Bioluminescence-Driven Optogenetics
Abstract
1. Introduction
2. Energy Transfer to Fluorescent Proteins
3. Delivering Light to Optogenetic Tools
4. Towards Complexity: Programming Intracellular and Cell-Cell Interactions with Light
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Oba, Y.; Stevani, C.V.; Oliveira, A.G.; Tsarkova, A.S.; Chepurnykh, T.V.; Yampolsky, I.V. Selected Least Studied but not Forgotten Bioluminescent Systems. Photochem. Photobiol. 2017, 93, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Haddock, S.H.D.; Moline, M.A.; Case, J.F. Bioluminescence in the Sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, A.; Sarkisyan, K.S. A brief review of bioluminescent systems (2019). Curr. Genet. 2019, 65, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Kotlobay, A.A.; Sarkisyan, K.S.; Mokrushina, Y.A.; Marcet-Houben, M.; Serebrovskaya, E.O.; Markina, N.M.; Somermeyer, L.G.; Gorokhovatsky, A.Y.; Vvedensky, A.; Purtov, K.V.; et al. Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. USA 2018, 115, 12728–12732. [Google Scholar] [CrossRef]
- Gregor, C.; Pape, J.K.; Gwosch, K.C.; Gilat, T.; Sahl, S.J.; Hell, S.W. Autonomous bioluminescence imaging of single mammalian cells with the bacterial bioluminescence system. Proc. Natl. Acad. Sci. USA 2019, 116, 26491–26496. [Google Scholar] [CrossRef]
- Conway, M.; Xu, T.; Kirkpatrick, A.; Ripp, S.; Sayler, G.; Close, D. Real-time tracking of stem cell viability, proliferation, and differentiation with autonomous bioluminescence imaging. BMC Biol. 2020, 18, 1–14. [Google Scholar] [CrossRef]
- Mitiouchkina, T.; Mishin, A.S.; Somermeyer, L.G.; Markina, N.M.; Chepurnyh, T.V.; Guglya, E.B.; Karataeva, T.A.; Palkina, K.A.; Shakhova, E.S.; Fakhranurova, L.I.; et al. Plants with genetically encoded autoluminescence. Nat. Biotechnol. 2020, 38, 944–946. [Google Scholar] [CrossRef]
- Khakhar, A.; Starker, C.G.; Chamness, J.C.; Lee, N.; Stokke, S.; Wang, C.; Swanson, R.; Rizvi, F.; Imaizumi, T.; Voytas, D.F. Building customizable auto-luminescent luciferase-based reporters in plants. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Fan, F.; Wood, K.V. Bioluminescent Assays for High-Throughput Screening. ASSAY Drug Dev. Technol. 2007, 5, 127–136. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Prescher, J.A. Building Biological Flashlights: Orthogonal Luciferases and Luciferins for in Vivo Imaging. Accounts Chem. Res. 2019, 52, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Jin, J.; Geng, Z.; Qi, Q.; Liang, Q. Programming Bacteria With Light—Sensors and Applications in Synthetic Biology. Front. Microbiol. 2018, 9, 2692. [Google Scholar] [CrossRef] [PubMed]
