Prognostic Role of FGFR3 Expression Status and Tumor-Related MicroRNAs Level in Association with PD-L1 Expression in Primary Luminal Non-Muscular Invasive Bladder Carcinoma
Abstract
1. Introduction
2. Results
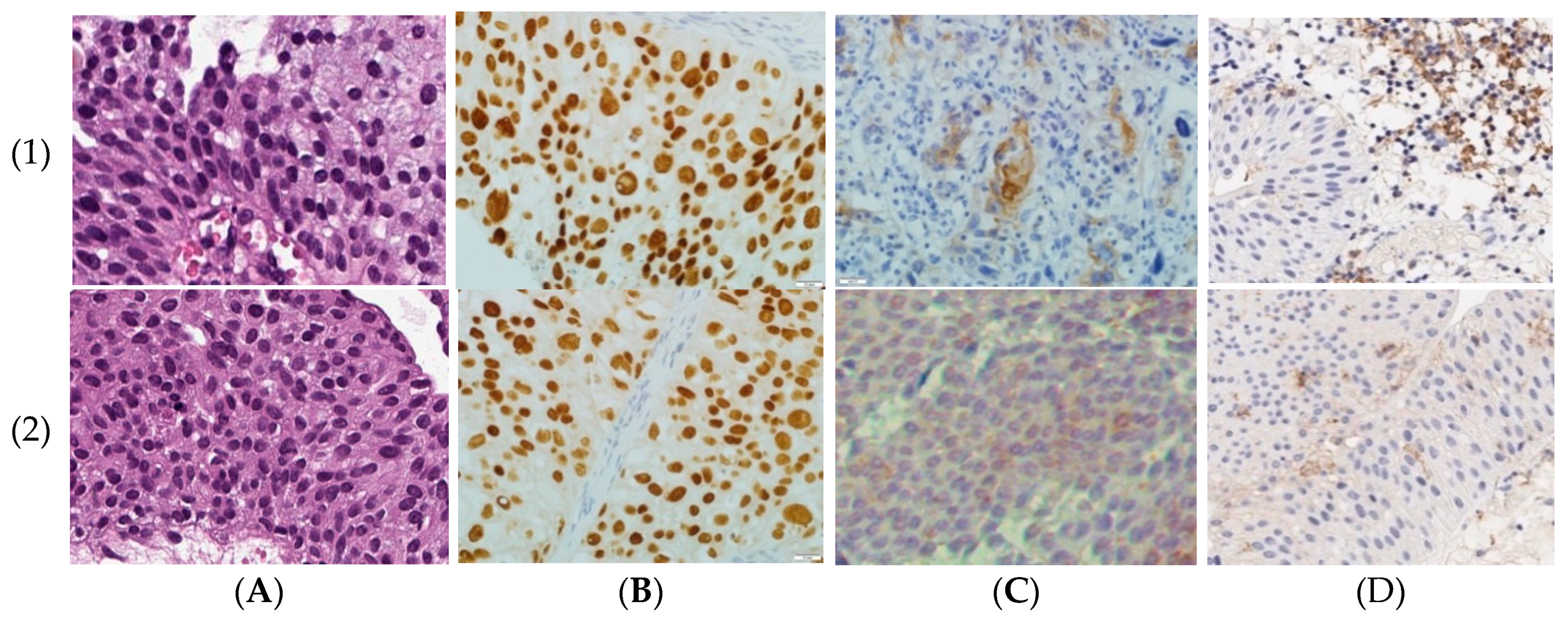
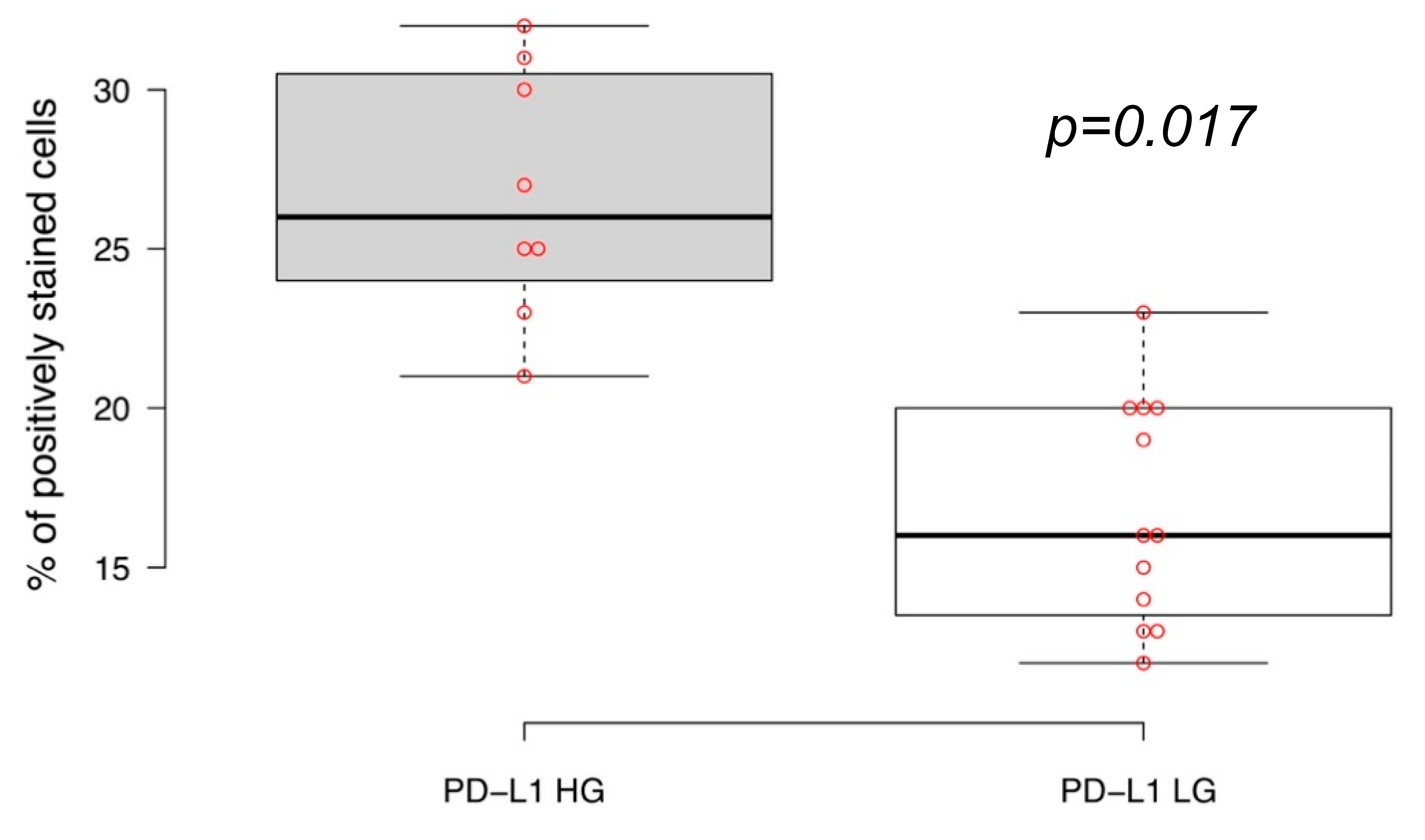
2.1. PD-L1 Expression in Primary Luminal NMIBC
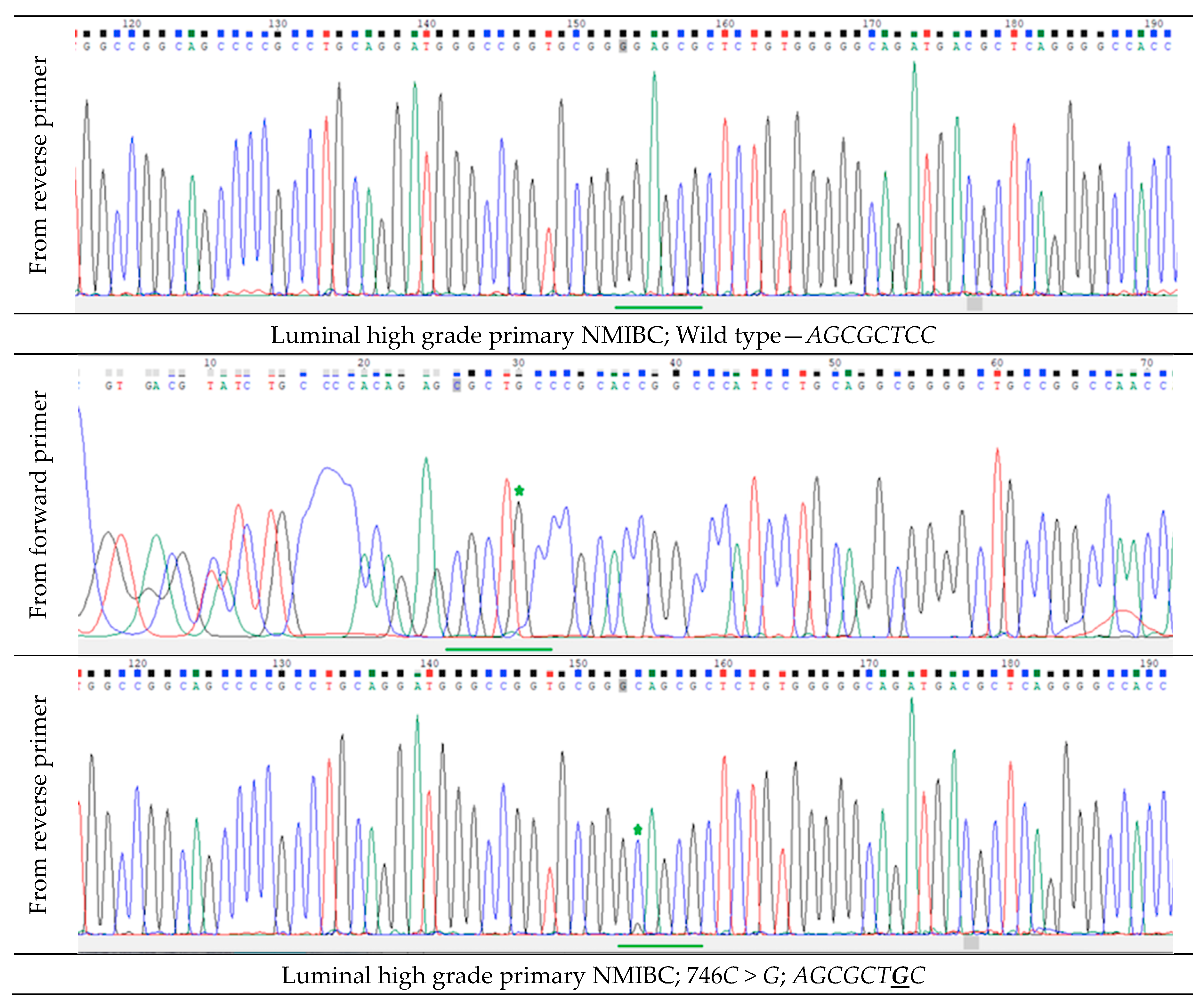
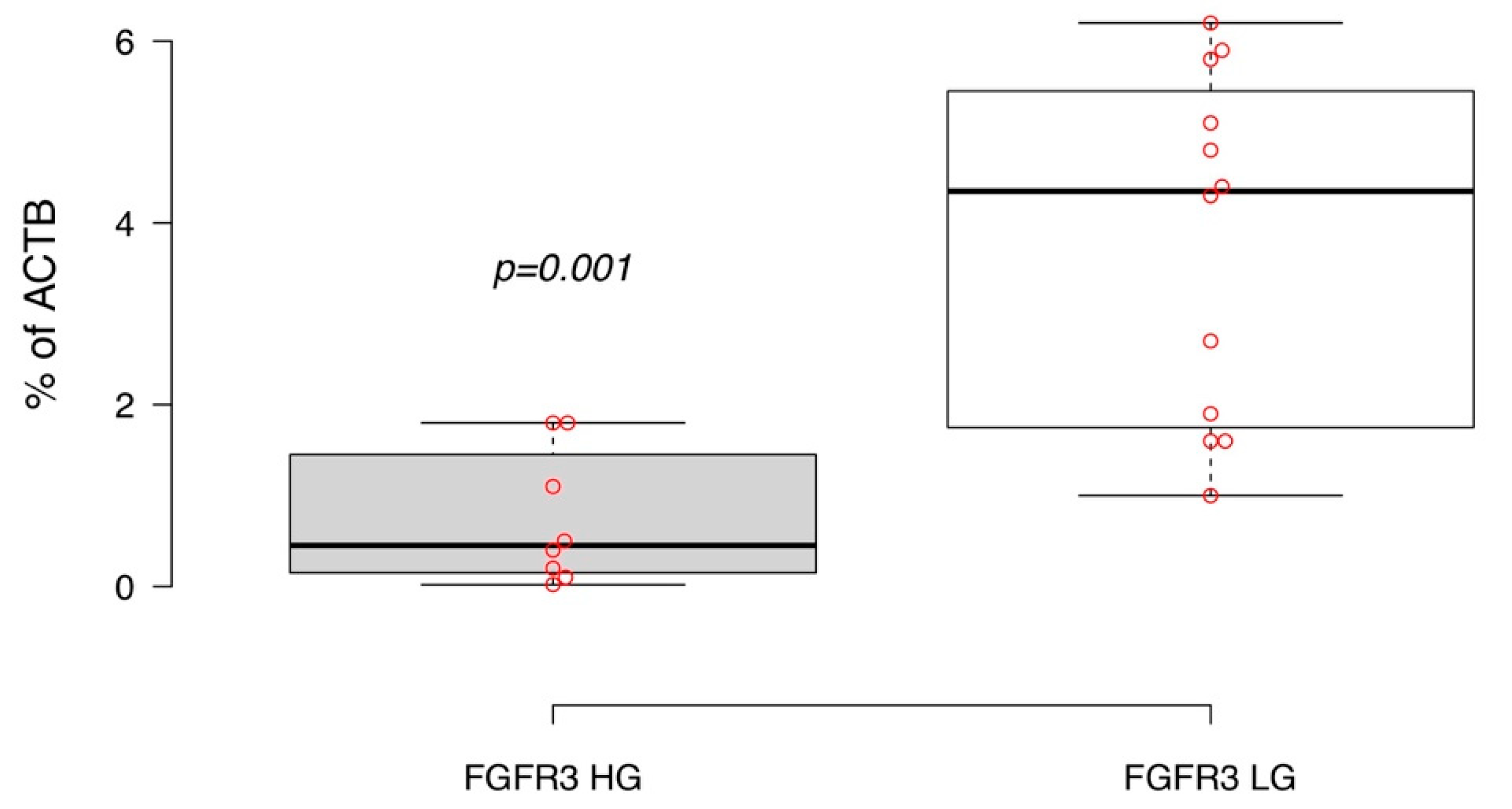
2.2. FGFR3 Expression and Hotspot Mutations in Primary Luminal NMIBC
2.3. MicroRNA-145 and MicroRNA-200a Expression in Primary Luminal NMIBC
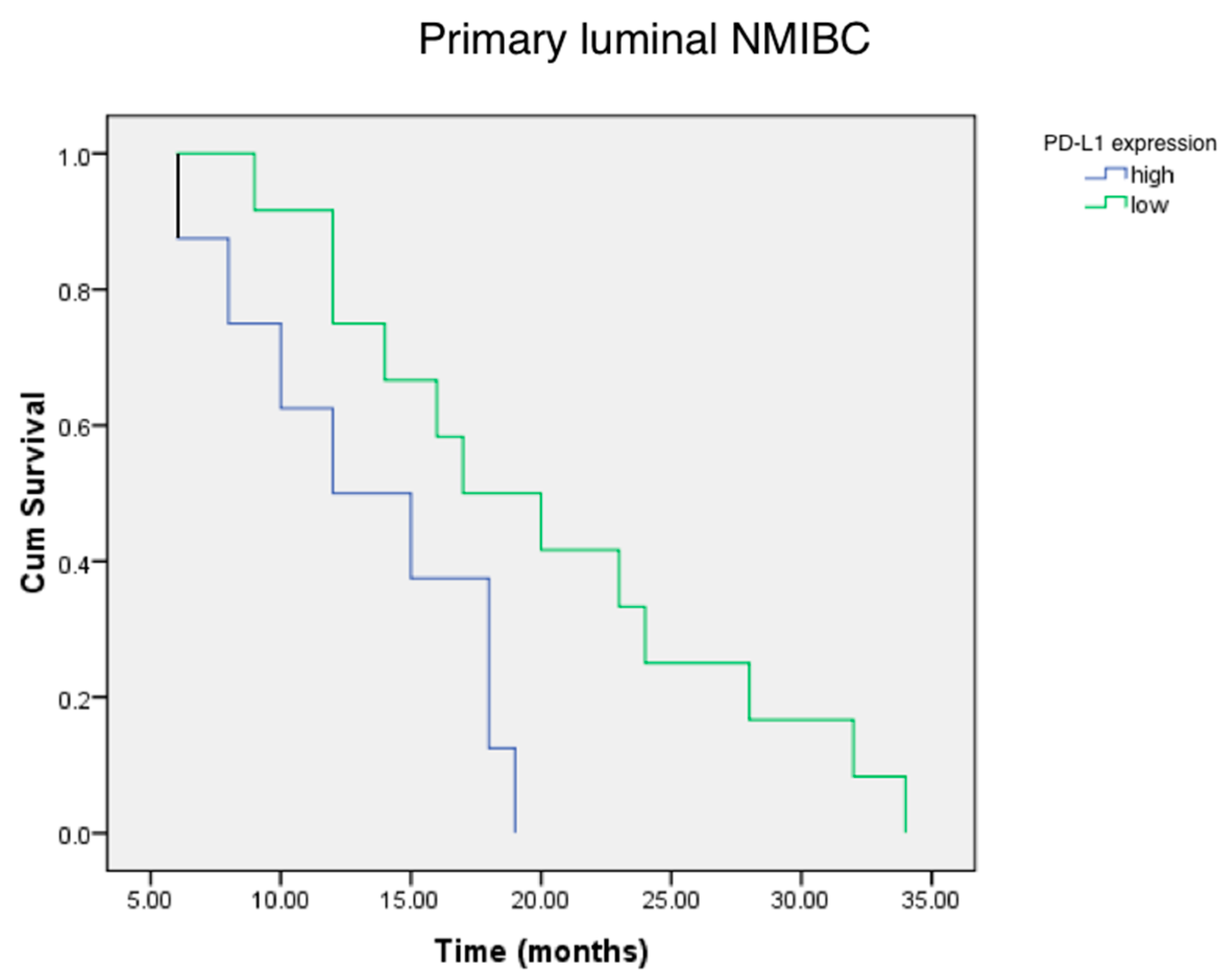
2.4. PD-L1, FGFR3 and MicroRNAs Expression, and Relapse-Free Survival in Luminal NMIBC
3. Discussion
4. Materials and Methods
4.1. Ethic Procedures
4.2. Study Population and Follow-Up Protocol
4.3. Tissue Sample Preparation and Processing
4.4. DNA and RNA Isolation
4.5. Reverse Transcription
4.6. Real-Time Polymerase Chain Reaction (PCR)
4.7. Detection of FGFR3 Gene Hotspot Mutations
4.8. Immunohistochemistry (IHC)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette-Guerin |
| EORTC | European Organization for Research and Treatment of Cancer |
| FGFR3 | Fibroblast growth factor receptor 3 |
| GATA3 | Transcription factor encoded by GATA3 gene |
| IHC | Immunohistochemistry |
| CR5/6 | Cytokeratin 5 and 6 |
| MIBC | Muscular-invasive bladder cancer |
| miR | microRNA |
| NMIBC | Non-muscular invasive bladder cancer |
| PCR | Polymerase chain reaction |
| PD1 | Programmed death receptor 1 |
