, Marios Theodoridis, Konstantinos Leivaditis, Stylianos Panagoutsos, and Vassilios Liakopoulos. 2020. "Red Blood Cell Distribution Width Is Associated with Deterioration of Renal Function and Cardiovascular Morbidity and Mortality in Patients with Diabetic Kidney Disease" Life 10, no. 11: 301. https://doi.org/10.3390/life10110301
APA StyleRoumeliotis, S., Stamou, A., Roumeliotis, A., Theodoridis, M., Leivaditis, K., Panagoutsos, S., & Liakopoulos, V. (2020). Red Blood Cell Distribution Width Is Associated with Deterioration of Renal Function and Cardiovascular Morbidity and Mortality in Patients with Diabetic Kidney Disease. Life, 10(11), 301. https://doi.org/10.3390/life10110301