- Kolar, K.; Knobloch, C.; Stork, H.; Žnidarič, M.; Weber, W. OptoBase: A Web Platform for Molecular Optogenetics. ACS Synth. Biol. 2018, 7, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Hongdusit, A.; Liechty, E.T.; Fox, J.M. Optogenetic interrogation and control of cell signaling. Curr. Opin. Biotechnol. 2020, 66, 195–206. [Google Scholar] [CrossRef]
- Brodl, E.; Winkler, A.; Macheroux, P. Molecular Mechanisms of Bacterial Bioluminescence. Comput. Struct. Biotechnol. J. 2018, 16, 551–564. [Google Scholar] [CrossRef]
- Gregor, C.; Gwosch, K.C.; Sahl, S.J.; Hell, S.W. Strongly enhanced bacterial bioluminescence with the ilux operon for single-cell imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 962–967. [Google Scholar] [CrossRef]
- Hollis, R.; Lagido, C.; Pettitt, J.; Porter, A.; Killham, K.; Paton, G.I.; Glover, L. Toxicity of the bacterial luciferase substrate, n -decyl aldehyde, to Saccharomyces cerevisiae and Caenorhabditis elegans. FEBS Lett. 2001, 506, 140–142. [Google Scholar] [CrossRef]
- Titushin, M.S.; Feng, Y.; Lee, J.; Vysotski, E.S.; Ouyang, S. Protein-protein complexation in bioluminescence. Protein Cell 2012, 2, 957–972. [Google Scholar] [CrossRef]
- Bhuckory, S.; Kays, J.C.; Dennis, A.M. In Vivo Biosensing Using Resonance Energy Transfer. Biosensors 2019, 9, 76. [Google Scholar] [CrossRef]
- Takai, A.; Nakano, M.; Saito, K.; Haruno, R.; Watanabe, T.M.; Ohyanagi, T.; Jin, T.; Okada, Y.; Nagai, T. Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 4352–4356. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kimura, T.; Shinoda, H.; Bai, G.; Daniels, M.J.; Arai, Y.; Nakano, M.; Nagai, T. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 2016, 7, 13718. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Oh, Y.-H.; Sens, A.; Ataie, N.; Dana, H.; Macklin, J.J.; Laviv, T.; Welf, E.S.; Dean, K.M.; Zhang, F.; et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 2016, 34, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Schaub, F.X.; Reza, S.; Flaveny, C.A.; Li, W.; Musicant, A.M.; Hoxha, S.; Guo, M.; Cleveland, J.L.; Amelio, A.L. Fluorophore-NanoLuc BRET Reporters Enable Sensitive In Vivo Optical Imaging and Flow Cytometry for Monitoring Tumorigenesis. Cancer Res. 2015, 75, 5023–5033. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Zylstra, J.; Fontaine, D.M.; Branchini, B.R.; Maye, M.M. Novel multistep BRET-FRET energy transfer using nanoconjugates of firefly proteins, quantum dots, and red fluorescent proteins. Nanoscale 2013, 5, 5303–5306. [Google Scholar] [CrossRef]
- Delbeke, J.; Hoffman, L.; Mols, K.; Braeken, D.; Prodanov, D. And Then There Was Light: Perspectives of Optogenetics for Deep Brain Stimulation and Neuromodulation. Front. Neurosci. 2017, 11, 663. [Google Scholar] [CrossRef]