| PD-L1 | Programmed death receptor ligand 1 |
| RFS | Relapse-free survival |
| TUR | Transurethral resection |
Appendix A
| Patient | Tumor Grade and Stage | Gender | Age, Years | Tumor Histology |
|---|---|---|---|---|
| 1 | T1, High grade | Male | 54 | UPC |
| 2 | T1, Low grade | Male | 57 | UPC |
| 3 | T1, High grade | Female | 61 | MC |
| 4 | T1, Low grade | Male | 60 | UPC |
| 5 | T1, Low grade | Female | 51 | UPC |
| 6 | T1, Low grade | Female | 64 | UPC |
| 7 | T1, High grade | Female | 67 | UPC |
| 8 | T1, High grade | Male | 59 | UPC |
| 9 | T1, Low grade | Female | 53 | UPC |
| 10 | T1, High grade | Male | 49 | MP |
| 11 | T1, Low grade | Male | 58 | UPC |
| 12 | T1, Low grade | Female | 50 | UPC |
| 13 | T1, Low grade | Male | 55 | UPC |
| 14 | T1, Low grade | Male | 48 | UPC |
| 15 | T1, Low grade | Male | 63 | UPC |
| 16 | T1, High grade | Female | 70 | UPC |
| 17 | T1, High grade | Male | 69 | UPC |
| 18 | T1, Low grade | Female | 47 | UPC |
| 19 | T1, Low grade | Female | 55 | UPC |
| 20 | T1, High grade | Male | 52 | UPC |
| Total | Low grade (12), High grade (8) | Male (11), Female (9) | Average (Mean ± SD) 57.1 ± 2.8 | UPC (18) MC (2) |
Appendix B

References
- Murphy, C.R.; Karnes, R.J. Bladder Cancer in Males: A Comprehensive Review of Urothelial Carcinoma of the Bladder. J. Men’s Health 2014, 11, 18–27. [Google Scholar] [CrossRef]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.L.; Hauser, R.A. The health economics of bladder cancer: A comprehensive review of published literature. PharmacoEconomics 2003, 21, 1315–1330. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, A.J.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Hurst, C.D.; Knowles, M.A. Bladder cancer: Multi-omic profiling refines the molecular view. Nat. Rev. Clin. Oncol. 2017, 15, 203–204. [Google Scholar] [CrossRef]
- Eifler, J.B.; Scarpato, K.R.; Clark, P.E. Management of noninvasive bladder cancers. Curr. Opin. Oncol. 2015, 27, 185–190. [Google Scholar] [CrossRef]
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdell, D.E.; Zhang, S.; et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. Oncotarget 2016, 12, 105–117. [Google Scholar]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: An update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti–Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients with Advanced Urothelial Bladder Cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Kawahara, T.; Ishiguro, Y.; Ohtake, S.; Kato, I.; Ito, Y.; Ito, H.; Makiyama, K.; Kondo, K.; Miyoshi, Y.; Yumura, Y.; et al. PD-1 and PD-L1 are more highly expressed in high-grade bladder cancer than in low-grade cases: PD-L1 might function as a mediator of stage progression in bladder cancer. BMC Urol. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Descamps-Dudez, O.; Louvet, E.; Bernard, M.; Auguste, A.; Gouy, S.; Scoazec, J.Y.; Genestie, C.; Adam, J. Heterogeneity in PD-L1 expression and CD8+ infiltrates in low grade versus high grade serous ovarian carcinomas. In Virchows Archiv; Springer: New York, NY, USA, 2016; Volume 469, p. S104. [Google Scholar]