- Banerjee, S.; Mitra, D. Structural Basis of Design and Engineering for Advanced Plant Optogenetics. Trends Plant. Sci. 2020, 25, 35–65. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The Development and Application of Optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in Neural Systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: Fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Al-Juboori, S.I.; Dondzillo, A.; Stubblefield, E.A.; Felsen, G.; Lei, T.C.; Klug, A. Light Scattering Properties Vary across Different Regions of the Adult Mouse Brain. PLoS ONE 2013, 8, e67626. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, Y.G.; Roshchin, M.V.; Lanin, A.A.; Balaban, P.M.; Zheltikov, A.M.; Belousov, V.V.; Nikitin, E.S. Thermogenetics as a New Direction in Controlling the Activity of Neural Networks. Neurosci. Behav. Physiol. 2020, 50, 1018–1023. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, X.; Chong, P.; Liu, J.; Andre, L.N.; Ong, K.S.; Jr, K.B.; Mahdi, A.I.; Li, J.; Fenno, L.E.; et al. Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl. Acad. Sci. USA 2019, 116, 26332–26342. [Google Scholar] [CrossRef]
- Sternson, S.M.; Roth, B.L. Chemogenetic Tools to Interrogate Brain Functions. Annu. Rev. Neurosci. 2014, 37, 387–407. [Google Scholar] [CrossRef]
- Berglund, K.; Birkner, E.; Augustine, G.J.; Hochgeschwender, U. Light-Emitting Channelrhodopsins for Combined Optogenetic and Chemical-Genetic Control of Neurons. PLoS ONE 2013, 8, e59759. [Google Scholar] [CrossRef]
- Berglund, K.; Clissold, K.; Li, H.E.; Wen, L.; Park, S.Y.; Gleixner, J.; Klein, M.E.; Lu, D.; Barter, J.W.; Rossi, M.A.; et al. Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation. Proc. Natl. Acad. Sci. USA 2016, 113, E358–E367. [Google Scholar] [CrossRef]
- Park, S.Y.; Song, S.-H.; Palmateer, B.; Pal, A.; Petersen, E.D.; Shall, G.P.; Welchko, R.M.; Ibata, K.; Miyawaki, A.; Augustine, G.J.; et al. Novel luciferase–opsin combinations for improved luminopsins. J. Neurosci. Res. 2017, 98, 410–421. [Google Scholar] [CrossRef]
- Berglund, K.; Fernandez, A.M.; Gutekunst, C.N.; Hochgeschwender, U.; Gross, R.E. Step-function luminopsins for bimodal prolonged neuromodulation. J. Neurosci. Res. 2020, 98, 422–436. [Google Scholar] [CrossRef]
- Medendorp, W.E.; Pal, A.; Waddell, M.; Björefeldt, A.; Moore, C.I.; Hochgeschwender, U. Selective postnatal excitation of neocortical pyramidal neurons results in distinctive behavioral and circuit deficits in adulthood. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tung, J.K.; Gutekunst, C.-A.; Gross, R.E. Inhibitory luminopsins: Genetically-encoded bioluminescent opsins for versatile, scalable and hardware-independent optogenetic inhibition. Sci. Rep. 2015, 5, 14366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Tung, J.K.; Wang, Z.; Yu, S.P.; Gross, R.E.; Wei, L.; Berglund, K. Improved trafficking and expression of luminopsins for more efficient optical and pharmacological control of neuronal activity. J. Neurosci. Res. 2020, 98, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, M.; More, A.I.; Friedman, N.G.; Hochgeschwender, U.; Moore, C.I. The BioLuminescent-OptoGenetic in vivo response to coelenterazine is proportional, sensitive, and specific in neocortex. J. Neurosci. Res. 2020, 98, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, M.L.; De Wergifosse, B.; Trouet, A.; Baguet, F.; Marchand-Brynaert, J.; Rees, J.-F. Antioxidative properties of natural coelenterazine and synthetic methyl coelenterazine in rat hepatocytes subjected to tert-butyl hydroperoxide-induced oxidative stress. Biochem. Pharmacol. 2000, 60, 471–478. [Google Scholar] [CrossRef]
- Dubuisson, M.L.N.; Rees, J.-F.; Marchand-Brynaert, J. Coelenterazine (Marine Bioluminescent Substrate): A Source of Inspiration for the Discovery of Novel Antioxidants. Drug Dev. Ind. Pharm. 2005, 31, 827–849. [Google Scholar] [CrossRef]
- Land, B.B.; Brayton, C.E.; Furman, K.E.; eLaPalombara, Z.; Dileone, R.J. Optogenetic inhibition of neurons by internal light production. Front. Behav. Neurosci. 2014, 8, 108. [Google Scholar] [CrossRef]
- Moore, C.I.; Berglund, K. BL-OG: BioLuminescent-OptoGenetics. J. Neurosci. Res. 2019, 98, 469–470. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Tung, J.K.; Gross, R.E.; English, A.W. Motoneuron activity is required for enhancements in functional recovery after peripheral nerve injury in exercised female mice. J. Neurosci. Res. 2020, 98, 448–457. [Google Scholar] [CrossRef]
- Tung, J.K.; Shiu, F.H.; Ding, K.; Gross, R.E. Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol. Dis. 2018, 109, 1–10. [Google Scholar] [CrossRef]
- Zenchak, J.R.; Palmateer, B.; Dorka, N.; Brown, T.M.; Wagner, L.; Medendorp, W.E.; Petersen, E.D.; Prakash, M.; Hochgeschwender, U. Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model. J. Neurosci. Res. 2020, 98, 458–468. [Google Scholar] [CrossRef]
- Yu, S.P.; Tung, J.K.; Wei, Z.Z.; Chen, D.; Berglund, K.; Zhong, W.; Zhang, J.Y.; Gu, X.; Song, M.; Gross, R.E.; et al. Optochemogenetic Stimulation of Transplanted iPS-NPCs Enhances Neuronal Repair and Functional Recovery after Ischemic Stroke. J. Neurosci. 2019, 39, 6571–6594. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.D.; Sharkey, E.D.; Pal, A.; Shafau, L.O.; Zenchak, J.R.; Peña, A.J.; Aggarwal, A.; Prakash, M.; Hochgeschwender, U. Restoring Function After Severe Spinal Cord Injury Through Bioluminescence-Driven Optogenetics. bioRxiv 2019. [Google Scholar] [CrossRef]
- Bulina, M.E.; Chudakov, D.M.; Britanova, O.V.; Yanushevich, Y.G.; Staroverov, D.B.; Chepurnykh, T.V.; Merzlyak, E.M.; Shkrob, M.A.; Lukyanov, S.; Lukyanov, K.A. A genetically encoded photosensitizer. Nat. Biotechnol. 2006, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sarkisyan, K.S.; Zlobovskaya, O.A.; Gorbachev, D.A.; Bozhanova, N.G.; Sharonov, G.; Staroverov, D.B.; Egorov, E.S.; Ryabova, A.V.; Solntsev, K.M.; Mishin, A.S.; et al. KillerOrange, a Genetically Encoded Photosensitizer Activated by Blue and Green Light. PLoS ONE 2015, 10, e0145287. [Google Scholar] [CrossRef]