- Blinova, E.; Enikeev, D.; Roshchin, D.; Samyshina, E.; Deryabina, O.; Swidsinski, A.; Blinov, D.; Kogan, E.A.; Dudina, M.; Barakat, H.; et al. Relapse-Free Survival and PD-L1 Expression in First High- and Low-Grade Relapsed Luminal, Basal and Double-Negative P53-Mutant Non-Muscular Invasive Bladder Cancer Depending on Previous Chemo- and Immunotherapy. Cancers 2020, 12, 1316. [Google Scholar] [CrossRef]
- Huang, T.; Li, B.-Q.; Cai, Y.-D. The Integrative Network of Gene Expression, MicroRNA, Methylation and Copy Number Variation in Colon and Rectal Cancer. Curr. Bioinform. 2016, 11, 59–65. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z.; Danilova, I.G.; Bolkov, M.A.; Tuzankina, I.A.; Liu, G. Identification of miR-200c and miR141-Mediated lncRNA-mRNA Crosstalks in Muscle-Invasive Bladder Cancer Subtypes. Front. Genet. 2018, 9, 422. [Google Scholar] [CrossRef]
- Fang, Z.; Dai, W.; Wang, X.; Chen, W.; Shen, C.; Ye, G.; Li, L. Circulating miR-205: A promising biomarker for the detection and prognosis evaluation of bladder cancer. Tumor Biol. 2015, 37, 8075–8082. [Google Scholar] [CrossRef]
- Yun, S.J.; Jeong, P.; Kim, W.T.; Kim, T.H.; Lee, Y.-S.; Song, P.H.; Choi, Y.H.; Kim, I.Y.; Moon, S.-K.; Kim, W.-J. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int. J. Oncol. 2012, 41, 1871–1878. [Google Scholar] [CrossRef]