- Byrne, L.C.; Khalid, F.; Lee, T.; Zin, E.A.; Greenberg, K.P.; Visel, M.; Schaffer, D.V.; Flannery, J.G. AAV-Mediated, Optogenetic Ablation of Müller Glia Leads to Structural and Functional Changes in the Mouse Retina. PLoS ONE 2013, 8, e76075. [Google Scholar] [CrossRef]
- Williams, D.C.; El Bejjani, R.; Ramirez, P.M.; Coakley, S.; Kim, S.A.; Lee, H.; Wen, Q.; Samuel, A.; Lu, H.; Hilliard, M.A.; et al. Rapid and Permanent Neuronal Inactivation In Vivo via Subcellular Generation of Reactive Oxygen with the Use of KillerRed. Cell Rep. 2013, 5, 553–563. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shidara, H.; Morisawa, Y.; Kawakami, M.; Tanahashi, Y.; Hotta, K.; Oka, K. A method for selective ablation of neurons in C. elegans using the phototoxic fluorescent protein, KillerRed. Neurosci. Lett. 2013, 548, 261–264. [Google Scholar] [CrossRef]
- Del Bene, F.; Wyart, C.; Robles, E.; Tran, A.; Looger, L.; Scott, E.K.; Isacoff, E.Y.; Baier, H. Filtering of Visual Information in the Tectum by an Identified Neural Circuit. Science 2010, 330, 669–673. [Google Scholar] [CrossRef]
- Jewhurst, K.; Levin, M.; McLaughlin, K.A. Optogenetic Control of Apoptosis in Targeted Tissues of Xenopus laevis Embryos. J. Cell Death 2014, 7, JCD–S18368. [Google Scholar] [CrossRef][Green Version]
- Liao, Z.-X.; Li, Y.-C.; Lu, H.-M.; Sung, H.-W. A genetically-encoded KillerRed protein as an intrinsically generated photosensitizer for photodynamic therapy. Biomaterials 2014, 35, 500–508. [Google Scholar] [CrossRef]
- Shirmanova, M.V.; Serebrovskaya, E.O.; Lukyanov, K.A.; Snopova, L.B.; Sirotkina, M.A.; Prodanetz, N.N.; Bugrova, M.; Minakova, E.A.; Turchin, I.V.; Kamensky, V.A.; et al. Phototoxic effects of fluorescent protein KillerRed on tumor cells in mice. J. Biophotonics 2013, 6, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.S.; Shirmanova, M.V.; Dudenkova, V.V.; Subochev, P.V.; Turchin, I.V.; Zagaynova, E.V.; Lukyanov, S.A.; Shakhov, B.E.; Kamensky, V.A. Photobleaching and phototoxicity of KillerRed in tumor spheroids induced by continuous wave and pulsed laser illumination. J. Biophotonics 2015, 8, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kanada, M.; Zhang, J.; Okazaki, S.; Terakawa, S. Photodynamic Treatment of Tumor with Bacteria Expressing KillerRed. PLoS ONE 2015, 10, e0131518. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.I.; Proshkina, G.M.; Chumakov, S.P.; Khodarovich, Y.; Deyev, S.M. Flavoprotein miniSOG Cytotoxisity Can Be Induced By Bioluminescence Resonance Energy Transfer. Acta Naturae 2016, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Shramova, E.I.; Proshkina, G.M.; Deyev, S.; Petrov, R.V. Flavoprotein miniSOG BRET-induced cytotoxicity depends on its intracellular localization. Dokl. Biochem. Biophys. 2017, 474, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Proshkina, G.M.; Shramova, E.; Shilova, O.; Ryabova, A.; Deyev, S. Phototoxicity of flavoprotein miniSOG induced by bioluminescence resonance energy transfer in genetically encoded system NanoLuc-miniSOG is comparable with its LED-excited phototoxicity. J. Photochem. Photobiol. B Biol. 2018, 188, 107–115. [Google Scholar] [CrossRef]
- Ishimoto, T.; Mori, H. A new bioluminescence-based tool for modulating target proteins in live cells. Sci. Rep. 2019, 9, 18239. [Google Scholar] [CrossRef]
- Gerhardt, K.P.; Olson, E.J.; Castillo-Hair, S.M.; Hartsough, L.A.; Landry, B.P.; Ekness, F.; Yokoo, R.; Gomez, E.J.; Ramakrishnan, P.; Suh, J.; et al. An open-hardware platform for optogenetics and photobiology. Sci. Rep. 2016, 6, 35363. [Google Scholar] [CrossRef]
- Agarwal, S.R.; Yang, P.-C.; Rice, M.; Singer, C.A.; Nikolaev, V.O.; Lohse, M.J.; Clancy, C.E.; Harvey, R.D. Role of Membrane Microdomains in Compartmentation of cAMP Signaling. PLoS ONE 2014, 9, e95835. [Google Scholar] [CrossRef]
- Rich, T.C.; Fagan, K.A.; Tse, T.E.; Schaack, J.; Cooper, D.M.F.; Karpen, J.W. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc. Natl. Acad. Sci. USA 2001, 98, 13049–13054. [Google Scholar] [CrossRef]
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research — still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Naim, N.; White, A.D.; Reece, J.M.; Wankhede, M.; Zhang, X.; Vilardaga, J.-P.; Altschuler, D.L. Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis. J. Biol. Chem. 2018, 294, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Recent advances and opportunities in synthetic logic gates engineering in living cells. Syst. Synth. Biol. 2014, 8, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qian, K.; Huang, Y.; Jin, N.; Lai, H.; Zhang, T.; Li, C.; Zhang, C.; Bi, X.; Wu, D.; et al. SynBioLGDB: A resource for experimentally validated logic gates in synthetic biology. Sci. Rep. 2015, 5, 8090. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Cho, K.F.; Kim, M.W.; Ting, A.Y. Luciferase-LOV BRET enables versatile and specific transcriptional readout of cellular protein-protein interactions. eLife 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Parag-Sharma, K.; O’Banion, C.P.; Henry, E.C.; Musicant, A.M.; Cleveland, J.L.; Lawrence, D.S.; Amelio, A.L. Engineered BRET-Based Biologic Light Sources Enable Spatiotemporal Control over Diverse Optogenetic Systems. ACS Synth. Biol. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Chen, F.; Warnock, R.L.; Van Der Meer, J.R.; Wegner, S. Bioluminescence-Triggered Photoswitchable Bacterial Adhesions Enable Higher Sensitivity and Dual-Readout Bacterial Biosensors for Mercury. ACS Sensors 2020, 5, 2205–2210. [Google Scholar] [CrossRef]