- Borkowska, E.M.; Konecki, T.; Pietrusiński, M.; Borowiec, M.; Jabłonowski, Z. MicroRNAs Which Can Prognosticate Aggressiveness of Bladder Cancer. Cancers 2019, 11, 1551. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Ryu, D.-S.; Kim, W.-J.; Kim, S.-J. Aberrantly expressed microRNAs in the context of bladder tumorigenesis. Investig. Clin. Urol. 2016, 57, S52–S59. [Google Scholar] [CrossRef]
- Kang, H.W.; Kim, Y.-H.; Jeong, P.; Park, C.; Kim, W.T.; Ryu, D.H.; Cha, E.-J.; Ha, Y.-S.; Kim, T.-H.; Kwon, T.G.; et al. Expression levels of FGFR3 as a prognostic marker for the progression of primary pT1 bladder cancer and its association with mutation status. Oncol. Lett. 2017, 14, 3817–3824. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Van Rhijn, B.W.G.; Prigoda, N.L.; Bapat, B.; Liu, L.; Boström, P.J.; Fleshner, N.E.; Gallie, B.; Zlotta, A.R.; Jewett, M.A.S.; et al. FGFR3 mutations, but not FGFR3 expression and FGFR3 copy-number variations, are associated with favourable non-muscle invasive bladder cancer. Virchows Archiv 2014, 465, 207–213. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Soria, F.; Krabbe, L.-M.; Todenhöfer, T.; Dobruch, J.; Mitra, A.P.; Inman, B.A.; Gust, K.M.; Lotan, Y.; Shariat, S.F. Molecular markers in bladder cancer. World J. Urol. 2019, 37, 31–40. [Google Scholar] [CrossRef]
- Mbeutcha, A.; Lucca, I.; Mathieu, R.; Lotan, Y.; Shariat, S.F. Current Status of Urinary Biomarkers for Detection and Surveillance of Bladder Cancer. Urol. Clin. N. Am. 2016, 43, 47–62. [Google Scholar] [CrossRef]