- Wood, K.V. The bioluminescence advantage. Promega Notes 2007, 96, 3–5. [Google Scholar]
- Landau, J.; Cameron, J.; Worthington, S.S. Avatar (Film); 20th Century Fox: Los Angeles, CA, USA, 2009. [Google Scholar]
- Chait, R.; Ruess, J.; Bergmiller, T.; Tkačik, G.; Guet, C.C. Shaping bacterial population behavior through computer-interfaced control of individual cells. Nat. Commun. 2017, 8, 1535. [Google Scholar] [CrossRef]
- Lugagne, J.-B.; Dunlop, M.J. Cell-machine interfaces for characterizing gene regulatory network dynamics. Curr. Opin. Syst. Biol. 2019, 14, 1–8. [Google Scholar] [CrossRef]
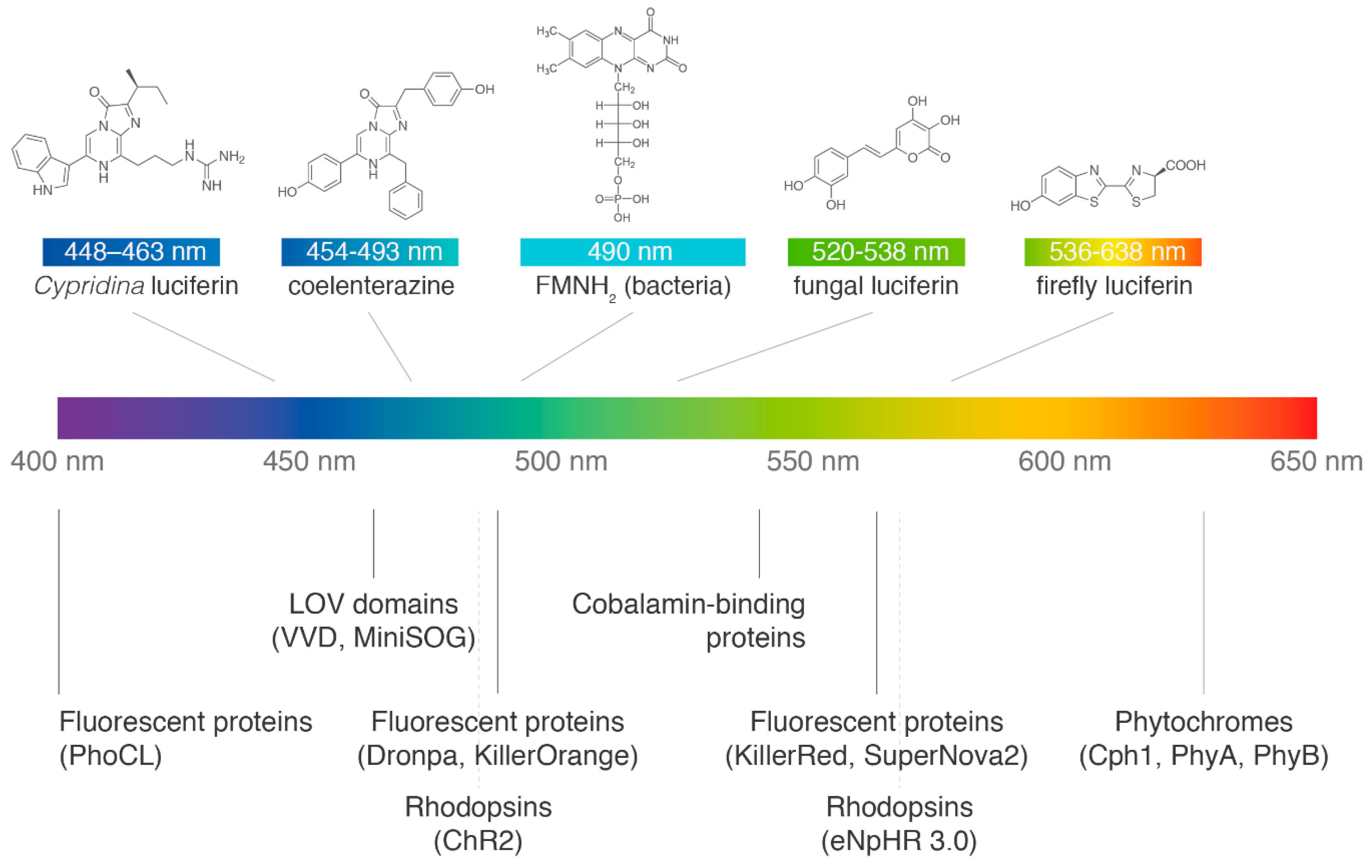

| Bioluminescence System | Summary |
|---|---|
| Lux Operon from Bacteria | The bacterial luciferase uses long-chain aldehydes and reduced flavin mononucleotide (FMNH2) to emit cyan light (490 nm). The bioluminescence pathway is encoded in a multicistronic lux operon that contains all the necessary genes to ensure a constant glow when transformed into other bacteria: two luciferase subunits, the three constituents of the fatty acid reductase complex, and a flavin reductase enzyme [16]. The brightest engineered version of the operon currently available is iLux [17]. While expression of the bacterial system in eukaryotic cells has been historically cumbersome [18], an improved version for mammalian cells has recently been reported [5]. |
| Caffeic Acid Cycle from Fungi | The fungal bioluminescence pathway was recently elucidated, becoming the first autonomous eukaryotic system available. The fungal luciferin is 3-hydroxyhispidin, a styryl pyrone that can be produced from caffeic acid in two enzymatic steps catalysed by the hispidin synthase (HispS) and hispidin-3-hydroxylase (H3H). Fungal luciferin emits green light (520 nm) upon oxidation by the luciferase Luz, and is recycled into caffeic acid by the fourth enzyme of the pathway, caffeoyl pyruvate hydrolase (CPH) [4]. In organisms lacking caffeic acid, fungal luciferin can be produced from tyrosine, with two extra enzymatic steps [4]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sureda-Vives, M.; Sarkisyan, K.S. Bioluminescence-Driven Optogenetics. Life 2020, 10, 318. https://doi.org/10.3390/life10120318
Sureda-Vives M, Sarkisyan KS. Bioluminescence-Driven Optogenetics. Life. 2020; 10(12):318. https://doi.org/10.3390/life10120318
Chicago/Turabian StyleSureda-Vives, Macià, and Karen S. Sarkisyan. 2020. "Bioluminescence-Driven Optogenetics" Life 10, no. 12: 318. https://doi.org/10.3390/life10120318
APA StyleSureda-Vives, M., & Sarkisyan, K. S. (2020). Bioluminescence-Driven Optogenetics. Life, 10(12), 318. https://doi.org/10.3390/life10120318