- Tabayoyong, W.; Kamat, A.M. Current Use and Promise of Urinary Markers for Urothelial Cancer. Curr. Urol. Rep. 2018, 19, 96. [Google Scholar] [CrossRef]
- Blinova, E.; Roshchin, D.; Kogan, E.A.; Samyshina, E.; Demura, T.; Deryabina, O.; Suslova, I.; Blinov, D.; Zhdanov, P.; Osmanov, U.; et al. Patient-Derived Non-Muscular Invasive Bladder Cancer Xenografts of Main Molecular Subtypes of the Tumor for Anti-Pd-l1 Treatment Assessment. Cells 2019, 8, 526. [Google Scholar] [CrossRef]
- Pashaei, E.; Guzel, E.; Ozgurses, M.E.; Demirel, G.; Aydin, N.; Ozen, M. A Meta-Analysis: Identification of Common Mir-145 Target Genes that have Similar Behavior in Different GEO Datasets. PLoS ONE 2016, 11, e0161491. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Luo, Y.; Xu, J.; Liufu, H.; Tian, Z.; Huang, C.; Li, J.; Huang, C. A Feedback Loop Formed by ATG7/Autophagy, FOXO3a/miR-145 and PD-L1 Regulates Stem-Like Properties and Invasion in Human Bladder Cancer. Cancers 2019, 11, 349. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Van Der Meijden, A.P.M.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Soukup, V.; Čapoun, O.; Cohen, D.; Hernández, V.; Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Lam, T.; MacLennan, S.; et al. Prognostic Performance and Reproducibility of the 1973 and 2004/2016 World Health Organization Grading Classification Systems in Non–muscle-invasive Bladder Cancer: A European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel Systematic Review. Eur. Urol. 2017, 72, 801–813. [Google Scholar] [CrossRef]
- Baust, J.M.; Snyder, K.K.; Van Buskirk, R. Integrating Molecular Control to Improve Cryopreservation Outcome. Biopreserv. Biobank. 2017, 15, 134–141. [Google Scholar] [CrossRef]
- Wang, C.C.; Tsai, Y.C.; Jeng, Y.M. Biological significance of GATA3, cytokeratin 20, cytokeratin 5/6 and p53 expression in muscle-invasive bladder cancer. PLoS ONE 2019, 14, e0221785. [Google Scholar] [CrossRef]
- Lerner, S.P.; McConkey, D.J.; Hoadley, K.A.; Chan, K.S.; Kim, W.Y.; Radvanyi, F.; Höglund, M.; Real, F.X. Bladder Cancer Molecular Taxonomy: Summary from a Consensus Meeting. Bladder Cancer 2016, 2, 37–47. [Google Scholar] [CrossRef]
- Ventana PD-L1 (SP263) Assay Staining in Urothelial Carcinoma. Interpretation Guide. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/p160046c.pdf (accessed on 26 December 2019).

| Variables | HR | 95% CI | p-Value |
|---|---|---|---|
| Tumor grade | 571.72 | 11.03–2.96 | 0.002 |
| PD-L1 expression | 2.33 | 0.92–1.92 | 0.012 |
| miR-200a expression | 0.98 | 0.95–1.01 | 0.40 |
| miR-145 expression | 0.99 | 0.93–1.06 | 0.96 |
| FGFR3 expression | 0.08 | 0.17–0.42 | 0.003 |
| FGFR3 gene mutations | 1.46 | 0.35–6.00 | 0.593 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinova, E.; Buzdin, A.; Enikeev, D.; Roshchin, D.; Suntsova, M.; Samyshina, E.; Drobyshev, A.; Deryabina, O.; Demura, T.; Blinov, D.; et al. Prognostic Role of FGFR3 Expression Status and Tumor-Related MicroRNAs Level in Association with PD-L1 Expression in Primary Luminal Non-Muscular Invasive Bladder Carcinoma. Life 2020, 10, 305. https://doi.org/10.3390/life10110305
Blinova E, Buzdin A, Enikeev D, Roshchin D, Suntsova M, Samyshina E, Drobyshev A, Deryabina O, Demura T, Blinov D, et al. Prognostic Role of FGFR3 Expression Status and Tumor-Related MicroRNAs Level in Association with PD-L1 Expression in Primary Luminal Non-Muscular Invasive Bladder Carcinoma. Life. 2020; 10(11):305. https://doi.org/10.3390/life10110305
Chicago/Turabian StyleBlinova, Ekaterina, Anton Buzdin, Dmitry Enikeev, Dmitry Roshchin, Maria Suntsova, Elena Samyshina, Aleksey Drobyshev, Olga Deryabina, Tatiana Demura, Dmitry Blinov, and et al. 2020. "Prognostic Role of FGFR3 Expression Status and Tumor-Related MicroRNAs Level in Association with PD-L1 Expression in Primary Luminal Non-Muscular Invasive Bladder Carcinoma" Life 10, no. 11: 305. https://doi.org/10.3390/life10110305
APA StyleBlinova, E., Buzdin, A., Enikeev, D., Roshchin, D., Suntsova, M., Samyshina, E., Drobyshev, A., Deryabina, O., Demura, T., Blinov, D., Shich, E., Barakat, H., Borger, P., Merinov, D., Kachmazov, A., Serebrianyi, S., Tumutolova, O., Potoldykova, N., Zhdanov, P., ... Perepechin, D. (2020). Prognostic Role of FGFR3 Expression Status and Tumor-Related MicroRNAs Level in Association with PD-L1 Expression in Primary Luminal Non-Muscular Invasive Bladder Carcinoma. Life, 10(11), 305. https://doi.org/10.3390/life10110305






